Nebivolol Polymeric Nanoparticles-Loaded In Situ Gel for Effective Treatment of Glaucoma: Optimization, Physicochemical Characterization, and Pharmacokinetic and Pharmacodynamic Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical and Bioanalytical Methods
2.3. Selection of Excipients and Process Parameters for the Preparation of NEB-PNPs
2.4. Formulation of NEB-PNPs
2.4.1. Optimization by Applying Design of Experiments (DoE) for the Preparation of NEB-PNPs
2.4.2. Optimization and Validation of the Regression Equations
2.5. Evaluation of Various Physical Characteristics of NEB-PNPs
2.5.1. Analysis of Particle Size and Zeta Potential of NEB-PNPs
2.5.2. Analysis of Drug Loading and Entrapment Efficiency of NEB-PNPs
2.6. Scanning Electron Microscopy (SEM) Imaging of the Optimized NEB-PNPs
2.7. Differential Scanning Calorimetry (DSC) of the Optimized NEB-PNPs
2.8. X-ray Diffractometric Analysis of the Optimized NEB-PNPs
2.9. Preparation of NEB-PNPs-Loaded Dual-Sensitive In Situ Gel
2.10. Rheological Evaluation of NEB-PNPs-ISG Formulation
2.11. In Vitro Drug-Release Studies of NEB-PNPs-Susp and NEB-PNPs-ISG Formulations
2.12. In Vivo Evaluation of Optimized NEB-PNPs and NEB-PNPs-ISG Formulations
2.12.1. Ocular Pharmacokinetic Studies
2.12.2. Ocular Pharmacodynamic Studies of NEB-PNP Formulations
3. Results and Discussion
3.1. Preliminary Trials for the Preparation of NEB-PNPs
3.2. Optimization of NEB-PNPs
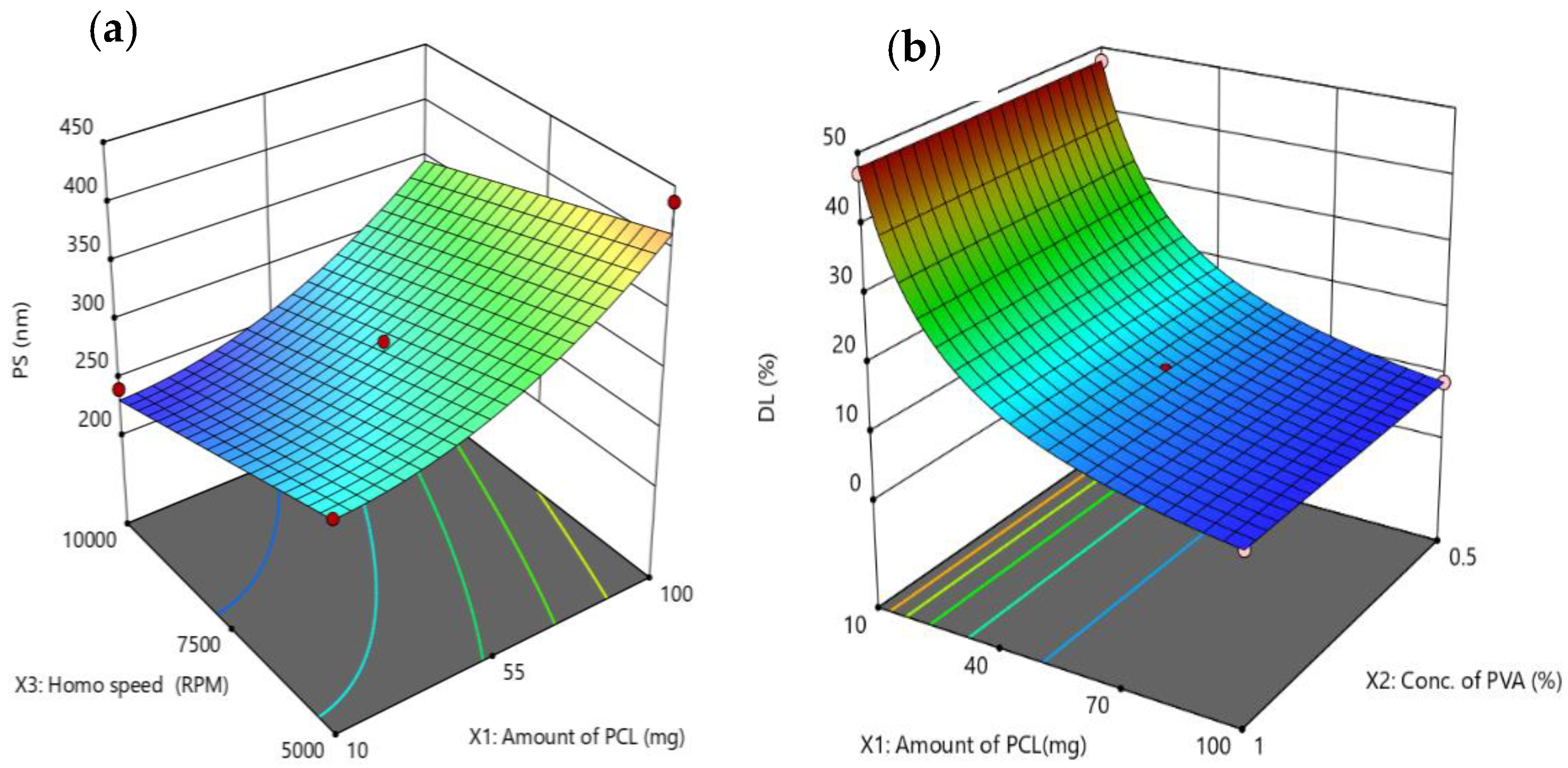
3.2.1. Impact of Critical Factors on Particle Size of NEB-PNPs
3.2.2. Impact of Critical Factors on Drug Loading of NEB-PNPs
3.2.3. Validation of the Regression Equations for Particle Size and Loading Efficiency
3.3. Physical Characterization of the Optimized NEB-PNPs Using Zeta-Sizer, SEM, DSC, and pXRD
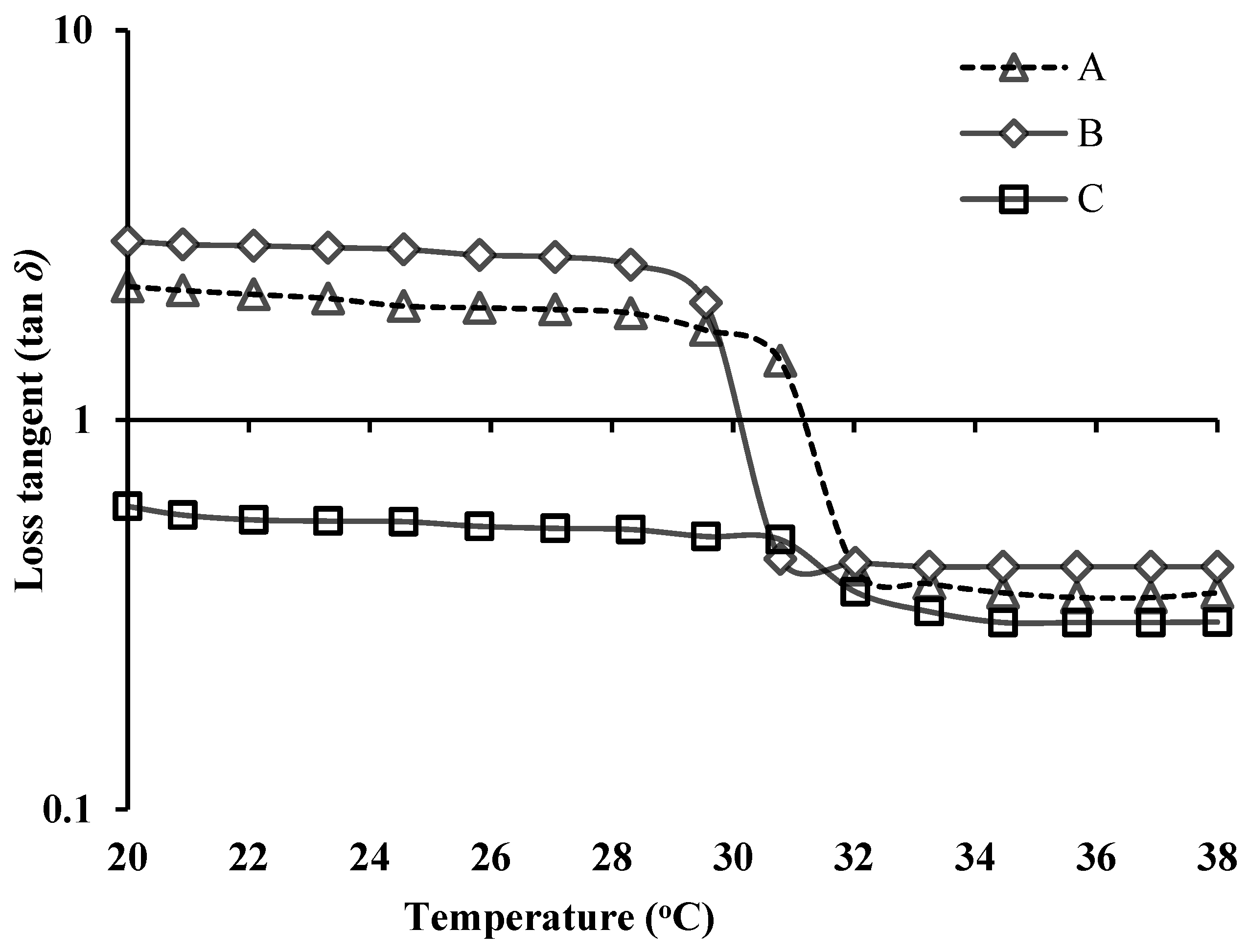
3.4. Rheological Evaluation of NEB-PNPs-ISG Formulation
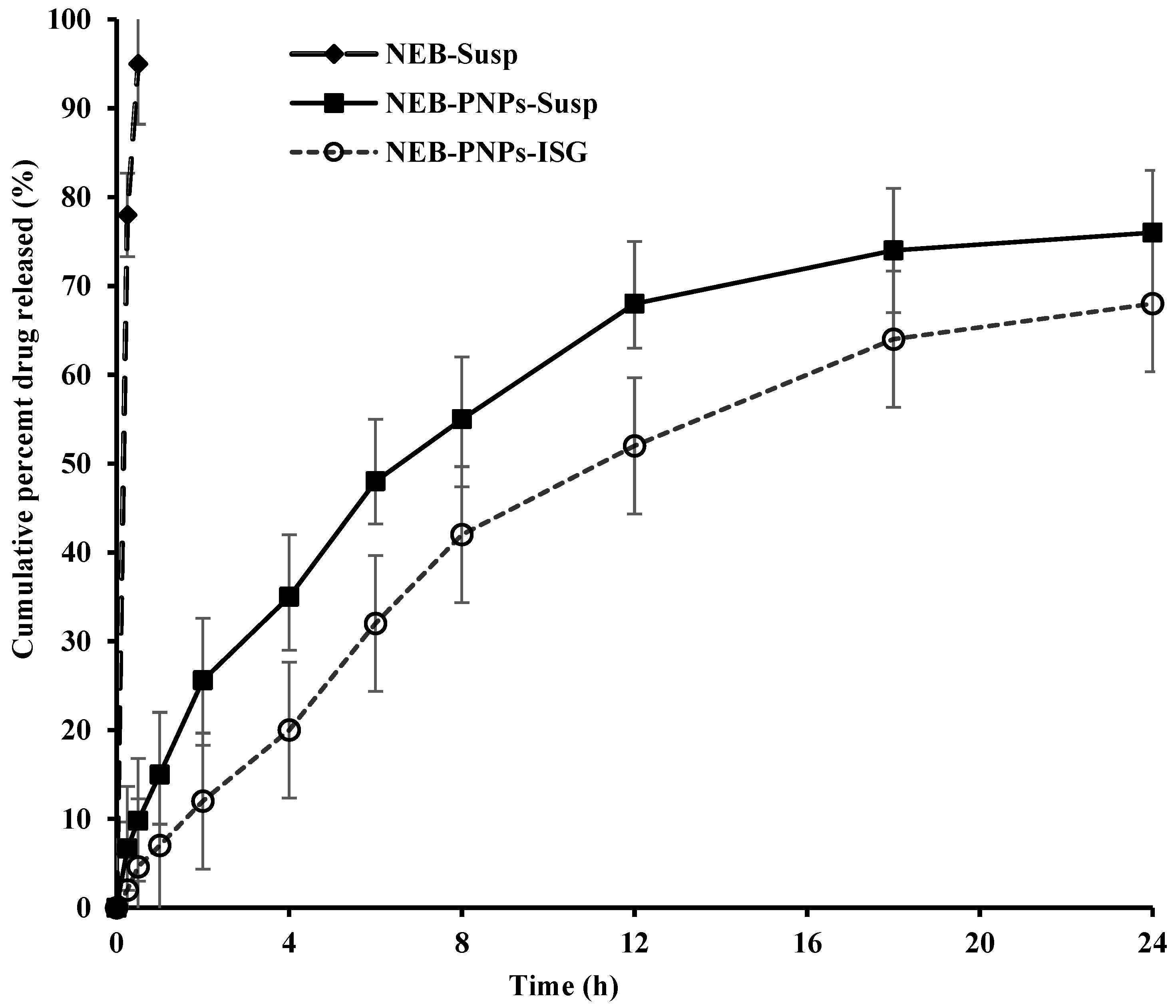
3.5. Drug-Release Studies of NEB-PNPs Formulations
3.6. Stability Studies of PNPs Formulations
3.7. In Vivo Studies of the NEB-PNPs Formulations
3.7.1. Ocular Pharmacokinetic Studies of NEB-PNPs Formulations
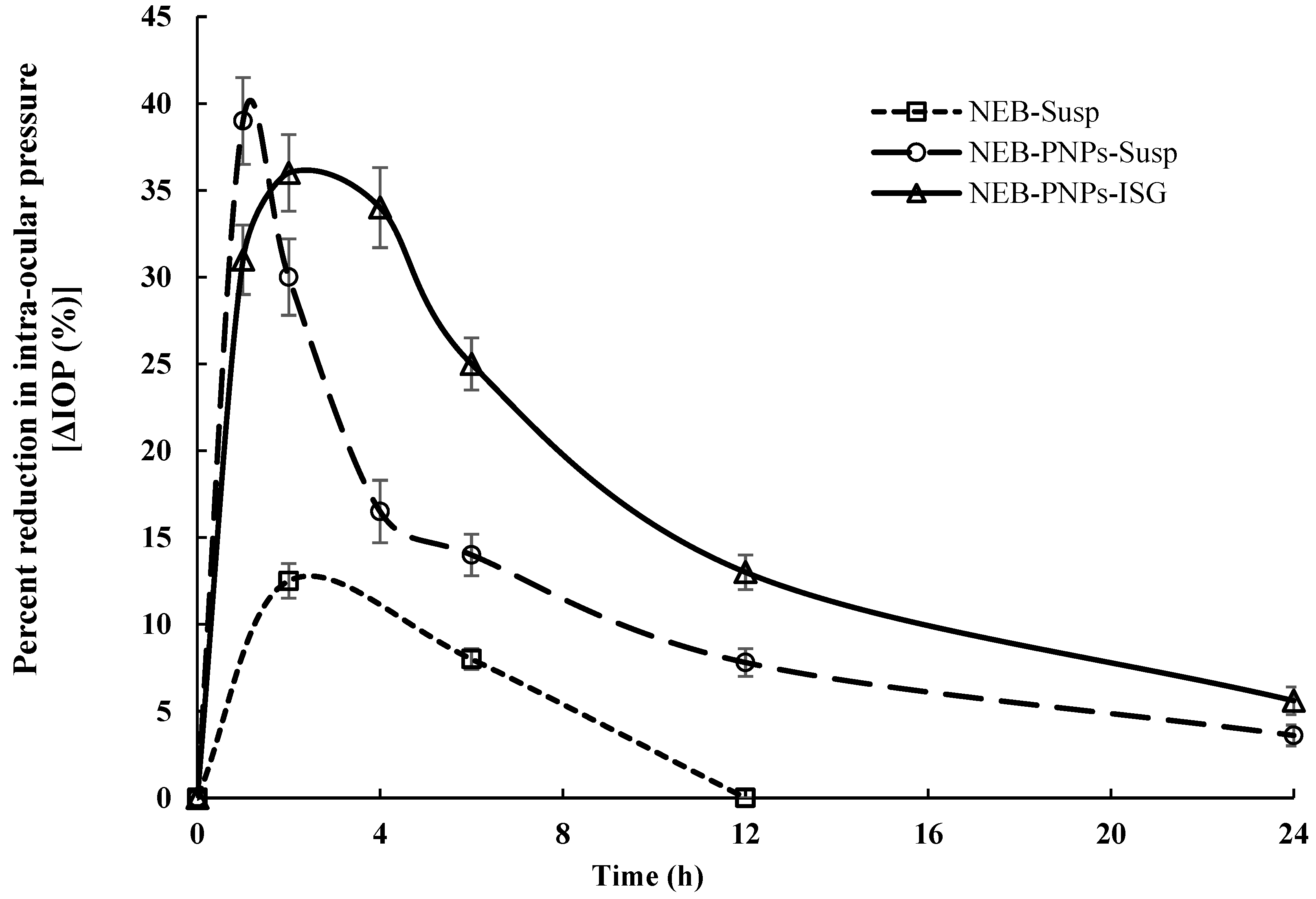
3.7.2. Ocular Pharmacodynamic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Geewoo, N.P.; Hyuck, J.L. Chemical Insights into Topical Agents in Intraocular Pressure Management: From Glaucoma Etiopathology to Therapeutic Approaches. Pharmaceutics 2024, 16, 274. [Google Scholar] [CrossRef] [PubMed]
- Maffei, A.; Lembo, G. Nitric oxide mechanisms of nebivolol. Ther. Adv. Cardiovasc. Dis. 2009, 3, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Pradeep Singh, R.; Punna Rao, R.; Shahid Iqbal, M.; Mohammed Shareef, K.; Himanshu, K.; Prasanna, K.; Upendra, B. Design, Characterization and Pharmacokinetic–Pharmacodynamic Evaluation of Poloxamer and Kappa-Carrageenan-Based Dual-Responsive In Situ Gel of Nebivolol for Treatment of Open-Angle Glaucoma. Pharmaceutics 2023, 15, 405. [Google Scholar] [CrossRef]
- Kolawole, M.O.; Cook, T., M. In situ gelling drug delivery systems for topical drug delivery. Eur. J. Pharm. Biopharm. 2023, 184, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Yumei, W.; Yuanyuan, L.; Xinyue, L.; Dereje, K.; Bing, Z.; Jing, R.; Jun, L.; Jiawei, L.; Shouying, D.; Zhidong, L. Research progress of in-situ gelling ophthalmic drug delivery system. Asian J. Pharm. Sci. 2019, 14, 1–15. [Google Scholar]
- Mohammed Shareef, K.; Punna Rao, R.; Divya Shrikant, D. Design, optimization and pharmacokinetic evaluation of PLGA phosphatidylcholine hybrid nanoparticles of triamcinolone acetonide loaded in situ gel for topical ocular delivery. Int. J. Pharm. 2023, 647, 123530. [Google Scholar]
- Almeida, H.; Amaral, H.M.; Lobão, P.; Silva, C.A.; Sousa Loboa, J.M. Applications of polymeric and lipid nanoparticles in ophthalmic pharmaceutical formulations: Present and future considerations. J. Pharm. Pharm. Sci. 2014, 17, 278–293. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Salama, A.H.; AbouSamra, M.M.; Awad, E.A.G.; Mansy, S.S. Promising bioadhesive ofloxacin-loaded polymeric nanoparticles for the treatment of ocular inflammation: Formulation and in vivo evaluation. Drug Deliv. Transl. Res. 2021, 11, 1943–1957. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Chauhan, M.K. Optimization and evaluation of encapsulated brimonidine tartrate-loaded nanoparticles incorporation in situ gel for efficient intraocular pressure reduction. J. Sol-Gel Sci. Technol. 2020, 95, 190–201. [Google Scholar] [CrossRef]
- Szabo, Z.I.; Szabo, T.; Emoke, R.; Sipos, E. Validated HPLC method for determination of nebivolol in pharmaceutical dosage form and in vitro dissolution studies. Stud. Univ. BabesBolyai. Chem. 2014, 59, 195–203. [Google Scholar]
- Rawat, P.S.; Ravi, P.R.; Kaswan, L.; Raghuvanshi, R.S. Development and validation of a bio-analytical method for simultaneous quantification of nebivolol and labetalol in aqueous humor and plasma using LC-MS/MS and its application to ocular pharmacokinetic studies. J. Chromatogr. B 2020, 1136, 121908. [Google Scholar] [CrossRef] [PubMed]
- Fereig, S.A.; El-Zaafarany, G.M.; Arafa, M.G.; Abdel-Mottaleb, M.M.A. Self-assembled tacrolimus-loaded lecithin-chitosan hybrid nanoparticles for in vivo management of psoriasis. Int. J. Pharm. 2021, 608, 121114. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Saraf, S.; Pradhan, M.; Patel, R.J.; Singhvi, G.; Alexander, A. Design and optimization of curcumin loaded nano lipid carrier system using Box-Behnken design. Biomed. Pharmacother. 2021, 141, 111919. [Google Scholar] [CrossRef]
- Garms, B.C.; Hamish, P.; Darcy, B.; Felicity, Y.H.; Andrew, K.W.; Anitha, A.; Lisbeth, G. Evaluating the effect of synthesis, isolation, and characterisation variables on reported particle size and dispersity of drug loaded PLGA nanoparticles. Mater. Adv. 2021, 2, 5657–5671. [Google Scholar] [CrossRef]
- Dalvi, A.; Ravi, P.R.; Uppuluri, C.T. Rufinamide-loaded chitosan nanoparticles in xyloglucan-based thermoresponsive in situ gel for direct nose to brain delivery. Front. Pharmacol. 2021, 12, 691936. [Google Scholar] [CrossRef] [PubMed]
- Murthy, A.; Ravi, P.R.; Kathuria, H.; Vats, R. Self-assembled lecithin-chitosan nanoparticles improve the oral bioavailability and alter the pharmacokinetics of raloxifene. Int. J. Pharm. 2020, 588, 119731. [Google Scholar] [CrossRef] [PubMed]
- Akbari, B.; Tavandashti, M.P.; Zandrahimi, M. Particle size characterization of nanoparticles–a practical approach. Iran. J. Mater. Sci. Eng. 2011, 8, 48–56. [Google Scholar]
- Ceulemans, J.; Ludwig, A. Optimisation of carbomer viscous eye drops: An in vitro experimental design approach using rheological techniques. Eur. J. Pharm. Biopharm. 2002, 54, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Gratieri, T.; Gelfuso, G.M.; Rocha, E.M.; Sarmento, V.H.; Freitas, D.O.; Lopez, R.F.V. A poloxamer/chitosan in situ forming gel with prolonged retention time for ocular delivery. Eur. J. Pharm. Biopharm. 2010, 75, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Singh, D.; Brar, V. Bioadhesive okra polymer based buccal patches as platform for controlled drug delivery. Int. J. Biol. Macromol. 2014, 70, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Azadi, S.; Ashrafi, H.; Azadi, A. Mathematical modeling of drug release from swellable polymeric nanoparticles. J. Appl. Pharm. Sci. 2017, 7, 125–133. [Google Scholar]
- Singhvi, G.; Singh, M. In-vitro drug release characterization models. Int. J. Pharm. Stud. Res. 2011, 2, 77–84. [Google Scholar]
- Javadzadeh, Y.; Hamedeyazdan, S. Floating Drug Delivery Systems for Eradication of Helicobacter pylori in Treatment of Peptic Ulcer Disease. In Trends in Helicobacter Pylori Infection; IntechOpen Limited: London, UK, 2014; p. 13. [Google Scholar] [CrossRef]
- Diwan, R.; Ravi, P.R.; Agarwal, S.I.; Aggarwal, V. Cilnidipine loaded poly (ε-caprolactone) nanoparticles for enhanced oral delivery: Optimization using DoE, physical characterization, pharmacokinetic, and pharmacodynamic evaluation. Pharm. Dev. Technol. 2021, 26, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Reddy, I.K.; Vaithiyalingam, S.R.; Khan, M.A.; Bodor, N.S. Intraocular pressure-lowering activity and in vivo disposition of dipivalyl terbutalone in rabbits. Drug Dev. Ind. Pharm. 2001, 27, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Genotoxicity and in vitro investigation of Gefitinib-loaded polycaprolactone fabricated nanoparticles for anticancer activity against NCI-H460 cell lines. J. Exp. Nanosci. 2022, 17, 214–246. [Google Scholar] [CrossRef]


| Design used for optimization | Box–Behnken design (BBD): A response surface methodology suitable to optimize and determine the mathematical equations relating three or four independent factors and one or more critical responses. |
| Criteria for selection the design | Though BBD and central composite design (CCD) are the two most popular optimization designs available, BBD was selected for the following reasons:
|
| No. of experimental runs used in the design | In the current work, which involves three critical factors, a total 17 runs were performed for optimization (12 factorial runs + 5 center-point runs) |
| Critical factors | X1—amount of PCL; X2—concentration of stabilizer; X3—homogenization speed |
| Critical responses | Y1—particle size; Y2—drug loading (%) |
| Method to determine optimal solution | Simultaneous optimization of both the critical responses based on the highest overall desirability value. |
| Source | Particle Size (Y1) | Drug Loading (Y2) | ||||||
|---|---|---|---|---|---|---|---|---|
| SS | DF | Fcal | Pcal | SS | DF | Fcal | Pcal | |
| Model | 38,605.75 | 3 | 36.68 | <0.0001 | 0.018 | 1 | 37,300 | <0.0001 |
| X1 | 27,097.92 | 1 | 77.23 | <0.0001 | 0.018 | 1 | 37,300 | <0.0001 |
| X3 | 8508.6 | 1 | 24.25 | 0.0003 | ||||
| X32 | 2999.23 | 1 | 8.55 | 0.0119 | ||||
| Residual | 4561.33 | 13 | 0.00001 | 15 | ||||
| Lack-of-Fit | 3913.55 | 9 | 2.69 | 0.1773 | 0.0001 | 11 | 1.16 | 0.482 |
| Pure Error | 647.78 | 4 | 0.000002 | 4 | ||||
| Total | 43,167.08 | 16 | 0.0177 | 16 | ||||
| Composition of Optimized NEB-PNPs | * Physicochemical Characteristics of Optimized NEB-PNPs | ||||
|---|---|---|---|---|---|
| PS (nm) | PDI | ZP (mV) | EE (%) | DL (%) | |
| Organic Phase: 10 mg of NEB + 25 mg of PCL dissolved in 1 mL of NMP Aqueous Phase: PVA (0.75% w/v in water) | 270.9 ± 6.3 | 0.24 ± 0.03 | −8.2 ± 1.2 | 96.7 ± 0.3 | 28.8 ± 2.4 |
| Biological Matrix | PK Parameters | Units | Treatments | ||
|---|---|---|---|---|---|
| NEB-Susp # | NEB-PNPs-Susp | NEB-PNPs-ISG | |||
| Aqueous humor | Cmax a | ng/mL | 28.2 ± 3.1 | 36.8 ± 3.2 | 30.2 ± 2.1 |
| Tmax b | h | 2.0 | 4.0 | 4.0 | |
| AUC0–t c | ng × h/mL | 189 | 204.4 | 329.2 | |
| MRT0–∞ c | h | 6.1 | 6.4 | 9.7 | |
| Plasma d | Cmax | ng/mL | 1.86 ± 0.1 | 1.15 ± 0.08 | 0.58 ± 0.03 |
| Tmax | h | 1.0 | 2.0 | 4.0 | |
| AUC0–t | ng × h/mL | 20.2 ± 2.7 | 12.1 ± 0.9 | 8.38 ± 0.56 | |
| MRT0–∞ | h | 25.8 ± 1.5 | 10.4 ± 1.1 | 4.6 ± 0.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rawat, P.S.; Ravi, P.R.; Khan, M.S.; Mahajan, R.R.; Szeleszczuk, Ł. Nebivolol Polymeric Nanoparticles-Loaded In Situ Gel for Effective Treatment of Glaucoma: Optimization, Physicochemical Characterization, and Pharmacokinetic and Pharmacodynamic Evaluation. Nanomaterials 2024, 14, 1347. https://doi.org/10.3390/nano14161347
Rawat PS, Ravi PR, Khan MS, Mahajan RR, Szeleszczuk Ł. Nebivolol Polymeric Nanoparticles-Loaded In Situ Gel for Effective Treatment of Glaucoma: Optimization, Physicochemical Characterization, and Pharmacokinetic and Pharmacodynamic Evaluation. Nanomaterials. 2024; 14(16):1347. https://doi.org/10.3390/nano14161347
Chicago/Turabian StyleRawat, Pradeep Singh, Punna Rao Ravi, Mohammed Shareef Khan, Radhika Rajiv Mahajan, and Łukasz Szeleszczuk. 2024. "Nebivolol Polymeric Nanoparticles-Loaded In Situ Gel for Effective Treatment of Glaucoma: Optimization, Physicochemical Characterization, and Pharmacokinetic and Pharmacodynamic Evaluation" Nanomaterials 14, no. 16: 1347. https://doi.org/10.3390/nano14161347
APA StyleRawat, P. S., Ravi, P. R., Khan, M. S., Mahajan, R. R., & Szeleszczuk, Ł. (2024). Nebivolol Polymeric Nanoparticles-Loaded In Situ Gel for Effective Treatment of Glaucoma: Optimization, Physicochemical Characterization, and Pharmacokinetic and Pharmacodynamic Evaluation. Nanomaterials, 14(16), 1347. https://doi.org/10.3390/nano14161347








