Enhanced Fenton Degradation of Tetracycline over Cerium-Doped MIL88-A/g-C3N4: Catalytic Performance and Mechanism
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of MIL-88A
2.3. Preparation of Ce-Doped MIL-88A
2.4. Synthesis of g-C3N3
2.5. Synthesis of Ce-Doped MIL-88A/g-C3N4 Composite
2.6. Characterization
2.7. Fenton-like Degradation Experiments
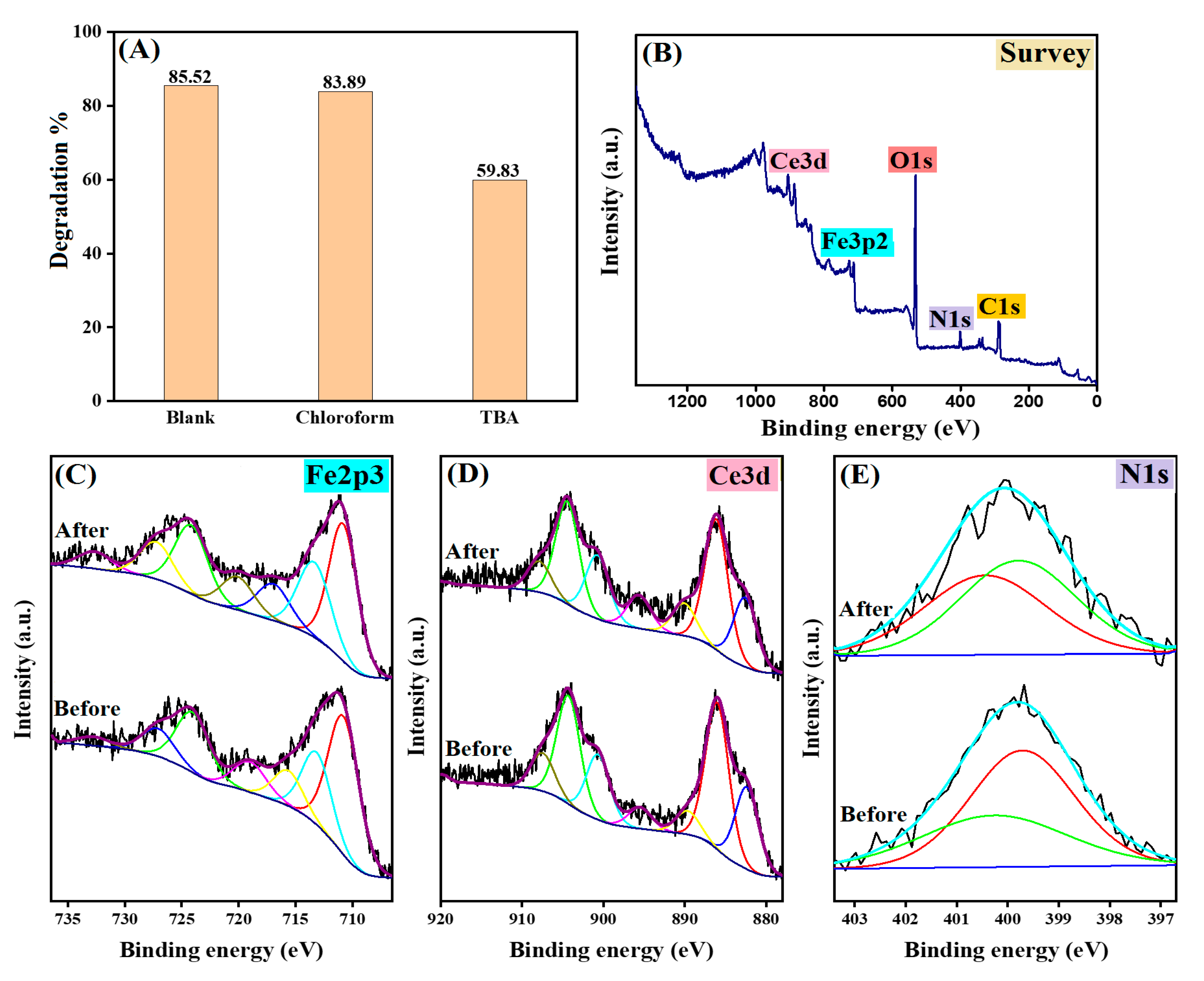
2.8. Quenching Test
2.9. Regeneration Test
3. Results and Discussion
3.1. Characterization of (Ce0.33Fe) MIL-88A/10%g-C3N4
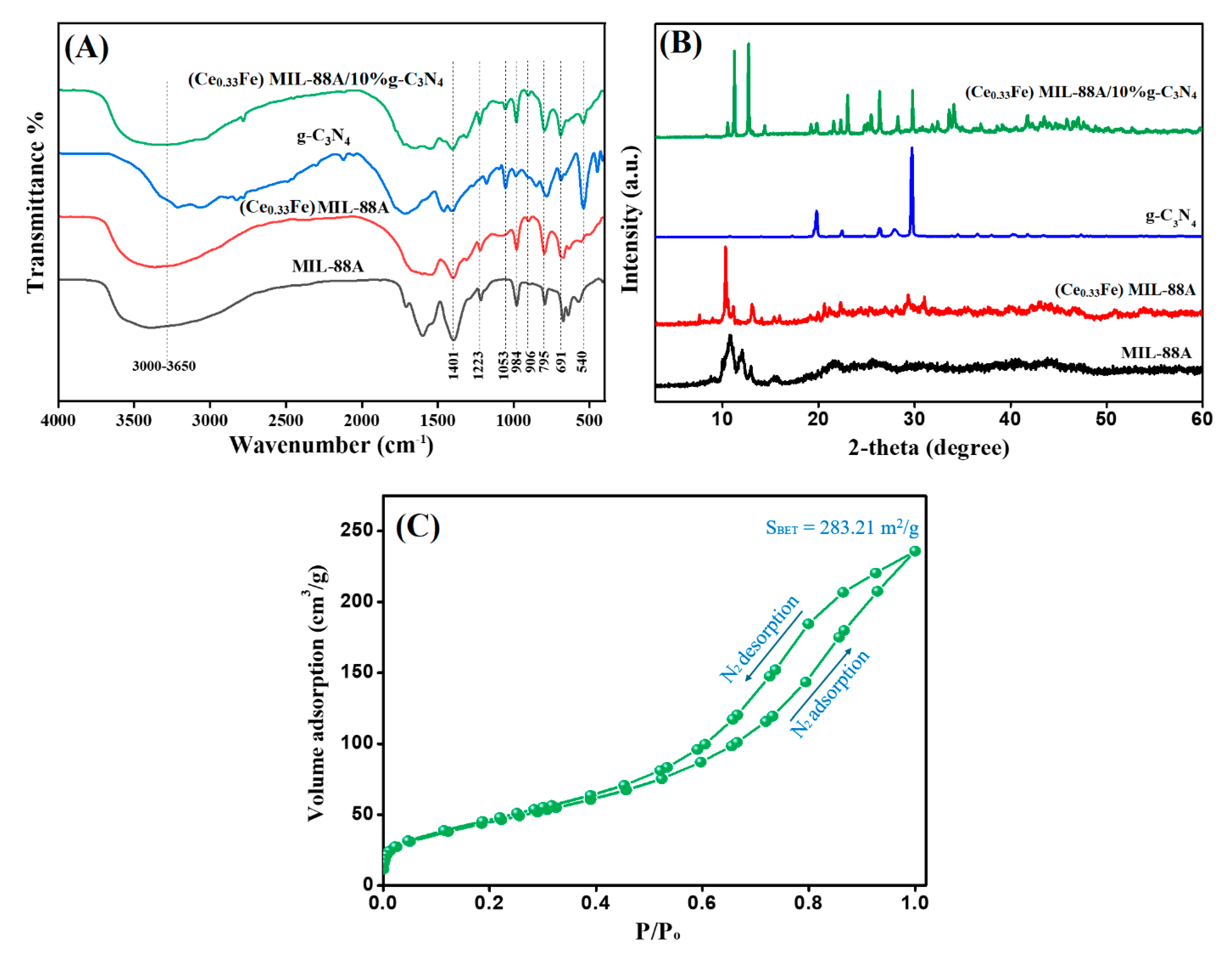
3.1.1. Fourier-Transform Infrared Spectra (FT-IR)
3.1.2. X-ray Diffraction (XRD)
3.1.3. Brunauer–Emmett–Teller (BET)
3.1.4. X-ray Photoelectron Spectroscopy (XPS)
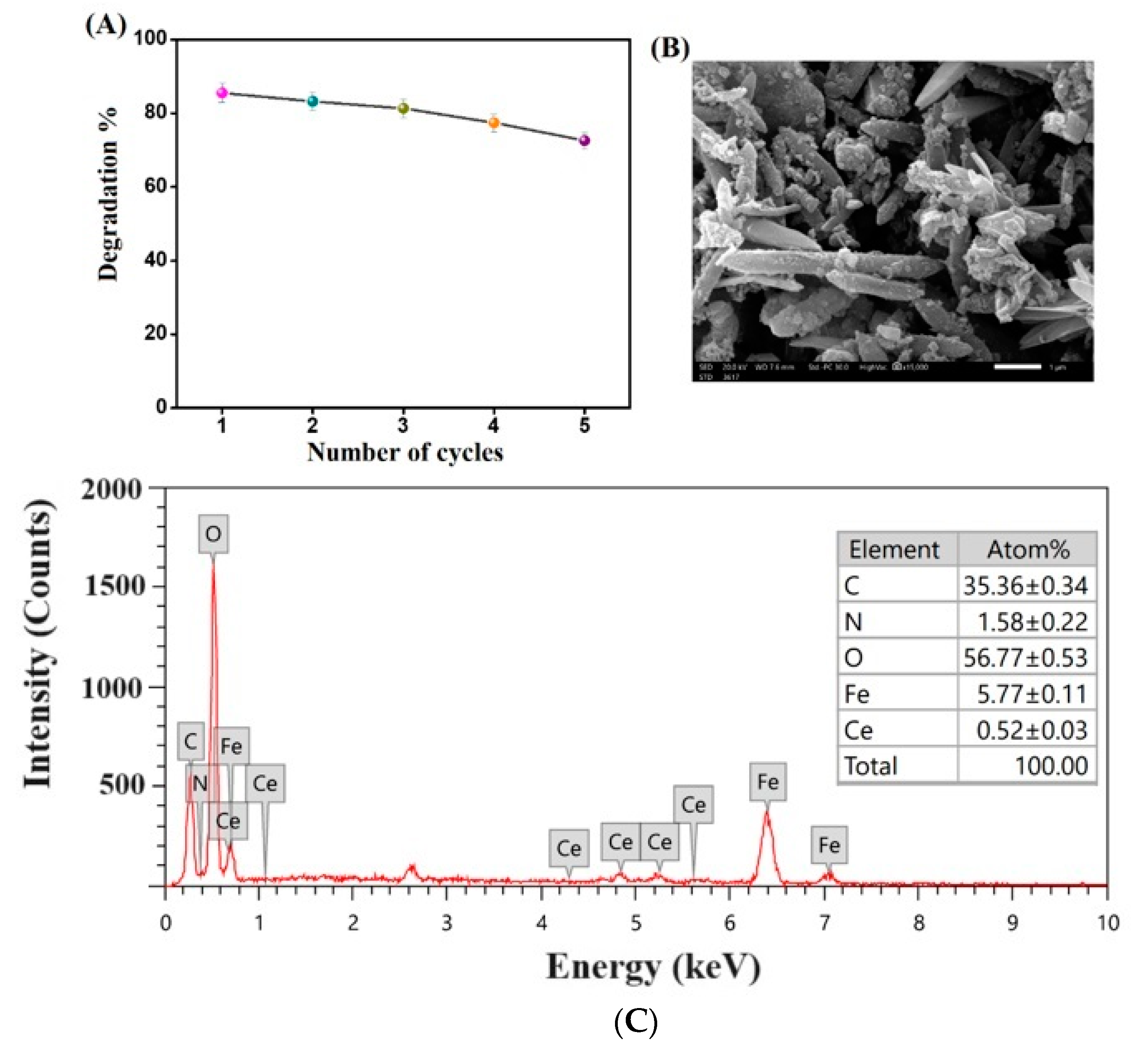
3.1.5. Scanning Electron Microscopy (SEM)
3.2. Fenton-like Degradation of Tetracycline Hydrochloride
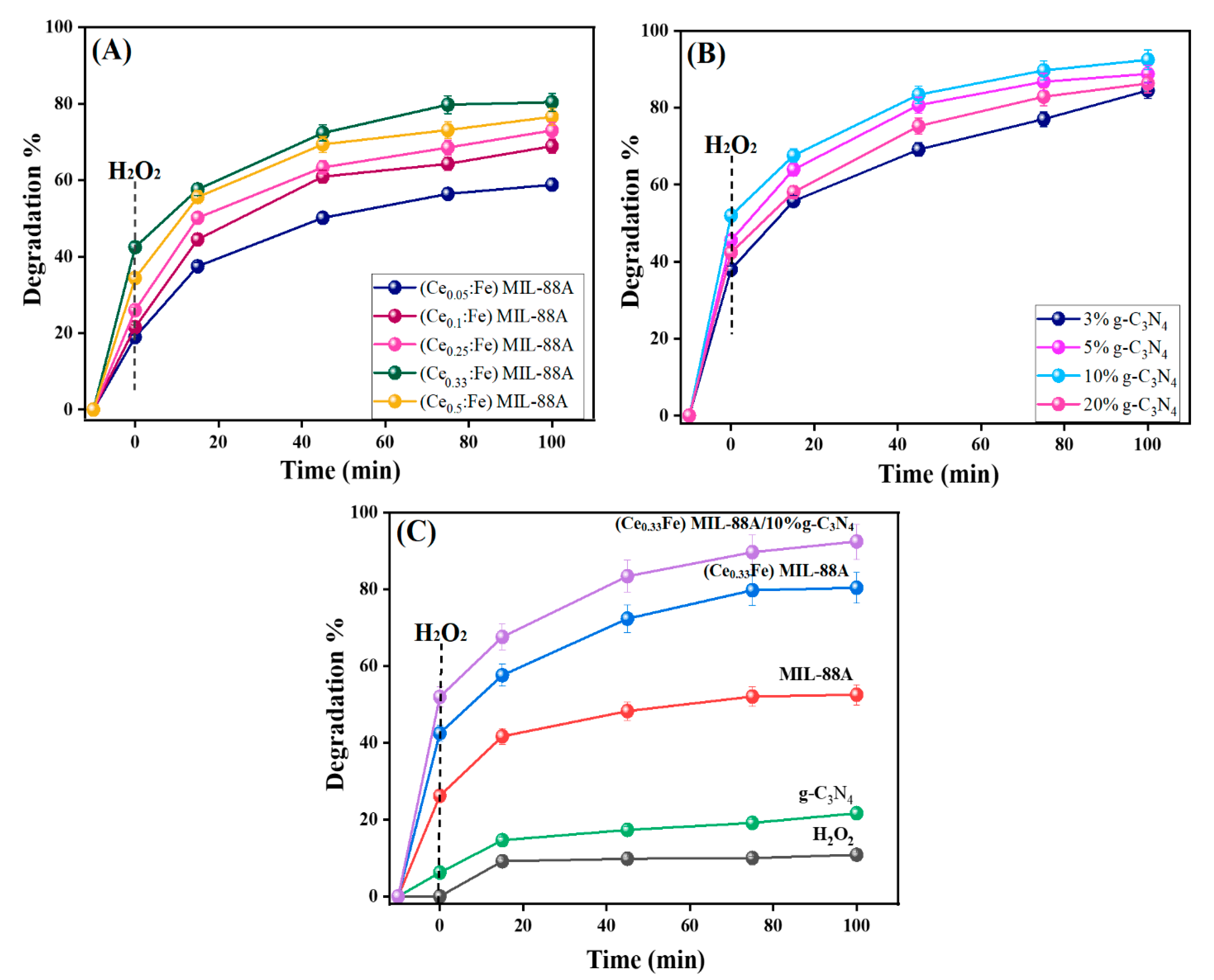
3.2.1. Optimization of the Components’ Ratio of Ce-Doped MIL-88A/g-C3N4
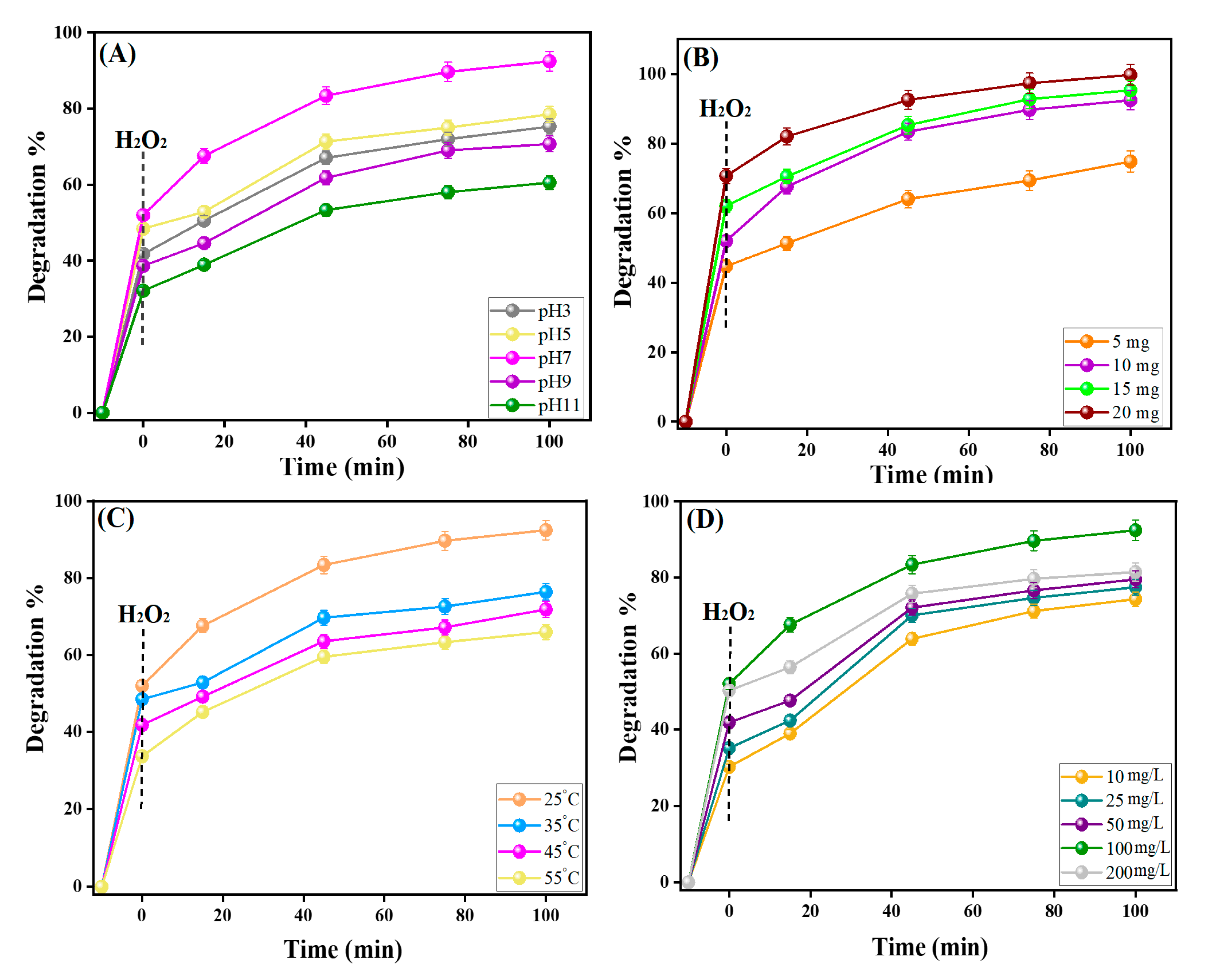
3.2.2. Effects of pH
3.2.3. Effects of Catalyst Dosage
3.2.4. Effects of Reaction Temperature
3.2.5. Effects of H2O2 Concentration
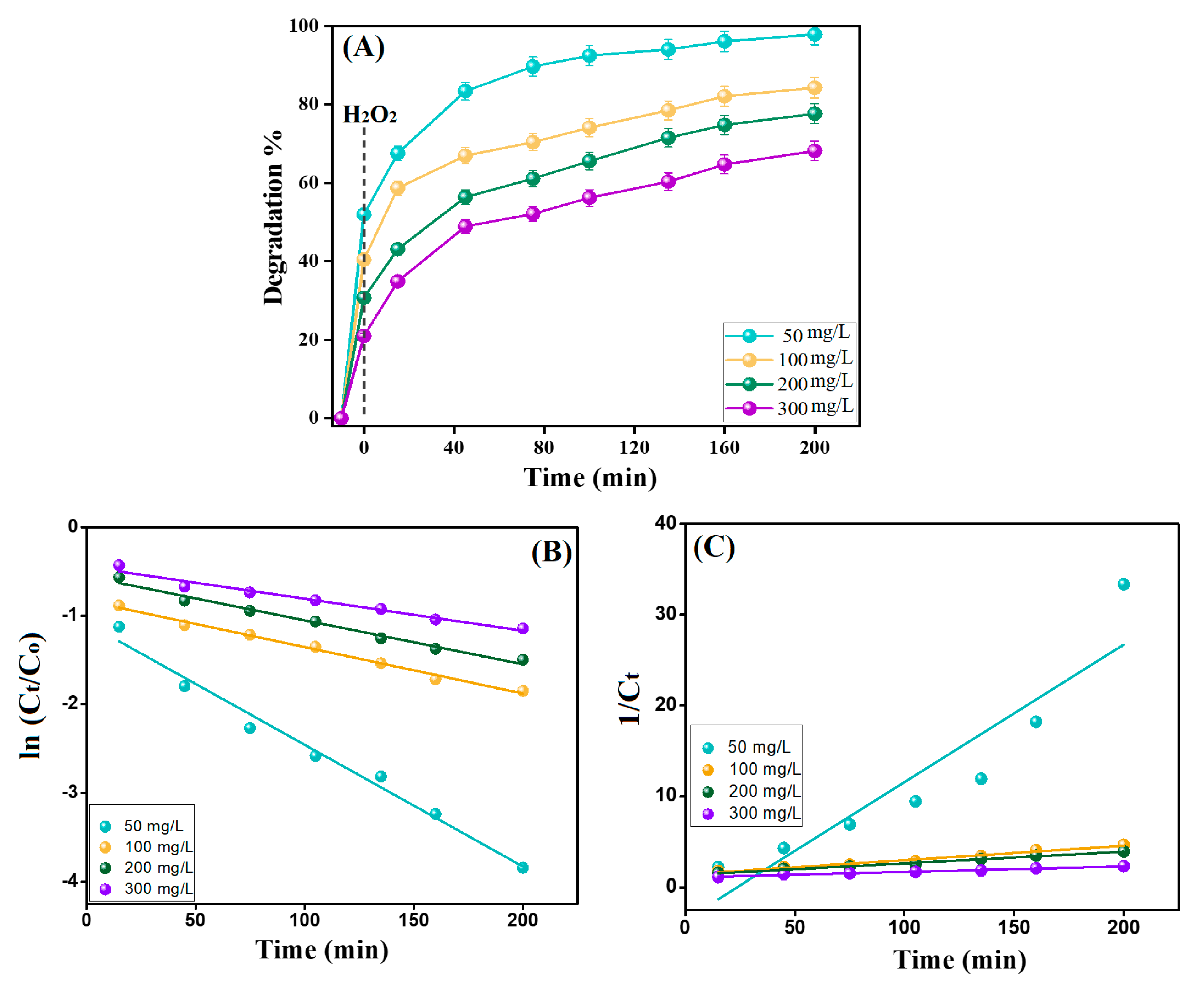
3.2.6. Effects of Initial Concentration
3.3. Kinetic Study
3.4. Identification of Reactive Species
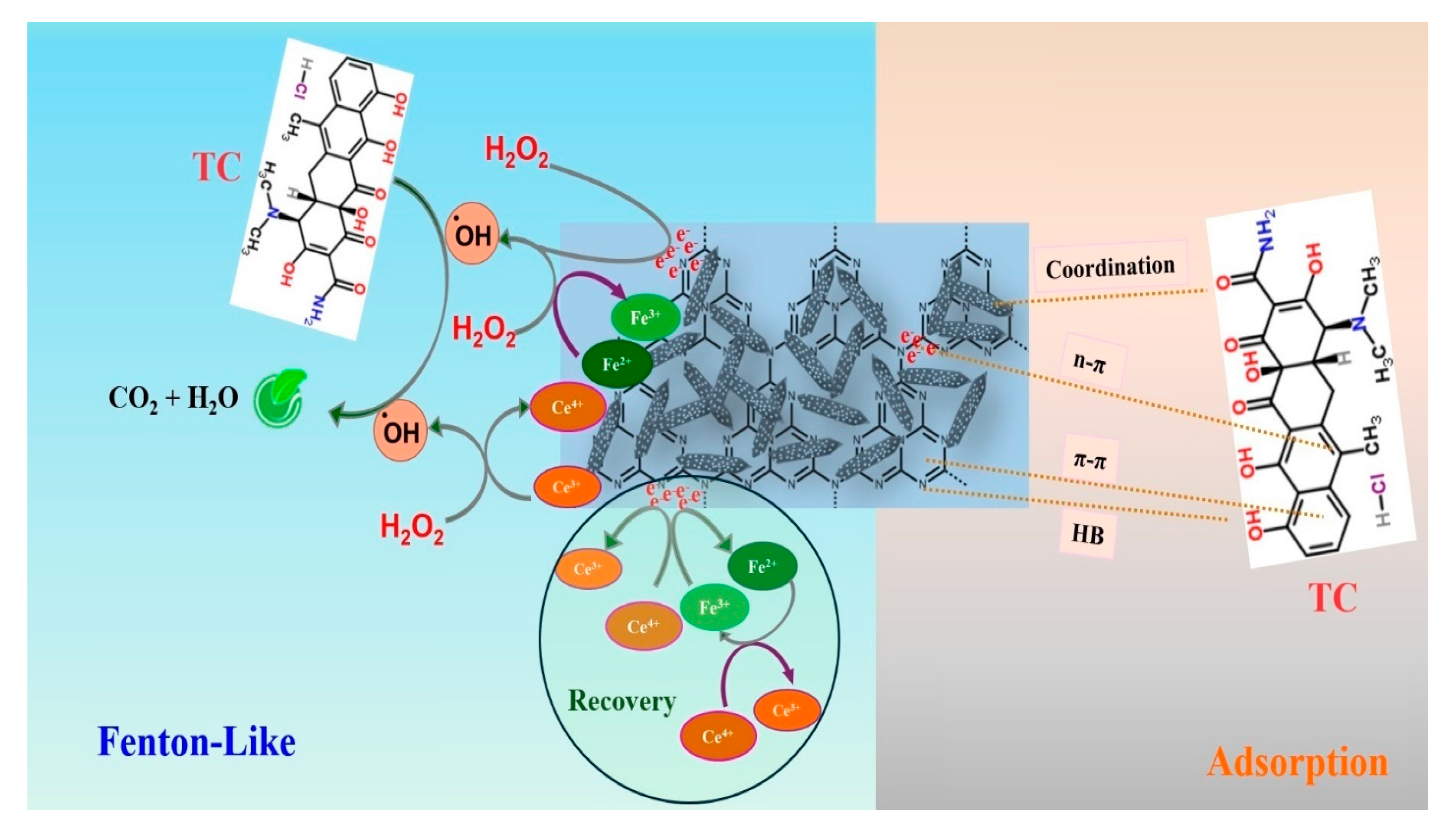
3.5. Degradation Mechanism
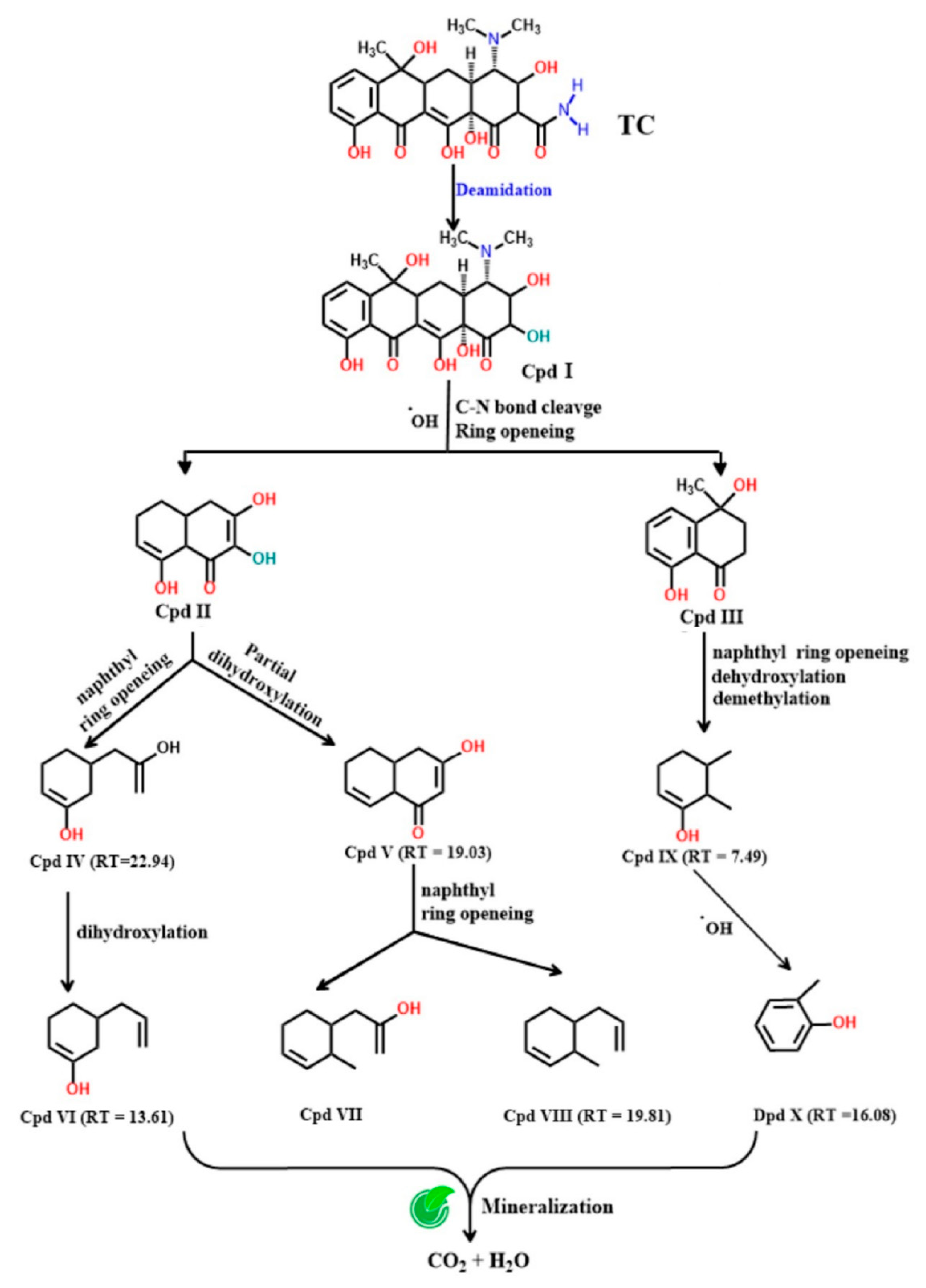
3.6. The Degradation Pathway of TC
3.7. Recycling Test
3.8. Catalyst Stability
3.9. Comparison Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Monaem, E.M.A.; Hosny, M.; Eltaweil, A.S. Synergistic effect between adsorption and Fenton-like degradation on CoNiFe-LDH/ZIF-8 composite for efficient degradation of doxycycline. Chem. Eng. Sci. 2024, 287, 119707. [Google Scholar] [CrossRef]
- Omer, A.M.; El-Monaem, E.M.A.; El-Subruiti, G.M.; El-Latif, M.M.A.; Eltaweil, A.S. Fabrication of easy separable and reusable MIL-125(Ti)/MIL-53(Fe) binary MOF/CNT/Alginate composite microbeads for tetracycline removal from water bodies. Sci. Rep. 2021, 11, 23818. [Google Scholar] [CrossRef] [PubMed]
- Abdelfatah, A.M.; Fawzy, M.; El-Khouly, M.E.; Eltaweil, A.S. Efficient adsorptive removal of tetracycline from aqueous solution using phytosynthesized nano-zero valent iron. J. Saudi Chem. Soc. 2021, 25, 101365. [Google Scholar] [CrossRef]
- El-Monaem, E.M.A.; Omer, A.M.; Khalifa, R.E.; Eltaweil, A.S. Floatable cellulose acetate beads embedded with flower-like zwitterionic binary MOF/PDA for efficient removal of tetracycline. J. Colloid Interface Sci. 2022, 620, 333–345. [Google Scholar] [CrossRef] [PubMed]
- El-Monaem, E.M.A.; Eltaweil, A.S.; Elshishini, H.M.; Hosny, M.; Alsoaud, M.M.A.; Attia, N.F.; El-Subruiti, G.M.; Omer, A.M. Sustainable adsorptive removal of antibiotic residues by chitosan composites: An insight into current developments and future recommendations. Arab. J. Chem. 2022, 15, 103743. [Google Scholar] [CrossRef] [PubMed]
- Fane, A.G.; Wang, R.; Hu, M.X. Synthetic Membranes for Water Purification: Status and Future. Angew. Chem. Int. Ed. 2015, 54, 3368–3386. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Wu, X. Microbial degradation of tetracycline in the aquatic environment: A review. Crit. Rev. Biotechnol. 2020, 40, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zhao, M.; Yuan, D.; Li, X.; Wang, Z.; Zhang, X.; Jiao, T.; Ke, J. Fe3O4 nanoparticles three-dimensional electro-peroxydisulfate for improving tetracycline degradation. Chemosphere 2021, 268, 129315. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Z.; Wu, K.; Dai, G.; Chen, Q.; Zhou, L.; Zheng, J.; Ma, L.; Li, G.; Wang, W.; et al. Novel Ag-bridged dual Z-scheme g-C3N4/BiOI/AgI plasmonic heterojunction: Exceptional photocatalytic activity towards tetracycline and the mechanism insight. J. Environ. Sci. 2023, 131, 123–140. [Google Scholar] [CrossRef]
- Teng, Y.; Li, W.; Wang, J.; Jia, S.; Zhang, H.; Yang, T.; Li, X.; Li, L.; Wang, C. A green hydrothermal synthesis of polyacrylonitrile@carbon/MIL-101(Fe) composite nanofiber membrane for efficient selective removal of tetracycline. Sep. Purif. Technol. 2023, 315, 123610. [Google Scholar] [CrossRef]
- Abbasifar, A.; Shahrabadi, F.; ValizadehKaji, B. Effects of green synthesized zinc and copper nano-fertilizers on the morphological and biochemical attributes of basil plant. J. Plant Nutr. 2020, 43, 1104–1118. [Google Scholar] [CrossRef]
- Liao, X.; Wang, F.; Wang, F.; Cai, Y.; Yao, Y.; Teng, B.-T.; Hao, Q.; Shuxiang, L. Synthesis of (100) surface oriented MIL-88A-Fe with rod-like structure and its enhanced fenton-like performance for phenol removal. Appl. Catal. B Environ. 2019, 259, 118064. [Google Scholar] [CrossRef]
- Ren, G.; Zhao, K.; Zhao, L. A Fenton-like method using ZnO doped MIL-88A for degradation of methylene blue dyes. RSC Adv. 2020, 10, 39973–39980. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhu, J.; Zhu, G.; He, H. Strategies for enhancing the heterogeneous Fenton catalytic reactivity: A review. Appl. Catal. B Environ. 2019, 255, 117739. [Google Scholar] [CrossRef]
- Liu, C.; Wang, P.; Huang, P.; Yang, Z.; Zhou, G. Photo-induced heterogeneous regeneration of Fe(II) in Fenton reaction for efficient polycyclic antibiotics removal and in-depth charge transfer mechanism. J. Colloid Interface Sci. 2023, 638, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yu, R.; Zhao, S.; Cao, X.; Zhang, X.; Bai, S. Heterogeneous Fenton system driven by iron-loaded sludge biochar for sulfamethoxazole-containing wastewater treatment. J. Environ. Manag. 2023, 335, 117576. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, J. Fenton-like degradation of sulfamethoxazole using Fe-based magnetic nanoparticles embedded into mesoporous carbon hybrid as an efficient catalyst. Chem. Eng. J. 2018, 351, 1085–1094. [Google Scholar] [CrossRef]
- Li, T.; Ge, L.; Peng, X.; Wang, W.; Zhang, W. Enhanced degradation of sulfamethoxazole by a novel Fenton-like system with significantly reduced consumption of H2O2 activated by g-C3N4/MgO composite. Water Res. 2021, 190, 116777. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, J. Fe-based Fenton-like catalysts for water treatment: Preparation, characterization and modification. Chemosphere 2021, 276, 130177. [Google Scholar] [CrossRef]
- Omer, A.M.; El-Monaem, E.M.A.; El-Latif, M.M.A.; El-Subruiti, G.M.; Eltaweil, A.S. Facile fabrication of novel magnetic ZIF-67 MOF@aminated chitosan composite beads for the adsorptive removal of Cr(VI) from aqueous solutions. Carbohydr. Polym. 2021, 265, 118084. [Google Scholar] [CrossRef]
- Zhao, B.; Gong, J.; Song, B.; Sang, F.; Zhou, C.; Zhang, C.; Cao, W.; Niu, Q.; Chen, Z. Effects of activated carbon, biochar, and carbon nanotubes on the heterogeneous Fenton oxidation catalyzed by pyrite for ciprofloxacin degradation. Chemosphere 2022, 308, 136427. [Google Scholar] [CrossRef] [PubMed]
- El-Monaem, E.M.A.; Eltaweil, A.S.; El-Subruiti, G.M.; Mohy-Eldin, M.S.; Omer, A.M. Adsorption of nitrophenol onto a novel Fe3O4-κ-carrageenan/MIL-125(Ti) composite: Process optimization, isotherms, kinetics, and mechanism. Environ. Sci. Pollut. Res. 2023, 30, 49301–49313. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Rashwan, A.K.; Osman, A.I.; El-Monaem, E.M.A.; Elgarahy, A.M.; Eltaweil, A.S.; Omar, M.; Li, Y.; Mehanni, A.-H.E.; Chen, W.; et al. Synthesis and potential applications of cyclodextrin-based metal–organic frameworks: A review. Environ. Chem. Lett. 2023, 21, 447–477. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Zhang, C.; Li, L.; Jiang, J. Iron terephthalate metal–organic framework: Revealing the effective activation of hydrogen peroxide for the degradation of organic dye under visible light irradiation. Appl. Catal. B Environ. 2014, 148–149, 191–200. [Google Scholar] [CrossRef]
- Lv, H.; Zhao, H.; Cao, T.; Qian, L.; Wang, Y.; Zhao, G. Efficient degradation of high concentration azo-dye wastewater by heterogeneous Fenton process with iron-based metal-organic framework. J. Mol. Catal. A Chem. 2015, 400, 81–89. [Google Scholar] [CrossRef]
- Akgün, D.; Dükkancı, M. g-C3N4 supported Ag/AgCl@MIL-88A MOF based triple composites for highly efficient diuron photodegradation under visible LED light irradiation. J. Water Process Eng. 2023, 51, 103469. [Google Scholar] [CrossRef]
- Ahmad, M.; Quan, X.; Chen, S.; Yu, H. Tuning Lewis acidity of MIL-88B-Fe with mix-valence coordinatively unsaturated iron centers on ultrathin Ti3C2 nanosheets for efficient photo-Fenton reaction. Appl. Catal. B Environ. 2020, 264, 118534. [Google Scholar] [CrossRef]
- Xu, H.; Shan, C.; Wu, X.; Sun, M.; Huang, B.; Tang, Y.; Yan, C.-H. Fabrication of layered double hydroxide microcapsules mediated by cerium doping in metal–organic frameworks for boosting water splitting. Energy Environ. Sci. 2020, 13, 2949–2956. [Google Scholar] [CrossRef]
- Yu, D.; Wang, L.; Yang, T.; Yang, G.; Wang, D.; Ni, H.; Wu, M. Tuning Lewis acidity of iron-based metal-organic frameworks for enhanced catalytic ozonation. Chem. Eng. J. 2021, 404, 127075. [Google Scholar] [CrossRef]
- Ge, L.; Peng, Z.; Wang, W.; Tan, F.; Wang, X.; Su, B.; Qiao, X.; Wong, P.K. g-C3N4/MgO nanosheets: Light-independent, metal-poisoning-free catalysts for the activation of hydrogen peroxide to degrade organics. J. Mater. Chem. A 2018, 6, 16421–16429. [Google Scholar] [CrossRef]
- Su, S.; Xing, Z.; Zhang, S.; Du, M.; Wang, Y.; Li, Z.; Chen, P.; Zhu, Q.; Zhou, W. Ultrathin mesoporous g-C3N4/NH2-MIL-101(Fe) octahedron heterojunctions as efficient photo-Fenton-like system for enhanced photo-thermal effect and promoted visible-light-driven photocatalytic performance. Appl. Surf. Sci. 2021, 537, 147890. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, Q.; Xie, Z.; Hu, C.; Lyu, L. Enhanced Fenton-like process via interfacial electron donating of pollutants over in situ Cobalt-doped graphitic carbon nitride. J. Colloid Interface Sci. 2022, 608, 673–682. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Iqbal, W.; Shi, M.; Yang, L.; Chang, N.; Qin, C. Morphology-effects of four different dimensional graphitic carbon nitrides on photocatalytic performance of dye degradation, water oxidation and splitting. J. Phys. Chem. Solids 2023, 173, 111109. [Google Scholar] [CrossRef]
- Gao, M.; Li, B.; Liu, J.; Hu, Y.; Cheng, H. Adsorption behavior and mechanism of p-arsanilic acid on a Fe-based metal–organic framework. J. Colloid Interface Sci. 2023, 629, 616–627. [Google Scholar] [CrossRef]
- Li, N.; Du, H.; Tan, M.; Yang, L.; Xue, B.; Zheng, S.; Wang, Q. Construction of Z-scheme CuBi2O4/MIL-88A(Fe) heterojunctions with enhanced LED light driven photocatalytic Cr(VI) reduction and antibacterial performance. Appl. Surf. Sci. 2023, 614, 156249. [Google Scholar] [CrossRef]
- Yang, B.; Dai, J.; Zhao, Y.; Wang, Z.; Wu, J.; Ji, C.; Zhang, Y.; Pu, X. Synergy effect between tetracycline and Cr(VI) on combined pollution systems driving biochar-templated Fe3O4@SiO2/TiO2/g-C3N4 composites for enhanced removal of pollutants. Biochar 2023, 5, 1. [Google Scholar] [CrossRef]
- Ke, Y.; You, Q.; Ai, J.; Yang, X.; Shang, Q.; Liu, Y.; Wang, D.; Liao, G. Improved performance of visible-light photocatalytic H2-production and Cr(VI) reduction by waste pigeon guano doped g-C3N4 nanosheets. J. Mater. Sci. Technol. 2023, 152, 37–49. [Google Scholar] [CrossRef]
- Li, M.; Li, C.; Chunrui, Z.; Li, T.; Jiang, J.; Han, Z.; Zhang, C.; Sun, H.; Dong, S. Citric acid-modified MIL-88A(Fe) for enhanced photo-Fenton oxidation in water decontamination. Sep. Purif. Technol. 2023, 308, 122945. [Google Scholar] [CrossRef]
- Yao, Y.; Zheng, H.; Tao, Z.; Wang, Y.; Ma, Z.; Qiu, Y.; Wang, S. Highly efficient oxidation of aqueous contaminants by mesoporous graphitic carbon nitride supported copper phosphide with dual active sites. Appl. Surf. Sci. 2023, 613, 155902. [Google Scholar] [CrossRef]
- Tang, C.; Ma, S.-Q.; Yi, X.-H.; Wang, C.-C.; Wang, P. MIL-88A(Fe)/TCNQ composites for boosted photo-Fenton sulfamethoxazole degradation under LED visible light. Mater. Res. Bull. 2023, 160, 112138. [Google Scholar] [CrossRef]
- Chen, J.; Qin, C.; Mou, Y.; Cao, Y.; Chen, H.; Yuan, X.; Wang, H. Linker regulation of iron-based MOFs for highly effective Fenton-like degradation of refractory organic contaminants. Chem. Eng. J. 2023, 459, 141588. [Google Scholar] [CrossRef]
- Gogoi, A.; Navgire, M.; Sarma, K.C.; Gogoi, P. Highly efficient heterogeneous Fenton activities of magnetic β-cyclodextrin (Fe) framework for Eriochrome black T degradation. Mater. Chem. Phys. 2019, 231, 233–243. [Google Scholar] [CrossRef]
- Seo, J.; Gowda, A.; Babu, S. Almost Complete Removal of Ceria Particles Down to 10 nm Size from Silicon Dioxide Surfaces. ECS J. Solid State Sci. Technol. 2018, 7, P243–P252. [Google Scholar] [CrossRef]
- Vazirov, R.A.; Sokovnin, S.Y.; Ilves, V.G.; Bazhukova, I.N.; Pizurova, N.; Kuznetsov, M.V. Physicochemical characterization and antioxidant properties of cerium oxide nanoparticles. J. Phys. Conf. Ser. 2018, 1115, 032094. [Google Scholar] [CrossRef]
- El-Monaem, E.M.A.; Ayoup, M.S.; Omer, A.M.; Hammad, E.N.; Eltaweil, A.S. Sandwich-like construction of a new aminated chitosan Schiff base for efficient removal of Congo red. Appl. Water Sci. 2023, 13, 67. [Google Scholar] [CrossRef]
- Li, L.; Yang, S.; Wang, Y.; Hui, S.; Xiao, T.; Kong, J.; Zhao, X. Nitrogen-doped carbon nanosheets for efficient degradation of bisphenol A by H2O2 activation at neutral pH values. Sep. Purif. Technol. 2023, 306, 122687. [Google Scholar] [CrossRef]
- Du, Z.; Zhou, C.; Chen, D.; Li, W.; Wang, H.; Wu, H.; Zhang, Z.; Yang, H. Coupling of gaseous toluene adsorption and heterogeneous oxidative degradation by iron-based porous polymeric resin LXQ-10. Fuel 2023, 337, 127130. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, M.; Chen, T.; Zhang, G.; Xu, H.; Sun, H.; Zhang, L. Facile fabrication of rGO/PPy/nZVI catalytic microreactor for ultrafast removal of p-nitrophenol from water. Appl. Catal. B Environ. 2023, 324, 122270. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Bakr, S.S.; El-Monaem, E.M.A.; El-Subruiti, G.M. Magnetic hierarchical flower-like Fe3O4@ZIF-67/CuNiMn-LDH catalyst with enhanced redox cycle for Fenton-like degradation of Congo red: Optimization and mechanism. Environ. Sci. Pollut. Res. 2023, 30, 75332–75348. [Google Scholar] [CrossRef]
- Ashrafi, M.; Farhadi, S. Polyoxometalate supported on a magnetic Fe3O4/MIL-88A rod-like nanocomposite as an adsorbent for the removal of ciprofloxacin, tetracycline and cationic organic dyes from aqueous solutions. RSC Adv. 2023, 13, 6356–6367. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Wang, Z.; Hu, C.; Ma, D.; Chen, W.; Ao, T. Effective and structure-controlled adsorption of tetracycline hydrochloride from aqueous solution by using Fe-based metal-organic frameworks. Appl. Surf. Sci. 2021, 542, 148662. [Google Scholar] [CrossRef]
- Su, L.; Wang, P.; Li, M.; Zhao, Z.; Li, Y.; Zhan, S. Synergistic enhancement of photocatalytic molecular oxygen activation by nitrogen defect and interfacial photoelectron transfer over Z-scheme α-Fe2O3/g-C3N4 heterojunction. Appl. Catal. B Environ. 2023, 335, 122890. [Google Scholar] [CrossRef]
- Ghorai, K.; Panda, A.; Bhattacharjee, M.; Mandal, D.; Hossain, A.; Bera, P.; Seikh, M.M.; Gayen, A. Facile synthesis of CuCr2O4/CeO2 nanocomposite: A new Fenton like catalyst with domestic LED light assisted improved photocatalytic activity for the degradation of RhB, MB and MO dyes. Appl. Surf. Sci. 2021, 536, 147604. [Google Scholar] [CrossRef]
- Balu, K.; Chicardi, E.; Sepúlveda, R.; Durai, M.; Ishaque, F.; Chauhan, D.; Ahn, Y.-H. BiOX (X = I or Cl?) modified Na-K2Ti6O13 nanostructured materials for efficient degradation of Tetracycline, Acid Black 1 dye and microbial disinfection in wastewater under Blue LED. Sep. Purif. Technol. 2023, 309, 122998. [Google Scholar] [CrossRef]
- Beena, J.; Joji, L.R. Analysis of the Essential Oil from the Leaves of Wrightia tinctoria R. Br. from South India. Int. J. Ayurveda Pharma Res. 2015, 2, 47–52. [Google Scholar]
- Limam, I.; Guenne, A.; Driss, M.; Mazeas, L. Simultaneous determination of phenol, methylphenols, chlorophenols and bisphenol-A by headspace solid-phase microextraction-gas chromatography-mass spectrometry in water samples and industrial effluents. Int. J. Environ. Anal. Chem. 2010, 90, 230–244. [Google Scholar] [CrossRef]
- Wang, C.; Sun, R.; Huang, R.; Wang, H. Superior fenton-like degradation of tetracycline by iron loaded graphitic carbon derived from microplastics: Synthesis, catalytic performance, and mechanism. Sep. Purif. Technol. 2021, 270, 118773. [Google Scholar] [CrossRef]
- Li, X.; Cui, K.; Guo, Z.; Yang, T.; Cao, Y.; Xiang, Y.; Chen, H.; Xi, M. Heterogeneous Fenton-like degradation of tetracyclines using porous magnetic chitosan microspheres as an efficient catalyst compared with two preparation methods. Chem. Eng. J. 2020, 379, 122324. [Google Scholar] [CrossRef]
- Zhang, A.; Shan, F.; Zhang, Z.; Wang, J.; Zhang, T.; Liu, M. Efficient decontamination of tetracycline via fenton-like process mediated by chitosan-based Fe-MOFs under wide pH range. Sep. Purif. Technol. 2024, 344, 127212. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, W.; Zhang, X.; Wang, Y.; Li, T.; Wang, H. Mechanochemical synthesis of sulfidated microscale zerovalent iron/graphene composite for enhanced degradation of tetracycline. Sep. Purif. Technol. 2024, 343, 126936. [Google Scholar] [CrossRef]
- Zhao, Y.; Gu, S.; Li, L.; Wang, M. From waste to catalyst: Growth mechanisms of ZSM-5 zeolite from coal fly ash & rice husk ash and its performance as catalyst for tetracycline degradation in fenton-like oxidation. Environ. Pollut. 2024, 345, 123509. [Google Scholar] [PubMed]
- Nie, M.; Li, Y.; He, J.; Xie, C.; Wu, Z.; Sun, B.; Zhang, K.; Kong, L.; Liu, J. Degradation of tetracycline in water using Fe3O4 nanospheres as Fenton-like catalysts: Kinetics, mechanisms and pathways. New J. Chem. 2020, 44, 2847–2857. [Google Scholar] [CrossRef]
- Gu, Y.-Y.; Wu, Z.; Shen, Y.; Lu, C.; Lu, L.; Bian, Z.; Zhang, X.; Zhao, C.; Fu, R.; Li, H. Efficient Fenton-like degradation of tetracycline by stalactite-like CuCo-LDO/CN catalysts: The overlooked contribution of dissolved oxygen. Chemosphere 2023, 338, 139540. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Nie, K.; Han, G.; Hua, X.; Zhang, K.; Dang, S.; Yao, B.; Meng, J. Iron ore tailings as efficient Fenton-like catalysts for degradation of tetracycline. Mater. Today Commun. 2023, 37, 107598. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, B. Fabrication of magnetic MnFe2O4@HL composites with an in situ Fenton-like reaction for enhancing tetracycline degradation. J. Colloid Interface Sci. 2024, 658, 997–1008. [Google Scholar] [CrossRef]
- Deng, S.; Guo, Z.; Chen, Y.-H.; Cui, K.-P.; Ding, Z.-G.; Wang, B.; Weerasooriya, R.; Chen, X. Fabrication of Cu0-composited CuFe2O4 magnetic nanoparticles on diatomite support for efficient degradation of tetracycline hydrochloride by a Fenton-like system. J. Environ. Chem. Eng. 2023, 11, 110045. [Google Scholar] [CrossRef]



| Kinetic Model | TC Concentrations (mg/L) | |||
|---|---|---|---|---|
| 50 | 100 | 200 | 300 | |
| First order | ||||
| k1 | 0.0137 | 0.0052 | 0.0049 | 0.0036 |
| R2 | 0.980 | 0.988 | 0.988 | 0.984 |
| Second order | ||||
| k2 | 0.1511 | 0.0157 | 0.0128 | 0.0061 |
| R2 | 0.828 | 0.973 | 0.979 | 0.962 |
| Catalysts | Parameters | Degradation % | Ref. |
|---|---|---|---|
| Fe-MPC | Time = 10 min, pH = 4.3, [H2O2] = 1 mM, [TC] = 40 mg/L, catalyst = 0.02 g. | 83% | [57] |
| Fe3O4-Cs | Time = 120 min, pH = 3, [H2O2] = 10 mM, [TC] = 48.09 mg/L, catalyst = 0.5 g. | 96% | [58] |
| MIL-101(Fe)@CS | Time = 140 min, pH = 5, [H2O2] = 0.09 mol/L, [TC] = 100 mg/L, catalyst = 2 g. | 87.36% | [59] |
| S-mZVI/rGO | Time = 60 min, pH = 5, [H2O2] = 2 mM, [TC] = 20 mg/L, catalyst = 0.2 g. | 97.2% | [60] |
| Fe/Cu-ZSM-5 | Time = 10 min, pH = 7, [H2O2] = 1.0 g/L, [TC] = 30 mg/L, catalyst = 0.9 g. | 99.02% | [61] |
| Fe3O4-S | Time = 110 min, pH = 7, [H2O2] = 50 mM, [TC] = 25 mg/L, catalyst = 0.5 g. | 82.6% | [62] |
| CuCo-LDO/CN | Time = 40 min, pH = 6.9, [H2O2] = 15 mM, [TC] = 20 mg/L, catalyst = 0.08 g. | 93.2% | [63] |
| IOT | Time = 360 min, pH = 3.6, [H2O2] = 1700 mg/L, [TC] = 200 mg/L, catalyst = 0.3 g. | 95% | [64] |
| MnFe2O4@HL | Time = 120 min, pH = 3, [H2O2] = 1 mM, [TC] = 0.1 mM, catalyst = 0.3 g. | 92.3% | [65] |
| Cu/CuFe2O4/DE | Time = 90 min, pH = 5, [H2O2] = 4.0 mM, [TC] = 50 mg/L, catalyst = 0.2 g. | 95.5% | [66] |
| (Ce0.33Fe) MIL-88A/10%g-C3N4 | Time = 100 min, pH = 7, [H2O2] = 100 mg/L, [TC] = 50 mg/L, catalyst = 0.01 g. | 92.44% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eltaweil, A.S.; Galal, A.M.; Abd El-Monaem, E.M.; Al Harby, N.; Batouti, M.E. Enhanced Fenton Degradation of Tetracycline over Cerium-Doped MIL88-A/g-C3N4: Catalytic Performance and Mechanism. Nanomaterials 2024, 14, 1282. https://doi.org/10.3390/nano14151282
Eltaweil AS, Galal AM, Abd El-Monaem EM, Al Harby N, Batouti ME. Enhanced Fenton Degradation of Tetracycline over Cerium-Doped MIL88-A/g-C3N4: Catalytic Performance and Mechanism. Nanomaterials. 2024; 14(15):1282. https://doi.org/10.3390/nano14151282
Chicago/Turabian StyleEltaweil, Abdelazeem S., Amira M. Galal, Eman M. Abd El-Monaem, Nouf Al Harby, and Mervette El Batouti. 2024. "Enhanced Fenton Degradation of Tetracycline over Cerium-Doped MIL88-A/g-C3N4: Catalytic Performance and Mechanism" Nanomaterials 14, no. 15: 1282. https://doi.org/10.3390/nano14151282





