Controlled Morphological Growth and Photonic Lasing in Cesium Lead Bromide Microcrystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis Procedure
2.3. Characterizations
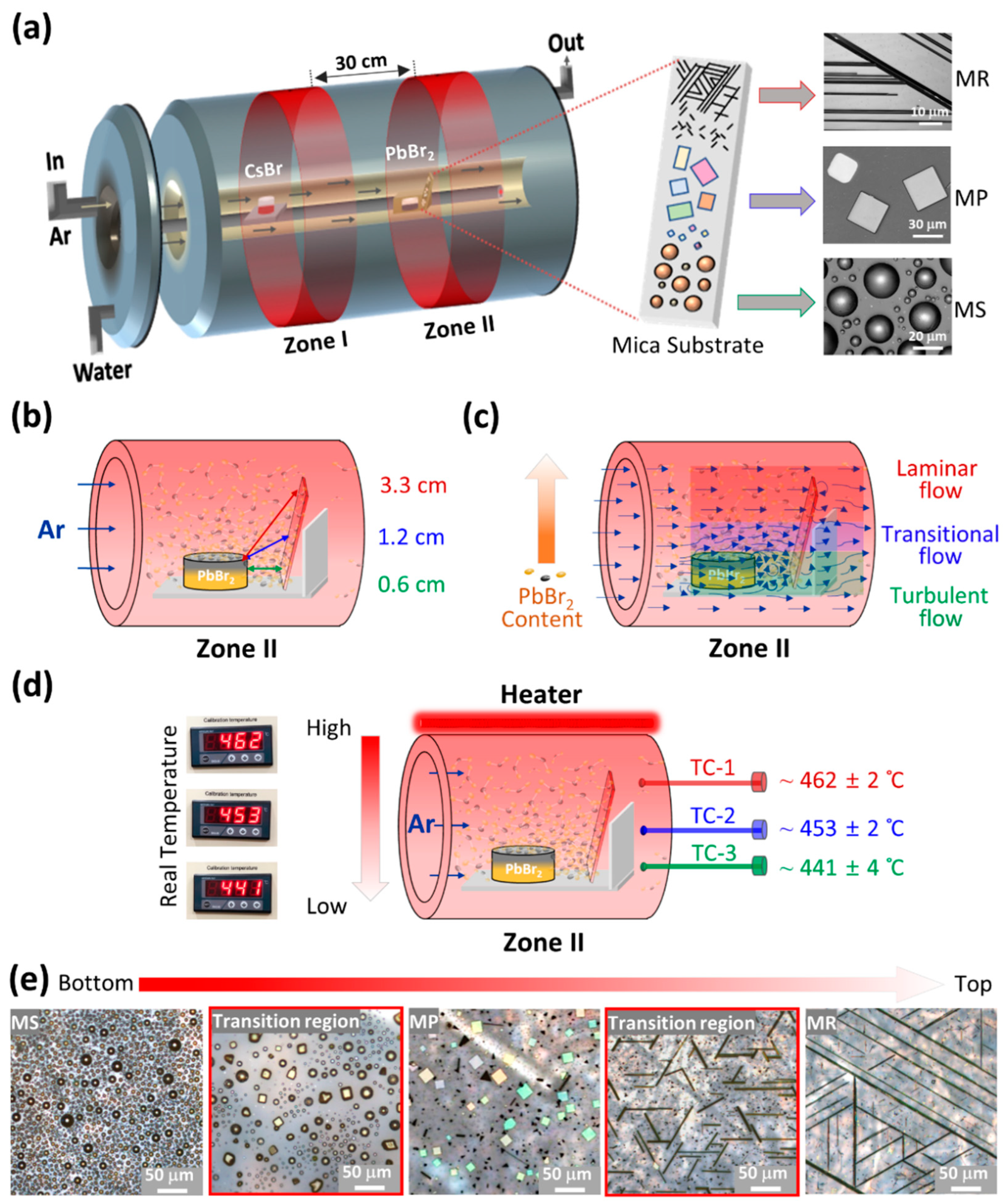
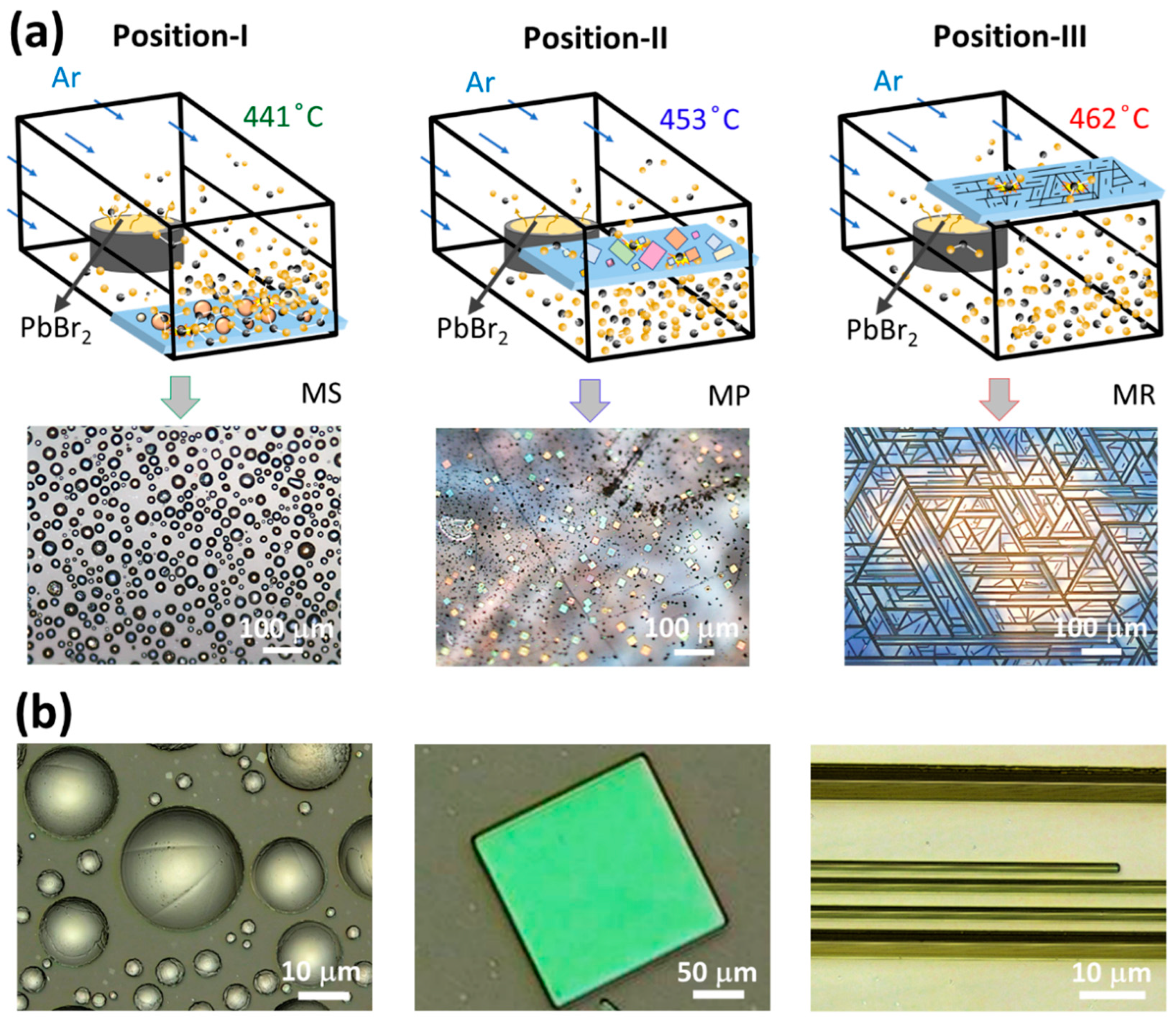
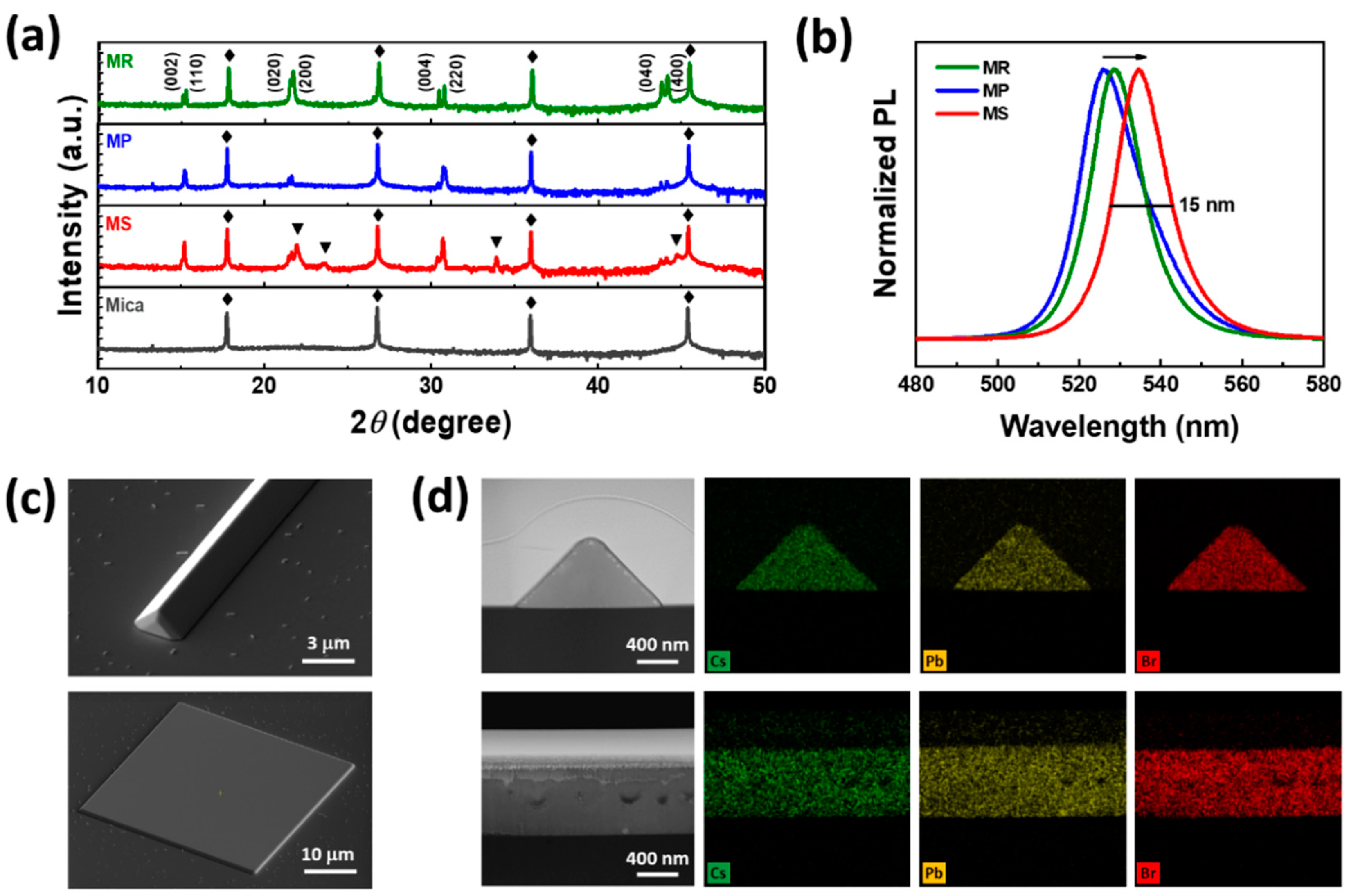
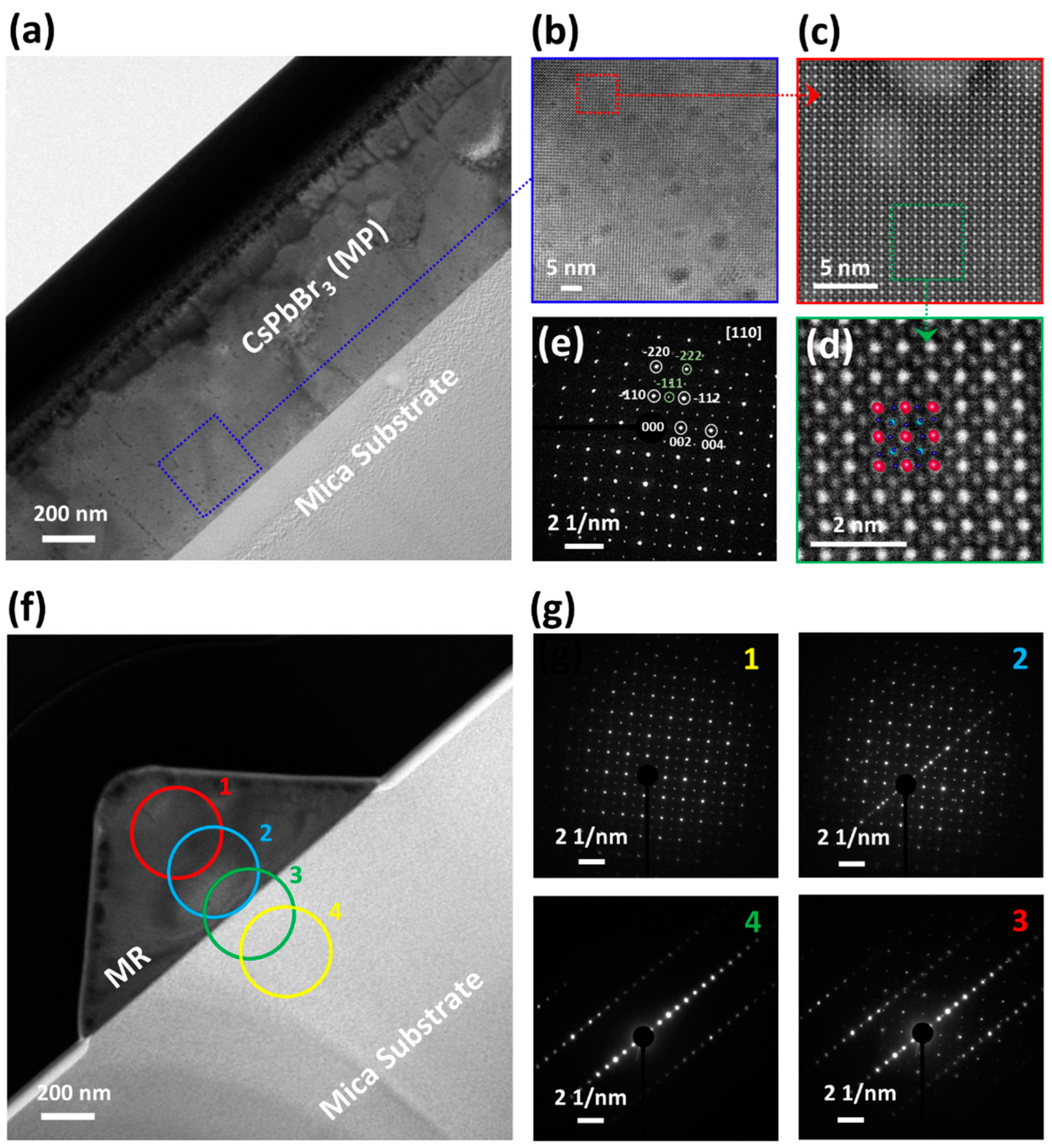
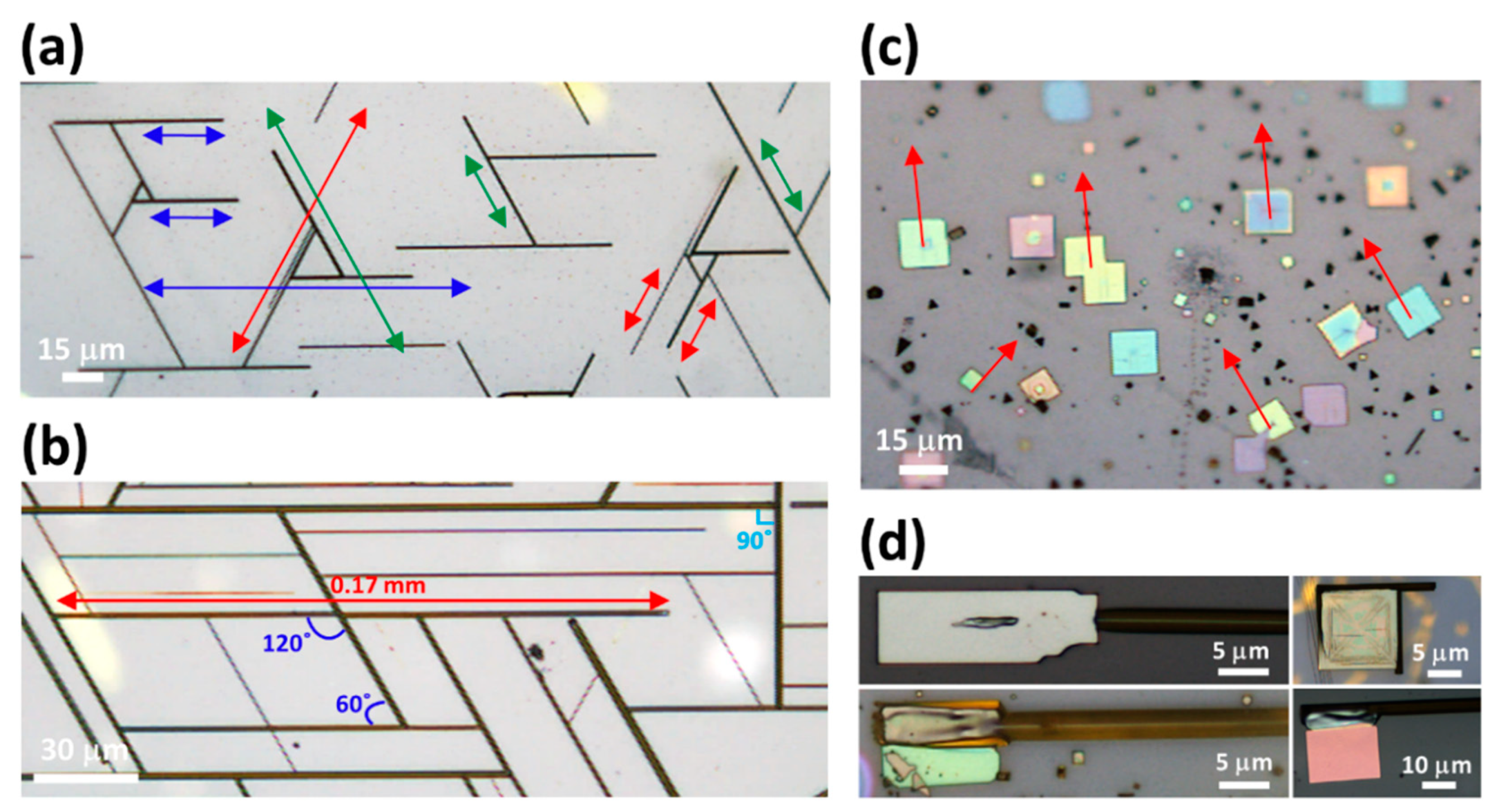
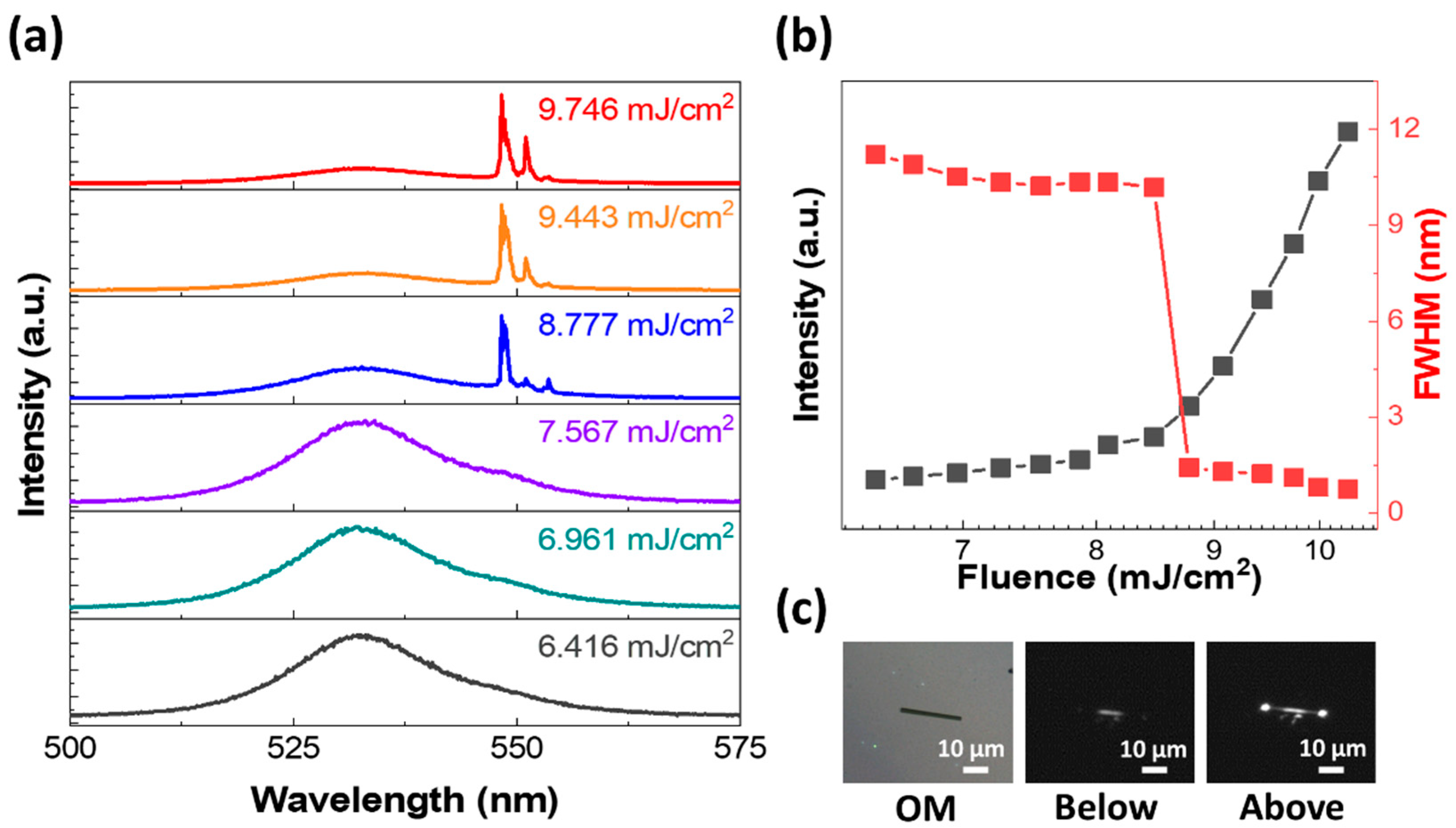
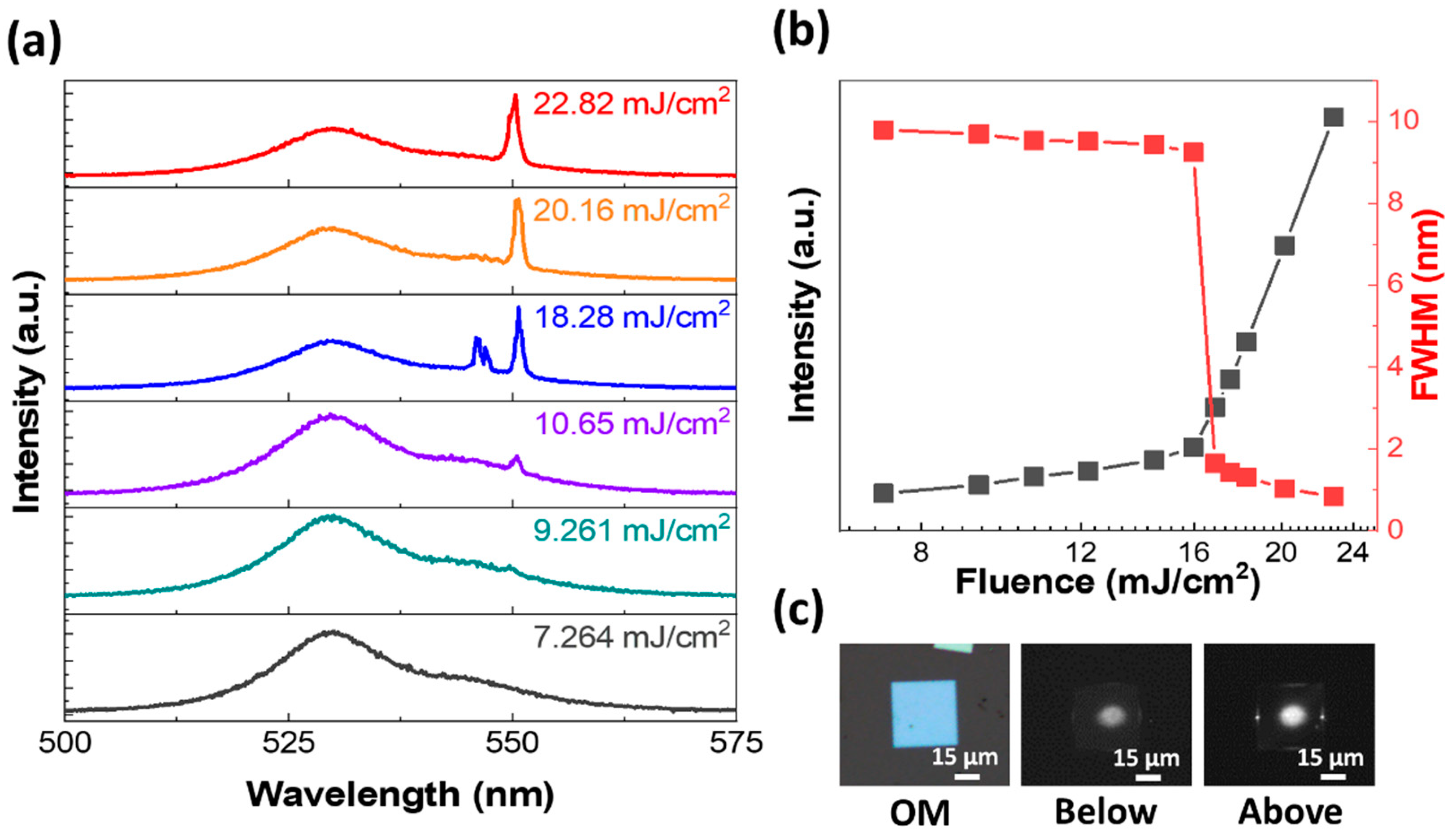
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Du, Z.; Jiang, X.; Cao, S.; Zou, B.; Zheng, J.; Zhao, J. Ultrastable photodetectors based on blue CsPbBr3 perovskite nanoplatelets via a surface engineering strategy. ACS Appl. Mater. Interfaces 2024, 16, 11694–11703. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, Z.; Huang, Q.; Ye, Y.; Ye, B.; Shen, Z.; Wu, W.; Zeng, Z.; Hong, Z.; Meng, Z.; et al. Achieving high-efficiency CsPbBr3 perovskite light emitting diode via GABr-didecyldimethyl ammonium bromide hybrid ligand passivation strategies. Org. Electron. 2023, 118, 106797. [Google Scholar] [CrossRef]
- Tahir, Z.; Jung, J.W.; Rashid, M.U.; Kim, S.; Dang, D.K.; Kang, J.W.; Cho, C.H.; Jang, J.I.; Kim, Y.S. Strong exciton-photon coupling in self-hybridized organic-inorganic lead halide perovskite microcavities. Nanophotonics 2023, 12, 4297–4306. [Google Scholar] [CrossRef]
- Zhang, Z.; Saparov, B. Charge carrier mobility of halide perovskite single crystals for ionizing radiation detection. Appl. Phys. Lett. 2021, 119, 030502. [Google Scholar] [CrossRef]
- Haruta, Y.; Ikenoue, T.; Miyake, M.; Hirato, T. Fabrication of (101)-oriented CsPbBr3 thick films with high carrier mobility using a mist deposition method. Appl. Phys. Express 2019, 12, 085505. [Google Scholar] [CrossRef]
- Alvarez, S.G.; Lin, W.; Abdellah, M.; Meng, J.; Zidek, K.; Pullerits, T.N.; Zheng, K. Charge carrier diffusion dynamics in multisized quaternary alkylammonium-capped CsPbBr3 perovskite nanocrystal solids. ACS Appl. Mater. Interface 2021, 13, 44742–44750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, G.; Zhang, Y.; Fang, Z.; He, H.; Zhu, H. Long-range transport and ultrafast interfacial charge transfer in perovskite/monolayer semiconductor heterostructure for enhanced light absorption and photocarrier lifetime. J. Chem. Phys. 2022, 156, 244701. [Google Scholar] [CrossRef] [PubMed]
- Straus, D.B.; Cava, R.J. Tuning the band gap in the halide perovskite CsPbBr3 through Sr substitution. ACS Appl. Mater. Interface 2022, 14, 34884–34890. [Google Scholar] [CrossRef] [PubMed]
- Rajeswarapalanichamy, R.; Amudhavalli, A.; Padmavathy, R.; Iyakutti, K. Band gap engineering in halide cubic perovskites CsPbBr3− yIy (y = 0, 1, 2, 3) – A DFT study. Mater. Sci. Eng. B 2020, 258, 114560. [Google Scholar] [CrossRef]
- Kulbak, M.; Gupta, S.; Kedem, N.; Levine, I.; Bendikov, T.; Hodes, G.; Cahen, D. Cesium enhances long-term stability of lead bromide perovskite-based solar cells. J. Phys. Chem. Lett. 2016, 7, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Kerner, R.A.; Zhao, L.; Tran, N.L.; Lee, K.M.; Koh, T.W.; Scholes, G.D.; Rand, B.P. Efficient perovskite light-emitting diodes featuring nanometre-sized crystallites. Nat. Photonics 2017, 11, 108–115. [Google Scholar] [CrossRef]
- Yuan, H.; Zhao, Y.; Duan, J.; Wang, Y.; Yang, X.; Tang, Q. All-inorganic CsPbBr3 perovskite solar cell with 10.26% efficiency by spectra engineering. J. Mater. Chem. A 2018, 6, 24324–24329. [Google Scholar] [CrossRef]
- Ou, Z.; Yi, Y.; Hu, Z.; Zhu, J.; Wang, W.; Meng, H.; Zhang, X.; Jing, S.; Xu, S.; Hong, F.; et al. Improvement of CsPbBr3 photodetector performance by tuning the morphology with PMMA additive. J. Alloys Compd. 2020, 821, 153344. [Google Scholar] [CrossRef]
- Huang, C.Y.; Zou, C.; Mao, C.; Corp, K.L.; Yao, Y.C.; Lee, Y.J.; Schlenker, C.W.; Jen, A.K.Y.; Lin, L.Y. CsPbBr3 perovskite quantum dot vertical cavity lasers with low threshold and high stability. ACS Photonics 2017, 4, 2281–2289. [Google Scholar] [CrossRef]
- Du, X.; Wu, G.; Cheng, J.; Dang, H.; Ma, K.; Zhang, Y.W.; Tan, P.F.; Chen, S. High-quality CsPbBr3 perovskite nanocrystals for quantum dot light-emitting diodes. RSC Adv. 2017, 7, 10391–10396. [Google Scholar] [CrossRef]
- Jin, B.; Zuo, N.; Hu, Z.Y.; Cui, W.; Wang, R.; Tendeloo, G.V.; Zhou, X.; Zhai, T. Excellent excitonic photovoltaic effect in 2D CsPbBr3/CdS heterostructures. Adv. Funct. Mater. 2020, 30, 2006166. [Google Scholar] [CrossRef]
- Yu, J.; Liu, G.; Chen, C.; Li, Y.; Xu, M.; Wang, T.; Zhao, G.; Zhang, L. Perovskite CsPbBr3 crystals: Growth and applications. J. Mater. Chem. C 2020, 8, 6326–6341. [Google Scholar] [CrossRef]
- Ananthakumar, S.; Kumar, J.R.; Babu, S.M. Cesium lead halide (CsPbX3, X = Cl, Br, I) perovskite quantum dots-synthesis, properties, and applications: A review of their present status. J. Photon. Energy 2016, 6, 042001. [Google Scholar] [CrossRef]
- Chaudhary, B.; Kshetri, Y.K.; Kim, H.S.; Lee, S.W.; Kim, T.H. Current status on synthesis, properties and applications of CsPbX3(X = Cl, Br, I) perovskite quantum dots/nanocrystals. Nanotechnology 2021, 32, 502007. [Google Scholar] [CrossRef]
- Chen, M.; Zou, Y.; Wu, L.; Pan, Q.; Yang, D.; Hu, H.; Tan, Y.; Zhong, Q.; Xu, Y.; Liu, H.; et al. Solvothermal synthesis of high-quality all-inorganic cesium lead halide perovskite nanocrystals: From nanocube to ultrathin nanowire. Adv. Funct. Mater. 2017, 27, 1701121. [Google Scholar] [CrossRef]
- Yan, J.; Hou, S.; Li, X.; Dong, J.; Zou, L.; Yang, M.; Xing, J.; Liu, H.; Hao, H. Preparation of highly efficient and stable CsPbBr3 perovskite solar cells based on an anti-solvent rinsing strategy. Sol. Energy. Mater. Sol. Cells 2022, 234, 111420. [Google Scholar] [CrossRef]
- Cleveland, I.J.; Tran, M.N.; Dey, A.; Aydil, E.S. Vapor deposition of CsPbBr3 thin films by evaporation of CsBr and PbBr2. J. Vac. Sci. Technol. 2021, 39, 043415. [Google Scholar] [CrossRef]
- Xiang, Y.; Mo, X.; Li, X.; Huang, K.; He, P.; Dai, G.; Yang, J. Progress on growth of metal halide perovskites by vapor-phase synthesis and their applications. J. Phys. D Appl. Phys. 2021, 55, 073001. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Zhang, C.; Dong, H.; Lin, W.; Zhuang, P.; Wen, Y.; Tian, B.; Cai, W.; Zhang, X. Fractal-theory-based control of the shape and quality of CVD-grown 2D materials. Adv. Funct. Mater. 2019, 31, 1902431. [Google Scholar] [CrossRef]
- Mo, X.; Li, X.; Dai, G.; He, P.; Sun, J.; Huang, H.; Yang, J. All-inorganic perovskite CsPbBr3 microstructures growth via chemical vapor deposition for high-performance photodetectors. Nanoscale 2019, 11, 21386–21393. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Xin, G.; Lian, J.; Shi, J. Two-dimensional van der waals epitaxy kinetics in a three-dimensional perovskite halide. Crys. Growth Des. 2015, 15, 4741–4749. [Google Scholar] [CrossRef]
- Chen, J.; Morrow, D.J.; Fu, Y.; Zheng, W.; Zhao, Y.; Dang, L.; Stolt, M.J.; Kohler, D.D.; Wang, X.; Czech, K.J.; et al. Single-crystal thin films of cesium lead bromide perovskite epitaxially grown on metal oxide perovskite (SrTiO3). J. Am. Chem. Soc. 2017, 139, 13525–13532. [Google Scholar] [CrossRef]
- Zhong, Y.; Liao, K.; Du, W.; Zhu, J.; Shang, Q.; Zhou, F.; Wu, X.; Sui, X.; Shi, J.; Yue, S.; et al. Large-scale thin CsPbBr3 single-crystal film grown on sapphire via chemical vapor deposition: Toward laser array application. ACS Nano 2020, 14, 15605–15615. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, R.; Liu, X.; Xing, J.; Sum, T.C.; Xiong, Q. High-quality whispering-gallery-mode lasing from cesium lead halide perovskite nanoplatelets. Adv. Funct. Mater. 2016, 26, 6238–6245. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Shivanna, R.; Yang, Y.; Chen, Z.; Guo, Y.; Wang, G.C.; Wertz, E.; Deschler, F.; Cai, Z.; et al. Photon transport in one-dimensional incommensurately epitaxial CsPbX3 arrays. Nano Lett. 2016, 16, 7974–7981. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fu, Y.; Samad, L.; Dang, L.; Zhao, Y.; Shen, S.; Guo, L.; Jin, S. Vapor-phase epitaxial growth of aligned nanowire networks of cesium lead halide perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2017, 17, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Oksenberg, E.; Merdasa, A.; Houben, L.; Ashiri, I.K.; Rothman, A.; Scheblykin, I.G.; Unger, E.L.; Joselevich, E. Large lattice distortions and size-dependent bandgap modulation in epitaxial halide perovskite nanowires. Nat. Commun. 2020, 11, 489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, C.; Chen, S.; Tian, J.; Yan, J.; Weng, G.; Hu, X.; Tao, J.; Pan, Y.; Chen, S.; et al. Lasing operation in the CsPbBr3 perovskite micron hemisphere cavity grown by chemical vapor deposition. J. Chem. Eng. 2020, 389, 124395. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Zhou, H.; Wang, X.; Yuan, S.; Yang, Z.; Zhu, X.; Ma, R.; Pan, A. Room-temperature high-performance CsPbBr3 perovskite tetrahedral microlasers. Nanoscale 2019, 11, 2393–2400. [Google Scholar] [CrossRef] [PubMed]
- Lan, S.; Li, W.; Wang, S.; Li, J.; Wang, J.; Wang, H.; Luo, H.; Li, D. Vapor-phase growth of CsPbBr3 microstructures for highly efficient pure green light emission. Adv. Opt. Mater. 2019, 7, 1801336. [Google Scholar] [CrossRef]
- Wang, X.; You, F.; Huang, J.; Yao, Y.; Xu, F. Effect of carrier gas flow rate on the morphology and luminescence properties of CsPbBr3 microcrystals. Crystals 2022, 12, 479. [Google Scholar] [CrossRef]
- LUXEL Vapor Pressure Chart. Available online: http://luxel.com/wp-content/uploads/2013/04/Luxel-Vapor-Pressure-Chart.pdf (accessed on 26 December 2023).
- Park, Y.C.; Park, B.C.; Romankov, S.; Park, K.J.; Yoo, J.H.; Lee, Y.B.; YANG, J.M. Use of permanent marker to deposit a protection layer against FIB damage in TEM specimen preparation. J. Microsc. 2014, 255, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Momeni, K.; AlSaud, M.A.; Azizi, A.; Hainey, M.F.; Redwing, J.M.; Chen, L.Q.; Alem, N. Controlled synthesis of 2D transition metal dichalcogenides: From vertical to planar MoS2. 2D Mater. 2017, 4, 025029. [Google Scholar] [CrossRef]
- Bai, M.; Wen, S.; Zhao, J.; Du, Y.; Xie, F.; Liu, H. Effect of carrier gas flow field on chemical vapor deposition of 2D MoS2 crystal. Coatings 2021, 11, 547. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, D.; Kisielowski, C.; Dou, L.; Kornienko, N.; Bekenstein, Y.; Wong, A.B.; Alivisatos, A.P.; Yang, P. Atomic resolution imaging of halide perovskites. Nano Lett. 2016, 16, 7530–7535. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, Z.; Fu, Q.; Chen, Z.; He, J.; Zhang, S.; Yan, L.; Hu, Y.; Luo, W. Growth and characterization of all-inorganic lead halide perovskite semiconductor CsPbBr3 single crystals. CrystEngComm 2017, 19, 6797–6803. [Google Scholar] [CrossRef]
- Yamada, T.; Yamada, Y.; Kanemitsu, Y. Photon recycling in perovskite CH3NH3PbX3 (X = I, Br, Cl) bulk single crystals and polycrystalline films. J. Lumin. 2020, 220, 116987. [Google Scholar] [CrossRef]
- Brennan, M.C.; Kuno, M.; Rouvimov, S. Crystal structure of individual CsPbBr3 perovskite nanocubes. Inorg. Chem. 2018, 58, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, F.; Li, X.; Ru, F.; Liu, P.; Wang, L.; Ji, W.; Xia, J.; Meng, X. Epitaxial growth of large-scale orthorhombic CsPbBr3 perovskite thin films with anisotropic photoresponse property. Adv. Funct. Mater. 2019, 29, 1904913. [Google Scholar] [CrossRef]
- Song, K.; Liu, L.; Zhang, D.; Hautzinger, M.P.; Jin, S.; Han, Y. Atomic-resolution imaging of halide perovskites using electron microscopy. Adv. Energy Mater. 2020, 10, 1904006. [Google Scholar] [CrossRef]
- Ji, Q.; Zhang, Y.; Gao, T.; Zhang, Y.; Ma, D.; Liu, M.; Chen, Y.; Qiao, X.; Tan, P.H.; Kan, M.; et al. Epitaxial monolayer MoS2 on mica with novel photoluminescence. Nano Lett. 2013, 13, 3870–3877. [Google Scholar] [CrossRef] [PubMed]
- Torres, O.; Gordillo, G.; Plazas, M.; Landínez Téllez, D.; Roa-Rojas, J. Optical features of PbBr2 semiconductor thin films for radiation attenuation application. J. Mater. Sci. Mater. Electron. 2021, 32, 16937–16944. [Google Scholar] [CrossRef]
- Ravikant, C.; Arun, P.; Kumar, K. SPR sensitivity of silver nanorods in CsBr-Ag nanocomposite thin films. Mater. Res. Express 2016, 3, 076403. [Google Scholar]
- Lalanne, P.; Mias, S.; Hugonin, J.P. Two physical mechanisms for boosting the quality factor to cavity volume ratio of photonic crystal microcavities. Opt. Express 2004, 12, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Khurgin, J.B.; Noginov, M.A. How Do the Purcell Factor, the Q-Factor, and the Beta Factor Affect the Laser Threshold? Laser Photonics Rev. 2021, 15, 2000250. [Google Scholar] [CrossRef]
- Srinivasan, K.; Borselli, M.; Painter, O.; Stintz, A.; Krishna, S. Cavity Q, mode volume, and lasing threshold in small diameter AlGaAs microdisks with embedded quantum dots. Opt. Express 2006, 14, 1094–1105. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guan, S.; Liu, Y.; Xu, G.; Cai, B. Lasing properties of cesium lead halide perovskite nanowires fabricated by one-drop self-assembly and ion-exchange methods. Opt. Express 2018, 26, 33856–33864. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Zhou, Z.; Huang, Z.; Wang, K.; Cai, Z.; Hu, X.; Li, L.; Li, M.; Zhao, Y.S.; Song, Y. Controllable growth of high-quality inorganic perovskite microplate arrays for functional optoelectronics. Adv. Mater. 2020, 32, 1908006. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Zhang, S.; Wu, Z.; Shang, Q.; Mi, Y.; Chen, J.; Qin, C.; Qiu, X.; Zhang, Q.; Liu, X. Unveiling lasing mechanism in CsPbBr3 microsphere cavities. Nanoscale 2019, 11, 3145–3153. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, T.; Min, Q.; Pi, C.; Li, F.; Yang, X.; Xu, X. Ultrahigh photo-stable all-inorganic perovskite nanocrystals and their robust random lasing. Nanoscale Adv. 2020, 2, 888–895. [Google Scholar] [CrossRef]
- Yakunin, S.; Protesescu, L.; Krieg, F.; Bodnarchuk, M.I.; Nedelcu, G.; Humer, M.; Luca, G.D.; Fiebig, M.; Heis, W.; Kovalenko, M.V. Low-threshold amplified spontaneous emission and lasing from colloidal nanocrystals of caesium lead halide perovskites. Nat. Commun. 2015, 6, 8056. [Google Scholar] [CrossRef]
- Wang, J.; Yu, H.; Liu, G.; Liu, W.; Duan, Y.; Cheng, X.; Dai, L.; Wang, S.; Gong, Q. Ultrafast lasing dynamics in a CsPbBr3 perovskite microplate. Adv. Photonics Res. 2022, 3, 2100182. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, M.U.; Tahir, Z.; Sheeraz, M.; Ullah, F.; Park, Y.C.; Maqbool, F.; Kim, Y.S. Controlled Morphological Growth and Photonic Lasing in Cesium Lead Bromide Microcrystals. Nanomaterials 2024, 14, 1248. https://doi.org/10.3390/nano14151248
Rashid MU, Tahir Z, Sheeraz M, Ullah F, Park YC, Maqbool F, Kim YS. Controlled Morphological Growth and Photonic Lasing in Cesium Lead Bromide Microcrystals. Nanomaterials. 2024; 14(15):1248. https://doi.org/10.3390/nano14151248
Chicago/Turabian StyleRashid, Mamoon Ur, Zeeshan Tahir, Muhammad Sheeraz, Farman Ullah, Yun Chang Park, Faisal Maqbool, and Yong Soo Kim. 2024. "Controlled Morphological Growth and Photonic Lasing in Cesium Lead Bromide Microcrystals" Nanomaterials 14, no. 15: 1248. https://doi.org/10.3390/nano14151248





