Nano-Bioremediation of Arsenic and Its Effect on the Biological Activity and Growth of Maize Plants Grown in Highly Arsenic-Contaminated Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Used in this Experimental
2.2. Bacteria Isolation and Purification
2.3. Bacterial Inoculums
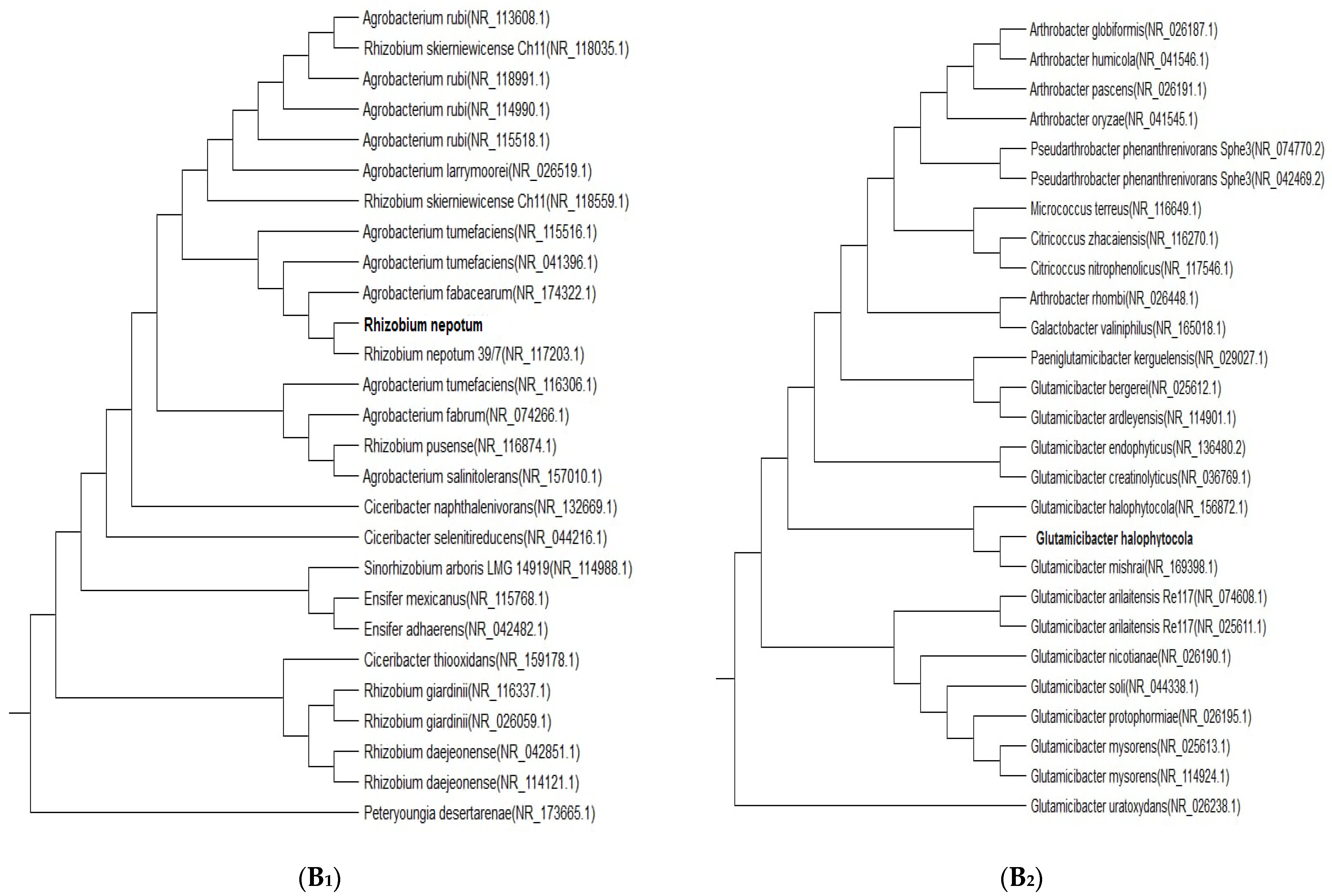
2.4. Molecular Identification of Two Isolates
2.5. Preparation of Nano-MgO
2.6. Pot Experimental Procedures
2.7. Biochemical Determination
2.8. Plant Analysis
2.9. Spectroscopic Analysis
2.10. Statistical Analysis
3. Results
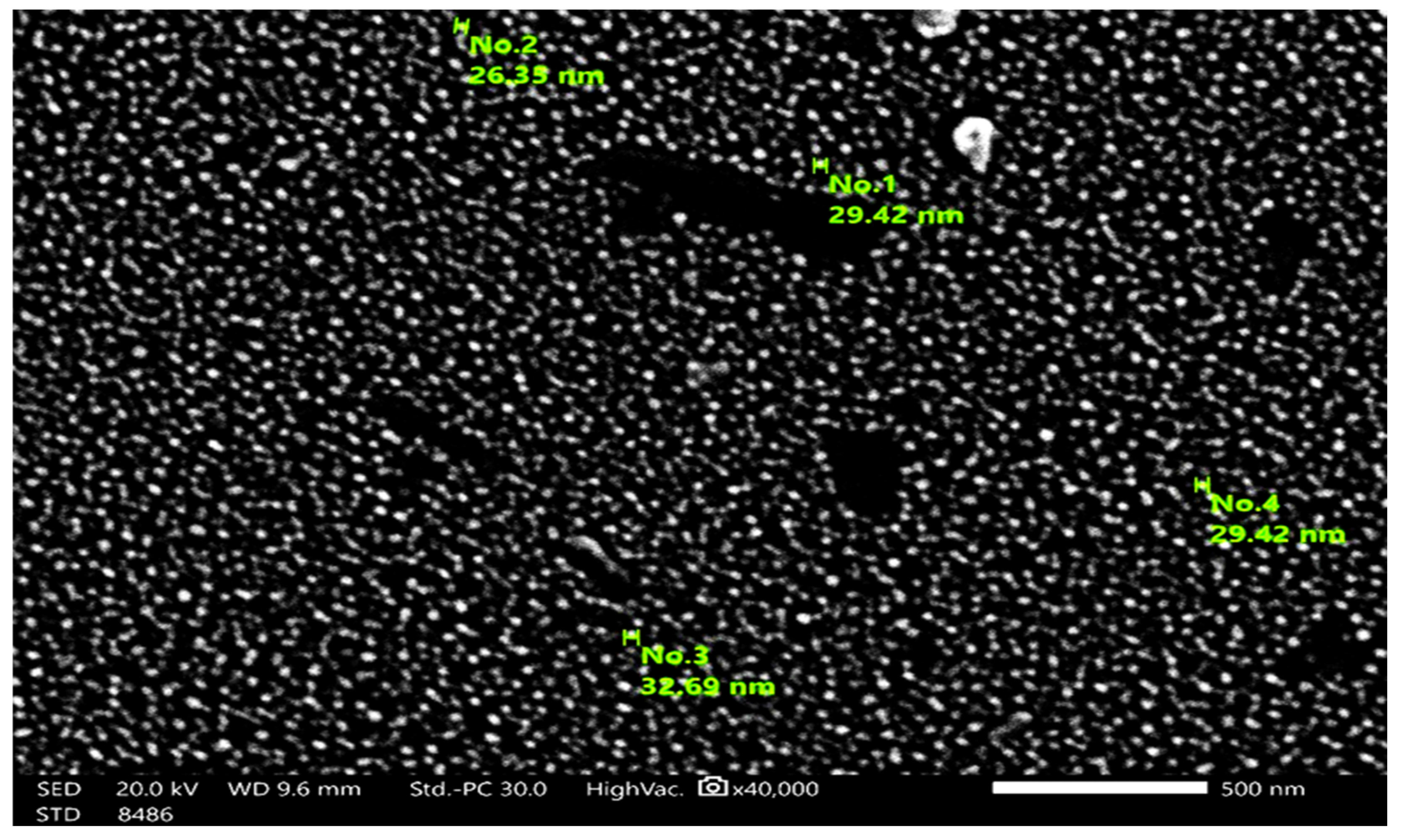
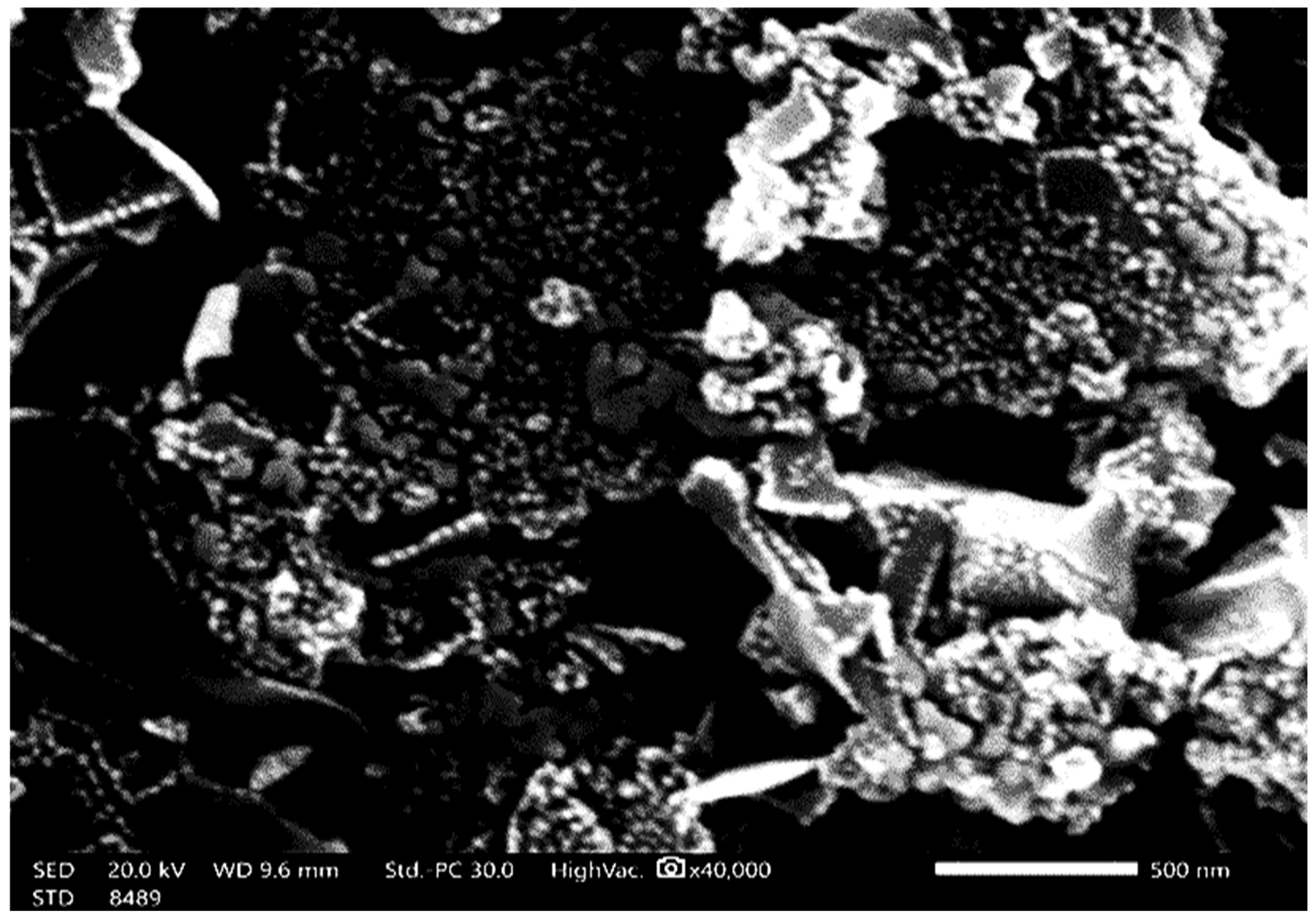
3.1. Spectroscopic Analysis of MgO-NPs
3.2. Maize Plant Biomass
3.3. Biochemical Characteristics
3.4. Arsenic Available in Soil
3.5. Arsenic in Maize Plants
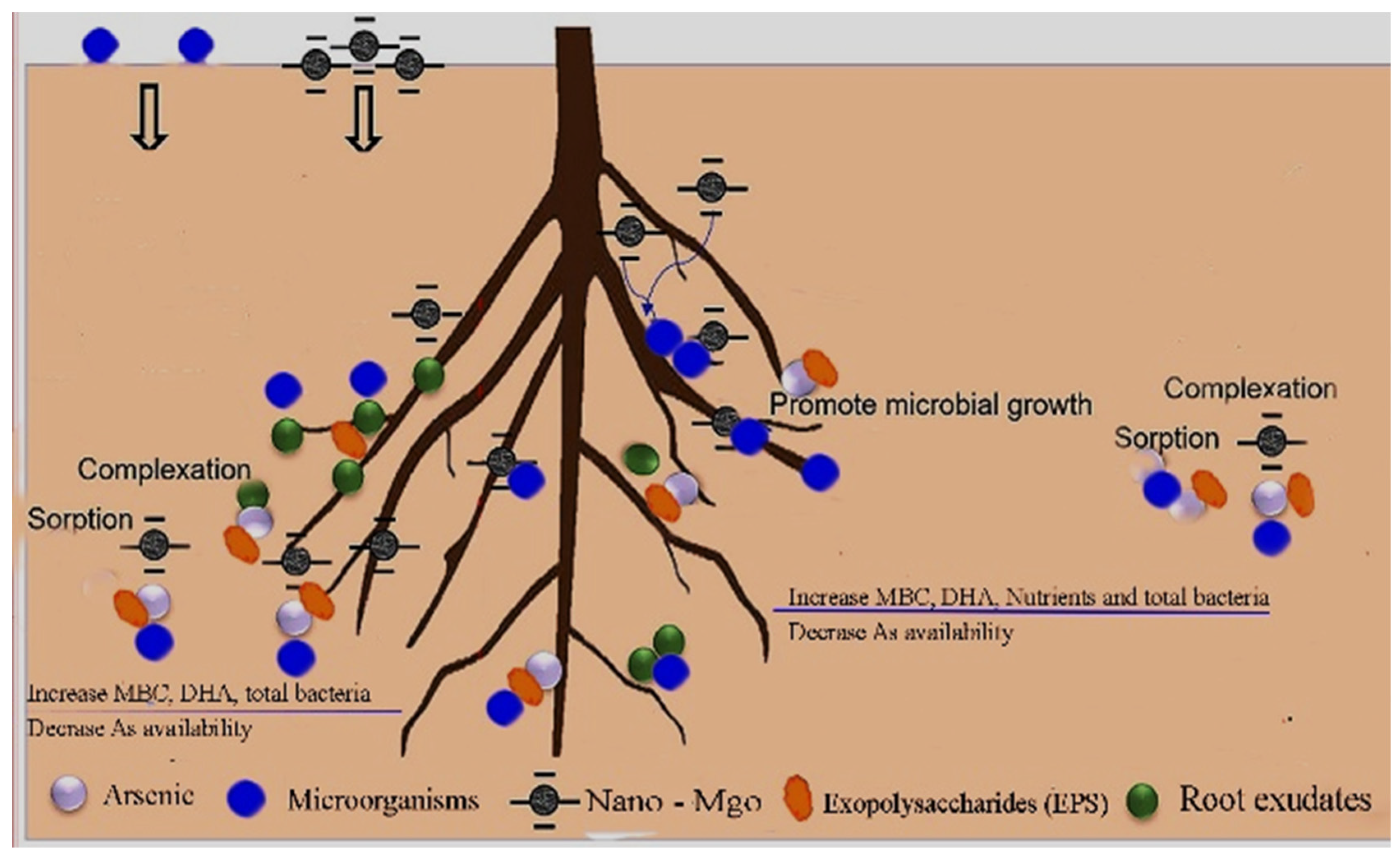
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L.J.E.I. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef]
- Duker, A.A.; Carranza, E.; Hale, M. Arsenic geochemistry and health. Environ. Int. 2005, 31, 631–641. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, L.; Zhang, J.; Shi, J.; Wang, Y.; Wang, S. Adverse Effects of Thermal Food Processing on the Structural, Nutritional, and Biological Properties of Proteins. Annu. Rev. Food Sci. Technol. 2021, 25, 259–286. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kumar, M.; Katul, G.G.; Porporato, A. Reduced resilience as an early warning signal of forest mortality. Nat. Clim. Chang. 2019, 9, 880–885. [Google Scholar] [CrossRef]
- Mahmoud, E.K.; Ghoneim, A.M. Effect of polluted water on soil and plant contamination by heavy metals in El-Mahla El-Kobra, Egypt. Solid Earth 2016, 7, 703–711. [Google Scholar] [CrossRef]
- Gupta, P.; Diwan, B. Bacterial Exopolysaccharide mediated heavy metal removal: A review on biosynthesis, mechanism and remediation strategies. Biotechnol. Rep. 2017, 13, 58–71. [Google Scholar] [CrossRef]
- Pulsawat, W.; Leksawasdi, N.; Rogers, P.L.; Foster, L.J.R. Anions effects on biosorption of Mn(II) by extracellular polymeric substance (EPS) from Rhizobium etli. Biotechnol. Lett. 2003, 25, 1267–1270. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.K.; Singh, J.S.; Singh, D.P. Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition. Pedosphere 2011, 21, 214–222. [Google Scholar] [CrossRef]
- Qin, S.; Xing, K.; Jiang, J.H.; Xu, L.H.; Li, W.J. Biodiversity, bioactive natural products and biotechnological potential of plant-associated endophytic actinobacteria. Appl. Microbiol. Biotechnol. 2011, 89, 457–473. [Google Scholar] [CrossRef]
- Santos-Medellín, C.; Edwards, J.; Liechty, Z.; Nguyen, B.; Sundaresan, V. Drought stress results in a compartment-specific restructuring of the rice root-associated microbiomes. mBio 2017, 8, e00764-17. [Google Scholar] [CrossRef]
- Titah, H.S.; Abdullah, S.R.S.; Idris, M.; Anuar, N.; Basri, H.; Mukhlisin, M.; Tangahu, B.V.; Purwanti, I.F.; Kurniawan, S.B. Arsenic resistance and biosorption by isolated rhizobacteria from the roots of Ludwigia octovalvis. Int. J. Microbiol. 2018, 2018, 3101498. [Google Scholar] [CrossRef]
- Laha, A.; Bhattacharyya, S.; Sengupta, S.; Bhattacharyya, K.; GuhaRoy, S. Rhizobium Leguminosarum: A Model Arsenic Resistant, Arsenite Oxidizing Bacterium Possessing Plant Growth Promoting Attributes. Curr. World Environ. 2021, 16, 31. [Google Scholar] [CrossRef]
- Singh, G.; Biswas, D.R.; Marwaha, T.S. Mobilization of potassium from waste mica by plant growth promoting rhizobacteria and its assimilation by maize (Zea mays) and wheat (Triticum aestivum L.): A hydroponics study under phytotron growth chamber. J. Plant Nutr. 2010, 33, 1236–1251. [Google Scholar] [CrossRef]
- Belimov, A.; Kunakova, A.M.; Safronova, V.I.; Stepanok, V.V.; Yudkin, L.; Alekseev, Y.V.; Kozhemyakov, A.P. Employment of rhizobacteria for the inoculation of barley plants cultivated in soil contaminated with lead and cadmium. Microbiology 2004, 73, 99–106. [Google Scholar]
- Qin, S.; Feng, W.; Zhang, Y.J.; Wang, T.; Xiong, Y.W.; Xing, K. Diversity of Bacterial Microbiota of Coastal Halophyte Limonium sinense and Amelioration of Salinity Stress Damage by Symbiotic Plant Growth-Promoting Actinobacterium glutamicibacter halophytocola KLBMP 5180. Appl. Environ. Microbiol. 2018, 84, e01533-18. [Google Scholar] [CrossRef] [PubMed]
- Qaralleh, H.; Khleifat, K.M.; Hajleh, M.N.A.; Al-Limoun, M.O.; Alshawawreh, R.; Magharbeh, M.K.; Alqaraleh, M. Plant growth-promoting rhizobium nepotum phenol utilization: Characterization and kinetics. J. Hunan Univ. Nat. Sci. 2022, 49. [Google Scholar] [CrossRef]
- Rincón-Molina, C.I.; Rincón-Molina, F.A.; Zenteno-Rojas, A.; Ruíz-Valdiviezo, V.M.; Culebro- Ricaldi, J.M.; Rincón-Rosales, R. Bacterial diversity with plant growth-promoting potential isolated from Agave americana rhizosphere. Agro Product. 2021, 3. [Google Scholar] [CrossRef]
- Herliana, O.; Harjoso, T.; Anwar, A.H.S.; Fauzi, A. The effect of Rhizobium and N fertilizer on growth and yield of black soybean (Glycine max (L.) Merril). Earth Environ. Sci. 2019, 255, 012015. [Google Scholar] [CrossRef]
- Biswal, T. Nanobioremediation and Its Application for Sustainable Environment. Phytoremediation Manag. Environ. Contam. 2018, 7, 463–485. [Google Scholar]
- Cao, X.; Alabresm, A.; Pin Chen, Y.; Decho, A.W.; Lead, J. Improved metal remediation using a combined bacterial and nanoscience approach. Sci. Total Environ. 2019, 704, 135378. [Google Scholar] [CrossRef]
- Němeček, J.; Pokorný, P.; Lhotský, O.; Knytl, V.; Najmanová, P.; Steinová, J.; Černík, M.; Filipová, A.; Filip, J.; Cajthaml, T. Combined nano-biotechnology for in-situ remediation of mixed contamination of groundwater by hexavalent chromium and chlorinated solvents. Sci. Total Environ. 2016, 563, 822–834. [Google Scholar] [CrossRef] [PubMed]
- Adikesavan, S.; Nilanjana, D. Degradation of cefdinir by C. candida sp. SMN04 and MgO nanoparticles—An integrated (Nano-Bio) approach. Environ. Prog. Sustain. Energy 2016, 35, 706–714. [Google Scholar] [CrossRef]
- Difco, M. Dehydrated Culture Media and Reagents for Microbiology, 10th ed.; Difco Laboratories: Defroit, MI, USA, 1985; pp. 487–623. [Google Scholar]
- Chandrappa, K.; Venkatesha, T.; Praveen, B.; Shylesha, B. Generation of nanostructured MgO particles by solution phase method. Res. J. Chem. Sci. 2015, 2231, 606X. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bartlett, R.J. Oxidation-reduction status of aerobic soils. Chem. Soil Environ. 1981, 40, 77–102. [Google Scholar] [CrossRef]
- Nelson, D.A.; Sommers, L. Total carbon, organic carbon, and organic matter. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1983, 9, 539–579. [Google Scholar] [CrossRef]
- Cottenie, A.M.; Velghe, G.; Kiekens, L. Biological and Analytical Aspects of Pollution; Laboratory of Analytical and Agrochemistry State University: Ghent, Belgium, 1982; 424p. [Google Scholar] [CrossRef]
- Allen, O.N. Experiments in Soil Bacteriology; Wisconsin Univeristy Press: Madison, WI, USA, 1959; p. 202. [Google Scholar]
- Casida, J.r.L.E.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Amato, M.; Ladd, J.N. Assay for microbial biomass based on ninhydrin-reactive nitrogen in extracts of fumigated soils. Soil Biol. Biochem. 1988, 20, 107–114. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Mueller, T. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kEN value. Soil Biol. Biochem. 1996, 28, 33–37. [Google Scholar] [CrossRef]
- SAS Institute Inc. JMP®, Version 9.0; SAS Institute Inc.: Cary, NC, USA, 2007. [Google Scholar]
- Faizan, M.; Bhat, J.A.; El-Serehy, H.A.; Moustakas, M.; Ahmad, P. Magnesium Oxide Nanoparticles (MgO-NPs) Alleviate Arsenic Toxicity in Soybean by Modulating Photosynthetic Function, Nutrient Uptake and Antioxidant Potential. Metals 2022, 12, 2030. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Adamakis, I.D.S.; Moustaka, J.; İşgören, S.; Şaş, B. Harnessing the role of foliar applied salicylic acid in decreasing chlorophyll content to reassess photosystem II photoprotection in crop plants. Int. J. Mol. Sci. 2022, 23, 7038. [Google Scholar] [CrossRef]
- Ahmed, T.; Noman, M.; Manzoor, N.; Shahid, M.; Hussaini, K.M.; Rizwan, M.; Ali, S.; Maqsood, A.; Li, B. Green magnesium oxide nanoparticles-based modulation of cellular oxidative repair mechanisms to reduce arsenic uptake and translocation in rice (Oryza sativa L.) plants. Environ. Pollut. 2021, 288, 117785. [Google Scholar] [CrossRef]
- Wu, F.; Fang, Q.; Yan, S.; Pan, L.; Tang, X.; Ye, W. Effects of zinc oxide nanoparticles on arsenic stress in rice (Oryza sativa L.): Germination, early growth, and arsenic uptake. Environ. Sci. Pollut. Res. 2020, 27, 26974–26981. [Google Scholar] [CrossRef]
- Bidi, H.; Fallah, H.; Niknejad, Y.; Barari Tari, D. Iron oxide nanoparticles alleviate arsenic phytotoxicity in rice by improving iron uptake, oxidative stress tolerance and diminishing arsenic accumulation. Plant Physiol. Biochem. 2021, 163, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Tresintsi, S.; Simeonidis, K.; Katsikini, M.; Paloura, E.C.; Bantsis, G.; Mitrakas, M. A novel approach for arsenic adsorbents regeneration using MgO. J. Hazard. Mater. 2014, 265, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Mehanathan, S.; Jaafar, J.; Nasir, A.M.; Ismail, A.F.; Matsuura, T.; Othman, M.D.; Rahman, M.A.; Yusof, N. Magnesium Oxide Nanoparticles for the Adsorption of Pentavalent Arsenic from Water: Effects of Calcination. Membranes 2023, 13, 475. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chi, F.; Shen, S.H.; Cheng, H.P.; Jing, Y.X.; Yanni, Y.G.; Dazzo, F.B. Ascending migration of endophytic rhizobia, from roots to leaves, inside rice plants and assessment of benefits to rice growth physiology. App. Environ. Microbiol. 2005, 71, 7271–7278. [Google Scholar] [CrossRef]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A fern that hyperaccumulates arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef] [PubMed]
- Welbaum, G.E.; Sturz, A.V.; Dong, Z.; Nowak, J. Managing soil microorganisms to improve productivity of agro-ecosystems. CRC Crit. Rev. Plant Sci. 2004, 23, 175–193. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, D.; Liu, Y.; Li, S.; Shen, Q.; Zhang, R. Effects of different plant root exudates and their organic acid components on chemotaxis, biofilm formation and colonization by beneficial rhizosphere-associated bacterial strains. Plant Soil 2014, 374, 689–700. [Google Scholar] [CrossRef]
- Mohite, B. Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J. Soil Sci. Plant Nutr. 2013, 13, 638–649. [Google Scholar] [CrossRef]
- Mohsin, H.; Shafique, M.; Zaid, M.; Rehman, Y. Microbial biochemical pathways of arsenic biotransformation and their application for bioremediation. Folia Microbiol. 2023, 68, 507–535. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jang, Y.J.; Lee, S.M.; Oh, B.T.; Chae, J.C.; Lee, K.J. Alleviation of salt stress by Enterobacter sp. EJ01 in tomato and Arabidopsis is accompanied by up-regulation of conserved salinity responsive factors in plants. Mol. Cells 2014, 37, 109–117. [Google Scholar] [CrossRef]
- Isolation, A. Screening of Arsenic Resistant Bacteria and Evaluation of Its Effect on Growth of Tomato (Solanum lycopersicum L.). Master’s Thesis, Faculty of Agriculture, University of Agricultural Sciences, Raichur, India, 2018. [Google Scholar]
- Mota, R.; Rossi, F.; Andrenelli, L.; Pereira, S.B.; De Philippis, R.; Tamagnini, P. Released polysaccharides (RPS) from Cyanothece sp. CCY 0110 as biosorbent for heavy metals bioremediation: Interactions between metals and RPS binding sites. Appl. Microbiol. Biotechnol. 2016, 100, 7765–7775. [Google Scholar] [CrossRef]
- Zhu, X.; Lv, B.; Shang, X.; Wang, J.; Li, M.; Yu, X. The immobilization effects on Pb, Cd and Cu by the inoculation of organic phosphorus-degrading bacteria (OPDB) with rapeseed dregs in acidic soil. Geoderma 2019, 350, 1–10. [Google Scholar] [CrossRef]
- Nicolaus, B.; Kambourova, M.; Oner, E.T. Exopolysaccharides from extremophiles: From fundamentals to biotechnology. Environ. Technol. 2010, 31, 1145–1158. [Google Scholar] [CrossRef]
- Gugliandolo, C.; Lentini, V.; Spanò, A.; Maugeri, T.L. New bacilli from shallow hydrothermal vents of Panarea Island (Italy) and their biotechnological potentialities. J. Appl. Microbiol. 2012, 112, 1102–1112. [Google Scholar] [CrossRef]
- Spanò, A.; Zammuto, V.; Macrì, A.; Agostino, E.; Nicolò, M.S.; Scala, A.; Trombetta, D.; Smeriglio, A.; Ingegneri, M.; Caccamo, M.T.; et al. Arsenic Adsorption and Toxicity Reduction of An Exopolysaccharide Produced by Bacillus licheniformis B3-15 of Shallow Hydrothermal Vent Origin. J. Mar. Sci. Eng. 2023, 11, 325. [Google Scholar] [CrossRef]
- Li, H.; Qiu, Y.; Yao, T.; Ma, Y.; Zhang, H.; Yang, X. Effects of PGPR microbial inoculants on the growth and soil properties of Avena sativa, Medicago sativa, and Cucumis sativus seedlings. Soil Tillage Res. 2020, 199, 104577. [Google Scholar] [CrossRef]



| Chemical Characteristics | Unit | Value | Physical Characteristics | Unit | Value |
|---|---|---|---|---|---|
| EC | dS m−1 | 0.225 | Particle size distribution | ||
| pH (Soil suspension 1:2.5) | 7.95 | Clay | % | 1.6 | |
| Soluble ions | Silt | % | 1.7 | ||
| Na+ | mmol L−1 | 0.42 | Sand | % | 96.70 |
| K+ | mmol L−1 | 0.24 | Texture class | Sandy | |
| Ca+2 | mmol L−1 | 1.00 | Field capacity | % | 11.6 |
| Mg+2 | mmol L−1 | 0.57 | Bulk density | g cm−3 | 1.48 |
| Cl− | mmol L−1 | 1.20 | |||
| HCO3− | mmol L−1 | 0.92 | |||
| SO4−2 | mmol L−1 | 0.11 | |||
| SOM a | % | 0.003 | |||
| MBC b | % | 0.002 | |||
| CaCO3 | % | 4.07 | |||
| CEC | cmol kg−1 | 4.65 | |||
| Total N | 0.02 | ||||
| Total P | 0.07 | ||||
| Total K | % | 0.0002 | |||
| Arsenic (As) | mg kg−1 | 0.05 | |||
| Treatments | Total Chlorophyll μmol m−2 | Root Length cm | Shoot Length cm | Fresh Weight g plant−1 | Dry Weight g plant−1 |
|---|---|---|---|---|---|
| A | 8.90 ± 1.25 g | 1.97 ± 0.25 e | 15.00 ± 2.65 f | 6.30 ± 0.06 c | 2.10 ± 0.02 b |
| AB1 | 18.75 ± 1.25 d | 2.10 ± 0.15 e | 17.67 ± 2.89 b | 7.41 ± 0.06 b | 2.47 ± 0.02 a |
| AB2 | 19.95 ± 0.15 c | 2.10 ± 0.10 e | 16.00 ± 2.65 e | 7.47 ± 0.01 ab | 2.49 ± 0.00 a |
| AN | 16.90 ± 0.80 f | 2.57 ± 0.31 d | 16.67 ± 0.58 d | 7.61 ± 0.02 a | 2.54 ± 0.01 a |
| AB1N | 17.57 ± 2.46 e | 3.17 ± 0.38 b | 16.67 ± 0.58 d | 7.37 ± 0.6 a | 2.54 ± 0.03 a |
| AB2N | 23.45 ± 1.05 b | 3.00 ± 0.00 c | 17.00 ± 3.00 c | 7.41 ± 0.03 b | 2.56 ± 0.02 a |
| AB1 B2 | 24.20 ± 1.10 a | 6.20 ± 0.20 a | 18.33 ± 1.53 a | 7.61 ± 0.08 a | 2.57 ± 0.01 a |
| LSD | 2.46 | 0.43 | 3.46 | 1.62 | 0.32 |
| Treatments | pH | SOM % | MBC mg Kg−1 | DHA mg TPF g−1 Soil day−1 | Total Count of Bacteria CFU, log 10 g−1 |
|---|---|---|---|---|---|
| A | 7.51 ± 0.06 b | 0.59 ± 0.03 d | 0.08 ± 0.02 d | 61.67 ± 3.51 g | 3.51 ± 0.30 e |
| AB1 | 7.34 ± 0.080 c | 0.76 ± 0.04 c | 0.12 ± 0.06 d | 139.67 ± 1.53 e | 5.08 ± 0.16 c |
| AB2 | 7.02 ± 0.02 d | 0.91 ± 0.03 a | 0.36 ± 0.05 b | 182.33 ± 5.69 c | 5.73 ± 0.43 b |
| AN | 7.73 ± 0.10 a | 0.77 ± 0.03 c | 0.10 ± 0.04 d | 121.00 ± 2.65 f | 4.51 ± 0.34 d |
| AB1N | 7.69 ± 0.02 a | 0.86 ± 0.04 b | 0.19 ± 0.09 c | 148.33 ± 4.04 d | 5.26 ± 0.13 c |
| AB2N | 7.67 ± 0.01 a | 0.87 ± 0.01 a b | 0.33 ± 0.04 b | 189.00 ± 4.00 b | 6.15 ± 0.13 ab |
| AB1 B2 | 6.81 ± 0.04 e | 0.92 ± 0.04 a | 0.87 ± 0.04 a | 206.33 ± 7.64 a | 6.35 ± 0.12 a |
| LSD | 0.07 | 0.05 | 0.09 | 5.07 | 0.44 |
| Treatments | As Available mg Kg−1 | As (mg/kg plant) | % | As Uptake (mg pots−1) | % |
|---|---|---|---|---|---|
| A | 46.33 ± 0.58 f | 17.75 ± 0.58 e | 0.224 e | ||
| AB1 | 48.67 ± 0.58 d | 14.44 ± 0.58 c | 18.64 | 0.214 d | 4.46 |
| AB2 | 51.00 ± 1.00 c | 13.52 ± 1.00 b | 23.83 | 0.202 c | 9.82 |
| AN | 33.67 ± 0.58 g | 11.75 ± 0.58 a | 33.80 | 0.18 a | 19.64 |
| AB1N | 49.67 ± 0.58 cd | 12.21 ± 0.58 ab | 31.21 | 0.186 b | 16.96 |
| AB2N | 52.33 ± 0.58 bc | 11.25 ± 1.00 a | 36.62 | 0.173 a | 22.76 |
| AB1B2 | 55.67 ± 0.58 a | 12.54 ± 0.58 ab | 29.35 | 0.193 bc | 13.83 |
| LSD | 1.14 | 1.26 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Sharkawy, M.; AL-Huqail, A.A.; Aljuaid, A.M.; Kamal, N.; Mahmoud, E.; Omara, A.E.-D.; El-Kader, N.A.; Li, J.; Mahmoud, N.N.; El Baroudy, A.A.; et al. Nano-Bioremediation of Arsenic and Its Effect on the Biological Activity and Growth of Maize Plants Grown in Highly Arsenic-Contaminated Soil. Nanomaterials 2024, 14, 1164. https://doi.org/10.3390/nano14131164
El Sharkawy M, AL-Huqail AA, Aljuaid AM, Kamal N, Mahmoud E, Omara AE-D, El-Kader NA, Li J, Mahmoud NN, El Baroudy AA, et al. Nano-Bioremediation of Arsenic and Its Effect on the Biological Activity and Growth of Maize Plants Grown in Highly Arsenic-Contaminated Soil. Nanomaterials. 2024; 14(13):1164. https://doi.org/10.3390/nano14131164
Chicago/Turabian StyleEl Sharkawy, Mahmoud, Arwa A. AL-Huqail, Alya M. Aljuaid, Nourhan Kamal, Esawy Mahmoud, Alaa El-Dein Omara, Nasser Abd El-Kader, Jian Li, Nashaat N. Mahmoud, Ahmed A. El Baroudy, and et al. 2024. "Nano-Bioremediation of Arsenic and Its Effect on the Biological Activity and Growth of Maize Plants Grown in Highly Arsenic-Contaminated Soil" Nanomaterials 14, no. 13: 1164. https://doi.org/10.3390/nano14131164
APA StyleEl Sharkawy, M., AL-Huqail, A. A., Aljuaid, A. M., Kamal, N., Mahmoud, E., Omara, A. E.-D., El-Kader, N. A., Li, J., Mahmoud, N. N., El Baroudy, A. A., Ghoneim, A. M., & Ismail, S. M. (2024). Nano-Bioremediation of Arsenic and Its Effect on the Biological Activity and Growth of Maize Plants Grown in Highly Arsenic-Contaminated Soil. Nanomaterials, 14(13), 1164. https://doi.org/10.3390/nano14131164









