The Preparation of a Polyamidoxime–Phosphorylated Cellulose Nanofibrils Composite Aerogel for the Selective Extraction of Uranium from Seawater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
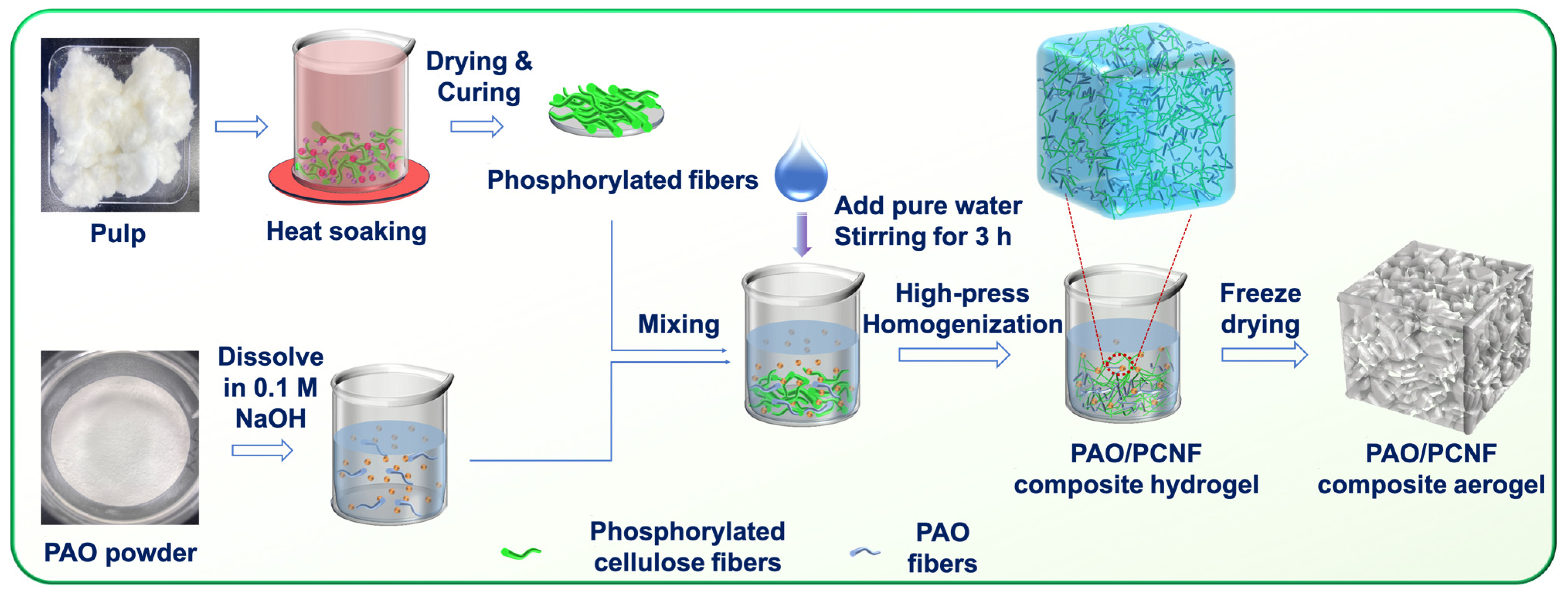
2.2. Synthesis of Composite Materials
2.2.1. Preparation of PCNFs
2.2.2. Synthesis of PAO
2.2.3. Preparation of Aerogel Adsorbents
2.3. Characterization
2.4. Determination of Uranium Adsorption
2.4.1. Preparation of Uranium-Spiked Simulated Seawater
2.4.2. Adsorption Kinetics of Uranium in Uranium-Spiked Pure Water
2.4.3. Equilibrium Adsorption Isotherm Test
2.4.4. Selective Adsorption from Uranium-Spiked Simulated Seawater at 100× Natural Seawater
2.4.5. Desorption Test
3. Results and Discussion
3.1. Preparation and Characterization of Adsorbents
3.1.1. Synthesis and Characterization of PAO
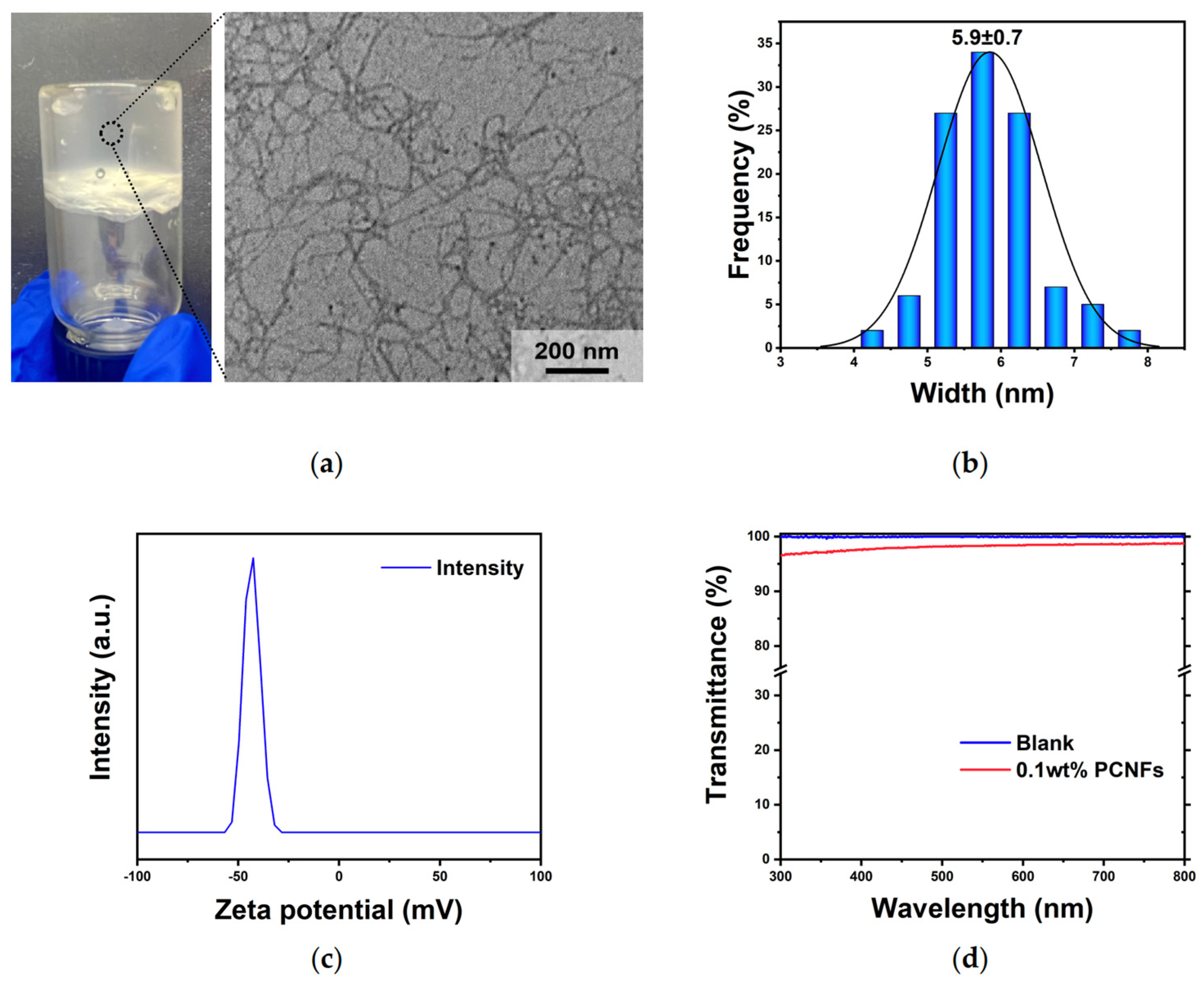
3.1.2. Preparation and Characterization of PCNFs
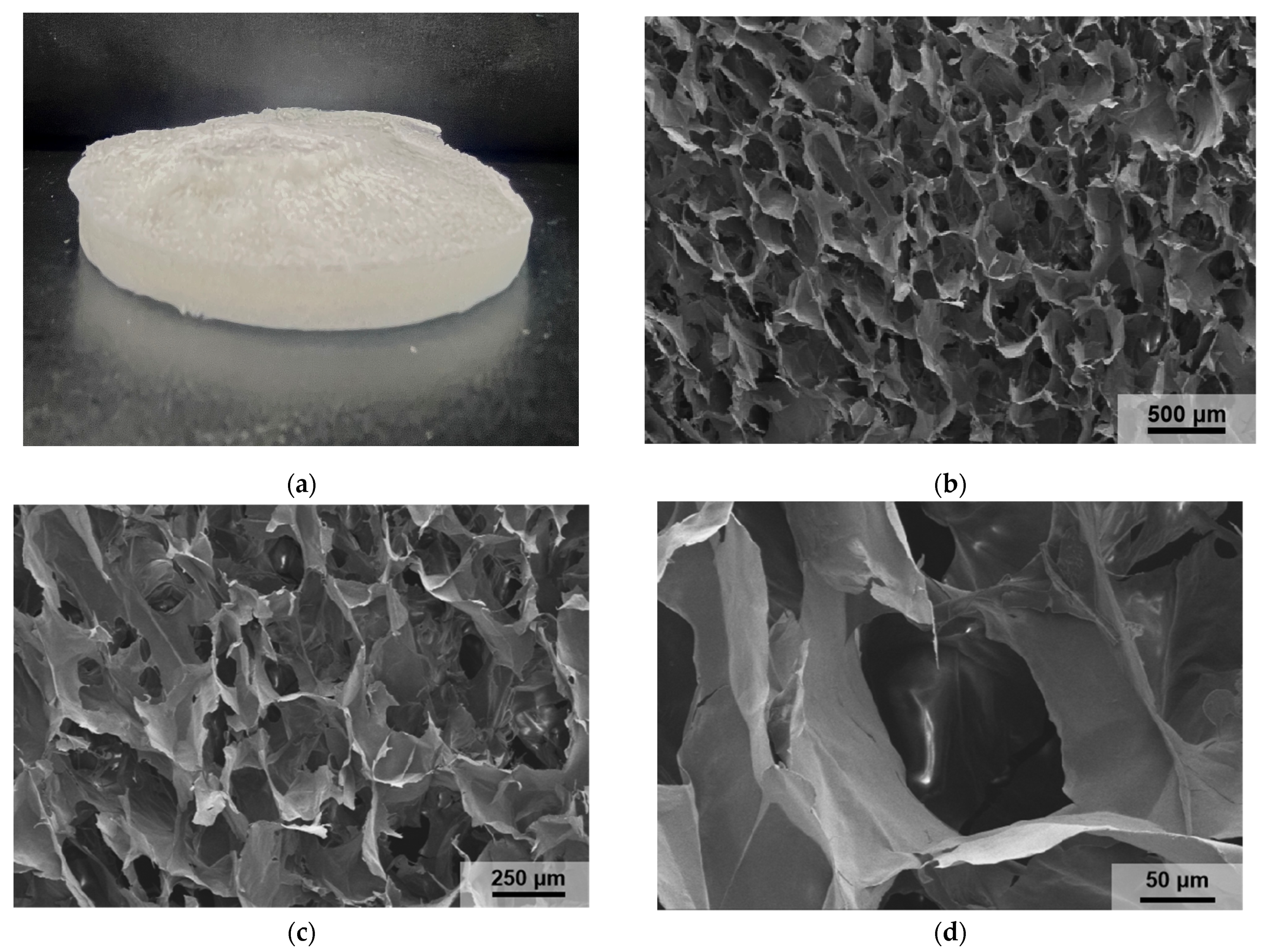
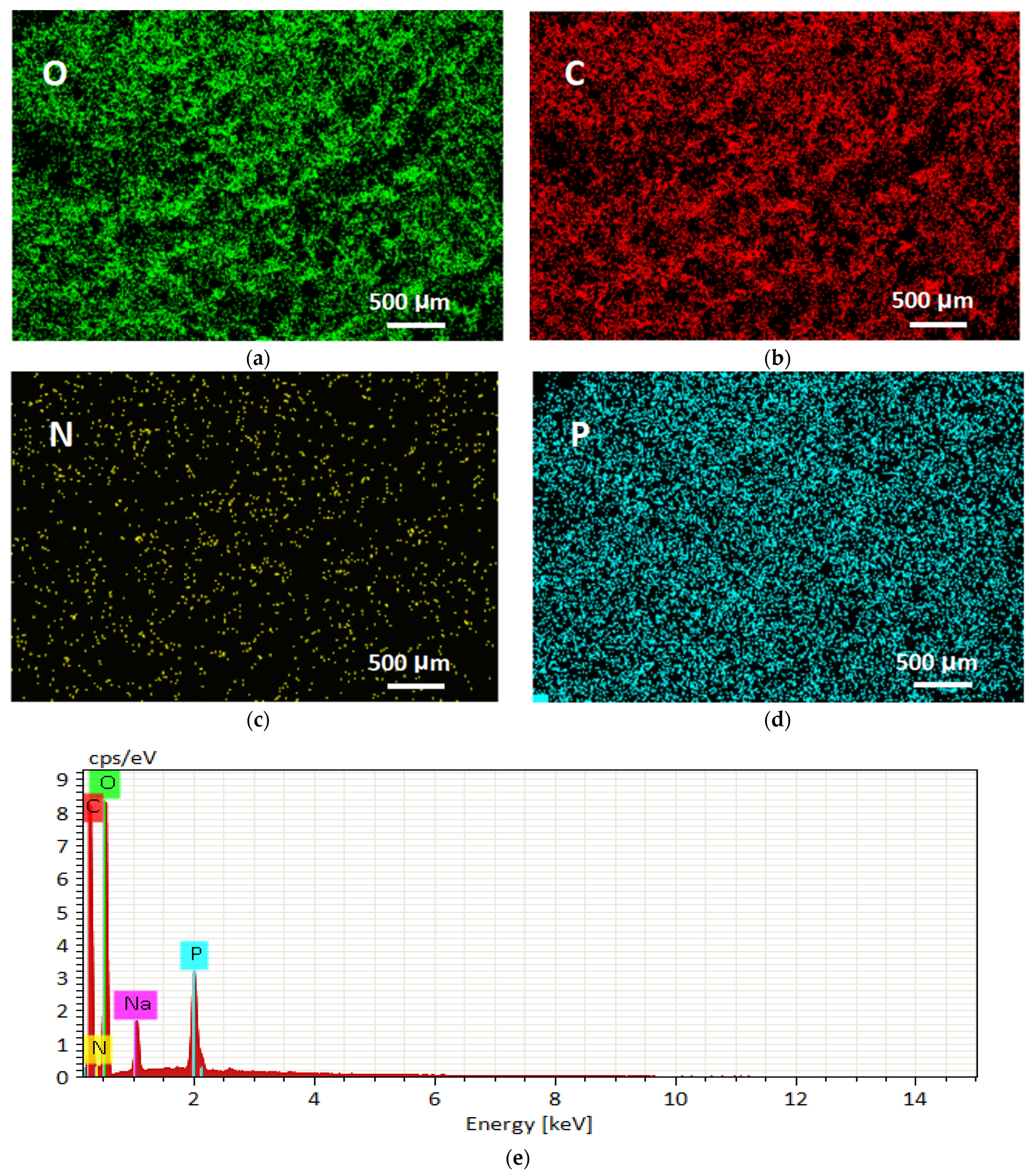
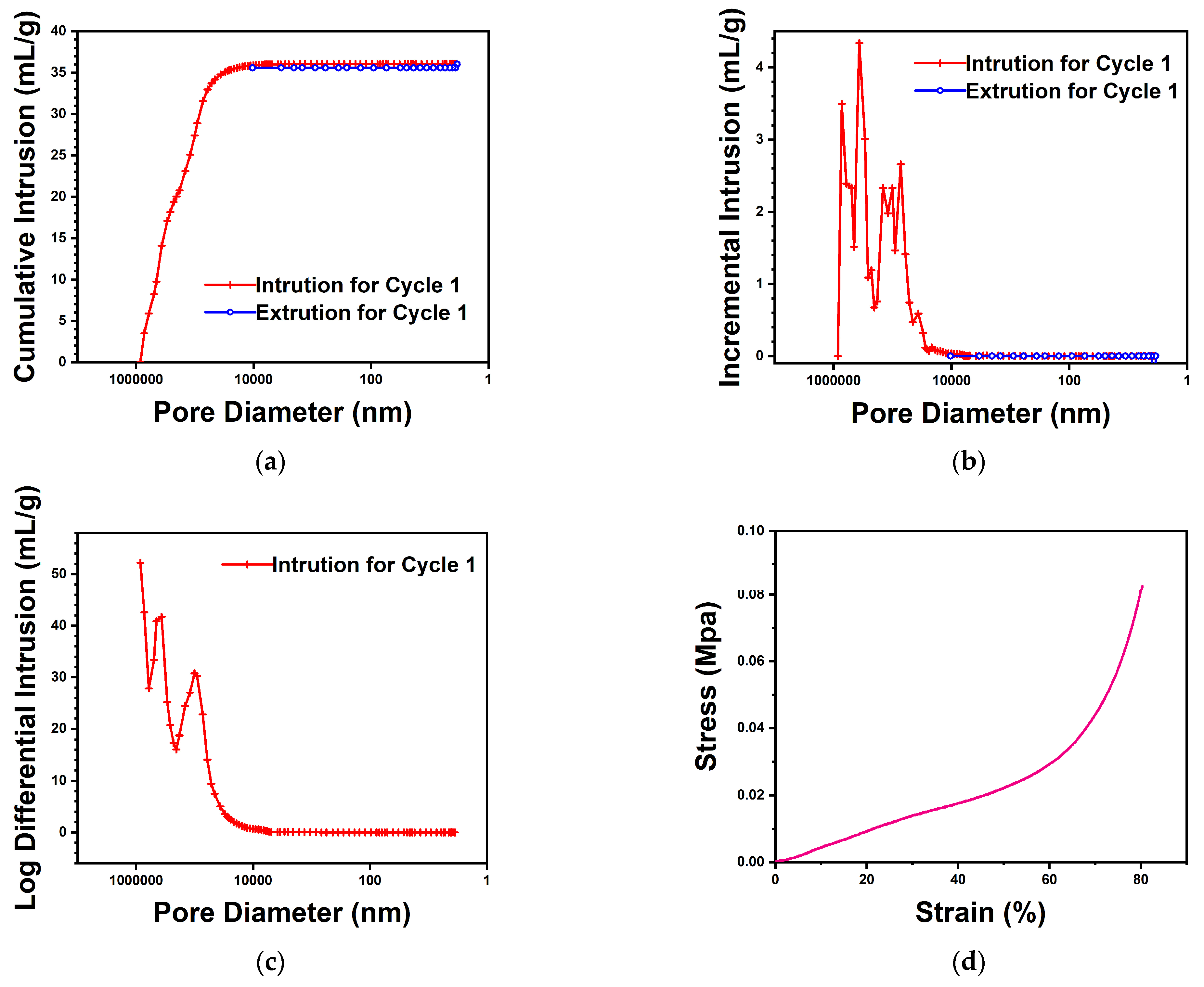
3.1.3. Characterization of PAO-PCNF Composite Aerogels
3.2. The Absorbance of Uranium-Spiked Seawater
3.3. The Effect of Composite Ratio on Uranium Adsorption
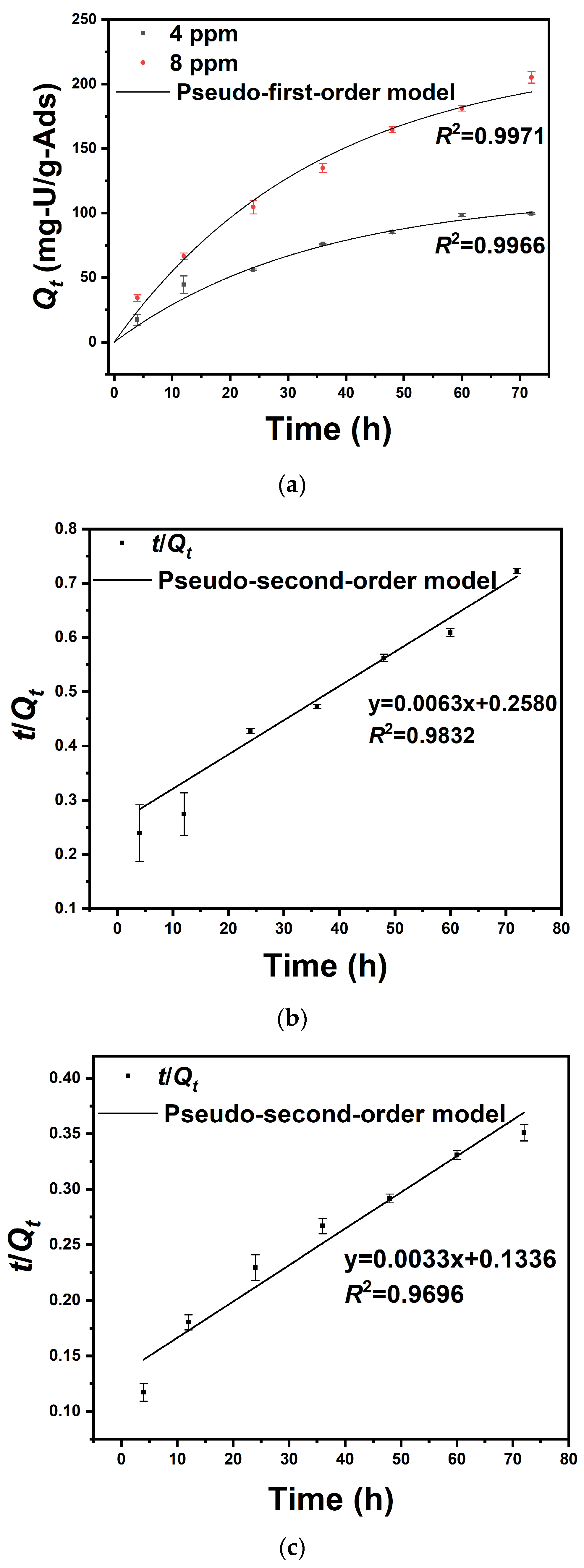
3.4. Uranium Adsorption Kinetics in Uranium-Spiked Pure Water
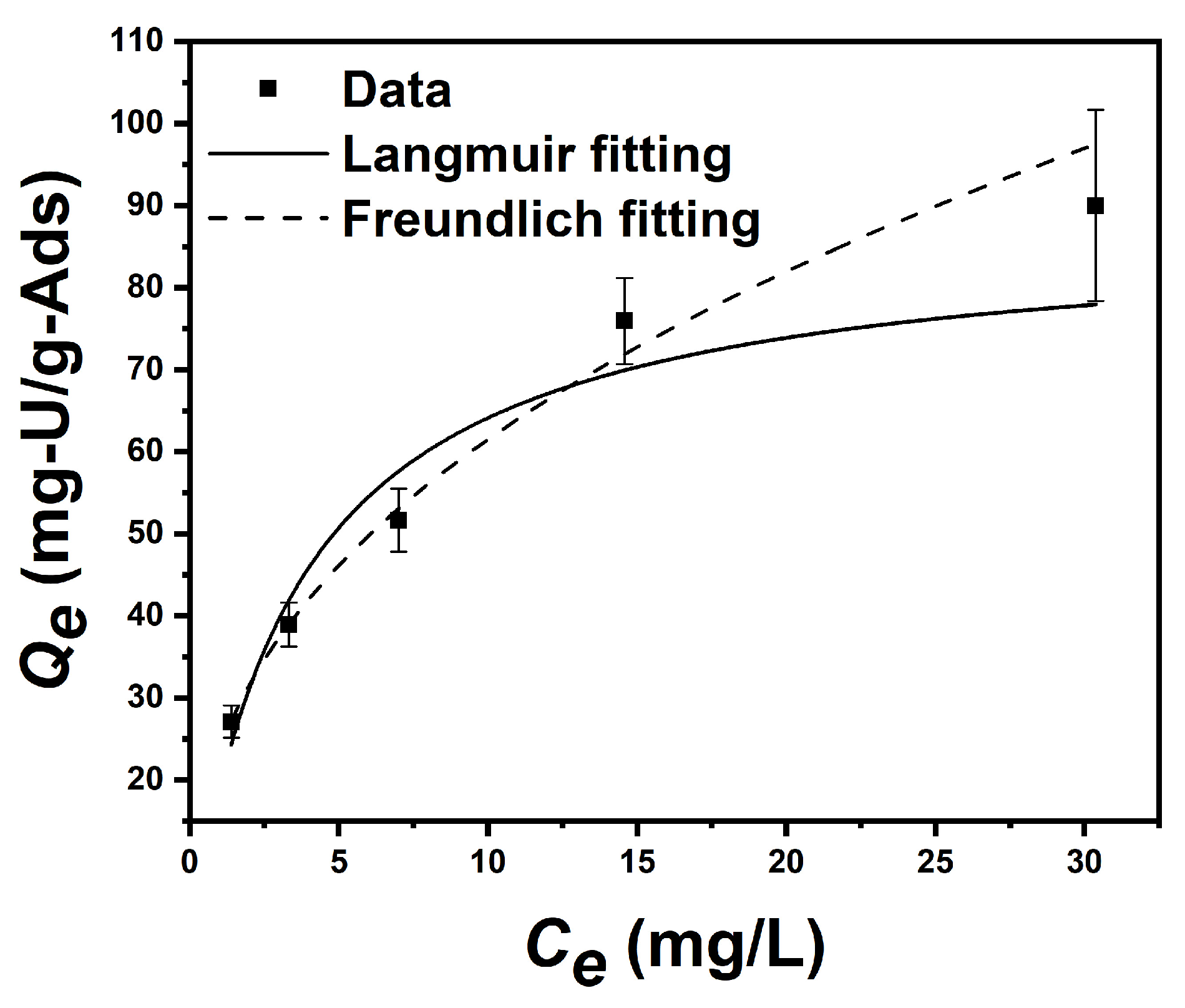
3.5. Equilibrium Adsorption Isotherm Test in Uranium-Spiked Simulated Seawater
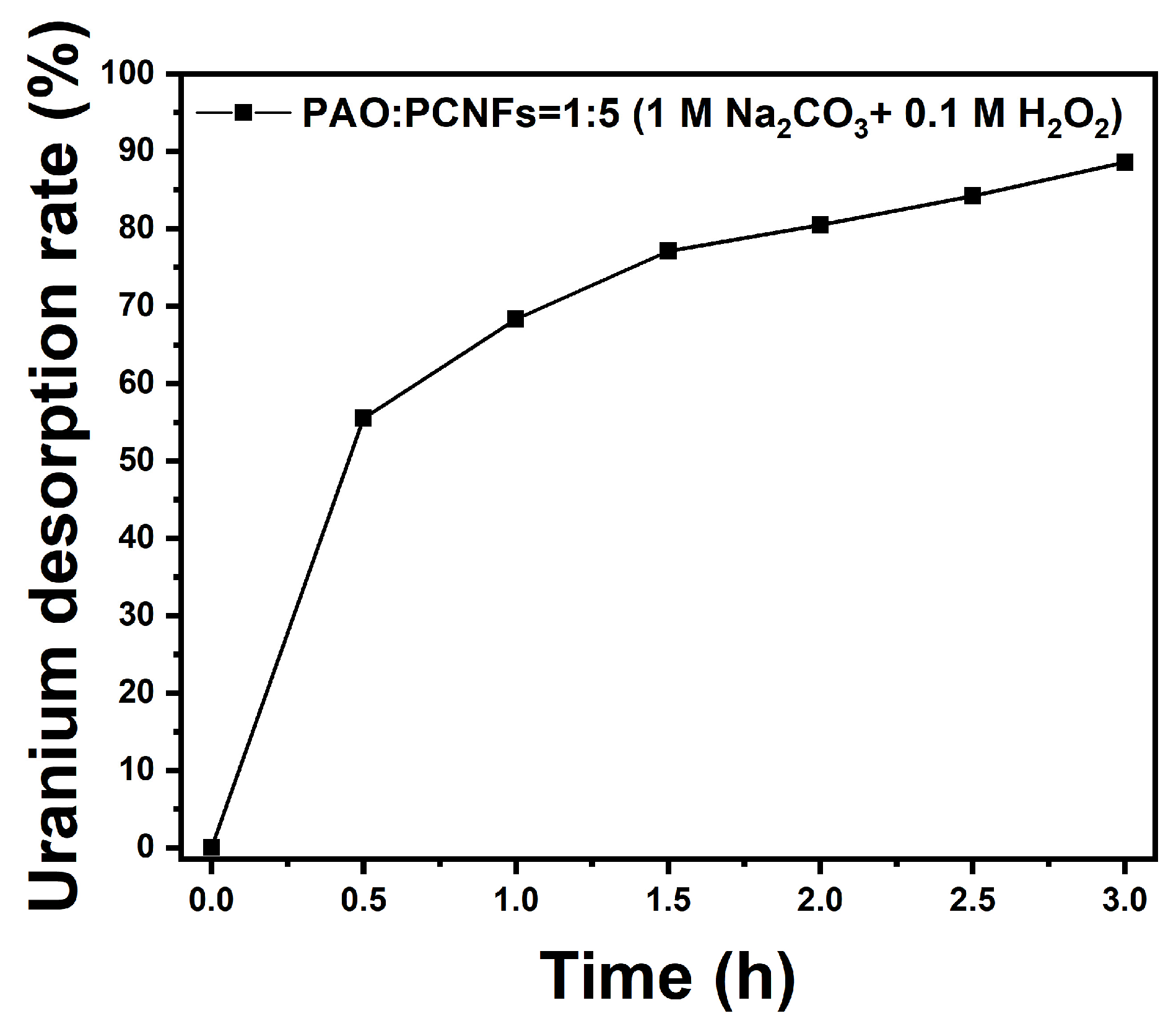
3.6. Desorption Test in Uranium-Spiked Simulated Seawater
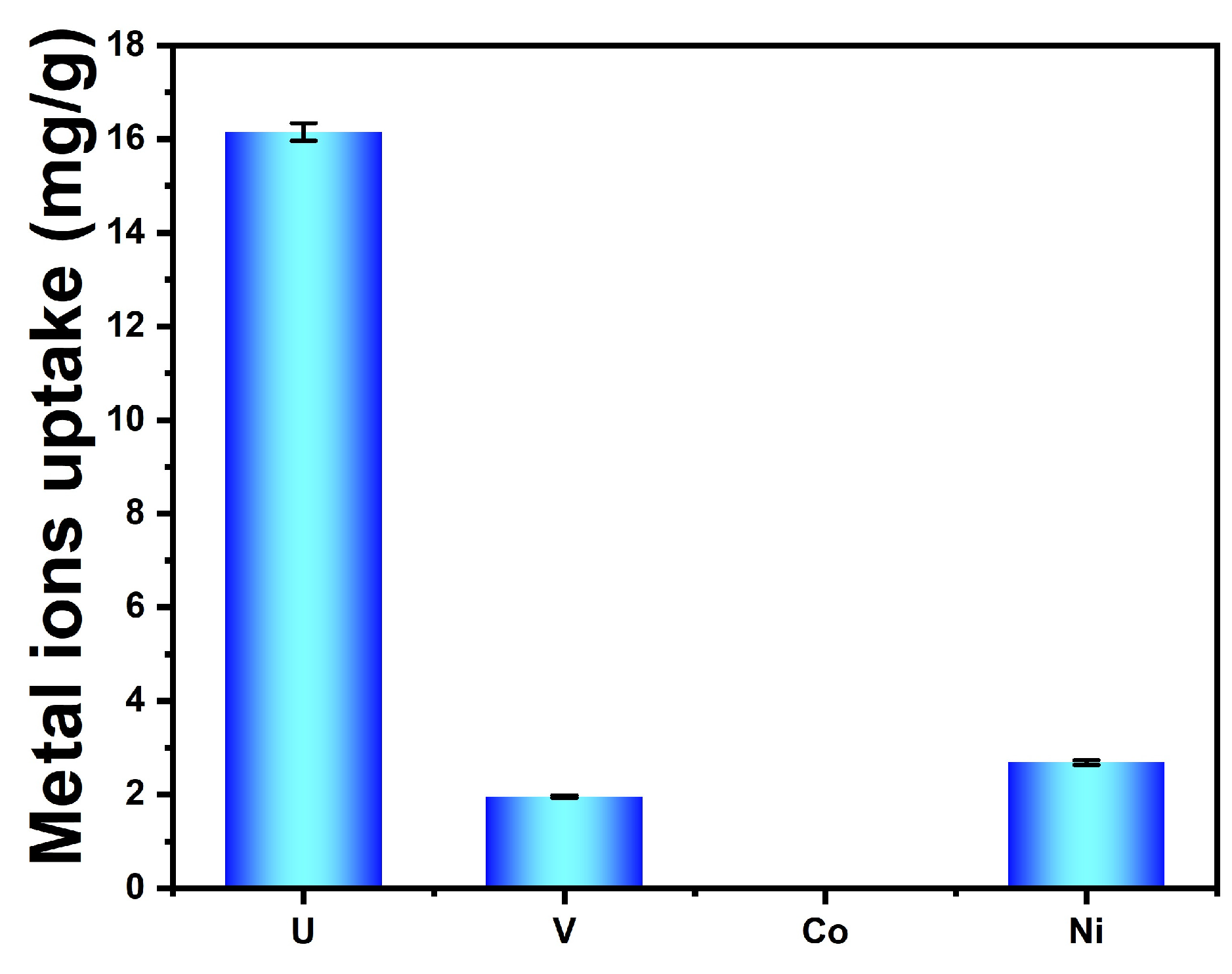
3.7. Selective Adsorption from Uranium-Spiked Simulated Seawater at 100× Natural Seawater
3.8. The Mechanism of Uranium Adsorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mei, D.C.; Liu, L.J.; Yan, B. Adsorption of uranium (VI) by metal-organic frameworks and covalent-organic frameworks from water. Coord. Chem. Rev. 2023, 475, 214917. [Google Scholar] [CrossRef]
- Li, Z.; Niu, Y.; Su, Y.; Song, Y.; Wang, F.; Gou, Y.; Wang, H.; Chen, S. Latest Research Progress of Uranium Extraction From Seawater. J. Nucl. Radiochem. 2022, 44, 233–245. [Google Scholar]
- Liu, X.L.; Xie, Y.H.; Hao, M.J.; Chen, Z.S.; Yang, H.; Waterhouse, G.I.N.; Ma, S.Q.; Wang, X.K. Highly Efficient Electrocatalytic Uranium Extraction from Seawater over an Amidoxime-Functionalized In-N-C Catalyst. Adv. Sci. 2022, 9, e2201735. [Google Scholar] [CrossRef] [PubMed]
- Naik, M.U.D. Adsorbents for the Uranium Capture from Seawater for a Clean Energy Source and Environmental Safety: A Review. ACS Omega 2024, 9, 12380–12402. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Qu, C.; Meng, X.; Wang, Z.; Mo, H.; Jiang, C.; Sun, R.; Wang, J.; Gong, T.; Chen, S. Preparation of porous amidoximated nanofibers with antibacterial properties, and experiments on uranium extraction from seawater. J. Radioanal. Nucl. Chem. 2023, 332, 669–682. [Google Scholar] [CrossRef]
- Gholap, H.; Warule, S.; Sangshetti, J.; Kulkarni, G.; Banpurkar, A.; Satpute, S.; Patil, R. Hierarchical nanostructures of Au@ZnO: Antibacterial and antibiofilm agent. Appl. Microbiol. Biotechnol. 2016, 100, 5849–5858. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Goh, K.; Goto, A.; Zhao, Y.L.; Wang, R. Uranium and lithium extraction from seawater: Challenges and opportunities for a sustainable energy future. J. Mater. Chem. A 2023, 11, 22551–22589. [Google Scholar] [CrossRef]
- Zou, W.; Zhao, L.; Han, R. Removal of Uranium (VI) by Fixed Bed Ion-exchange Column Using Natural Zeolite Coated with Manganese Oxide. Chin. J. Chem. Eng. 2009, 17, 585–593. [Google Scholar] [CrossRef]
- Zhu, Z.; Pranolo, Y.; Cheng, C.Y. Uranium recovery from strong acidic solutions by solvent extraction with Cyanex 923 and a modifier. Miner. Eng. 2016, 89, 77–83. [Google Scholar] [CrossRef]
- Tan, L.; Liu, Q.; Jing, X.; Liu, J.; Song, D.; Hu, S.; Liu, L.; Wang, J. Removal of uranium(VI) ions from aqueous solution by magnetic cobalt ferrite/multiwalled carbon nanotubes composites. Chem. Eng. J. 2015, 273, 307–315. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, R.; Chen, M.; Liu, Y.; Xie, Z.; Tang, S.; Yuan, Y.; Wang, N. Vertically Aligned Polyamidoxime/Graphene Oxide Hybrid Sheets’ Membrane for Ultrafast and Selective Extraction of Uranium from Seawater. Adv. Funct. Mater. 2021, 32, 2111049. [Google Scholar] [CrossRef]
- Rånby, B.G.; Banderet, A.; Sillén, L.G. Aqueous Colloidal Solutions of Cellulose Micelles. Acta Chem. Scand. 1949, 3, 649–650. [Google Scholar] [CrossRef]
- Wang, R.; Yuan, C.; Tao, J. Modification of Cellulose Nanofibrils and Its Applicationin Flexible Electronics. Mater. Rev. 2019, 33, 2949–2957. [Google Scholar]
- Cherian, B.M.; Leão, A.L.; Souza, S.F.d.; Costa, L.M.M.; Olyveira, G.M.d.; Kottaisamy, M.; Nagarajan, E.R.; Thomas, S. Cellulose nanocomposites with nanofibres isolated from pineapple leaf fibers for medical applications. Carbohydr. Polym. 2011, 86, 1790–1798. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, W.B.; Sharma, S.; Tong, G.L.; Deng, Y.L. Synthesis of Cyclodextrin-functionalized Cellulose Nanofibril Aerogel as a Highly Effective Adsorbent for Phenol Pollutant Removal. Bioresources 2015, 10, 7555–7568. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, Y.Y.; Zhang, A.; Song, Y.X.; Xu, J.C.; Ni, Y.R.; Pu, A.L.; Yang, L.; Chi, F.T. High selectivity of oxime-modified ZIFs to uranium. J. Radioanal. Nucl. Chem. 2022, 331, 1237–1247. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.; Liu, L.; Zhang, X. Research progress of amidoxime functional materials for uranium adsorption. J. Funct. Mater. 2021, 52, 5050–5056+5075. [Google Scholar]
- Zhang, J.; Tian, B.; Li, J.; Li, Z.; Zhang, N.; Li, T.; Liu, Z.; Sun, Y.; Zhao, H. Research progress of amidoxime uranium adsorption materials. J. Radiat. Res. Radiat. Process. 2023, 41, 1–15. [Google Scholar]
- Wongjaikham, W.; Wongsawaeng, D.; Hosemann, P. Synthesis of amidoxime polymer gel to extract uranium compound from seawater by UV radiation curing. J. Nucl. Sci. Technol. 2019, 56, 541–552. [Google Scholar] [CrossRef]
- Cheng, G.; Zhang, A.R.; Zhao, Z.W.; Chai, Z.M.; Hu, B.W.; Han, B.; Ai, Y.J.; Wang, X.K. Extremely stable amidoxime functionalized covalent organic frameworks for uranium extraction from seawater with high efficiency and selectivity. Sci. Bull. 2021, 66, 1994–2001. [Google Scholar] [CrossRef]
- Zhao, L.; Yin, X.; Qin, Z. Adsorption Behavior of Amidoximated PAN for Uranium. Nucl. Phys. Rev. 2012, 29, 395–398. [Google Scholar]
- Wang, Y.; Lin, Z.; Liu, Q.; Zhu, J.; Liu, J.; Yu, J.; Chen, R.; Liu, P.; Wang, J. Simple one-step synthesis of woven amidoximated natural material bamboo strips for uranium extraction from seawater. Chem. Eng. J. 2021, 425, 131538. [Google Scholar] [CrossRef]
- Pu, Y.; Qiang, T.; Ren, L. Anti-biofouling bio-adsorbent with ultrahigh uranium extraction capacity: One uranium resource recycling solution. Desalination 2022, 531, 115721. [Google Scholar] [CrossRef]
- Wu, M.-B.; Ye, H.; Liu, S.-C.; Huang, Y.-Q.; Jin, J.-M.; Yao, J. Wooden composite separators with ultrahigh uranium/vanadium selectivity and antibacterial property for capturing uranium from seawater. Compos. Commun. 2022, 32, 101159. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, J.B.; Wang, X.L.; Sui, Y.Y.; Fan, W.T.; Zhang, H.X. Preparation of amidoxime modified imidazole copolymers and the efficient extraction of U(VI) from the alkaline aqueous solution. Sep. Purif. Technol. 2022, 280, 119982. [Google Scholar] [CrossRef]
- Gan, J.L.; Zhang, L.Y.; Wang, Q.L.; Xin, Q.; Xiong, Y.; Hu, E.M.; Lei, Z.W.; Wang, H.Q.; Wang, H.Q. Phosphorylation improved the competitive U/V adsorption on chitosan-based adsorbent containing amidoxime for rapid uranium extraction from seawater. Int. J. Biol. Macromol. 2023, 238, 124074. [Google Scholar] [CrossRef] [PubMed]
- Jackson, V.E.; Gutowski, K.E.; Dixon, D.A. Density functional theory study of the complexation of the uranyl dication with anionic phosphate ligands with and without water molecules. J. Phys. Chem. A 2013, 117, 8939–8957. [Google Scholar] [CrossRef] [PubMed]
- Montes, E.; Vázquez, H. Calculation of Energy Level Alignment and Interface Electronic Structure in Molecular Junctions beyond DFT. J. Phys. Chem. C 2021, 125, 25825–25831. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Nakano, T.; Komeiji, Y.; Yamashita, K.; Okiyama, Y.; Yoshikawa, H.; Yamataka, H. Fragment molecular orbital-based molecular dynamics (FMO-MD) method with MP2 gradient. Chem. Phys. Lett. 2011, 504, 95–99. [Google Scholar] [CrossRef]
- Yue, C.T.; Liu, R.J.; Yu, Y.H.; Wan, Q.Y.; Wang, H.; Liu, L.C.; Zhang, X. Synthesis of novel phosphate-based hypercrosslinked polymers for efficient uranium extraction from radioactive wastewater. J. Water Process Eng. 2023, 53, 103582. [Google Scholar] [CrossRef]
- Nomura, S.; Hara, R. The effect of organic substituents and structure of organophosphorus compounds on their extraction abilities for uranium. Anal. Chim. Acta 1961, 25, 212–218. [Google Scholar] [CrossRef]
- Bunus, F.; Dumitrescu, P. Uranium(VI) extraction from acid mixtures with organophosphorus esters. Hydrometallurgy 1986, 16, 167–175. [Google Scholar] [CrossRef]
- Chen, S.; Ren, N.; Cui, M.; Huang, R.; Qi, W.; He, Z.; Su, R. Heat Soaking Pretreatment for Greener Production of Phosphorylated Cellulose Nanofibrils with Higher Charge Density. ACS Sustain. Chem. Eng. 2022, 10, 8876–8884. [Google Scholar] [CrossRef]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.W.; Liu, T.; Zhang, X.B.; Zhang, R.Q.; Tang, S.; Yuan, Y.H.; Xie, Z.J.; Liu, Y.J.; Wang, H.; Fedorovich, K.V.; et al. Photoinduced Enhancement of Uranium Extraction from Seawater by MOF/Black Phosphorus Quantum Dots Heterojunction Anchored on Cellulose Nanofiber Aerogel. Adv. Funct. Mater. 2021, 31, 2100106. [Google Scholar] [CrossRef]
- Yuan, Y.H.; Feng, S.W.; Feng, L.J.; Yu, Q.H.; Liu, T.T.; Wang, N. A Bio-inspired Nano-pocket Spatial Structure for Targeting Uranyl Capture. Angew. Chem.-Int. Ed. 2020, 59, 4262–4268. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.Q.; Yuan, L.Y.; Zhu, L.; Liu, Z.R.; Chu, S.Q.; Zheng, L.R.; Zhang, J.; Chai, Z.F.; Shi, W.Q. Introduction of amino groups into acid-resistant MOFs for enhanced U(VI) sorption. J. Mater. Chem. A 2015, 3, 525–534. [Google Scholar] [CrossRef]
- Meng, J.; Lin, X.Y.; Li, H.N.; Zhang, Y.D.; Zhou, J.; Chen, Y.; Shang, R.; Luo, X.G. Adsorption capacity of kelp- like electrospun nanofibers immobilized with bayberry tannin for uranium(VI) extraction from seawater. Rsc Adv. 2019, 9, 8091–8103. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.S.; Xiao, H.Y.; Qian, Y.C.; Zhao, X.L.; Kong, X.Y.; Liu, P.; Xin, W.W.; Fu, L.; Jiang, L.; Wen, L.P. Bioinspired hierarchical porous membrane for efficient uranium extraction from seawater. Nat. Sustain. 2022, 5, 71–80. [Google Scholar] [CrossRef]
- Shao, D.; Li, Y.; Wang, X.; Hu, S.; Wen, J.; Xiong, J.; Asiri, A.M.; Marwani, H.M. Phosphate-Functionalized Polyethylene with High Adsorption of Uranium(VI). ACS Omega 2017, 2, 3267–3275. [Google Scholar] [CrossRef]
- Du, Z.; Ni, Y.; Peng, H.; Tang, M.; Li, Y.; Lv, L.; Li, X.; Chi, F. Adsorption of U(VI) by chitosan crosslinked PAO aerogel. J. Radioanal. Nucl. Chem. 2024, 333, 71–84. [Google Scholar] [CrossRef]
- Dai, Z.; Sun, Y.; Zhang, H.; Ding, D.; Li, L. Highly Efficient Removal of Uranium(VI) from Wastewater by Polyamidoxime/Polyethyleneimine Magnetic Graphene Oxide. J. Chem. Eng. Data 2019, 64, 5797–5805. [Google Scholar] [CrossRef]



| Reagent | Specification | Manufacturer |
|---|---|---|
| Polyacrylonitrile (PAN) | Mw = 150,000, 99%, AR | Macklin |
| Hydroxylamine hydrochloride (NH2OH·HCl) | 99%, AR | Macklin |
| N,N-Dimethylformamide (DMF) | ≥99.8%, ACS | Macklin |
| Arsenazo III | 95%, AR | Macklin |
| Sodium hydroxide (NaOH) | 99.5%, AR | Kermel |
| Sodium chloride (NaCl) | ≥99.5%, AR | Macklin |
| Sodium bicarbonate (NaHCO3) | ≥99.8%, AR | Aladdin |
| Uranium hexahydrate nitrate (UO2(NO3)2·6H2O) | AR | Macklin |
| Ammonium phosphate monobasic (NH4H2PO4) | 99%, AR | Meryer |
| Urea (99%) | AR | Meryer |
| Hydrogen peroxide (H2O2) | 30%, GR | DAMAO |
| Anhydrous sodium carbonate (Na2CO3) | ≥99.8%, GR | Feng Chuan |
| Hydrochloric acid (HCl) | 0.1 M | Tianjin Jinke Fine Chemical Research Institute |
| Nickel chloride titration solution (NiCl2) | 0.5 M | Aladdin |
| Sodium metavanadate (NaVO3) | 99.0%, AR | Macklin |
| Cobalt chloride (CoCl2∙6H2O) | 99.0%, AR | Feng Chuan |
| Concentrated hydrochloric acid (HCl) | AR | Feng Chuan |
| PAO/PCNFs | Mass of PAO (mg) | Mass of PCNFs (mg) | Volume of 0.1 M NaOH (mL) | Total Mass (kg) |
|---|---|---|---|---|
| 1:1 | 20 | 20 | 700 | 1.5 |
| 1:5 | 4 | 20 | 200 | 1.5 |
| 1:10 | 2 | 20 | 100 | 1.5 |
| 1:20 | 1 | 20 | 50 | 1.5 |
| Element | Atomicity | Normalized Mass (%) | Atom (%) |
|---|---|---|---|
| O | 8 | 47.03 | 42.68 |
| C | 6 | 39.45 | 47.70 |
| N | 7 | 4.75 | 4.93 |
| Na | 11 | 3.58 | 2.26 |
| P | 15 | 5.18 | 2.43 |
| Total | 100.00 | 100.00 | |
| Adsorbent | Initial Uranium Concentration (ppm) | Volume of Solution (L) | Adsorbent Dose (g) | pH | Qe (mg/g) | References |
|---|---|---|---|---|---|---|
| Kelp-like electrospun nanofibers immobilized with bayberry tannin | 80 | 0.05 | 0.02 | 5.5 | 170.1 | [38] |
| Bioinspired hierarchical porous membrane | 8 | 0.2 | 0.002 | 5.5 | 124.2 | [39] |
| Phosphate-functionalized polyethylene | 50 | 1 | 0.2 | 8.2 | 173.8 | [40] |
| Chitosan crosslinked PAO aerogel | 100 | 0.025 | 0.01 | 6 | 203.4 | [41] |
| PAO-PCNF composite aerogel | 8 | 0.5 | 0.01 | 7 | 204.3 | This work |
| Pseudo-First-Order Kinetic | Initial Uranium Concentration (ppm) | K1 (min−1) | Qe (mg/g) | R2 |
| 4 | 2.9 × 10−2 | 114.4 | 0.9966 | |
| 8 | 2.8 × 10−2 | 223.2 | 0.9971 | |
| Pseudo-second-order kinetic | Initial uranium concentration (ppm) | K2 (g mg−1 min−1) | Qe (mg/g) | R2 |
| 4 | 1.5 × 10−4 | 158.5 | 0.9832 | |
| 8 | 8.0 × 10−5 | 305.8 | 0.9696 |
| Langmuir | k3 (L/mg) | Qm (mg/g) | R2 |
| 0.278 | 87.14 | 0.9305 | |
| Freundlich | k4 (mg/g/(ppm)n) | 1/n | R2 |
| 23.681 | 0.414 | 0.9902 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Cui, M.; Su, R.; Huang, R. The Preparation of a Polyamidoxime–Phosphorylated Cellulose Nanofibrils Composite Aerogel for the Selective Extraction of Uranium from Seawater. Nanomaterials 2024, 14, 1297. https://doi.org/10.3390/nano14151297
Yang X, Cui M, Su R, Huang R. The Preparation of a Polyamidoxime–Phosphorylated Cellulose Nanofibrils Composite Aerogel for the Selective Extraction of Uranium from Seawater. Nanomaterials. 2024; 14(15):1297. https://doi.org/10.3390/nano14151297
Chicago/Turabian StyleYang, Xiaoying, Mei Cui, Rongxin Su, and Renliang Huang. 2024. "The Preparation of a Polyamidoxime–Phosphorylated Cellulose Nanofibrils Composite Aerogel for the Selective Extraction of Uranium from Seawater" Nanomaterials 14, no. 15: 1297. https://doi.org/10.3390/nano14151297





