What Are the Key Factors for the Detection of Peptides Using Mass Spectrometry on Boron-Doped Diamond Surfaces?
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
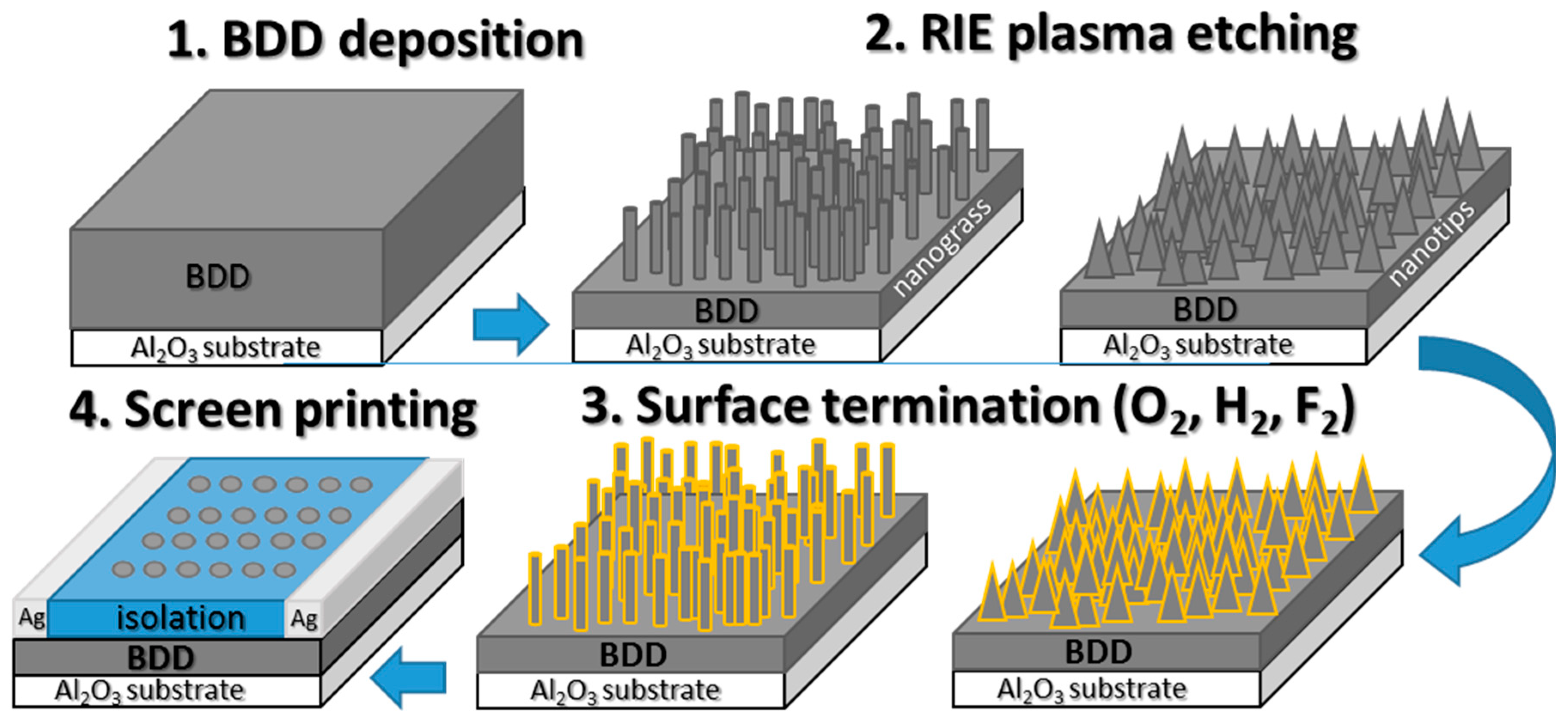
2.2. Diamond Growth and Structuring
2.3. Substrate Preparation for MALDI-MS Analysis
2.4. Scanning Electron Microscopy
2.5. Raman Spectroscopy
2.6. Contact Angle Measurements
2.7. Photoelectron Spectroscopy
2.8. Sample Preparation
2.9. MALDI-MS Analysis
3. Results and Discussion
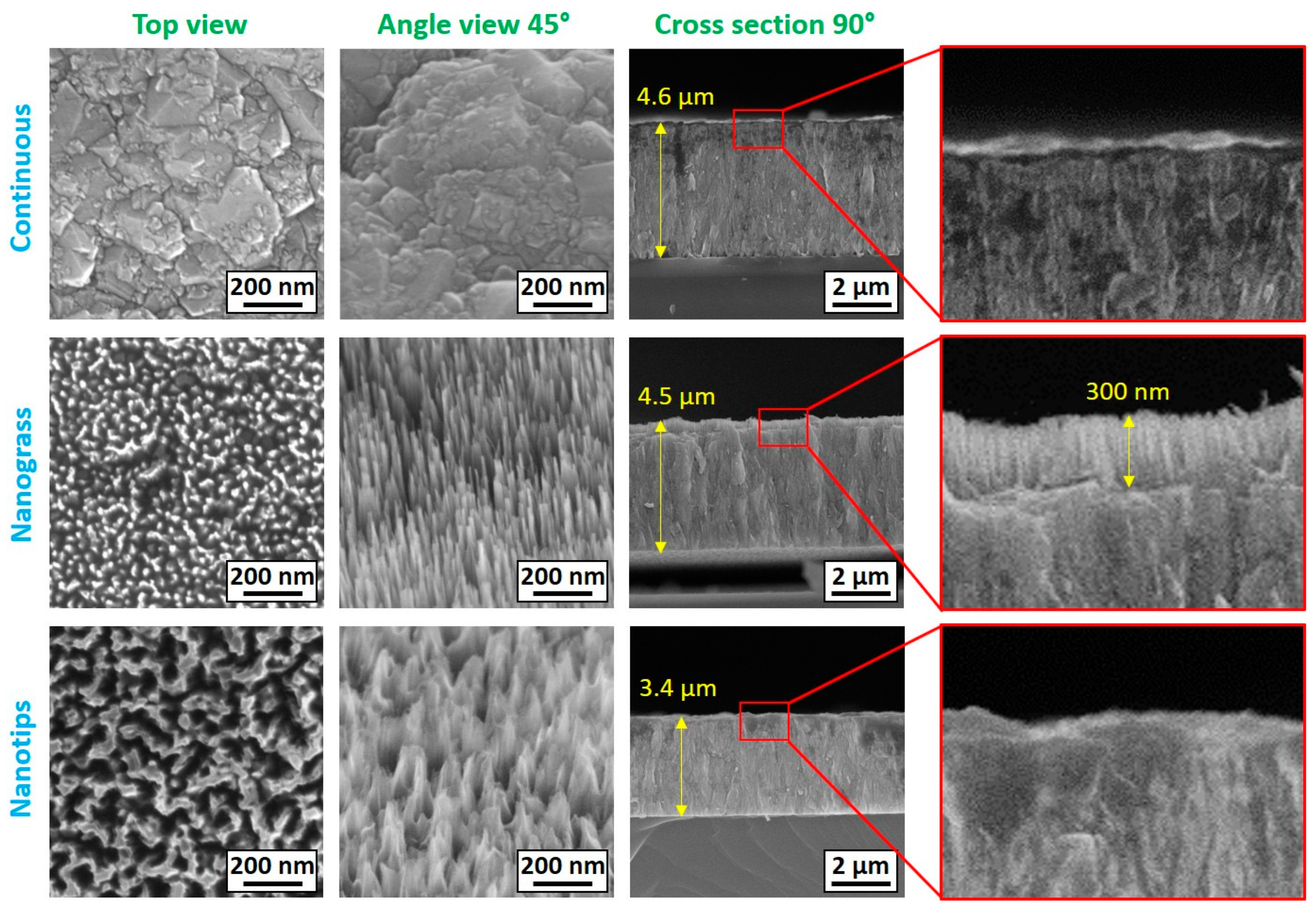
3.1. SEM Analysis of BDD
3.2. Raman Analysis of BDD
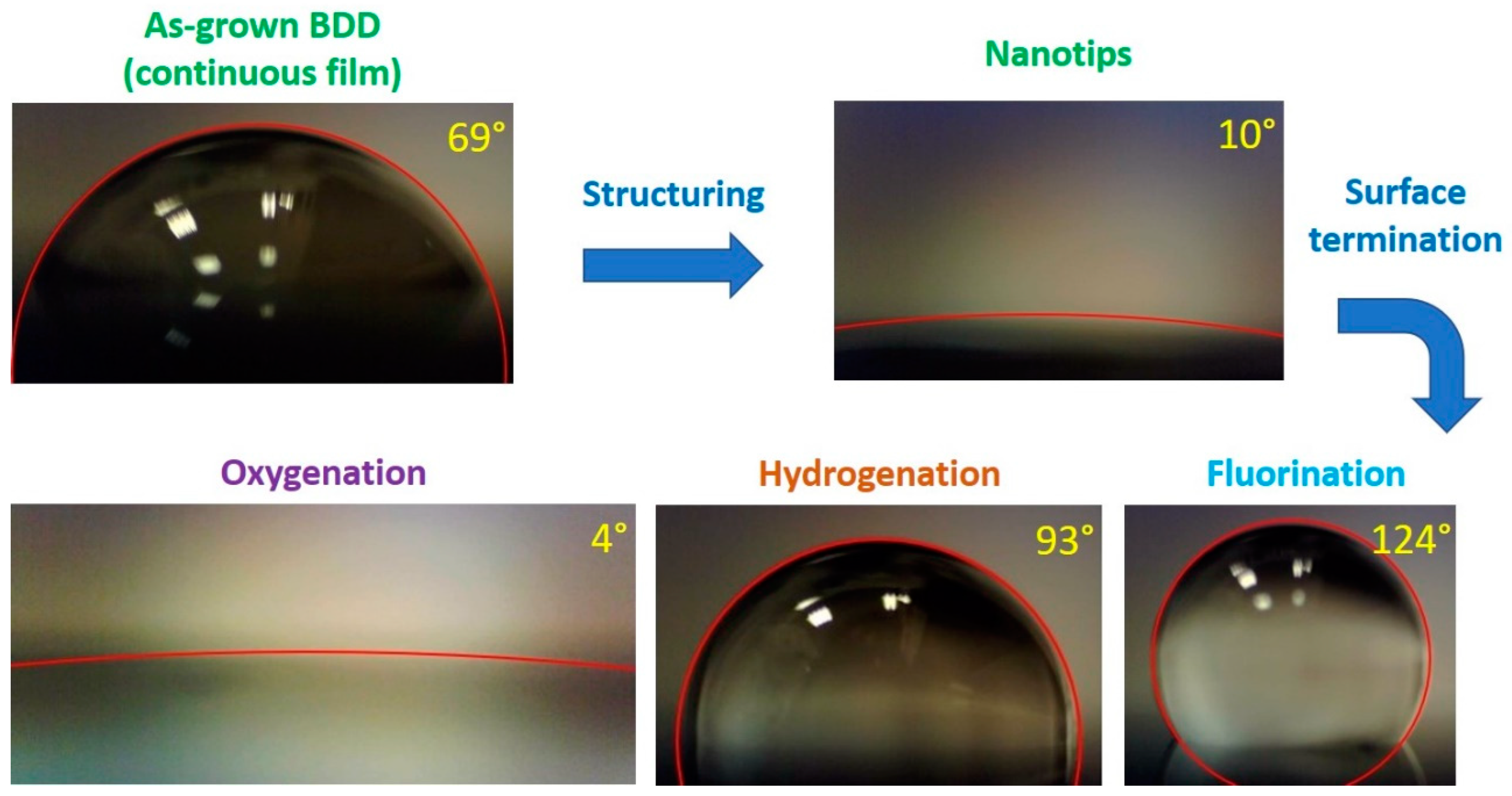
3.3. Contact Angle Measurements of BDD
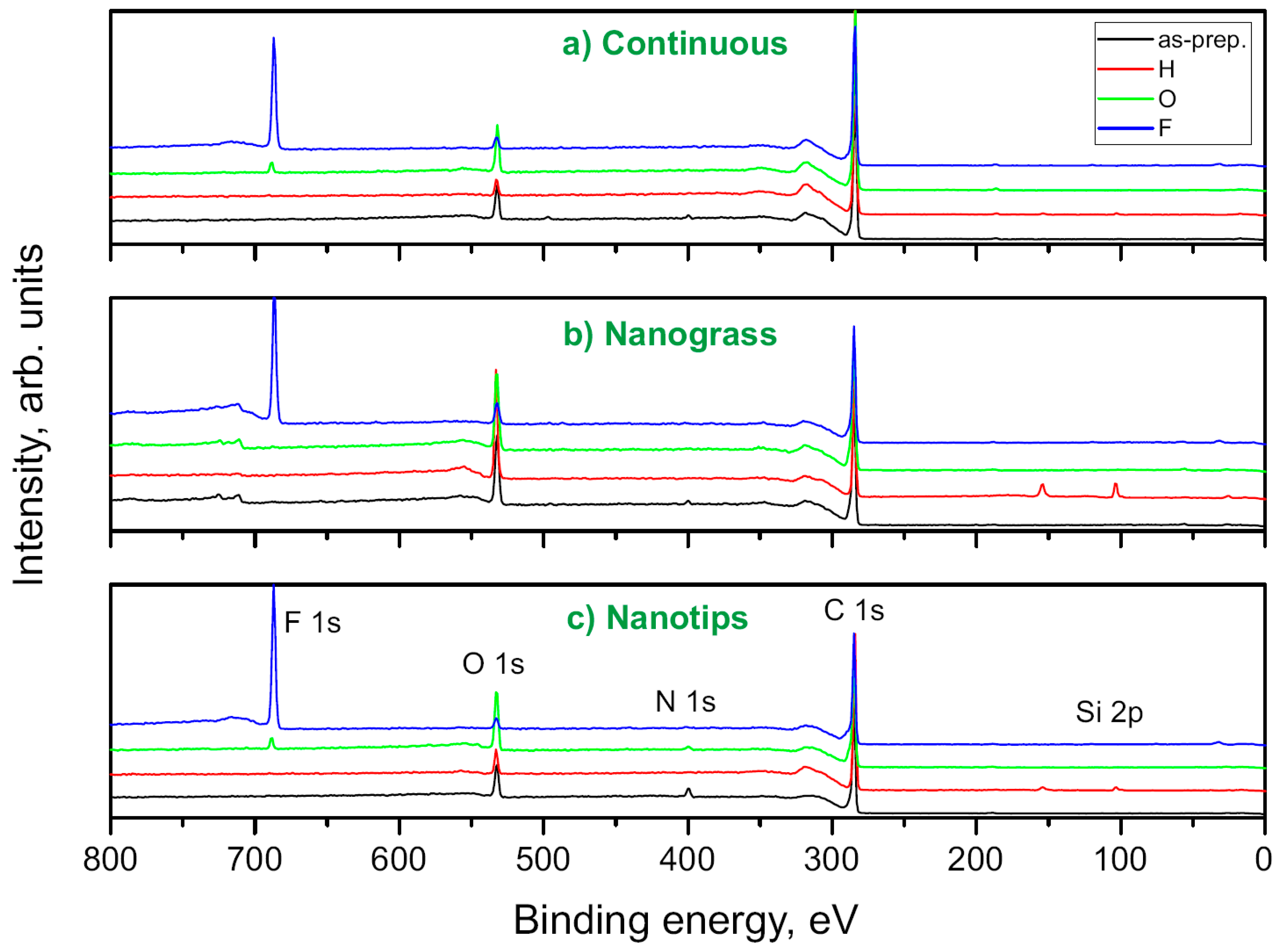
3.4. XPS Measurements of BDD
3.5. UPS Measurements of BDD
3.6. MALDI-MS Analysis of Peptides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cournut, A.; Hosu, I.S.; Braud, F.; Moustiez, P.; Coffinier, Y.; Enjalbal, C.; Bich, C. Development of nanomaterial enabling highly sensitive surface-assisted laser desorption/ionization mass spectrometry peptide analysis. Rapid Commun. Mass Spectrom. 2023, 37, e9476. [Google Scholar] [CrossRef] [PubMed]
- Darie-Ion, L.; Whitham, D.; Jayathirtha, M.; Rai, Y.; Neagu, A.N.; Darie, C.C.; Petre, B.A. Applications of MALDI-MS/MS-Based Proteomics in Biomedical Research. Molecules 2022, 27, 6196. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Yi, J.; Han, G.B.; Qiao, L. MALDI-TOF Mass Spectrometry in Clinical Analysis and Research. ACS Meas. Sci. Au 2022, 2, 385–404. [Google Scholar] [CrossRef] [PubMed]
- Greive, S.J.; Bacri, L.; Cressiot, B.; Pelta, J. Identification of Conformational Variants for Bradykinin Biomarker Peptides from a Biofluid Using a Nanopore and Machine Learning. ACS Nano 2023, 18, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kim, Y.-H.; Jo, G.; Yoo, J.; Park, S.-M.; Jun, B.-H.; Yeo, W.-S. Efficient Analysis of Small Molecules via Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (LDI–TOF MS) Using Gold Nanoshells with Nanogaps. Nanomaterials 2023, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Buriak, J.M.; Siuzdak, G. Desorption–ionization mass spectrometry on porous silicon. Nature 1999, 399, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Sunner, J. An activated carbon substrate surface for laser desorption mass spectrometry. J. Am. Soc. Mass. Spectrom. 2000, 11, 644–649. [Google Scholar] [CrossRef]
- Wang, S.; Niu, H.; Cai, Y.; Cao, D. Multifunctional Au NPs-polydopamine-polyvinylidene fluoride membrane chips as probe for enrichment and rapid detection of organic contaminants. Talanta 2018, 181, 340–345. [Google Scholar] [CrossRef]
- Du, J.; Zhu, Q.; Teng, F.; Wang, Y.; Lu, N. Ag nanoparticles/ZnO nanorods for highly sensitive detection of small molecules with laser desorption/ionization mass spectrometry. Talanta 2019, 192, 79–85. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Q.; Lu, X.; Deng, C.; Sun, N.; Yang, X. Magnetic metal-organic framework nanocomposites for enrichment and direct detection of environmental pollutants by negative-ion matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Talanta 2019, 194, 329–335. [Google Scholar] [CrossRef]
- Pei, C.; Liu, C.; Wang, Y.; Cheng, D.; Li, R.; Shu, W.; Zhang, C.; Hu, W.; Jin, A.; Yang, Y. FeOOH@ metal–organic framework core–satellite nanocomposites for the serum metabolic fingerprinting of gynecological cancers. Angew. Chem. 2020, 132, 10923–10927. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, Y.; Wu, J.; Li, R.; Hu, D.; Lin, Z.; Cai, Z. A magnetic covalent organic framework as an adsorbent and a new matrix for enrichment and rapid determination of PAHs and their derivatives in PM 2.5 by surface-assisted laser desorption/ionization-time of flight-mass spectrometry. Chem. Commun. 2019, 55, 3745–3748. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, Q.; Huang, X.; Nie, Z.; Ruan, T.; Du, Y.; Jiang, G. Fluorographene as a mass spectrometry probe for high-throughput identification and screening of emerging chemical contaminants in complex samples. Anal. Chem. 2017, 89, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Hosu, I.; Sobaszek, M.; Ficek, M.; Bogdanowicz, R.; Drobecq, H.; Boussekey, L.; Barras, A.; Melnyk, O.; Boukherroub, R.; Coffinier, Y. Carbon nanowalls: A new versatile graphene based interface for the laser desorption/ionization-mass spectrometry detection of small compounds in real samples. Nanoscale 2017, 9, 9701–9715. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Li, Y.; Li, R.; Shuang, S.; Dong, C.; Cai, Z. Facile Synthesis of N-Doped Carbon Dots as a New Matrix for Detection of Hydroxy-Polycyclic Aromatic Hydrocarbons by Negative-Ion Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry. ACS Appl. Mater. Interf. 2016, 8, 12976–12984. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Singh, R. A review of nucleation, growth and low temperature synthesis of diamond thin films. Int. Mater. Rev. 2007, 52, 29–64. [Google Scholar] [CrossRef]
- Yang, N.; Yu, S.; Macpherson, J.V.; Einaga, Y.; Zhao, H.; Zhao, G.; Swain, G.M.; Jiang, X. Conductive diamond: Synthesis, properties, and electrochemical applications. Chem. Soc. Rev. 2019, 48, 157–204. [Google Scholar] [CrossRef] [PubMed]
- Kraft, A. Doped diamond: A compact review on a new, versatile electrode material. Int. J. Electrochem. Sci. 2007, 2, 355–385. [Google Scholar] [CrossRef]
- Marton, M.; Ritomsky, M.; Michniak, P.; Behul, M.; Rehacek, V.; Redhammer, R.; Vincze, A.; Papula, M.; Vojs, M. Study of self-masking nanostructuring of boron doped diamond films by RF plasma etching. Vacuum 2019, 170, 108954. [Google Scholar] [CrossRef]
- Toros, A.; Kiss, M.K.; Graziosi, T.; Mi, S.; Berrazouane, R.; Naamoun, M.; Vukajlovic Plestina, J.; Gallo, P.; Quack, N. Reactive ion etching of single crystal diamond by inductively coupled plasma: State of the art and catalog of recipes. Diamond Relat. Mater. 2020, 108, 107839. [Google Scholar] [CrossRef]
- Pfeifer, R.; Szabó, O.; Potocký, S.; Lorinčík, J.; Stehlík, S.; Marton, M.; Vojs, M.; Kromka, A. Generation of Nanoporous Diamond Electrodes Fabricated by a Low-Cost Process at Moderate Temperatures. ACS Appl. Eng. Mater. 2023, 1, 1446–1454. [Google Scholar] [CrossRef]
- Artemenko, A.; Ižák, T.; Marton, M.; Ukraintsev, E.; Stuchlík, J.; Hruška, K.; Vojs, M.; Kromka, A. Stability of the surface termination of nanocrystalline diamond and diamond-like carbon films exposed to open air conditions. Diamond Relat. Mater. 2019, 100, 107562. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Lin, G.; Wang, Z.; Zhang, J.; Shi, H. Advances in deposition of diamond films on cemented carbide and progress of diamond coated cutting tools. Vacuum 2023, 217, 112562. [Google Scholar] [CrossRef]
- Marton, M.; Vojs, M.; Michniak, P.; Behúl, M.; Rehacek, V.; Pifko, M.; Stehlík, Š.; Kromka, A. New chemical pathway for large-area deposition of doped diamond films by linear antenna microwave plasma chemical vapor deposition. Diamond Relat. Mater. 2022, 126, 109111. [Google Scholar] [CrossRef]
- 2024. Available online: https://www.protpi.ch/Calculator/ProteinTool#AminoAcidComposition (accessed on 6 January 2024).
- Yang, J.; Zhang, Y. I-TASSER server: New development for protein structure and function predictions. Nucleic Acids Res. 2015, 43, W174–W181. [Google Scholar] [CrossRef]
- Zhang, C.; Freddolino, P.L.; Zhang, Y. COFACTOR: Improved protein function prediction by combining structure, sequence and protein–protein interaction information. Nucleic Acids Res. 2017, 45, W291–W299. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, C.; Li, Y.; Pearce, R.; Bell, E.W.; Zhang, Y. Folding non-homologous proteins by coupling deep-learning contact maps with I-TASSER assembly simulations. Cell Rep. Methods 2021, 1, 3. [Google Scholar] [CrossRef]
- Available online: https://zhanggroup.org/I-TASSER/ (accessed on 6 January 2024).
- Lu, J.; Xu, D.; Huang, N.; Jiang, X.; Yang, B. One-dimensional diamond nanostructures: Fabrication, properties and applications. Carbon 2024, 223, 119020. [Google Scholar] [CrossRef]
- Sartori, A.F.; Orlando, S.; Bellucci, A.; Trucchi, D.M.; Abrahami, S.; Boehme, T.; Hantschel, T.; Vandervorst, W.; Buijnsters, J.G. Laser-Induced Periodic Surface Structures (LIPSS) on Heavily Boron-Doped Diamond for Electrode Applications. ACS Appl. Mater. Interf. 2018, 10, 43236–43251. [Google Scholar] [CrossRef]
- Mortet, V.; Gregora, I.; Taylor, A.; Lambert, N.; Ashcheulov, P.; Gedeonova, Z.; Hubik, P. New perspectives for heavily boron-doped diamond Raman spectrum analysis. Carbon 2020, 168, 319–327. [Google Scholar] [CrossRef]
- Zhai, Z.; Chen, B.; Zhang, C.; Liu, L.; Song, H.; Zheng, Z.; Li, J.; Yang, B.; Jiang, X.; Huang, N. Construction of porous diamond film with enhanced electric double-layer capacitance via regrowth of diamond nanoplatelets. Funct. Diam. 2023, 3, 2263468. [Google Scholar] [CrossRef]
- Mortet, V.; Taylor, A.; Živcová, Z.V.; Machon, D.; Frank, O.; Hubík, P.; Trémouilles, D.; Kavan, L. Analysis of heavily boron-doped diamond Raman spectrum. Diamond Relat. Mater. 2018, 88, 163–166. [Google Scholar] [CrossRef]
- Romanyuk, O.; Bartoš, I.; Gordeev, I.; Artemenko, A.; Varga, M.; Ižák, T.; Marton, M.; Jiříček, P.; Kromka, A. Electron affinity of undoped and boron-doped polycrystalline diamond films. Diamond Relat. Mater. 2018, 87, 208–214. [Google Scholar] [CrossRef]
- Crawford, K.G.; Maini, I.; Macdonald, D.A.; Moran, D.A.J. Surface transfer doping of diamond: A review. Prog. Surf. Sci. 2021, 96, 100613. [Google Scholar] [CrossRef]
- Ozkan, S.; Ipekci, A. Serum Angiotensin II as a Biomarker in COVID-19. In Biomarkers in Trauma, Injury and Critical Care; Rajendram, R., Preedy, V.R., Patel, V.B., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–24. [Google Scholar]
- Ramchand, J.; Burrell, L.M. Circulating ACE2: A novel biomarker of cardiovascular risk. Lancet 2020, 396, 937–939. [Google Scholar] [CrossRef]
- Arisido, M.W.; Foco, L.; Shoemaker, R.; Melotti, R.; Delles, C.; Gögele, M.; Barolo, S.; Baron, S.; Azizi, M.; Dominiczak, A.F.; et al. Cluster analysis of angiotensin biomarkers to identify antihypertensive drug treatment in population studies. BMC Med. Res. Methodol. 2023, 23, 131. [Google Scholar] [CrossRef]
- Ferrario, C.M.; Groban, L.; Wang, H.; Sun, X.; VonCannon, J.L.; Wright, K.N.; Ahmad, S. The renin–angiotensin system biomolecular cascade: A 2022 update of newer insights and concepts. Kidney Int. Suppl. 2022, 12, 36–47. [Google Scholar] [CrossRef]
- Alasag, N.; Sener, E.; Dogrukol-Ak, D. Simultaneous Determination of Substance P and CGRP in Rat Brainstem Tissue Using LC-ESI-MS/MS Method. Curr. Anal. Chem. 2018, 14, 583–590. [Google Scholar] [CrossRef]
- Fang, H.Y.; Liu, Y.; Wang, S.F. Analysis of Peptides in Plasma by High Performance Liquid Chromatography coupled with Electrospray Ionization Mass Spectrometry. In Proceedings of the International Conference on Mechanical Properties of Materials and Information Technology (ICMPMIT 2011), Chongqing, China, 10–11 September 2011; pp. 273–279. [Google Scholar]
- Pereira, A.S.; DiLeone, L.; Souza, F.H.; Lilla, S.; Richter, M.; Schwartsmann, G.; De Nucci, G. Quantification of the bombesin/gastrin releasing peptide antagonist RC-3095 by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2005, 816, 321–326. [Google Scholar] [CrossRef]
- Schirinzi, T.; Maftei, D.; Ralli, M.; Greco, A.; Mercuri, N.B.; Lattanzi, R.; Severini, C. Serum Substance P Is Increased in Parkinson’s Disease and Correlates with Motor Impairment. Mov. Disord. 2022, 37, 228–230. [Google Scholar] [CrossRef]
- Gorshkov, M.V.; Frankevich, V.E.; Zenobi, R. Characteristics of photoelectrons emitted in matrix-assisted laser desorption/ionization Fourier transform ion cyclotron resonance experiments. Eur. J. Mass Spectrom. 2002, 8, 67–69. [Google Scholar] [CrossRef]
- Frankevich, V.; Knochenmuss, R.; Zenobi, R. The origin of electrons in MALDI and their use for sympathetic cooling of negative ions in FTICR. Int. J. Mass Spectrom. 2002, 220, 11–19. [Google Scholar] [CrossRef]




| Peptide N° | Sequence | Mass (Da) * | [M+H]+ | pI # | Charge at pH 7.4 # |
|---|---|---|---|---|---|
| Bradykinin 1–7 (Brad) | RPPGFSP | 757.4 | 758.3992 | 9.1 | +0.19 |
| Angiotensin II (Ang II) | DRVYIHPF | 1046.5 | 1046.5418 | 6.9 | −0.29 |
| Angiotensin I (Ang I) | DRVYIHPFHL | 1296.7 | 1296.6848 | 7.1 | −0.25 |
| Substance P (Sub P) | RPKPQQFFGLM | 1347.7 | 1347.7354 | 10.7 | +1.18 |
| Bombesin (Bomb) ¶ | OQRLGNQWAVGHLM | 1619.8 | 1619.8223 | 11.1 | +1.04 |
| Renin Substrate (Ren S) | DRVYIHPFHLVIHN | 1758.9 | 1758.9326 | 7.2 | −0.20 |
| ACTH 1–17 (AC1) | SYSMEHFRWGKPVGKKR | 2093.1 | 2093.0862 | 10.3 | +3.26 |
| ACTH 18–39 (AC18) | RPVKVYPNGAEDESAEAFPLEF | 2465.2 | 2465.1983 | 4.3 | −3.82 |
| Somatostatin | SANSNPAMAPRERKAGCKNFFWKTFTSC | 3147.5 | 3147.4710 | 9.6 | +3.07 |
| Atomic Concentration, at.% | |||||||
|---|---|---|---|---|---|---|---|
| Sample | C | O | B | F | N | Si | Al |
| Continuous | 88.1 | 8.3 | 2.1 | 0.0 | 1.1 | 0.4 | 0.0 |
| Nanograss | 77.0 | 18.9 | 1.9 | 0.0 | 1.7 | 0.5 | 0.0 |
| Nanotips | 81.0 | 11.8 | 2.1 | 0.0 | 5.1 | 0.0 | 0.0 |
| Continuous-H | 94.2 | 3.0 | 1.8 | 0.0 | 0.0 | 1.0 | 0.0 |
| Nanograss-H | 64.1 | 21.9 | 1.0 | 0.0 | 0.0 | 13.0 | 0.0 |
| Nanotip-H | 89.2 | 6.4 | 1.2 | 0.0 | 0.0 | 3.2 | 0.0 |
| Continuous-F | 75.6 | 2.8 | 2.3 | 17.5 | 0.9 | 0.0 | 0.9 |
| Nanograss-F | 66.2 | 6.2 | 2.0 | 24.0 | 0.8 | 0.0 | 0.8 |
| Nanotip-F | 65.8 | 3.2 | 1.8 | 27.8 | 0.7 | 0.0 | 0.7 |
| Continuous-O | 85.9 | 9.0 | 3.0 | 1.6 | 0.5 | 0.0 | 0.0 |
| Nanograss-O | 75.6 | 20.4 | 2.4 | 0.7 | 0.9 | 0.0 | 0.0 |
| Nanotip-O | 75.5 | 17.4 | 2.4 | 2.5 | 2.2 | 0.0 | 0.0 |
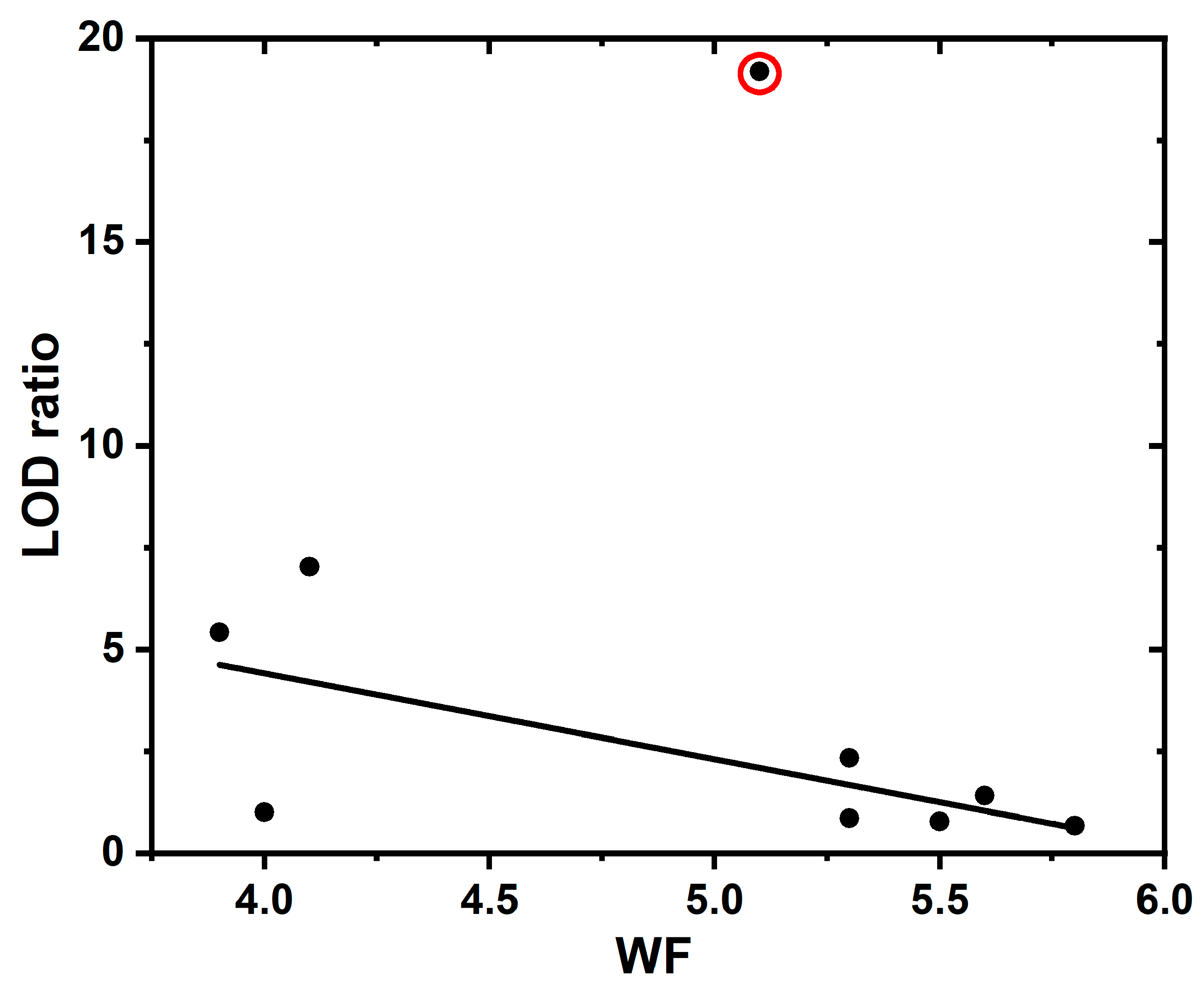
| Energy, eV | WF, eV | EA, eV | ||||
|---|---|---|---|---|---|---|
| Sample | EVBM | Ecutoff | ECBM | EBG | ||
| Continuous | 2.3 | 17.0 | −3.2 | 5.5 | 4.2 | 1.0 |
| Nanograss | 1.5 | 16.3 | −4.0 | 5.5 | 4.9 | 0.9 |
| Nanotips | 1.9 | 16.8 | −3.6 | 5.5 | 4.4 | 0.8 |
| NEA | ||||||
| Continuous-H | 0.7 | 17.1 | −4.8 | 5.5 | 4.1 | −0.7 |
| Nanograss-H | 1.2 | 17.2 | −4.3 | 5.5 | 4.0 | −0.3 |
| Nanotips-H | 1.1 | 17.3 | −4.4 | 5.5 | 3.9 | −0.5 |
| Continuous-F | 1.0 | 15.7 | −4.5 | 5.5 | 5.5 | 1.0 |
| Nanograss-F | 1.1 | 15.4 | −4.4 | 5.5 | 5.8 | 1.4 |
| Nanotips-F | 1.7 | 15.6 | −3.8 | 5.5 | 5.6 | 1.8 |
| Continuous-O | 1.2 | 16.1 | −4.3 | 5.5 | 5.1 | 0.8 |
| Nanograss-O | 1.8 | 15.9 | −3.7 | 5.5 | 5.3 | 1.6 |
| Nanotips-O | 1.9 | 15.9 | −3.6 | 5.5 | 5.3 | 1.7 |
| Peptide | Surface/ Termination | Continuous BDD | Nanograss BDD | Nanotip BDD | |||
|---|---|---|---|---|---|---|---|
| LOD (pM) | LODratio * | LOD (pM) | LODratio * | LOD (pM) | LODratio * | ||
| Sub P | BDD/H | 6.2 | 7.03 | 43.7 | 1.00 | 8.1 | 5.42 |
| BDD/O | 2.3 | 19.19 | 18.7 | 2.34 | 51.0 | 0.86 | |
| BDD/F | 56.1 | 0.78 | 63.9 | 0.68 | 31.0 | 1.41 | |
| Steel chip | 43.8 | ||||||
| Ang II | BDD/H | 27.3 | 1.24 | 33.6 | 1.01 | 12.2 | 2.78 |
| BDD/O | 29.1 | 1.17 | 57.6 | 0.59 | 316.3 | 0.11 | |
| BDD/F | 422.0 | 0.08 | 31.5 | 1.08 | 51.3 | 0.66 | |
| Steel chip | 34.0 | ||||||
| AC 1 | BDD/H | 19.4 | 2.78 | 42.7 | 1.27 | 54.5 | 0.99 |
| BDD/O | ND | ND | 63.9 | 0.85 | 63.9 | 0.85 | |
| BDD/F | 63.9 | 0.85 | 63.9 | 0.85 | 63.9 | 0.85 | |
| Steel chip | 54.1 | ||||||
| AC 18 | BDD/H | 54.1 | 1.64 | 51.7 | 1.72 | 54.3 | 1.64 |
| BDD/O | 21.1 | 4.21 | 63.9 | 1.39 | 63.9 | 1.39 | |
| BDD/F | 63.9 | 1.39 | 63.9 | 1.39 | 63.9 | 1.39 | |
| Steel chip | 88.9 | ||||||
| Ang I | BDD/H | 141.4 | 0.22 | 47.6 | 0.66 | 16.0 | 1.95 |
| BDD/O | 84.8 | 0.37 | 63.9 | 0.49 | 33.2 | 0.94 | |
| BDD/F | 49.9 | 0.63 | 63.9 | 0.49 | 37.6 | 0.83 | |
| Steel chip | 31.2 | ||||||
| Bomb | BDD/H | 31.8 | 1.87 | 266.6 | 0.22 | 35.6 | 1.67 |
| BDD/O | 40.4 | 1.48 | 753.8 | 0.08 | 40.4 | 1.48 | |
| BDD/F | 63.9 | 0.93 | 63.9 | 0.93 | 63.9 | 0.93 | |
| Steel chip | 59.6 | ||||||
| Brad | BDD/H | 51.9 | 1.73 | 63.9 | 1.41 | 723.8 | 0.12 |
| BDD/O | 18.9 | 4.76 | 63.9 | 1.41 | 25.8 | 3.49 | |
| BDD/F | 15.2 | 5.92 | 247.2 | 0.36 | 63.9 | 1.41 | |
| Steel chip | 90.0 | ||||||
| Ren S | BDD/H | 21.6 | 2.14 | 3.1 | 14.94 | 51.1 | 0.91 |
| BDD/O | 54.9 | 0.84 | 63.9 | 0.72 | 16.8 | 2.76 | |
| BDD/F | 63.9 | 0.72 | 63.9 | 0.72 | 63.9 | 0.72 | |
| Steel chip | 46.3 | ||||||
| Som | BDD/H | 63.9 | 1.00 | 63.9 | 1.00 | 63.9 | 1.00 |
| BDD/O | ND | ND | 63.9 | 1.00 | ND | ND | |
| BDD/F | ND | ND | ND | ND | ND | ND | |
| Steel chip | 63.9 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguedo, J.; Vojs, M.; Vrška, M.; Nemcovic, M.; Pakanova, Z.; Dragounova, K.A.; Romanyuk, O.; Kromka, A.; Varga, M.; Hatala, M.; et al. What Are the Key Factors for the Detection of Peptides Using Mass Spectrometry on Boron-Doped Diamond Surfaces? Nanomaterials 2024, 14, 1241. https://doi.org/10.3390/nano14151241
Aguedo J, Vojs M, Vrška M, Nemcovic M, Pakanova Z, Dragounova KA, Romanyuk O, Kromka A, Varga M, Hatala M, et al. What Are the Key Factors for the Detection of Peptides Using Mass Spectrometry on Boron-Doped Diamond Surfaces? Nanomaterials. 2024; 14(15):1241. https://doi.org/10.3390/nano14151241
Chicago/Turabian StyleAguedo, Juvissan, Marian Vojs, Martin Vrška, Marek Nemcovic, Zuzana Pakanova, Katerina Aubrechtova Dragounova, Oleksandr Romanyuk, Alexander Kromka, Marian Varga, Michal Hatala, and et al. 2024. "What Are the Key Factors for the Detection of Peptides Using Mass Spectrometry on Boron-Doped Diamond Surfaces?" Nanomaterials 14, no. 15: 1241. https://doi.org/10.3390/nano14151241






