Plant-Derived Extracellular Vesicles as a Novel Frontier in Cancer Therapeutics
Abstract
1. Introduction
2. Isolation and Characterization of PDEVs from Plants and Fruits
3. Therapeutic Applications of PDEVs in Multiple Types of Cancer
3.1. Citrus Fruits
3.2. Other Fruits
3.3. Ginseng
3.4. Ginger
3.5. Tea Leaves and Flowers
| PDEVs | Type of Study | Type of Cells and/or Mice Models | Results | Ref. |
|---|---|---|---|---|
| Grape fruits and C. paradisi | In vitro | A375 human melanoma cell line, MCF7 breast adenocarcinoma cell line, A549 human lung carcinoma cell line. | Grapefruit-derived EVs, alongside those from C. paradisi, exhibited strong cytotoxicity against tumor cells, especially A375 melanoma cells, resulting in substantial viability reduction, cell cycle arrest, the downregulation of phosphorylated ERK and AKT, and a decrease in key mediators (ICAM1 and cathepsin) associated with cancer progression. | [49] |
| Lemon-derived extracellular vesicles (LDEVs) | In vitro and in vivo | Gastric cancer cell line AGS, BGC-823, and SGC-7901. SGC-7901 xenograft models. | LDEVs induced S-phase cell cycle arrest and apoptosis in gastric cancer cells through the generation of ROS in vitro and suppressed gastric cancer growth in vivo with no toxicities in major organs. | [23] |
| Citrus limon juice L.-derived nanovesicles | In vitro and in vivo | A549 human lung carcinoma cell line, SW480 human colorectal adenocarcinoma cell line, LAMA 84 human chronic myeloid leukemia cell line. LAMA84 xenograft models. | Citrus limon juice L.-derived nanovesicles inhibit the proliferation of diverse cancer cell lines in vitro and suppress tumor growth in vivo by inducing TRAIL-mediated apoptotic cell death. | [24] |
| Romina fruit and its anthocyanin fraction | In vitro | Patient-derived primary myometrial and leiomyoma cells. | Romina (R) and Romina anthocyanin (RA) treatment significantly inhibited the expression of ECM components including collagen 1A1, fibronectin, and versican in leiomyoma cells. | [51] |
| Grape exosome-like nanoparticles (GELNs) | In vitro and in vivo | Intestinal epithelial CT26 cell line, Crypt cells, EGFPhi cells, and Lgr5-EGFP+ cells. Lgr5-EGFP-IRES-CreERT2 mice, B6.Cg-Tg(BAT-lacZ)3Picc/J mice. | The oral administration of GELNs can promote intestinal stem cell proliferation and organoid formation, stimulates Wnt/β-catenin pathway activation, and enhances the expression of stem cell growth-related genes. Lgr5-EGFP-IRES-CreERT2 mice were effectively prevented from DSS-induced colitis at a daily dose of 2 mg. | [41] |
| Panax ginseng C.A. Mey.-derived nanoparticles (GDNPs) | In vitro and in vivo | Murine melanoma cell line (B16F10), breast cancer cell line (4T1), and human embryonic kidney cell line (HEK293T). MyD88-, TLR4-, and TLR2-deficient C57/BL6 mice. | GDNPs altered M2 polarization and significantly suppressed melanoma growth in vitro and in vivo in tumor-bearing mice. | [57] |
| Panax ginseng-derived exosome-like nanoparticles (GENs) | In vitro and in vivo | C6 rat glioma cells, mouse embryonic fibroblast NIH3T3 cells, brain capillary endothelial cells (BCEC). Male Wistar rats (8 weeks old, 200–250 g) and male Balb/C mice (6–8 weeks old, 18–20 g). | GENs penetrated the blood–brain barrier (BBB) in C6 glioma cells and accumulated in mice brain tumors. The intracranial (IC) and intravenous (IV) injection of GENs effectively inhibited tumor growth in mice. GENs with anti-glioma effects can influence the tumor microenvironment (TME) by regulating tumor-associated macrophages (TAMs). | [32] |
| Ginger-derived nanovesicles (GDNs) | In vitro and in vivo | RAW 264.7 cells, Caco-2BBE, and Colon-26 cells. DSS-induced colitis mouse model, IL-10 knockout (IL10−/−) mice, and chemically induced colorectal cancer (CRC) models. | GDNPs did not affect cell viability, and do not cause local or systemic side effects. Anti-inflammatory effects were observed by increased levels of anti-inflammatory cytokines and decreased levels of pro-inflammatory cytokines. | [62] |
| Tea leaf-derived nanotherapeutics (NTs) | In vitro and in vivo | RAW 264.7 macrophages. C57BL/6 mice (12 weeks of age). | Treatment with tea leaf-derived NTs reduced pro-inflammatory cytokines and increased anti-inflammatory cytokines in RAW 264.7 macrophages. The oral administration of tea-derived NTs to mice during DSS treatment showed excellent biocompatibility, protected organs, and significantly reduced colon tumors. | [64] |
| Tea flowers-derived exosome-like NPs (TFENs) | In vitro and in vivo | MCF-7 cells, 4T1 cells, A549 cells, and HeLa cells Human breast cancer MCF-7 xenograft tumor model and Lung metastasis mice model | The anti-proliferative effects of TFENs resulted in mitochondria damages in MCF-7 and 4T1 cells and triggered cell cycle arrest. A significant inhibition of breast tumor growth and mitigation of lung metastasis were observed in mice administered intravenously or orally at doses of 1.5 or 3 mg TFENs/kg. | [25] |
| Bitter melon-derived extracellular vesicles (BMEVs) | In vitro and in vivo | WSU-HN6 and CAL27 oral squamous cell carcinoma (OSCC) cell line. Female BALB/c nude mice (4–6 weeks old). | The synergistic effect of BMEV + 5-FU resulted in the downregulation of NLRP3 and IL-1β expression in mouse OSCC tumors. | [56] |
4. Nano-Delivery Systems of PDEVs for Cancer Treatment
4.1. Grapefruit
4.2. Other Fruits
4.3. Ginger
4.4. Other Plants
| ORIGINS | Type of Study | Type of Cells and/or Mice Models | Findings | Ref. |
|---|---|---|---|---|
| Grapefruit-derived nanovectors (GNVs) | In vitro and in vivo | Mouse glioma 261 (Gl261) cells, murine mammary carcinoma cell line 4T1, lung carcinoma cell line A549, murine colorectal carcinoma cell line CT26, human colorectal adenocarcinoma cells SW620, and primary lymphocytes. C57BL/6j mice, BALB/c mice, and NOD/SCID mice 6–8 weeks of age. | GNVs, as therapeutic delivery vectors, transport biotinylated molecules and achieve targeted gene expression with minimal toxicity. The intranasal injection of GNVs encapsulated with the Stat3 inhibitor JSI-124 into GL26 tumor-bearing mice showed a significant inhibition of tumor growth. The intravenous administration of GNVs-folate (FA)-paclitaxel (PTX) showed enhanced targeted delivery and a significant reduction in tumor growth in the mouse CT26 colon cancer model and the human SW620 colon cancer SCID mouse model. No adverse reactions or major organ lesions were observed. | [70] |
| Grapefruit-derived nanovectors (GNVs) | In vitro and in vivo | Mouse T lymphoma EL4 cells, mouse 4T1, 4TO7 breast cancer cell lines, mouse NMuMG mammary gland epithelial cells, CT26 colon cancer and human umbilical vein endothelial cells (HUVECs), and CT26 cells. DSS-induced colitis mice; CT26 tumor model and 4T1 tumor model. | IGNVs exhibit advanced homing properties to inflammatory tissues. IGNV-DOX was highly accumulated in the tumor site and the I.V. administration of IGNV-DOX to the mice led to notable decreases in the growth of breast and colon tumors in tumor-bearing mice. In mice with DSS-induced colitis, IGNV-Cur treatment showed better therapeutic efficacy in inhibiting colitis than curcumin-loaded GNV or curcumin alone. | [71] |
| Grapefruit-derived nanovesicles (GDNs) | In vitro and in vivo | Macrophages, intestinal epithelial cells, intestinal leukocytes, RAW264.7 cells. DSS-induced colitis mouse model. | Orally administered GDNs deliver methotrexate (GMTX) to intestinal macrophages, significantly reducing MTX toxicity while enhancing its anti-inflammatory effects in a DSS-induced mouse colitis model. | [72] |
| Orange-derived extracellular vesicle-based nanodrugs (DN@OEV) | In vitro and in vivo | Human ovarian cancer cell line SKOV3 and mouse breast cancer cell line 4T-1. Orthotopic SKOV3-Luc ovarian cancer xenograft nude mouse model. | DN@OEV induced an excellent transcytosis process in SKOV3 and 4T-1 cells. Upon intraperitoneal injections, DN@OEV showed high penetration and accumulation in the tumor tissue of mice with orthotopic ovarian cancer, effectively reducing tumor growth and preventing metastasis. | [73] |
| Kiwifruit-derived extracellular vesicles’ (KEVs) targeted delivery of sorafenib (KEVs-SFB) | In vitro and in vivo | Human hepatocyte LO2 cells, HepG2 human hepatoblastoma cells. Orthotopic HepG2 liver cancer xenograft nude mouse models. | KEVs-SFB remained stable in the gastrointestinal tract and accumulated in the liver after oral administration to orthotopic HepG2 liver cancer xenograft mice. The anti-tumor ability of KEVs-SFB was demonstrated both in vitro and in vivo. | [33] |
| Systemic delivery of siRNA by ginger-derived exosome-like nanovesicles (GDENs) | In vitro and in vivo | HEK-293, Raw 264.7 macrophages, and KB cells (human epithelial carcinoma cells). KB cell xenograft mice model. | Ligand displayed on the surface of GDENs facilitated cellular uptake, and improved the systemic delivery of siRNAs to the cancer cells. KB cell xenograft mice that received FA-3WJ/GDENs/siRNA complexes via intravenous injection effectively inhibited tumor growth. | [74] |
| Ginger-derived lipid vehicles (GDLVs) | In vitro and in vivo | Caco-2BBE, Colon-26 cells, and RAW 267.4 macrophages. Six-week-old female FVB mice. | GDLVs loaded with siRNA-CD98 efficiently decreased CD98 expression in colon-26 and RAW 264.7 cells, as well as in the mouse colon, following two oral doses of the siRNA-CD98/GDLV complexes. | [75] |
| Ginger-derived nanovectors (GDNVs) | In vitro and in vivo | Human Colorectal Adenocarcinoma Stable Cell Line HT29, Caco2-BBE cells, and mouse colon adenocarcinoma cell line colon-26. Colon-26 subcutaneous xenograft model. | GDNVs are taken up by Colon-26 and HT-29 cancer cells via endocytosis. DOX-GDNVs exhibit significant cytotoxic activity against Colon-26 and HT-29 cells in vitro. In the Colon-26 xenograft mouse model, the intravenous injection of DOX-FA-GDNVs effectively targeted tumors and significantly inhibited tumor growth and reduced tumor volume. No obvious histopathological damage to major organs was observed. | [62] |
| Broccoli EVs loaded with exogenous miRNAs | In vitro | Human colorectal adenocarcinoma cells (Caco-2). | Exogenous miRNA-loaded broccoli EVs protect miRNAs from RNase degradation and gastrointestinal digestion, resulting in 30% cytotoxicity in Caco-2 cells. | [76] |
| Aloe-derived nanovesicles (ADNVs) | In vitro and in vivo | Murine melanoma cell line B16F10 cells, 4T1 cells, and MCF-10A cells. Male BALB/c mice. | gADNVs possess good stability and antioxidant properties. Their safety profiles were validated both in vitro and in vivo. Moreover, ICG-loaded gADNVs are stable and exhibit dose-dependent cytotoxicity in vitro and significant tumor-suppressive efficacy in vivo. | [77] |
5. Clinical Trials of PDEVs as Anti-Tumor Agents
6. Advantages and Challenges of PDEVs Compared to MDEVs
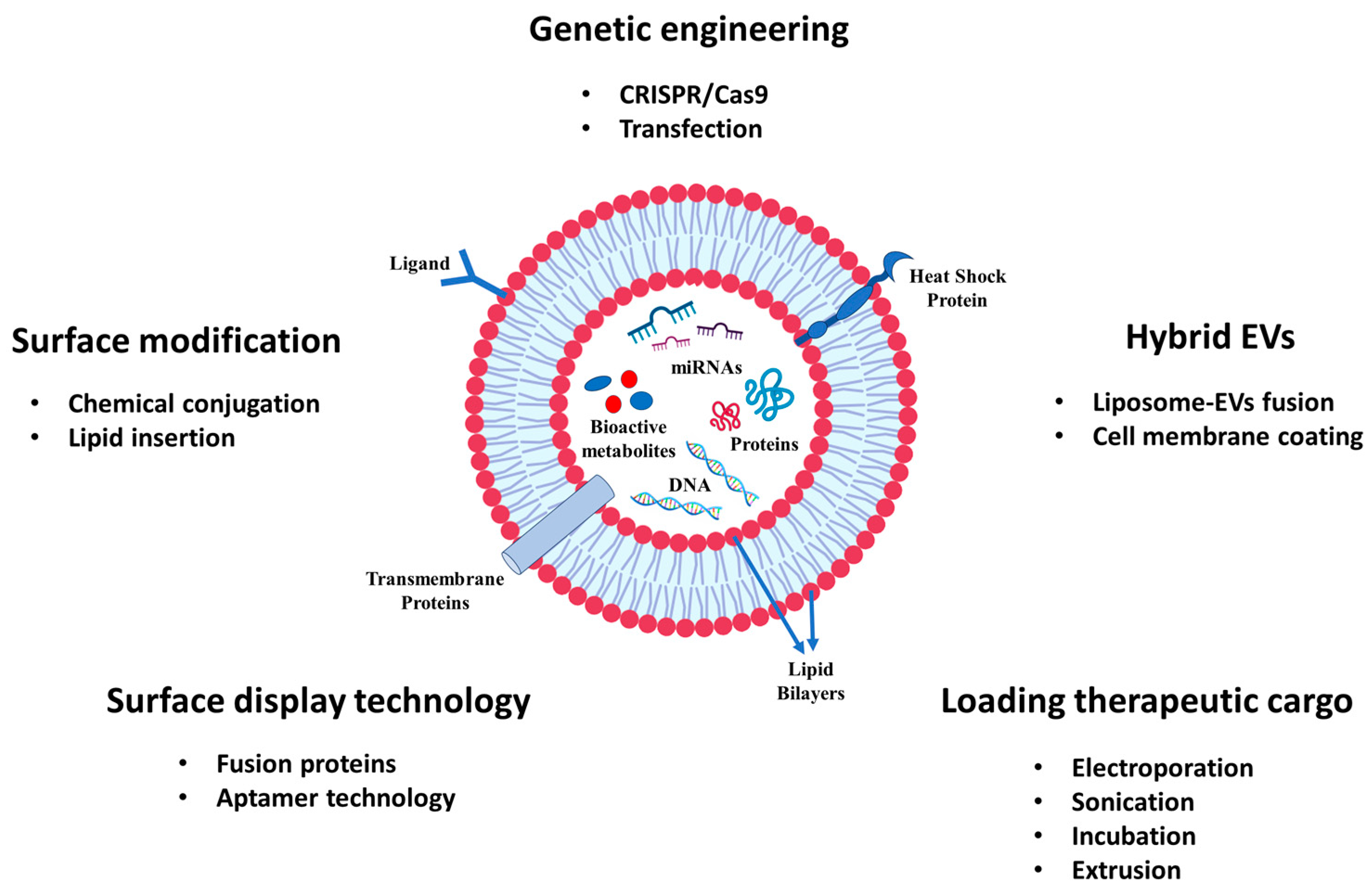
7. Future Perspective
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Global Burden of Disease 2019 Cancer Collaboration. Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life Years for 29 Cancer Groups from 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar] [CrossRef]
- Ilbawi, A.M.; Anderson, B.O. Cancer in global health: How do prevention and early detection strategies relate? Sci. Transl. Med. 2015, 7, 278cm1. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Wang, F.X.; Jia, K.K.; Kong, L.D. Natural Product Interventions for Chemotherapy and Radiotherapy-Induced Side Effects. Front. Pharmacol. 2018, 9, 1253. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Wang, X.L.; He, D.H.; Cheng, Y.X. Protection against chemotherapy- and radiotherapy-induced side effects: A review based on the mechanisms and therapeutic opportunities of phytochemicals. Phytomedicine 2021, 80, 153402. [Google Scholar] [CrossRef]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Harding, C.V.; Heuser, J.E.; Stahl, P.D. Exosomes: Looking back three decades and into the future. J. Cell Biol. 2013, 200, 367–371. [Google Scholar] [CrossRef]
- Rashed, M.H.; Bayraktar, E.; Helal, G.K.; Abd-Ellah, M.F.; Amero, P.; Chavez-Reyes, A.; Rodriguez-Aguayo, C. Exosomes: From Garbage Bins to Promising Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 538. [Google Scholar] [CrossRef]
- Yuana, Y.; Sturk, A.; Nieuwland, R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013, 27, 31–39. [Google Scholar] [CrossRef]
- Pitt, J.M.; Kroemer, G.; Zitvogel, L. Extracellular vesicles: Masters of intercellular communication and potential clinical interventions. J. Clin. Investig. 2016, 126, 1139–1143. [Google Scholar] [CrossRef]
- Ambattu, L.A.; Ramesan, S.; Dekiwadia, C.; Hanssen, E.; Li, H.; Yeo, L.Y. High frequency acoustic cell stimulation promotes exosome generation regulated by a calcium-dependent mechanism. Commun. Biol. 2020, 3, 553. [Google Scholar] [CrossRef]
- Baek, G.; Choi, H.; Kim, Y.; Lee, H.C.; Choi, C. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Therapeutics and as a Drug Delivery Platform. Stem Cells Transl. Med. 2019, 8, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell. Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: A next generation therapeutic tool? Cell Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef]
- Turturici, G.; Tinnirello, R.; Sconzo, G.; Geraci, F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: Advantages and disadvantages. Am. J. Physiol. Cell Physiol. 2014, 306, C621–C633. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef]
- Sarasati, A.; Syahruddin, M.H.; Nuryanti, A.; Ana, I.D.; Barlian, A.; Wijaya, C.H.; Ratnadewi, D.; Wungu, T.D.K.; Takemori, H. Plant-Derived Exosome-like Nanoparticles for Biomedical Applications and Regenerative Therapy. Biomedicines 2023, 11, 1053. [Google Scholar] [CrossRef] [PubMed]
- Kameli, N.; Dragojlovic-Kerkache, A.; Savelkoul, P.; Stassen, F.R. Plant-Derived Extracellular Vesicles: Current Findings, Challenges, and Future Applications. Membranes 2021, 11, 411. [Google Scholar] [CrossRef]
- Karamanidou, T.; Tsouknidas, A. Plant-Derived Extracellular Vesicles as Therapeutic Nanocarriers. Int. J. Mol. Sci. 2021, 23, 191. [Google Scholar] [CrossRef]
- Chun, Y.; Wenjing, Z.; Muran, B.; Qiyuan, L.; Qing, Z.; Yao, X.; Xiaoya, L.; Robert, H.; Cheng, J.; William, C.C.; et al. Edible Plant-Derived Extracellular Vesicles Serve as Promising Therapeutic Systems. Nano TransMed 2023, 2, 100004. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, Y.; Zhang, K.; Liu, Y.; Liang, Q.; Thakur, A.; Liu, W.; Yan, Y. Plant-derived extracellular vesicles (PDEVs) in nanomedicine for human disease and therapeutic modalities. J. Nanobiotechnology 2023, 21, 114. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, X.; Luo, Q.; Xu, L.; Chen, F. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J. Nanobiotechnology 2020, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Lo Dico, A.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A.; et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, Q.; Liang, Y.; Zu, M.; Chen, N.; Canup, B.S.B.; Luo, L.; Wang, C.; Zeng, L.; Xiao, B. Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta Pharm Sin. B 2022, 12, 907–923. [Google Scholar] [CrossRef] [PubMed]
- Study Investigating the Ability of Plant Exosomes to Deliver Curcumin to Normal and Colon Cancer Tissue. ClinicalTrials.gov Identifier (NCT Number): NCT01294072. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT01294072 (accessed on 1 January 2022).
- Edible Plant Exosome Ability to Prevent Oral Mucositis Associated with Chemoradiation Treatment of Head and Neck Cancer. ClinicalTrials.gov Identifier (NCT Number): NCT01668849. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT01668849 (accessed on 1 January 2022).
- Sall, I.M.; Flaviu, T.A. Plant and mammalian-derived extracellular vesicles: A new therapeutic approach for the future. Front. Bioeng. Biotechnol. 2023, 11, 1215650. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, C.; Bruce, K.D.; Burgy, O.; Boyd, T.D.; Michel, C.R.; Garcia-Perez, J.E.; Adame, V.; Anton, P.; Bettcher, B.M.; Chial, H.J.; et al. Exosome Isolation by Ultracentrifugation and Precipitation and Techniques for Downstream Analyses. Curr. Protoc. Cell Biol. 2020, 88, e110. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Jeong, H.; Jang, E.; Kim, E.; Yoon, Y.; Jang, S.; Jeong, H.-S.; Jang, G. Isolation of high-purity and high-stability exosomes from ginseng. Front. Plant Sci. 2023, 13, 1064412. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.G.; Choi, S.Y.; Kim, H.; Choi, E.J.; Lee, E.J.; Park, P.J.; Ko, J.; Kim, K.P.; Baek, H.S. Panax ginseng-Derived Extracellular Vesicles Facilitate Anti-Senescence Effects in Human Skin Cells: An Eco-Friendly and Sustainable Way to Use Ginseng Substances. Cells 2021, 10, 486. [Google Scholar] [CrossRef]
- Kim, J.; Zhu, Y.; Chen, S.; Wang, D.; Zhang, S.; Xia, J.; Li, S.; Qiu, Q.; Lee, H.; Wang, J. Anti-glioma effect of ginseng-derived exosomes-like nanoparticles by active blood–brain-barrier penetration and tumor microenvironment modulation. J. Nanobiotechnology 2023, 21, 253. [Google Scholar] [CrossRef]
- Fang, Z.; Song, M.; Lai, K.; Cui, M.; Yin, M.; Liu, K. Kiwi-derived extracellular vesicles for oral delivery of sorafenib. Eur. J. Pharm. Sci. 2023, 191, 106604. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.; Wang, X.; Dong, X.; Wang, C.; Wang, L.; Yang, X.; Li, T. Broccoli-Derived Exosome-like Nanoparticles Alleviate Loperamide-Induced Constipation, in Correlation with Regulation on Gut Microbiota and Tryptophan Metabolism. J. Agric. Food Chem. 2023, 71, 16568–16580. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, V.; Lucchetti, D.; Gatto, I.; Maiorana, A.; Marcantoni, M.; Maulucci, G.; Papi, M.; Pola, R.; De Spirito, M.; Sgambato, A. Dynamic light scattering for the characterization and counting of extracellular vesicles: A powerful noninvasive tool. J. Nanopart. Res. 2014, 16, 2583. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef] [PubMed]
- Nemati, M.; Singh, B.; Mir, R.A.; Nemati, M.; Babaei, A.; Ahmadi, M.; Rasmi, Y.; Golezani, A.G.; Rezaie, J. Plant-derived extracellular vesicles: A novel nanomedicine approach with advantages and challenges. Cell Commun. Signal. 2022, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jang, H.; Kim, W.; Kim, D.; Park, J.H. Therapeutic Applications of Plant-Derived Extracellular Vesicles as Antioxidants for Oxidative Stress-Related Diseases. Antioxidants 2023, 12, 1286. [Google Scholar] [CrossRef] [PubMed]
- Li, D.F.; Tang, Q.; Yang, M.F.; Xu, H.M.; Zhu, M.Z.; Zhang, Y.; Tian, C.M.; Nie, Y.Q.; Wang, J.Y.; Liang, Y.J.; et al. Plant-derived exosomal nanoparticles: Potential therapeutic for inflammatory bowel disease. Nanoscale Adv. 2023, 5, 3575–3588. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Y.; Zhu, G.; Zeng, L.; Xu, S.; Cheng, H.; Ouyang, Z.; Chen, J.; Pathak, J.L.; Wu, L.; et al. The Emerging Role of Plant-Derived Exosomes-Like Nanoparticles in Immune Regulation and Periodontitis Treatment. Front. Immunol. 2022, 13, 896745. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.B.; Wang, B.; Zhang, L.; et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef]
- Cho, J.H.; Hong, Y.D.; Kim, D.; Park, S.J.; Kim, J.S.; Kim, H.-M.; Yoon, E.J.; Cho, J.-S. Confirmation of plant-derived exosomes as bioactive substances for skin application through comparative analysis of keratinocyte transcriptome. Appl. Biol. Chem. 2022, 65, 8. [Google Scholar] [CrossRef]
- Niu, G.; Jian, T.; Gai, Y.; Chen, J. Microbiota and plant-derived vesicles that serve as therapeutic agents and delivery carriers to regulate metabolic syndrome. Adv. Drug Del. Rev. 2023, 196, 114774. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Félix, F.; Graciano-Verdugo, A.Z.; Moreno-Vásquez, M.J.; Lagarda-Díaz, I.; Barreras-Urbina, C.G.; Armenta-Villegas, L.; Olguín-Moreno, A.; Tapia-Hernández, J.A. Trends in Sustainable Green Synthesis of Silver Nanoparticles Using Agri-Food Waste Extracts and Their Applications in Health. J. Nanomater. 2022, 2022, 8874003. [Google Scholar] [CrossRef]
- Garcia-Larez, F.L.; Esquer, J.; Guzmán, H.; Zepeda-Quintana, D.S.; Moreno-Vásquez, M.J.; Rodríguez-Félix, F.; Del-Toro-Sánchez, C.L.; López-Corona, B.E.; Tapia-Hernández, J.A. Effect of Ultrasound-Assisted Extraction (UAE) parameters on the recovery of polyphenols from pecan nutshell waste biomass and its antioxidant activity. Biomass Convers. Biorefinery 2024. [Google Scholar] [CrossRef]
- Del-Toro-Sánchez, C.L.; Rodríguez-Félix, F.; Cinco-Moroyoqui, F.J.; Juárez, J.; Ruiz-Cruz, S.; Wong-Corral, F.J.; Borboa-Flores, J.; Castro-Enríquez, D.D.; Barreras-Urbina, C.G.; Tapia-Hernández, J.A. Recovery of phytochemical from three safflower (Carthamus tinctorius L.) by-products: Antioxidant properties, protective effect of human erythrocytes and profile by UPLC-DAD-MS. J. Food Process. Preserv. 2021, 45, e15765. [Google Scholar] [CrossRef]
- Richa, R.; Kohli, D.; Vishwakarma, D.; Mishra, A.; Kabdal, B.; Kothakota, A.; Richa, S.; Sirohi, R.; Kumar, R.; Naik, B. Citrus fruit: Classification, value addition, nutritional and medicinal values, and relation with pandemic and hidden hunger. J. Agric. Food Res. 2023, 14, 100718. [Google Scholar] [CrossRef]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. Antiproliferative effects of the readily extractable fractions prepared from various citrus juices on several cancer cell lines. J. Agric. Food Chem. 1999, 47, 2509–2512. [Google Scholar] [CrossRef] [PubMed]
- Stanly, C.; Alfieri, M.; Ambrosone, A.; Leone, A.; Fiume, I.; Pocsfalvi, G. Grapefruit-Derived Micro and Nanovesicles Show Distinct Metabolome Profiles and Anticancer Activities in the A375 Human Melanoma Cell Line. Cells 2020, 9, 2722. [Google Scholar] [CrossRef]
- Raimondo, S.; Saieva, L.; Cristaldi, M.; Monteleone, F.; Fontana, S.; Alessandro, R. Label-free quantitative proteomic profiling of colon cancer cells identifies acetyl-CoA carboxylase alpha as antitumor target of Citrus limon-derived nanovesicles. J. Proteom. 2018, 173, 1–11. [Google Scholar] [CrossRef]
- Giampieri, F.; Islam, M.S.; Greco, S.; Gasparrini, M.; Forbes Hernandez, T.Y.; Delli Carpini, G.; Giannubilo, S.R.; Ciavattini, A.; Mezzetti, B.; Mazzoni, L.; et al. Romina: A powerful strawberry with in vitro efficacy against uterine leiomyoma cells. J. Cell. Physiol. 2019, 234, 7622–7633. [Google Scholar] [CrossRef]
- Ray, R.B.; Raychoudhuri, A.; Steele, R.; Nerurkar, P. Bitter melon (Momordica charantia) extract inhibits breast cancer cell proliferation by modulating cell cycle regulatory genes and promotes apoptosis. Cancer Res. 2010, 70, 1925–1931. [Google Scholar] [CrossRef]
- Ru, P.; Steele, R.; Nerurkar, P.V.; Phillips, N.; Ray, R.B. Bitter melon extract impairs prostate cancer cell-cycle progression and delays prostatic intraepithelial neoplasia in TRAMP model. Cancer Prev. Res. 2011, 4, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Deep, G.; Jain, A.K.; Raina, K.; Agarwal, C.; Wempe, M.F.; Agarwal, R. Bitter melon juice activates cellular energy sensor AMP-activated protein kinase causing apoptotic death of human pancreatic carcinoma cells. Carcinogenesis 2013, 34, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Rajamoorthi, A.; Shrivastava, S.; Steele, R.; Nerurkar, P.; Gonzalez, J.G.; Crawford, S.; Varvares, M.; Ray, R.B. Bitter melon reduces head and neck squamous cell carcinoma growth by targeting c-Met signaling. PLoS ONE 2013, 8, e78006. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Luo, Q.; Chen, X.; Chen, F. Bitter melon derived extracellular vesicles enhance the therapeutic effects and reduce the drug resistance of 5-fluorouracil on oral squamous cell carcinoma. J. Nanobiotechnology 2021, 19, 259. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Yan, H.; Han, X.; Weng, L.; Wei, Q.; Sun, X.; Lu, W.; Wei, Q.; Ye, J.; Cai, X.; et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J. ImmunoTherapy Cancer 2019, 7, 326. [Google Scholar] [CrossRef] [PubMed]
- Spence, C. Ginger: The pungent spice. Int. J. Gastron. Food Sci. 2023, 33, 100793. [Google Scholar] [CrossRef]
- Yin, L.; Yan, L.; Yu, Q.; Wang, J.; Liu, C.; Wang, L.; Zheng, L. Characterization of the MicroRNA Profile of Ginger Exosome-like Nanoparticles and Their Anti-Inflammatory Effects in Intestinal Caco-2 Cells. J. Agric. Food Chem. 2022, 70, 4725–4734. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, Y.; Yu, J. Exosome-like Nanoparticles from Ginger Rhizomes Inhibited NLRP3 Inflammasome Activation. Mol. Pharm. 2019, 16, 2690–2699. [Google Scholar] [CrossRef] [PubMed]
- Anusha, R.; Ashin, M.; Priya, S. Ginger exosome-like nanoparticles (GELNs) induced apoptosis, cell cycle arrest, and anti-metastatic effects in triple-negative breast cancer MDA-MB-231 cells. Food Chem. Toxicol. 2023, 182, 114102. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef]
- Frei, B.; Higdon, J.V. Antioxidant Activity of Tea Polyphenols In Vivo: Evidence from Animal Studies. J. Nutr. 2003, 133, 3275S–3284S. [Google Scholar] [CrossRef] [PubMed]
- Zu, M.; Xie, D.; Canup, B.S.B.; Chen, N.; Wang, Y.; Sun, R.; Zhang, Z.; Fu, Y.; Dai, F.; Xiao, B. ‘Green’ nanotherapeutics from tea leaves for orally targeted prevention and alleviation of colon diseases. Biomaterials 2021, 279, 121178. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Ding, Y.; Chen, G.; Sun, Y.; Zeng, X.; Ye, H. Components identification and nutritional value exploration of tea (Camellia sinensis L.) flower extract: Evidence for functional food. Food Res. Int. 2020, 132, 109100. [Google Scholar] [CrossRef] [PubMed]
- Way, T.-D.; Lin, H.-Y.; Hua, K.-T.; Lee, J.-C.; Li, W.-H.; Lee, M.-R.; Shuang, C.-H.; Lin, J.-K. Beneficial effects of different tea flowers against human breast cancer MCF-7 cells. Food Chem. 2009, 114, 1231–1236. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, N.; Rankin, G.O.; Li, B.; Rojanasakul, Y.; Tu, Y.; Chen, Y.C. Anti-proliferative effect and cell cycle arrest induced by saponins extracted from tea (Camellia sinensis) flower in human ovarian cancer cells. J. Funct. Foods 2017, 37, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hou, X.; Li, M.; Ji, R.; Li, Z.; Wang, Y.; Guo, Y.; Liu, D.; Huang, B.; Du, H. Active fractions of golden-flowered tea (Camellia nitidissima Chi) inhibit epidermal growth factor receptor mutated non-small cell lung cancer via multiple pathways and targets in vitro and in vivo. Front. Nutr. 2022, 9, 1014414. [Google Scholar] [CrossRef] [PubMed]
- Jisu, K.; Shiyi, L.; Shuya, Z.; Jianxin, W. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J. Pharm. Sci. 2022, 17, 53–69. [Google Scholar] [CrossRef]
- Wang, Q.; Zhuang, X.; Mu, J.; Deng, Z.-B.; Jiang, H.; Zhang, L.; Xiang, X.; Wang, B.; Yan, J.; Miller, D.; et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat. Commun. 2013, 4, 1867. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ren, Y.; Mu, J.; Egilmez, N.K.; Zhuang, X.; Deng, Z.; Zhang, L.; Yan, J.; Miller, D.; Zhang, H.G. Grapefruit-Derived Nanovectors Use an Activated Leukocyte Trafficking Pathway to Deliver Therapeutic Agents to Inflammatory Tumor Sites. Cancer Res. 2015, 75, 2520–2529. [Google Scholar] [CrossRef]
- Wang, B.; Zhuang, X.; Deng, Z.B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef]
- Long, F.; Pan, Y.; Li, J.; Sha, S.; Shi, X.; Guo, H.; Huang, C.; Xiao, Q.; Fan, C.; Zhang, X.; et al. Orange-derived extracellular vesicles nanodrugs for efficient treatment of ovarian cancer assisted by transcytosis effect. Acta Pharm. Sin. B 2023, 13, 5121–5134. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, H.; Yin, H.; Bennett, C.; Zhang, H.-g.; Guo, P. Arrowtail RNA for Ligand Display on Ginger Exosome-like Nanovesicles to Systemic Deliver siRNA for Cancer Suppression. Sci. Rep. 2018, 8, 14644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, X.; Han, M.K.; Collins, J.F.; Merlin, D. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine 2017, 12, 1927–1943. [Google Scholar] [CrossRef] [PubMed]
- del Pozo-Acebo, L.; López de las Hazas, M.-C.; Tomé-Carneiro, J.; del Saz-Lara, A.; Gil-Zamorano, J.; Balaguer, L.; Chapado, L.A.; Busto, R.; Visioli, F.; Dávalos, A. Therapeutic potential of broccoli-derived extracellular vesicles as nanocarriers of exogenous miRNAs. Pharmacol. Res. 2022, 185, 106472. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Wang, H.; Shi, W.; Chen, L.; Chen, T.; Chen, G.; Wang, W.; Lan, J.; Huang, Z.; Zhang, J.; et al. Aloe derived nanovesicle as a functional carrier for indocyanine green encapsulation and phototherapy. J. Nanobiotechnology 2021, 19, 439. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.A.; Adeyemo, T.R.; Rotimi, D.; Batiha, G.E.; Mostafa-Hedeab, G.; Iyobhebhe, M.E.; Elebiyo, T.C.; Atunwa, B.; Ojo, A.B.; Lima, C.M.G.; et al. Anticancer Properties of Curcumin Against Colorectal Cancer: A Review. Front. Oncol. 2022, 12, 881641. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef] [PubMed]
- Perini, G.; Palmieri, V.; D’Ascenzo, M.; Colussi, C.; Grassi, C.; Friggeri, G.; Augello, A.; Cui, L.; Papi, M.; Spirito, M.D. Near-infrared controlled release of mesenchymal stem cells secretome from bioprinted graphene-based microbeads for nerve regeneration. Int. J. Bioprinting 2023, 10, 10-36922. [Google Scholar] [CrossRef]
- Weng, Z.; Zhang, B.; Wu, C.; Yu, F.; Han, B.; Li, B.; Li, L. Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. J. Hematol. Oncol. 2021, 14, 136. [Google Scholar] [CrossRef]
- Luo, H.; Jin, J.; Jin, J.; Lou, K.; He, H.; Feng, S.; Zeng, F.; Zou, J. Emerging applications of extracellular vesicles in tumor therapy. Cancer Nanotechnol. 2023, 14, 63. [Google Scholar] [CrossRef]
- Lou, G.; Song, X.; Yang, F.; Wu, S.; Wang, J.; Chen, Z.; Liu, Y. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J. Hematol. Oncol. 2015, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.P.; Khan, S.; Gilligan, K.E.; Zafar, H.; Lalor, P.; Glynn, C.; O’Flatharta, C.; Ingoldsby, H.; Dockery, P.; De Bhulbh, A.; et al. Employing mesenchymal stem cells to support tumor-targeted delivery of extracellular vesicle (EV)-encapsulated microRNA-379. Oncogene 2018, 37, 2137–2149. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.E.; de Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular vesicle-based therapeutics: Natural versus engineered targeting and trafficking. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]

| PDEVs | Clinical Phase | Intervention/Treatment | Outcome Measures | References |
|---|---|---|---|---|
| Grape-derived EVs | Phase I | Head and neck cancer | Effectiveness of grape-derived EVs in reducing oral mucositis in patients undergoing chemoradiotherapy for head and neck cancer. | [27] |
| Plant-derived EVs delivering curcumin | Phase I | Colon cancer tissue | Ability of plant-derived extracellular vesicles to deliver curcumin to normal and colon cancer tissue. | [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, L.; Perini, G.; Palmieri, V.; De Spirito, M.; Papi, M. Plant-Derived Extracellular Vesicles as a Novel Frontier in Cancer Therapeutics. Nanomaterials 2024, 14, 1331. https://doi.org/10.3390/nano14161331
Cui L, Perini G, Palmieri V, De Spirito M, Papi M. Plant-Derived Extracellular Vesicles as a Novel Frontier in Cancer Therapeutics. Nanomaterials. 2024; 14(16):1331. https://doi.org/10.3390/nano14161331
Chicago/Turabian StyleCui, Lishan, Giordano Perini, Valentina Palmieri, Marco De Spirito, and Massimiliano Papi. 2024. "Plant-Derived Extracellular Vesicles as a Novel Frontier in Cancer Therapeutics" Nanomaterials 14, no. 16: 1331. https://doi.org/10.3390/nano14161331
APA StyleCui, L., Perini, G., Palmieri, V., De Spirito, M., & Papi, M. (2024). Plant-Derived Extracellular Vesicles as a Novel Frontier in Cancer Therapeutics. Nanomaterials, 14(16), 1331. https://doi.org/10.3390/nano14161331









