Investigating the Potential of CO2 Nanobubble Systems for Enhanced Oil Recovery in Extra-Low-Permeability Reservoirs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
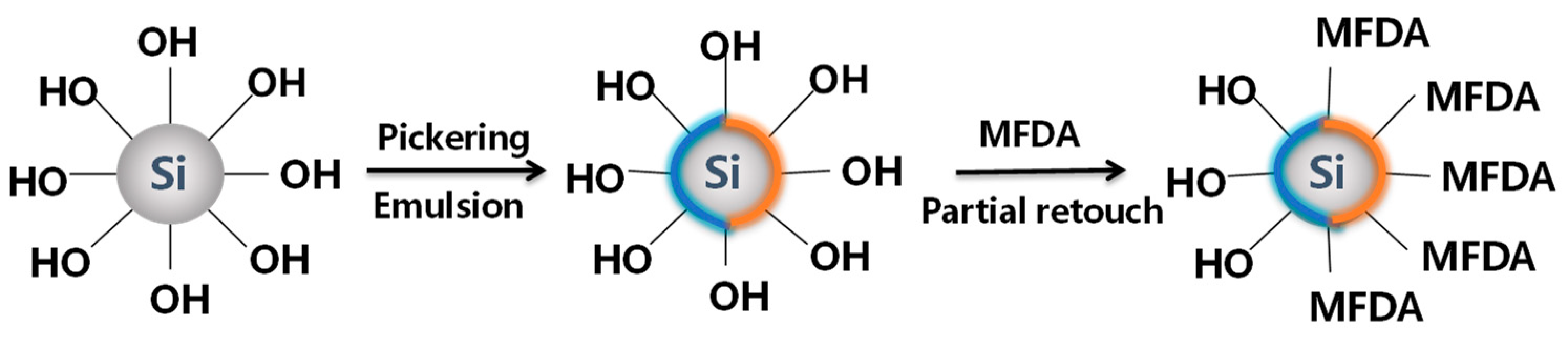
2.2. Preparation and Characterization of Modified Nanoparticles
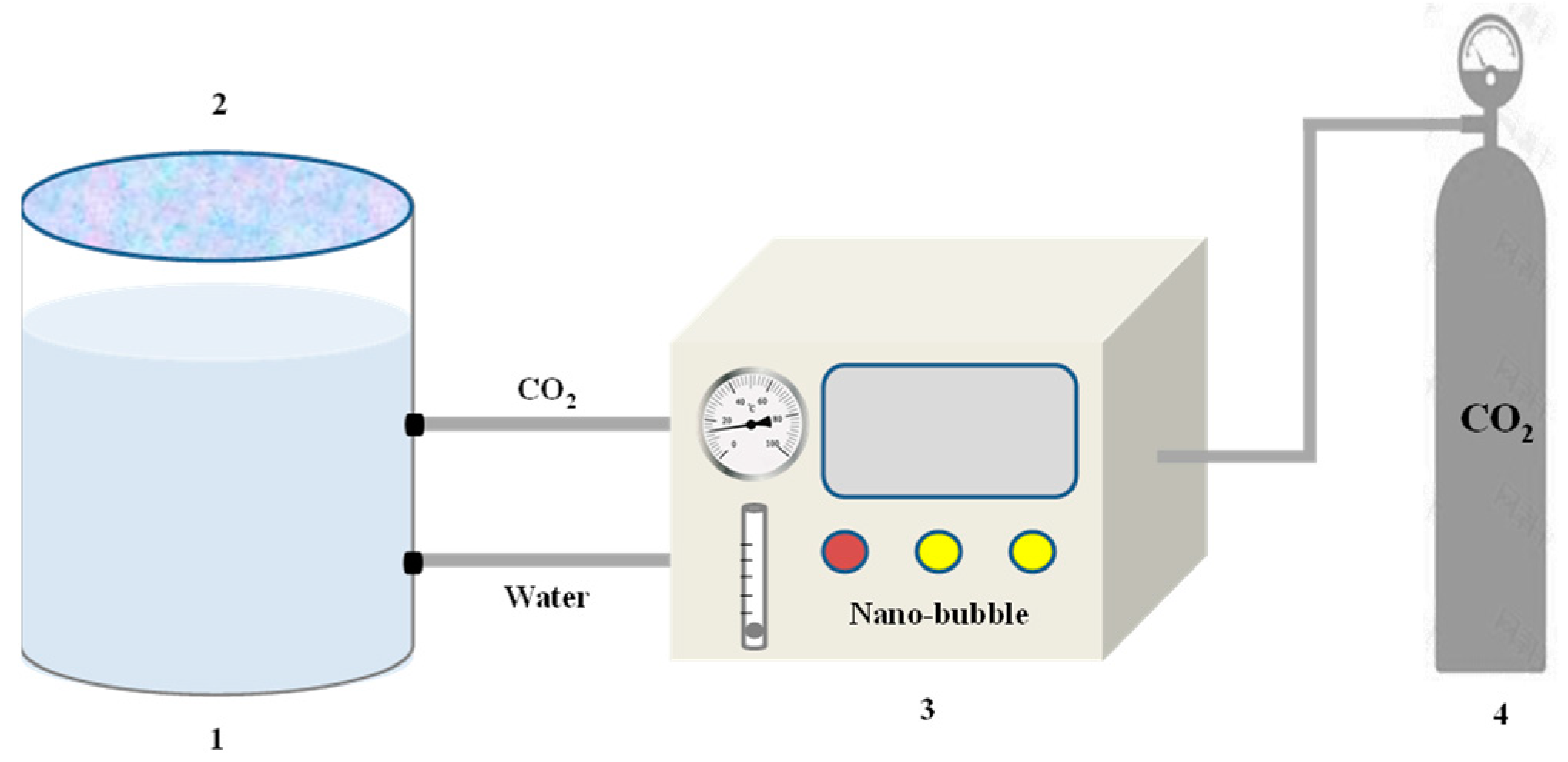
2.3. CO2 Nanobubble System Preparation
2.4. CO2 Nanobubble System Number Concentration
2.5. CO2 Nanobubble System Stability
2.6. CO2 Nanobubble System Wettability
2.7. CO2 Nanobubble System Interface Features
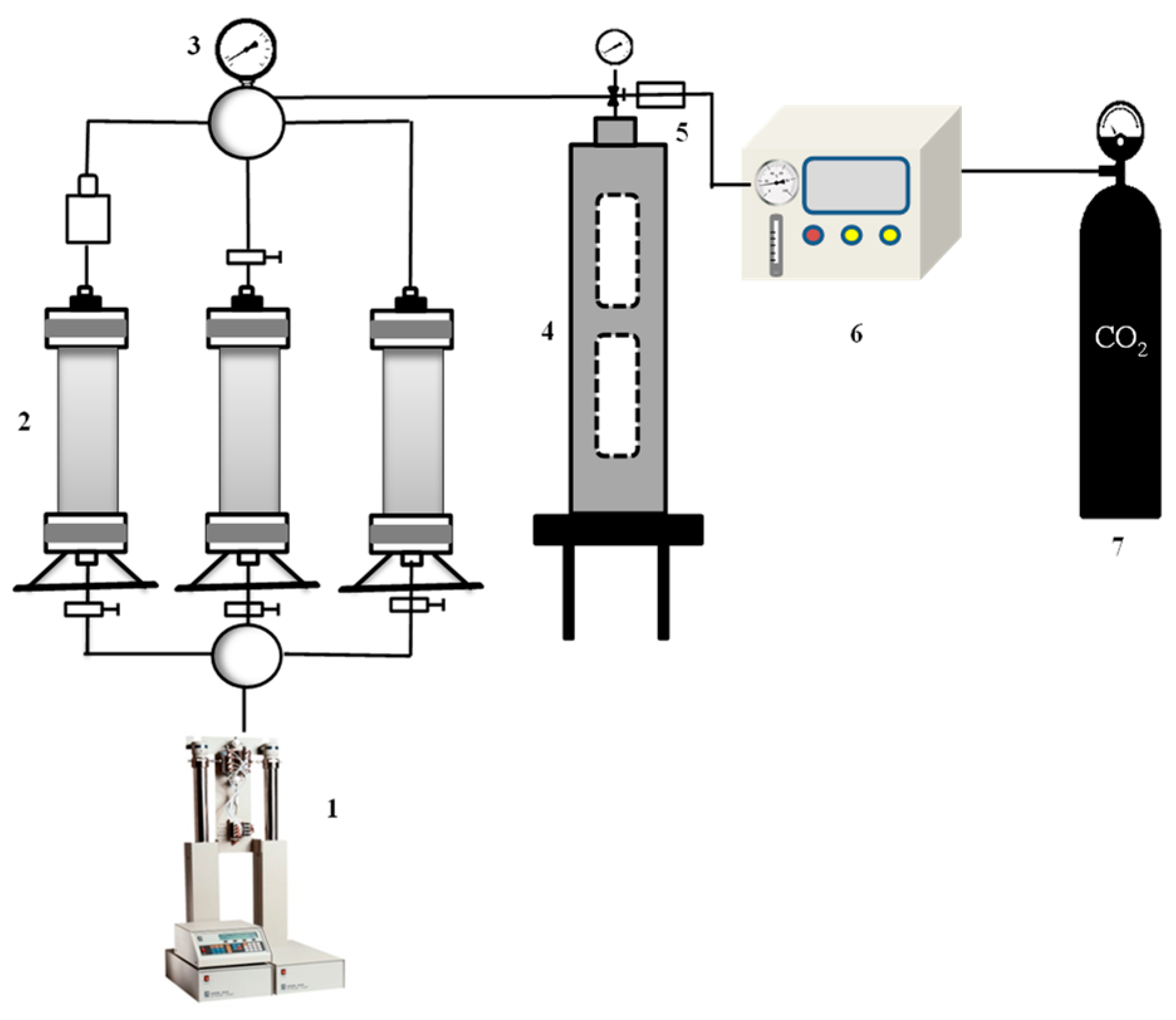
2.8. Core Flooding Experiment
3. Results and Discussion
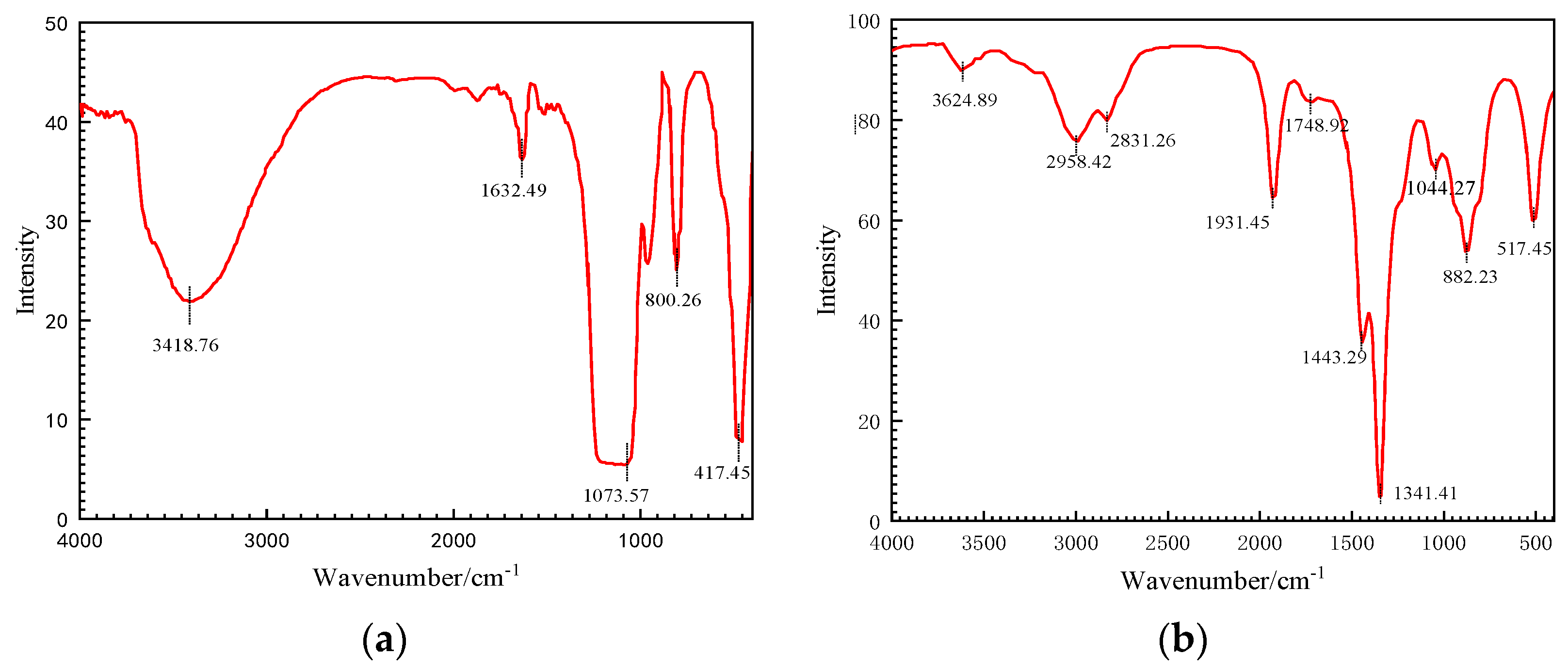
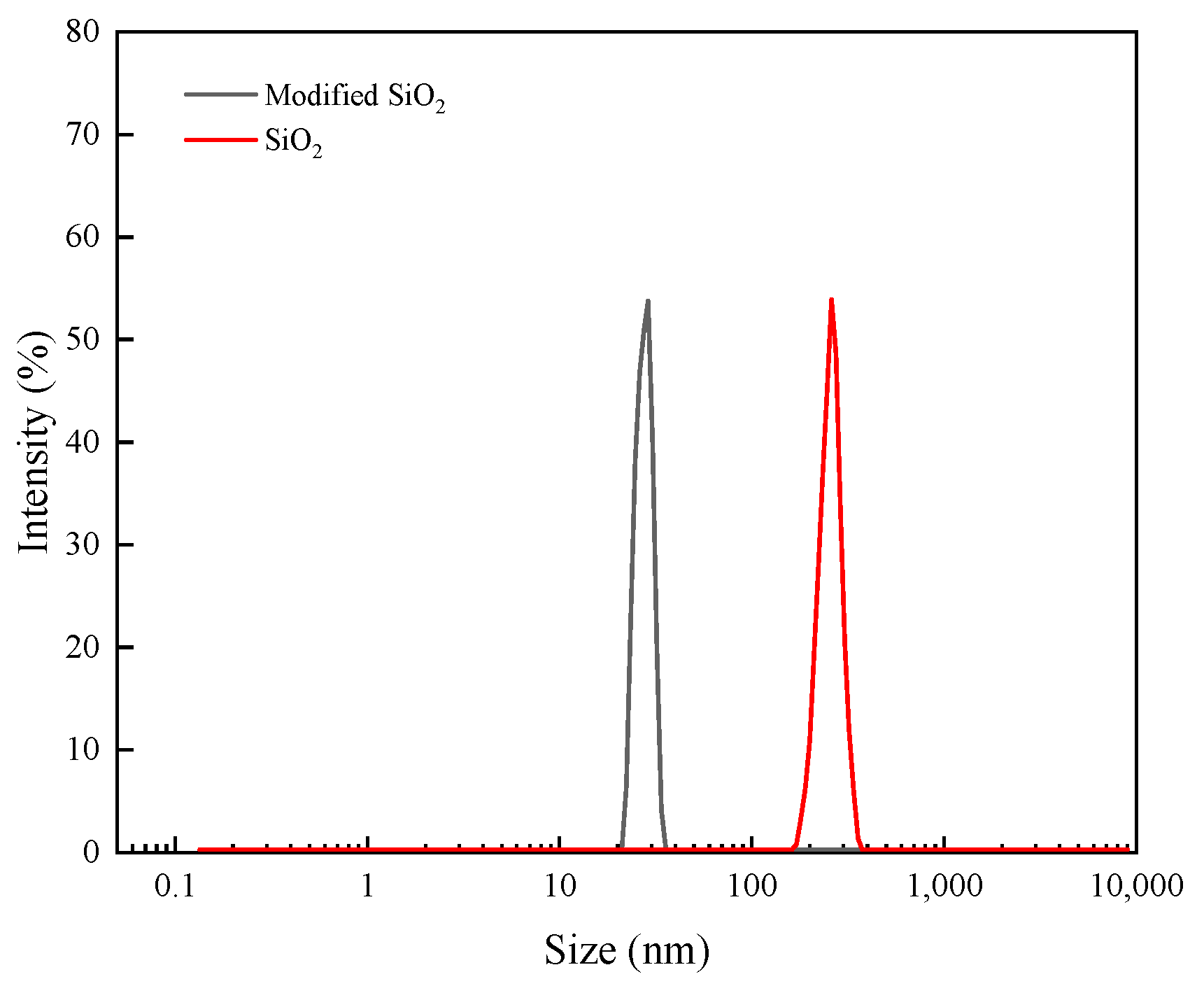
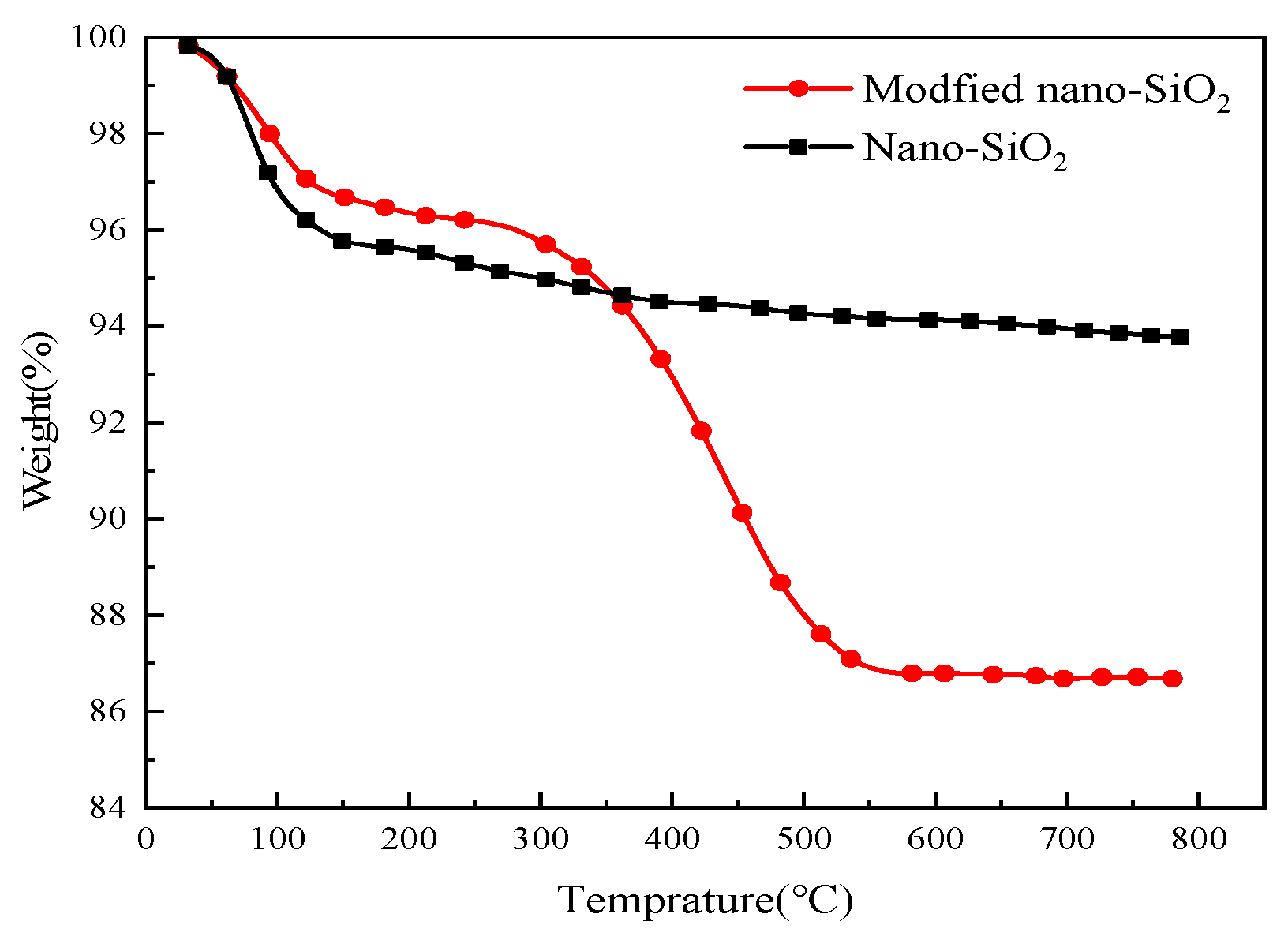
3.1. Structural Characterization of Modified Nanoparticles
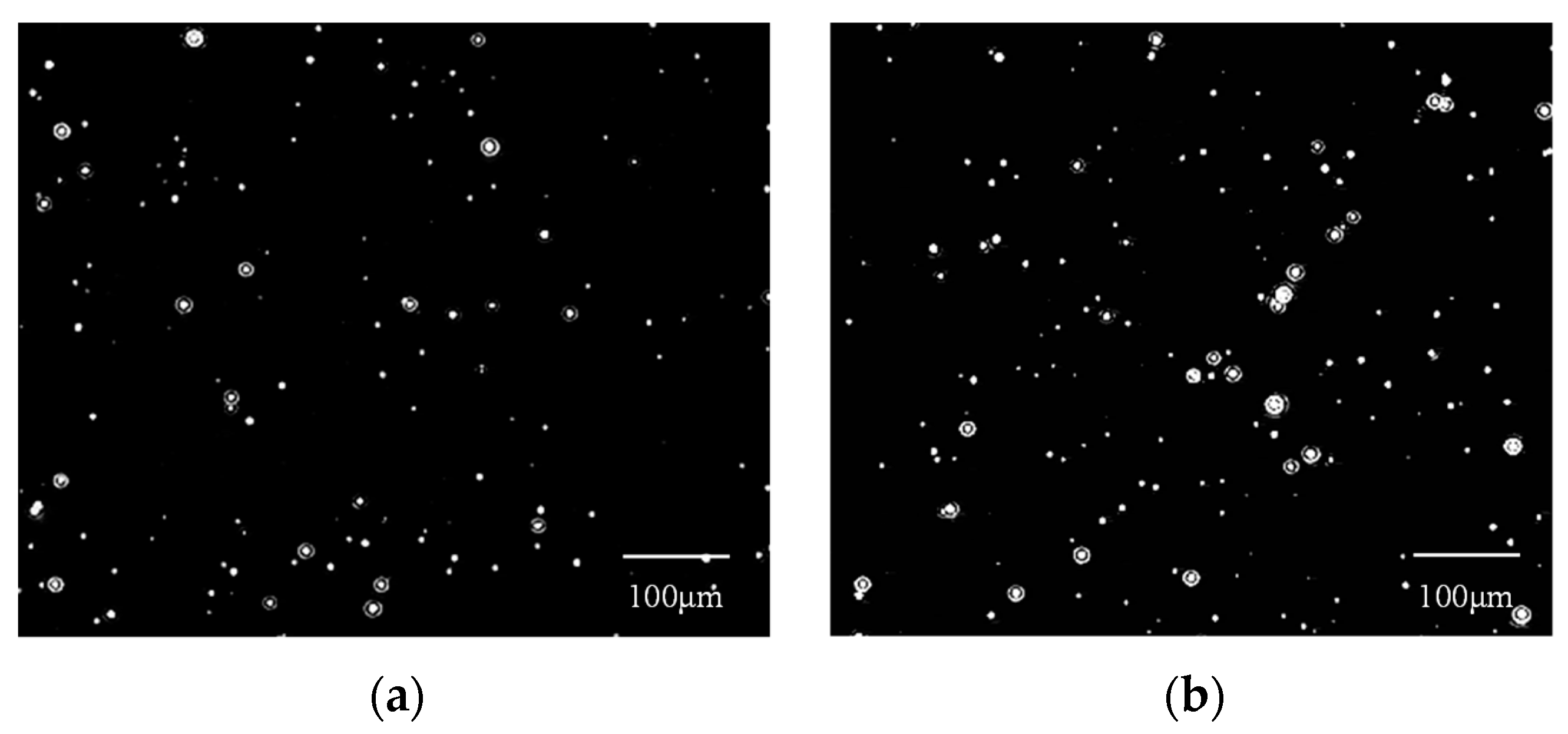
3.2. Formation and Characterization of CO2 Nanobubbles
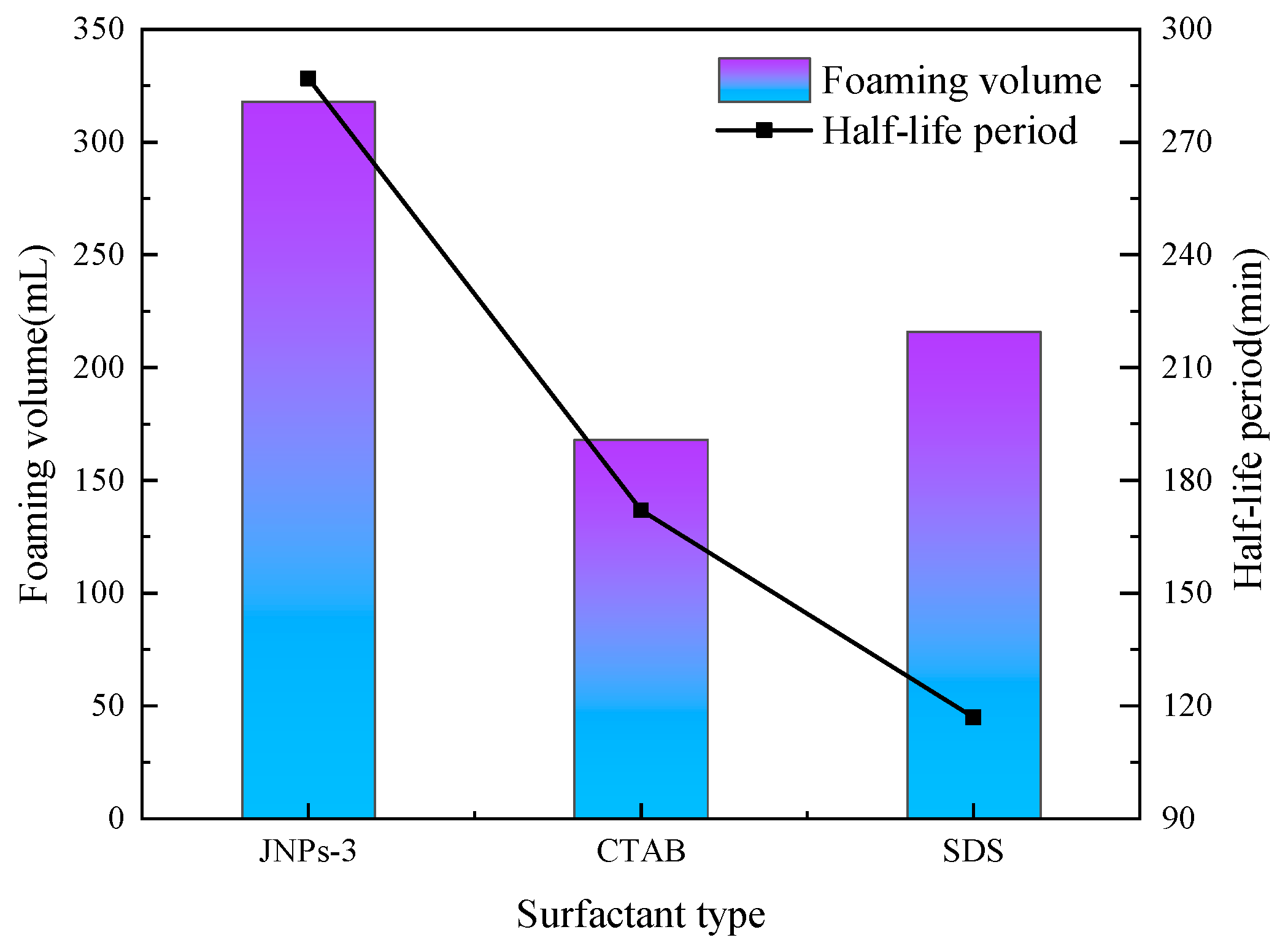
3.3. Surfactant Effect on CO2 Nanobubble System
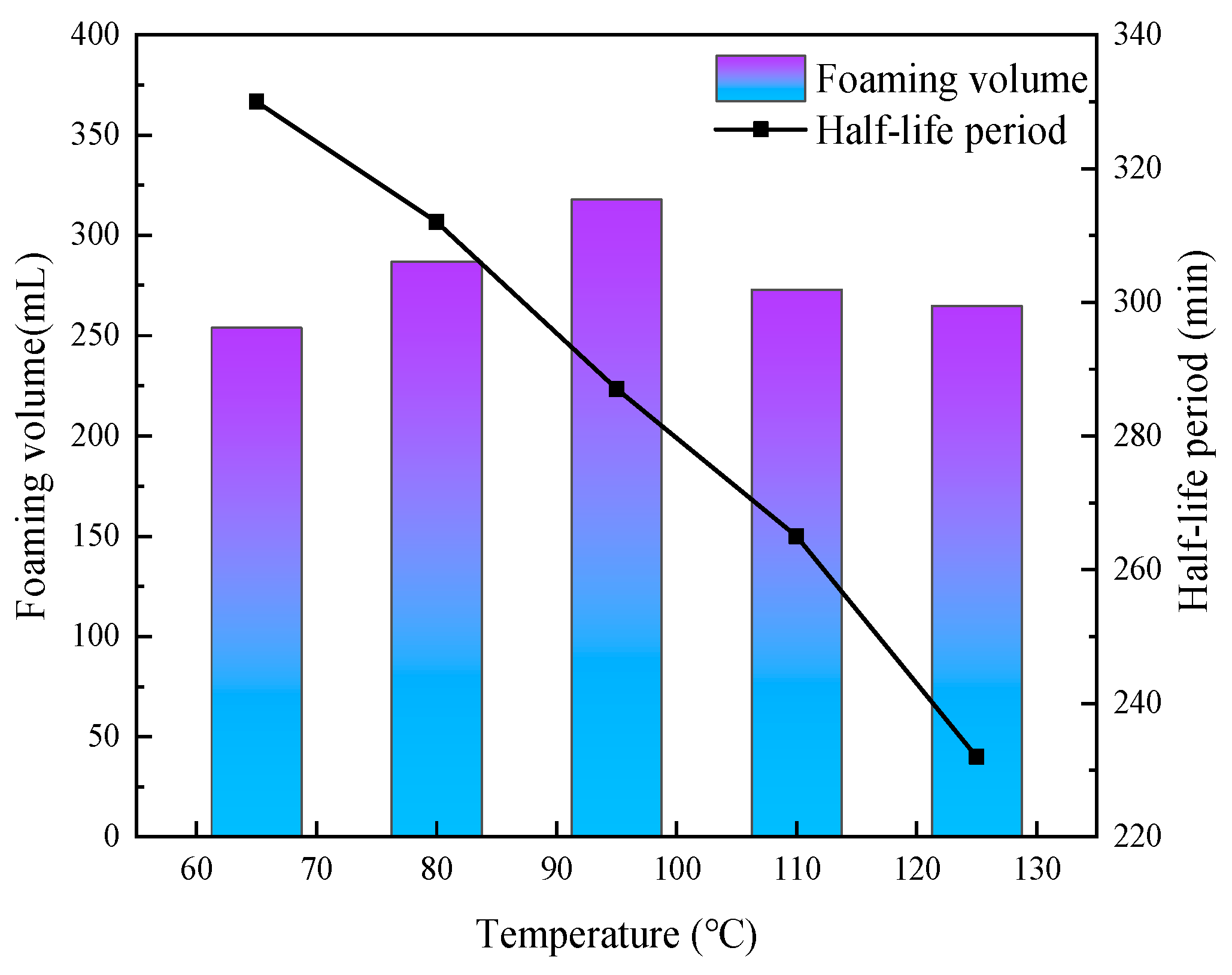
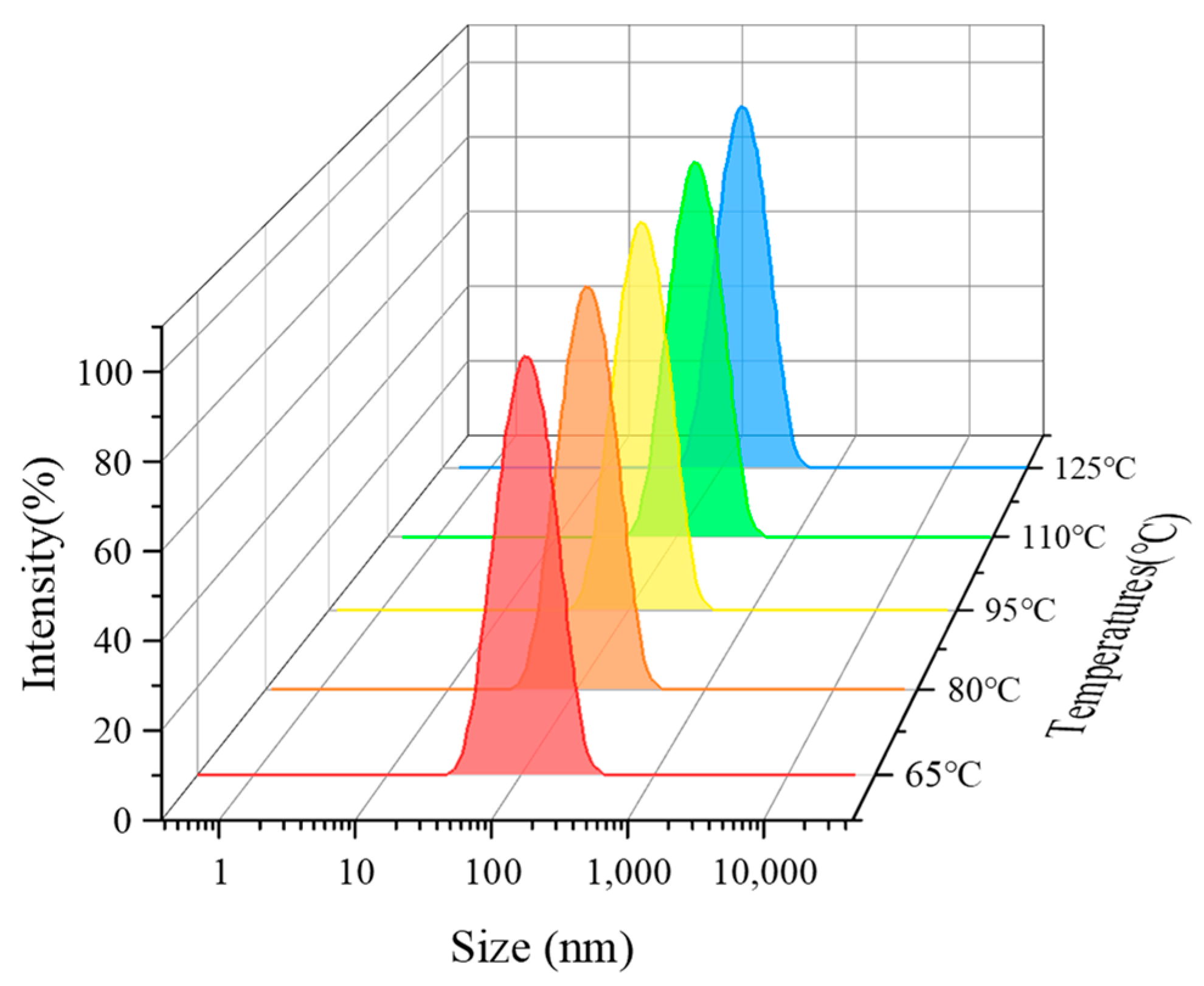
3.4. Effect of Temperature on the Properties of CO2 Nanobubble System
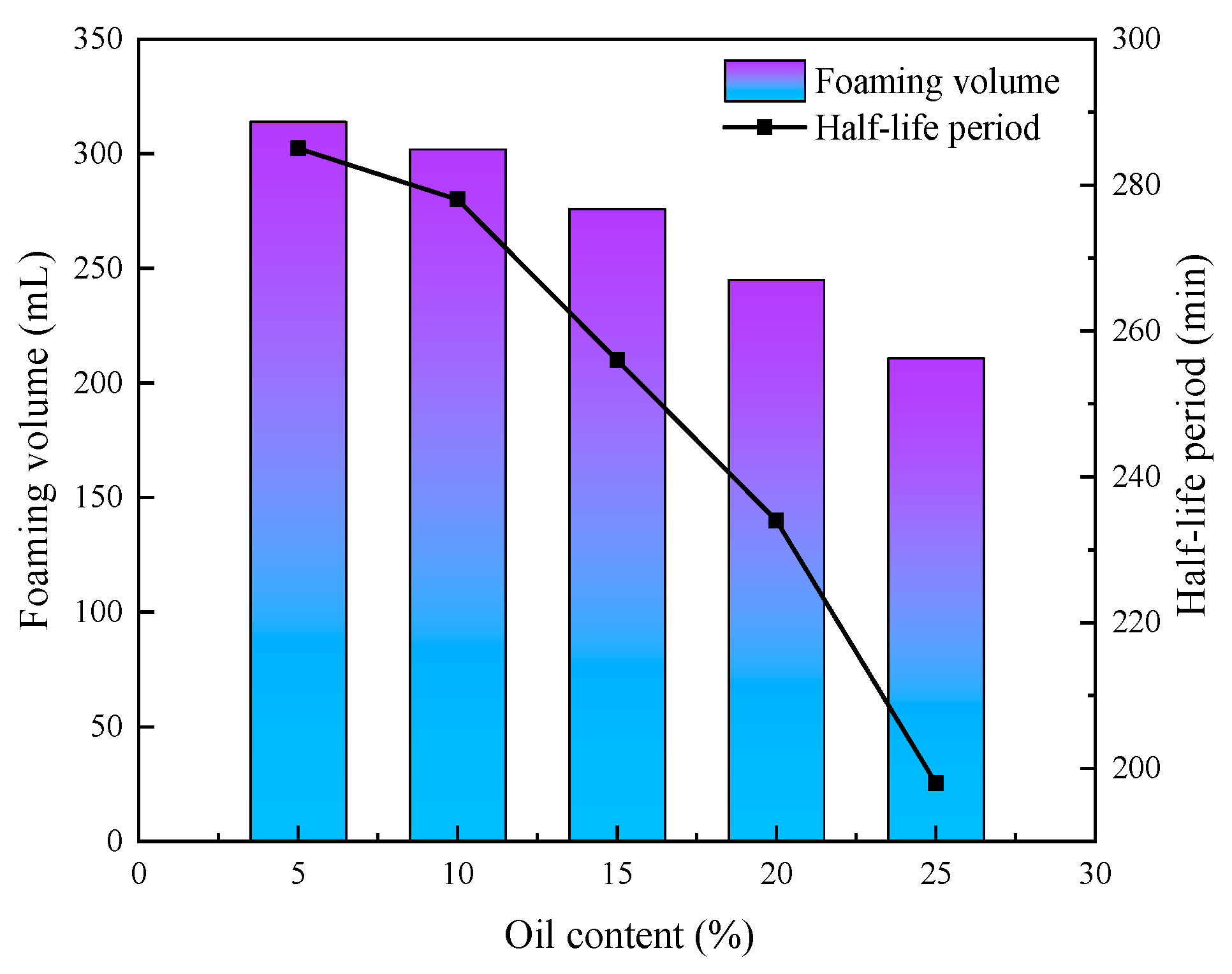
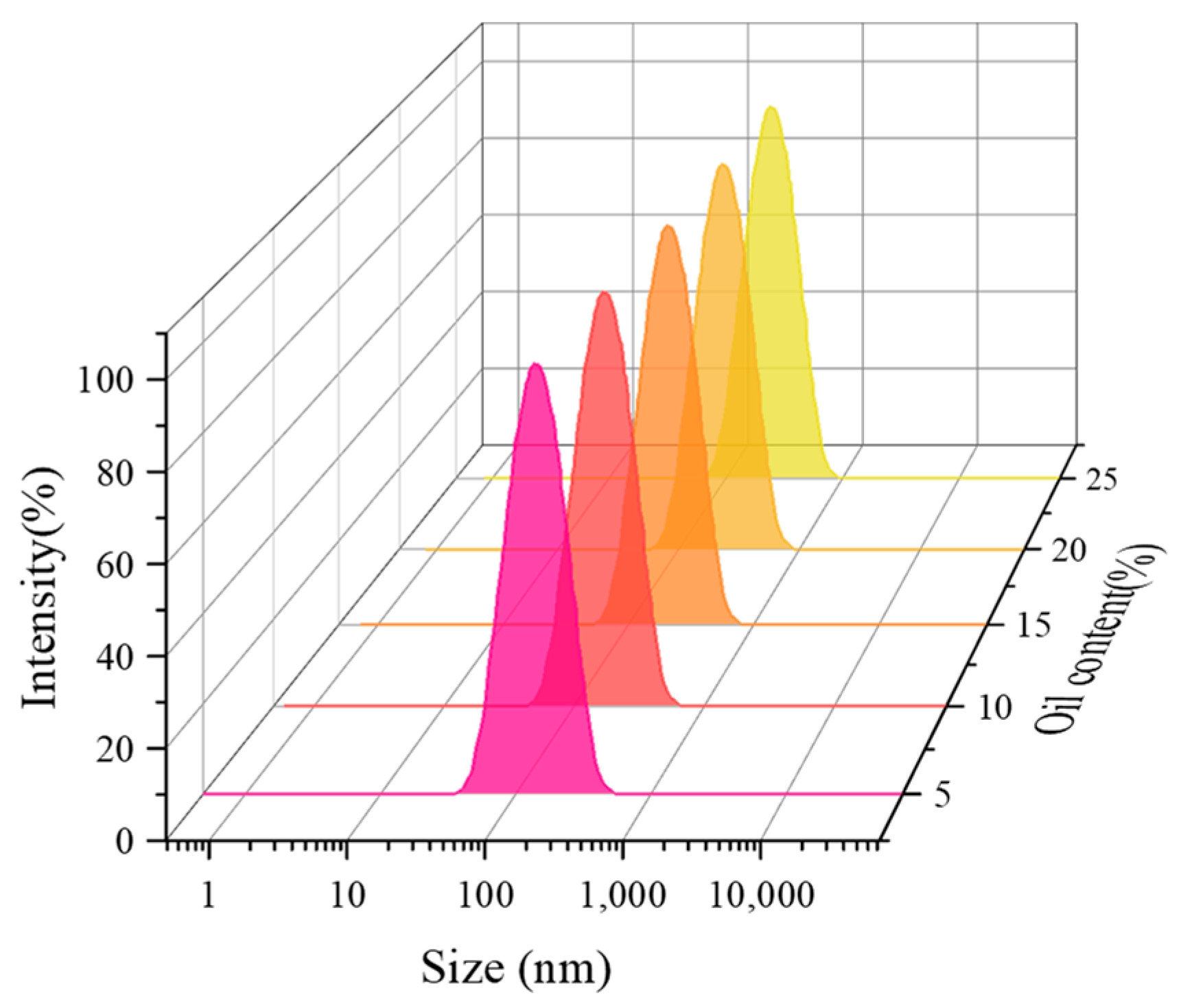
3.5. Effect of Oil Content on the Properties of CO2 Nanobubble System
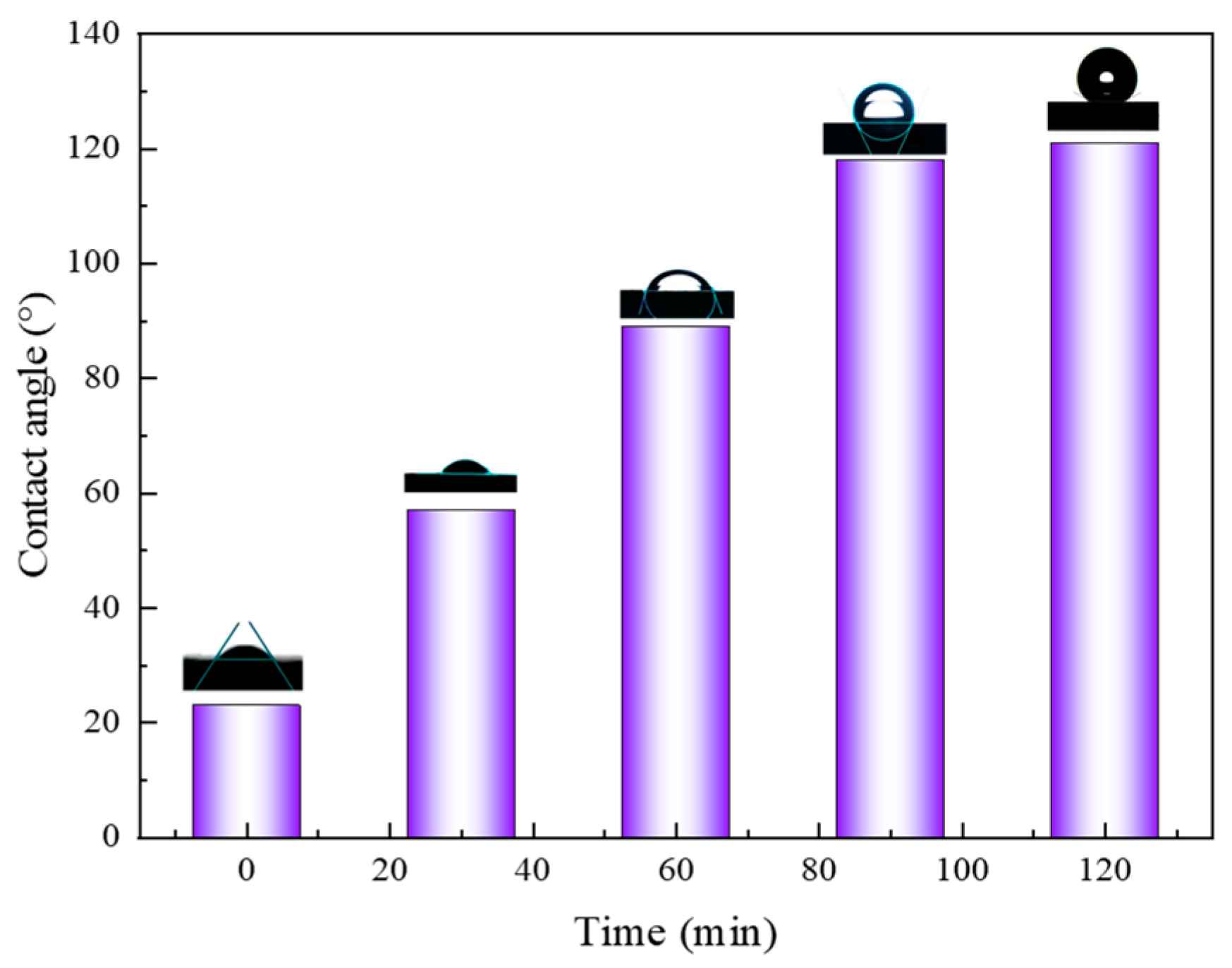
3.6. Contact Angle of CO2 Nanobubble System
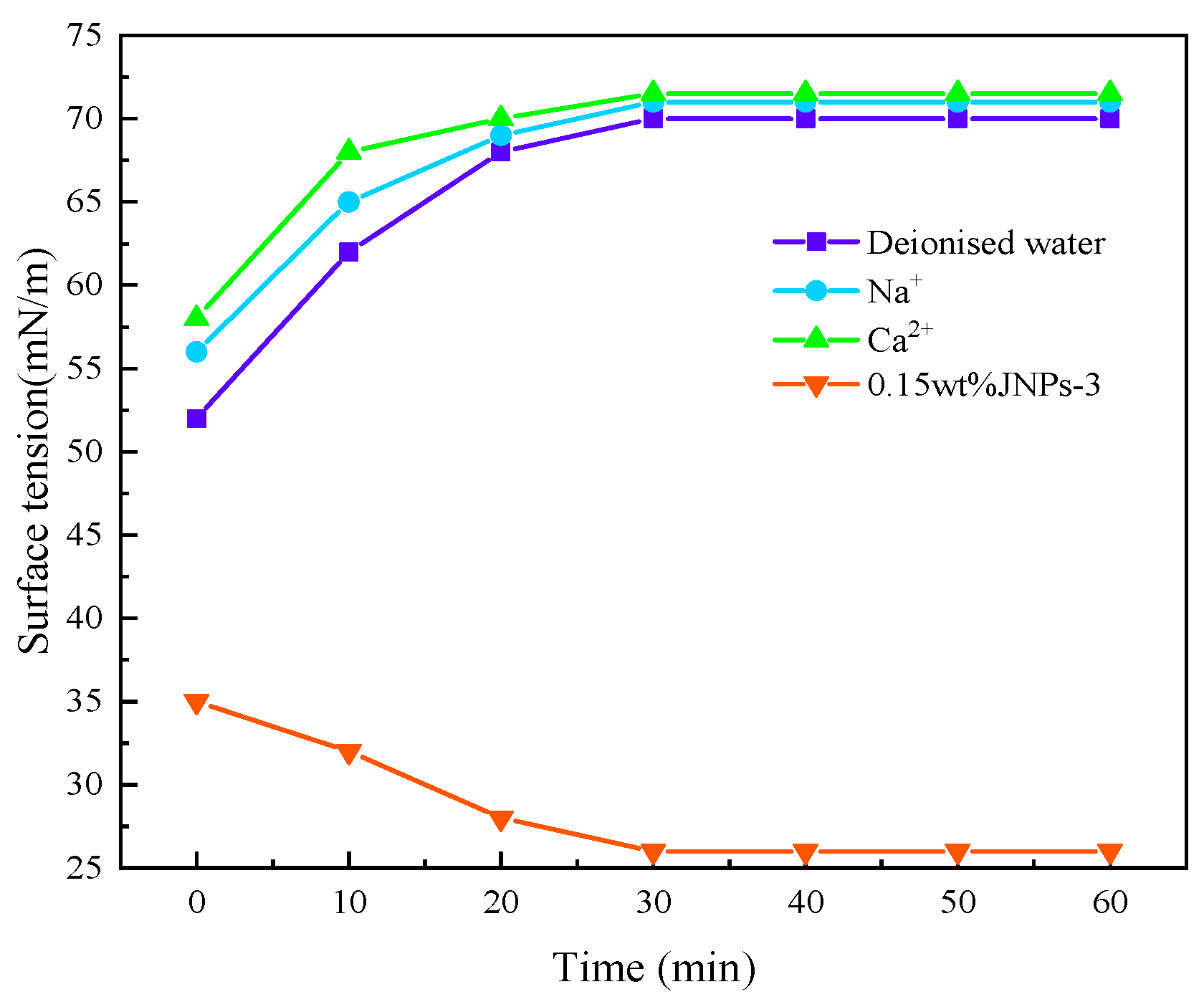
3.7. Interfacial Tension of CO2 Nanobubble Systems
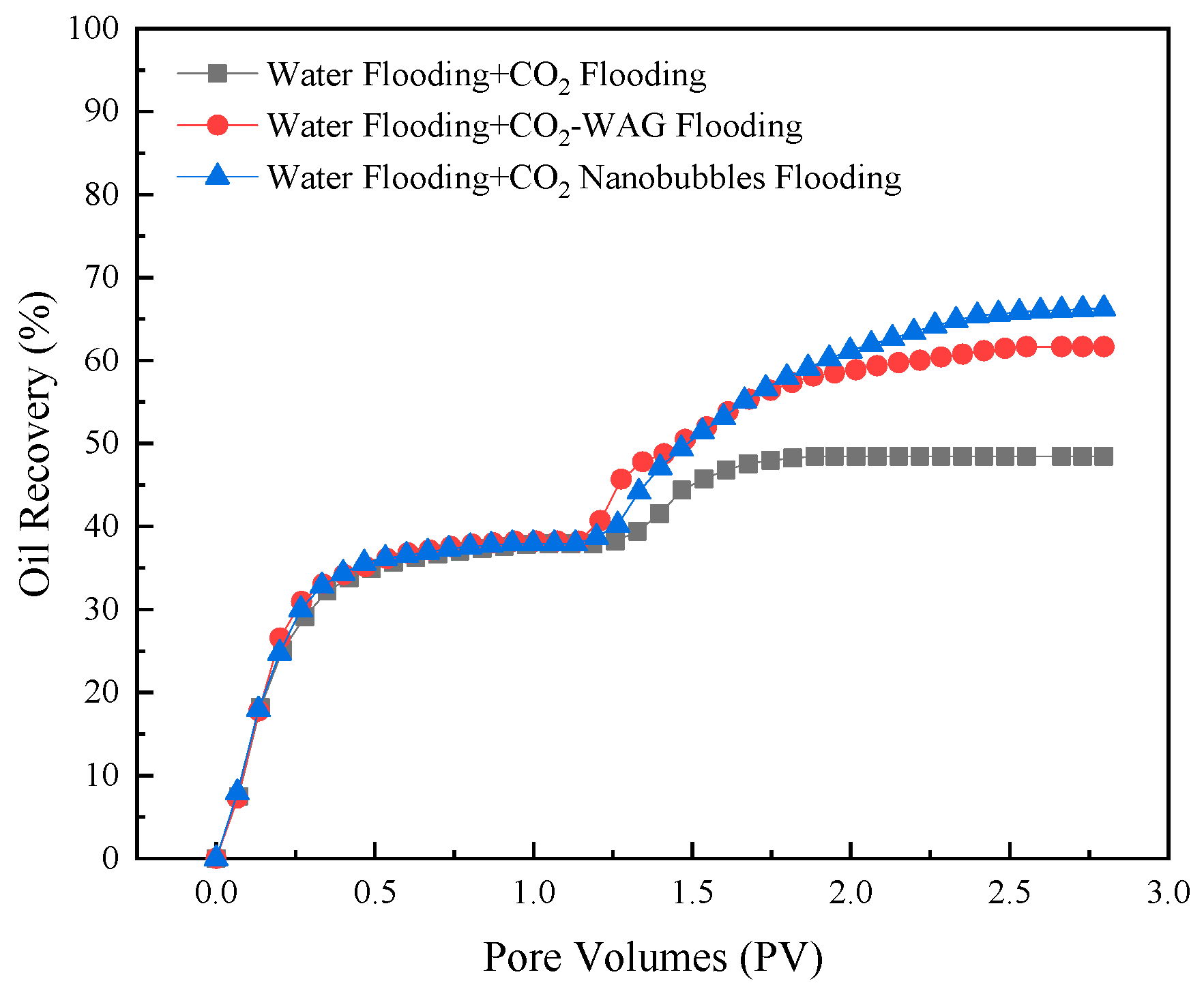
3.8. Core Flooding Experiment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Yang, Z.; Li, S.; Huang, W.; Zhan, J.; Lin, W. Reservoir Characteristics and Effective Development Technology in Typical Low-Permeability to Ultralow-Permeability Reservoirs of China National Petroleum Corporation. Energy Explor. Exploit. 2021, 39, 1713–1726. [Google Scholar] [CrossRef]
- Ding, S.; Wen, F.; Wang, N.; Zhang, Y.; Lu, R.; Gao, Y.; Yu, H. Multi-Objective Optimization of CO2 Enhanced Oil Recovery and Storage Processes in Low Permeability Reservoirs. Int. J. Greenh. Gas Control 2022, 121, 103802. [Google Scholar] [CrossRef]
- Wang, J.; Xie, H.-P.; Matthai, S.K.; Hu, J.-J.; Li, C.-B. The Role of Natural Fracture Activation in Hydraulic Fracturing for Deep Unconventional Geo-Energy Reservoir Stimulation. Pet. Sci. 2023, 20, 2141–2164. [Google Scholar] [CrossRef]
- Jian, G.; Fernandez, C.A.; Puerto, M.; Sarathi, R.; Bonneville, A.; Biswal, S.L. Advances and Challenges in CO2 Foam Technologies for Enhanced Oil Recovery in Carbonate Reservoirs. J. Petrol. Sci. Eng. 2021, 202, 108447. [Google Scholar] [CrossRef]
- Xiong, R.; Xu, R.X.; Huang, C.; De Smedt, S.; Braeckmans, K. Stimuli-Responsive Nanobubbles for Biomedical Applications. Chem. Soc. Rev. 2021, 50, 5746–5776. [Google Scholar] [CrossRef] [PubMed]
- Paknahad, A.A.; Kerr, L.; Wong, D.A.; Kolios, M.C.; Tsai, S.S.H. Biomedical Nanobubbles and Opportunities for Microfluidics. RSC Adv. 2021, 11, 32750–32774. [Google Scholar] [CrossRef]
- Resnick, W.; Gal-Or, B. Gas-Liquid Dispersions. In Advances in Chemical Engineering; Drew, T.B., Cokelet, G.R., Hoopes, J.W., Vermeulen, T., Eds.; Academic Press: Cambridge, MA, USA, 1968; Volume 7, pp. 295–395. [Google Scholar]
- Rao, N.; Singh, R.; Bashambu, L. Carbon-Based Nanomaterials: Synthesis and Prospective Applications. Mater. Today Proc. 2021, 44, 608–614. [Google Scholar] [CrossRef]
- Du, S.; Shi, Y. Nanostructures in Tight Oil Reservoirs: Multiple Perspectives. Int. J. Hydrogen Energy 2024, 49, 884–896. [Google Scholar] [CrossRef]
- Lee, J.I.; Yim, B.-S.; Kim, J.-M. Effect of Dissolved-Gas Concentration on Bulk Nanobubbles Generation Using Ultrasonication. Sci. Rep. 2020, 10, 18816. [Google Scholar] [CrossRef]
- Favvas, E.P.; Kyzas, G.Z.; Efthimiadou, E.K.; Mitropoulos, A.C. Bulk Nanobubbles, Generation Methods and Potential Applications. Curr. Opin. Colloid Interface Sci. 2021, 54, 101455. [Google Scholar] [CrossRef]
- Müller, F.; Peukert, W.; Polke, R.; Stenger, F. Dispersing Nanoparticles in Liquids. Int. J. Miner. Process. 2004, 74, S31–S41. [Google Scholar] [CrossRef]
- Li, X.; Peng, B.; Liu, Q.; Liu, J.; Shang, L. Micro and Nanobubbles Technologies as a New Horizon for CO2-EOR and CO2 Geological Storage Techniques: A Review. Fuel 2023, 341, 127661. [Google Scholar] [CrossRef]
- Porter, R.T.J.; Fairweather, M.; Pourkashanian, M.; Woolley, R.M. The Range and Level of Impurities in CO2 Streams from Different Carbon Capture Sources. Int. J. Greenh. Gas Control 2015, 36, 161–174. [Google Scholar] [CrossRef]
- Kang, W.-L.; Zhou, B.-B.; Issakhov, M.; Gabdullin, M. Advances in Enhanced Oil Recovery Technologies for Low Permeability Reservoirs. Pet. Sci. 2022, 19, 1622–1640. [Google Scholar] [CrossRef]
- Han, G.; Chen, S.; Su, S.; Huang, Y.; Liu, B.; Sun, H. A Review and Perspective on Micro and Nanobubbles: What They Are and Why They Matter. Miner. Eng. 2022, 189, 107906. [Google Scholar] [CrossRef]
- Agarwal, A.; Ng, W.J.; Liu, Y. Principle and Applications of Microbubble and Nanobubble Technology for Water Treatment. Chemosphere 2011, 84, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xia, Z. Application of Ozone Micro-Nano-Bubbles to Groundwater Remediation. J. Hazard. Mater. 2018, 342, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Jian, G.; Fernandez, C.A.; Puerto, M.; Sarathi, R.; Bonneville, A.; Biswal, S.L. Briefing: In Situ Decontamination of Sediments Using Ozone Nanobubbles and Ultrasound. J. Environ. Eng. Sci. 2017, 12, 1–3. [Google Scholar]
- Liu, S.; Oshita, S.; Makino, Y.; Wang, Q.; Kawagoe, Y.; Uchida, T. Oxidative Capacity of Nanobubbles and Its Effect on Seed Germination. ACS Sustain. Chem. Eng. 2016, 4, 1347–1353. [Google Scholar] [CrossRef]
- Lukianova-Hleb, E.Y.; Belyanin, A.; Kashinath, S.; Wu, X.; Lapotko, D.O. Plasmonic Nanobubble-Enhanced Endosomal Escape Processes for Selective and Guided Intracellular Delivery of Chemotherapy to Drug-Resistant Cancer Cells. Biomaterials 2012, 33, 1821–1826. [Google Scholar] [CrossRef]
- Alheshibri, M.; Jehannin, M.; Coleman, V.A.; Craig, V.S.J. Does Gas Supersaturation by a Chemical Reaction Produce Bulk Nanobubbles? J. Colloid Interface Sci. 2019, 554, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Etchepare, R.; Oliveira, H.; Nicknig, M.; Azevedo, A.; Rubio, J. Nanobubbles: Generation Using a Multiphase Pump, Properties and Features in Flotation. Miner. Eng. 2017, 112, 19–26. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, S.; Zhang, L.; Hu, J. Generation and Stability of Bulk Nanobubbles: A Review and Perspective. Curr. Opin. Colloid Interface Sci. 2021, 53, 101439. [Google Scholar] [CrossRef]
- Thi Phan, K.K.; Truong, T.; Wang, Y.; Bhandari, B. Nanobubbles: Fundamental Characteristics and Applications in Food Processing. Trends Food Sci. Technol. 2020, 95, 118–130. [Google Scholar] [CrossRef]
- Ma, X.; Li, M.; Pfeiffer, P.; Eisener, J.; Ohl, C.-D.; Sun, C. Ion Adsorption Stabilizes Bulk Nanobubbles. J. Colloid Interface Sci. 2022, 606, 1380–1394. [Google Scholar] [CrossRef]
- Phan, K.; Truong, T.; Wang, Y.; Bhandari, B. Effect of Electrolytes and Surfactants on Generation and Longevity of Carbon Dioxide Nanobubbles. Food Chem. 2021, 363, 130299. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Mastiani, M.; Hafez, M.; Kunkel, G.; Ghattas Asfour, C.; Garcia-Ocampo, K.I.; Linares, N.; Saldana, C.; Yang, K.; Kim, M. Injection of In-Situ Generated CO2 Microbubbles into Deep Saline Aquifers for Enhanced Carbon Sequestration. Int. J. Greenh. Gas Control 2019, 83, 256–264. [Google Scholar] [CrossRef]
- Hampton, M.A.; Nguyen, A.V. Nanobubbles and the Nanobubble Bridging Capillary Force. Adv. Colloid Interface Sci. 2010, 154, 30–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, H.; Qi, N.; Qin, Y.; Zhang, X.; Li, Y. Generation and Stability of Size-Adjustable Bulk Nanobubbles Based on Periodic Pressure Change. Sci. Rep. 2019, 9, 1118. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K.; Tuziuti, T.; Kanematsu, W. High Temperature and Pressure inside a Dissolving Oxygen Nanobubble. Ultrason. Sonochem. 2019, 55, 308–312. [Google Scholar] [CrossRef]
- Cao, X.; Feng, Q.; Ji, Y. Effect of Interaction between Carbon Dioxide and Fluid Phase/Rock Interface on Carbon Dioxide Storage. Processes 2023, 11, 3331. [Google Scholar] [CrossRef]
- Wang, H.; Cai, J.; Su, Y.; Jin, Z.; Zhang, M.; Wang, W.; Li, G. Pore-Scale Study on Shale Oil–CO2–Water Miscibility, Competitive Adsorption, and Multiphase Flow Behaviors. Langmuir 2023, 39, 12226–12234. [Google Scholar] [CrossRef]
- Liang, S.; Luo, W.; Luo, Z.; Wang, W.; Xue, X.; Dong, B. Research of CO2-Soluble Surfactants for Enhanced Oil Recovery: Review and Outlook. Molecules 2023, 28, 8042. [Google Scholar] [CrossRef]
- Lawal, T.; Wang, H.; Mirzaei-Paiaman, A.; Okuno, R. Aqueous Nanobubble Dispersion of CO2 for Enhanced Oil Recovery—Coreflooding and Huff-n-Puff Experiments. OnePetro, 22 April 2024. [Google Scholar]
- Wang, Z.; An, C.; Lee, K.; Feng, Q.; Zhang, B. Exploring the Role of Nanobubbles in the Fate and Transport of Spilled Oil on Shorelines. ACS EST Water 2023, 3, 30–40. [Google Scholar] [CrossRef]
- Nassabeh, M.; Iglauer, S.; Keshavarz, A.; You, Z. Advancements, Challenges, and Perspectives of Flue Gas Injection in Subsurface Formations: A Comprehensive Review. Energy Fuels 2023, 37, 16282–16310. [Google Scholar] [CrossRef]
- Nassabeh, M.; You, Z.; Keshavarz, A.; Iglauer, S. Sub-Surface Geospatial Intelligence in Carbon Capture, Utilization and Storage: A Machine Learning Approach for Offshore Storage Site Selection. Energy 2024, 305, 132086. [Google Scholar] [CrossRef]
- Michailidi, E.D.; Bomis, G.; Varoutoglou, A.; Efthimiadou, E.K.; Mitropoulos, A.C.; Favvas, E.P. Chapter 4—Fundamentals and Applications of Nanobubbles. In Interface Science and Technology; Kyzas, G.Z., Mitropoulos, A.C., Eds.; Advanced Low-Cost Separation Techniques in Interface Science; Elsevier: Amsterdam, The Netherlands, 2019; Volume 30, pp. 69–99. [Google Scholar]
- Kanematsu, W.; Tuziuti, T.; Yasui, K. The Influence of Storage Conditions and Container Materials on the Long Term Stability of Bulk Nanobubbles—Consideration from a Perspective of Interactions between Bubbles and Surroundings. Chem. Eng. Sci. 2020, 219, 115594. [Google Scholar] [CrossRef]
- Wagner, T.; Lipinski, H.-G.; Wiemann, M. Dark Field Nanoparticle Tracking Analysis for Size Characterization of Plasmonic and Non-Plasmonic Particles. J. Nanopart. Res. Interdiscip. Forum Nanoscale Sci. Technol. 2014, 16, 2419. [Google Scholar] [CrossRef]
- Ulatowski, K.; Sobieszuk, P.; Mróz, A.; Ciach, T. Stability of Nanobubbles Generated in Water Using Porous Membrane System. Chem. Eng. Process. Process Intensif. 2019, 136, 62–71. [Google Scholar] [CrossRef]
- Tomar, D.S.; Baingne, M.; Sharma, G. Stability of Gravity-Driven Free Surface Flow of Surfactant-Laden Liquid Film Flowing down a Flexible Inclined Plane. Chem. Eng. Sci. 2017, 165, 216–228. [Google Scholar] [CrossRef]
- Du, D.; Zhang, X.; Li, Y.; Zhao, D.; Wang, F.; Sun, Z. Experimental Study on Rheological Properties of Nanoparticle-Stabilized Carbon Dioxide Foam. J. Nat. Gas Sci. Eng. 2020, 75, 103140. [Google Scholar] [CrossRef]
- Bu, X.; Zhou, S.; Tian, X.; Ni, C.; Nazari, S.; Alheshibri, M. Effect of Aging Time, Airflow Rate, and Nonionic Surfactants on the Surface Tension of Bulk Nanobubbles Water. J. Mol. Liq. 2022, 359, 119274. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Albarico, F.P.J.B.; Pandey, A.; Chen, C.-W.; Dong, C.-D. Organic Wastes Bioremediation and Its Changing Prospects. Sci. Total Environ. 2022, 824, 153889. [Google Scholar] [CrossRef]





| Core Number | Core Size | Permeability (10−3 μm2) | Experimental Program |
|---|---|---|---|
| L-1 | 99.82 | 21.67 | Water flooding to 98% water content + CO2 flooding to 1500 m3/t gas/oil ratio end |
| L-2 | 98.65 | 24.19 | Water flooding to 98% water content + CO2/water flooding to 1500 m3/t gas/oil ratio end |
| L-3 | 99.73 | 22.36 | Water flooding to 98% water content + CO2 nanobubbles to 1500 m3/t gas/oil ratio end |
| Na+ | K+ | Ca2+ | Mg2+ | HCO3− | Cl− |
|---|---|---|---|---|---|
| 581.85 | 10.49 | 22.75 | 8.04 | 898.38 | 448.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, L.; Wu, J.; Zhang, M.; Wang, K.; Li, B.; Yu, X.; Hou, Y.; Zhao, Y. Investigating the Potential of CO2 Nanobubble Systems for Enhanced Oil Recovery in Extra-Low-Permeability Reservoirs. Nanomaterials 2024, 14, 1280. https://doi.org/10.3390/nano14151280
Cai L, Wu J, Zhang M, Wang K, Li B, Yu X, Hou Y, Zhao Y. Investigating the Potential of CO2 Nanobubble Systems for Enhanced Oil Recovery in Extra-Low-Permeability Reservoirs. Nanomaterials. 2024; 14(15):1280. https://doi.org/10.3390/nano14151280
Chicago/Turabian StyleCai, Liyuan, Jingchun Wu, Miaoxin Zhang, Keliang Wang, Bo Li, Xin Yu, Yangyang Hou, and Yang Zhao. 2024. "Investigating the Potential of CO2 Nanobubble Systems for Enhanced Oil Recovery in Extra-Low-Permeability Reservoirs" Nanomaterials 14, no. 15: 1280. https://doi.org/10.3390/nano14151280
APA StyleCai, L., Wu, J., Zhang, M., Wang, K., Li, B., Yu, X., Hou, Y., & Zhao, Y. (2024). Investigating the Potential of CO2 Nanobubble Systems for Enhanced Oil Recovery in Extra-Low-Permeability Reservoirs. Nanomaterials, 14(15), 1280. https://doi.org/10.3390/nano14151280







