Synthesis, Structural Analysis, and Peroxidase-Mimicking Activity of AuPt Branched Nanoparticles
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Synthesis of NPs
2.3. Functionalization of NPs with PAcMA
2.4. Enzymatic Activity Studies
2.5. Characterization
3. Results and Discussion
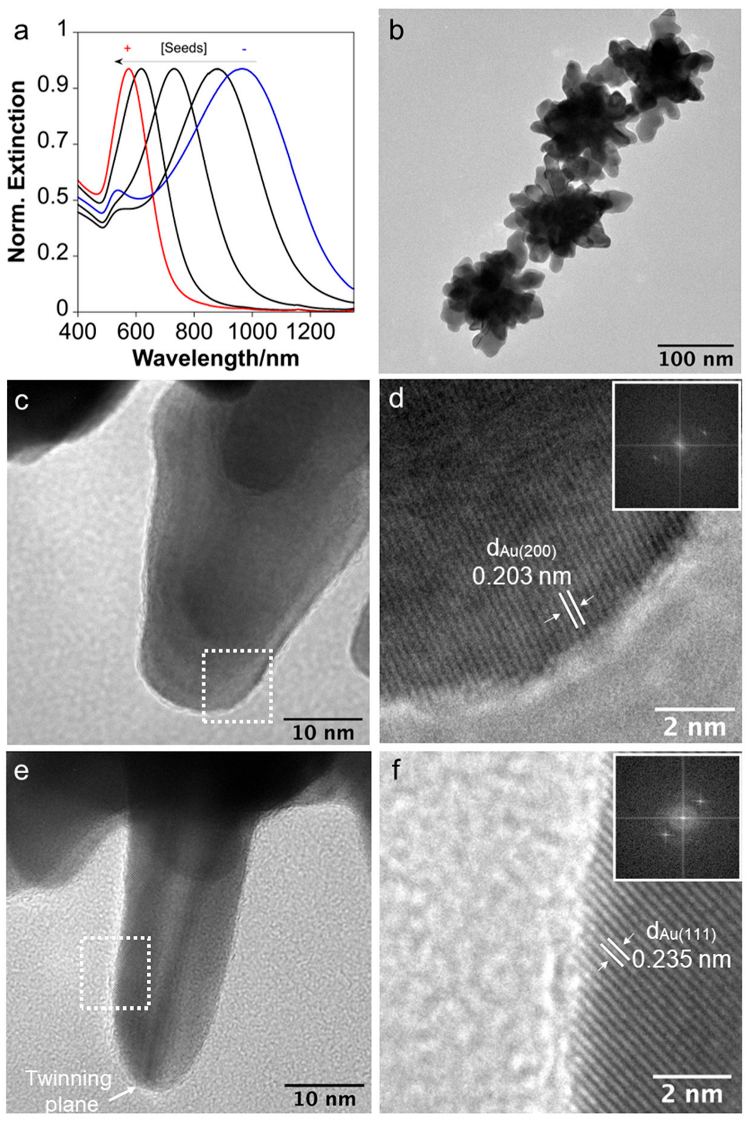
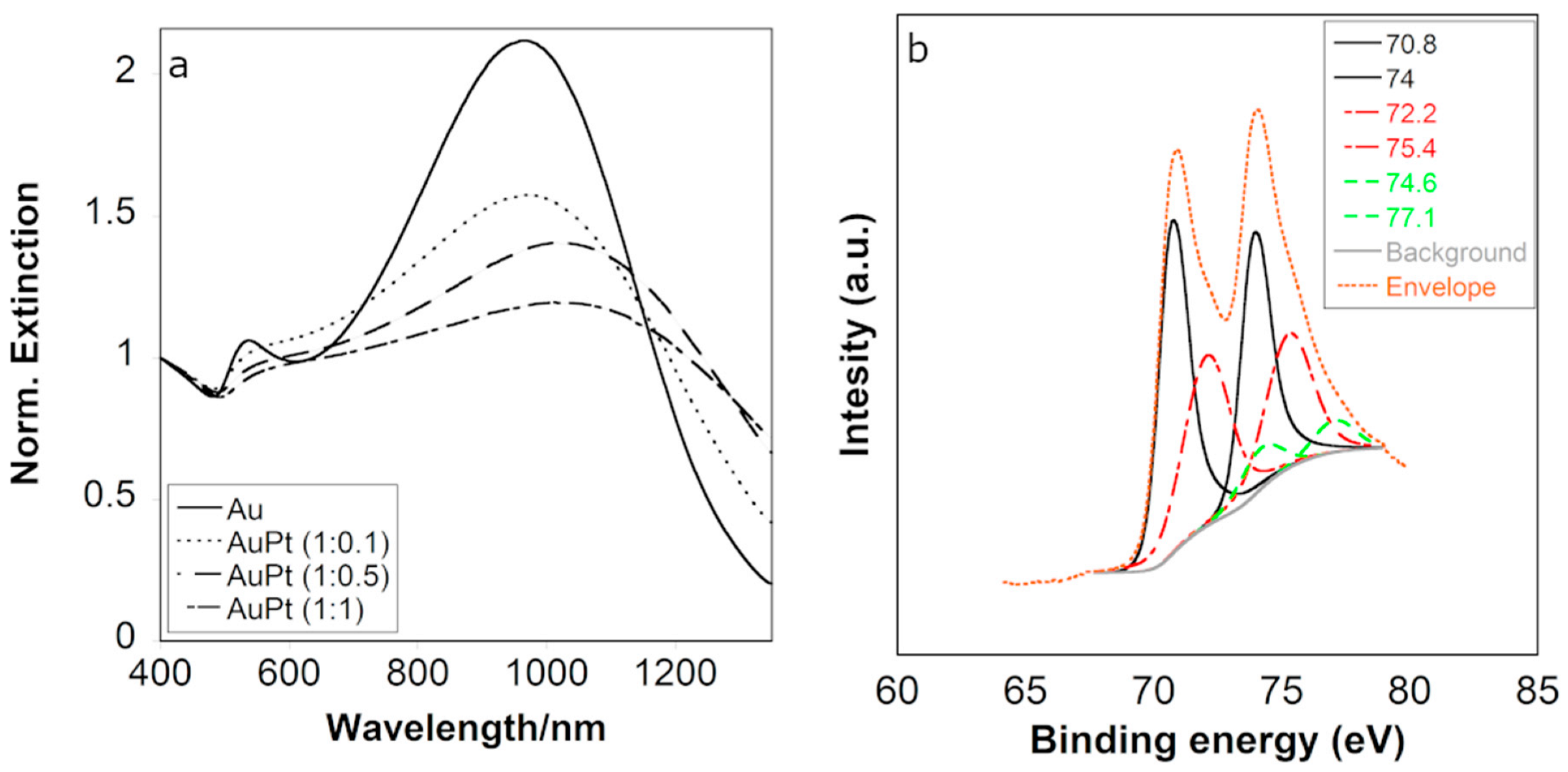
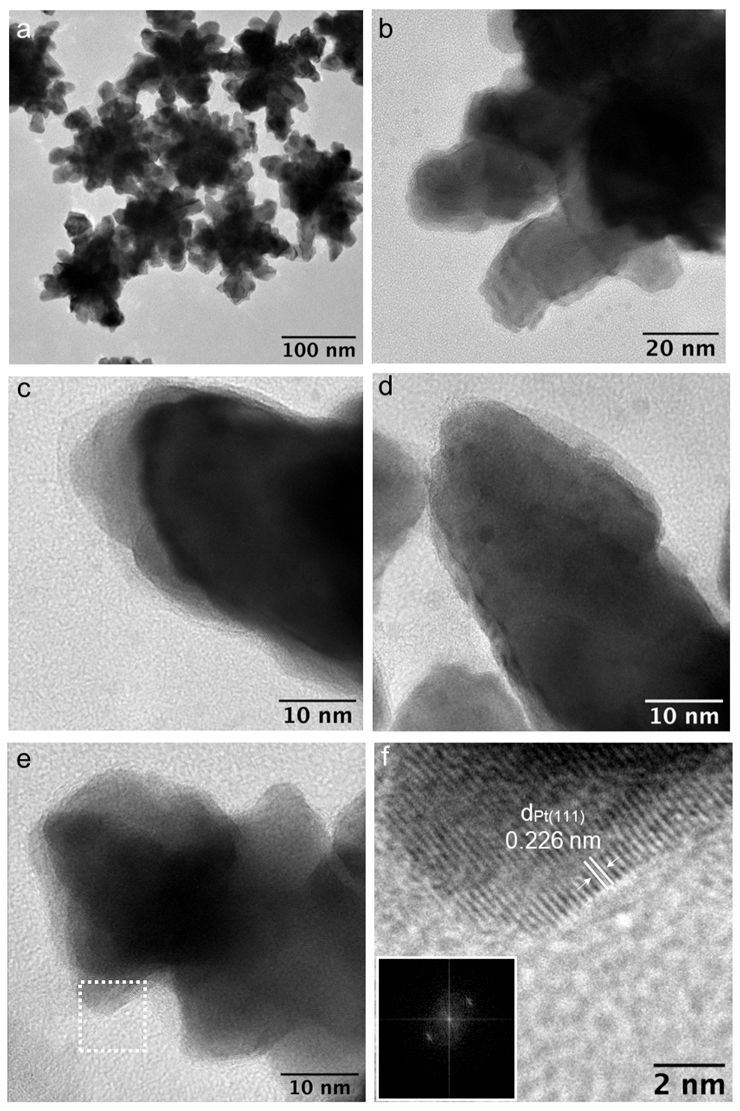
3.1. Synthesis and Characterization of Nanoparticles
3.2. Catalysis Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melián-Cabrera, I. Catalytic Materials: Concepts to Understand the Pathway to Implementation. Ind. Eng. Chem. Res. 2021, 60, 18545–18559. [Google Scholar] [CrossRef]
- Olveira, S.; Forster, S.P.; Seeger, S. Nanocatalysis: Academic Discipline and Industrial Realities. J. Nanotechnol. 2014, 2014, 1–19. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Wang, H.; Uemura, Y.; Nakamura, T.; Yu, L.; Sekizawa, O.; Uruga, T.; Tada, M.; Samjeské, G.; Iwasawa, Y.; et al. In Situ Study of Oxidation States of Platinum Nanoparticles on a Polymer Electrolyte Fuel Cell Electrode by near Ambient Pressure Hard X-Ray Photoelectron Spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 6013–6021. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Liu, C.; Su, D.; Xin, H.L.; Fang, H.T.; Eren, B.; Zhang, S.; Murray, C.B.; Salmeron, M.B. Bimetallic Synergy in Cobalt–Palladium Nanocatalysts for CO Oxidation. Nat. Catal. 2019, 2, 78–85. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, X.; Zhang, G.; Li, J.; Liu, Y.; Yan, X. Novel Targeted PH-Responsive Drug Delivery Systems Based on PEGMA-Modified Bimetallic Prussian Blue Analogs for Breast Cancer Chemotherapy. RSC Adv. 2023, 13, 1684–1700. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.-Q.; Zi, T.-Q.; Liu, C.; Cui, D.-P.; Wu, D.; Li, A.-D. Co–Pt Bimetallic Nanoparticles with Tunable Magnetic and Electrocatalytic Properties Prepared by Atomic Layer Deposition. Chem. Commun. 2020, 56, 8675–8678. [Google Scholar] [CrossRef] [PubMed]
- Zaleska-Medynska, A.; Marchelek, M.; Diak, M.; Grabowska, E. Noble Metal-Based Bimetallic Nanoparticles: The Effect of the Structure on the Optical, Catalytic and Photocatalytic Properties. Adv. Colloid Interface Sci. 2016, 229, 80–107. [Google Scholar] [CrossRef]
- Pearce, A.K.; Wilks, T.R.; Arno, M.C.; O’Reilly, R.K. Synthesis and Applications of Anisotropic Nanoparticles with Precisely Defined Dimensions. Nat. Rev. Chem. 2020, 5, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Nuti, S.; Fernández-Lodeiro, C.; Fernández-Lodeiro, J.; Fernández-Lodeiro, A.; Pérez-Juste, J.; Pastoriza-Santos, I.; LaGrow, A.P.; Schraidt, O.; Luis Capelo-Martínez, J.; Lodeiro, C. Polyallylamine Assisted Synthesis of 3D Branched AuNPs with Plasmon Tunability in the Vis-NIR Region as Refractive Index Sensitivity Probes. J. Colloid Interface Sci. 2022, 611, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lodeiro, C.; Fernández-Lodeiro, J.; Fernández-Lodeiro, A.; Nuti, S.; Lodeiro, C.; LaGrow, A.; Pérez-Juste, I.; Pérez-Juste, J.; Pastoriza-Santos, I. Synthesis of Tuneable Gold Nanostars: The Role of Adenosine Monophosphate. J. Mater. Chem. C 2023, 11, 12626–12636. [Google Scholar] [CrossRef]
- Tsoulos, T.V.; Fabris, L. Interface and Bulk Standing Waves Drive the Coupling of Plasmonic Nanostar Antennas. J. Phys. Chem. C 2018, 122, 28949–28957. [Google Scholar] [CrossRef]
- An, H.; Lv, Z.; Zhang, K.; Deng, C.; Wang, H.; Xu, Z.; Wang, M.; Yin, Z. Plasmonic Coupling Enhancement of Core-Shell Au@Pt Assemblies on ZnIn2S4 Nanosheets towards Photocatalytic H2 Production. Appl. Surf. Sci. 2021, 536, 147934. [Google Scholar] [CrossRef]
- Zhao, Y.; Fu, Q.; Cui, X.; Chi, H.; Lu, Y.; Liu, X.; Yu, M.; Fei, Q.; Feng, G.; Shan, H.; et al. A Colorimetric Sensor for Detecting Thiourea Based on Inhibiting Peroxidase-like Activity of Gold-Platinum Nanoparticles. Anal. Methods 2021, 13, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulou, A.P.; Theodosiou, M.; Sakellis, E.; Boukos, N.; Papanastasiou, G.; Wang, C.; Tavares, A.; Corral, C.A.; Gournis, D.; Chalmpes, N.; et al. Bimetallic Gold-Platinum Nanoparticles as a Drug Delivery System Coated with a New Drug to Target Glioblastoma. Colloids Surf. B Biointerfaces 2022, 214, 112463. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Peng, J.; Xiao, Y.; Li, W.; Tan, L.; Xu, X.; Qian, Z. Porous Au@Pt Nanoparticles: Therapeutic Platform for Tumor Chemo-Photothermal Co-Therapy and Alleviating Doxorubicin-Induced Oxidative Damage. ACS Appl. Mater. Interfaces 2018, 10, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xu, J.; Wang, X.; Zhu, X.; Kan, C. Preparation of Gold Nanostars Covered with Platinum Particles and Their Photoelectrocatalysis Properties. CrystEngComm 2023, 25, 6814–6821. [Google Scholar] [CrossRef]
- Bazán-Díaz, L.; Mendoza-Cruz, R.; Liao, C.-K.; Mahmoud, M.A. Asymmetric Deposition of Platinum Atoms on Gold Nanorods Reduced the Plasmon Field Distortion Induced by the Substrate. J. Phys. Chem. C 2019, 123, 30509–30518. [Google Scholar] [CrossRef]
- Grzelczak, M.; Pérez-Juste, J.; De Abajo, F.J.G.; Liz-Marzán, L.M. Optical Properties of Platinum-Coated Gold Nanorods. J. Phys. Chem. C 2007, 111, 6183–6188. [Google Scholar] [CrossRef]
- Pedrazo-Tardajos, A.; Irmak, E.A.; Kumar, V.; Sánchez-Iglesias, A.; Chen, Q.; Wirix, M.; Freitag, B.; Albrecht, W.; Van Aert, S.; Liz-Marzán, L.M.; et al. Thermal Activation of Gold Atom Diffusion in Au@Pt Nanorods. ACS Nano 2022, 16, 9608–9619. [Google Scholar] [CrossRef] [PubMed]
- Bian, T.; Sun, B.; Luo, S.; Huang, L.; Su, S.; Meng, C.; Su, S.; Yuan, A.; Zhang, H. Seed-Mediated Synthesis of Au@PtCu Nanostars with Rich Twin Defects as Efficient and Stable Electrocatalysts for Methanol Oxidation Reaction. RSC Adv. 2019, 9, 35887–35894. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.; Mai, H.D.; Yoo, H. Platinum Overgrowth on Gold Multipod Nanoparticles: Investigation of Synergistic Catalytic Effects in a Bimetallic Nanosystem. ChemNanoMat 2017, 3, 196–203. [Google Scholar] [CrossRef]
- Peng, Y.; Li, L.; Tao, R.; Tan, L.; Qiu, M.; Guo, L. One-Pot Synthesis of Au@Pt Star-like Nanocrystals and Their Enhanced Electrocatalytic Performance for Formic Acid and Ethanol Oxidation. Nano Res. 2018, 11, 3222–3232. [Google Scholar] [CrossRef]
- Jiang, B.; Duan, D.; Gao, L.; Zhou, M.; Fan, K.; Tang, Y.; Xi, J.; Bi, Y.; Tong, Z.; Gao, G.F.; et al. Standardized Assays for Determining the Catalytic Activity and Kinetics of Peroxidase-like Nanozymes. Nat. Protoc. 2018, 13, 1506–1520. [Google Scholar] [CrossRef]
- Nuti, S.; Fernández-Lodeiro, A.; Chinchilla, L.E.; Hungría, A.B.; Capelo-Martinez, J.-L.; Lodeiro, C.; Fernández-Lodeiro, J. Synthesis and Structural Characterization of Branched Bimetallic AuPd Nanoparticles with a Highly Tunable Optical Response. J. Phys. Chem. Lett. 2023, 14, 6315–6320. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Adhikari, C.R.; Kawakita, H.; Ohto, K.; Inoue, K.; Harada, H. Selective Recovery of Precious Metals by Persimmon Waste Chemically Modified with Dimethylamine. Bioresour. Technol. 2009, 100, 4083–4089. [Google Scholar] [CrossRef]
- Ooi, M.D.J.; Aziz, A.A. Enhanced Pt Surface Activation: A Strategy for Catalyst Application. Int. J. Hydrog. Energy 2019, 44, 30532–30542. [Google Scholar] [CrossRef]
- Navaee, A.; Salimi, A. Anodic Platinum Dissolution, Entrapping by Amine Functionalized-Reduced Graphene Oxide: A Simple Approach to Derive the Uniform Distribution of Platinum Nanoparticles with Efficient Electrocatalytic Activity for Durable Hydrogen Evolution and Ethanol Oxid. Electrochim. Acta 2016, 211, 322–330. [Google Scholar] [CrossRef]
- Huang, X.Y.; Wang, A.J.; Zhang, L.; Fang, K.M.; Wu, L.J.; Feng, J.J. Melamine-Assisted Solvothermal Synthesis of PtNi Nanodentrites as Highly Efficient and Durable Electrocatalyst for Hydrogen Evolution Reaction. J. Colloid. Interface Sci. 2018, 531, 578–584. [Google Scholar] [CrossRef]
- Rónavári, A.; Bélteky, P.; Boka, E.; Zakupszky, D.; Igaz, N.; Szerencsés, B.; Pfeiffer, I.; Kónya, Z.; Kiricsi, M. Polyvinyl-Pyrrolidone-Coated Silver Nanoparticles—The Colloidal, Chemical and Biological Consequences of Steric Stabilization under Biorelevant Conditions. Int. J. Mol. Sci. 2021, 22, 8673. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Buchman, J.T.; Rodriguez, R.S.; Ring, H.L.; He, J.; Bantz, K.C.; Haynes, C.L. Stabilization of Silver and Gold Nanoparticles: Preservation and Improvement of Plasmonic Functionalities. Chem. Rev. 2019, 119, 664–699. [Google Scholar] [CrossRef] [PubMed]
- Sylvestre, J.P.; Poulin, S.; Kabashin, A.V.; Sacher, E.; Meunier, M.; Luong, J.H.T. Surface Chemistry of Gold Nanoparticles Produced by Laser Ablation in Aqueous Media. J. Phys. Chem. B 2004, 108, 16864–16869. [Google Scholar] [CrossRef]
- Shao, J.; Liu, M.; Wang, Z.; Li, K.; Bao, B.; Zhao, S.; Zhou, S. Controllable Synthesis of Surface Pt-Rich Bimetallic AuPt Nanocatalysts for Selective Hydrogenation Reactions. ACS Omega 2019, 4, 15621–15627. [Google Scholar] [CrossRef] [PubMed]
- Muelas-Ramos, V.; Belver, C.; Rodriguez, J.J.; Bedia, J. Synthesis of Noble Metal-Decorated NH2-MIL-125 Titanium MOF for the Photocatalytic Degradation of Acetaminophen under Solar Irradiation. Sep. Purif. Technol. 2021, 272, 118896. [Google Scholar] [CrossRef]
- Shyu, J. Characterization of Pt/$gamma;-Alumina Catalysts Containing Ceria. J. Catal. 1989, 115, 16–23. [Google Scholar] [CrossRef]
- Bera, P.; Priolkar, K.R.; Gayen, A.; Sarode, P.R.; Hegde, M.S.; Emura, S.; Kumashiro, R.; Jayaram, V.; Subbanna, G.N. Ionic Dispersion of Pt over CeO2 by the Combustion Method: Structural Investigation by XRD, TEM, XPS, and EXAFS. Chem. Mater. 2003, 15, 2049–2060. [Google Scholar] [CrossRef]
- Bonet-Aleta, J.; Garcia-Peiro, J.I.; Irusta, S.; Hueso, J.L. Gold-Platinum Nanoparticles with Core-Shell Configuration as Efficient Oxidase-like Nanosensors for Glutathione Detection. Nanomaterials 2022, 12, 755. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.-R.; Liu, D.-Y.; Wu, Y.-F.; Duan, S.; Xie, Z.-X.; Jiang, Z.-Y.; Tian, Z.-Q. Epitaxial Growth of Heterogeneous Metal Nanocrystals: From Gold Nano-Octahedra to Palladium and Silver Nanocubes. J. Am. Chem. Soc. 2008, 130, 6949–6951. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Wu, K.; Jiang, X.; Tao, L.; Chen, Y.; Lin, J.; Zhou, Y.; Wei, S.; Tang, Y.; Lu, T.; et al. Polyallylamine-Directed Green Synthesis of Platinum Nanocubes. Shape and Electronic Effect Codependent Enhanced Electrocatalytic Activity. Phys. Chem. Chem. Phys. 2013, 15, 3793–3802. [Google Scholar] [CrossRef] [PubMed]
- Divi, S.; Chatterjee, A. Understanding Segregation Behavior in AuPt, NiPt, and AgAu Bimetallic Nanoparticles Using Distribution Coefficients. J. Phys. Chem. C 2016, 120, 27296–27306. [Google Scholar] [CrossRef]
- Danielis, N.; Vega, L.; Fronzoni, G.; Stener, M.; Bruix, A.; Neyman, K.M. AgPd, AuPd, and AuPt Nanoalloys with Ag- or Au-Rich Compositions: Modeling Chemical Ordering and Optical Properties. J. Phys. Chem. C 2021, 125, 17372–17384. [Google Scholar] [CrossRef] [PubMed]
- Higareda, A.; Kumar-Krishnan, S.; García-Ruiz, A.F.; Maya-Cornejo, J.; Lopez-Miranda, J.L.; Bahena, D.; Rosas, G.; Pérez, R.; Esparza, R. Synthesis of Au@Pt Core–Shell Nanoparticles as Efficient Electrocatalyst for Methanol Electro-Oxidation. Nanomaterials 2019, 9, 1644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; Gao, Y.C.; Li, H.W.; Wu, Y. Gold-Platinum Bimetallic Nanoclusters for Oxidase-like Catalysis. ACS Appl. Nano Mater. 2020, 3, 9318–9328. [Google Scholar] [CrossRef]
- Fu, Z.; Zeng, W.; Cai, S.; Li, H.; Ding, J.; Wang, C.; Chen, Y.; Han, N.; Yang, R. Porous Au@Pt Nanoparticles with Superior Peroxidase-like Activity for Colorimetric Detection of Spike Protein of SARS-CoV-2. J. Colloid Interface Sci. 2021, 604, 113–121. [Google Scholar] [CrossRef]
- Bao, Y.-W.; Hua, X.-W.; Ran, H.-H.; Zeng, J.; Wu, F.-G. Metal-Doped Carbon Nanoparticles with Intrinsic Peroxidase-like Activity for Colorimetric Detection of H 2 O 2 and Glucose. J. Mater. Chem. B 2019, 7, 296–304. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Han, X.; Jia, H.; Cai, J.; Zhou, Y.; Zheng, Z. AuPt Alloy Nanostructures with Tunable Composition and Enzyme-like Activities for Colorimetric Detection of Bisulfide. Sci. Rep. 2017, 7, 40103. [Google Scholar] [CrossRef] [PubMed]
- Navyatha, B.; Singh, S.; Nara, S. AuPeroxidase Nanozymes: Promises and Applications in Biosensing. Biosens. Bioelectron. 2021, 175, 112882. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, R.; Liu, X.; Shan, G.; Chen, Y.; Tong, T.; Liu, Y. Laser-Induced Formation of Au/Pt Nanorods with Peroxidase Mimicking and SERS Enhancement Properties for Application to the Colorimetric Determination of H2O2. Microchim. Acta 2018, 185, 445. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic Peroxidase-like Activity of Ferromagnetic Nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-J.; Chen, T.-H.; Jiang, J.-Y.; Tseng, W.-L. Lysozyme-Directed Synthesis of Platinum Nanoclusters as a Mimic Oxidase. Nanoscale 2014, 6, 9618–9624. [Google Scholar] [CrossRef]
- Cui, Y.; Lai, X.; Liang, B.; Liang, Y.; Sun, H.; Wang, L. Polyethyleneimine-Stabilized Platinum Nanoparticles as Peroxidase Mimic for Colorimetric Detection of Glucose. ACS Omega 2020, 5, 6800–6808. [Google Scholar] [CrossRef] [PubMed]
- Son, S.E.; Gupta, P.K.; Hur, W.; Lee, H.B.; Park, Y.; Park, J.; Kim, S.N.; Seong, G.H. Citric Acid-Functionalized Rhodium–Platinum Nanoparticles as Peroxidase Mimics for Determination of Cholesterol. ACS Appl. Nano Mater. 2021, 4, 8282–8291. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, X.; Zhang, J.; Li, W. DNA-Based Platinum Nanozymes for Peroxidase. J. Phys. Chem. C 2014, 118, 18116–18125. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Liu, H.; Younis, M.R.; Lei, S.; Yang, C.; Lin, J.; Qu, J.; Huang, P. Ultrasmall Platinum Nanozymes as Broad-Spectrum Antioxidants for Theranostic Application in Acute Kidney Injury. Chem. Eng. J. 2021, 409, 127371. [Google Scholar] [CrossRef]
- Fan, L.; Ji, X.; Lin, G.; Liu, K.; Chen, S.; Ma, G.; Xue, W.; Zhang, X.; Wang, L. Green Synthesis of Stable Platinum Nanoclusters with Enhanced Peroxidase-like Activity for Sensitive Detection of Glucose and Glutathione. Microchem. J. 2021, 166, 106202. [Google Scholar] [CrossRef]
- Deng, H.-H.; Lin, X.-L.; Liu, Y.-H.; Li, K.-L.; Zhuang, Q.-Q.; Peng, H.-P.; Liu, A.-L.; Xia, X.H.; Chen, W. Chitosan-Stabilized Platinum Nanoparticles as Effective Oxidase Mimics for Colorimetric Detection of Acid Phosphatase. Nanoscale 2017, 9, 10292–10300. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, G.; Liu, W.; Zhang, Y.; Zhu, C.; Sun, Q.; Zhang, M.; Wei, H. Platinum– Nickel Nanoparticles with Enhanced Oxidase-like Activity for Total Antioxidant Capacity. Anal. Chem. 2023, 95, 5937–5945. [Google Scholar] [CrossRef] [PubMed]
- Pham, X.-H.; Tran, V.-K.; Hahm, E.; Kim, Y.-H.; Kim, J.; Kim, W.; Jun, B.-H. Synthesis of Gold-Platinum Core-Shell Nanoparticles Assembled on a Silica Template and Their Peroxidase Nanozyme Properties. Int. J. Mol. Sci. 2022, 23, 6424. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, K.; Gao, M.; Zhang, T.; Wang, L.; Cui, Y.; Ji, X.; Ma, G.; Hu, J. VancomycinStabilized Platinum Nanoparticles with Oxidase-like Activity for Sensitive Dopamine Detection. Biomolecules 2023, 13, 1312. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.C.; Wang, C.; Zhang, C.X.; Li, H.W.; Wu, Y. Controlled Preparation and Application of Glutathione Capped Gold and Platinum Alloy Nanoclusters with High Peroxidase-like Activity. J. Mater. Sci. Technol. 2022, 109, 140–146. [Google Scholar] [CrossRef]
- Shah, J.; Singh, S. Unveiling the Role of ATP in Amplification of Intrinsic Peroxidase-like of Gold Nanoparticles. 3 Biotech 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]

| TMB | H2O2 | |||
|---|---|---|---|---|
| Nanoparticle | Km (mM) | Vmax (10−8 M·s−1) | Km (mM) | Vmax (10−8 M·s−1) |
| Au NPs | 0.21 | 1.80 | 3229 | 1.72 |
| AuPt NPs | 0.42 | 73.2 | 1100.6 | 71.7 |
| Au@PACMA NPs | 0.22 | 2.78 | 3141 | 5.66 |
| AuPt@PACMA NPs | 0.80 | 146.4 | 291.5 | 58.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuti, S.; Fernández-Lodeiro, J.; Palomo, J.M.; Capelo-Martinez, J.-L.; Lodeiro, C.; Fernández-Lodeiro, A. Synthesis, Structural Analysis, and Peroxidase-Mimicking Activity of AuPt Branched Nanoparticles. Nanomaterials 2024, 14, 1166. https://doi.org/10.3390/nano14131166
Nuti S, Fernández-Lodeiro J, Palomo JM, Capelo-Martinez J-L, Lodeiro C, Fernández-Lodeiro A. Synthesis, Structural Analysis, and Peroxidase-Mimicking Activity of AuPt Branched Nanoparticles. Nanomaterials. 2024; 14(13):1166. https://doi.org/10.3390/nano14131166
Chicago/Turabian StyleNuti, Silvia, Javier Fernández-Lodeiro, Jose M. Palomo, José-Luis Capelo-Martinez, Carlos Lodeiro, and Adrián Fernández-Lodeiro. 2024. "Synthesis, Structural Analysis, and Peroxidase-Mimicking Activity of AuPt Branched Nanoparticles" Nanomaterials 14, no. 13: 1166. https://doi.org/10.3390/nano14131166
APA StyleNuti, S., Fernández-Lodeiro, J., Palomo, J. M., Capelo-Martinez, J.-L., Lodeiro, C., & Fernández-Lodeiro, A. (2024). Synthesis, Structural Analysis, and Peroxidase-Mimicking Activity of AuPt Branched Nanoparticles. Nanomaterials, 14(13), 1166. https://doi.org/10.3390/nano14131166








