Synthetic or Natural (Bio-Based) Hydroxyapatite? A Systematic Comparison between Biomimetic Nanostructured Coatings Produced by Ionized Jet Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Targets Characterization
2.2.2. IJD
2.2.3. Morphological Characterization of the Films
2.2.4. Physiochemical Characterization of the Films
2.2.5. The Effect of Thermal Treatments
2.2.6. Stability Profile
3. Results
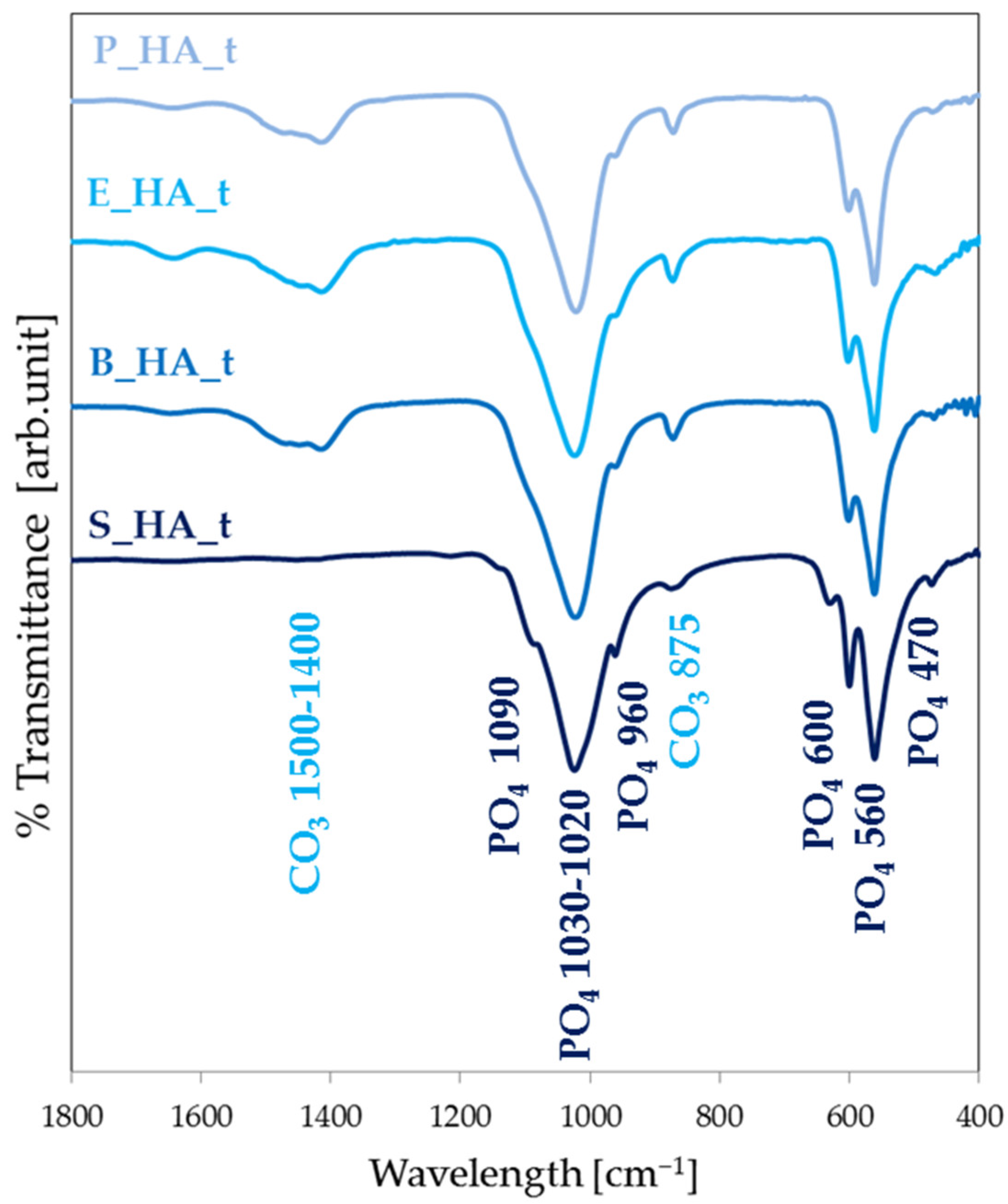
3.1. Target Characterization
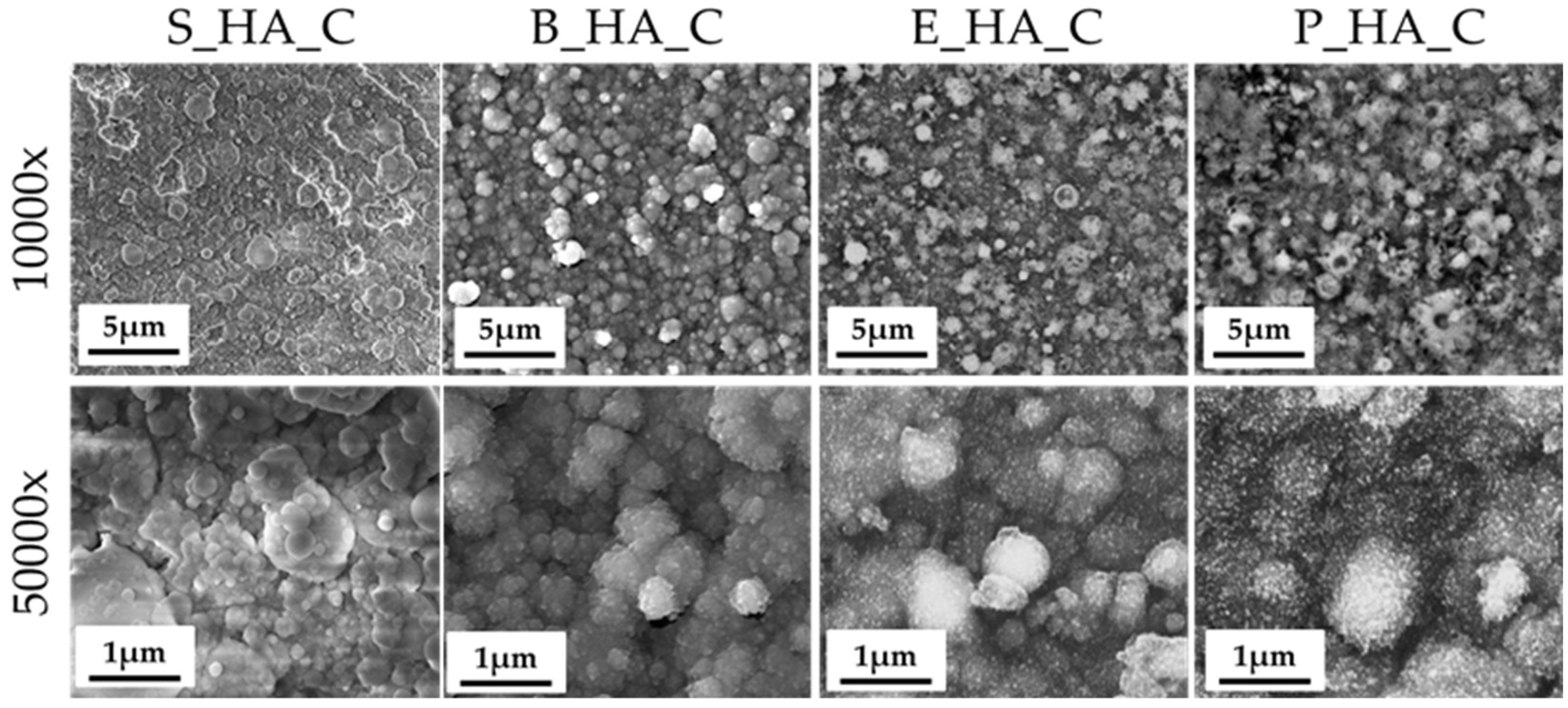
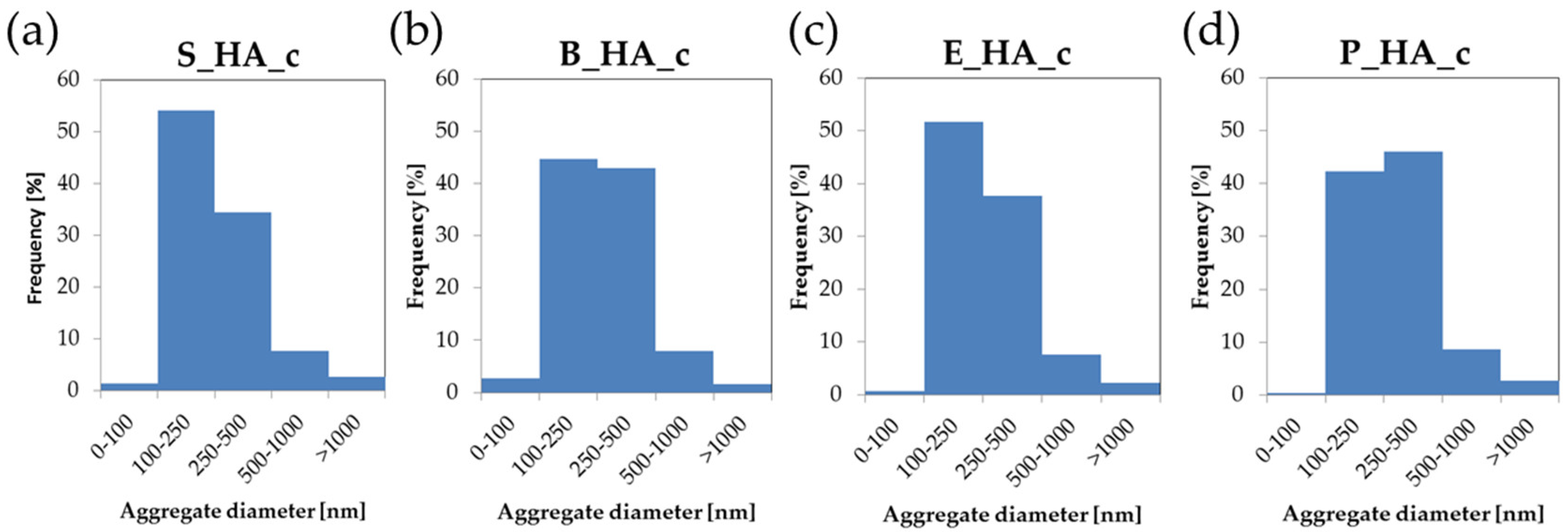
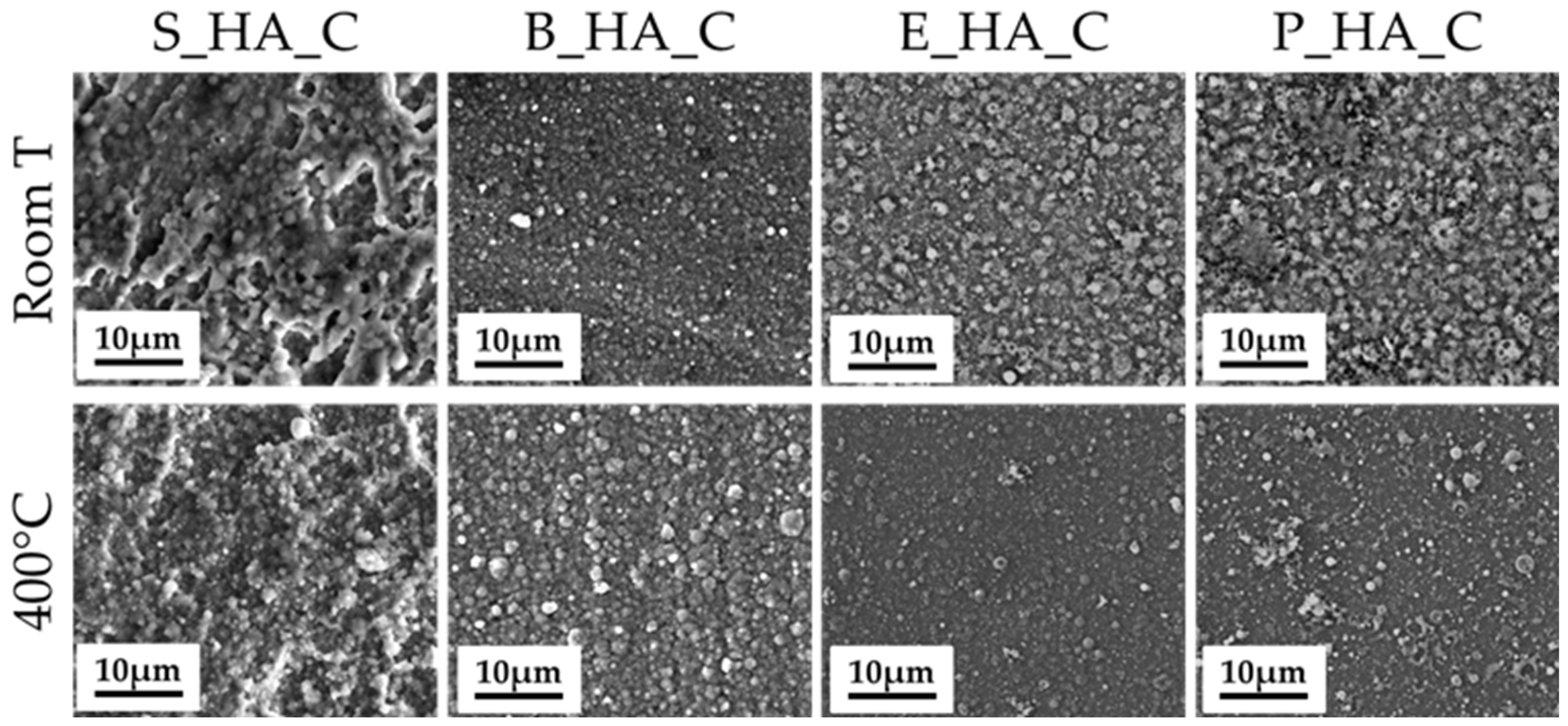
3.2. Coatings Morphology
3.3. Physiochemical Coatings Characterization
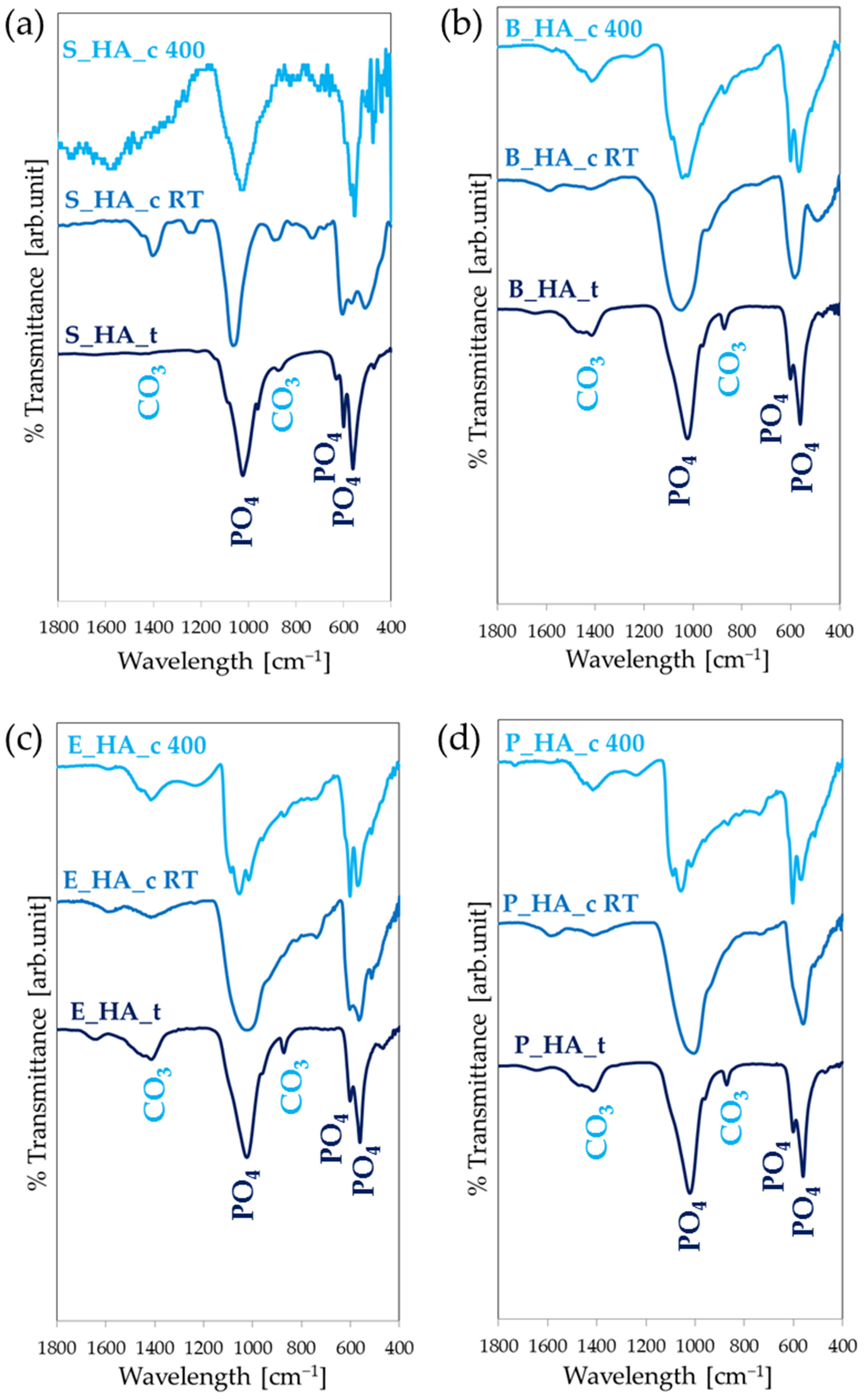
3.4. The Effect of Thermal Treatments
3.5. Stability Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, P.; Zhang, Y.; Hu, R.; Shi, B.; Zhang, L.; Huang, Q.; Yang, Y.; Tang, P.; Lin, C. Advanced Surface Engineering of Titanium Materials for Biomedical Applications: From Static Modification to Dynamic Responsive Regulation. Bioact. Mater. 2023, 27, 15–57. [Google Scholar] [CrossRef]
- Bigi, A.; Boanini, E. Functionalized Biomimetic Calcium Phosphates for Bone Tissue Repair. J. Appl. Biomater. Funct. Mater. 2017, 15, e313–e325. [Google Scholar] [CrossRef] [PubMed]
- Surmenev, R.A. A Review of Plasma-Assisted Methods for Calcium Phosphate-Based Coatings Fabrication. Surf. Coat. Technol. 2012, 206, 2035–2056. [Google Scholar] [CrossRef]
- Ielo, I.; Calabrese, G.; De Luca, G.; Conoci, S. Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics. Int. J. Mol. Sci. 2022, 23, 9721. [Google Scholar] [CrossRef]
- Montazerian, M.; Hosseinzadeh, F.; Migneco, C.; Fook, M.V.L.; Baino, F. Bioceramic Coatings on Metallic Implants: An Overview. Ceram. Int. 2022, 48, 8987–9005. [Google Scholar] [CrossRef]
- Gupta, K.; Meena, K. Artificial Bone Scaffolds and Bone Joints by Additive Manufacturing: A Review. Bioprinting 2023, 31, e00268. [Google Scholar] [CrossRef]
- Szczes, A.; Holysz, L.; Chibowski, E. Synthesis of Hydroxyapatite for Biomedical Applications. Adv. Colloid. Interface Sci. 2017, 249, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Boanini, E.; Gazzano, M.; Bigi, A. Ionic Substitutions in Calcium Phosphates Synthesized at Low Temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, M.; Zhu, Y.; Yeung, K.W.K.; Chu, P.K.; Wu, S. The Modulation of Stem Cell Behaviors by Functionalized Nanoceramic Coatings on Ti-Based Implants. Bioact. Mater. 2016, 1, 65–76. [Google Scholar] [CrossRef]
- von Erlach, T.C.; Bertazzo, S.; Wozniak, M.A.; Horejs, C.-M.; Maynard, S.A.; Attwood, S.; Robinson, B.K.; Autefage, H.; Kallepitis, C.; del Río Hernández, A.; et al. Cell-Geometry-Dependent Changes in Plasma Membrane Order Direct Stem Cell Signalling and Fate. Nat. Mater. 2018, 17, 237–242. [Google Scholar] [CrossRef]
- de Peppo, G.M.; Agheli, H.; Karlsson, C.; Ekstrom, K.; Brisby, H.; Lenneras, M.; Gustafsson, S.; Sjövall, P.; Johansson, A.; Olsson, E.; et al. Osteogenic Response of Human Mesenchymal Stem Cells to Well-Defined Nanoscale Topography in Vitro. Int. J. Nanomed. 2014, 9, 2499. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yu, L.; Xie, W.; Camacho, L.C.; Zhang, M.; Chu, Z.; Wei, Q.; Haag, R. Surface Roughness and Substrate Stiffness Synergize To Drive Cellular Mechanoresponse. Nano Lett. 2020, 20, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Sartori, M.; Graziani, G.; Sassoni, E.; Pagani, S.; Boi, M.; Maltarello, M.C.; Baldini, N.; Fini, M. Nanostructure and Biomimetics Orchestrate Mesenchymal Stromal Cell Differentiation: An in Vitro Bioactivity Study on New Coatings for Orthopedic Applications. Mater. Sci. Eng. C 2021, 123, 112031. [Google Scholar] [CrossRef] [PubMed]
- Litak, J.; Czyzewski, W.; Szymoniuk, M.; Pastuszak, B.; Litak, J.; Litak, G.; Grochowski, C.; Rahnama-Hezavah, M.; Kamieniak, P. Hydroxyapatite Use in Spine Surgery—Molecular and Clinical Aspect. Materials 2022, 15, 2906. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.J.; Pelletier, M.H.; Walsh, W.R.; Mobbs, R.J. Spine Interbody Implants: Material Selection and Modification, Functionalization and Bioactivation of Surfaces to Improve Osseointegration. Orthop. Surg. 2014, 6, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, S.; Rahman, M.; Kamruzzaman, M.; Khatun, H.; Ali, M.O.; Haque, M.M. Recent Developments in Hydroxyapatite Coating on Magnesium Alloys for Clinical Applications. Results Eng. 2023, 17, 101002. [Google Scholar] [CrossRef]
- Liu, X.; He, D.; Zhou, Z.; Wang, G.; Wang, Z.; Wu, X.; Tan, Z. Characteristics of (002) Oriented Hydroxyapatite Coatings Deposited by Atmospheric Plasma Spraying. Coatings 2018, 8, 258. [Google Scholar] [CrossRef]
- Duta, L.; Popescu, A. Current Status on Pulsed Laser Deposition of Coatings from Animal-Origin Calcium Phosphate Sources. Coatings 2019, 9, 335. [Google Scholar] [CrossRef]
- Graziani, G.; Bianchi, M.; Sassoni, E.; Russo, A.; Marcacci, M. Ion-Substituted Calcium Phosphate Coatings Deposited by Plasma-Assisted Techniques: A Review. Mater. Sci. Eng. C 2017, 74, 219–229. [Google Scholar] [CrossRef]
- Graziani, G.; Boi, M.; Bianchi, M. A Review on Ionic Substitutions in Hydroxyapatite Thin Films: Towards Complete Biomimetism. Coatings 2018, 8, 269. [Google Scholar] [CrossRef]
- Mehta, A.; Singh, G. Consequences of Hydroxyapatite Doping Using Plasma Spray to Implant Biomaterials. J. Electrochem. Sci. Eng. 2023, 13, 5–23. [Google Scholar] [CrossRef]
- Suska, F.; Omar, O.; Emanuelsson, L.; Taylor, M.; Gruner, P.; Kinbrum, A.; Hunt, D.; Hunt, T.; Taylor, A.; Palmquist, A. Enhancement of CRF-PEEK Osseointegration by Plasma-Sprayed Hydroxyapatite: A Rabbit Model. J. Biomater. Appl. 2014, 29, 234–242. [Google Scholar] [CrossRef]
- Safavi, M.S.; Surmeneva, M.A.; Surmenev, R.A.; Khalil-Allafi, J. RF-Magnetron Sputter Deposited Hydroxyapatite-Based Composite & Multilayer Coatings: A Systematic Review from Mechanical, Corrosion, and Biological Points of View. Ceram. Int. 2021, 47, 3031–3053. [Google Scholar] [CrossRef]
- Buyuksungur, S.; Huri, P.Y.; Schmidt, J.; Pana, I.; Dinu, M.; Vitelaru, C.; Kiss, A.E.; Tamay, D.G.; Hasirci, V.; Vladescu, A.; et al. In Vitro Cytotoxicity, Corrosion and Antibacterial Efficiencies of Zn Doped Hydroxyapatite Coated Ti Based Implant Materials. Ceram. Int. 2023, 49, 12570–12584. [Google Scholar] [CrossRef]
- Liguori, A.; Gualandi, C.; Focarete, M.L.; Biscarini, F.; Bianchi, M. The Pulsed Electron Deposition Technique for Biomedical Applications: A Review. Coatings 2019, 10, 16. [Google Scholar] [CrossRef]
- Skočdopole, J.; Kalvoda, L.; Nozar, P.; Netopilík, M. Preparation of Polymeric Coatings by Ionized Jet Deposition Method. Chem. Pap. 2018, 72, 1735–1739. [Google Scholar] [CrossRef]
- Bianchi, M.; Pisciotta, A.; Bertoni, L.; Berni, M.; Gambardella, A.; Visani, A.; Russo, A.; de Pol, A.; Carnevale, G. Osteogenic Differentiation of HDPSCs on Biogenic Bone Apatite Thin Films. Stem Cells Int. 2017, 2017, 3579283. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; Berni, M.; Gambardella, A.; De Carolis, M.; Maltarello, M.C.; Boi, M.; Carnevale, G.; Bianchi, M. Fabrication and Characterization of Biomimetic Hydroxyapatite Thin Films for Bone Implants by Direct Ablation of a Biogenic Source. Mater. Sci. Eng. C 2019, 99, 853–862. [Google Scholar] [CrossRef]
- Montesissa, M.; Borciani, G.; Rubini, K.; Valle, F.; Boi, M.; Baldini, N.; Boanini, E.; Graziani, G. Ionized Jet Deposition of Calcium Phosphates-Based Nanocoatings: Tuning Coating Properties and Cell Behavior by Target Composition and Substrate Heating. Nanomaterials 2023, 13, 1758. [Google Scholar] [CrossRef]
- Di Pompo, G.; Liguori, A.; Carlini, M.; Avnet, S.; Boi, M.; Baldini, N.; Focarete, M.L.; Bianchi, M.; Gualandi, C.; Graziani, G. Electrospun Fibers Coated with Nanostructured Biomimetic Hydroxyapatite: A New Platform for Regeneration at the Bone Interfaces. Biomater. Adv. 2023, 144, 213231. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, S.; Matinlinna, J.P.; Chen, Z.; Pan, H. Insight into Biological Apatite: Physiochemical Properties and Preparation Approaches. Biomed. Res. Int. 2013, 2013, 929748. [Google Scholar] [CrossRef] [PubMed]
- Mohd Pu’ad, N.A.S.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Syntheses of Hydroxyapatite from Natural Sources. Heliyon 2019, 5, e01588. [Google Scholar] [CrossRef] [PubMed]
- Dorcioman, G.; Grumezescu, V.; Stan, G.E.; Chifiriuc, M.C.; Gradisteanu, G.P.; Miculescu, F.; Matei, E.; Popescu-Pelin, G.; Zgura, I.; Craciun, V.; et al. Hydroxyapatite Thin Films of Marine Origin as Sustainable Candidates for Dental Implants. Pharmaceutics 2023, 15, 1294. [Google Scholar] [CrossRef] [PubMed]
- Oktar, F.N.; Unal, S.; Gunduz, O.; Nissan, B.B.; Macha, I.J.; Akyol, S.; Duta, L.; Ekren, N.; Altan, E.; Yetmez, M. Marine-Derived Bioceramics for Orthopedic, Reconstructive and Dental Surgery Applications. J. Aust. Ceram. Soc. 2023, 59, 57–81. [Google Scholar] [CrossRef]
- Chioibasu, D.; Duta, L.; Popescu-Pelin, G.; Popa, N.; Milodin, N.; Iosub, S.; Balescu, L.M.; Catalin Galca, A.; Claudiu Popa, A.; Oktar, F.N.; et al. Animal Origin Bioactive Hydroxyapatite Thin Films Synthesized by RF-Magnetron Sputtering on 3D Printed Cranial Implants. Metals 2019, 9, 1332. [Google Scholar] [CrossRef]
- Firdaus Hussin, M.S.; Abdullah, H.Z.; Idris, M.I.; Abdul Wahap, M.A. Extraction of Natural Hydroxyapatite for Biomedical Applications—A Review. Heliyon 2022, 8, e10356. [Google Scholar] [CrossRef] [PubMed]
- Borciani, G.; Fischetti, T.; Ciapetti, G.; Montesissa, M.; Baldini, N.; Graziani, G. Marine Biological Waste as a Source of Hydroxyapatite for Bone Tissue Engineering Applications. Ceram. Int. 2023, 49, 1572–1584. [Google Scholar] [CrossRef]
- Khurshid, Z.; Alfarhan, M.F.; Mazher, J.; Bayan, Y.; Cooper, P.R.; Dias, G.J.; Adanir, N.; Ratnayake, J. Extraction of Hydroxyapatite from Camel Bone for Bone Tissue Engineering Application. Molecules 2022, 27, 7946. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; Govoni, M.; Vivarelli, L.; Boi, M.; De Carolis, M.; Bianchi, M.; Sassoni, E.; Bignozzi, M.C.; Carnevale, G.; Marmi, F.; et al. A Comprehensive Microstructural and Compositional Characterization of Allogenic and Xenogenic Bone: Application to Bone Grafts and Nanostructured Biomimetic Coatings. Coatings 2020, 10, 522. [Google Scholar] [CrossRef]
- Antonakos, A.; Liarokapis, E.; Leventouri, T. Micro-Raman and FTIR Studies of Synthetic and Natural Apatites. Biomaterials 2007, 28, 3043–3054. [Google Scholar] [CrossRef]
- Tao, J. FTIR and Raman Studies of Structure and Bonding in Mineral and Organic–Mineral Composites. In Methods in Enzymology; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 532, pp. 533–556. ISBN 9780124166172. [Google Scholar]
- Rehman, I.; Bonfield, W. Characterization of Hydroxyapatite and Carbonated Apatite by Photo Acoustic FTIR Spectroscopy. J. Mater. Sci. Mater. Med. 1997, 8, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Koutsopoulos, S. Synthesis and Characterization of Hydroxyapatite Crystals: A Review Study on the Analytical Methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.; Li, Y.; Wen, C. Ion-Substituted Calcium Phosphate Coatings by Physical Vapor Deposition Magnetron Sputtering for Biomedical Applications: A Review. Acta Biomater. 2019, 89, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Bolbasov, E.N.; Lapin, I.N.; Svetlichnyi, V.A.; Lenivtseva, Y.D.; Malashicheva, A.; Malashichev, Y.; Golovkin, A.S.; Anissimov, Y.G.; Tverdokhlebov, S.I. The Formation of Calcium Phosphate Coatings by Pulse Laser Deposition on the Surface of Polymeric Ferroelectric. Appl. Surf. Sci. 2015, 349, 420–429. [Google Scholar] [CrossRef]
- Lenis, J.A.; Hurtado, F.M.; Gómez, M.A.; Bolívar, F.J. Effect of Thermal Treatment on Structure, Phase and Mechanical Properties of Hydroxyapatite Thin Films Grown by RF Magnetron Sputtering. Thin Solid. Films 2019, 669, 571–578. [Google Scholar] [CrossRef]
- Rau, J.V.; Generosi, A.; Laureti, S.; Komlev, V.S.; Ferro, D.; Cesaro, S.N.; Paci, B.; Albertini, V.R.; Agostinelli, E.; Barinov, S.M. Physicochemical Investigation of Pulsed Laser Deposited Carbonated Hydroxyapatite Films on Titanium. ACS Appl. Mater. Interfaces 2009, 1, 1813–1820. [Google Scholar] [CrossRef]

| Ca10(PO4)6(OH)2 |  |  |
|---|---|---|
| Synthetic Hydroxyapatite | Natural Derived-Hydroxyapatite | Human Bone Hydroxyapatite |
| Ca/P ratio 1.67 | Ca/P lower than 1.67 | Ca/P lower than 1.67 |
| Absence of ion-doping | Different ion substitutions Carbonate rich | Different ion substitutions Carbonate rich |
| Lower solubility | Higher solubility | Higher solubility |
| Higher crystallinity | Lower crystallinity with respect to synthetic HA | Lower crystallinity with respect to synthetic HA |
| S_HA | B_HA | E_HA | P_HA | |||||
|---|---|---|---|---|---|---|---|---|
| Target | Coating | Target | Coating | Target | Coating | Target | Coating | |
| Mg (wt.% ± s.d.) | - | - | 0.57 ± 0.02 | 0.15 ± 0.02 | 0.30 ± 0.01 | 0.07 ± 0.04 | 0.52 ± 0.09 | 0.16 ± 0.08 |
| Na (wt.% ± s.d.) | - | - | 0.69 ± 0.44 | 0.21 ± 0.03 | 0.86 ± 0.06 | 0.24 ± 0.20 | 0.68 ± 0.01 | 0.53 ± 0.26 |
| Ca/P (at.) | 1.67 | 1.91 | 1.57 | 1.84 | 1.46 | 1.95 | 1.36 | 1.85 |
| (Ca + Mg)/P | - | - | 1.63 | 1.91 | 1.51 | 1.90 | 1.43 | 1.77 |
| S_HA_c | B_HA_c | E_HA_c | P_HA_c | |
|---|---|---|---|---|
| Dm [nm] | 79 ± 25 | 66 ± 35 | 80 ± 40 | 92 ± 9 |
| DM [nm] | 1372 ± 221 | 1329 ± 30 | 1328 ± 336 | 1432 ± 90 |
| Da [nm] | 298 ± 208 | 304 ± 191 | 304 ± 192 | 329 ± 215 |
| S_HA_c | B_HA_c | E_HA_c | P_HA_c | |
|---|---|---|---|---|
| Room T | 127 ± 11 | 111 ± 22 | 103 ± 18 | 119 ± 24 |
| 400 °C | 122 ± 34 | 118 ± 31 | 121 ± 42 | 129 ± 44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montesissa, M.; Sassoni, E.; Boi, M.; Borciani, G.; Boanini, E.; Graziani, G. Synthetic or Natural (Bio-Based) Hydroxyapatite? A Systematic Comparison between Biomimetic Nanostructured Coatings Produced by Ionized Jet Deposition. Nanomaterials 2024, 14, 1332. https://doi.org/10.3390/nano14161332
Montesissa M, Sassoni E, Boi M, Borciani G, Boanini E, Graziani G. Synthetic or Natural (Bio-Based) Hydroxyapatite? A Systematic Comparison between Biomimetic Nanostructured Coatings Produced by Ionized Jet Deposition. Nanomaterials. 2024; 14(16):1332. https://doi.org/10.3390/nano14161332
Chicago/Turabian StyleMontesissa, Matteo, Enrico Sassoni, Marco Boi, Giorgia Borciani, Elisa Boanini, and Gabriela Graziani. 2024. "Synthetic or Natural (Bio-Based) Hydroxyapatite? A Systematic Comparison between Biomimetic Nanostructured Coatings Produced by Ionized Jet Deposition" Nanomaterials 14, no. 16: 1332. https://doi.org/10.3390/nano14161332







