Engineering of LiTaO3 Nanoparticles by Flame Spray Pyrolysis: Understanding In Situ Li-Incorporation into the Ta2O5 Lattice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of LiTaO3 Nanoparticles via Flame Spray Pyrolysis (FSP)
2.2. Structural Characterization Techniques
2.3. Photocatalytic Evaluation
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, P.; Zhang, J.; Gong, J. Tantalum-Based Semiconductors for Solar Water Splitting. Chem. Soc. Rev. 2014, 43, 4395–4422. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous Photocatalyst Materials for Water Splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Zhu, J.; Li, H.; Zhong, L.; Xiao, P.; Xu, X.; Yang, X.; Zhao, Z.; Li, J. Perovskite Oxides: Preparation, Characterizations, and Applications in Heterogeneous Catalysis. ACS Catal. 2014, 4, 2917–2940. [Google Scholar] [CrossRef]
- Jiao, A.; Zhang, Y.; Yang, L.; Zhao, X.; Wu, C.; Chen, T.; Zhan, R.; Huang, Z.; Lin, H. Enhanced CO2 Response of La1−xFeO3−δ Perovskites with A-Site Deficiency Synthesized by Flame Spray Pyrolysis. Ceram. Int. 2023, 49, 591–599. [Google Scholar] [CrossRef]
- Ahmad, T.; Farooq, U.; Phul, R. Fabrication and Photocatalytic Applications of Perovskite Materials with Special Emphasis on Alkali-Metal-Based Niobates and Tantalates. Ind. Eng. Chem. Res. 2018, 57, 18–41. [Google Scholar] [CrossRef]
- Ishihara, T.; Nishiguchi, H.; Fukamachi, K.; Takita, Y. Effects of Acceptor Doping to KTaO3 on Photocatalytic Decomposition of Pure H2O. J. Phys. Chem. B 1999, 103, 1–3. [Google Scholar] [CrossRef]
- Bienkowski, K.; Solarska, R.; Trinh, L.; Widera-Kalinowska, J.; Al-Anesi, B.; Liu, M.; Grandhi, G.K.; Vivo, P.; Oral, B.; Yılmaz, B.; et al. Halide Perovskites for Photoelectrochemical Water Splitting and CO2 Reduction: Challenges and Opportunities. ACS Catal. 2024, 14, 6603–6622. [Google Scholar] [CrossRef]
- Yan, S.C.; Wang, Z.Q.; Li, Z.S.; Zou, Z.G. Photocatalytic Activities for Water Splitting of La-Doped-NaTaO3 Fabricated by Microwave Synthesis. Solid State Ion. 2009, 180, 1539–1542. [Google Scholar] [CrossRef]
- Takasugi, S.; Tomita, K.; Iwaoka, M.; Kato, H.; Kakihana, M. The Hydrothermal and Solvothermal Synthesis of LiTaO3 Photocatalyst: Suppressing the Deterioration of the Water Splitting Activity without Using a Cocatalyst. Int. J. Hydrogen Energy 2015, 40, 5638–5643. [Google Scholar] [CrossRef]
- Zlotnik, S.; Tobaldi, D.M.; Seabra, P.; Labrincha, J.A.; Vilarinho, P.M. Alkali Niobate and Tantalate Perovskites as Alternative Photocatalysts. ChemPhysChem 2016, 17, 3570–3575. [Google Scholar] [CrossRef]
- Kato, H.; Kudo, A. Water Splitting into H2 and O2 on Alkali Tantalate Photocatalysts ATaO3 (A = Li, Na, and K). J. Phys. Chem. B 2001, 105, 4285–4292. [Google Scholar] [CrossRef]
- Nyman, M.; Anderson, T.M.; Provencio, P.P. Comparison of Aqueous and Non-Aqueous Soft-Chemical Syntheses of Lithium Niobate and Lithium Tantalate Powders. Cryst. Growth Des. 2009, 9, 1036–1040. [Google Scholar] [CrossRef]
- Kato, H.; Kudo, A. New Tantalate Photocatalysts for Water Decomposition into H2 and O2. Chem. Phys. Lett. 1998, 295, 487–492. [Google Scholar] [CrossRef]
- Kato, H.; Kudo, A. Highly Efficient Decomposition of Pure Water into H2 and O2 over NaTaO3 Photocatalysts. Catal. Lett. 1999, 58, 153–155. [Google Scholar] [CrossRef]
- Ashiri, R.; Nemati, A.; Sasani Ghamsari, M.; Sanjabi, S.; Aalipour, M. A Modified Method for Barium Titanate Nanoparticles Synthesis. Mater. Res. Bull. 2011, 46, 2291–2295. [Google Scholar] [CrossRef]
- Joshi, U.A.; Lee, J.S. Template-Free Hydrothermal Synthesis of Single-Crystalline Barium Titanate and Strontium Titanate Nanowires. Small 2005, 1, 1172–1176. [Google Scholar] [CrossRef]
- Pan, Q.; Hu, H.; Zou, Y.; Chen, M.; Wu, L.; Yang, D.; Yuan, X.; Fan, J.; Sun, B.; Zhang, Q. Microwave-Assisted Synthesis of High-Quality “All-Inorganic” CsPbX3 (X = Cl, Br, I) Perovskite Nanocrystals and Their Application in Light Emitting Diodes. J. Mater. Chem. C 2017, 5, 10947–10954. [Google Scholar] [CrossRef]
- Sonia; Chandrasekhar, M.; Kumar, P. Microwave Sintered Sol–Gel Derived BaTiO3 and Ba0.95La0.05TiO3 Ceramic Samples for Capacitor Applications. Ceram. Int. 2016, 42, 10587–10592. [Google Scholar] [CrossRef]
- Chiarello, G.; Rossetti, I.; Forni, L. Flame-Spray Pyrolysis Preparation of Perovskites for Methane Catalytic Combustion. J. Catal. 2005, 236, 251–261. [Google Scholar] [CrossRef]
- Zeyrek Ongun, M.; Oguzlar, S.; Akalin, S.A.; Yildirim, S. Characterization and Optical Gas Sensing Properties of BaSnO3 Synthesized by Novel Technique: Flame Spray Pyrolysis. J. Mater. Sci. Mater. Electron. 2021, 32, 15160–15170. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Amal, R.; Mädler, L. Flame Spray Pyrolysis: An Enabling Technology for Nanoparticles Design and Fabrication. Nanoscale 2010, 2, 1324. [Google Scholar] [CrossRef]
- Angel, S.; Schneider, F.; Apazeller, S.; Kaziur-Cegla, W.; Schmidt, T.C.; Schulz, C.; Wiggers, H. Spray-Flame Synthesis of LaMO3 (M = Mn, Fe, Co) Perovskite Nanomaterials: Effect of Spray Droplet Size and Esterification on Particle Size Distribution. Proc. Combust. Inst. 2021, 38, 1279–1287. [Google Scholar] [CrossRef]
- Yuan, X.; Meng, L.; Xu, Z.; Zheng, C.; Zhao, H. CuO Quantum Dots Supported by SrTiO3 Perovskite Using the Flame Spray Pyrolysis Method: Enhanced Activity and Excellent Thermal Resistance for Catalytic Combustion of CO and CH4. Environ. Sci. Technol. 2021, 55, 14080–14086. [Google Scholar] [CrossRef] [PubMed]
- Psathas, P.; Georgiou, Y.; Moularas, C.; Armatas, G.S.; Deligiannakis, Y. Controlled-Phase Synthesis of Bi2Fe4O9 & BiFeO3 by Flame Spray Pyrolysis and Their Evaluation as Non-Noble Metal Catalysts for Efficient Reduction of 4-Nitrophenol. Powder Technol. 2020, 368, 268–277. [Google Scholar] [CrossRef]
- Psathas, P.; Solakidou, M.; Mantzanis, A.; Deligiannakis, Y. Flame Spray Pyrolysis Engineering of Nanosized Mullite-Bi2Fe4O9 and Perovskite-BiFeO3 as Highly Efficient Photocatalysts for O2 Production from H2O Splitting. Energies 2021, 14, 5235. [Google Scholar] [CrossRef]
- Kho, Y.K.; Teoh, W.Y.; Iwase, A.; Mädler, L.; Kudo, A.; Amal, R. Flame Preparation of Visible-Light-Responsive BiVO4 Oxygen Evolution Photocatalysts with Subsequent Activation via Aqueous Route. ACS Appl. Mater. Interfaces 2011, 3, 1997–2004. [Google Scholar] [CrossRef]
- Stathi, P.; Solakidou, M.; Deligiannakis, Y. Lattice Defects Engineering in W-, Zr-Doped BiVO4 by Flame Spray Pyrolysis: Enhancing Photocatalytic O2 Evolution. Nanomaterials 2021, 11, 501. [Google Scholar] [CrossRef] [PubMed]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi (B) 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Koirala, R.; Pratsinis, S.E.; Baiker, A. Synthesis of Catalytic Materials in Flames: Opportunities and Challenges. Chem. Soc. Rev. 2016, 45, 3053–3068. [Google Scholar] [CrossRef]
- Pratsinis, S.E.; Groehn, A.J.; Waser, O.; Brenner, O. Process Design for Size-Controlled Flame Spray Synthesis of Li4Ti5O12 and Electrochemical Performance. Chem. Process Eng. 2017, 38, 51–66. [Google Scholar]
- Waser, O.; Groehn, A.J.; Eggersdorfer, M.L.; Pratsinis, S.E. Air Entrainment During Flame Aerosol Synthesis of Nanoparticles. Aerosol Sci. Technol. 2014, 48, 1195–1206. [Google Scholar] [CrossRef]
- Knausenberger, H. Selected Properties of Pyrolytic Ta2O5 Films. J. Electrochem. Soc. 1973, 120, 927–931. [Google Scholar] [CrossRef]
- Satapathy, S.; Gupta, P.K.; Srivastava, H.; Srivastava, A.K.; Wadhawan, V.K.; Varma, K.B.R.; Sathe, V.G. Effect of Capping Ligands on the Synthesis and on the Physical Properties of the Nanoparticles of LiTaO3. J. Cryst. Growth 2007, 307, 185–191. [Google Scholar] [CrossRef]
- Djohan, N.; Harsono, B.; Liman, J.; Hardhienata, H. Structural, Optical Properties and Raman Spectroscopy of In2O3 Doped LiTaO3 Thin Films. Int. J. Nanoelectron. Mater. 2022, 15, 17–26. [Google Scholar]
- Halas, S.; Durakiewicz, T. Work Functions of Elements Expressed in Terms of the Fermi Energy and the Density of Free Electrons. J. Phys. Condens. Matter 1998, 10, 10815–10826. [Google Scholar] [CrossRef]
- Shi, J.; Guo, L. ABO3-Based Photocatalysts for Water Splitting. Prog. Nat. Sci. Mater. Int. 2012, 22, 592–615. [Google Scholar] [CrossRef]
- Kato, H.; Kudo, A. Photocatalytic Water Splitting into H2 and O2 over Various Tantalate Photocatalysts. Catal. Today 2003, 78, 561–569. [Google Scholar] [CrossRef]
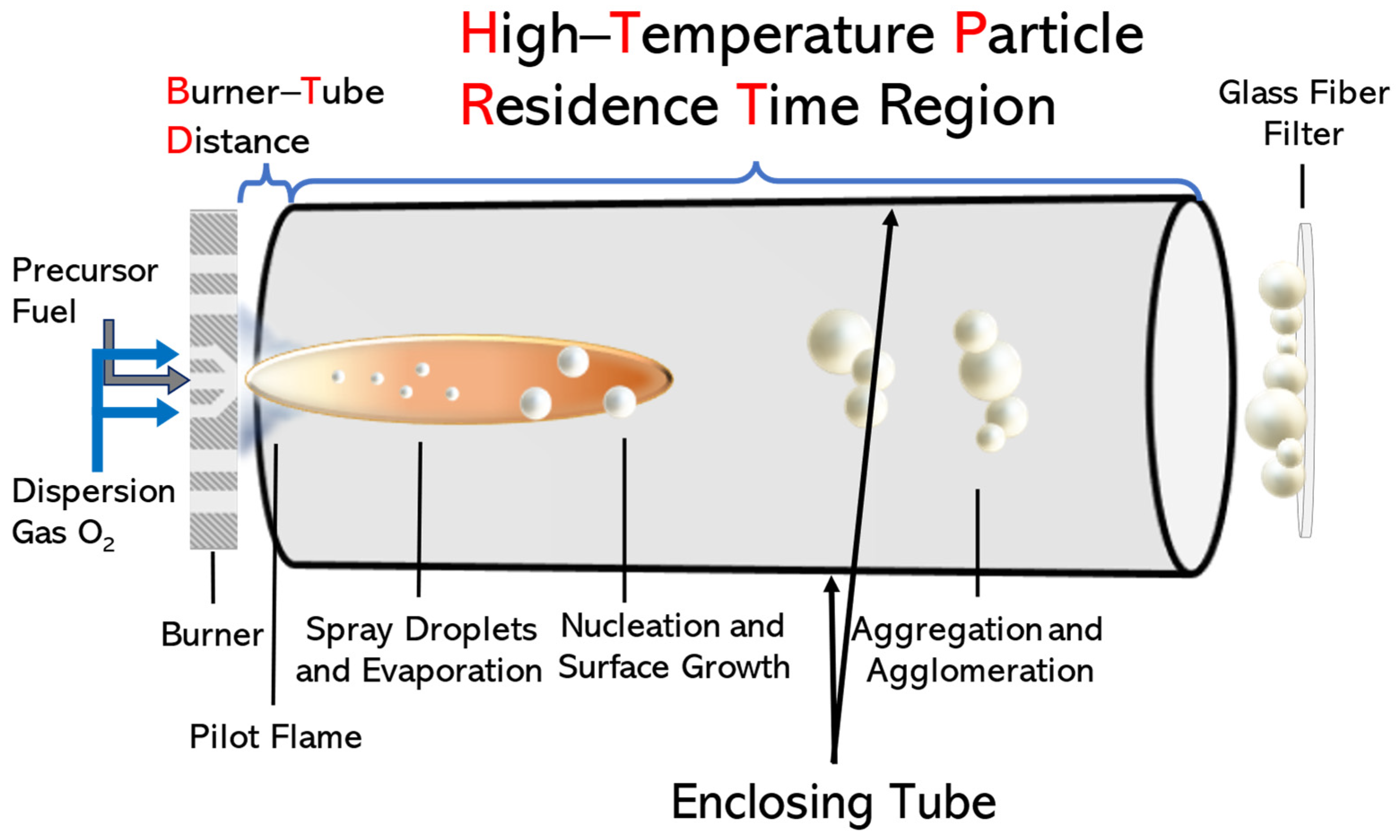
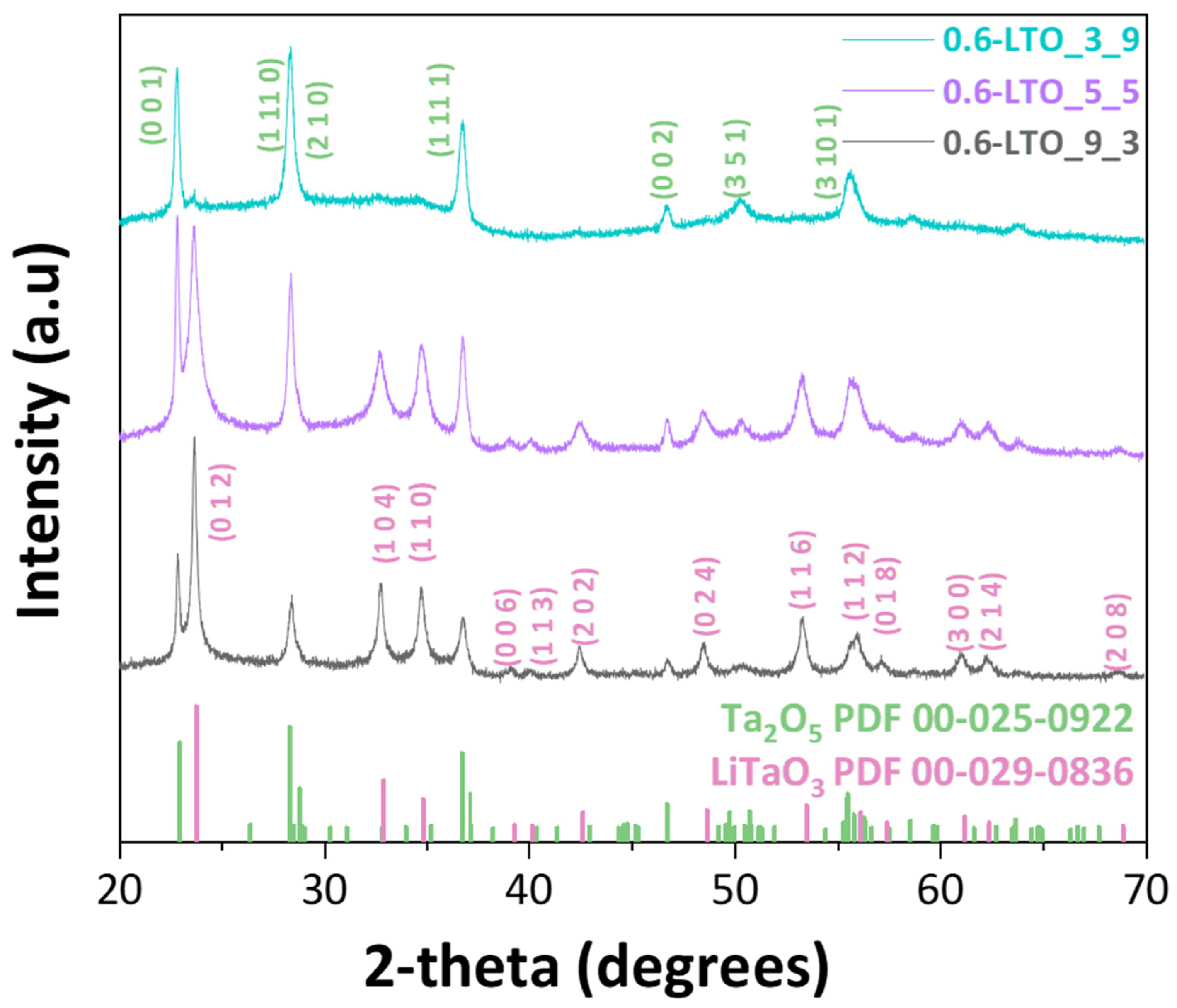
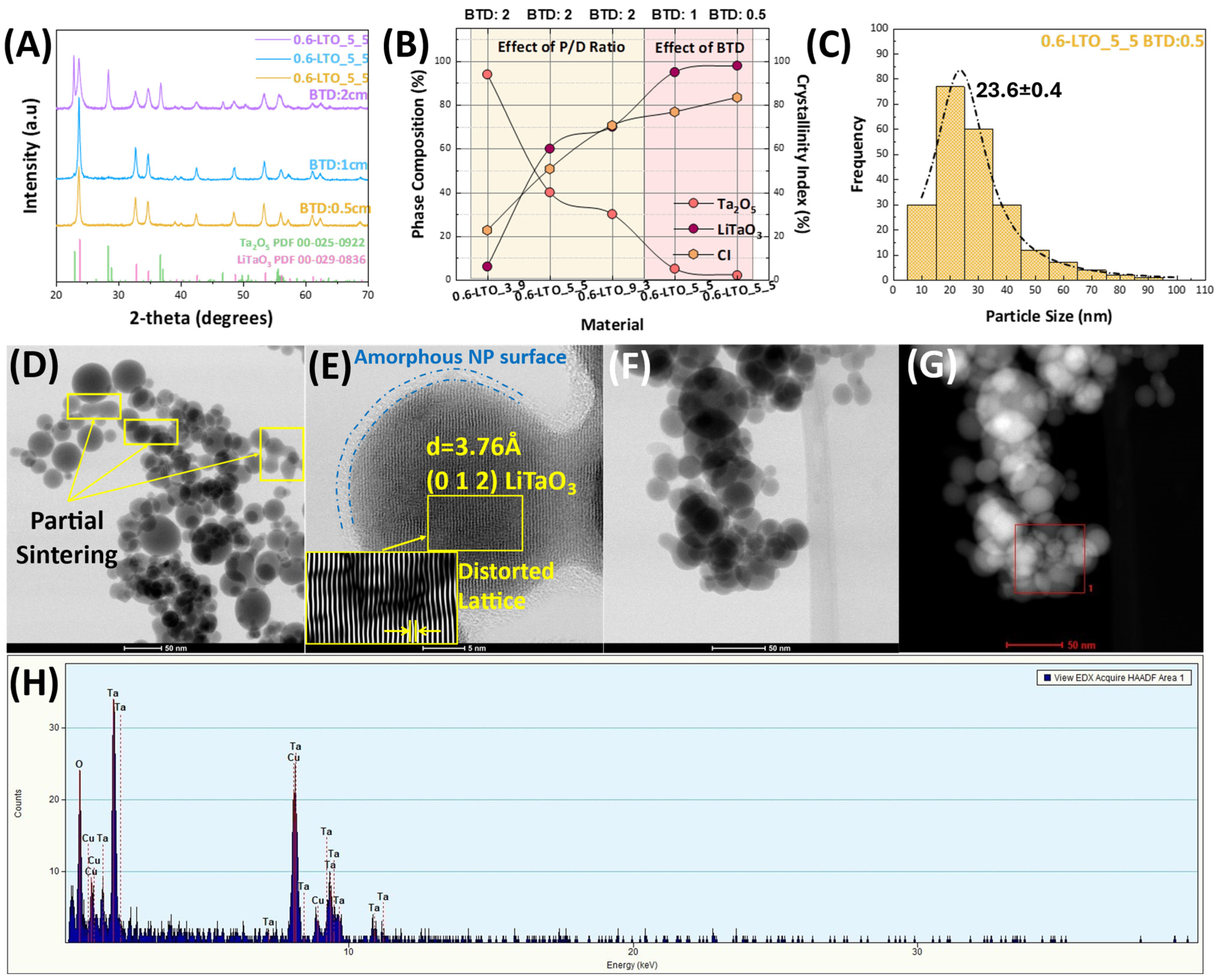
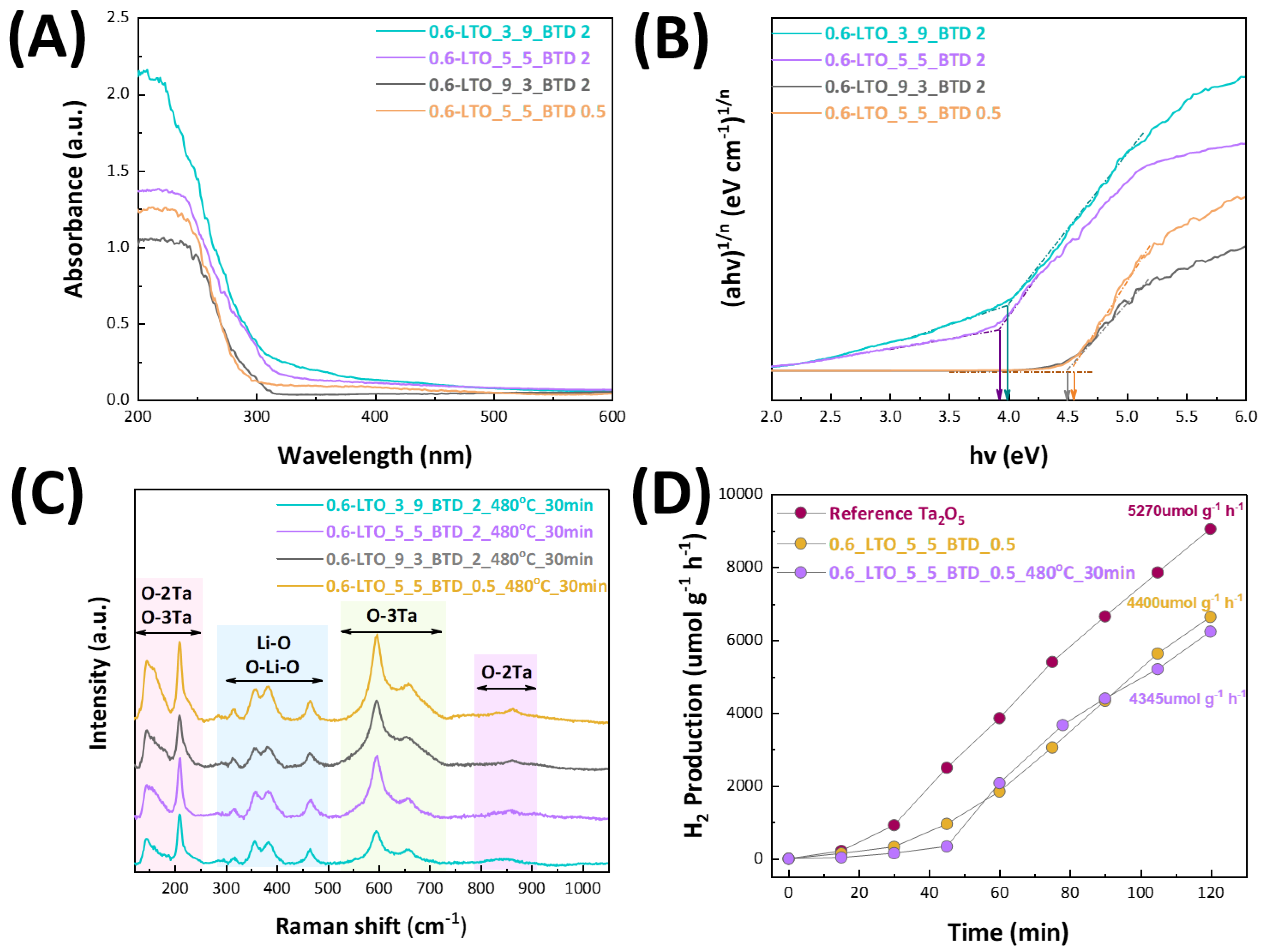
| Nanomaterial | P/D | [Li/Ta] Concentration (mM) | BTD (cm) | HTPRT (cm) | % Phase (±5%) | Crystallite Size (nm) (±0.5 nm) 1 | CI (%) 2 | ||
|---|---|---|---|---|---|---|---|---|---|
| Ta2O5 (%) | LiTaO3 (%) | dXRD Ta2O5 | dXRD LiTaO3 | ||||||
| 0.6-LTO_3_9 | 3/9 | 300:300 | 2 | 44 | 94 | 6 | 33.8 | - | 22.6 |
| 0.6-LTO_5_5 | 5/5 | 300:300 | 2 | 44 | 40 | 60 | 26.7 | 16.6 | 50.7 |
| 0.6-LTO_9_3 | 9/3 | 300:300 | 2 | 44 | 30 | 70 | 24 | 27.9 | 70.6 |
| 0.6-LTO_5_5 | 5/5 | 300:300 | 1 | 44 | 5 | 95 | - | 15.2 | 76.8 |
| 0.6-LTO_5_5 | 5/5 | 300:300 | 0.5 | 44 | 2 | 98 | - | 25 | 83.4 |
| Phonon Branch | Assignment | Raman Shift (cm−1) | Raman Shift (cm−1) (Literature) |
|---|---|---|---|
| E (TO1) | O-3Ta and O-2Ta deformation | 145 | 143 [34], 144 [35] |
| A1 (TO1) | 209 | 208 [34], 209 [35] | |
| E (TO4) | Li-O stretching and O-Li-O bending | 317 | 315 [34,35] |
| A1 (LO2) | 357 | 356 [34,35] | |
| E (LO5) | 381 | 383 [34], 380 [35] | |
| E (TO7) | 466 | 465 [34], 463 [35] | |
| A1 (TO4) | O-3Ta stretching | 597 | 596 [34,35] |
| E (LO8) | 659 | 661 [34,35] | |
| A1 (LO4) | O-2Ta stretching | 863 | 861 [34], 863 [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psathas, P.; Zindrou, A.; Spyrou, A.V.; Deligiannakis, Y. Engineering of LiTaO3 Nanoparticles by Flame Spray Pyrolysis: Understanding In Situ Li-Incorporation into the Ta2O5 Lattice. Nanomaterials 2024, 14, 1257. https://doi.org/10.3390/nano14151257
Psathas P, Zindrou A, Spyrou AV, Deligiannakis Y. Engineering of LiTaO3 Nanoparticles by Flame Spray Pyrolysis: Understanding In Situ Li-Incorporation into the Ta2O5 Lattice. Nanomaterials. 2024; 14(15):1257. https://doi.org/10.3390/nano14151257
Chicago/Turabian StylePsathas, Pavlos, Areti Zindrou, Anastasia V. Spyrou, and Yiannis Deligiannakis. 2024. "Engineering of LiTaO3 Nanoparticles by Flame Spray Pyrolysis: Understanding In Situ Li-Incorporation into the Ta2O5 Lattice" Nanomaterials 14, no. 15: 1257. https://doi.org/10.3390/nano14151257





