Influence of Long Milling Time on the Electrical Resistivity of Nanocrystalline Ni2MnSn Heusler Alloy Obtained by Mechanosynthesis
Abstract
:1. Introduction
2. Materials and Methods
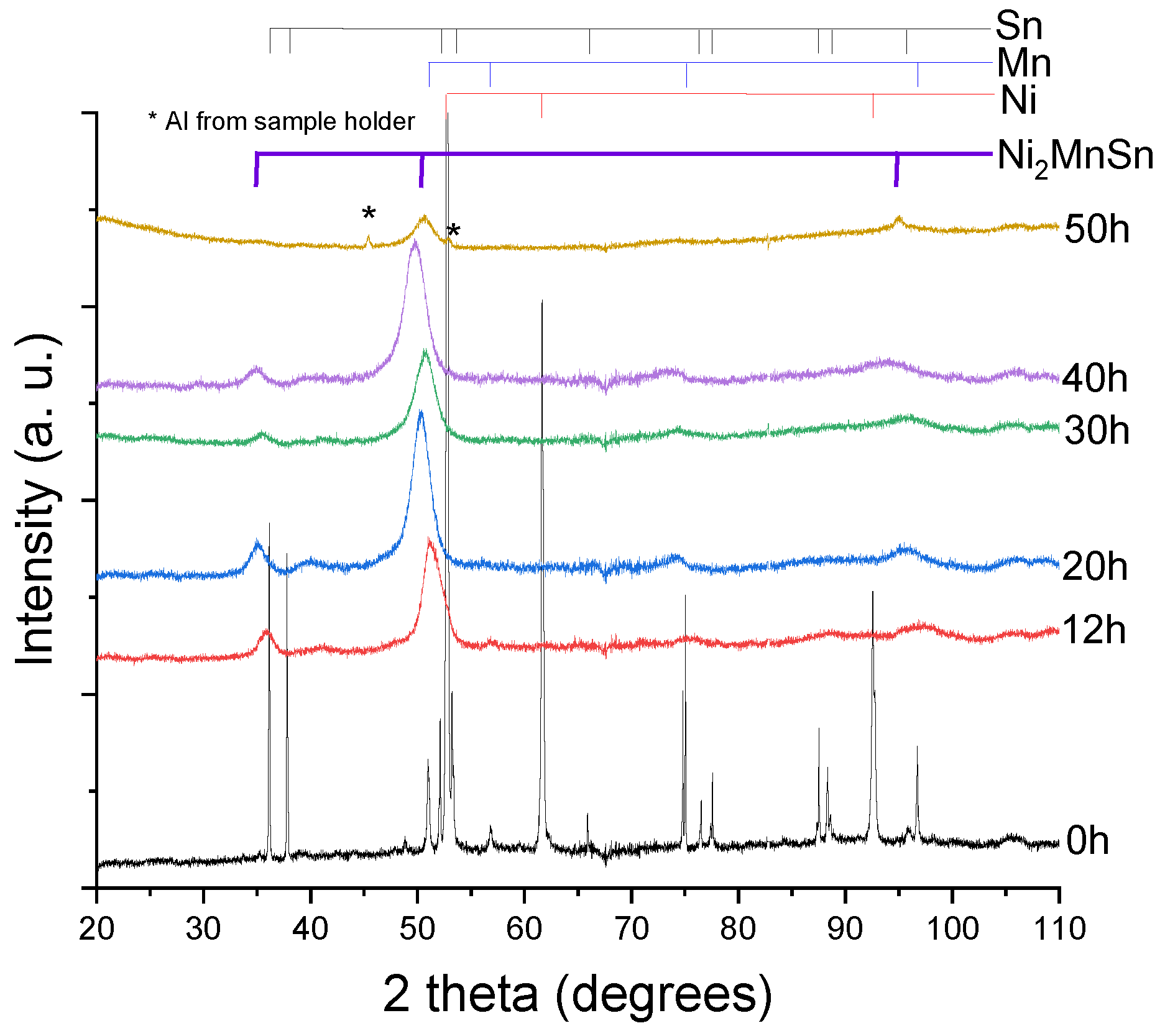
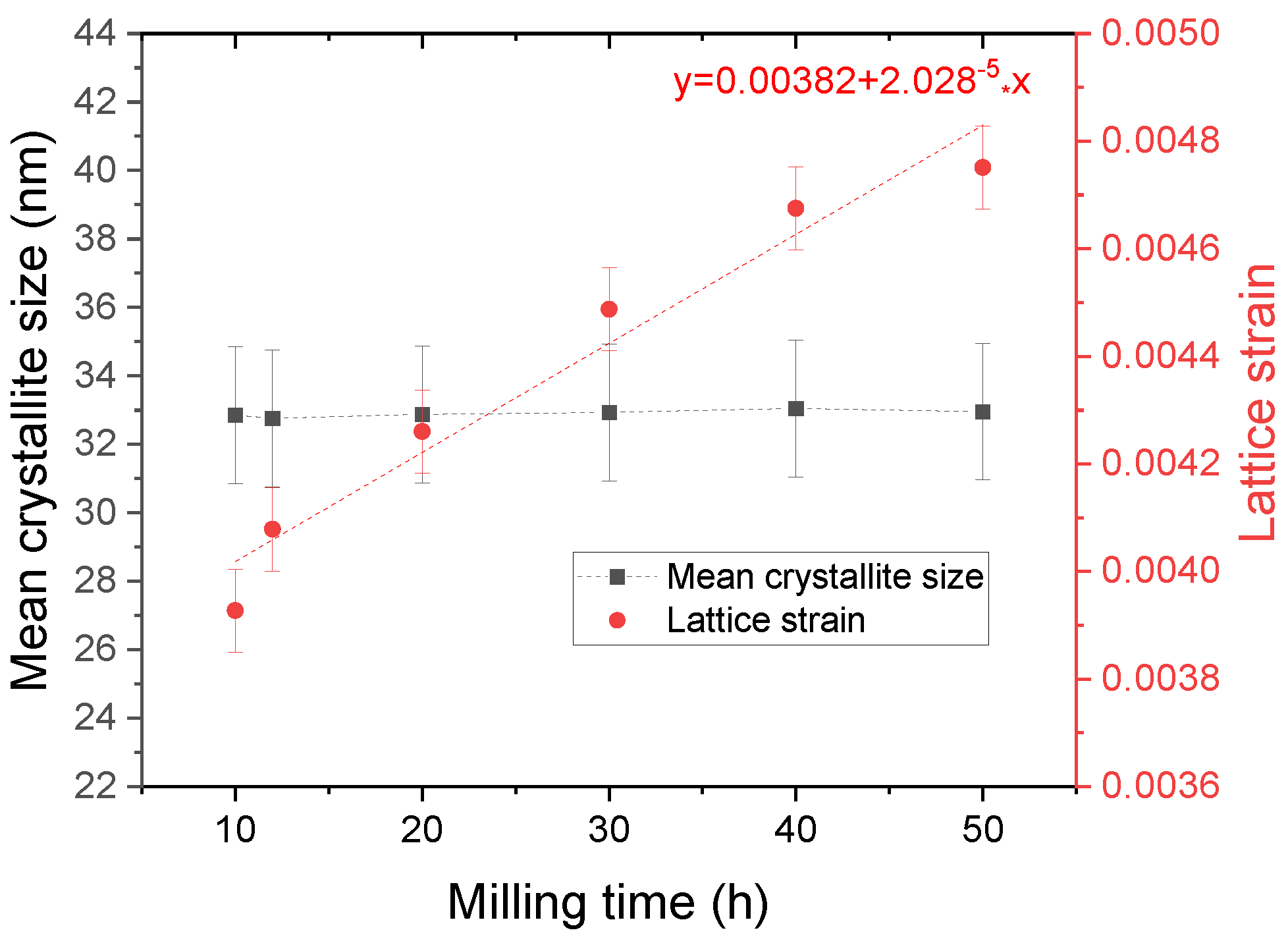
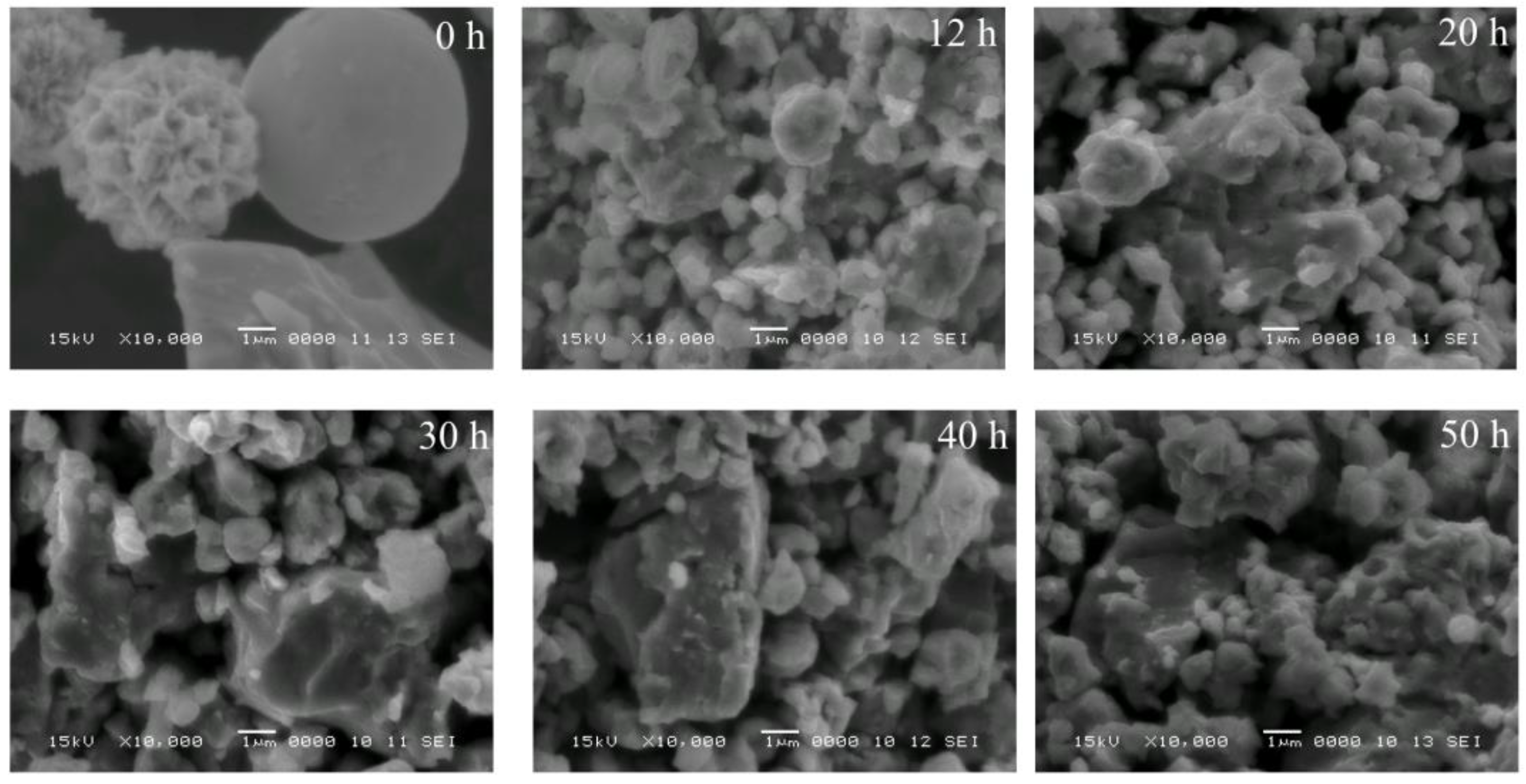
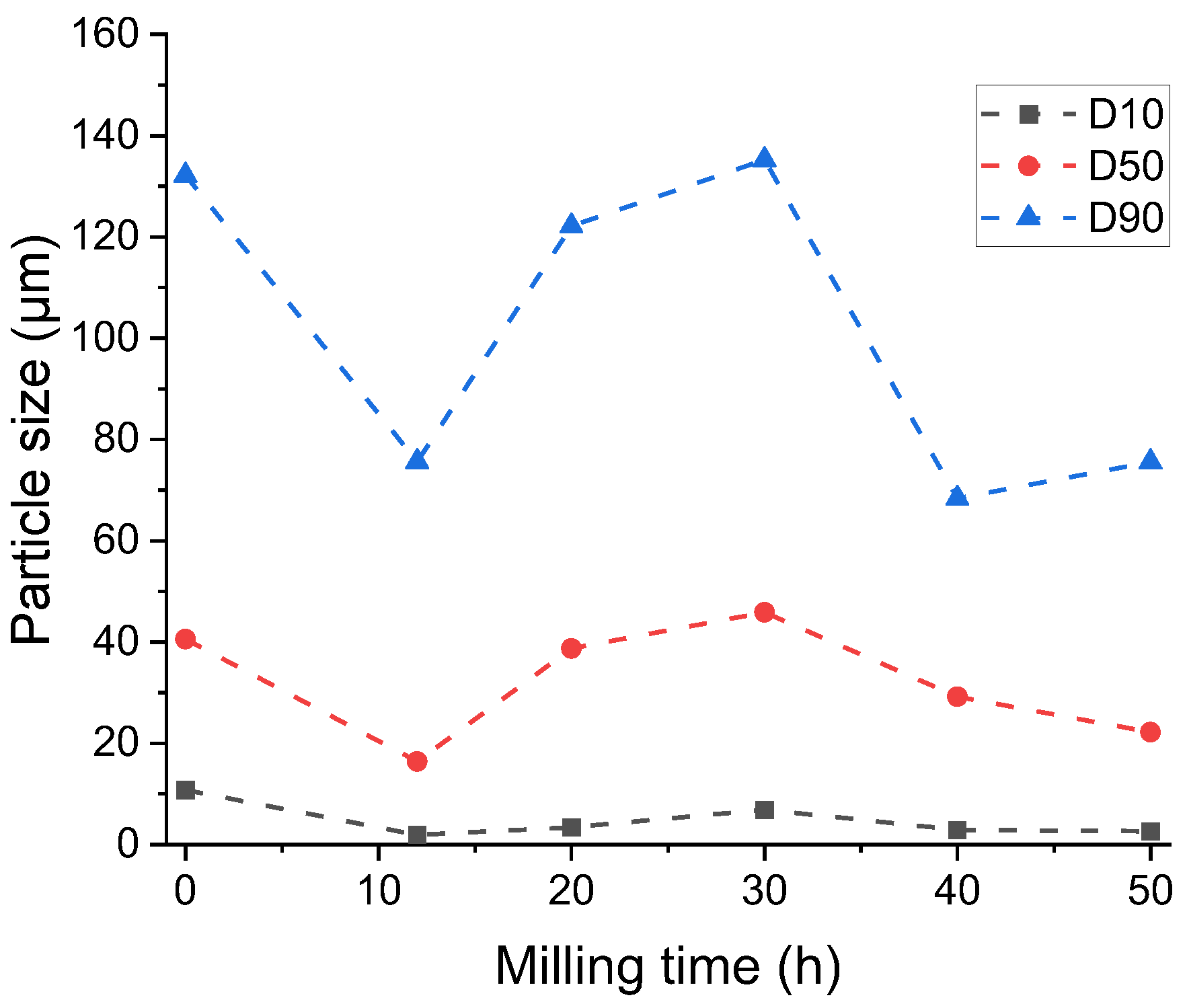
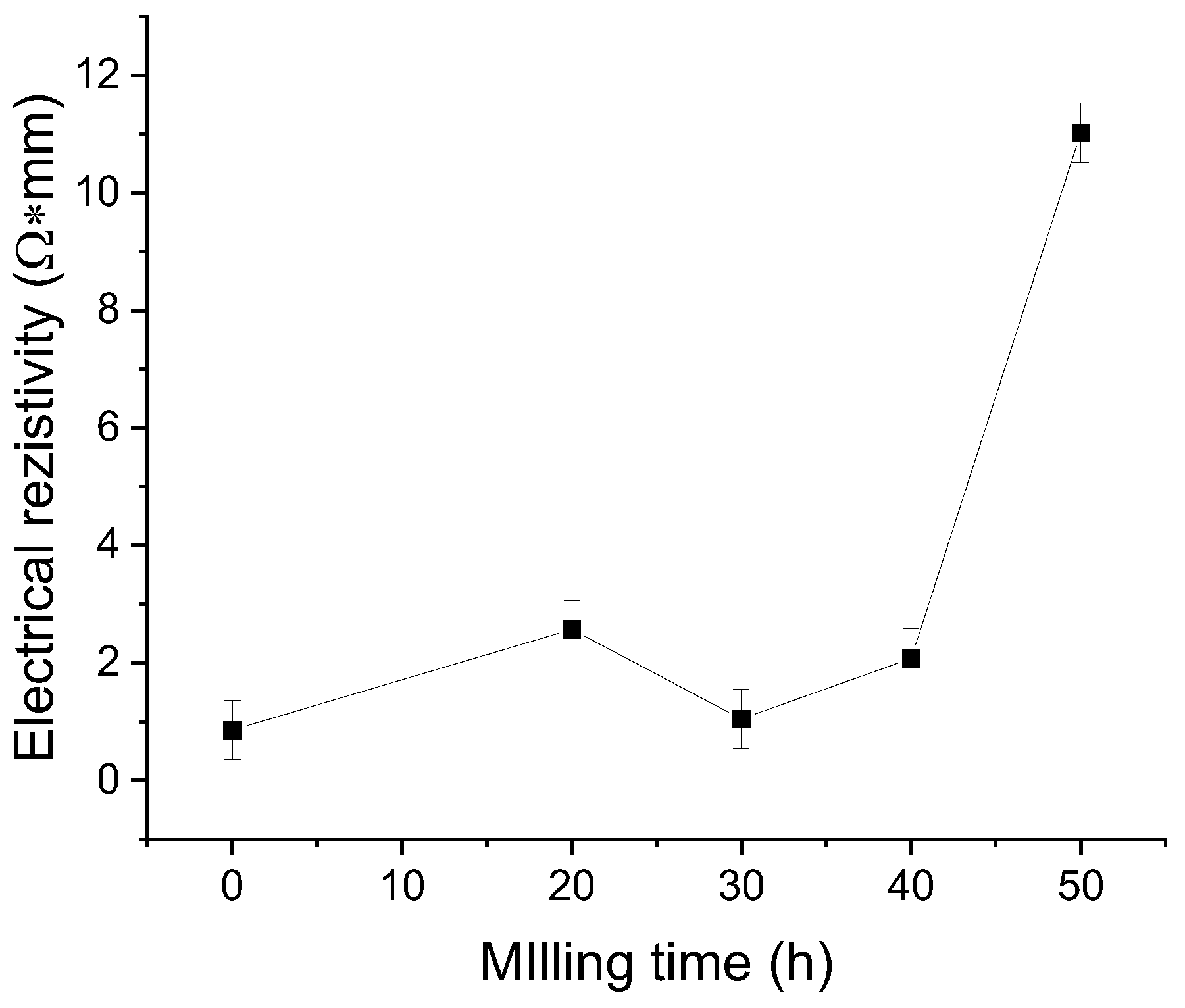
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Felser, C.; Hirohata, A. Heusler Alloys: Properties, Growth, Applications; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Ahn, K. Ni-Mn based conventional full Heusler alloys, all-d-metal full Heusler alloys, and their promising functional properties to solid state cooling by magnetocaloric effect. J. Alloys Compd. 2024, 978, 173378. [Google Scholar] [CrossRef]
- Chulist, R.; Czaja, P.; Tokarski, T.; Faryna, M. Martensite stabilisation in single crystalline Ni-Mn-Ga and Ni-Mn-Sn magnetic shape memory alloys. Mater. Lett. 2018, 230, 266–269. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, W.; Xue, S.; Zhai, Q.; Frenzel, J.; Luo, Z. Composition-dependent crystal structure and martensitic transformation in Heusler Ni-Mn-Sn alloys. Acta Mater. 2013, 61, 4648–4656. [Google Scholar] [CrossRef]
- Sivaprakash, P.; Muthu, S.E.; Singh, A.K.; Dubey, K.K.; Kannan, M.; Muthukumaran, S.; Guha, S.; Kar, M.; Singh, S.; Arumugam, S. Effect of chemical and external hydrostatic pressure on magnetic and magnetocaloric properties of Pt doped Ni2MnGa shape memory Heusler alloys. J. Magn. Magn. Mater. 2020, 514, 167136. [Google Scholar] [CrossRef]
- Devarajan, U.; Singh, S.; Muthu, S.E.; Selvan, G.K.; Sivaprakash, P.; Barman, S.R.; Arumugam, S. Investigations on the electronic transport and piezoresistivity properties of Ni2-XMn1+XGa (X = 0 and 0.15) Heusler alloys under hydrostatic pressure. Appl. Phys. Lett. 2014, 105, 1–5. [Google Scholar] [CrossRef]
- Tavares, S.; Yang, K.; Meyers, M.A. Heusler alloys: Past, properties, new alloys, and prospects. Prog. Mater. Sci. 2023, 132, 101017. [Google Scholar] [CrossRef]
- Thoene, J.; Chadov, S.; Fecher, G.; Felser, C.; Kübler, J. Exchange energies, Curie temperatures and magnons in Heusler compounds. J. Phys. D. Appl. Phys. 2009, 42, 084013. [Google Scholar] [CrossRef]
- Şaşioǧlu, E.; Sandratskii, L.M.; Bruno, P. Pressure dependence of the Curie temperature in Ni2MnSn Heusler alloy: A first-principles study. Phys. Rev. B Condens. Matter Mater. Phys. 2005, 71, 214412. [Google Scholar] [CrossRef]
- Helmholdt, R.B.; Buschow, K.H.J. Crystallographic and magnetic structure of Ni, MnSn and NiMnSn. J. Less-Common Met. 1987, 128, 167–171. [Google Scholar] [CrossRef]
- Graf, T.; Felser, C.; Parkin, S.S.P. Simple rules for the understanding of Heusler compounds. Prog. Solid State Chem. 2011, 39, 1–50. [Google Scholar] [CrossRef]
- Benguerine, O.; Nabi, Z.; Benichou, B.; Bouabdallah, B.; Bouchenafa, H.; Maachou, M.; Ahuja, R. Structural, elastic, electronic, and magnetic properties of Ni2MnSb, Ni2MnSn, and Ni2MnSb0.5Sn0.5 magnetic shape memory alloys. Rev. Mex. Física 2020, 66, 121–126. [Google Scholar] [CrossRef]
- Brown, P.J.; Gandy, A.P.; Kanomata, T.; Matsumoto, M.; Neumann, K.; Neumann, K.U.; Sheikh, A.; Ziebeck, K.R.A. The crystal structures and transformation mechanisms in stoichiometric Ni2MnGa. Mater. Sci. Forum 2008, 583, 285–301. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Ghomashchi, R.; Xie, Z.; Chen, L. Ferromagnetic shape memory Heusler materials: Synthesis, microstructure characterization and magnetostructural properties. Materials 2018, 11, 988. [Google Scholar] [CrossRef]
- Nazmunnahar, M.; Ryba, T.; Del Val, J.J.; Ipatov, M.; González, J.; Hašková, V.; Szabó, P.; Samuely, P.; Kravcak, J.; Vargova, Z.; et al. Half-metallic Ni2MnSn Heusler alloy prepared by rapid quenching. J. Magn. Magn. Mater. 2015, 386, 98–101. [Google Scholar] [CrossRef]
- Elphick, K.; Frost, W.; Samiepour, M.; Kubota, T.; Takanashi, K.; Sukegawa, H.; Mitani, S.; Hirohata, A. Heusler alloys for spintronic devices: Review on recent development and future perspectives. Sci. Technol. Adv. Mater. 2021, 22, 235–271. [Google Scholar] [CrossRef] [PubMed]
- Kamarád, J.; Kaštil, J.; Friák, M.; Turek, I.; Kubíčková, L.; Kaman, O.; Schneeweiss, O.; Arnold, Z. Magnetization and exchange-bias effect in powders of the Heusler Ni2MnSn-based alloys. J. Alloys Compd. 2024, 976, 173157. [Google Scholar] [CrossRef]
- Schleicher, B.; Klar, D.; Ollefs, K.; Diestel, A.; Walecki, D.; Weschke, E.; Schultz, L.; Nielsch, K.; Fähler, S.; Wende, H.; et al. Electronic structure and magnetism of epitaxial Ni-Mn-Ga(-Co) thin films with partial disorder: A view across the phase transition. J. Phys. D. Appl. Phys. 2017, 50, 465005. [Google Scholar] [CrossRef]
- Eggert, B.; Çakır, A.; Günzing, D.; Josten, N.; Scheibel, F.; Brand, R.A.; Farle, M.; Acet, M.; Wende, H.; Ollefs, K. Formation of precipitates in off-stoichiometric Ni-Mn-Sn Heusler alloys probed through the induced Sn-moment. RSC Adv. 2023, 13, 18217–18222. [Google Scholar] [CrossRef]
- Smith, R.; Gercsi, Z.; Zhang, R.; Siewierska, K.E.; Rode, K.; Coey, J.M.D. Effects of disorder on the magnetic properties of the Heusler alloy V2FeAl. Acta Mater. 2024, 267, 119733. [Google Scholar] [CrossRef]
- Datta, S.; Guha, S.; Panda, S.K.; Kar, M. Structural and magnetic property analysis of bulk and nanocrystalline Ni1.8Mn1.2Sn Heusler alloy. J. Magn. Magn. Mater. 2022, 544, 168656. [Google Scholar] [CrossRef]
- Kamarád, J.; Kaštil, J.; Friák, M.; Mazalová, M.; Schneeweiss, O.; Míšek, M.; Kaman, O.; Arnold, Z. Pressure study of magnetism in off-stoichiometric Ni2MnSn-based alloys. J. Magn. Magn. Mater. 2021, 539, 168345. [Google Scholar] [CrossRef]
- Dhanal, S.V.; Ghaste, A.; Akkimardi, V.G.; Kori, S.A. Study of the effect of mechanical alloying on the structure of Ni-Mn-Sn Heusler alloy. J. Mech. Sci. Technol. 2020, 34, 149–154. [Google Scholar] [CrossRef]
- Popa, F.; Marinca, T.F.; Chicinaş, H.F.; Isnard, O.; Chicinaş, I. Ni-Mn-Sn Heusler: Milling and annealing effect on structural and magnetic properties. J. Phys. Conf. Ser. 2017, 903, 2–5. [Google Scholar] [CrossRef]
- Popa, F.; Chicinaş, H.F.; Marinca, T.F.; Chicinaş, I. Influence of mechanical alloying and heat treatment processing on the Ni2MnSn Heusler alloy structure. J. Alloys Compd. 2017, 716, 137–143. [Google Scholar] [CrossRef]
- Popa, F.; Cebotari, V.; Marinca, T.F.; Isnard, O.; Chicinaș, I. Crystallographic and magnetic analysis of ordered-disordered Ni51Mn34Sn15 Heusler alloy obtained by mechanical alloying and annealing. J. Alloys Compd. 2023, 964, 171275. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying: A critical review. Mater. Res. Lett. 2022, 10, 619–647. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Phys. Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Roisnel, T.; Rodríguez-Carvajal, J. WinPLOTR: A windows tool for powder diffraction pattern analysis. Mater. Sci. Forum 2001, 378–381, 118–123. [Google Scholar] [CrossRef]
- Langford, J.I. The use of the Voigt function in determining microstructural properties from diffraction data by means of pattern decomposition. In Proceedings of the International Conference. Accuracy in Powder Diffraction II, NIST, Gaithersburg, MD, USA, 26–29 May 1992; pp. 110–126. [Google Scholar]
- Vives, S.; Gaffet, E.; Meunier, C. X-ray diffraction line profile analysis of iron ball milled powders. Mater. Sci. Eng. A 2004, 366, 229–238. [Google Scholar] [CrossRef]
- Çakır, A.; Acet, M.; Farle, M. A High-Entropy B2 Heusler Alloy. High Entropy Alloys Mater. 2023, 1, 266–270. [Google Scholar] [CrossRef]
- Bose, S.; Kudrnovský, J.; Drchal, V. Magnetism and electronic transport in (Ni, Cu)2 MnSn Heusler alloys under ambient and elevated pressures. World J. Eng. 2012, 9, 13–22. [Google Scholar] [CrossRef]
- Maziarz, W.; Wójcik, A.; Grzegorek, J.; Żywczak, A.; Czaja, P.; Szczerba, M.J.; Dutkiewicz, J.; Cesari, E. Microstructure, magneto-structural transformations and mechanical properties of Ni50Mn37.5Sn12.5-xInx (x=0, 2, 4, 6 % at.) metamagnetic shape memory alloys sintered by vacuum hot pressing. J. Alloys Compd. 2017, 715, 445–453. [Google Scholar] [CrossRef]
- Matyja, E.; Prusik, K.; Zubko, M.; Dercz, G.; Glowka, K. Structure of the Ni-Co-Mn-In alloy obtained by mechanical alloying and sintering. J. Alloys Compd. 2019, 801, 529–535. [Google Scholar] [CrossRef]





| Milling Time (h) | Rp (%) | Rwp (%) | χ2 |
|---|---|---|---|
| 12 | 3.12 | 4.04 | 3.68 |
| 20 | 3.15 | 4.06 | 4.78 |
| 30 | 2.92 | 3.78 | 2.93 |
| 40 | 3.27 | 4.13 | 4.79 |
| 50 | 3.27 | 4.3 | 2.12 |
| Element (at. %) | 20 h | 30 h | 40 h | 50 h |
|---|---|---|---|---|
| Ni | 49.6 | 49.0 | 48.6 | 48.5 |
| Mn | 26.3 | 25.6 | 25.8 | 25.8 |
| Sn | 24.2 | 25.4 | 25.7 | 25.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, F.; Marinca, T.F.; Sechel, N.A.; Frunzӑ, D.I.; Chicinaș, I. Influence of Long Milling Time on the Electrical Resistivity of Nanocrystalline Ni2MnSn Heusler Alloy Obtained by Mechanosynthesis. Nanomaterials 2024, 14, 1156. https://doi.org/10.3390/nano14131156
Popa F, Marinca TF, Sechel NA, Frunzӑ DI, Chicinaș I. Influence of Long Milling Time on the Electrical Resistivity of Nanocrystalline Ni2MnSn Heusler Alloy Obtained by Mechanosynthesis. Nanomaterials. 2024; 14(13):1156. https://doi.org/10.3390/nano14131156
Chicago/Turabian StylePopa, Florin, Traian Florin Marinca, Niculina Argentina Sechel, Dan Ioan Frunzӑ, and Ionel Chicinaș. 2024. "Influence of Long Milling Time on the Electrical Resistivity of Nanocrystalline Ni2MnSn Heusler Alloy Obtained by Mechanosynthesis" Nanomaterials 14, no. 13: 1156. https://doi.org/10.3390/nano14131156
APA StylePopa, F., Marinca, T. F., Sechel, N. A., Frunzӑ, D. I., & Chicinaș, I. (2024). Influence of Long Milling Time on the Electrical Resistivity of Nanocrystalline Ni2MnSn Heusler Alloy Obtained by Mechanosynthesis. Nanomaterials, 14(13), 1156. https://doi.org/10.3390/nano14131156






