Rheological Behavior of an Aqueous Suspension of Oxidized Carbon Nanohorn (CNHox)
Abstract
:1. Introduction
2. Materials and Methods
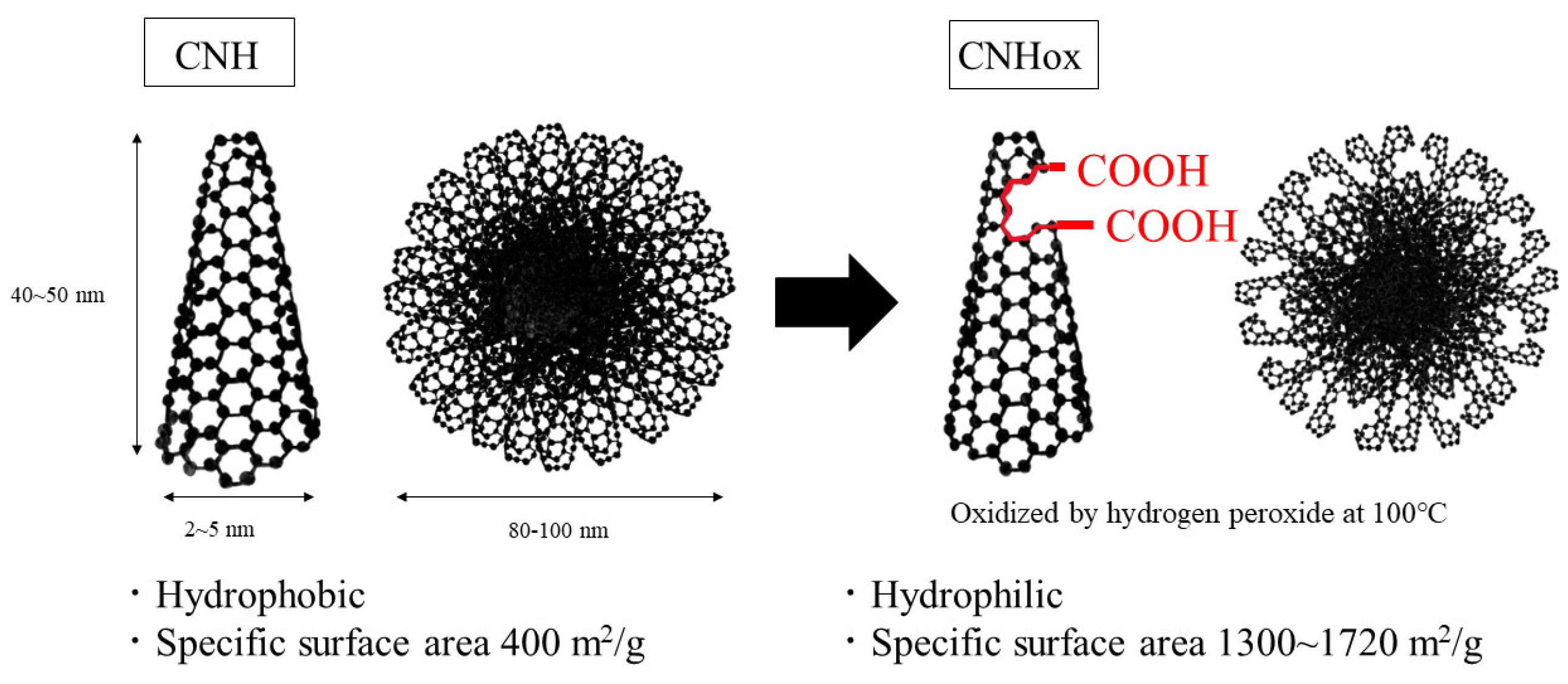
2.1. Materials
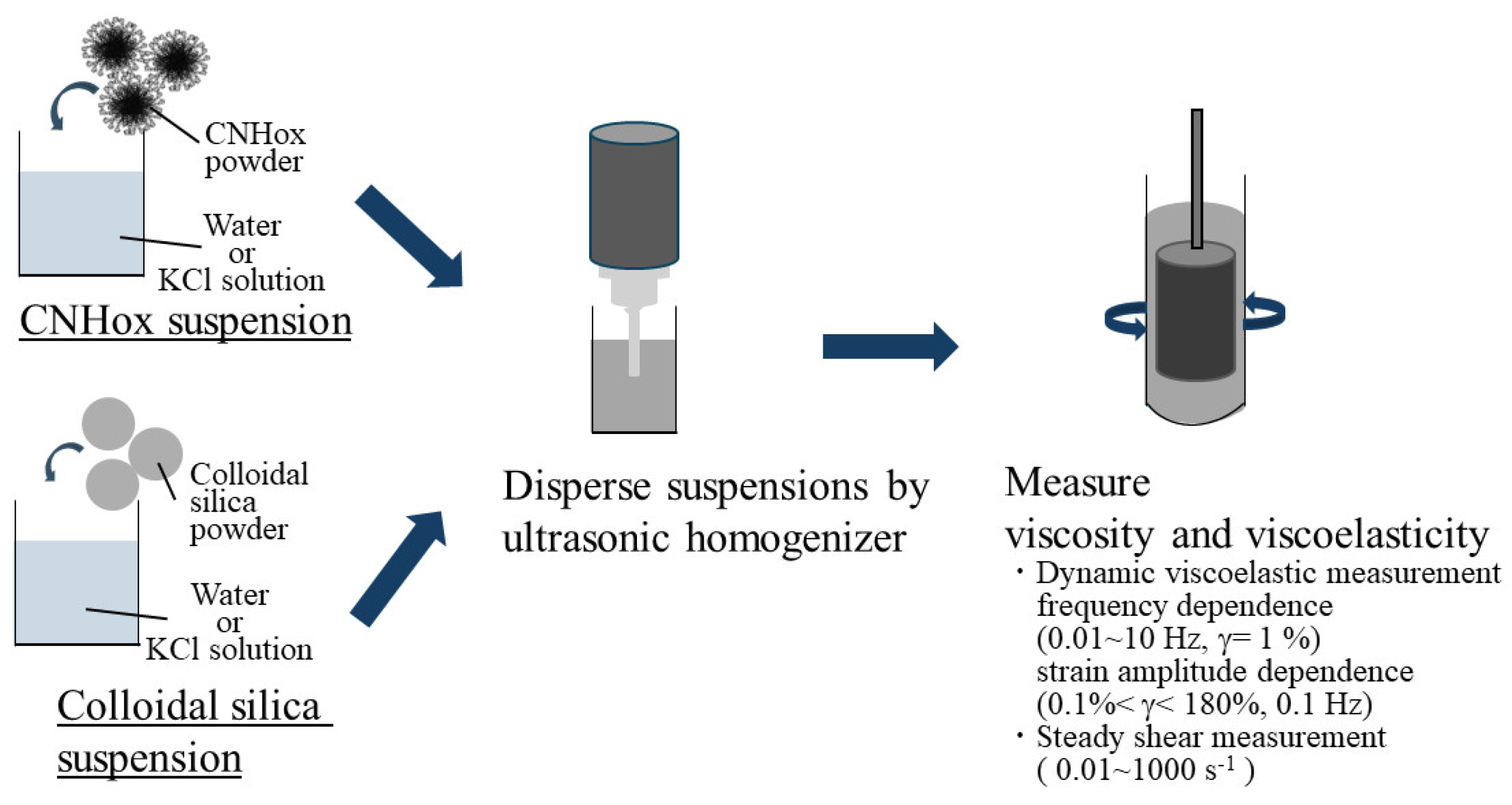
2.2. Rheological Measurements
3. Results & Discussion
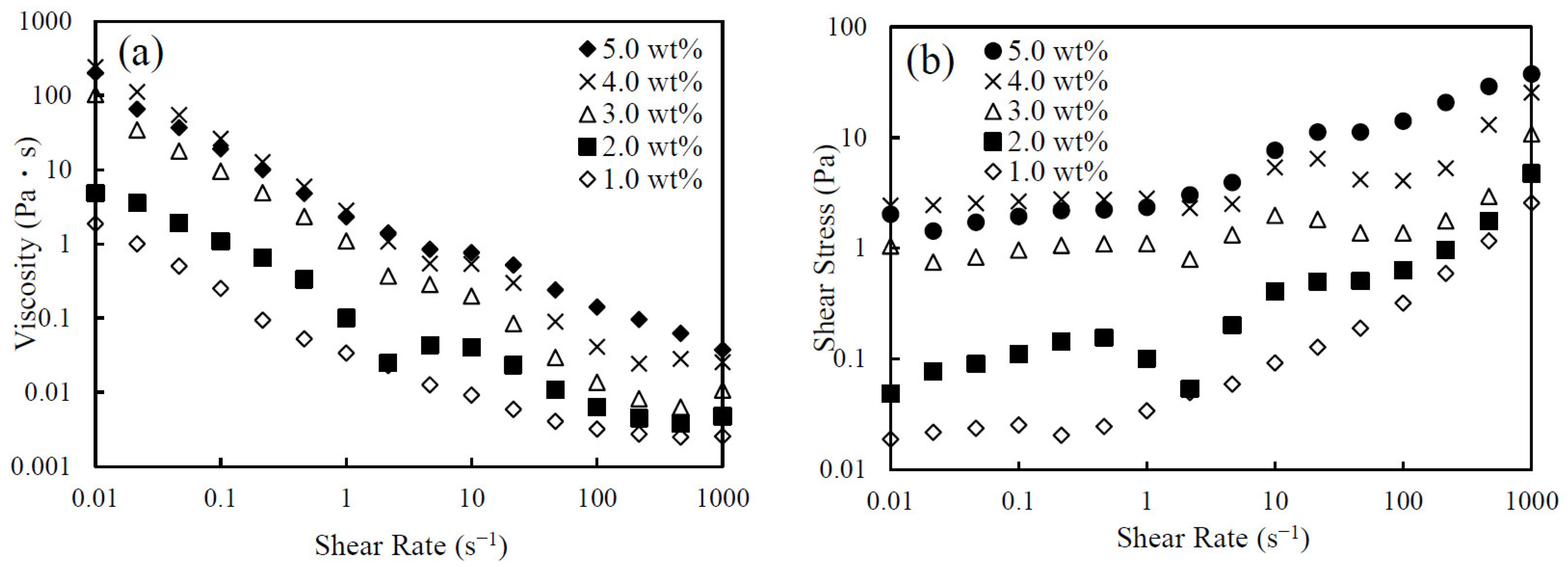
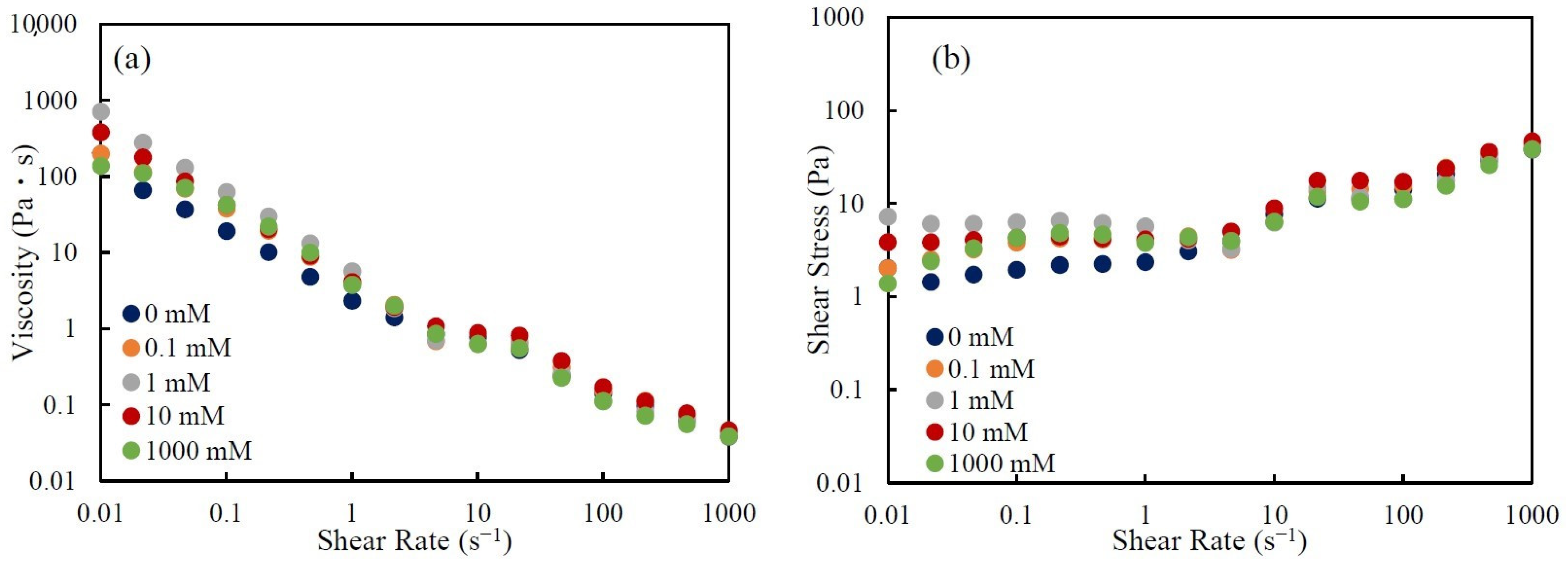
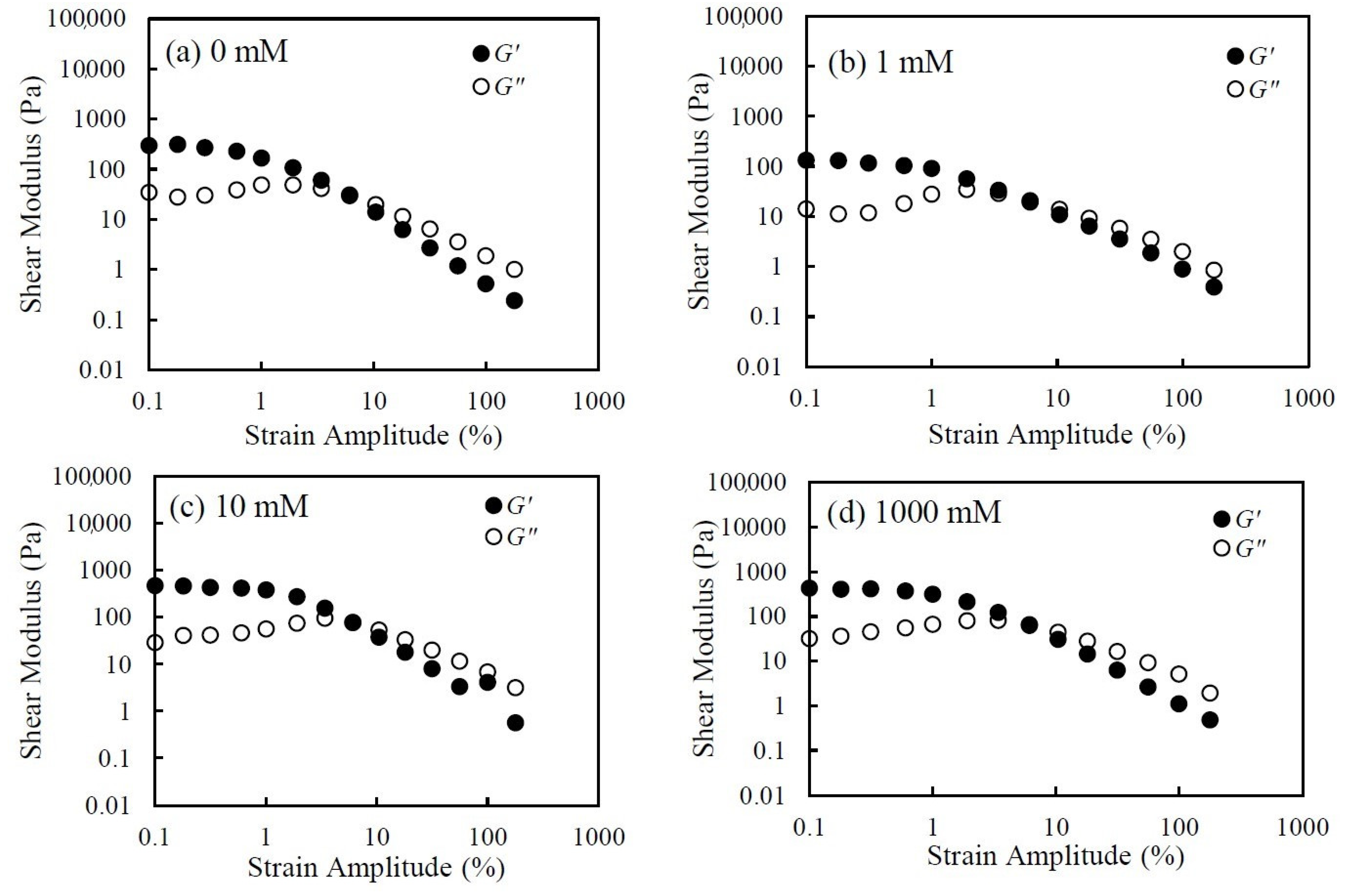
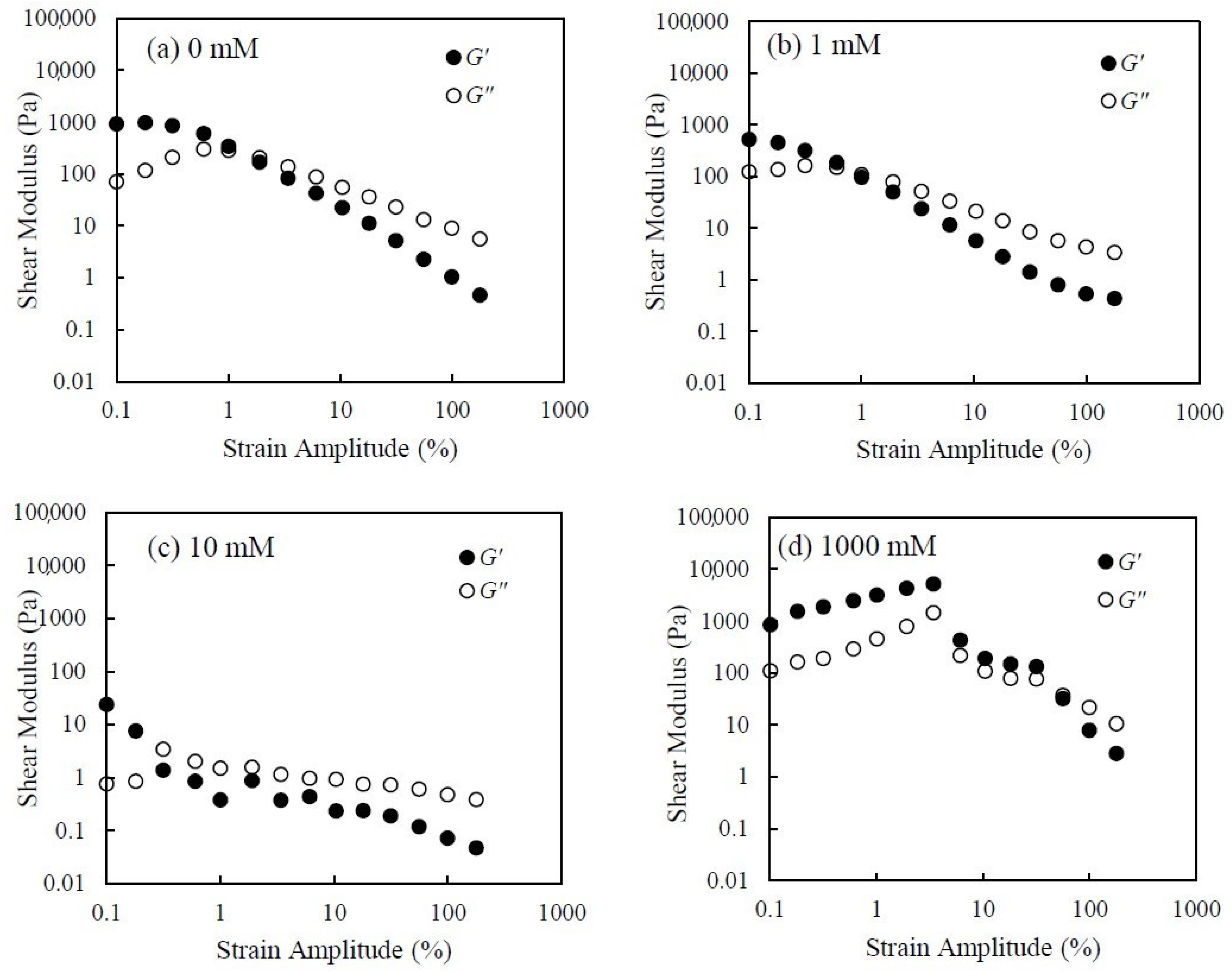
3.1. Rheological Behavior of CNHox Suspensions
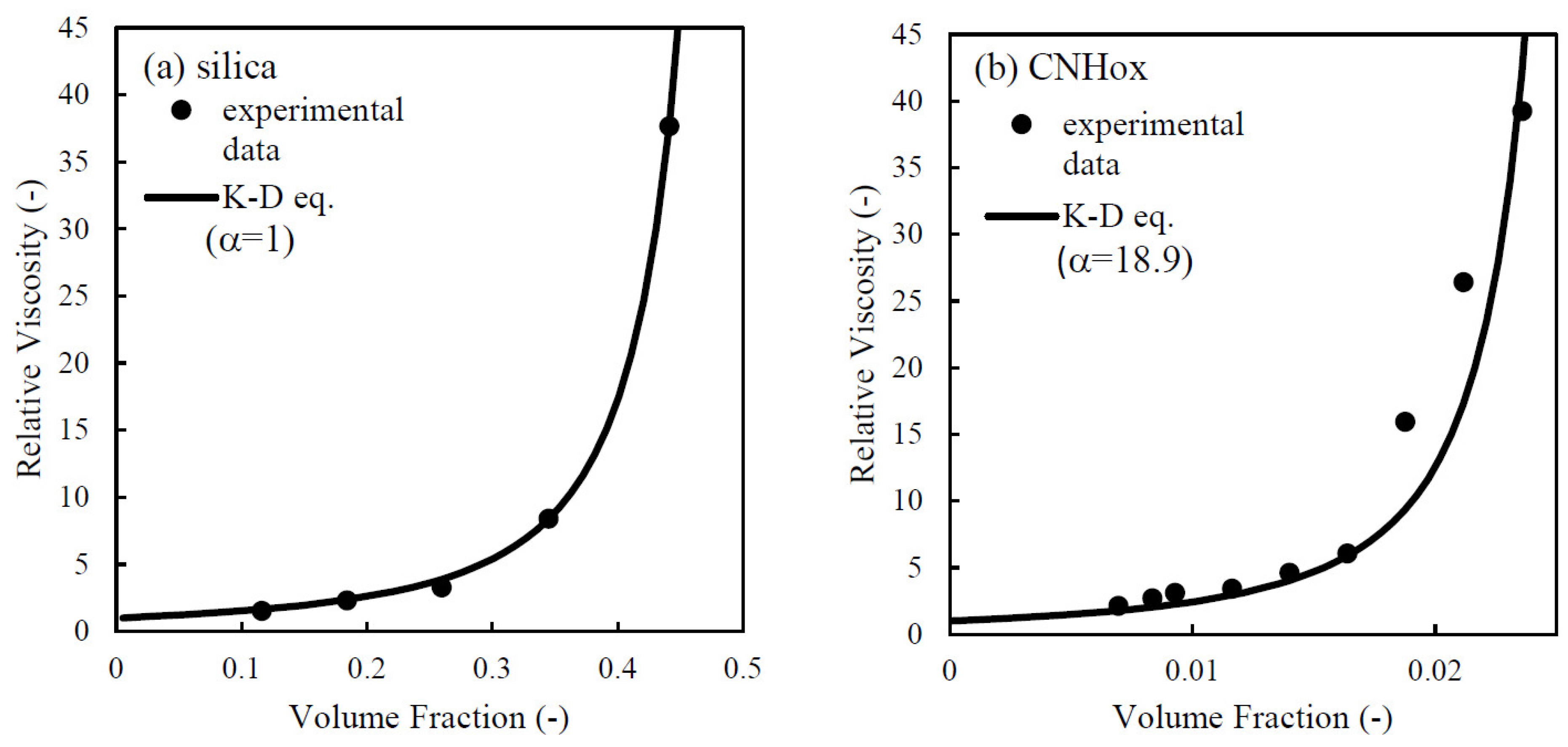
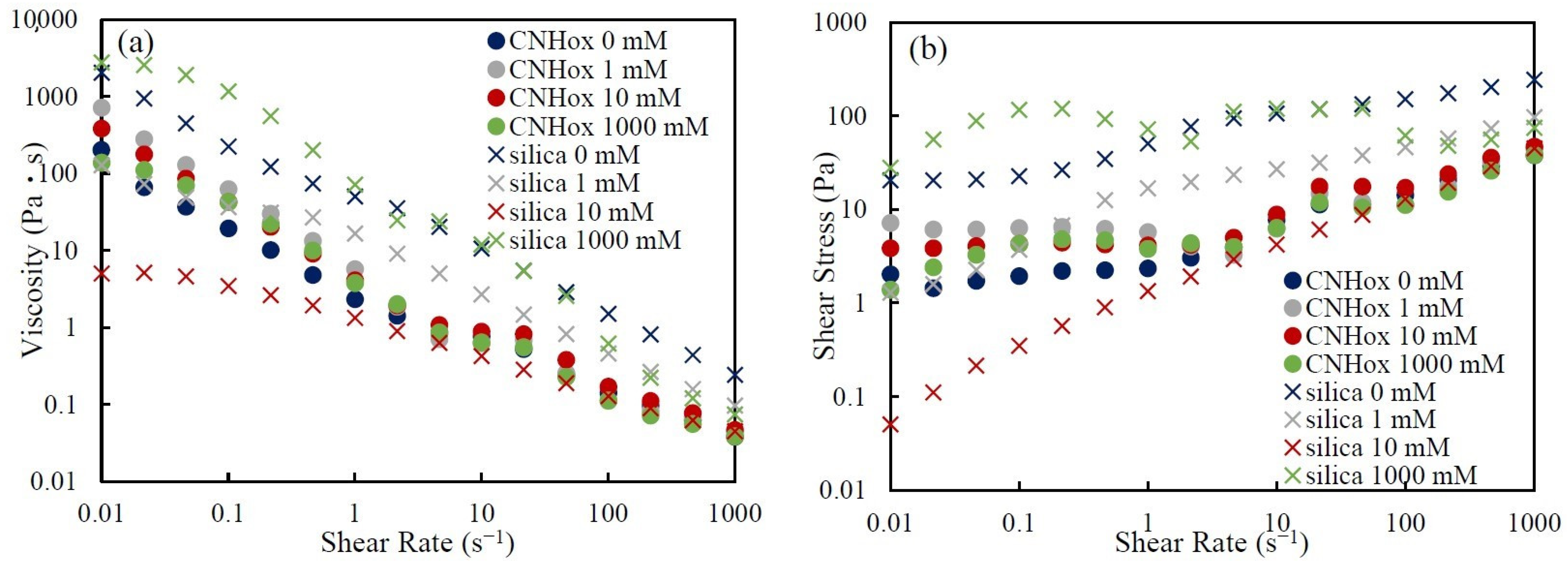
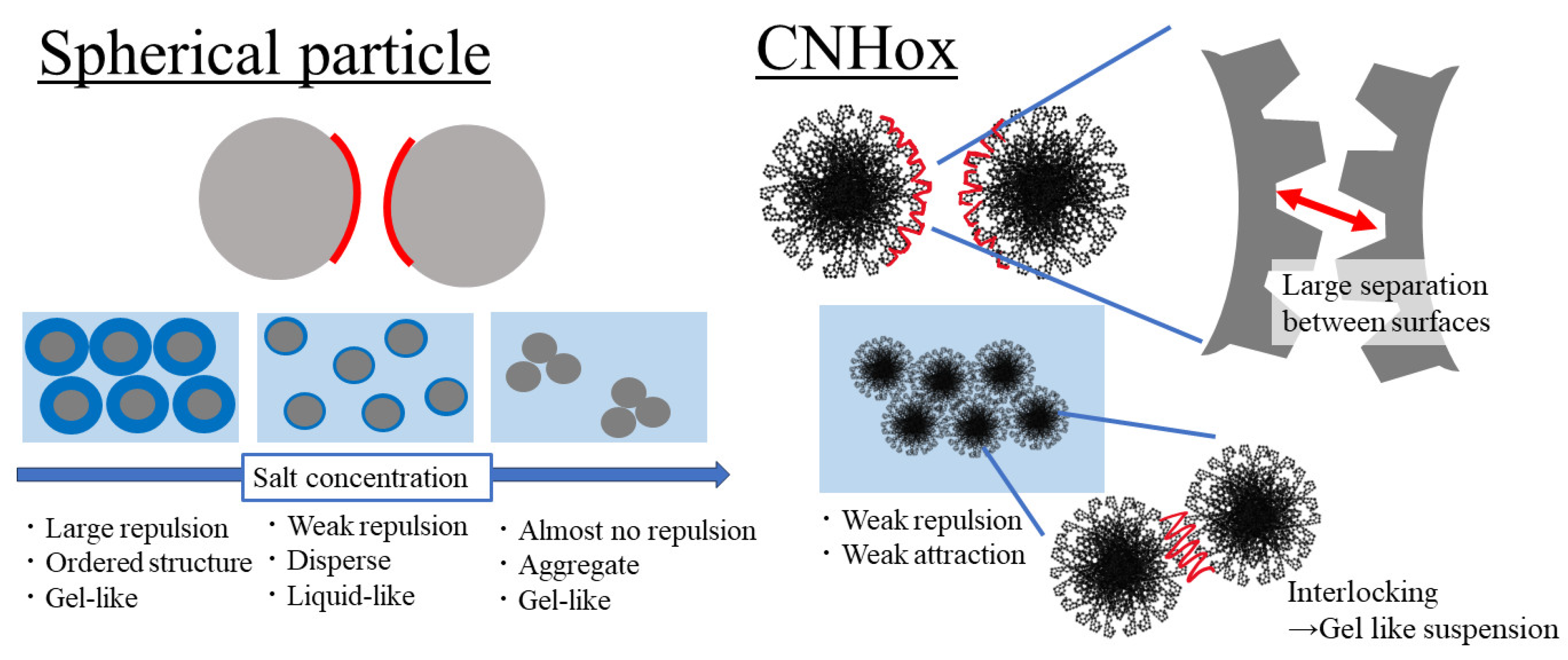
3.2. Effects of Particle Structure on Suspension Viscosity
3.3. Effect of Salt Concentration on Rheological Behavior
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Popv, V.N. Carbon nanotubes: Properties and application. Mater. Sci. Eng. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Yamada, T.; Ishihara, M.; Kim, J.; Iijima, S. A roll-to-roll microwave plasma chemical vapor deposition process for the production of 294 mm width graphene films at low temperature. Carbon 2012, 50, 2615–2619. [Google Scholar] [CrossRef]
- Chen, K.L.; Elimelech, M. Relating Colloidal Stability of Fullerene (C60) Nanoparticles to Nanoparticle Charge and Electrokinetic Properties. Environ. Sci. Technol. 2009, 43, 7270–7276. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S.; Yudasaka, M.; Yamada, R.; Bandow, S.; Suenaga, K.; Kokai, F.; Takahashi, K. Nano-aggregates of single-walled graphitic carbon nano-horns. Chem. Phys. Lett. 1999, 309, 165–170. [Google Scholar] [CrossRef]
- Hawelek, L.; Brodka, A.; Dore, J.C.; Hannon, A.C.; Iijima, S.; Yudasaka, M.; Ohba, T.; kaneko, K.; Burian, A. Structural Modeling of Dahilia-Type Single Walled Carbon Nanohorn Aggregates by Molecular Dynamics. J. Phys. Chem. A 2013, 117, 9057–9061. [Google Scholar] [CrossRef]
- Guldi, D.M.; Martin, N. Carbon Nanotubes and Related Structures: Synthesis, Characterization, Functionalization, and Applications; John Wiley & Sons: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Miyawaki, J.; Yudasaka, M.; Azami, T.; Iijima, S. Toxicity of Single-Walled Carbon Nanohorns. ACS Nano 2008, 2, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Obara, R.; Sasaki, H.; Chonan, Y.; Komiyama, T.; Kotani, K.; Yamaguchi, H.; Yamauchi, S.; Sugawara, Y.; Sekine, T.; Sugiyama, S.; et al. Characteristics of spark plasma sintered nanocarbon materials. Nihon Sozai Bussei Gakkaishi 2022, 32, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Yuge, R.; Manako, T.; Nakahara, K.; Yasui, M.; Iwasa, S.; Yoshitake, T. The Production of an Electrochemical Capacitor Electrode Using Holey Single-Wall Carbon Nanohorns with high Specific Surface Area. Carbon 2012, 50, 5569–5573. [Google Scholar] [CrossRef]
- Utsumi, S.; Miyawaki, K.; Tanaka, H.; Hattori, Y.; Itoi, T.; Ichikuni, N.; Kanoh, H.; Yudasaka, M.; Iijima, S.; Kaneko, K. Opening Mechanism of Internal Nanoporosity of Single-Wall Carbon Nanohorn. J. Phys. Chem. B 2005, 109, 14319–14324. [Google Scholar] [CrossRef]
- Miyawaki, J.; Yudasaka, M.; Iijima, S. Solvent Effects on Hole-Edge structure for single-Wall Carbon Nanotubes and Single-Wall Carbon Nanohorns. J. Phys. Chem. B 2004, 108, 10732–10735. [Google Scholar] [CrossRef]
- Zhang, M.; Yudasaka, M.; Ajima, K.; Miyawaki, J.; Iijima, S. Light-Assisted Oxidation of Single-Wall Carbon Nanohorns for Abundant Creation of Oxygenated Groups That Enable Chemical Modifications with Proteins to Enhance Biocompatibility. ACS Nano 2007, 1, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Whitney, J.R.; Sarkar, S.; Zhang, J.; Do, T.; Young, T.; Manson, M.K.; Campbell, T.A.; Puretzky, A.A.; Rouleau, C.M.; More, K.L.; et al. Single Walled Carbon Nanohorns as Photothermal Cancer Agents. Lasers Surg. Med. 2011, 43, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Miyako, E.; Deguchi, T.; Nakajima, Y.; Iijima, S. Photothermic regulation of gene expression triggered by laser-induced carbon nanohorns. Biol. Sci. 2012, 109, 7523–7528. [Google Scholar] [CrossRef] [PubMed]
- Selvam, C.; Harish, S.; Lal, D.M. Effective thermal conductivity and rheological characteristics of ethylene glycol-based nanofluids with single-walled carbon nanohorn inclusions. Fuller. Nanotub. Carbon Nanostruct. 2016, 25, 86–93. [Google Scholar] [CrossRef]
- Zin, V.; Agreti, F.; Barison, S.; Colla, L.; Mercadelli, E.; Fabrizio, M.; Pagura, C. Tribological Properties of Engine Oil with Carbon Nano-horns as Nano-additives. Tribol. Lett. 2014, 55, 45–53. [Google Scholar] [CrossRef]
- Basson, N.; Peng, C.H.S.; Geoghegan, P.; van der Lecq, T.; Steven, D.; Williams, S.; Lim, A.E.; Ho, W.H. A computational fluid dynamics investigation of endothelial cell damage from glaucoma drainage devices. Sci. Rep. 2024, 14, 3777. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, Y.; Xu, J.; Yoshino, F.; Xu, H.; Chen, X.; Zhao, L. Surface-engineered carbon nanohorns as a theranostic nanodevice for photoacoustic imaging and effective radiochemotherapy of cancer. Carbon 2021, 180, 185–196. [Google Scholar] [CrossRef]
- Zhang, M.; Yudasaka, M. Carbon nanohorns and their high potential in biological applications. In Carbon Nanostructures; Springer: Cham, Switzerland, 2016; pp. 77–107. [Google Scholar]
- Hsu, C.P.; Ramakrishna, S.N.; Zanini, M.; Spencer, N.D.; Isa, L. Roughness-dependent tribology effects on discontinuous shear thickening. Proc. Natl. Acad. Sci. USA 2018, 115, 5117–5122. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Hsu, C.P.; Isa, L. Particle Surface Roughness as a Design Tool for Colloidal Systems. Langmuir 2020, 36, 11171–11182. [Google Scholar] [CrossRef]
- More, R.V.; Ardekani, A.M. A constitutive model for sheared dense suspensions of rough particles. J. Rheol. 2020, 64, 1107–1120. [Google Scholar] [CrossRef]
- Blair, M.; Ness, C. Shear thickening in dense suspensions driven by particle interlocking. J. Fluid Mech. 2022, 948, A48. [Google Scholar] [CrossRef]
- Hsiao, L.C.; Pradeep, S. Experimental synthesis and characterization of rough particles for colloidal and granular rheology. Curr. Opin. Colloid Interface Sci. 2019, 43, 94–112. [Google Scholar] [CrossRef]
- Kobayashi, M.; Adachi, Y.; Ooi, S. On the steady shear viscosity of coagulated suspensions. Nihon Reoroji Gakkaishi 2000, 28, 143–144. [Google Scholar] [CrossRef]
- Murata, K.; Kaneko, K.; Steele, W.A.; Kokai, F.; Takahashi, K.; Kasuya, D.; Hirahara, K.; Yudasaka, M.; Iijima, S. Molecular Potential Structures of Heat-Treated Single-Wall Carbon Nanohorm Assemblies. J. Phys. Chem. B 2001, 105, 10210–10216. [Google Scholar] [CrossRef]
- Derjaguin, B.; Landau, L. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Acta Physicochim. 1941, 14, 633–662. [Google Scholar] [CrossRef]
- Verwey, E.J.W. Theory of the stability of lyophobic colloids. J. Phys. Chem. 1947, 51, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Skarba, M.; Galletto, P.; Cakara, D.; Borkovec, M. Effects of heat treatment on the aggregation and charging of Stöber-type silica. J. Colloid Interface Sci. 2005, 292, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Kusaka, Y.; Kobayashi, M. Charging and aggregation behavior of cellulose nanofibers in aqueous solution. Langmuir 2017, 33, 12660–12669. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, C.; Masuda, K.; Kobayashi, M. The effect of monovalent anion species on the aggregation and charging of allophane clay nanoparticles. Colloids Surf. A 2019, 577, 103–109. [Google Scholar] [CrossRef]
- Omija, K.; Hakim, A.; Masuda, K.; Yamaguchi, A.; Kobayashi, M. Effect of counter ion valence and pH on the aggregation and charging of oxidized carbon nanohorn (CNHox) in aqueous solution. Colloids Surf. A 2021, 619, 126552. [Google Scholar] [CrossRef]
- Nakamura, H.; Makino, S.; Ishii, M. Effects of electrostatic interaction on rheological behavior and microstructure of concentrated colloidal suspensions. Colloids Surf. A 2021, 623, 126576. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Ko, C.H.; Elimelech, A. DLVO Interaction between Rough Surfaces. Langmuir 1998, 14, 3365–3375. [Google Scholar] [CrossRef]
- Considine, R.F.; Drummond, A.J. Surface Roughness and Surface Force Measurement: A Comparison of Electrostatic Potentials Derived from Atomic Force Microscopy and Electrophoretic Mobility Measurements. Langmuir 2001, 17, 7777–7783. [Google Scholar] [CrossRef]
- Tian, Z.; Li, M.; Sugimoto, T.; Kobayashi, M. The Effect of Lysozyme on the Aggregation and Charging of Oxidized Carbon Nanohorn (CNHox) in Aqueous Solution. Appl. Sci. 2024, 14, 2645. [Google Scholar] [CrossRef]
- Nakamura, H.; Ishii, M. Rheological Behavior of Concentrated Monodispersed Colloidal Suspensions. Nihon Reoroji Gakkaishi. 2019, 47, 001–007. [Google Scholar] [CrossRef]
- Senchenko, V.N.; Belikov, R.S. Experimental investigation of density of pyrolytic graphite up to melting point. J. Phys. Conf. Ser. 2018, 946, 012105. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moteki, A.; Kobayashi, M. Rheological Behavior of an Aqueous Suspension of Oxidized Carbon Nanohorn (CNHox). Nanomaterials 2024, 14, 1247. https://doi.org/10.3390/nano14151247
Moteki A, Kobayashi M. Rheological Behavior of an Aqueous Suspension of Oxidized Carbon Nanohorn (CNHox). Nanomaterials. 2024; 14(15):1247. https://doi.org/10.3390/nano14151247
Chicago/Turabian StyleMoteki, Ayumi, and Motoyoshi Kobayashi. 2024. "Rheological Behavior of an Aqueous Suspension of Oxidized Carbon Nanohorn (CNHox)" Nanomaterials 14, no. 15: 1247. https://doi.org/10.3390/nano14151247





