Enhancement in Heat Transfer Performance of Water Vapor Condensation on Graphene-Coated Copper Surfaces: A Molecular Dynamics Study
Abstract
:1. Introduction
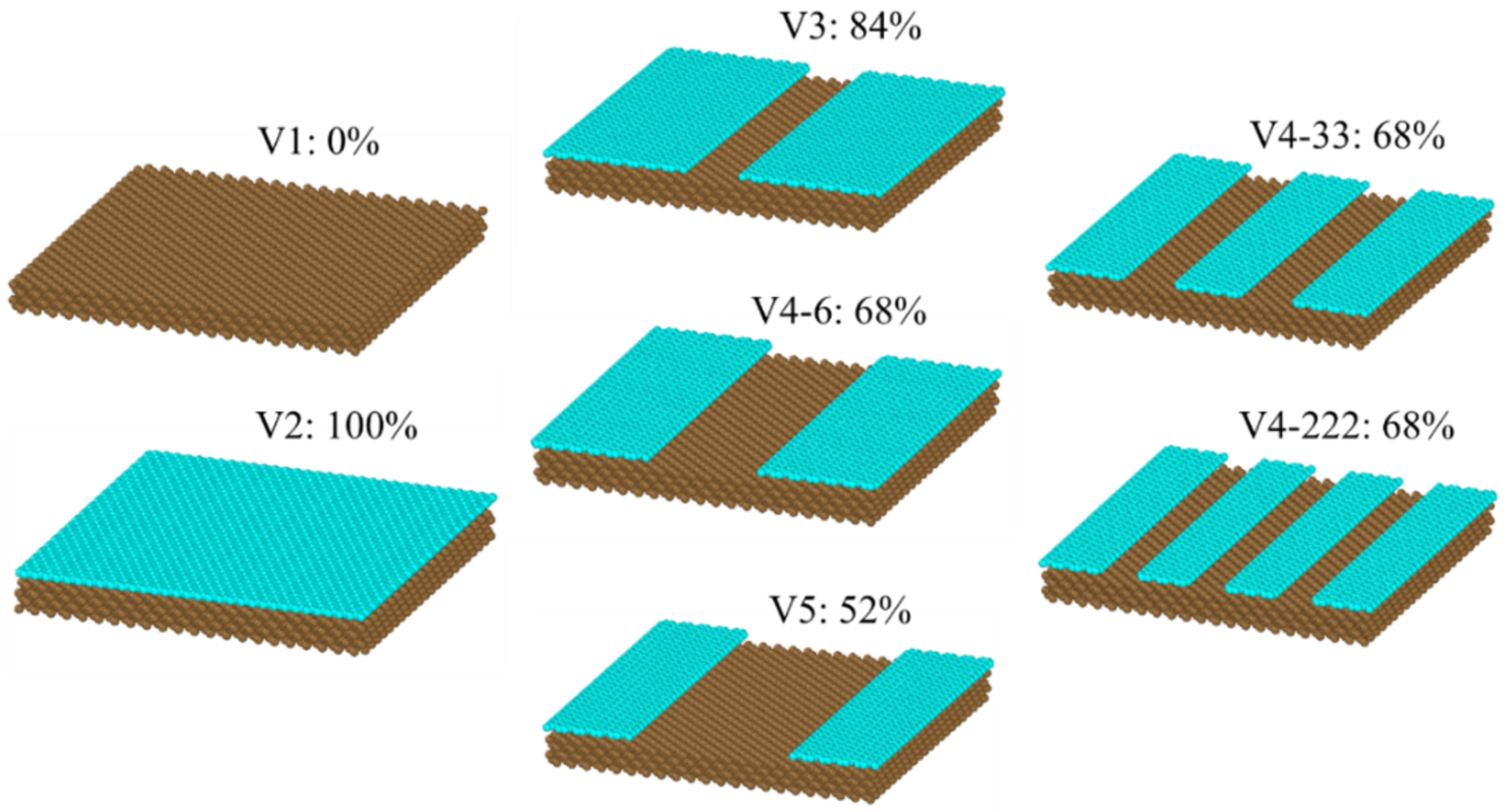
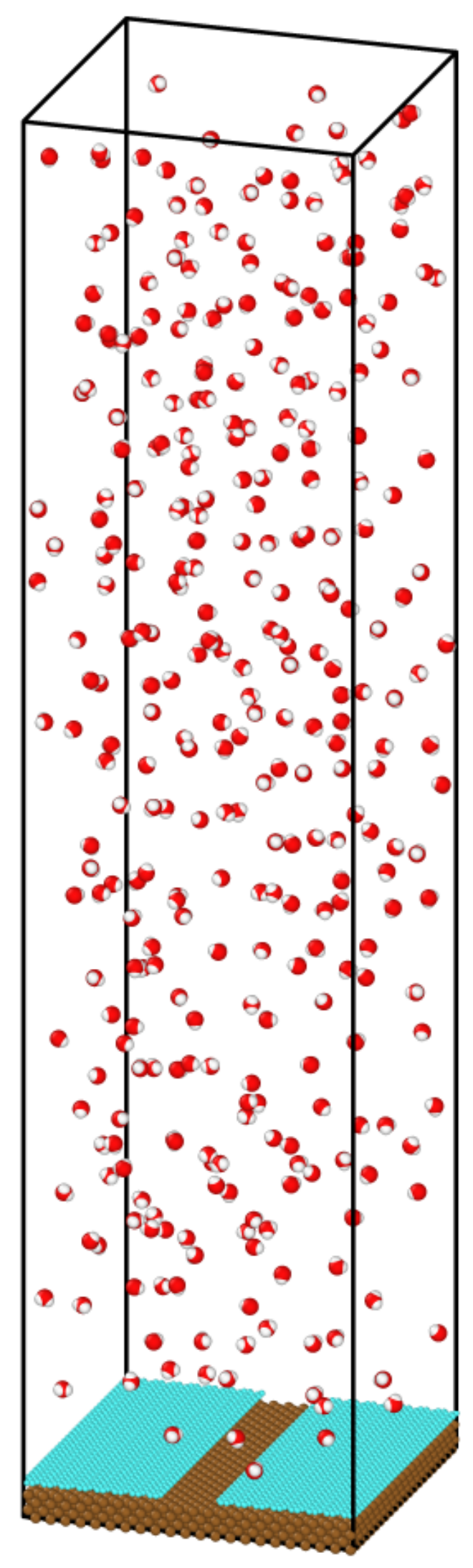
2. Methodology
3. Results
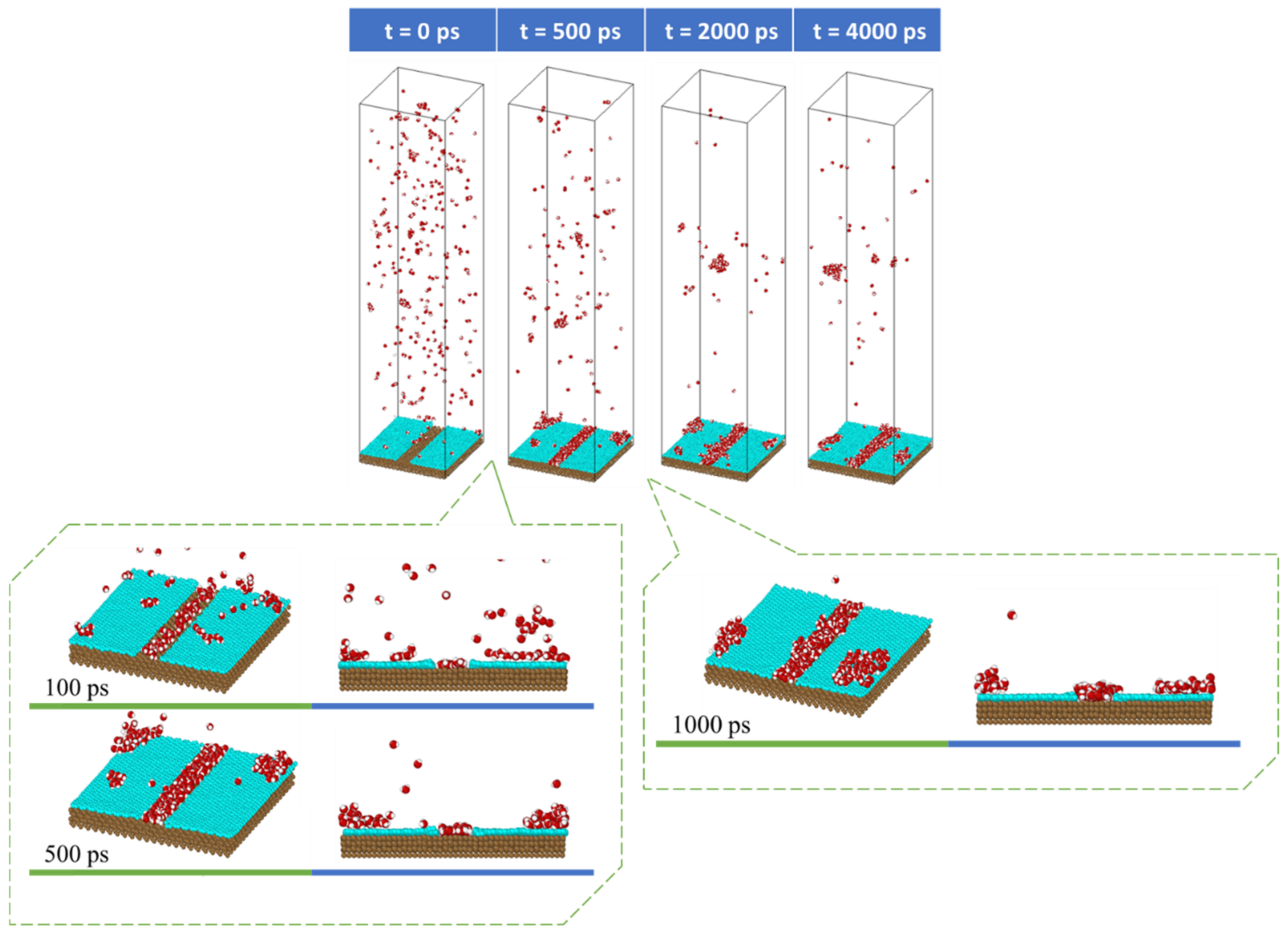
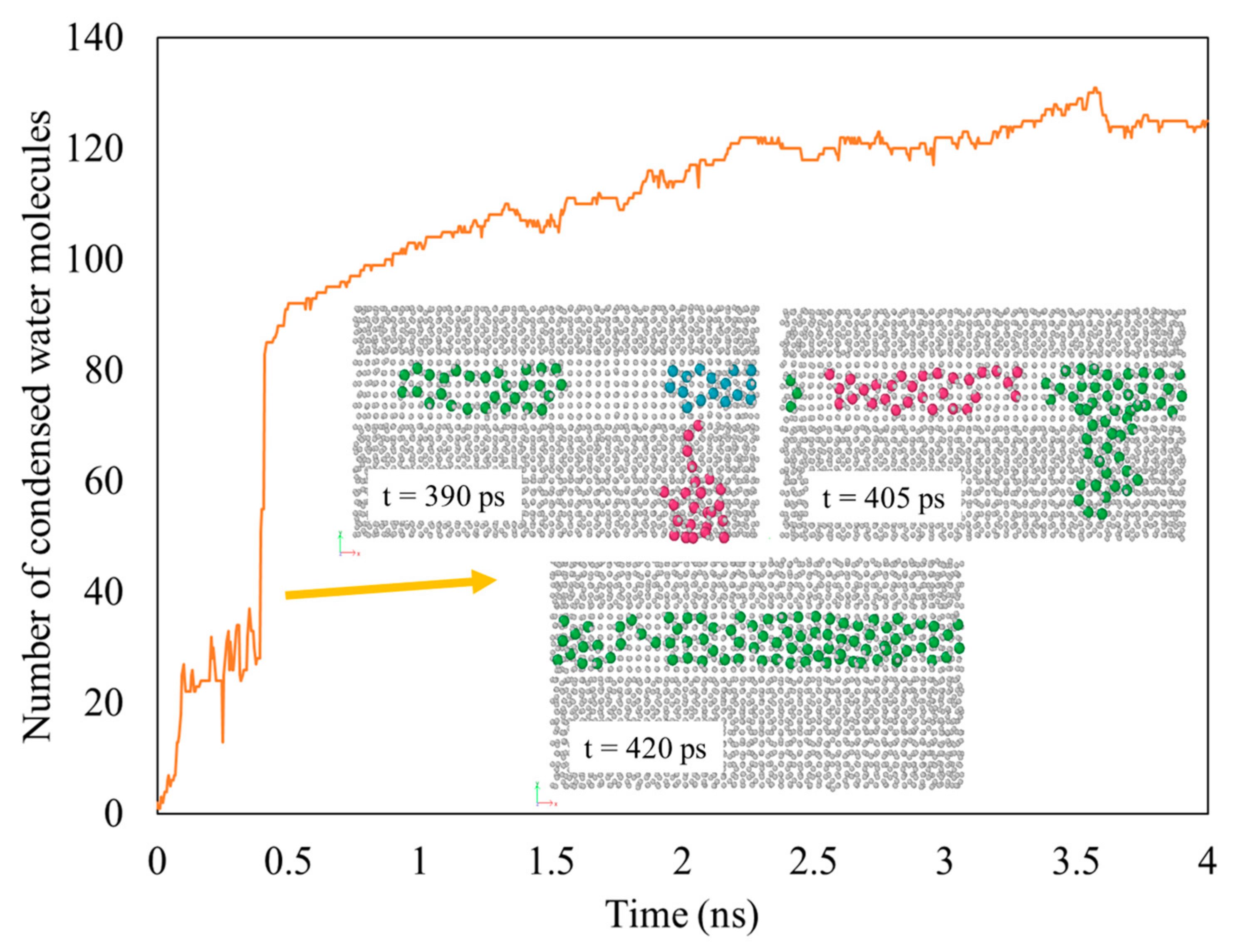
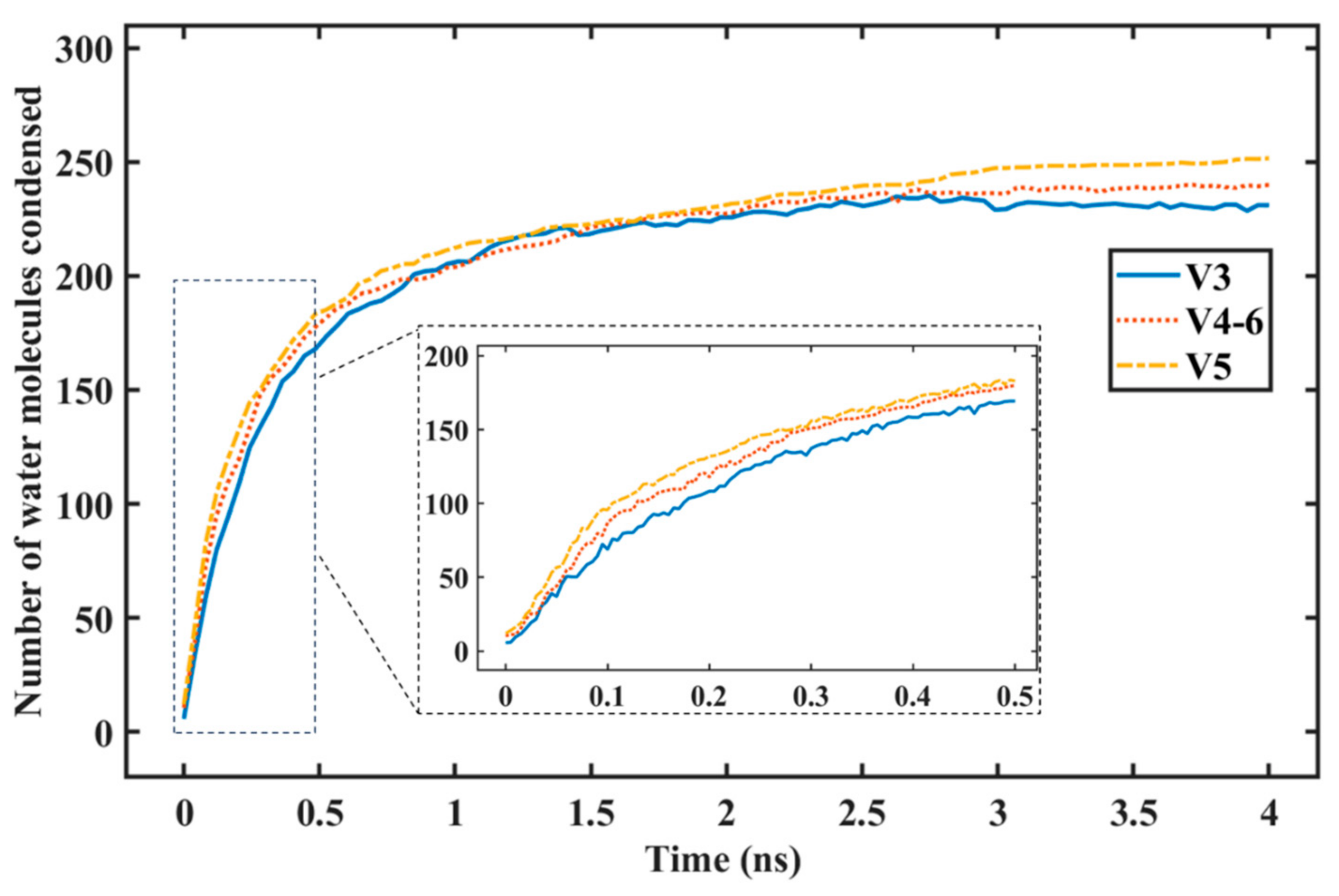
3.1. Rate of Condensation
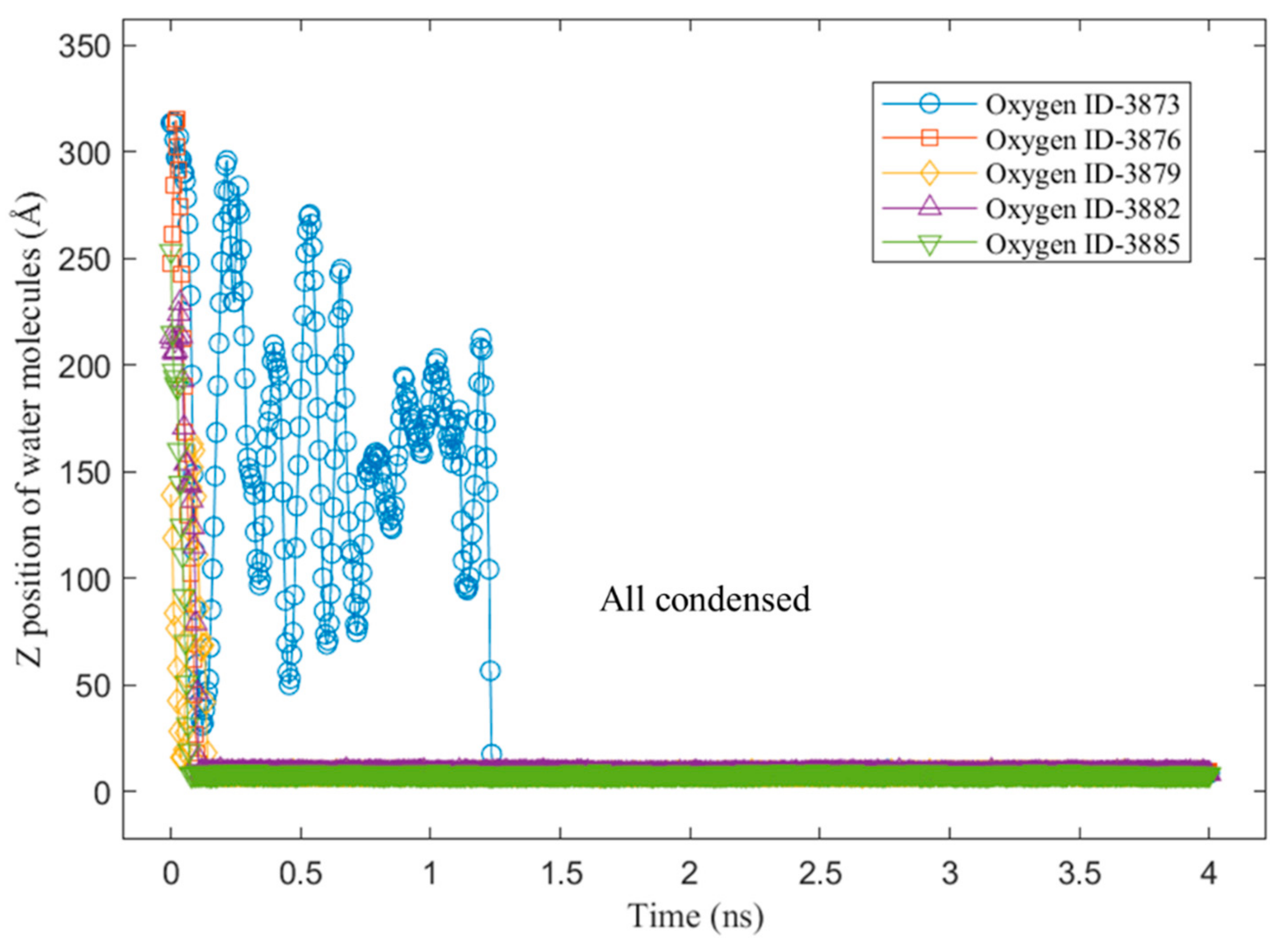
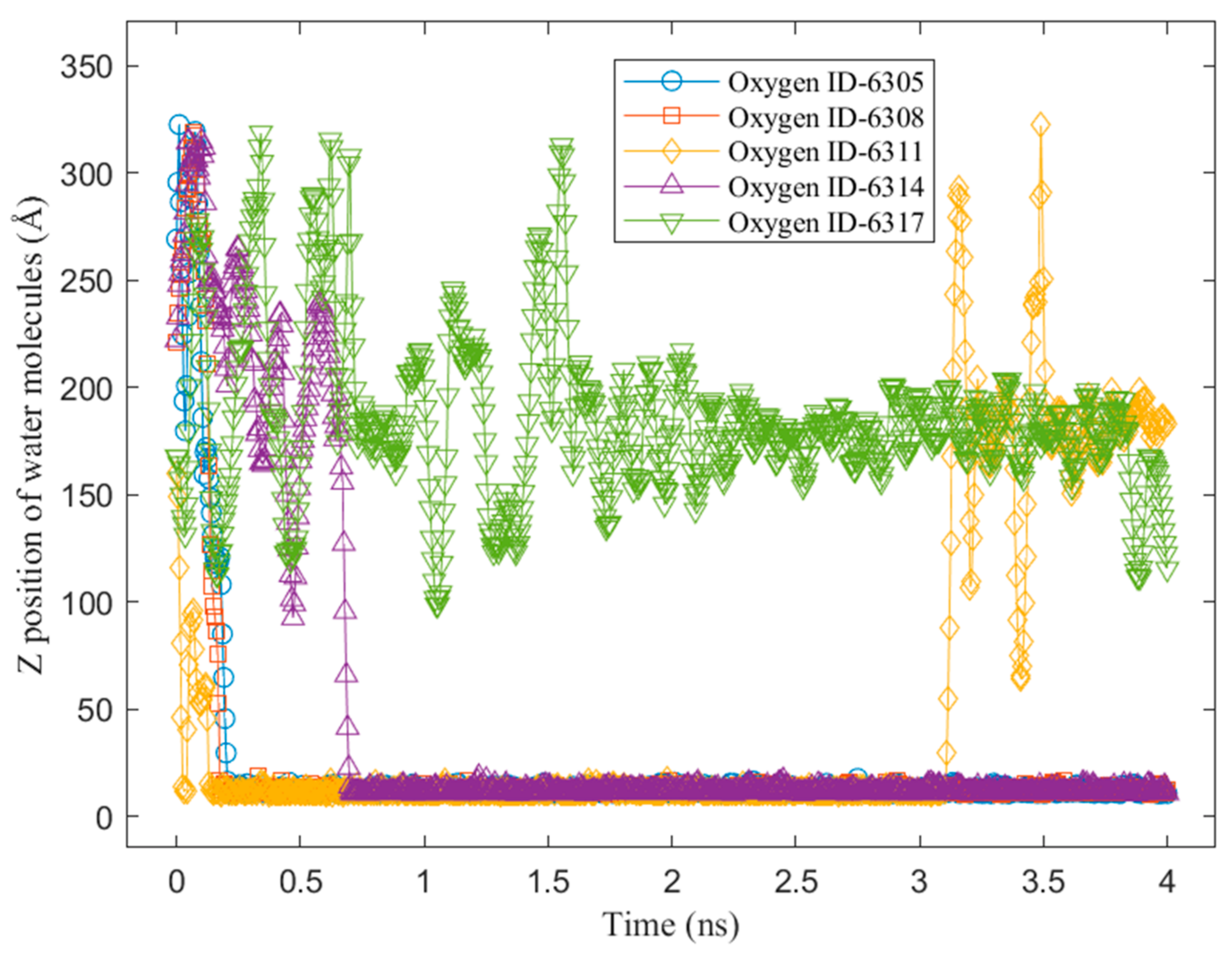
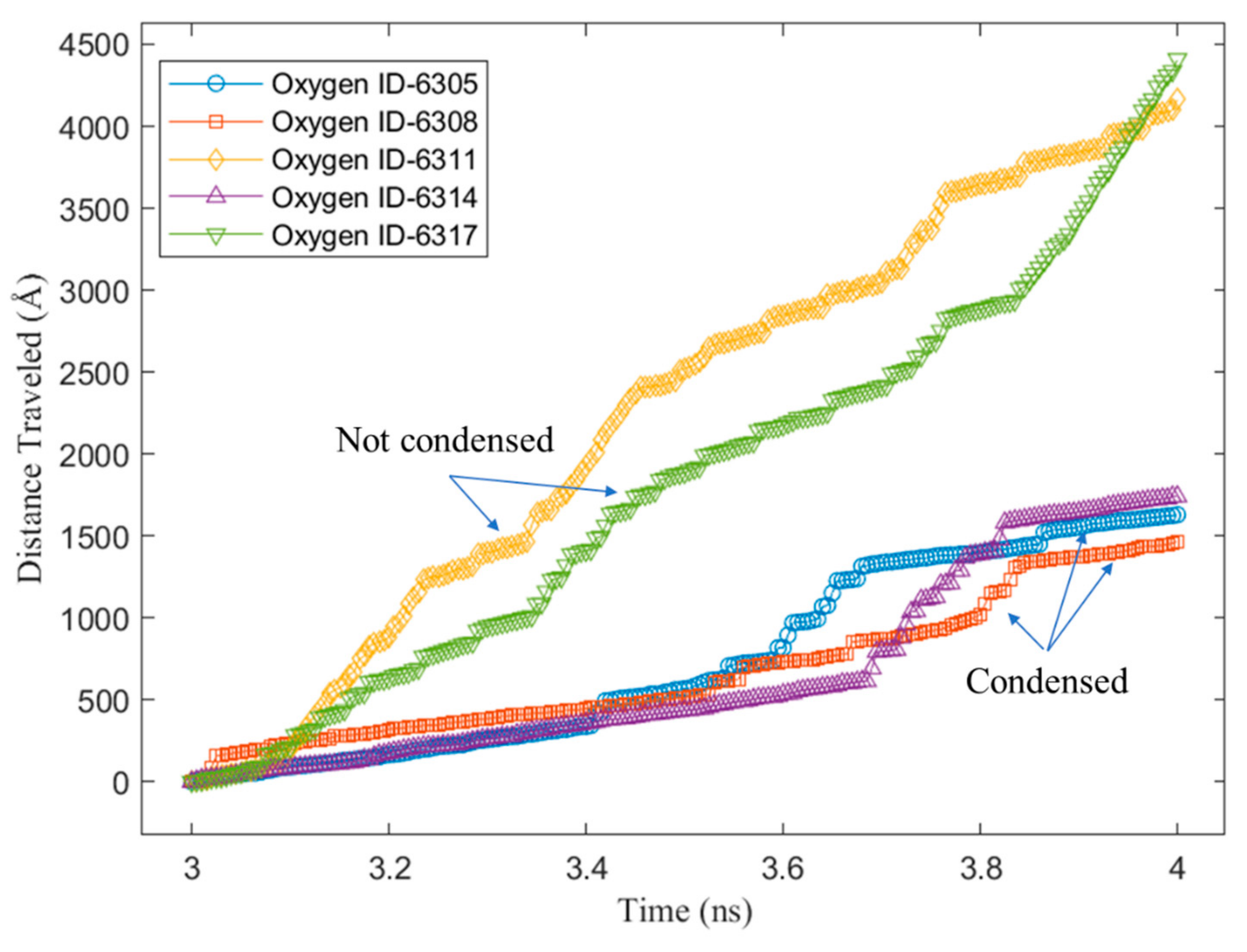
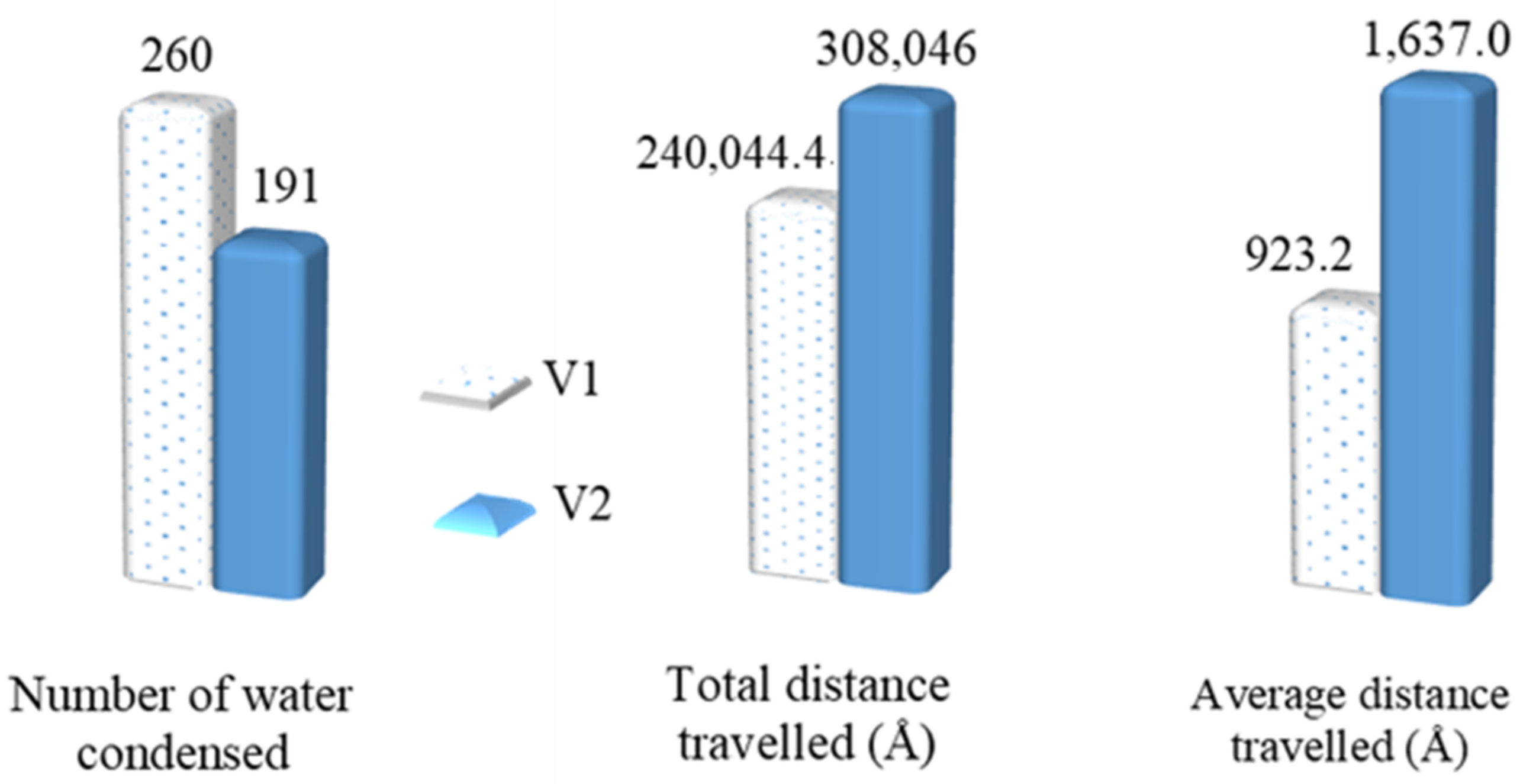
3.2. Mobility of Condensed Water Molecules on Hydrophilic and Hydrophobic Surfaces
- Tracking the water molecules to determine which water molecules were condensed and never returned to the vapor phase during the last 1 ns of the simulation time. This was performed by plotting the z-positions of the oxygen molecules over time.
- Calculating the total x–y distance of those particles in point a) over the last 1 ns of the simulation time.
- Averaging the calculated total x–y distance of all the water molecules during the last 1 ns of the simulation time.
3.3. Heat Transfer Rate
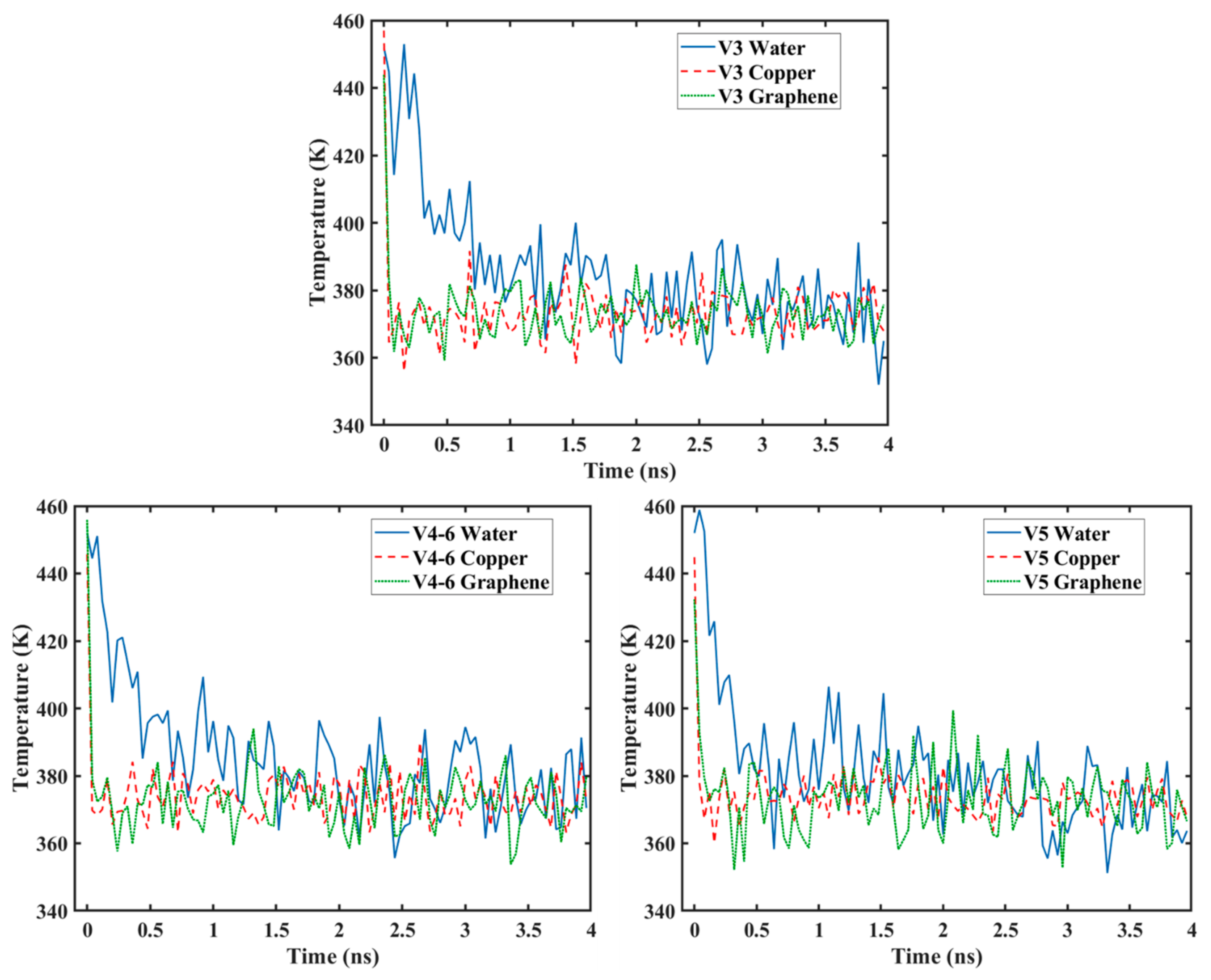
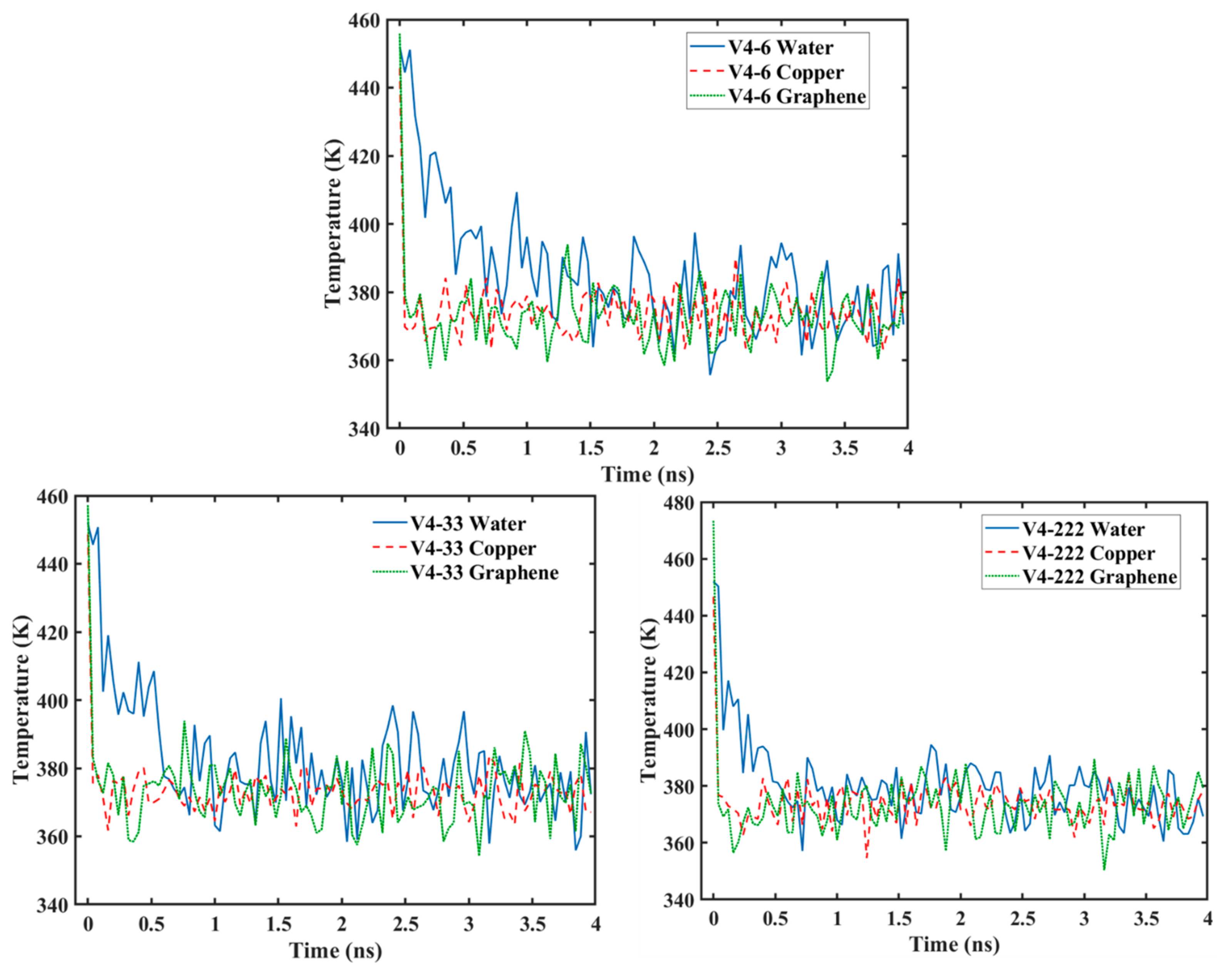
3.4. Temperature Variation
3.5. Vibrational Density of States
4. Conclusions
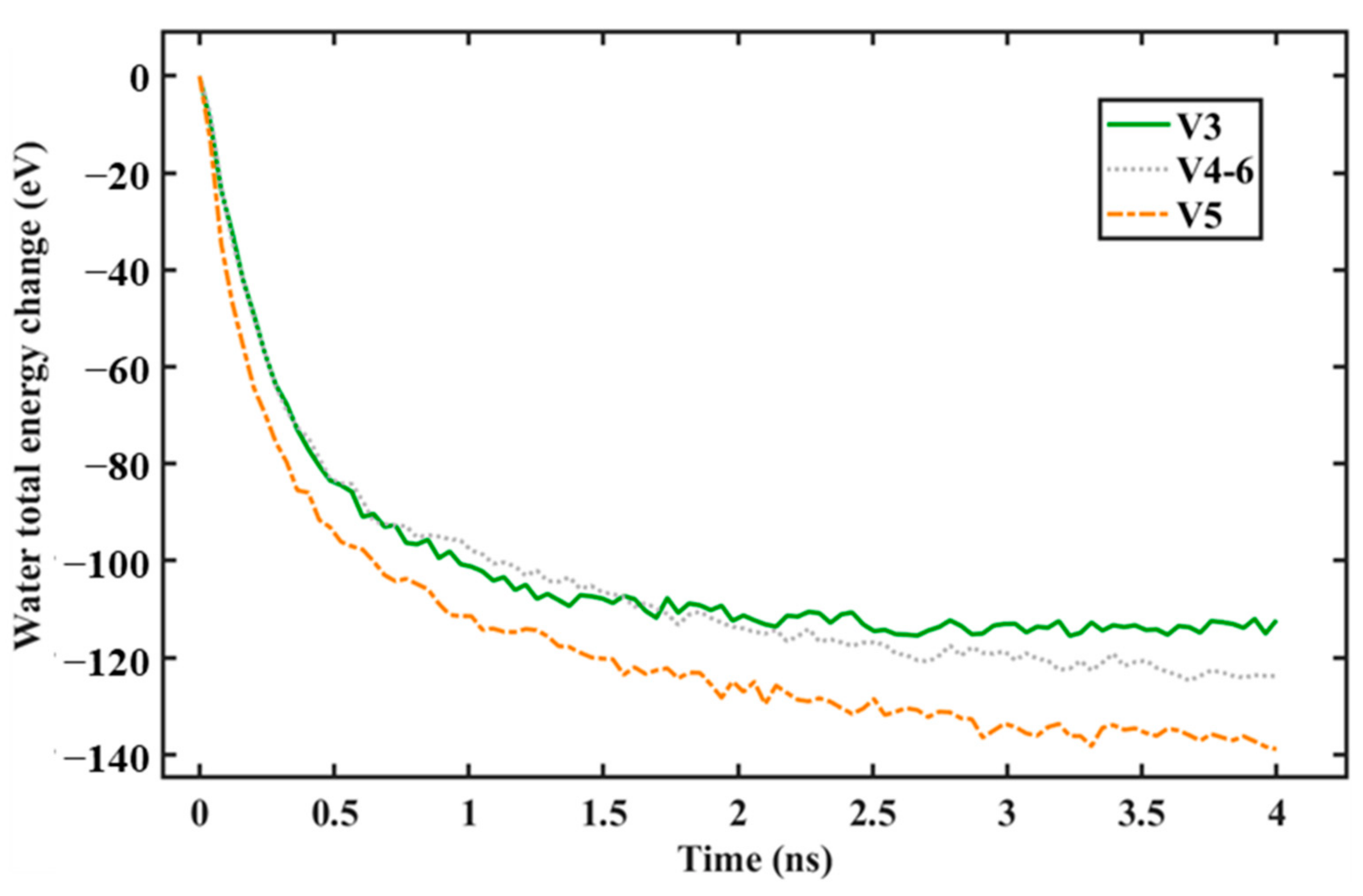
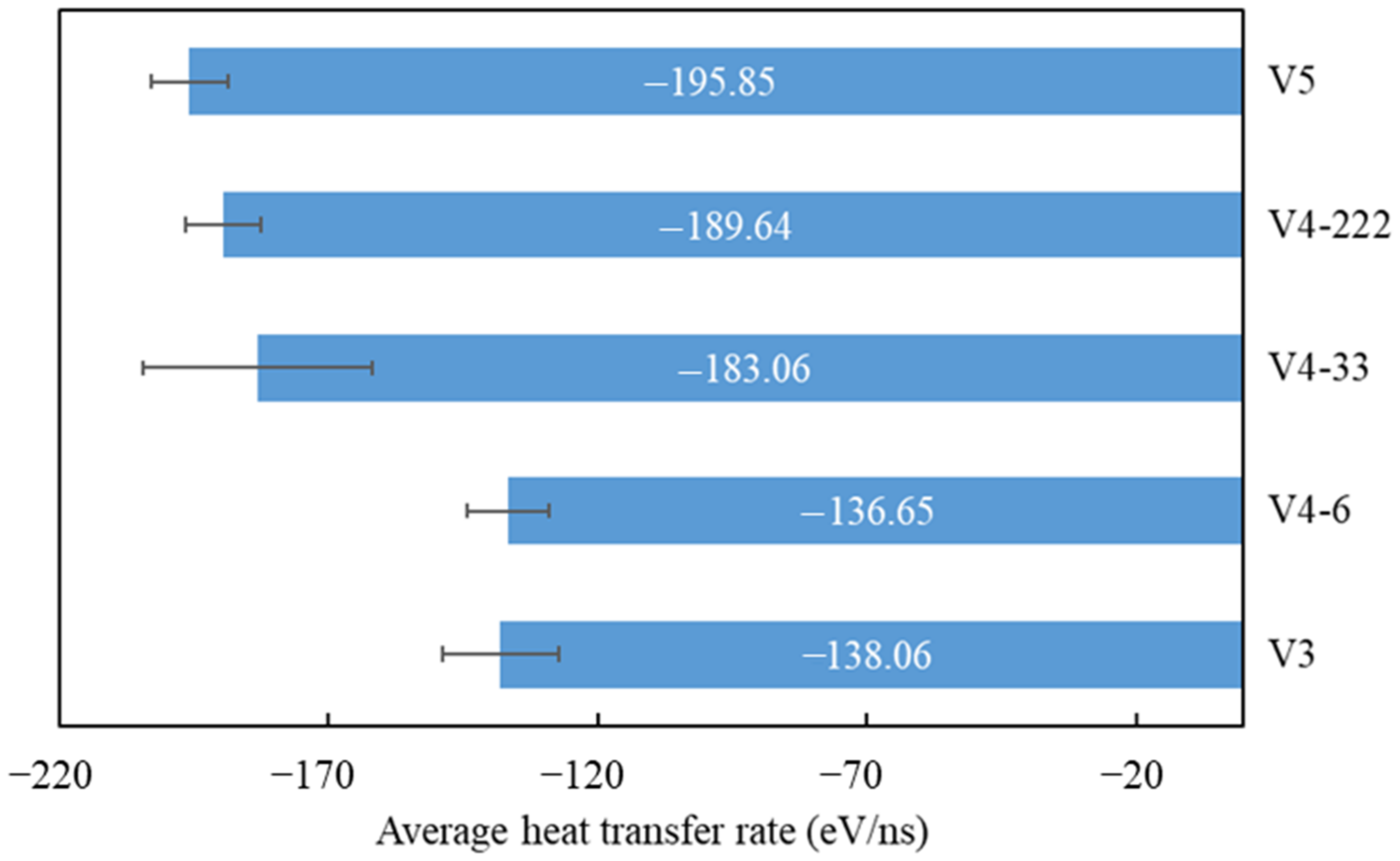
- As the graphene coverage decreased, the water condensation and heat transfer rate increased. By using graphene coverages of 84%, 68%, and 52%, the numbers of condensed water molecules on the surface were 664, 735, and 880, respectively, while the heat transfer rates tended to have an increasing trend, which were −138.06, −136.65, and −195.85 eV/ns, respectively.
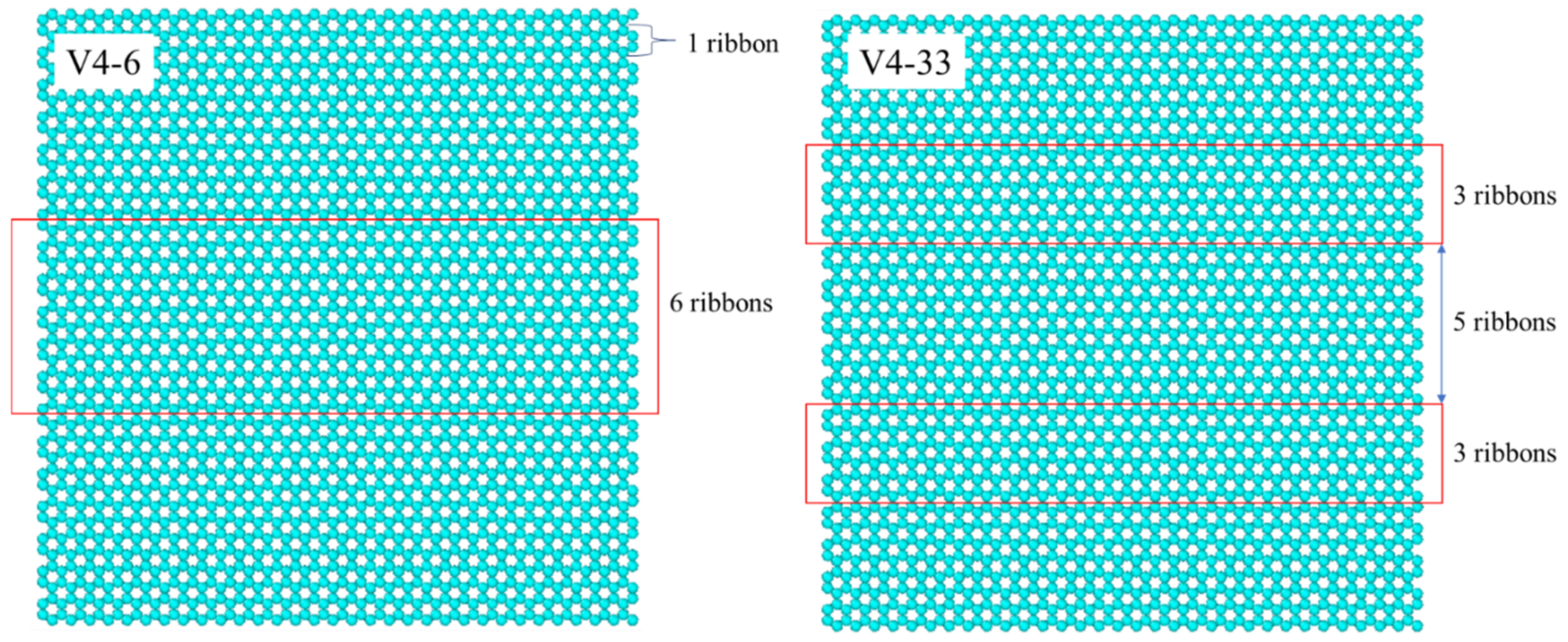
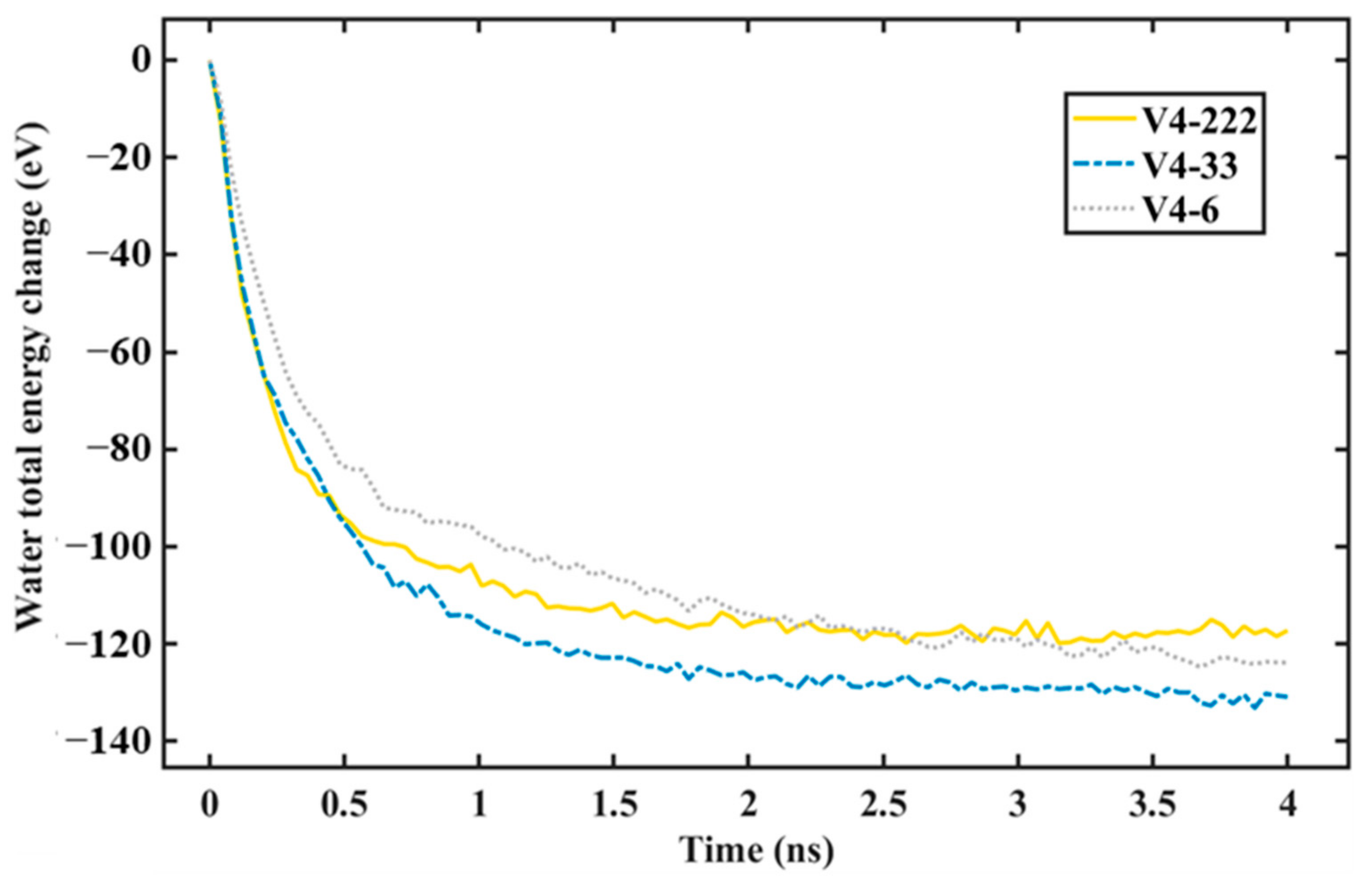
- As the graphene coverage was more distributed, the water condensation and heat transfer rate increased. By using the same graphene coverage of 68% with one, two, and three exposed copper regions in the middle, the numbers of condensed water molecules on the surface were 735, 868, and 898, respectively, while the heat transfer rates had an increasing trend, with values of −136.65, −183.06, and −189.64 eV/ns, respectively.
- As the graphene coverage decreased or became more distributed, the temperature of the water molecules dropped faster.
- The results also showed that the water mobility on the hydrophobic surface contributed to faster droplet growth on the hydrophilic region.
- The VDOS analysis showed that the overall condensation performance was strongly related to the heat transfer between the graphene coating and the copper surface. By using graphene coverages of 84%, 68%, and 52%, the overlap factors were 0.559, 0.564, and 0.567, respectively. By using a more distributed graphene coating with one, two, and three exposed copper regions in the middle, the overlap factors were 0.564, 0.594, and 0.620, respectively. These values show that distribution played a more important role than coverage.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozkan, O.; Wikramanayake, E.D.; Bahadur, V. Modeling humid air condensation in waste natural gas-powered atmospheric water harvesting systems. Appl. Therm. Eng. 2017, 118, 224–232. [Google Scholar] [CrossRef]
- Baghel, V.; Sikarwar, B.S.; Muralidhar, K. Dropwise condensation from moist air over a hydrophobic metallic substrate. Appl. Therm. Eng. 2020, 181, 115733. [Google Scholar] [CrossRef]
- Zhong, Z.; Ma, W.; Yao, S.; Niu, J.; Xu, X. Condensation heat transfer between a vertical superhydrophobic aluminum surface and moist air under natural convection. Appl. Therm. Eng. 2023, 229, 120591. [Google Scholar] [CrossRef]
- Wang, M.; Liu, E.; Jin, T.; Zafar, S.; Mei, X.; Fauconnier, M.-L.; De Clerck, C. Towards a better understanding of atmospheric water harvesting (AWH) technology. Water Res. 2024, 250, 121052. [Google Scholar] [CrossRef]
- Brunetti, A.; Macedonio, F.; Barbieri, G.; Drioli, E. Membrane condenser as emerging technology for water recovery and gas pre-treatment: Current status and perspectives. BMC Chem. Eng. 2019, 1, 19. [Google Scholar] [CrossRef]
- Frappa, M.; Brunetti, A.; Drioli, E.; Cui, Z.; Pan, J.; Macedonio, F. Membrane condenser for particulate abatement from waste-gaseous streams. J. Membr. Sci. Res. 2020, 6, 81–89. [Google Scholar] [CrossRef]
- Qaterji, A.M.; Salilih, E.M.; Siddiqui, M.E.; Almatrafi, E.; Nurrohman, N.; Abulkhair, H.; Alsaiari, A.; Macedonio, F.; Wang, Z.; Albeirutty, M.; et al. Development of an integrated membrane condenser system with LNG cold energy for water recovery from humid flue gases in power plants. Int. J. Hydrogen Energy 2023, 48, 30791–30803. [Google Scholar] [CrossRef]
- Salilih, E.M.; Bamaga, O.; Almatrafi, E.; Abulkhair, H.; Alhumade, H.; Bamasag, A.; Ahmed, I.; Alsaiari, A.; Nurrohman; Albeirutty, M. Performance analysis of a novel absorption-refrigeration driven membrane condenser system for recovery of water and waste heat from flue gas combustion. Int. J. Refrig. 2023, 156, 219–231. [Google Scholar] [CrossRef]
- Ji, C.; Li, L. Improving heat transfer and water recovery performance in high-moisture flue gas condensation using silicon carbide membranes. Int. J. Energy Res. 2021, 45, 10974–10988. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Chen, H.; Gao, D. Advances, challenges and perspectives of using transport membrane condenser to recover moisture and waste heat from flue gas. Sep. Purif. Technol. 2022, 285, 120331. [Google Scholar] [CrossRef]
- Macedonio, F.; Frappa, M.; Brunetti, A.; Barbieri, G.; Drioli, E. Recovery of water and contaminants from cooling tower plume. Environ. Eng. Res. 2020, 25, 222–229. [Google Scholar] [CrossRef]
- Kim, J.F.; Park, A.; Kim, S.-J.; Lee, P.; Cho, Y.; Park, H.; Nam, S.; Park, Y. Harnessing Clean Water from Power Plant Emissions Using Membrane Condenser Technology. ACS Sustain. Chem. Eng. 2018, 6, 6425–6433. [Google Scholar] [CrossRef]
- Iulianelli, A.; Drioli, E. Membrane engineering: Latest advancements in gas separation and pre-treatment processes, petrochemical industry and refinery, and future perspectives in emerging applications. Fuel Process. Technol. 2020, 206, 106464. [Google Scholar] [CrossRef]
- Kim, J.F.; Drioli, E. Transport membrane condenser heat exchangers to break the water-energy nexus—A critical review. Membranes 2020, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Mejri, A.; Arroyo, N.; Herlem, G.; Palmeri, J.; Manghi, M.; Henn, F.; Picaud, F. Impact of Single-Walled Carbon Nanotube Functionalization on Ion and Water Molecule Transport at the Nanoscale. Nanomaterials 2024, 14, 117. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xiao, Y.; Shi, X.; Song, S. Rapid evaporation of water on graphene/graphene-oxide: A molecular dynamics study. Nanomaterials 2017, 7, 265. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lin, D.; Zhao, W. Electric field induced dewetting of hydrophobic nanocavities at ambient temperature. Nanomaterials 2020, 10, 736. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Lan, Z.; Peng, B.L.; Wen, R.F.; Ma, X.H. Effect of surface free energies on the heterogeneous nucleation of water droplet: A molecular dynamics simulation approach. J. Chem. Phys. 2015, 142, 054701. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Tang, G.H. The effect of surface wettability on water vapor condensation in nanoscale. Sci. Rep. 2016, 6, 19192. [Google Scholar] [CrossRef]
- Gao, S.; Liao, Q.; Liu, W.; Liu, Z. Effects of Solid Fraction on Droplet Wetting and Vapor Condensation: A Molecular Dynamic Simulation Study. Langmuir 2017, 33, 12379–12388. [Google Scholar] [CrossRef]
- Ou, X.; Wang, X.; Lin, Z.; Li, J. Heterogeneous Condensation of Water on the Mica (001) Surface: A Molecular Dynamics Simulation Work. J. Phys. Chem. C 2017, 121, 6813–6819. [Google Scholar] [CrossRef]
- Hiratsuka, M.; Emoto, M.; Konno, A.; Ito, S. Molecular dynamics simulation of the influence of nanoscale structure on waterwetting and condensation. Micromachines 2019, 10, 587. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xie, H.; Hu, Z.; Liu, C. The impact of the electric field on surface condensation of water vapor: Insight from molecular dynamics simulation. Nanomaterials 2019, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, Y.; Chen, L. Molecular dynamics study on the effect of surface wettability on the performance of water vapor condensation. AIP Adv. 2019, 9, 025031. [Google Scholar] [CrossRef]
- Ranathunga, D.T.S.; Shamir, A.; Dai, X.; Nielsen, S.O. Molecular Dynamics Simulations of Water Condensation on Surfaces with Tunable Wettability. Langmuir 2020, 36, 7383–7391. [Google Scholar] [CrossRef]
- Qiang, W.; Lan, Z.; Du, B.; Ren, W.; Xu, W.; Wen, R.; Ma, X. Enrichment Effects Induced by Non-uniform Wettability Surfaces in the Presence of Non-condensable Gas: A Molecular Dynamics Simulation. Langmuir 2022, 38, 10192–10201. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, H.; Jiang, H.; Yang, Y.; Feng, S.; Liang, C.; Jia, Y. A study on the mechanism of water vapour condensation inhibition by nanostructures on the copper surface. J. Mater. Sci. 2022, 57, 20615–20630. [Google Scholar] [CrossRef]
- Nurrohman, N.; Almisbahi, H.; Albeirutty, M.; Bamaga, O.; Almatrafi, E.; Tocci, E. Graphene coating reduces the heat transfer performance of water vapor condensation on copper surfaces: A molecular simulation study. Alexandria Eng. J. 2023, 82, 101–125. [Google Scholar] [CrossRef]
- Xu, B.; Chen, Z. Molecular dynamics study of water vapor condensation on a composite wedge-shaped surface with multi wettability gradients. Int. Commun. Heat Mass Transf. 2019, 105, 65–72. [Google Scholar] [CrossRef]
- Xu, B.; Chen, Z. Condensation on Composite V-Shaped Surface with Different Gravity in Nanoscale. Microgravity Sci. Technol. 2019, 31, 603–613. [Google Scholar] [CrossRef]
- Wang, Z.J.; Wang, S.Y.; Wang, D.Q.; Yang, Y.R.; Wang, X.D.; Lee, D.J. Water vapor condensation on binary mixed substrates: A molecular dynamics study. Int. J. Heat Mass Transf. 2022, 184, 122281. [Google Scholar] [CrossRef]
- Niu, D.; Tang, G. Molecular dynamics simulation of droplet nucleation and growth on a rough surface: Revealing the microscopic mechanism of the flooding mode. RSC Adv. 2018, 8, 24517–24524. [Google Scholar] [CrossRef] [PubMed]
- Preston, D.J.; Mafra, D.L.; Miljkovic, N.; Kong, J.; Wang, E.N. Scalable graphene coatings for enhanced condensation heat transfer. Nano Lett. 2015, 15, 2902–2909. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, J.; Mi, X.; Gullapalli, H.; Thomas, A.V.; Yavari, F.; Shi, Y.; Ajayan, P.M.; Koratkar, N.A. Wetting transparency of graphene. Nat. Mater. 2012, 11, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Singh, E.; Thomas, A.V.; Mukherjee, R.; Mi, X.; Houshmand, F.; Peles, Y.; Shi, Y.; Koratkar, N. Graphene drape minimizes the pinning and hysteresis of water drops on nanotextured rough surfaces. ACS Nano 2013, 7, 3512–3521. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Wu, F.; Wang, R.; Zhu, J.; Gao, X. Microdrop-Assisted Microdomain Hydrophilicization of Superhydrophobic Surfaces for High-Efficiency Nucleation and Self-Removal of Condensate Microdrops. ACS Appl. Mater. Interfaces 2019, 11, 7553–7558. [Google Scholar] [CrossRef]
- Mishchenko, L.; Khan, M.; Aizenberg, J.; Hatton, B.D. Spatial Control of Condensation and Freezing on Superhydrophobic Surfaces with Hydrophilic Patches. Adv. Funct. Mater. 2013, 23, 4577–4584. [Google Scholar] [CrossRef]
- Bai, H.; Wang, L.; Ju, J.; Sun, R.; Zheng, Y.; Jiang, L. Efficient Water Collection on Integrative Bioinspired Surfaces with Star-Shaped Wettability Patterns. Adv. Mater. 2014, 26, 5025–5030. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B.; Mac Giolla Eain, M.; Xu, Q.F.; Egan, V.M.; Punch, J.; Lyons, A.M. Design and Fabrication of a Hybrid Superhydrophobic-Hydrophilic Surface That Exhibits Stable Dropwise Condensation. ACS Appl. Mater. Interfaces 2015, 7, 23575–23588. [Google Scholar] [CrossRef]
- Sun, R.; Zhao, J.; Liu, C.; Yu, N.; Mo, J.; Pan, Y.; Luo, D. Design and optimization of hybrid superhydrophobic–hydrophilic pattern surfaces for improving fog harvesting efficiency. Prog. Org. Coatings 2022, 171, 107016. [Google Scholar] [CrossRef]
- Wenting, K.; Shaohai, F.; Yunlu, P.; Xuezeng, Z. Ultraviolet modificated hydrophobic glass on-demand hydrophobic/superhydrophilic patterned surface for fog harvesting and underwater oil resistance. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 673, 131733. [Google Scholar] [CrossRef]
- Goswami, A.; Pillai, S.C.; McGranaghan, G. Surface modifications to enhance dropwise condensation. Surf. Interfaces 2021, 25, 101143. [Google Scholar] [CrossRef]
- Chen, C.H.; Cai, Q.; Tsai, C.; Chen, C.L.; Xiong, G.; Yu, Y.; Ren, Z. Dropwise condensation on superhydrophobic surfaces with two-tier roughness. Appl. Phys. Lett. 2007, 90, 173108. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, X.; Wang, J.; Wang, Z.; Wang, D.; Tian, J. Enhanced dropwise condensation on heterogeneously hybrid patterned surfaces. Case Stud. Therm. Eng. 2021, 27, 101319. [Google Scholar] [CrossRef]
- Cheng, J.; Vandadi, A.; Chen, C.L. Condensation Heat Transfer on Two-Tier Superhydrophobic Surfaces. ASME Int. Mech. Eng. Congr. Expo. Proc. 2013, 7, 2649–2653. [Google Scholar] [CrossRef]
- Chung, S.; Kadala, K.; Taylor, H. Stable dropwise condensation observed on a hierarchically structured superhydrophobic surface incorporating micro-domes. Microelectron. Eng. 2020, 225, 111252. [Google Scholar] [CrossRef]
- Ma, J.; Sett, S.; Cha, H.; Yan, X.; Miljkovic, N. Recent developments, challenges, and pathways to stable dropwise condensation: A perspective. Appl. Phys. Lett. 2020, 116, 260501. [Google Scholar] [CrossRef]
- Khan, S.A.; Tahir, F.; Baloch, A.A.B.; Koc, M. Review of micro-nanoscale surface coatings application for sustaining dropwise condensation. Coatings 2019, 9, 117. [Google Scholar] [CrossRef]
- Raj, R.; Maroo, S.C.; Wang, E.N. Wettability of graphene. Nano Lett. 2013, 13, 1509–1515. [Google Scholar] [CrossRef]
- Tancon, M.; Mirafiori, M.; Bortolin, S.; Martucci, A.; Del Col, D. Droplet sweeping to enhance heat transfer during dropwise condensation. J. Phys. Conf. Ser. 2021, 2116, 012013. [Google Scholar] [CrossRef]
- Xu, X.; Yi, D.; Wang, Z.; Yu, J.; Zhang, Z.; Qiao, R.; Sun, Z.; Hu, Z.; Gao, P.; Peng, H.; et al. Greatly Enhanced Anticorrosion of Cu by Commensurate Graphene Coating. Adv. Mater. 2018, 30, 1702944. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kim, D.; Kwak, K.; Nagata, Y.; Bonn, M.; Cho, M. Wettability of graphene, water contact angle, and interfacial water structure. Chem 2022, 8, 1187–1200. [Google Scholar] [CrossRef]
- Andersson, P.U.; Suter, M.T.; Marković, N.; Pettersson, J.B.C. Water condensation on graphite studied by elastic helium scattering and molecular dynamics simulations. J. Phys. Chem. C 2007, 111, 15258–15266. [Google Scholar] [CrossRef]
- Sun, N.; Li, J.; Ren, J.; Xu, Z.; Sun, H.; Du, Z.; Zhao, H.; Ettelatie, R.; Cheng, F. Insights into the enhanced flux of graphene oxide composite membrane in direct contact membrane distillation: The different role at evaporation and condensation interfaces. Water Res. 2022, 212, 118091. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Zhang, J.; Nshimiyimana, J.P.; Hu, X.; Wu, P.; Liu, S.; Liu, J.; Chu, W.; Sun, L. Wettability of monolayer graphene/single-walled carbon nanotube hybrid films. RSC Adv. 2017, 7, 48184–48188. [Google Scholar] [CrossRef]
- Sergi, D.; Scocchi, G.; Ortona, A. Molecular dynamics simulations of the contact angle between water droplets and graphite surfaces. Fluid Phase Equilib. 2012, 332, 173–177. [Google Scholar] [CrossRef]
- Ho, T.A.; Striolo, A. Molecular dynamics simulation of the graphene-water interface: Comparing water models. Mol. Simul. 2014, 40, 1190–1200. [Google Scholar] [CrossRef]
- Dutta, R.C.; Khan, S.; Singh, J.K. Wetting transition of water on graphite and boron-nitride surfaces: A molecular dynamics study. Fluid Phase Equilib. 2011, 302, 310–315. [Google Scholar] [CrossRef]
- Alexeev, D.; Chen, J.; Walther, J.H.; Giapis, K.P.; Angelikopoulos, P.; Koumoutsakos, P. Kapitza Resistance between Few-Layer Graphene and Water: Liquid Layering Effects. Nano Lett. 2015, 15, 5744–5749. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Kim, B.H. Stress and surface tension analyses of water on graphene-coated copper surfaces. Int. J. Precis. Eng. Manuf. 2016, 17, 503–510. [Google Scholar] [CrossRef]
- Wang, Z.J.; Wang, S.Y.; Wang, D.Q.; Yang, Y.R.; Wang, X.D.; Lee, D.J. Water vapor condensation on substrates with nanoscale hydrophilic spots: A molecular dynamics study. Int. J. Heat Mass Transf. 2023, 205, 123929. [Google Scholar] [CrossRef]
- López-Lemus, J.; Chapela, G.A.; Alejandre, J. Effect of flexibility on surface tension and coexisting densities of water. J. Chem. Phys. 2008, 128, 174703. [Google Scholar] [CrossRef] [PubMed]
- Frenkle, D.; Smit, B. Understanding Molecular Simulation: From Algorithms to Applications, 2nd ed.; Elsevier Inc.: San Diego, CA, USA, 2002. [Google Scholar]
- Jones, J. On the determination of molecular fields—II. From the equation of state of a gas. In Proceedings of the Royal Society of London; Series A, Containing Papers of a Mathematical and Physical Character; The Royal Society: London, UK, 1924; Volume 106, pp. 463–477. [Google Scholar] [CrossRef]
- Daw, M.S.; Baskes, M.I. Embedded-atom method: Derivation and application to impurities, surfaces, and other defects in metals. Phys. Rev. B 1984, 29, 6443–6453. [Google Scholar] [CrossRef]
- Liu, A.; Stuart, S.J. Empirical bond-order potential for hydrocarbons: Adaptive treatment of van der Waals interactions. J. Comput. Chem. 2008, 29, 601–611. [Google Scholar] [CrossRef]
- Schrader, M.E. Ultrahigh vacuum techniques in the measurement of contact angles. III. Water on copper and silver. J. Phys. Chem. 1974, 78, 87–89. [Google Scholar] [CrossRef]
- Hung, S.W.; Hsiao, P.Y.; Chen, C.P.; Chieng, C.C. Wettability of graphene-coated surface: Free energy investigations using molecular dynamics simulation. J. Phys. Chem. C 2015, 119, 8103–8111. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Chen, Y.; Chan, P.K.L. Investigation of interfacial thermal transport across graphene and an organic semiconductor using molecular dynamics simulations. Phys. Chem. Chem. Phys. 2017, 19, 15933–15941. [Google Scholar] [CrossRef] [PubMed]
- Broitman, E.; Gueorguiev, G.K.; Furlan, A.; Son, N.T.; Gellman, A.J.; Stafström, S.; Hultman, L. Water adsorption on fullerene-like carbon nitride overcoats. Thin Solid Films 2008, 517, 1106–1110. [Google Scholar] [CrossRef]
- Broitman, E.; Furlan, A.; Gueorguiev, G.K.; Czigány, Z.; Tarditi, A.M.; Gellman, A.J.; Stafström, S.; Hultman, L. Water adsorption on phosphorous-carbide thin films. Surf. Coatings Technol. 2009, 204, 1035–1039. [Google Scholar] [CrossRef]




| Interaction | (eV) | (Å) |
|---|---|---|
| Cu-C | 0.0262511 | 2.313 |
| Cu-H | 0.0 | 0.0 |
| Cu-O | 0.0525021 | 2.753 |
| C-H | 0.0 | 0.0 |
| C-O | 0.0054695159 | 3.279 |
| Variant | Rate of Condensation (#Molecules/ns) | Standard Deviation | Coeff. of Variation | |||
|---|---|---|---|---|---|---|
| Seed 1 | Seed 2 | Seed 3 | Average | |||
| V3 | 716 | 586 | 689 | 664 | 56 | 0.084 |
| V4-6 | 694 | 675 | 836 | 735 | 72 | 0.097 |
| V4-33 | 988 | 784 | 832 | 868 | 87 | 0.100 |
| V4-222 | 889 | 898 | 906 | 898 | 7 | 0.007 |
| V5 | 897 | 985 | 757 | 880 | 94 | 0.106 |
| Variant | Heat Transfer Rate (eV/ns) | Standard Deviation | Coeff. of Variation | |||
|---|---|---|---|---|---|---|
| Seed 1 | Seed 2 | Seed 3 | Average | |||
| V3 | −147.77 | −123.2 | −143.21 | −138.06 | 10.67 | −0.077 |
| V4-6 | −127.1 | −137.37 | −145.49 | −136.65 | 7.52 | −0.055 |
| V4-33 | −211.75 | −176.65 | −160.8 | −183.06 | 21.28 | −0.116 |
| V4-222 | −195.76 | −193.16 | −180.02 | −189.64 | 6.88 | −0.036 |
| V5 | −200.39 | −201.49 | −185.69 | −195.85 | 7.20 | −0.036 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurrohman, N.; Almisbahi, H.; Tocci, E.; Abulkhair, H.; Albeirutty, M.; Othman, R.; Bamaga, O. Enhancement in Heat Transfer Performance of Water Vapor Condensation on Graphene-Coated Copper Surfaces: A Molecular Dynamics Study. Nanomaterials 2024, 14, 1137. https://doi.org/10.3390/nano14131137
Nurrohman N, Almisbahi H, Tocci E, Abulkhair H, Albeirutty M, Othman R, Bamaga O. Enhancement in Heat Transfer Performance of Water Vapor Condensation on Graphene-Coated Copper Surfaces: A Molecular Dynamics Study. Nanomaterials. 2024; 14(13):1137. https://doi.org/10.3390/nano14131137
Chicago/Turabian StyleNurrohman, Nurrohman, Hind Almisbahi, Elena Tocci, Hani Abulkhair, Mohammed Albeirutty, Ramzi Othman, and Omar Bamaga. 2024. "Enhancement in Heat Transfer Performance of Water Vapor Condensation on Graphene-Coated Copper Surfaces: A Molecular Dynamics Study" Nanomaterials 14, no. 13: 1137. https://doi.org/10.3390/nano14131137
APA StyleNurrohman, N., Almisbahi, H., Tocci, E., Abulkhair, H., Albeirutty, M., Othman, R., & Bamaga, O. (2024). Enhancement in Heat Transfer Performance of Water Vapor Condensation on Graphene-Coated Copper Surfaces: A Molecular Dynamics Study. Nanomaterials, 14(13), 1137. https://doi.org/10.3390/nano14131137







