Influence of Polypyrrole on Phosphorus- and TiO2-Based Anode Nanomaterials for Li-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
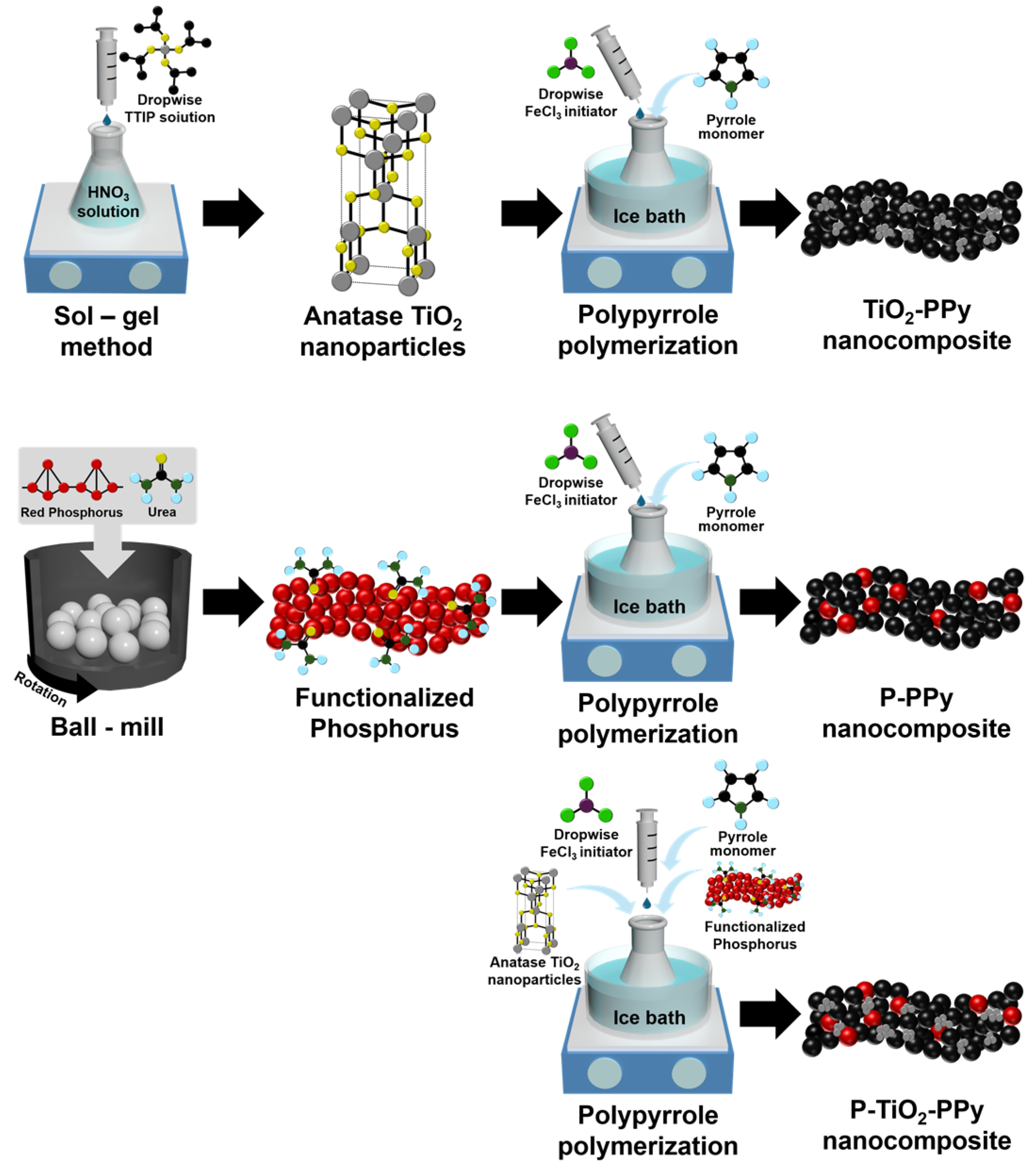
2.2. Synthesis of Urea-Functionalized Phosphorus (P)
2.3. Preparation of TiO2 Nanoparticles
2.4. Synthesis of TiO2–PPy and P–PPy Nanocomposites
2.5. Fabrication of Ternary P–TiO2–PPy Nanocomposite
2.6. Characterization
2.7. Li-Ion Battery Performance
3. Results and Discussion
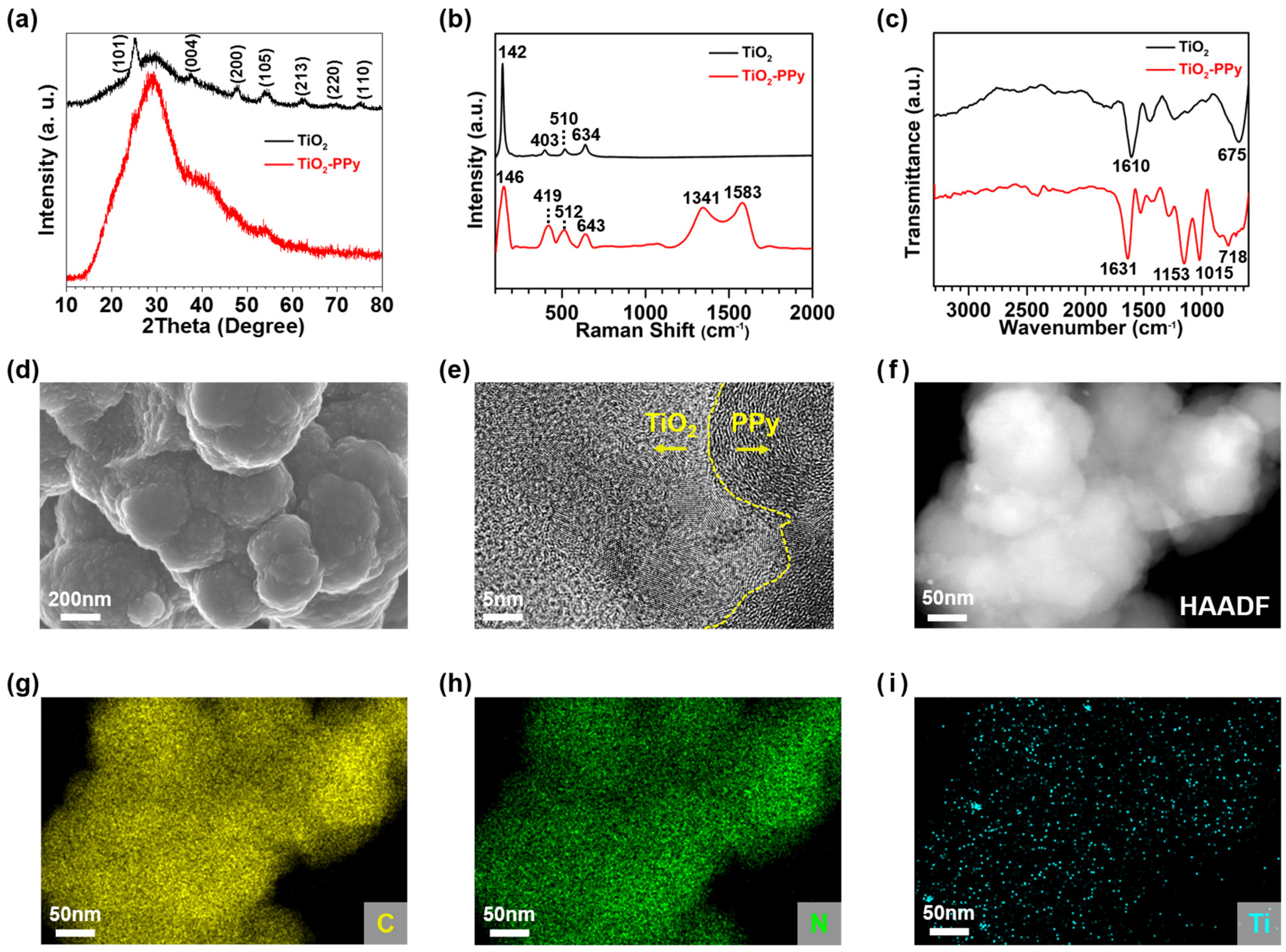
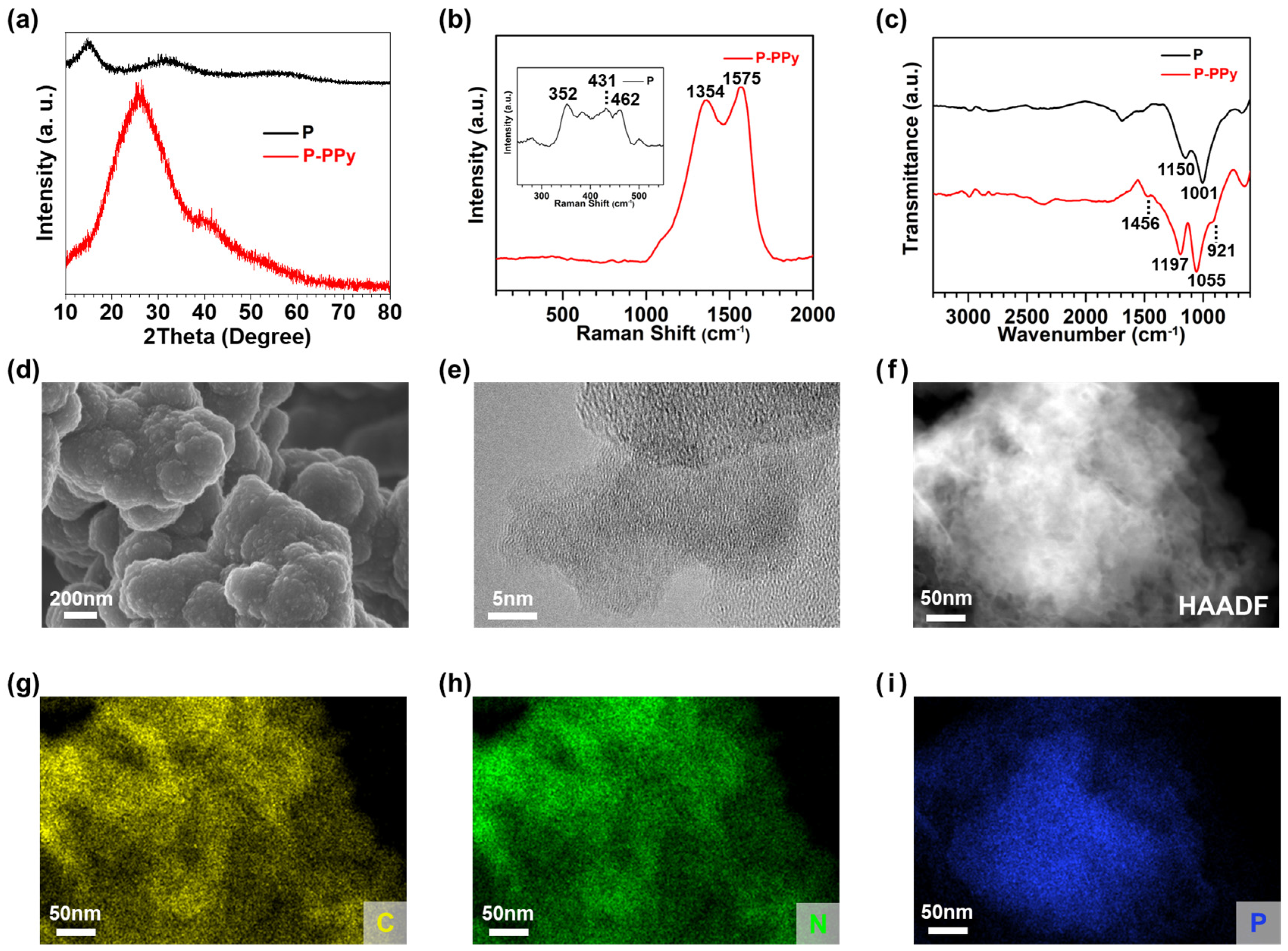
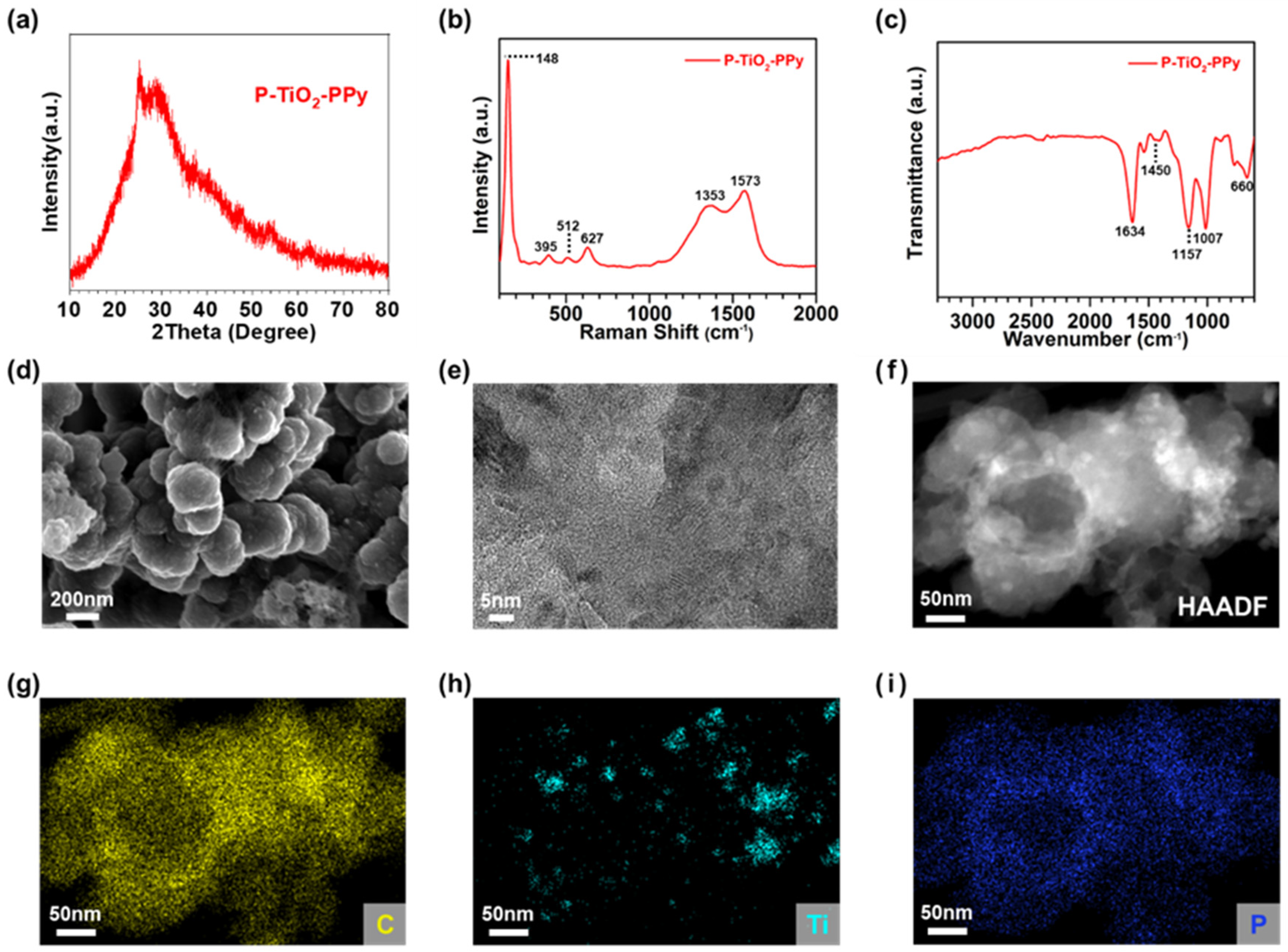
3.1. Synthesis and Characterization of Anode Materials
3.1.1. Synthesis of Single-Component Anodes
3.1.2. Synthesis and Characterization of PPy-Based Composite Anodes
3.2. Li-Ion Battery Performance of PPy-Based Nanocomposite Anodes
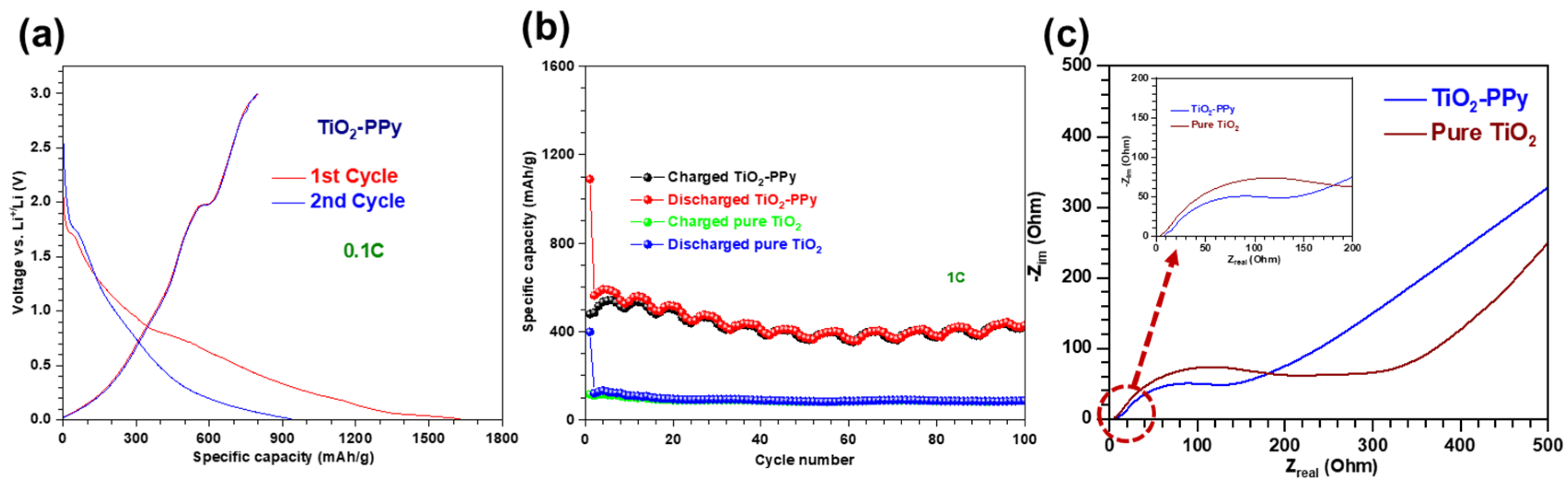
3.2.1. Li-Ion Battery Performance of TiO2–PPy Anodes
3.2.2. Li-Ion Battery Performance of P–PPy
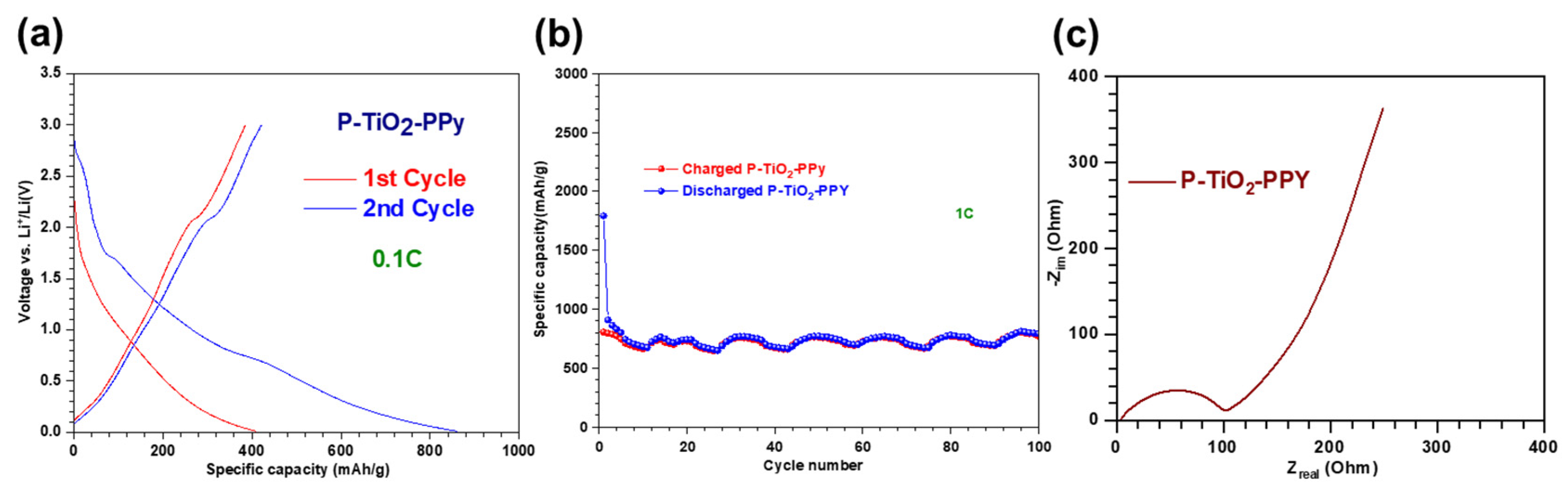
3.2.3. Li-Ion Battery Performance of P–TiO2–PPy
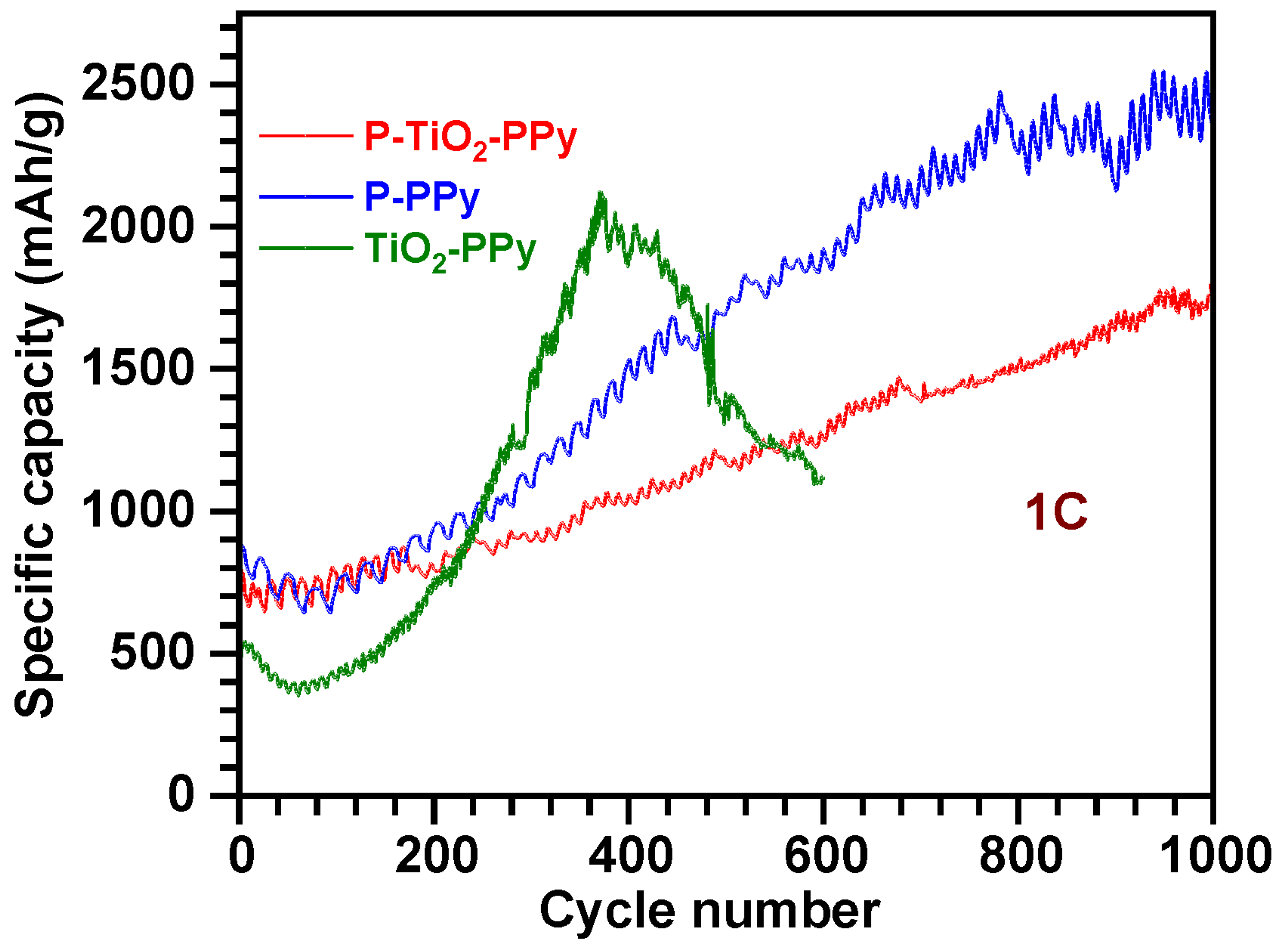
3.2.4. Long-Term Stability of PPy-Based Composite Anodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, M.-C.; Lee, Y.-Y.; Xu, B.; Powers, K.; Meng, Y.S. TiO2 flakes as anode materials for Li-ion-batteries. J. Power Sources 2012, 207, 166–172. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, W.; Luo, Z.-Z.; Zheng, Y.; Yu, Z.; Zhang, D.; Yang, J.; Tan, H.T.; Zhu, J.; Wang, X. Functionalized few-layer black phosphorus with super-wettability towards enhanced reaction kinetics for rechargeable batteries. Nano Energy 2017, 40, 576–586. [Google Scholar] [CrossRef]
- Sun, J.; Liu, C.; Wang, H.; Cao, Y.; Han, X.; Zhang, S.; Wang, H.; Zhang, Y.; Chen, A.; Yang, Z. Core–Shell Structure of a Polypyrrole-Coated Phosphorus/Carbon Nanotube Anode for High-Performance Lithium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 4112–4118. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Y.; Li, W.; Chen, X. Nanostructured TiO2-Based Anode Materials for High-Performance Rechargeable Lithium-Ion Batteries. ChemNanoMat 2016, 2, 764–775. [Google Scholar] [CrossRef]
- Jia, X.; Ge, Y.; Shao, L.; Wang, C.; Wallace, G.G. Tunable conducting polymers: Toward sustainable and versatile batteries. ACS Sustain. Chem. Eng. 2019, 7, 14321–14340. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, J.; Liu, H.; Dou, S. Study of silicon/polypyrrole composite as anode materials for Li-ion batteries. J. Power Sources 2005, 146, 448–451. [Google Scholar] [CrossRef]
- Park, C.M.; Sohn, H.J. Black phosphorus and its composite for lithium rechargeable batteries. Adv. Mater. 2007, 19, 2465–2468. [Google Scholar] [CrossRef]
- Kang, B.-H.; Shin, S.; Nam, K.; Bae, J.; Oh, J.-M.; Koo, S.-M.; Sohn, H.; Park, S.-H.; Shin, W.H. Exfoliated NbSe 2 nanosheet@ polypyrrole hybrid nanocomposites as a high performance anode of lithium-ion batteries. J. Mater. Chem. A 2023, 11, 19083–19090. [Google Scholar] [CrossRef]
- Yi, T.-F.; Qiu, L.-Y.; Mei, J.; Qi, S.-Y.; Cui, P.; Luo, S.; Zhu, Y.-R.; Xie, Y.; He, Y.-B. Porous spherical NiO@ NiMoO4@ PPy nanoarchitectures as advanced electrochemical pseudocapacitor materials. Sci. Bull. 2020, 65, 546–556. [Google Scholar] [CrossRef]
- Zhan, L.; Chen, H.; Fang, J.; Wang, S.; Ding, L.-X.; Li, Z.; Ashman, P.J.; Wang, H. Coaxial Co3O4@ polypyrrole core-shell nanowire arrays for high performance lithium ion batteries. Electrochim. Acta 2016, 209, 192–200. [Google Scholar] [CrossRef]
- Huang, H.; Wu, J.; Lin, X.; Li, L.; Shang, S.; Yuen, M.C.-w.; Yan, G. Self-assembly of polypyrrole/chitosan composite hydrogels. Carbohydr. Polym. 2013, 95, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ballester, N.M.; Rydzek, G.; Pakdel, A.; Oruganti, A.; Hasegawa, K.; Mitome, M.; Golberg, D.; Hill, J.P.; Abe, H.; Ariga, K. Nanostructured polymeric yolk–shell capsules: A versatile tool for hierarchical nanocatalyst design. J. Mater. Chem. A 2016, 4, 9850–9857. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.; Wang, R.; Zhang, Y.; Bai, W.; Ji, S.; Xuan, Z.; Yang, J.; Zheng, Z.; Guan, H. Preparation of PPy-coated MnO2 hybrid micromaterials and their improved cyclic performance as anode for lithium-ion batteries. Nanoscale Res. Lett. 2017, 12, 518. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, W.; Lai, L.; Yang, H.; Lim, S.H.; Zhen, Y.; Yu, T.; Shen, Z.; Lin, J. Three dimensionals α-Fe2O3/polypyrrole (Ppy) nanoarray as anode for micro lithium ion batteries. Nano Energy 2013, 2, 726–732. [Google Scholar] [CrossRef]
- Mahajan, M.; Singla, G.; Ogale, S. Polypyrrole-encapsulated polyoxomolybdate decorated MXene as a functional 2D/3D nanohybrid for a robust and high performance Li-ion battery. ACS Appl. Energy Mater. 2021, 4, 4541–4550. [Google Scholar] [CrossRef]
- Wang, K.; Huang, S.-P.; Wu, Y.; Cai, N.-N.; Li, N.; Xiao, Q.; Sun, Z. Critical thickness of a surface-functionalized coating for enhanced lithium storage: A case study of nanoscale polypyrrole-coated FeS2 as a cathode for Li-ion batteries. Nanoscale 2019, 11, 16277–16283. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, G.; Zhang, D.; Fan, J.; Chen, D.; Ge, Y.; Lin, F.; Zheng, C.; Li, L. Unveiling the impact of the polypyrrole coating layer thickness on the electrochemical performances of LiNi0.5Co0.2Mn0.3O2 in Li–ion battery. ChemistrySelect 2019, 4, 6354–6360. [Google Scholar] [CrossRef]
- Yi, T.-F.; Mei, J.; Peng, P.-P.; Luo, S. Facile synthesis of polypyrrole-modified Li5Cr7Ti6O25 with improved rate performance as negative electrode material for Li-ion batteries. Compos. Part B Eng. 2019, 167, 566–572. [Google Scholar] [CrossRef]
- Hong, J.Y.; Yoon, H.; Jang, J. Kinetic study of the formation of polypyrrole nanoparticles in water-soluble polymer/metal cation systems: A light-scattering analysis. Small 2010, 6, 679–686. [Google Scholar] [CrossRef]
- Shao, L.; Sun, H.; Miao, L.; Chen, X.; Han, M.; Sun, J.; Liu, S.; Li, L.; Cheng, F.; Chen, J. Facile preparation of NH2-functionalized black phosphorene for the electrocatalytic hydrogen evolution reaction. J. Mater. Chem. A 2018, 6, 2494–2499. [Google Scholar] [CrossRef]
- Kang, H.C.; Geckeler, K. Enhanced electrical conductivity of polypyrrole prepared by chemical oxidative polymerization: Effect of the preparation technique and polymer additive. Polymer 2000, 41, 6931–6934. [Google Scholar] [CrossRef]
- Wu, L.; Buchholz, D.; Bresser, D.; Chagas, L.G.; Passerini, S. Anatase TiO2 nanoparticles for high power sodium-ion anodes. J. Power Sources 2014, 251, 379–385. [Google Scholar] [CrossRef]
- Ahmed, K.; Kanwal, F.; Ramay, S.M.; Atiq, S.; Rehman, R.; Ali, S.M.; Alzayed, N.S. Synthesis and characterization of BaTiO3/polypyrrole composites with exceptional dielectric behaviour. Polymers 2018, 10, 1273. [Google Scholar] [CrossRef] [PubMed]
- Challagulla, S.; Tarafder, K.; Ganesan, R.; Roy, S. Structure sensitive photocatalytic reduction of nitroarenes over TiO2. Sci. Rep. 2017, 7, 8783. [Google Scholar] [CrossRef] [PubMed]
- Farea, M.A.; Mohammed, H.Y.; Sayyad, P.W.; Ingle, N.N.; Al-Gahouari, T.; Mahadik, M.M.; Bodkhe, G.A.; Shirsat, S.M.; Shirsat, M.D. Carbon monoxide sensor based on polypyrrole–graphene oxide composite: A cost-effective approach. Appl. Phys. A 2021, 127, 681. [Google Scholar] [CrossRef]
- Chougala, L.; Yatnatti, M.; Linganagoudar, R.; Kamble, R.; Kadadevarmath, J. A simple approach on synthesis of TiO2 nanoparticles and its application in dye sensitized solar cells. J. Nano-Electron. Phys. 2017, 9, 04005(6cc). [Google Scholar] [CrossRef] [PubMed]
- Turczyn, R.; Krukiewicz, K.; Katunin, A.; Sroka, J.; Sul, P. Fabrication and application of electrically conducting composites for electromagnetic interference shielding of remotely piloted aircraft systems. Compos. Struct. 2020, 232, 111498. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, S.; Qian, Y.; Han, Y.; Zhou, J.; Li, T.; Xi, L.; Lin, N.; Qian, Y. Amine-induced phase transition from white phosphorus to red/black phosphorus for Li/K-ion storage. Chem. Commun. 2019, 55, 6751–6754. [Google Scholar] [CrossRef] [PubMed]
- Johra, F.T.; Jung, W.-G. Synthesis of black phosphorus via a facile vapor transfer method. Electron. Mater. Lett. 2019, 15, 639–644. [Google Scholar] [CrossRef]
- Song, S.-J.; Raja, I.S.; Lee, Y.B.; Kang, M.S.; Seo, H.J.; Lee, H.U.; Han, D.-W. Comparison of cytotoxicity of black phosphorus nanosheets in different types of fibroblasts. Biomater. Res. 2019, 23, 23. [Google Scholar] [CrossRef]
- Kim, Y.K.; Shin, K.-Y. Functionalized phosphorene/polypyrrole hybrid nanomaterial by covalent bonding and its supercapacitor application. J. Ind. Eng. Chem. 2021, 94, 122–126. [Google Scholar] [CrossRef]
- Ansari, R. Polypyrrole conducting electroactive polymers: Synthesis and stability studies. J. Chem. 2006, 3, 186–201. [Google Scholar] [CrossRef]
- Lee, D.-H.; Lee, B.-H.; Sinha, A.K.; Park, J.-H.; Kim, M.-S.; Park, J.; Shin, H.; Lee, K.-S.; Sung, Y.-E.; Hyeon, T. Engineering titanium dioxide nanostructures for enhanced lithium-ion storage. J. Am. Chem. Soc. 2018, 140, 16676–16684. [Google Scholar] [CrossRef]
- Yang, F.; Wang, D.; Zhao, Y.; Tsui, K.-L.; Bae, S.J. A study of the relationship between coulombic efficiency and capacity degradation of commercial lithium-ion batteries. Energy 2018, 145, 486–495. [Google Scholar] [CrossRef]
- Shen, C.; Feng, W.; Yu, Y.; Wang, H.; Cheng, Y.; Dong, C.; Gu, J.; Zheng, A.; Liao, X.; Xu, X. In Situ Polymerization Inhibiting Electron Localization in Hybrid Electrolyte for Room-Temperature Solid-State Lithium Metal Batteries. Adv. Energy Mater. 2024, 14, 2304511. [Google Scholar] [CrossRef]
- Shen, C.; Yan, M.; Liao, X.; Xu, R.; Wang, H.; Feng, W.; Yang, W.; Li, Y.; Zhou, C.; Wang, H. Nanotrench Superfilling Facilitates Embedded Lithium Anode for High-Areal-Capacity Solid-State Batteries. ACS Nano 2024, 18, 5068–5078. [Google Scholar] [CrossRef]
- Jow, T.R.; Delp, S.A.; Allen, J.L.; Jones, J.-P.; Smart, M.C. Factors limiting Li+ charge transfer kinetics in Li-ion batteries. J. Electrochem. Soc. 2018, 165, A361. [Google Scholar] [CrossRef]
- Li, S.E.; Wang, B.; Peng, H.; Hu, X. An electrochemistry-based impedance model for lithium-ion batteries. J. Power Sources 2014, 258, 9–18. [Google Scholar] [CrossRef]
- Zheng, W.; Yan, Z.; Dai, Y.; Du, N.; Jiang, X.; Dai, H.; Li, X.; He, G. Interpenetrated networks between graphitic carbon infilling and ultrafine TiO2 nanocrystals with patterned macroporous structure for high-performance lithium ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 20491–20500. [Google Scholar] [CrossRef]
- Zhong, X.-B.; Wang, H.-Y.; Yang, Z.-Z.; Jin, B.; Jiang, Q.-C. Facile synthesis of mesoporous ZnCo2O4 coated with polypyrrole as an anode material for lithium-ion batteries. J. Power Sources 2015, 296, 298–304. [Google Scholar] [CrossRef]
- Zhou, J.; Shi, Q.; Ullah, S.; Yang, X.; Bachmatiuk, A.; Yang, R.; Rummeli, M.H. Phosphorus-based composites as anode materials for advanced alkali metal ion batteries. Adv. Funct. Mater. 2020, 30, 2004648. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, C.; Song, K.; Ha, S.; Sung, Y.; Kim, Y.; Shin, K.-Y.; Kim, B.H. Influence of Polypyrrole on Phosphorus- and TiO2-Based Anode Nanomaterials for Li-Ion Batteries. Nanomaterials 2024, 14, 1138. https://doi.org/10.3390/nano14131138
Kang C, Song K, Ha S, Sung Y, Kim Y, Shin K-Y, Kim BH. Influence of Polypyrrole on Phosphorus- and TiO2-Based Anode Nanomaterials for Li-Ion Batteries. Nanomaterials. 2024; 14(13):1138. https://doi.org/10.3390/nano14131138
Chicago/Turabian StyleKang, Chiwon, Kibum Song, Seungho Ha, Yujin Sung, Yejin Kim, Keun-Young Shin, and Byung Hyo Kim. 2024. "Influence of Polypyrrole on Phosphorus- and TiO2-Based Anode Nanomaterials for Li-Ion Batteries" Nanomaterials 14, no. 13: 1138. https://doi.org/10.3390/nano14131138
APA StyleKang, C., Song, K., Ha, S., Sung, Y., Kim, Y., Shin, K.-Y., & Kim, B. H. (2024). Influence of Polypyrrole on Phosphorus- and TiO2-Based Anode Nanomaterials for Li-Ion Batteries. Nanomaterials, 14(13), 1138. https://doi.org/10.3390/nano14131138






