Achieving Tunable Mechanoluminescence in CaZnOS:Tb3+, Sm3+ for Multicolor Stress Sensing
Abstract
1. Introduction
2. Experiments
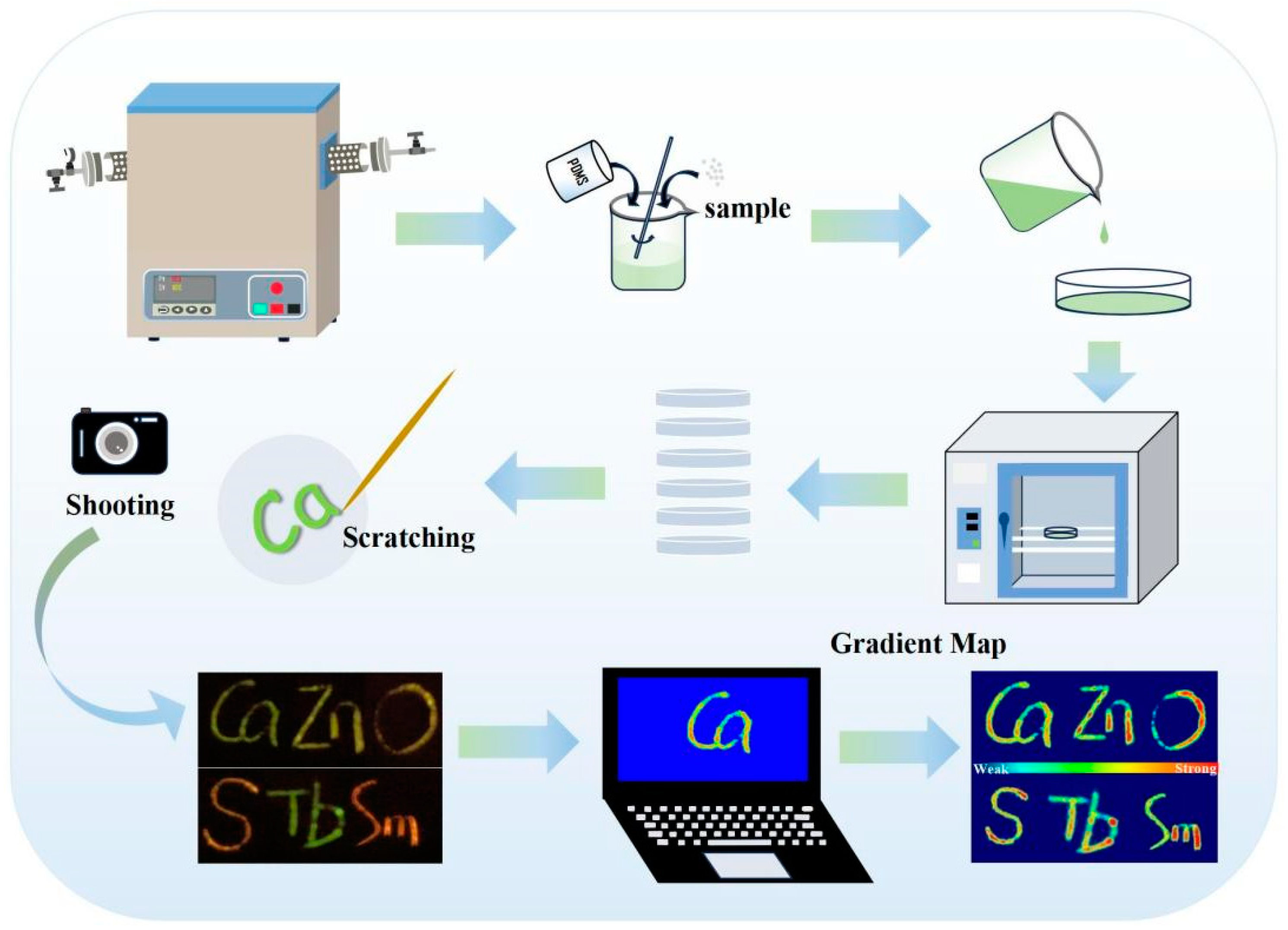
2.1. Sample Preparation
2.2. Fabrication of CaZnOS:Tb3+,Sm3+/PDMS Composite
2.3. Measurements and Characterization
3. Results and Discussion
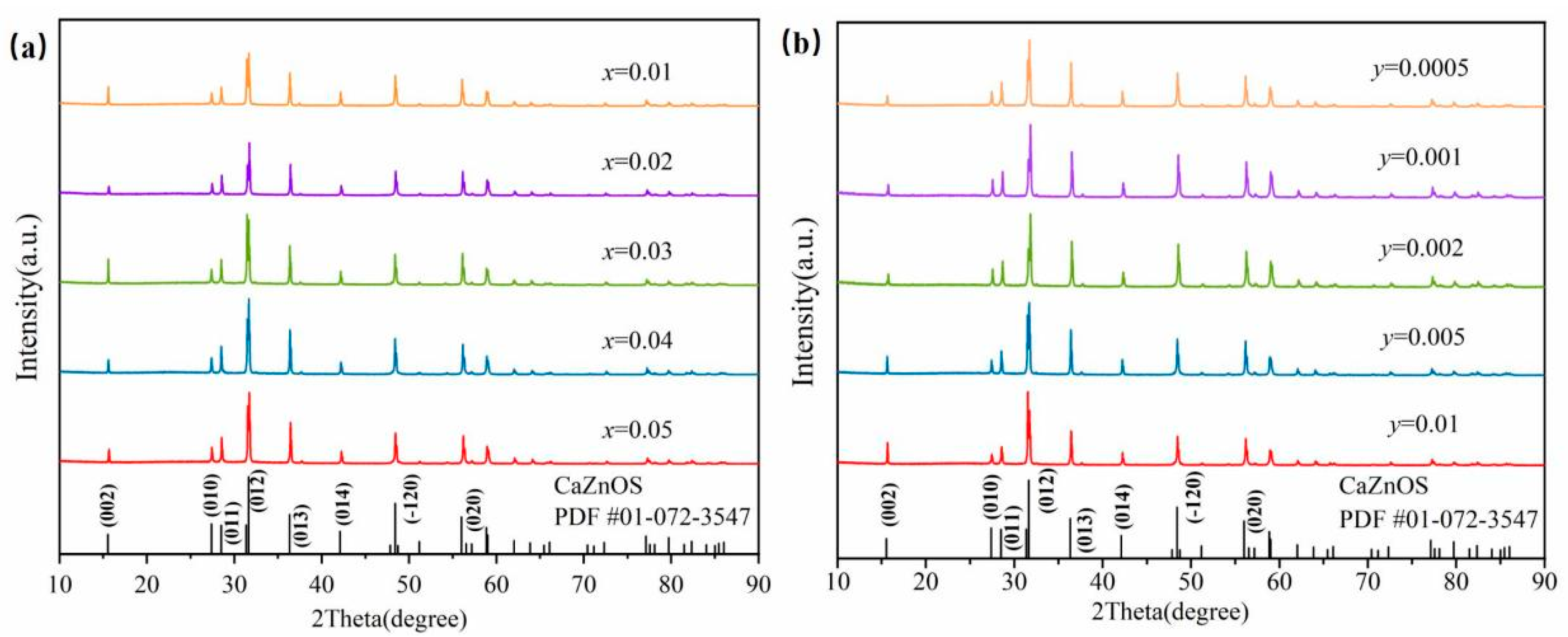
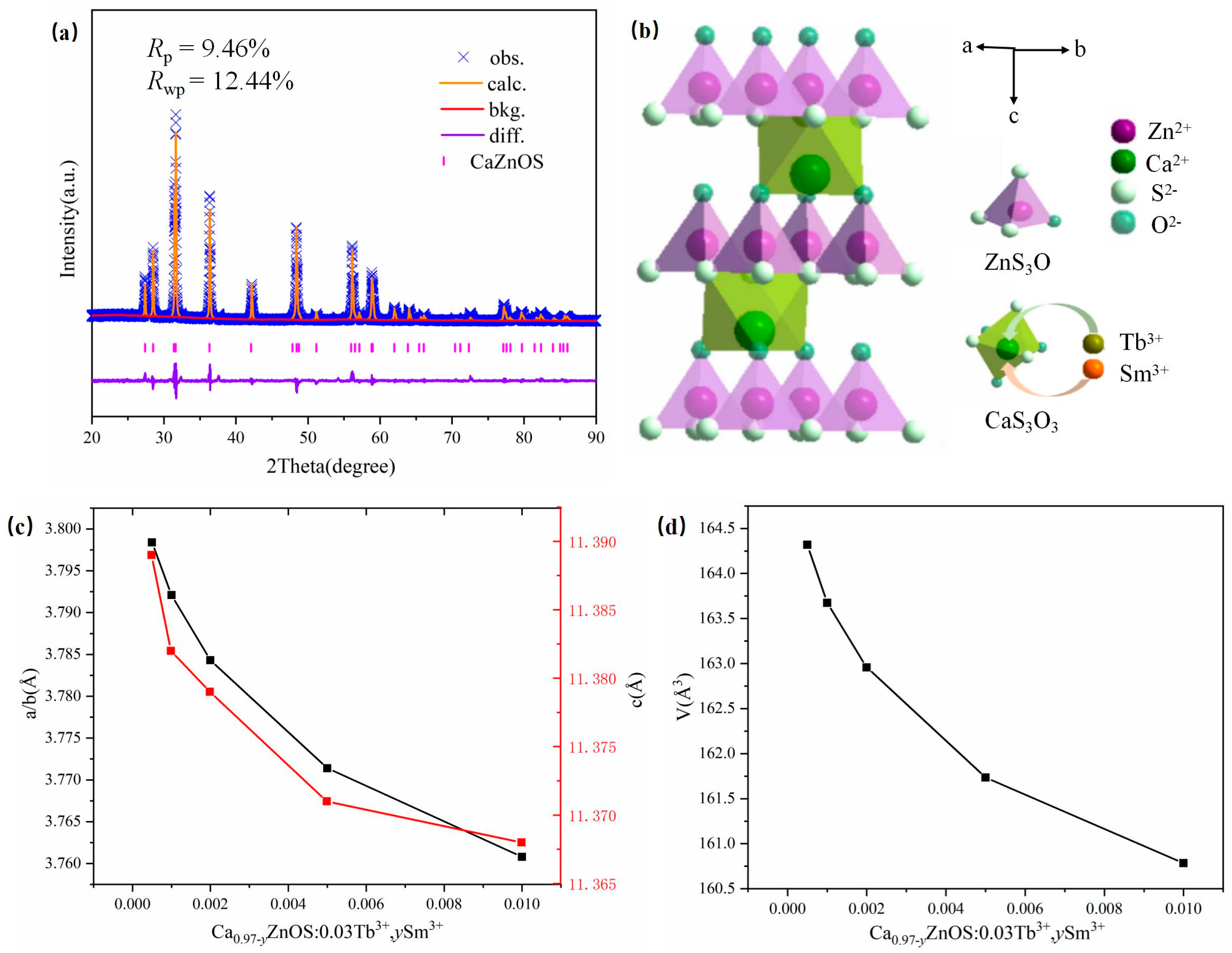
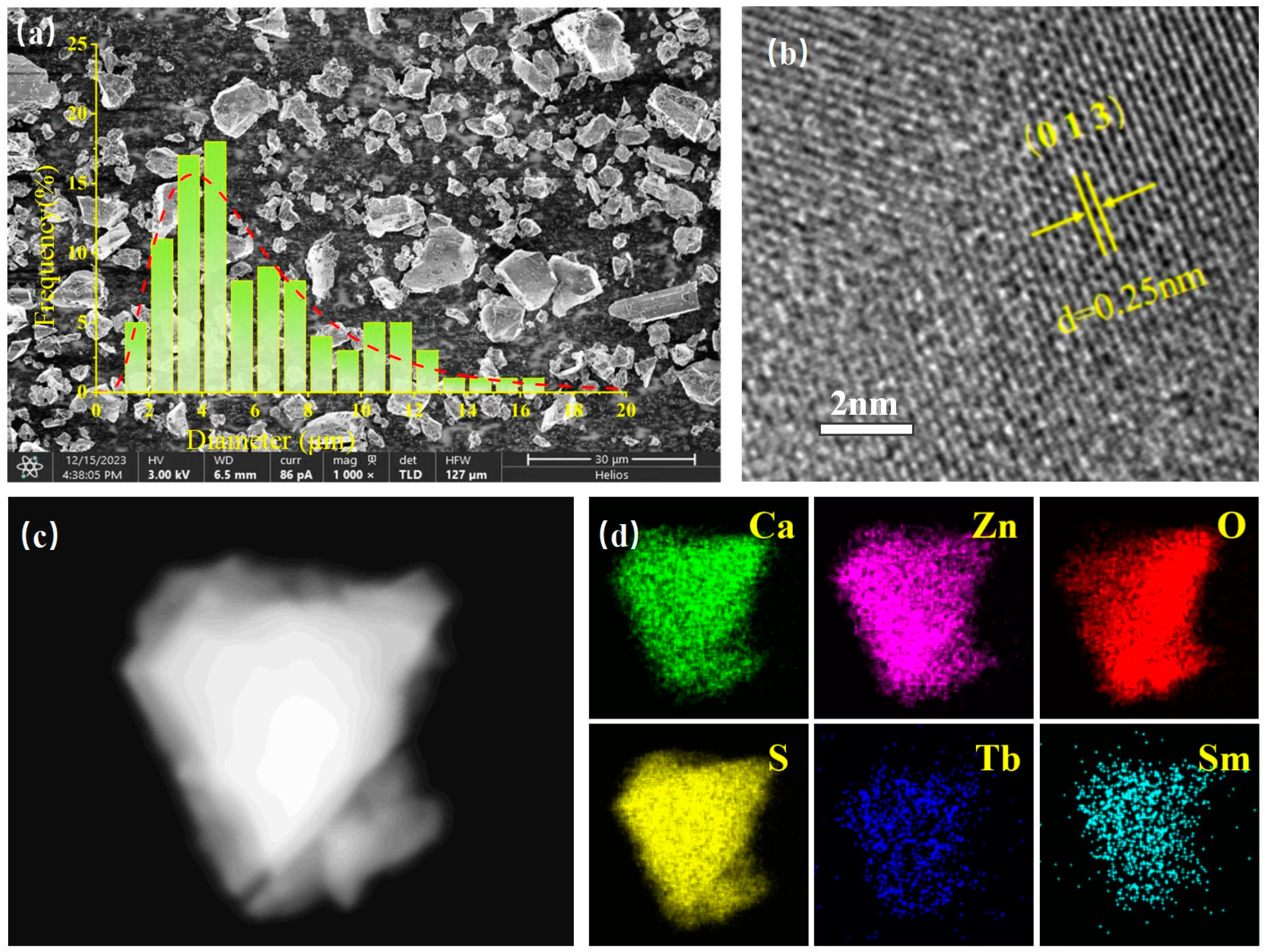
3.1. Phase and Crystal Structure Identification
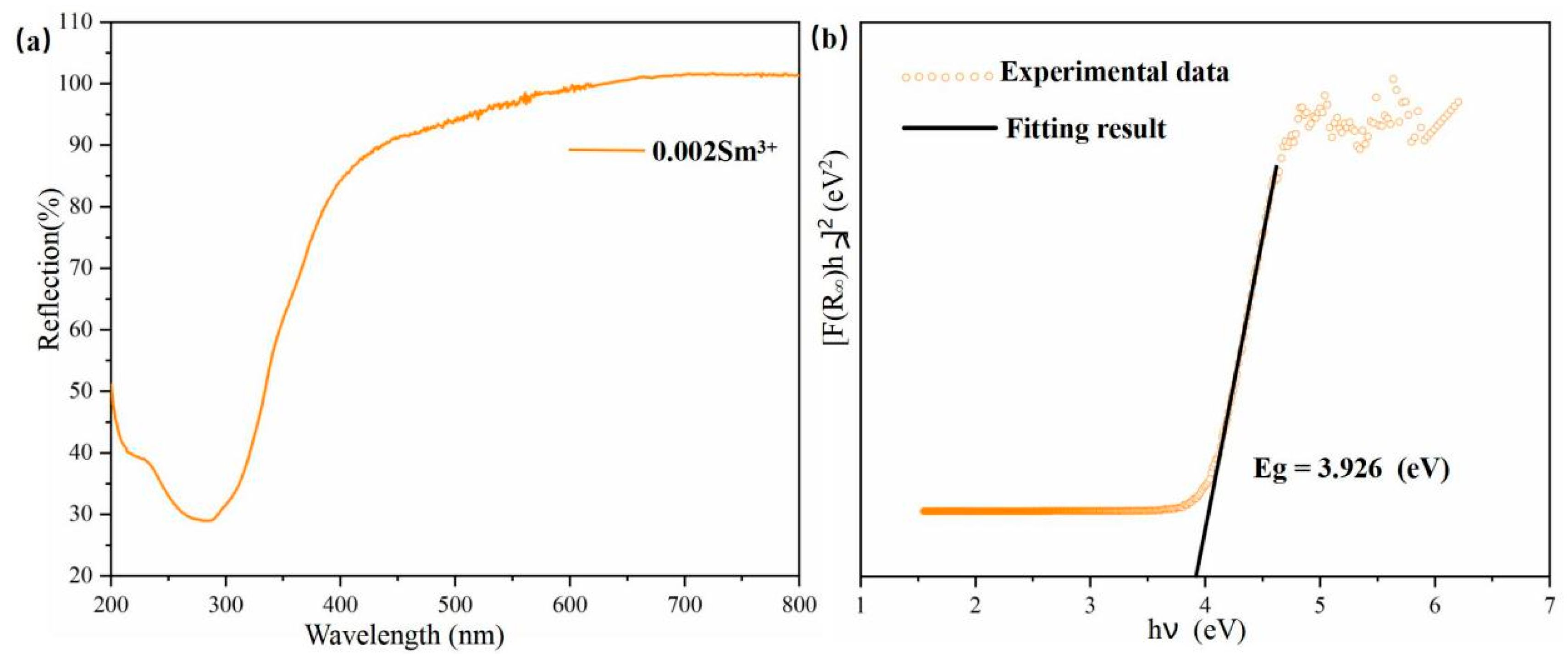
3.2. Diffuse Reflection Spectrum and Band Gap Calculation
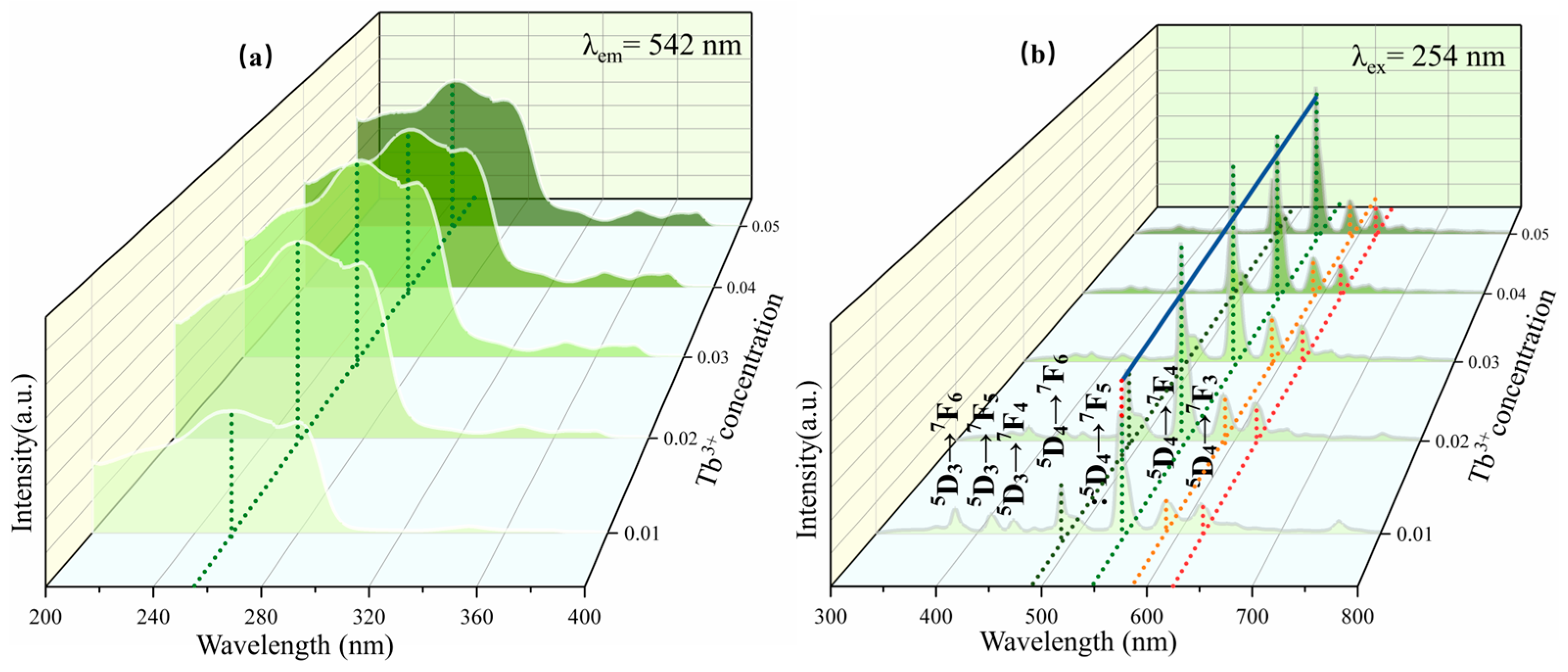
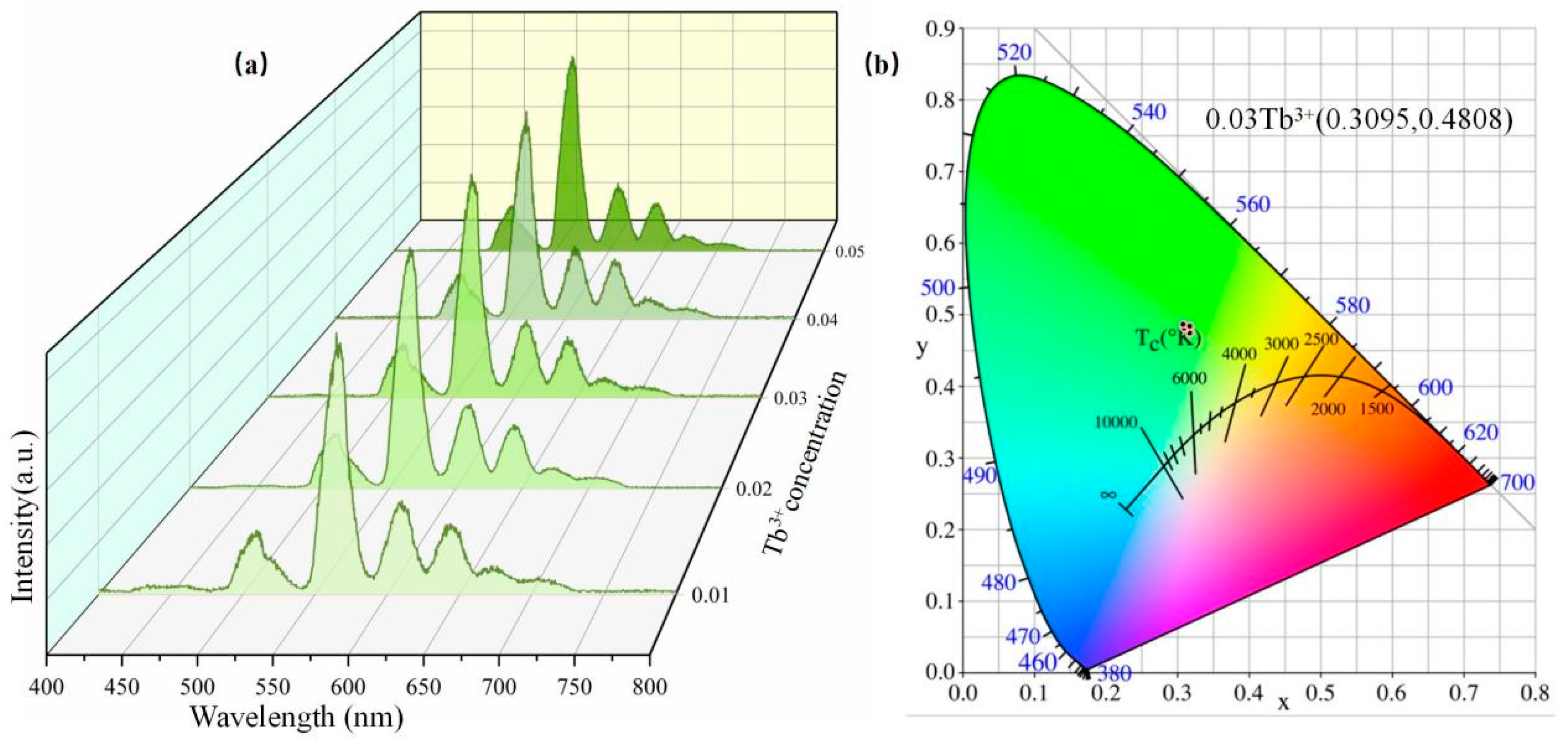
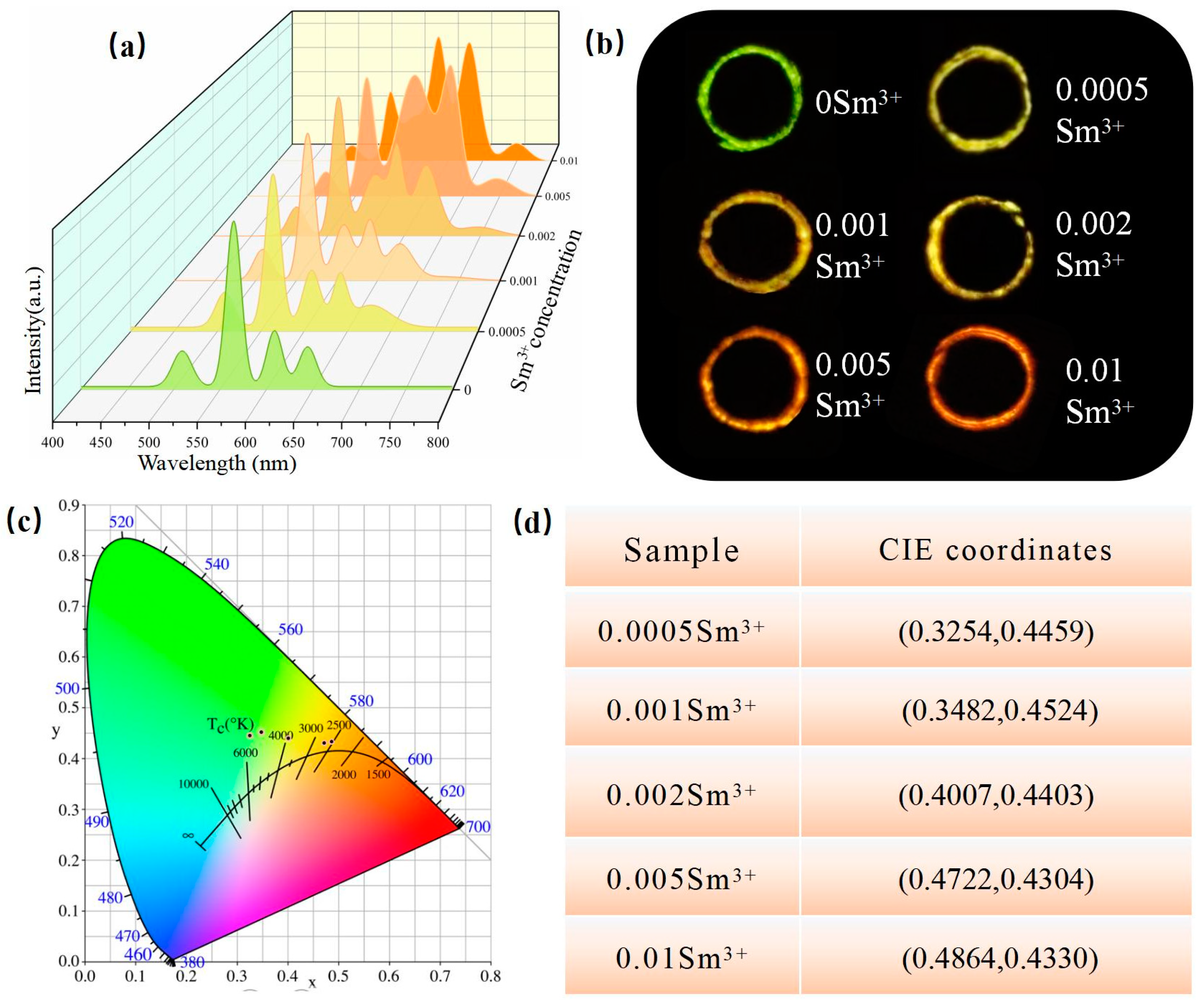
3.3. Excitation Spectrum and Emission Spectrum
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pan, X.; Zhuang, Y.; He, W.; Lin, C.; Mei, L.; Chen, C.; Xue, H.; Sun, Z.; Wang, C.; Peng, D.; et al. Quantifying the Interfacial Triboelectricity in Inorganic-organic Composite Mechanoluminescent Materials. Nat. Commun. 2024, 15, 2673. [Google Scholar] [CrossRef]
- Luo, J.; Ren, B.; Zhang, X.; Zhu, M.; Liang, T.; Huang, Z.; Zheng, Y.; Li, X.; Li, J.; Zheng, Z.; et al. Modulating Smart Mechanoluminescent Phosphors for Multistimuli Responsive Optical Wood. Adv. Sci. 2024, 11, 2305066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Wang, H.; Tian, Y.; Pan, H.; Zhang, L.; Wang, F.; Chang, J. Remote Regulation of Optogenetic Proteins by A Magneto-luminescence Microdevice. Adv. Funct. Mater. 2021, 31, 2006357. [Google Scholar] [CrossRef]
- Shin, H.; Timilsina, S.; Sohn, K.; Kim, J. Digital Image Correlation Compatible Mechanoluminescent Skin for Structural Health Monitoring. Adv. Sci. 2022, 9, 2105889. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wei, Y.; Ju, H.; Huang, X.; Li, J.; Wang, W.; Peng, D.; Tu, D.; Li, G. Microstrain-Stimulated Elastico-Mechanoluminescence with Dual-Mode Stress Sensing. Adv. Mater. 2024, 36, 2401296. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Zhang, K.; Peng, D.; Deng, Y.; Shan, C.; Dong, L. Mechanoluminescent Functional Devices: Developments, Applications and Prospects. Nano Energy 2024, 122, 109325. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, H.; Guo, J.; Zhao, Y.; Du, X.; Zhang, Q.; Chen, W.; Bian, T.; Zhang, Z.; Shen, J.; et al. Unrevealing Temporal Mechanoluminescence Behaviors at High Frequency via Piezoelectric Actuation. Small 2023, 19, 2207089. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, Q.; Wang, Y.; Wang, Z.; Wang, Z.; Annapooranan, R.; Latz, M.; Cai, S. Highly Robust and Soft Biohybrid Mechanoluminescence for Optical Signaling and Illumination. Nat. Commun. 2022, 13, 3914. [Google Scholar] [CrossRef]
- Bai, Y.; Guo, X.; Tian, B.; Liang, Y.; Peng, D.; Wang, Z. Self-charging Persistent Mechanoluminescence with Mechanics Storage and Visualization Activities. Adv. Sci. 2022, 9, 2203249. [Google Scholar] [CrossRef]
- Zhuang, Y.; Xie, R. Mechanoluminescence Rebrightening the Prospects of Stress Sensing: A Review. Adv. Mater. 2021, 33, 2005925. [Google Scholar]
- Chen, C.; Zhuang, Y.; Tu, D.; Wang, X.; Pan, C.; Xie, R. Creating Visible-to-near-infrared Mechanoluminescence in Mixed-anion Compounds SrZn2S2O and SrZnSO. Nano Energy 2020, 68, 104329. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, J.; Chen, B.; Sun, T.; Ma, R.; Wang, Y.; Zhu, H.; Peng, D.; Wang, F. Tuning Multimode Luminescence in Lanthanide (III) and Manganese (II) Co-Doped CaZnOS Crystals. Adv. Opt. Mater. 2020, 8, 2000274. [Google Scholar] [CrossRef]
- Du, Y.; Jiang, Y.; Sun, T.; Zhao, J.; Huang, B.; Peng, D.; Wang, F. Mechanically Excited Multicolor Luminescence in Lanthanide Ions. Adv. Mater. 2019, 31, 1807062. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, X.; Xiao, J.; Zhang, S.; Yang, X.; Xiao, J.; Li, X.; Peng, Q.; Luo, S.; Ba, H.; et al. Tunable Full-Color Mechanoluminescence in Rare Earth-Doped Transparent Amorphous Glass. Adv. Funct. Mater. 2024, 2404439. [Google Scholar] [CrossRef]
- Xiao, Y.; Xiong, P.; Le, Y.; Lun, Z.; Chen, K.; Wang, Z.; Shao, P.; Chen, Z.; Chen, D.; Yang, Z. Defect-management-induced Multi-stimulus-responsive Mechanoluminescence in Mn2+ Doped Gallate Compound. Nano Energy 2024, 120, 109086. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Wang, D.; Wang, D.; Shen, H.; Wu, H.; Wu, H.; Pan, G.; Hao, Z.; Liu, F.; et al. Liquid Nitrogen Temperature Mechanoluminescence and Persistent Luminescence. Adv. Funct. Mater. 2023, 33, 2305275. [Google Scholar] [CrossRef]
- Wu, S.; Xiao, B.; Xiao, Y.; Shao, P.; Wang, Y.; Xiong, P. Cr3+-activated Broadband Near-infrared Mechanoluminescence in Garnet Compound. Nano Energy 2023, 116, 108811. [Google Scholar] [CrossRef]
- Li, L.; Wondraczek, L.; Li, L.; Zhang, Y.; Zhu, Y.; Peng, M.; Mao, C. CaZnOS:Nd3+ Emits Tissue-penetrating Near-infrared Light Upon Force Loading. ACS Appl. Mater. Interfaces 2018, 10, 14509–14516. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xia, P.; Wang, T.; Yakovlev, A.; Hu, T.; Zhao, F.; Wang, Q.; Yu, X. Visual Representation of the Stress Distribution with a Color-Manipulated Mechanoluminescence of Fluoride for Structural Mechanics. Adv. Funct. Mater. 2023, 33, 2306875. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, X.; Xiao, Y.; Wang, H.; Shen, M.; Zhang, S.; Sun, H.; Huang, R.; Yu, T.; Huang, W. Realizing photoswitchable mechanoluminescence in organic crystals based on photochromism. Adv. Mater. 2023, 35, 2212273. [Google Scholar]
- Yang, X.; Cheng, Y.; Liu, Z.; Zhang, J.; Xu, J.; Lin, H.; Wang, Y. Designing Ratiometric Mechanoluminescence via Threshold Manipulation in Sandwich Structures. Laser Photonics Rev. 2024, 18, 2301004. [Google Scholar] [CrossRef]
- Qin, S.; Bian, J.; Han, Y.; Ma, Z.; Liu, B.; Zhang, J.; Xu, X.; Wang, Z. Intense and Efficient Green Mechanoluminescence in CaLaAl3O7 Through Tb3+ Doping. Mater. Res. Bull. 2022, 145, 111535. [Google Scholar] [CrossRef]
- Tu, D.; Xu, C.; Fujio, Y.; Yoshida, A. Mechanism of Mechanical Quenching and Mechanoluminescence in Phosphorescent CaZnOS:Cu. Light Sci. Appl. 2015, 4, e356. [Google Scholar] [CrossRef]
- Wang, W.; Peng, D.; Zhang, H.; Yang, X.; Pan, C. Mechanically Induced Strong Red Emission in Samarium Ions doped Piezoelectric Semiconductor CaZnOS for Dynamic Pressure Sensing and Imaging. Opt. Commun. 2017, 395, 24–28. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, X.; Ma, R.; Huang, Z.; Wang, C.; Zhu, M.; Du, Y.; Chen, X.; Pan, C.; Wang, B.; et al. Molten Salt Shielded Synthesis of Monodisperse Layered CaZnOS-Based Semiconductors for Piezophotonic and X-Ray Detection Applications. Small 2022, 18, 2107437. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xia, Z.; Lei, B.; Liu, Q. Full Color Control and White Emission from CaZnOS:Ce3+,Na+,Mn2+ Phosphors via Energy Transfer. J. Mater. Chem. C 2016, 4, 9711–9716. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Q.; Yang, X.; Yang, W.; An, R.; Li, T.; Zhou, Y.; Zhang, H.; Zhao, J.; Zhang, Z. Color Manipulation of Bi3+-activated CaZnOS Under Stress With Ultra-high Efficiency and Low Threshold for Anticounterfeiting Applications. J. Mater. Chem. C 2020, 8, 3308–3315. [Google Scholar]
- Yang, Y.; Zhou, Y.; Pan, D.; Zhang, Z.; Zhao, J. The Luminescence Properties of CaZnOS:Bi3+,Sm3+,Li+ Phosphors with Tunable Emissions and Energy Transfer for White Emission. J. Lumin. 2019, 206, 578–584. [Google Scholar] [CrossRef]
- Li, W.; Cai, Y.; Chang, J.; Wang, S.; Liu, J.; Zhou, L.; Wu, M.; Zhang, J. Unraveling Mechanoluminescent Mechanisms in Doped CaZnOS Materials: Co-Mediation of Trap-Controlled and Non-Trap-Controlled Processes. Adv. Funct. Mater. 2023, 33, 2305482. [Google Scholar]
- Peng, D.; Jiang, Y.; Huang, B.; Du, Y.; Zhao, J.; Zhang, X.; Ma, R.; Golovynskyi, S.; Chen, B.; Wang, F. A ZnS/CaZnOS Heterojunction for Efficient Mechanical-to-optical Energy Conversion by Conduction Band Offset. Adv. Mater. 2020, 32, 1907747. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, A.; Chen, X.; Zhao, J. Photoluminescence Properties and Energy Levels of RE (RE = Pr, Sm, Er, Tm) in Layered-CaZnOS Oxysulfide. J. Appl. Phys. 2013, 114, 213518. [Google Scholar]
- Wang, W.; Wang, Z.; Zhang, J.; Zhou, J.; Dong, W.; Wang, Y. Contact electrification induced Mechanoluminescence. Nano Energy 2022, 94, 106920. [Google Scholar]
- Zhang, J.; Zhao, L.; Long, Y.; Zhang, H.; Sun, B.; Han, W.; Yan, X.; Wang, X. Color manipulation of intense multiluminescence from CaZnOS:Mn2+ by Mn2+ concentration effect. Chem. Mater. 2015, 27, 7481–7489. [Google Scholar] [CrossRef]
- Lyu, T.; Dorenbos, P.; Wei, Z. Versatile and High-Performance LiTaO3:Tb3+,Gd3+ Perovskite for Multimode Anti-counterfeiting, Flexible X-ray Imaging, Continuous Stress Sensing, and Non-Real-Time Recording. Laser Photonics Rev. 2023, 17, 2300323. [Google Scholar] [CrossRef]
- Qiao, J.; Wang, L.; Liu, Y.; Huang, P.; Shi, Q.; Tian, Y.; Cui, C. Preparation, Photoluminescence and Thermally Stable Luminescence of High Brightness Red LiY5P2O13:Eu3+ Phosphor for White LEDs. J. Alloys Compd. 2016, 686, 601–607. [Google Scholar]
- Zheng, T.; Runowski, M.; Martín, I.; Soler-Carracedo, K.; Peng, L.; Skwierczyńska, M.; Sójka, M.; Barzowska, J.; Mahlik, S.; Hemmerich, H.; et al. Mechanoluminescence and Photoluminescence Heterojunction for Superior Multimode Sensing Platform of Friction, Force, Pressure, and Temperature in Fibers and 3D-printed Polymers. Adv. Mater. 2023, 35, 2304140. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, S.; Gu, Y.; Zhou, J.; Zhang, J. Contact-separation-induced Self-recoverable Mechanoluminescence of CaF2:Tb3+/PDMS Elastomer. Nat. Commun. 2024, 15, 2014. [Google Scholar] [PubMed]
- Hu, T.; Gao, Y.; Wang, B.; Yu, T.; Wen, D.; Chen, Y.; Zeng, Q. A New Class of Battery-free, Mechanically Powered, Piezoelectric Ca5Ga6O14:Tb3+ Phosphors with Self-recoverable Luminescence. J. Mater. Chem. C 2022, 10, 9554–9562. [Google Scholar]
- Li, J.; Xu, C.; Tu, D.; Chai, X.; Wang, X.; Liu, L.; Kawasaki, E. Tailoring Bandgap and Trap Distribution via Si or Ge Substitution for Sn to Improve Mechanoluminescence in Sr3Sn2O7:Sm3+ Layered Perovskite Oxide. Acta Mater. 2018, 145, 462–469. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, S.; Zhao, L.; Wang, C.; Lv, H.; Liu, B.; Qiu, J.; Xu, X.; Yu, X. Delayed Stress Memory by CaAl2O4:Eu2+ Mechanoluminescent Phosphor with Defect Engineering Regulation. J. Adv. Ceram. 2022, 11, 1319–1329. [Google Scholar]
- Shao, P.; Xiong, P.; Jiang, D.; Chen, Z.; Xiao, Y.; Sun, Y.; Chen, D.; Yang, Z. Tunable and Enhanced Mechanoluminescence in LiYGeO4:Tb3+ via Bi3+ → Tb3+ Energy Transfer. J. Mater. Chem. C 2023, 11, 2120–2128. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, F.; Zhang, L.; Wang, D.; Liang, Y.; Yang, S.; Wang, Z. Interfacial Triboelectrification-modulated Self-recoverable and Thermally Stable Mechanoluminescence in Mixed-anion Compounds. Nano Energy 2022, 96, 107075. [Google Scholar]
- Lyu, T.; Dorenbos, P.; Xiong, P.; Wei, Z. LiTaO3:Bi3+,Tb3+,Ga3+,Ge4+: A Smart Perovskite with High Charge Carrier Storage Capacity for X-ray Imaging, Stress sensing, and Non-real-time Recording. Adv. Funct. Mater. 2022, 32, 2206024. [Google Scholar] [CrossRef]
- Tong, J.; Huan, J.; Yu, X.; Cheng, J.; Zhang, Z.; Xing, J.; Zhao, J.; Yang, X. Significantly Enhanced Mechanoluminescence from Nb5+ Co-doped ZrO2:Sm3+ via a High Valence Ion doping Strategy. J. Mater. Chem. C 2023, 11, 11597–11605. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Li, Z.; Wang, Z.; Xiang, Z.; Wang, Z.; Li, S.; Zhang, M.; Liu, W. Achieving Tunable Mechanoluminescence in CaZnOS:Tb3+, Sm3+ for Multicolor Stress Sensing. Nanomaterials 2024, 14, 1279. https://doi.org/10.3390/nano14151279
Wang W, Li Z, Wang Z, Xiang Z, Wang Z, Li S, Zhang M, Liu W. Achieving Tunable Mechanoluminescence in CaZnOS:Tb3+, Sm3+ for Multicolor Stress Sensing. Nanomaterials. 2024; 14(15):1279. https://doi.org/10.3390/nano14151279
Chicago/Turabian StyleWang, Wenqi, Zihui Li, Ziying Wang, Zhizhi Xiang, Zhenbin Wang, Sixia Li, Mingjin Zhang, and Weisheng Liu. 2024. "Achieving Tunable Mechanoluminescence in CaZnOS:Tb3+, Sm3+ for Multicolor Stress Sensing" Nanomaterials 14, no. 15: 1279. https://doi.org/10.3390/nano14151279
APA StyleWang, W., Li, Z., Wang, Z., Xiang, Z., Wang, Z., Li, S., Zhang, M., & Liu, W. (2024). Achieving Tunable Mechanoluminescence in CaZnOS:Tb3+, Sm3+ for Multicolor Stress Sensing. Nanomaterials, 14(15), 1279. https://doi.org/10.3390/nano14151279






