Melanin-Based Nanoparticles for Lymph Node Tattooing: Experimental, Histopathological and Ultrastructural Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis, Characterization and In Vitro Studies of Nanoparticles
2.1.1. Materials
2.1.2. Synthesis of Nanoparticles with Encapsulated Melanin
2.1.3. Electron Microscopy Studies
2.1.4. Quantification of Melanin Encapsulation
2.1.5. In Vitro Release Studies
2.1.6. Cell Viability Assays
2.2. In Vivo Studies of Mel-NPs
2.2.1. Animals and Surgical Procedures
2.2.2. Histopathological Studies
2.2.3. Statistical Analysis
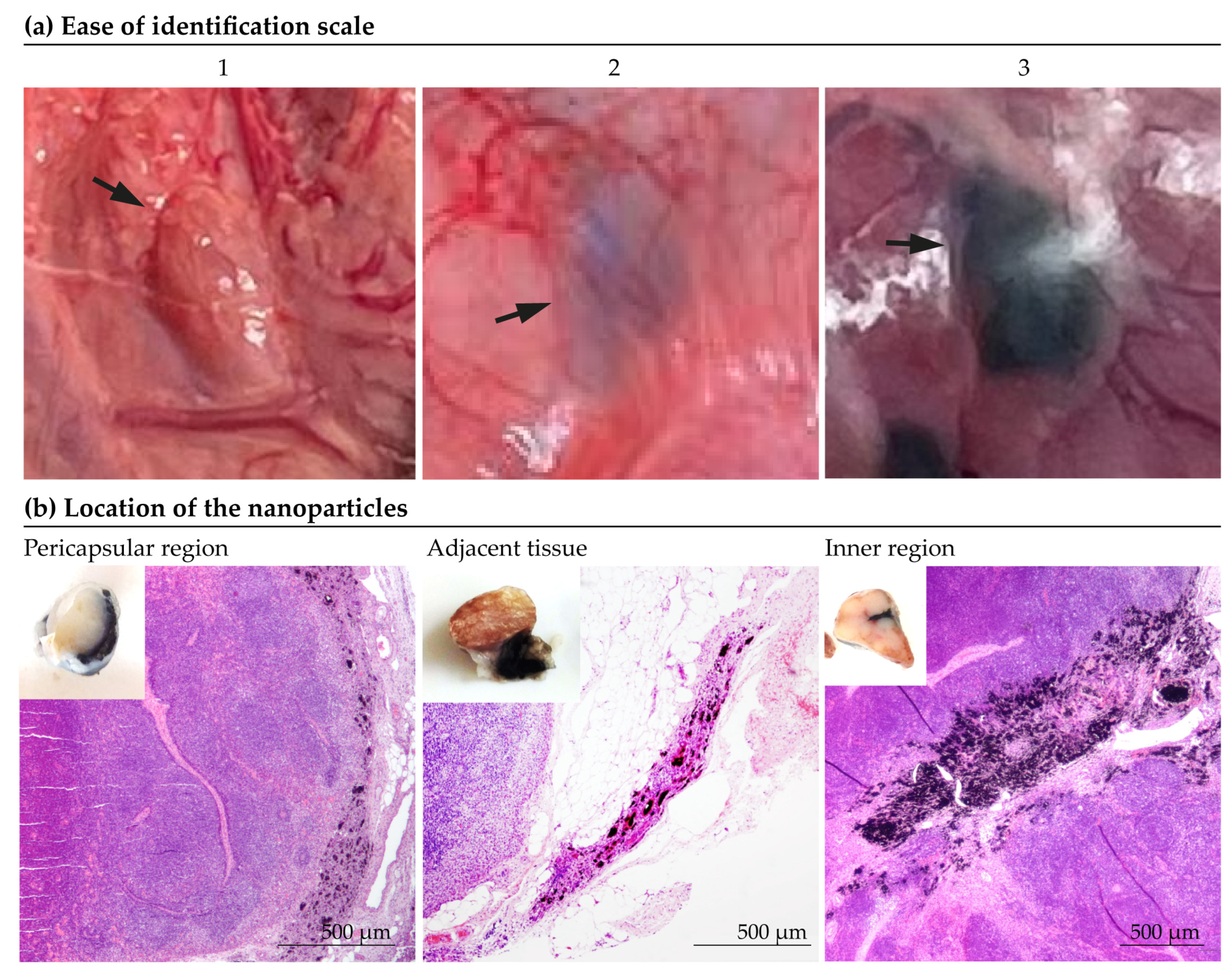
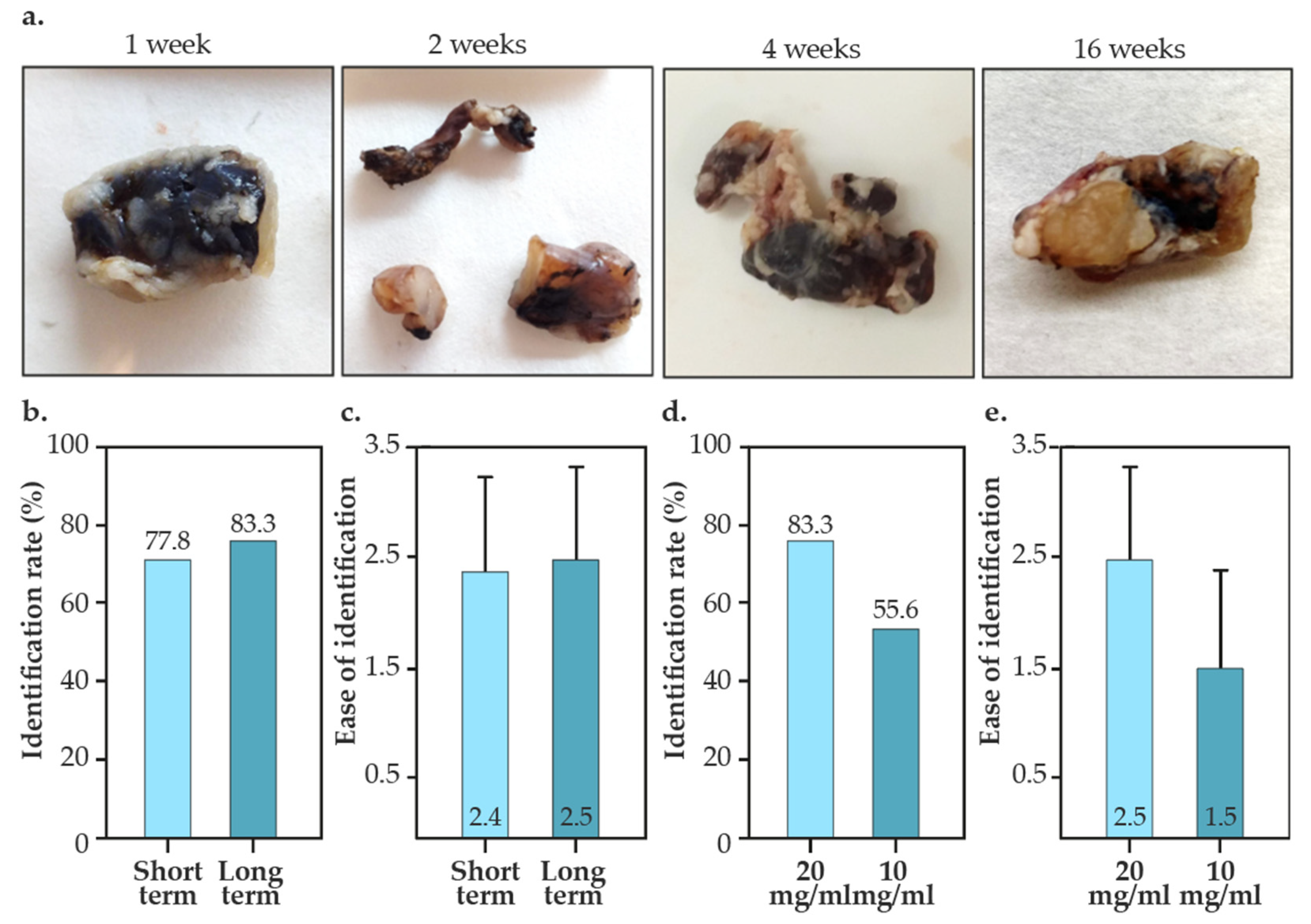
- Ease of identification. Before dissection surgery, the tattooed lymph nodes were evaluated in situ and the “ease of visual identification” was subjectively assessed by experienced surgeons on a scale from 1 to 3 (1 being ‘indistinguishable’ and 3 “optimal”), as depicted in Figure 2a.
- Location of the nanoparticles. The determination of the location of the particles was made subjectively according to the predominant location of the marking (Figure 2b). As shown in Figure 2b, the location of the marking was classified as: inner zone, pericapsular region, adjacent tissue, inner zone/pericapsular region, inner zone/adjacent tissue, pericapsular region/adjacent tissue or all locations.
- Extent of inflammation. The degree of intensity of the inflammatory response was quantified according to the extent of the inflamed tissue. The most extensive section of inflammatory tissue in the lymph node was manually measured using the ImageJ software 2024 [46].
2.2.4. Ultrastructural Studies
3. Results
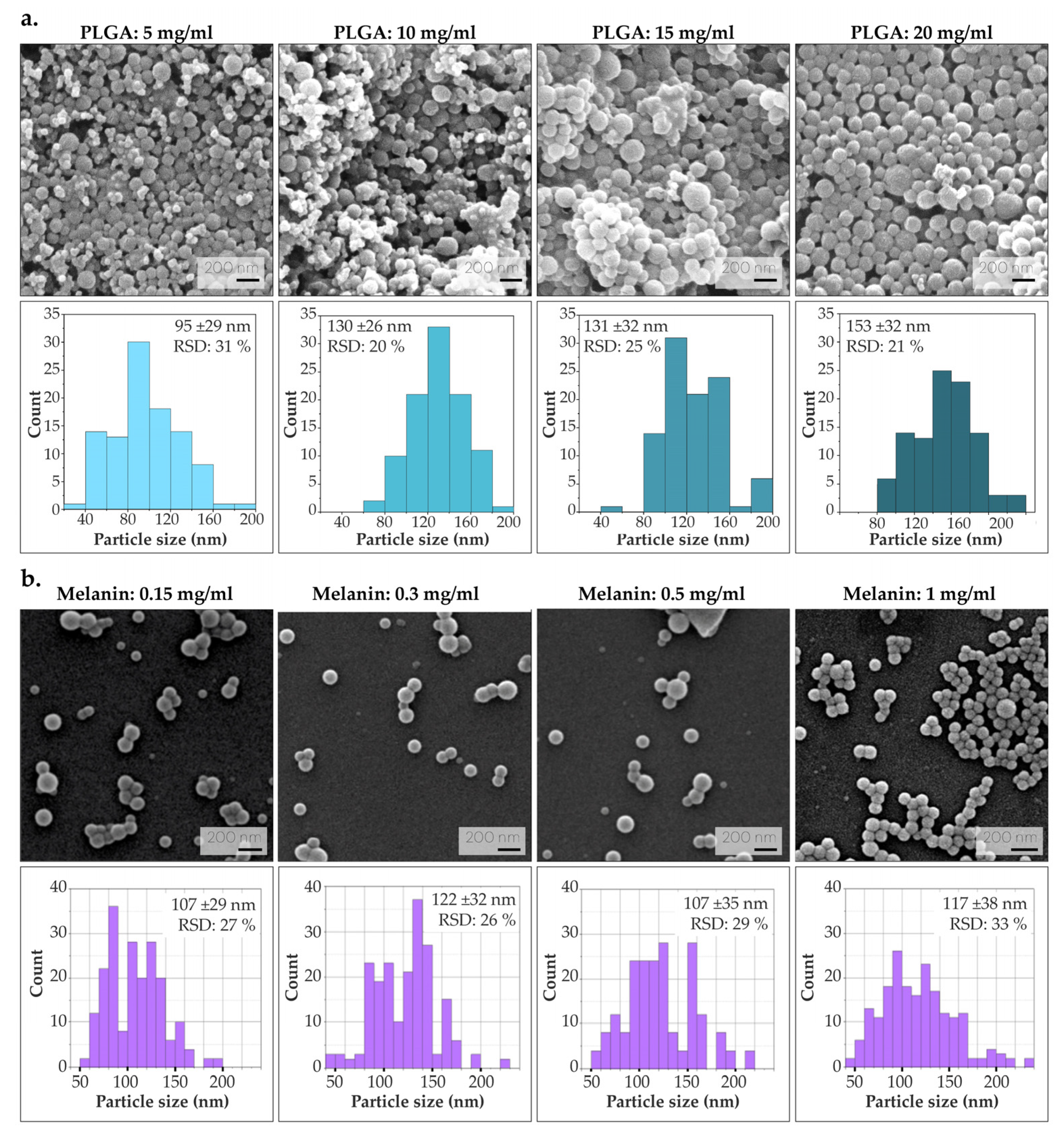
3.1. Synthesis and Characterization of Melanin-Loaded Nanoparticles
3.1.1. Optimization of PLGA and Melanin Concentration
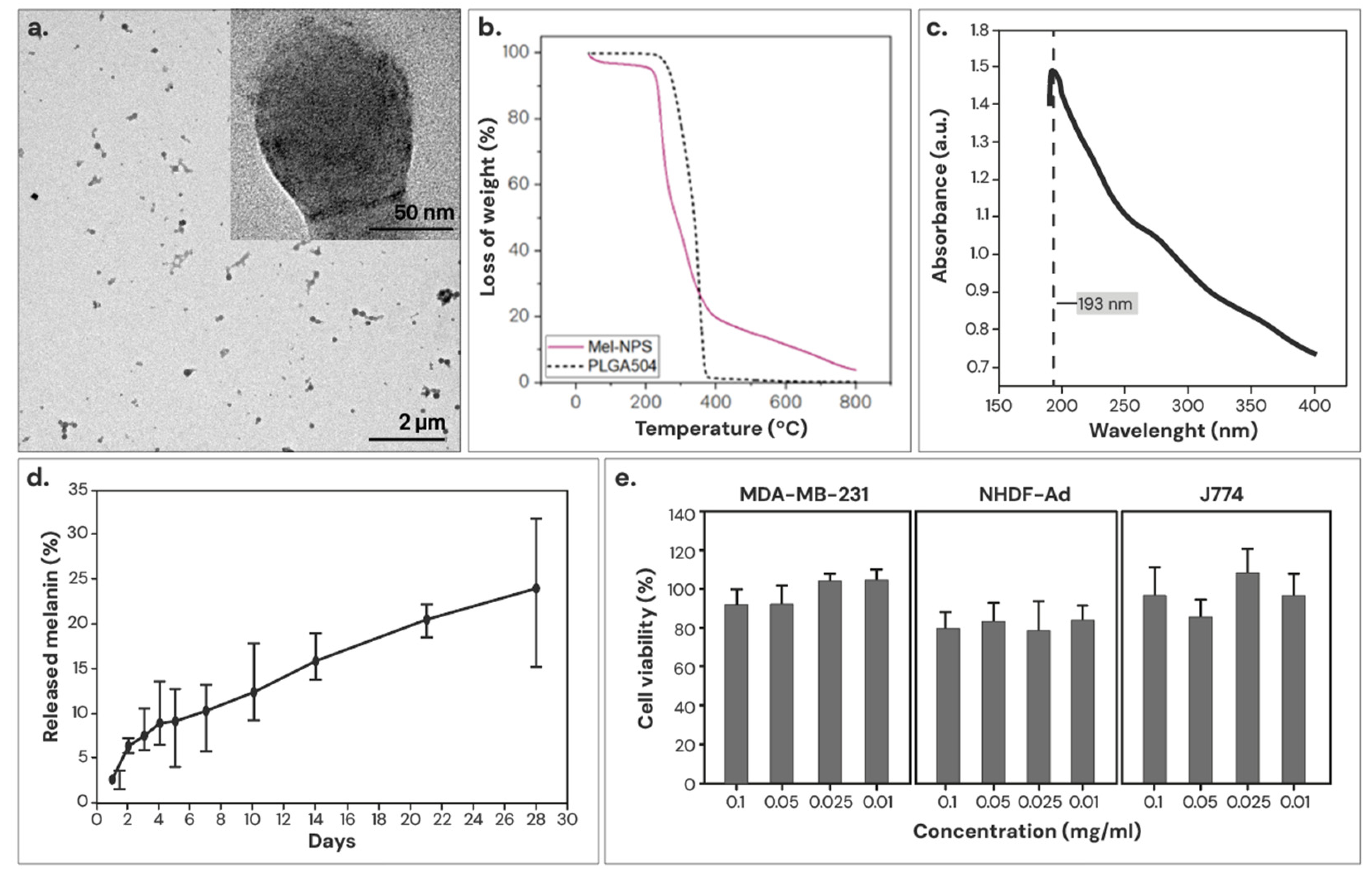
3.1.2. Characterization of the Optimized Mel-NPs
3.2. Intraoperative Identification of Tattooed Lymph Nodes
3.3. Histological Studies
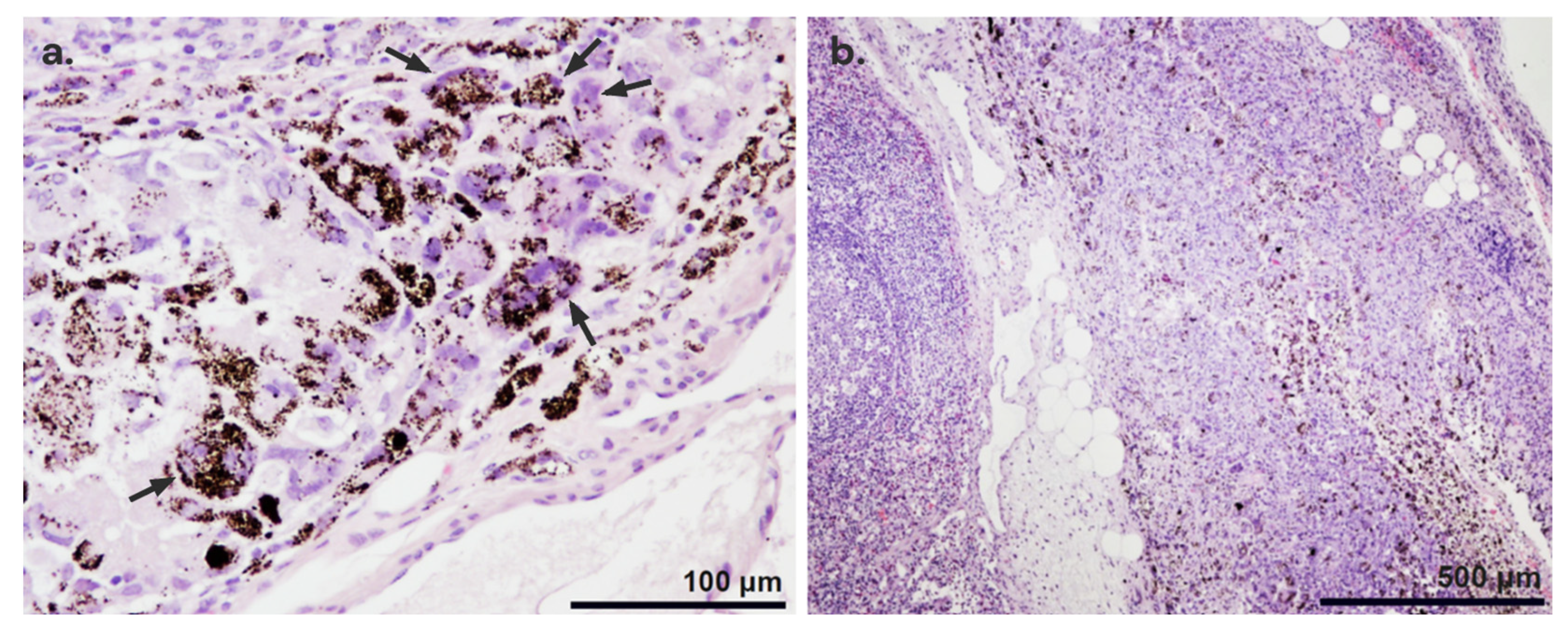
3.3.1. Histopathological Findings
3.3.2. Location of the Nanoparticles
3.3.3. Extent of Inflammation
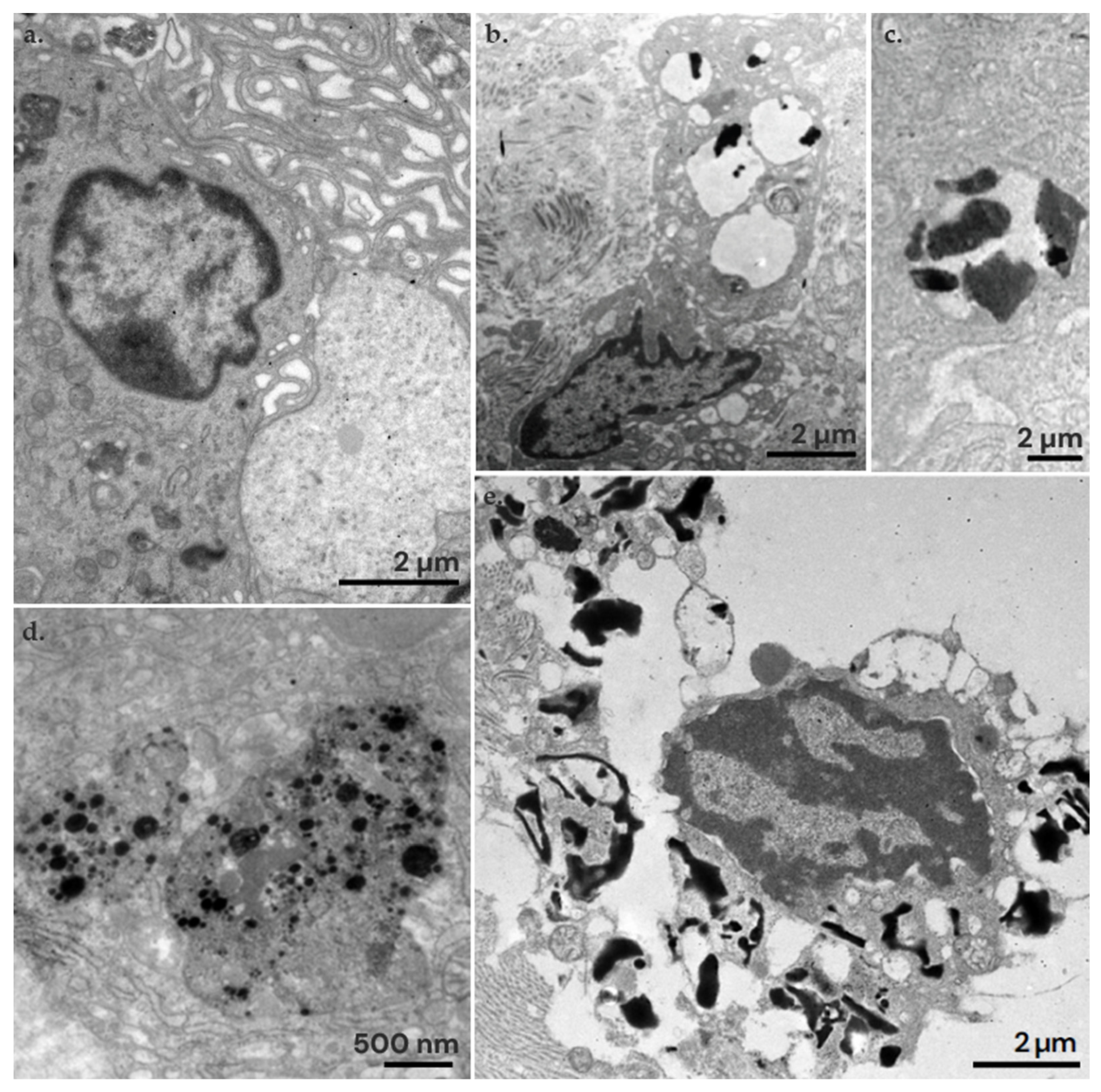
3.4. Ultrastructural Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Massagué, J.; Obenauf, A.C. Metastatic Colonization by Circulating Tumour Cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society Breast Cancer. Facts & Figures 2019–2020. 2020. Available online: https://www.cancer.org/cancer/types/breast-cancer.html (accessed on 7 March 2024).
- Turner, R.; Olilla, D.; Krasne, D.; Giuliano, A. Histopathologic Validation of the Sentinel Lymph Node Hypothesis for Breast Carcinoma. Ann. Surg. 1997, 226, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.; Kirgan, D.; Guenther, J.; Morton, D. Lymphatic Mapping and Sentinel Lymphadenectomy for Breast Cancer. Ann. Surg. 1994, 220, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Zhang-Yin, J.; Mauel, E.; Talpe, S. Update on Sentinel Lymph Node Methods and Pathology in Breast Cancer. Diagnostics 2024, 14, 252. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, T.L. Axillary Nodal Staging in Breast Cancer: What Have We Learned? Clin. Exp. Metastasis 2024. [Google Scholar] [CrossRef] [PubMed]
- Bernet, L.; Piñero, A.; Vidal-Sicart, S.; Peg, V.; Giménez, J.; Algara, M.; Dueñas, B.; Tresserra, F.; Cano, R.; Cordero, J.M.; et al. Consenso Sobre La Biopsia Selectiva Del Ganglio Centinela En El Cáncer de Mama. Revisión 2013 de La Sociedad Española de Senología y Patología Mamaria. Rev. Esp. Patol. 2014, 47, 22–32. [Google Scholar] [CrossRef]
- Boughey, J.; Suman, V.; Mittendorf, E.; Ahrendt, G.; Wilke, L.; Taback, G.; Leitch, A.; Kuerer, H.; Bowling, M.; Flippo-Morton, T.; et al. Sentinel Node Surgery after Neoadyuvant Chemotherapy in Patients with Node-Positive Breast Cancer: The American College of Surgeons Oncology Group. Z1071 Clinical Trial. JAMA 2013, 310, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, T.; Bauerfeind, I.; Fehm, T.; Fleige, B.; Hauschild, M.; Helms, G.; Lebeau, A.; Liedtke, C.; von Minchwitz, G.; Nekjudova, V.; et al. Sentinel-Lymph-Node Biopsy in Patients with Breast Cancer before and after Neoadjuvant Chemotherapy (SENTINA): A Prospective, Multicenter Cohort Study. Lancet Oncol. 2013, 14, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Boileau, J.; Porier, B.; Basik, M.; Holloway, C.; Gaboury, L.; Sideris, L.; Meterissian, S.; Arnaout, A.; Brackstone, M.; McCready, D.; et al. Sentinel Node Biopsy after Neoadyuvant Chemotherapy in Biopsy-Proven Node-Positive Breast Cancer: The SN FNAC Study. J. Clin. Oncol. 2015, 20, 258–264. [Google Scholar]
- Cao, S.; Liu, X.; Cui, J.; Liu, X.; Zhong, J.; Yang, Z.; Sun, D.; Wei, W. Feasibility and Reliability of Sentinel Lymph Node Biopsy after Neoadjuvant Chemotherapy in Breast Cancer Patients with Positive Axillary Nodes at Initial Diagnosis: An up-to-Date Meta-Analysis of 3578 Patients. Breast 2021, 59, 256–269. [Google Scholar] [CrossRef]
- Plecha, D.; Bai, S.; Patterson, H.; Thompson, C.; Shenk, R. Improving the Accuracy of Axillary Lymph Node Surgery in Breast Cancer with Ultrasound-Guided Wire Localization of Biopsy Proven Metastatic Lymph Nodes. Breast Oncol. 2015, 22, 4241–4246. [Google Scholar] [CrossRef] [PubMed]
- Man, V.; Kwong, A. Different Strategies in Marking Axillary Lymph Nodes in Breast Cancer Patients Undergoing Neoadjuvant Medical Treatment: A Systematic Review. Breast Cancer Res. Treat. 2021, 186, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, S.; Banys-Paluchowski, M.; Stickeler, E.; de Boniface, J.; Gentilini, O.D.; Kontos, M.; Seitz, S.; Kaltenecker, G.; Wärnberg, F.; Zetterlund, L.H.; et al. Applicability of Magnetic Seeds for Target Lymph Node Biopsy after Neoadjuvant Chemotherapy in Initially Node-Positive Breast Cancer Patients: Data from the AXSANA Study. Breast Cancer Res. Treat. 2023, 202, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Donker, M.; Straver, M.E.; Wesseling, J.; Loo, C.E.; Schot, M.; Drukker, C.A.; van Tinteren, H.; Sonke, G.S.; Rutgers, E.J.T.; Vrancken Peeters, M.-J.T.F.D. Marking Axillary Lymph Nodes With Radioactive Iodine Seeds for Axillary Staging After Neoadjuvant Systemic Treatment in Breast Cancer Patients. Ann. Surg. 2015, 261, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Caudle, A.S.; Yang, W.T.; Krishnamurthy, S.; Mittendorf, E.A.; Black, D.M.; Gilcrease, M.Z.; Bedrosian, I.; Hobbs, B.P.; DeSnyder, S.M.; Hwang, R.F.; et al. Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients With Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection. J. Clin. Oncol. 2016, 34, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.; Young, J.; Tokumaru, Y.; Quinn, M.; Edge, S.B.; Takabe, K. Options to Determine Pathological Response of Axillary Lymph Node Metastasis after Neoadjuvant Chemotherapy in Advanced Breast Cancer. Cancers 2021, 13, 4167. [Google Scholar] [CrossRef] [PubMed]
- Alamoodi, M.; Wazir, U.; Venkataraman, J.; Almukbel, R.; Mokbel, K. Assessing the Efficacy of Radioactive Iodine Seed Localisation in Targeted Axillary Dissection for Node-Positive Early Breast Cancer Patients Undergoing Neoadjuvant Systemic Therapy: A Systematic Review and Pooled Analysis. Diagnostics 2024, 14, 1175. [Google Scholar] [CrossRef] [PubMed]
- Wazir, U.; Michell, M.J.; Alamoodi, M.; Mokbel, K. Evaluating Radar Reflector Localisation in Targeted Axillary Dissection in Patients Undergoing Neoadjuvant Systemic Therapy for Node-Positive Early Breast Cancer: A Systematic Review and Pooled Analysis. Cancers 2024, 16, 1345. [Google Scholar] [CrossRef]
- Choy, N.; Lipson, J.; Porter, C.; Ozawa, M.; Kieryn, A.; Pal, S.; Kao, J.; Trinh, L.; Wheeler, A.; Ikeda, D.; et al. Initial Results with Preoperative Tattooing of Biopsied Axillary Lymph Nodes and Correlation to Sentinel Lymph Nodes in Breast Cancer Patients. Ann. Surg. Oncol. 2015, 22, 377–382. [Google Scholar] [CrossRef]
- Natsiopoulos, I.; Intzes, S.; Liappis, T.; Zarampoukas, K.; Zarampoukas, T.; Zacharopoulou, V.; Papazisis, K. Axillary Lymph Node Tattooing and Targeted Axillary Dissection in Breast Cancer Patients Who Presented as CN+ Before Neoadjuvant Chemotherapy and Became CN0 After Treatment. Clin. Breast Cancer 2019, 19, 208–215. [Google Scholar] [CrossRef]
- Allweis, T.M.; Menes, T.; Rotbart, N.; Rapson, Y.; Cernik, H.; Bokov, I.; Diment, J.; Magen, A.; Golan, O.; Levi-Bendet, N.; et al. Ultrasound Guided Tattooing of Axillary Lymph Nodes in Breast Cancer Patients Prior to Neoadjuvant Therapy, and Identification of Tattooed Nodes at the Time of Surgery. Eur. J. Surg. Oncol. 2020, 46, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; MacKerricher, W.; Tsai, J.; Choy, N.; Lipson, J.; Ikeda, D.; Pal, S.; De Martini, W.; Allison, K.H.; Wapnir, I.L. Pretreatment Tattoo Marking of Suspicious Axillary Lymph Nodes: Reliability and Correlation with Sentinel Lymph Node. Ann. Surg. Oncol. 2019, 26, 2452–2458. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, S.; Kühn, T.; de Boniface, J.; Stachs, A.; Winckelmann, A.; Frisell, J.; Wiklander-Bråkenhielm, I.; Stubert, J.; Gerber, B.; Reimer, T. Carbon Tattooing for Targeted Lymph Node Biopsy after Primary Systemic Therapy in Breast Cancer: Prospective Multicentre TATTOO Trial. Br. J. Surg. 2021, 108, 302–307. [Google Scholar] [CrossRef]
- Goyal, A.; Puri, S.; Marshall, A.; Valassiadou, K.; Hoosein, M.M.; Carmichael, A.R.; Erdelyi, G.; Sharma, N.; Dunn, J.; York, J. A Multicentre Prospective Feasibility Study of Carbon Dye Tattooing of Biopsied Axillary Node and Surgical Localisation in Breast Cancer Patients. Breast Cancer Res. Treat. 2021, 185, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Gatek, J.; Petru, V.; Kosac, P.; Ratajsky, M.; Duben, J.; Dudesek, B.; Jancik, P.; Zabojnikova, M.; Katrusak, J.; Opelova, P.; et al. Targeted Axillary Dissection with Preoperative Tattooing of Biopsied Positive Axillary Lymph Nodes in Breast Cancer. Neoplasma 2020, 67, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Incel Uysal, P.; Gurel, M.S.; Behzatoglu, K. A Tattoo-Associated Complication: Foreign Body Granulomatous Reaction. Am. J. Dermatopathol. 2016, 38, 936–937. [Google Scholar] [CrossRef] [PubMed]
- Bassi, A.; Campolmi, P.; Cannarozzo, G.; Conti, R.; Bruscino, N.; Gola, M.; Ermini, S.; Massi, D.; Moretti, S. Tattoo-Associated Skin Reaction: The Importance of an Early Diagnosis and Proper Treatment. BioMed Res. Int. 2014, 2014, 354608. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Yang, S.-T.; He, T.; Yang, S.; Tang, X.-H. Bioaccumulation and Toxicity of Carbon Nanoparticles Suspension Injection in Intravenously Exposed Mice. Int. J. Mol. Sci. 2017, 18, 2562. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.-L.; Yang, S.-T.; Xing, G. Molecular Toxicity of Nanomaterials. J. Biomed. Nanotechnol. 2014, 10, 2828–2851. [Google Scholar] [CrossRef]
- Liu, J.-H.; Wang, T.; Wang, H.; Gu, Y.; Xu, Y.; Tang, H.; Jia, G.; Liu, Y. Biocompatibility of Graphene Oxide Intravenously Administrated in Mice—Effects of Dose, Size and Exposure Protocols. Toxicol. Res. 2015, 4, 83–91. [Google Scholar] [CrossRef]
- Duch, M.C.; Budinger, G.R.S.; Liang, Y.T.; Soberanes, S.; Urich, D.; Chiarella, S.E.; Campochiaro, L.A.; Gonzalez, A.; Chandel, N.S.; Hersam, M.C.; et al. Minimizing Oxidation and Stable Nanoscale Dispersion Improves the Biocompatibility of Graphene in the Lung. Nano Lett. 2011, 11, 5201–5207. [Google Scholar] [CrossRef]
- Kuwahata, A.; Tanaka, R.; Matsuda, S.; Amada, E.; Irino, T.; Mayanagi, S.; Chikaki, S.; Saito, I.; Tanabe, N.; Kawakubo, H.; et al. Development of Magnetic Probe for Sentinel Lymph Node Detection in Laparoscopic Navigation for Gastric Cancer Patients. Sci. Rep. 2020, 10, 1798. [Google Scholar] [CrossRef] [PubMed]
- Piñero, A.; Torró, J.; León, J.; Castro, G.; Fuster, C.; Pardo, R. Superparamagnetic Iron Oxide as a Tracer for Sentinel Node Biopsy in Breast Cancer: A Comparative Non-Inferiority Study. Eur. J. Surg. Oncol. 2015, 41, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Rubio, I.T.; Diaz-Botero, S.; Esgueva, A.; Rodriguez, R.; Cortadellas, T.; Cordoba, O.; Espinosa-Bravo, M. The Superparamagnetic Iron Oxide Is Equivalent to the Tc99 Radiotracer Method for Identifying the Sentinel Lymph Node in Breast Cancer. Eur. J. Surg. Oncol. 2015, 41, 46–51. [Google Scholar] [CrossRef]
- Douek, M.; Klaase, J.; Monypenny, I.; Kothari, A.; Zechmeister, K.; Brown, D.; Wyld, L.; Drew, P.; Garmo, H.; Agbaje, O.; et al. Sentinel Node Biopsy Using a Magnetic Tracer versus Standard Technique: The SentiMAG Multicentre Trial. Ann. Surg. Oncol. 2014, 21, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.; Gerion, D. Fluorescence Imaging in Biology Using Nanoprobes. In Nanosystem Characterization Tools in the Life Sciences, 1st ed.; Wiley-VCH: Weinheim, Germany, 2006; pp. 1–37. [Google Scholar]
- Robe, A.; Pic, E.; Lassalle, H.P.; Bezdetnaya, L.; Guillemin, F.; Marchal, F. Quantum Dots in Axillary Lymph Node Mapping: Biodistribution Study in Healthy Mice. BMC Cancer 2008, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Ogawa, M.; Sato, N.; Choyke, P.L.; Kobayashi, H. In Vivo Real-Time, Multicolor, Quantum Dot Lymphatic Imaging. J. Invest. Dermatol. 2009, 129, 2818–2822. [Google Scholar] [CrossRef]
- Liu, Z.; Rong, P.; Yu, L.; Zhang, X.; Yang, C.; Guo, F.; Zhao, Y.; Zhou, K.; Wang, W.; Zeng, W. Dual-Modality Noninvasive Mapping of Sentinel Lymph Node by Photoacoustic and Near-Infrared Fluorescent Imaging Using Dye-Loaded Mesoporous Silica Nanoparticles. Mol. Pharm. 2015, 12, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Kojima, C.; Ogawa, M.; Magata, Y. Dendrimer-Based Sentinel Lymph Node Imaging. In Proceedings of the 10th World Biomaterials Congress, Montréal, QC, Canada, 17–22 May 2016. [Google Scholar]
- Yang, R.; Xia, S.; Ye, T.; Yao, J.; Zhang, R.; Wang, S.; Wang, S. Synthesis of a Novel Polyamidoamine Dendrimer Conjugating with Alkali Blue as a Lymphatic Tracer and Study on the Lymphatic Targeting in Vivo. Drug Deliv. 2016, 23, 2298–2308. [Google Scholar] [CrossRef]
- Gabriele, V.; Mazhabi, R.; Alexander, N.; Mukherjee, P.; Seyfried, T.N.; Nwaji, N.; Akinoglu, E.; Mackiewicz, A.; Zhou, G.; Giersig, M.; et al. Light- and Melanin Nanoparticle-Induced Cytotoxicity in Metastatic Cancer Cells. Pharmaceutics 2021, 13, 965. [Google Scholar] [CrossRef]
- Moreiras, H.; Seabra, M.C.; Barral, D.C. Melanin Transfer in the Epidermis: The Pursuit of Skin Pigmentation Control Mechanisms. Int. J. Mol. Sci. 2021, 22, 4466. [Google Scholar] [CrossRef] [PubMed]
- López-Royo, T.; Sebastián, V.; Moreno-Martínez, L.; Uson, L.; Yus, C.; Alejo, T.; Zaragoza, P.; Osta, R.; Arruebo, M.; Manzano, R. Encapsulation of Large-Size Plasmids in PLGA Nanoparticles for Gene Editing: Comparison of Three Different Synthesis Methods. Nanomaterials 2021, 11, 2723. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Larrea, A.; Clemente, A.; Luque-Michel, E.; Sebastian, V. Efficient Production of Hybrid Bio-Nanomaterials by Continuous Microchannel Emulsification: Dye-Doped SiO2 and Au-PLGA Nanoparticles. Chem. Eng. J. 2017, 316, 663–672. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices (Part 5: Tests for In Vitro Cytotoxicity). ISO: Geneva, Switzerland, 2009.
- Dostalek, L.; Cerny, A.; Saskova, P.; Pavlista, D. Selective Extirpation of Tattooed Lymph Node in Combination with Sentinel Lymph Node Biopsy in the Management of Node-Positive Breast Cancer Patients after Neoadjuvant Systemic Therapy. Breast Care 2021, 16, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; Kim, H.J.; Jung, J.H.; Park, H.Y.; Lee, J.; Kim, W.W.; Park, J.Y.; Chae, Y.S.; Lee, S.J. Ultrasound-Guided Restaging and Localization of Axillary Lymph Nodes After Neoadjuvant Chemotherapy for Guidance of Axillary Surgery in Breast Cancer Patients: Experience with Activated Charcoal. Ann. Surg. Oncol. 2018, 25, 494–500. [Google Scholar] [CrossRef]
- Park, S.; Koo, J.S.; Kim, G.M.; Sohn, J.; Kim, S.I.; Cho, Y.U.; Park, B.-W.; Park, V.Y.; Yoon, J.H.; Moon, H.J.; et al. Feasibility of Charcoal Tattooing of Cytology-Proven Metastatic Axillary Lymph Node at Diagnosis and Sentinel Lymph Node Biopsy after Neoadjuvant Chemotherapy in Breast Cancer Patients. Cancer Res. Treat. 2018, 50, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Batista, E.; Gouveia, P.; Mavioso, C.; Anacleto, J.; Ribeiro, J.; Sousa, B.; Gouveia, H.; Ferreira, A.; Chumbo, M.; et al. Targeted Axillary Dissection after Chemotherapy: Feasibility Study with Clip and Carbon Dye Tattoo—Neotarget Trial. Breast Care 2022, 17, 166–171. [Google Scholar] [CrossRef]
- Li, J.; Jia, S.; Wang, Y.; Zhang, Y.; Kong, L.; Cao, Y.; Liu, Y.; Zhang, Y.; Chen, B. Long-Term Tracing and Staining of Carbon Nanoparticles for Axillary Lymph Nodes in Patients with Locally Advanced Breast Cancer Treated with Neoadjuvant Chemotherapy. Asian J. Surg. 2022, 45, 89–96. [Google Scholar] [CrossRef]
- Pajcini, M.; Wapnir, I.; Tsai, J.; Edquilang, J.; DeMartini, W.; Ikeda, D. Influence of Imaging Features and Technique on US-Guided Tattoo Ink Marking of Axillary Lymph Nodes Removed at Sentinel Lymph Node Biopsy in Women with Breast Cancer. J. Breast Imaging 2021, 3, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xu, Z.; Liang, M.; Liu, Z.; Hou, J.; Chen, X.; Xu, D.; Fei, Y.; Tang, J. Diagnostic Accuracy of De-escalated Surgical Procedure in Axilla for Node-positive Breast Cancer Patients Treated with Neoadjuvant Systemic Therapy: A Systematic Review and Meta-analysis. Cancer Med. 2022, 11, 4085–4103. [Google Scholar] [CrossRef] [PubMed]
- Marcovici, I.; Coricovac, D.; Pinzaru, I.; Macasoi, I.; Popescu, R.; Chioibas, R.; Zupko, I.; Dehelean, C. Melanin and Melanin-Functionalized Nanoparticles as Promising Tools in Cancer Research—A Review. Cancers 2022, 14, 1838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Ling, Y.; Chen, C.; Liang, A.; Peng, Y.; Chang, H.; Su, P.; Huang, D. Sustained Release of Melatonin from Poly (Lactic-co-glycolic Acid) (PLGA) Microspheres to Induce Osteogenesis of Human Mesenchymal Stem Cells in Vitro. J. Pineal Res. 2013, 54, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kim, C.-S.; Saylor, D.M.; Koo, D. Polymer Degradation and Drug Delivery in PLGA-Based Drug-Polymer Applications: A Review of Experiments and Theories. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1692–1716. [Google Scholar] [CrossRef] [PubMed]
- Bhogal, R.H.; Thomas, S.S. Necrotizing Black Tattoo Reaction. Am. J. Clin. Dermatol. 2009, 10, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Bălăceanu-Gurău, B.; Apostol, E.; Caraivan, M.; Ion, A.; Tatar, R.; Mihai, M.M.; Popa, L.G.; Gurău, C.-D.; Orzan, O.A. Cutaneous Adverse Reactions Associated with Tattoos and Permanent Makeup Pigments. J. Clin. Med. 2024, 13, 503. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Jäättelä, M.; Liu, B. Lysosome as a Central Hub for Rewiring PH Homeostasis in Tumors. Cancers 2020, 12, 2437. [Google Scholar] [CrossRef]
- del Moral, M.; Loeck, M.; Muntimadugu, E.; Vives, G.; Pham, V.; Pfeifer, P.; Battaglia, G.; Muro, S. Role of the Lactide:Glycolide Ratio in PLGA Nanoparticle Stability and Release under Lysosomal Conditions for Enzyme Replacement Therapy of Lysosomal Storage Disorders. J. Funct. Biomater. 2023, 14, 440. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baselga, M.; Güemes, A.; Yus, C.; Alejo, T.; Sebastián, V.; Arribas, D.; Mendoza, G.; Monleón, E.; Arruebo, M. Melanin-Based Nanoparticles for Lymph Node Tattooing: Experimental, Histopathological and Ultrastructural Study. Nanomaterials 2024, 14, 1149. https://doi.org/10.3390/nano14131149
Baselga M, Güemes A, Yus C, Alejo T, Sebastián V, Arribas D, Mendoza G, Monleón E, Arruebo M. Melanin-Based Nanoparticles for Lymph Node Tattooing: Experimental, Histopathological and Ultrastructural Study. Nanomaterials. 2024; 14(13):1149. https://doi.org/10.3390/nano14131149
Chicago/Turabian StyleBaselga, Marta, Antonio Güemes, Cristina Yus, Teresa Alejo, Víctor Sebastián, Dolores Arribas, Gracia Mendoza, Eva Monleón, and Manuel Arruebo. 2024. "Melanin-Based Nanoparticles for Lymph Node Tattooing: Experimental, Histopathological and Ultrastructural Study" Nanomaterials 14, no. 13: 1149. https://doi.org/10.3390/nano14131149
APA StyleBaselga, M., Güemes, A., Yus, C., Alejo, T., Sebastián, V., Arribas, D., Mendoza, G., Monleón, E., & Arruebo, M. (2024). Melanin-Based Nanoparticles for Lymph Node Tattooing: Experimental, Histopathological and Ultrastructural Study. Nanomaterials, 14(13), 1149. https://doi.org/10.3390/nano14131149








