Hybrid Cellulosic Substrates Impregnated with Meta-PBI-Stabilized Carbon Nanotubes/Plant Extract-Synthesized Zinc Oxide—Antibacterial and Photocatalytic Dye Degradation Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Green Synthesis of Zinc Oxide Nanoparticles
2.3. Preparation of Polybenzimidazole-Stabilized Multi-Walled Carbon Nanotubes/Zinc Oxide Hybrid Dispersions
2.4. Surface Impregnation of Cellulosic Filter Paper Substrate with PBI@CNTs and PBI@CNTs/ZnO Dispersions
2.5. Physicochemical Characterization of Prepared PBI-Stabilized Carbon Nanotubes/Green Synthesized ZnO Composites
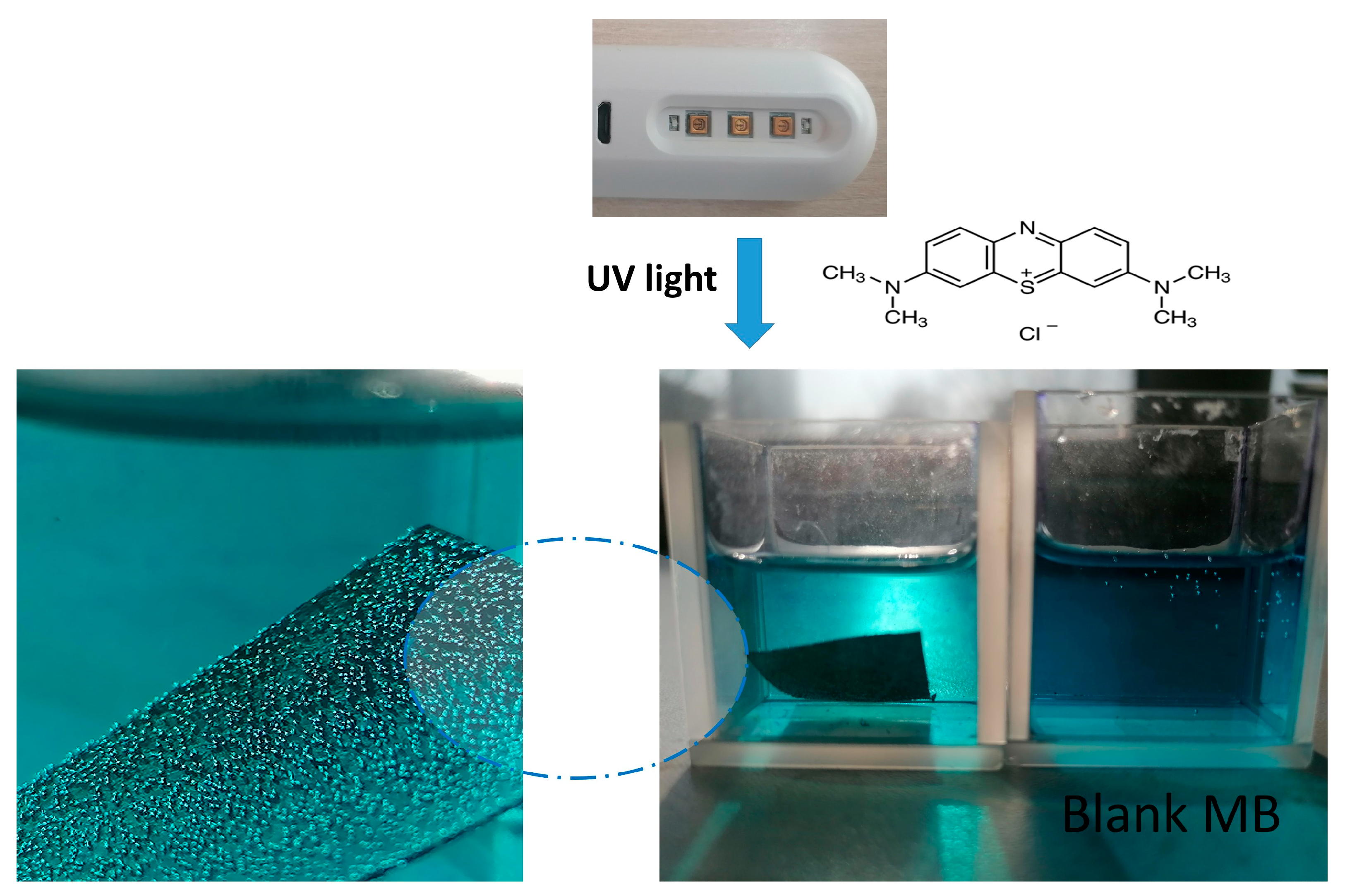
2.6. Photocatalytic Tests
2.7. Antibacterial Activity
2.7.1. Media and Test Microorganisms
2.7.2. Antibacterial Properties
Minimum Inhibitory Concentration (MIC) of ZnO and CNT Nanoparticles
2.7.3. Photocatalytic Antibacterial Activity of Bare and PBI-Stabilized ZnO Nanoparticles
2.7.4. Photocatalytic Antibacterial Activity of the Hybrid Materials
3. Results and Discussion
3.1. Preparation of PBI-Stabilized CNTs and/orZnO Impregnation Dispersions
3.2. Characterization of Prepared PBI-Stabilized Carbon Nanotubes/ZnO Dispersions and Impregnated Cellulose Substrates
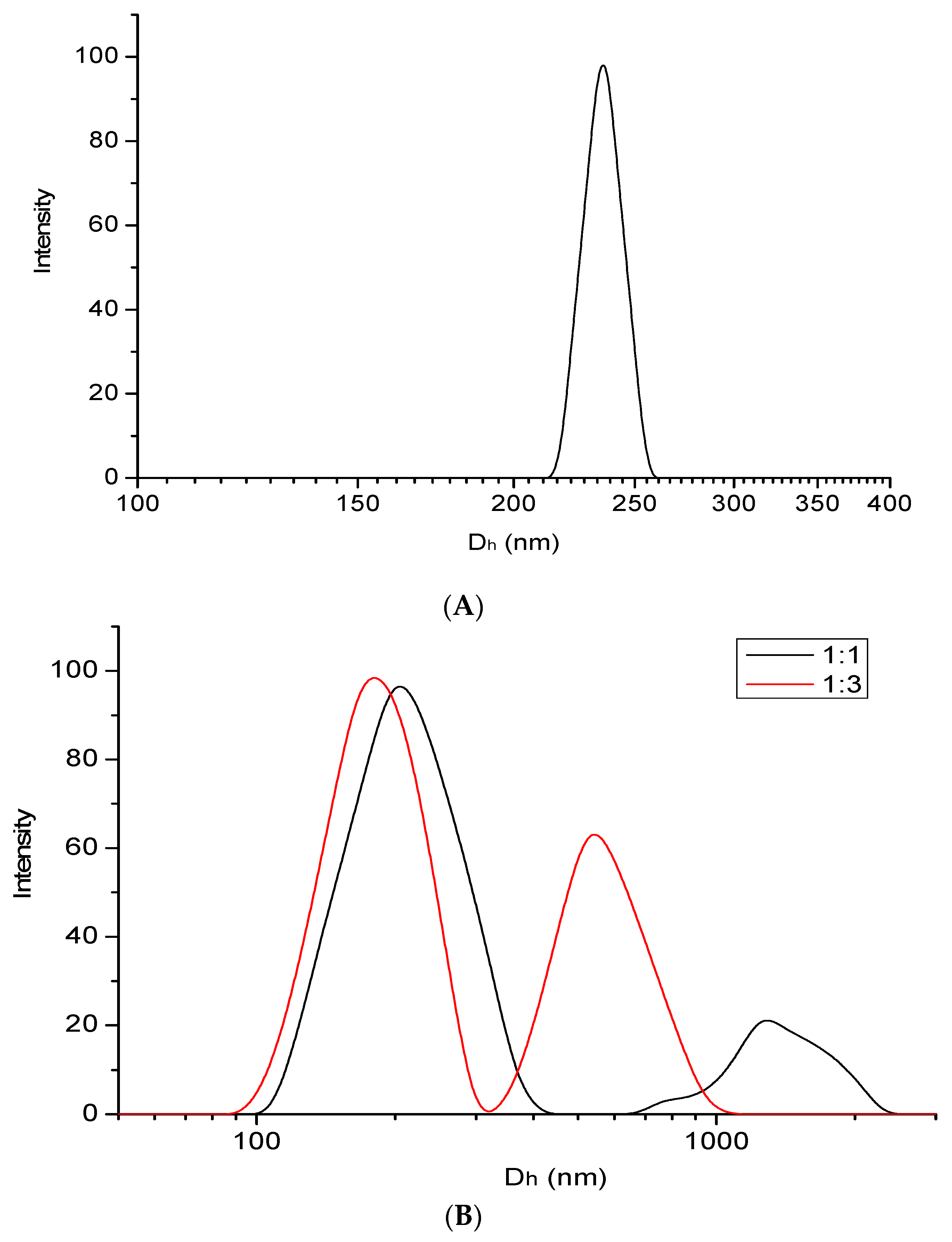
3.2.1. DLS Measurement of PBI@CNTs and PBI@CNTs/ZnO Dispersions
3.2.2. Impregnation of Cellulose Substrates with PBI@CNTs and/or ZnO Dispersions
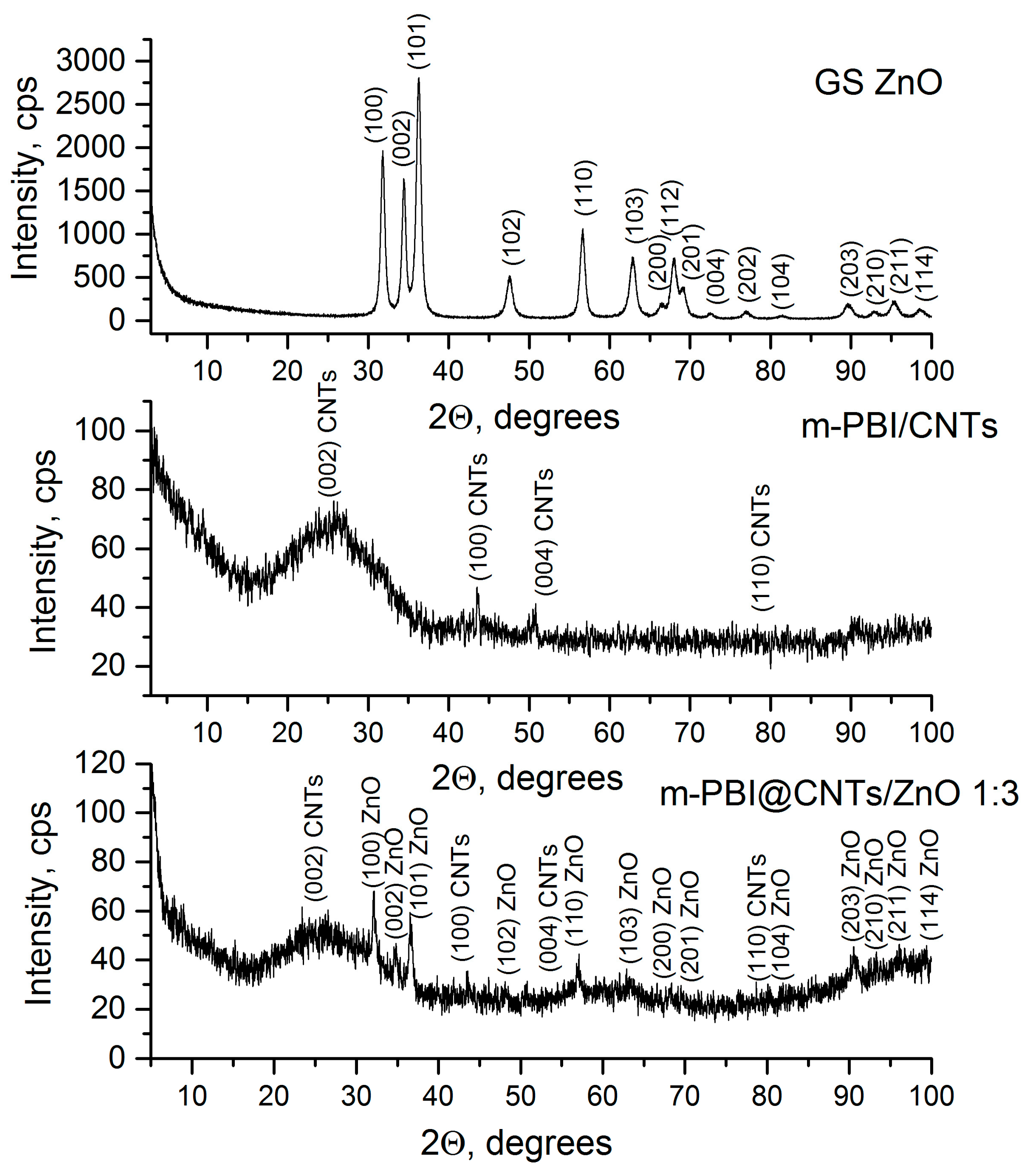
3.2.3. XRD Study
3.2.4. XPS Study of Impregnated Cellulosic Substrates
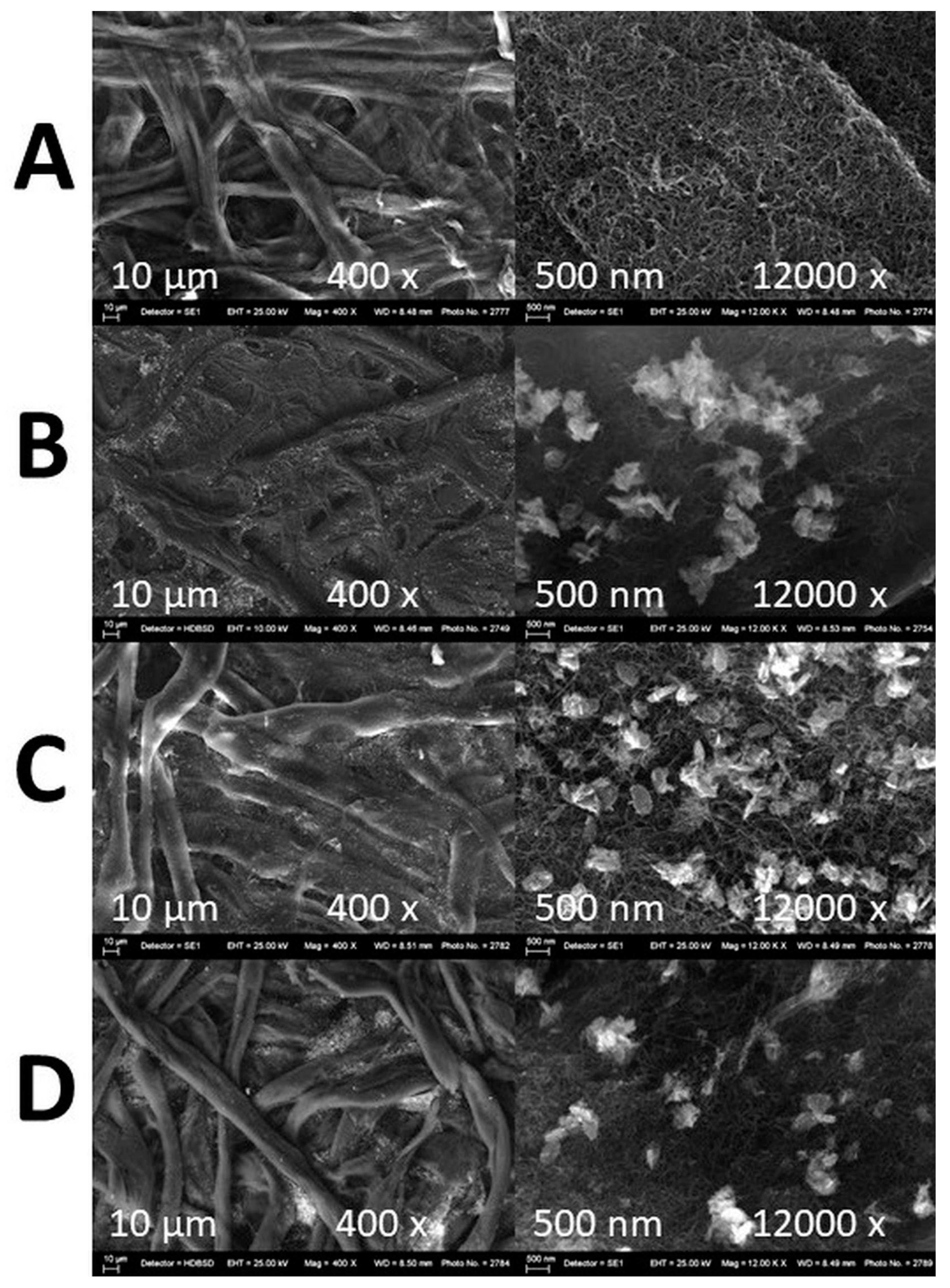
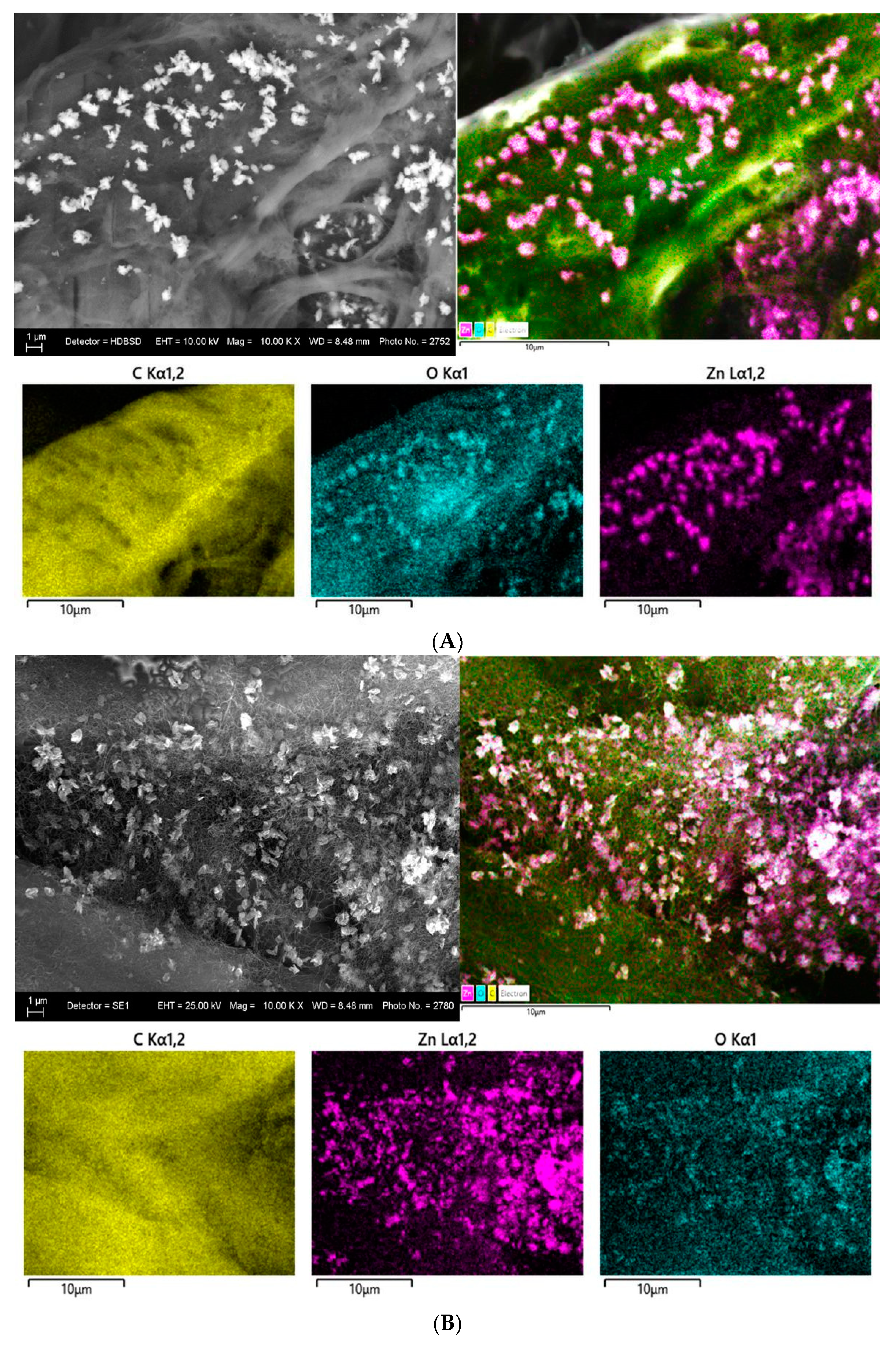
3.2.5. SEM and EDS Mapping/Spectra Analysis of the Photocatalyst Impregnated Cellulosic Scaffolds
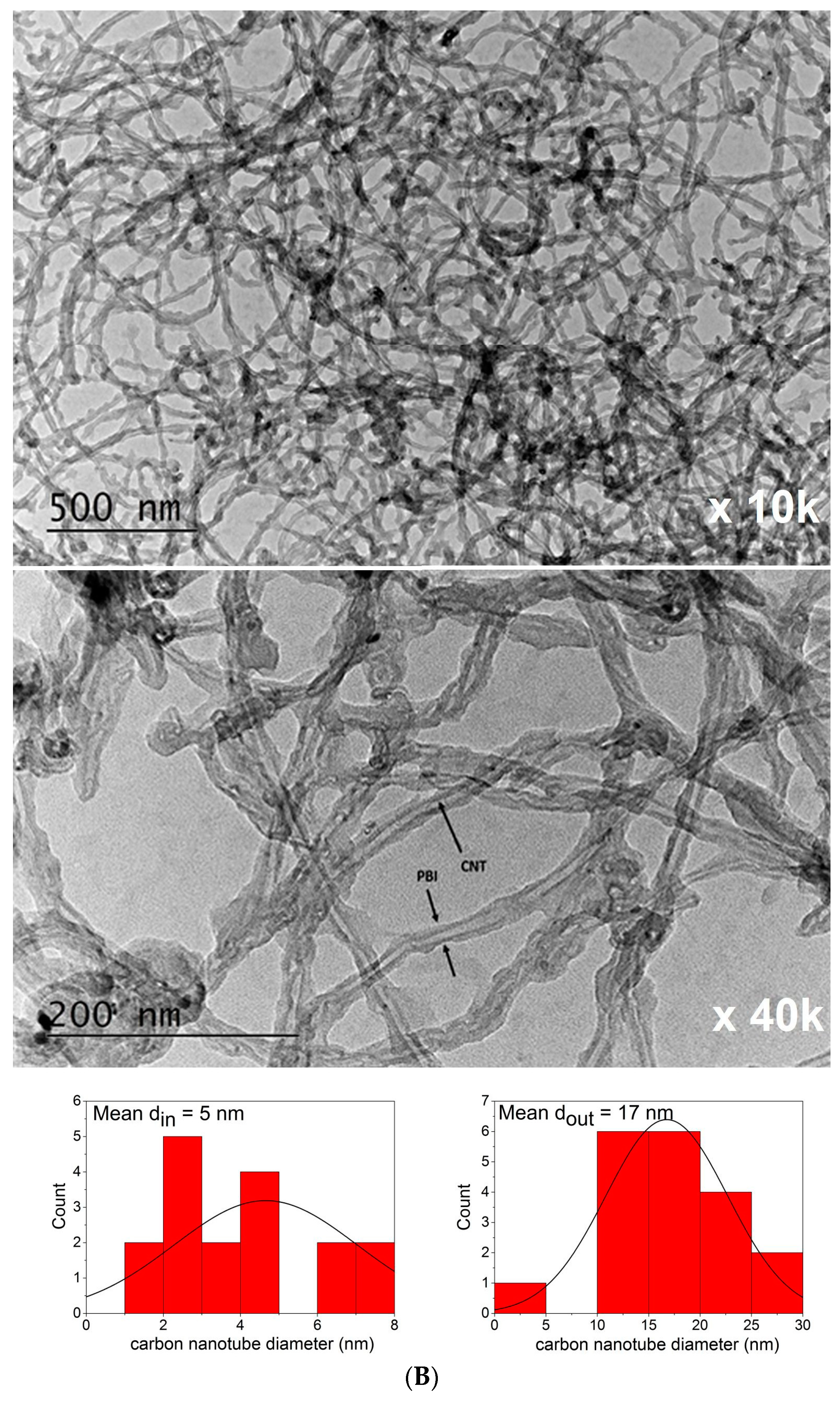
3.2.6. AFM and TEM Analysis
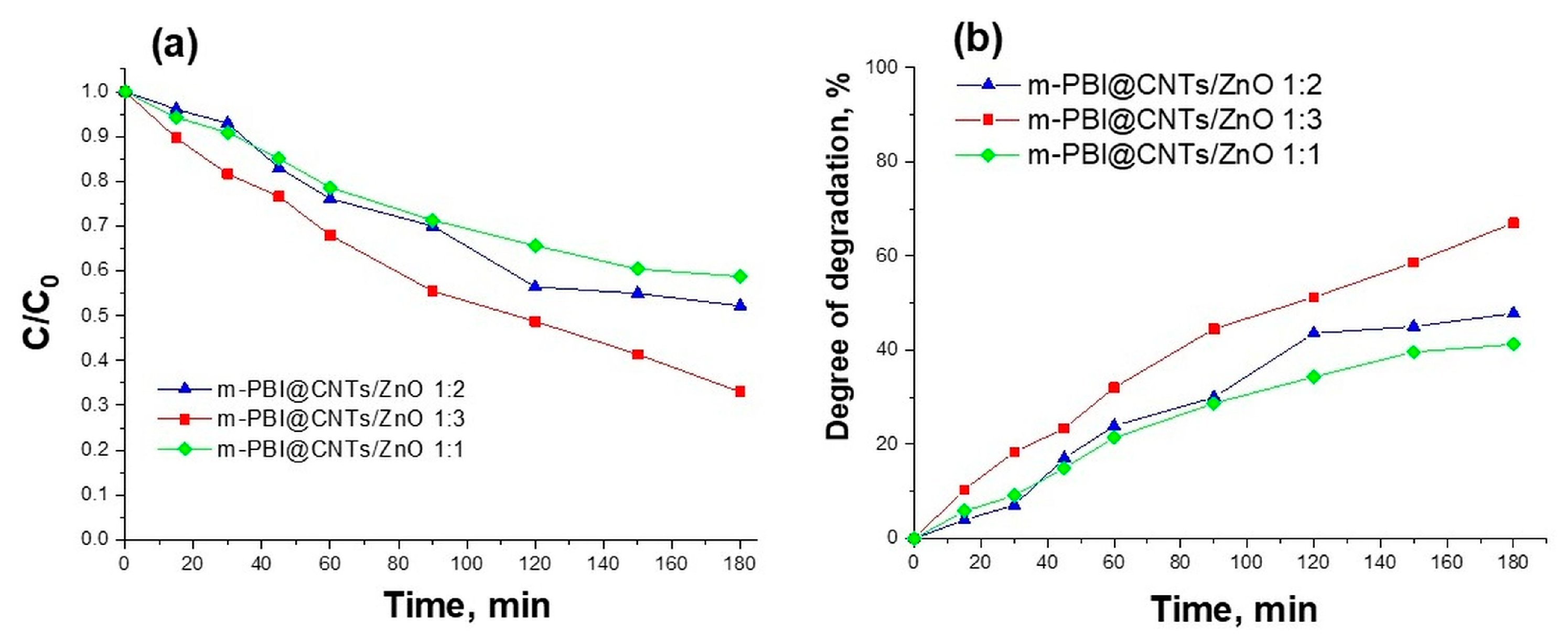
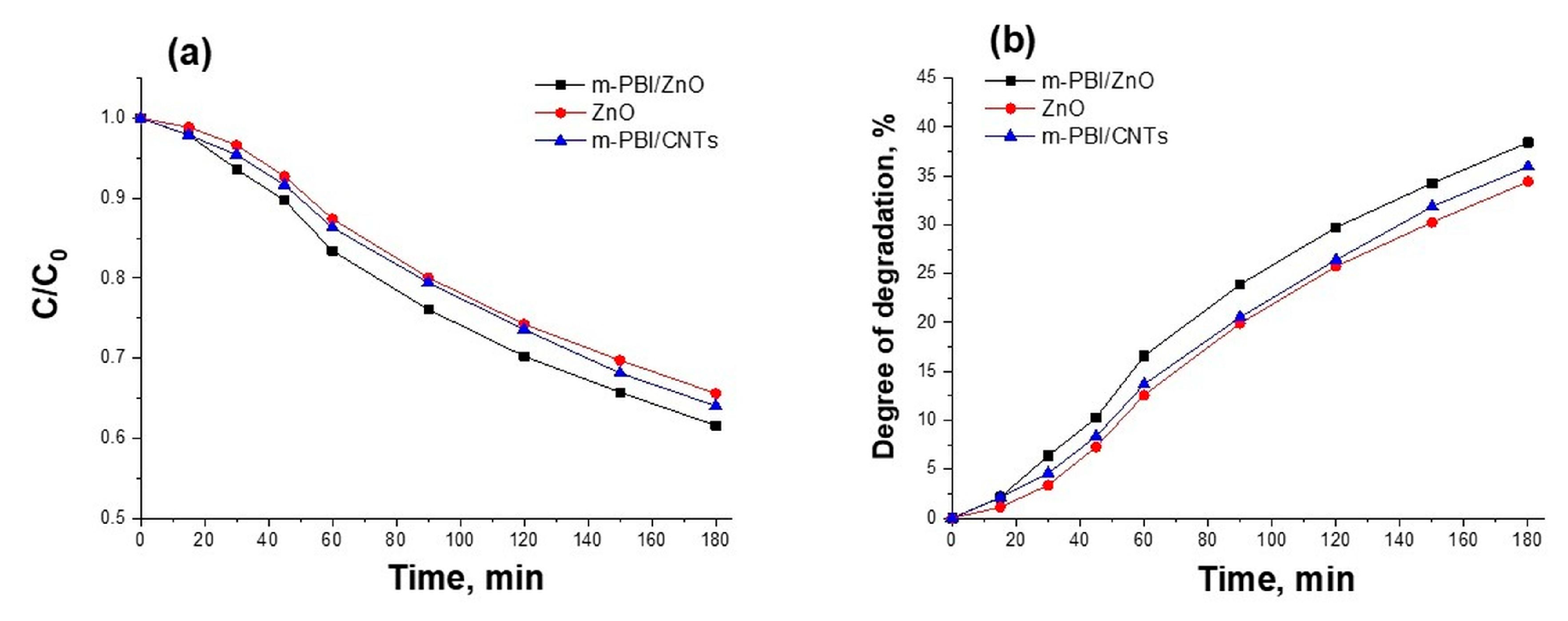
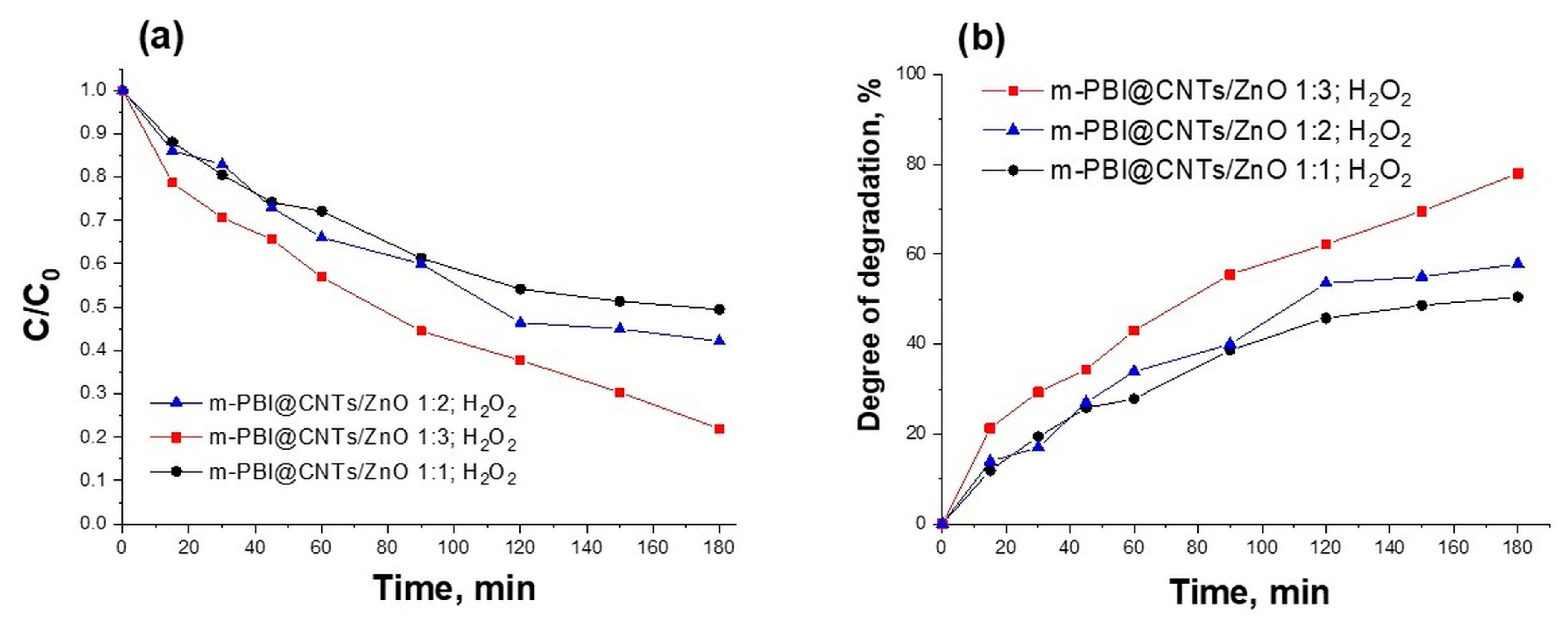
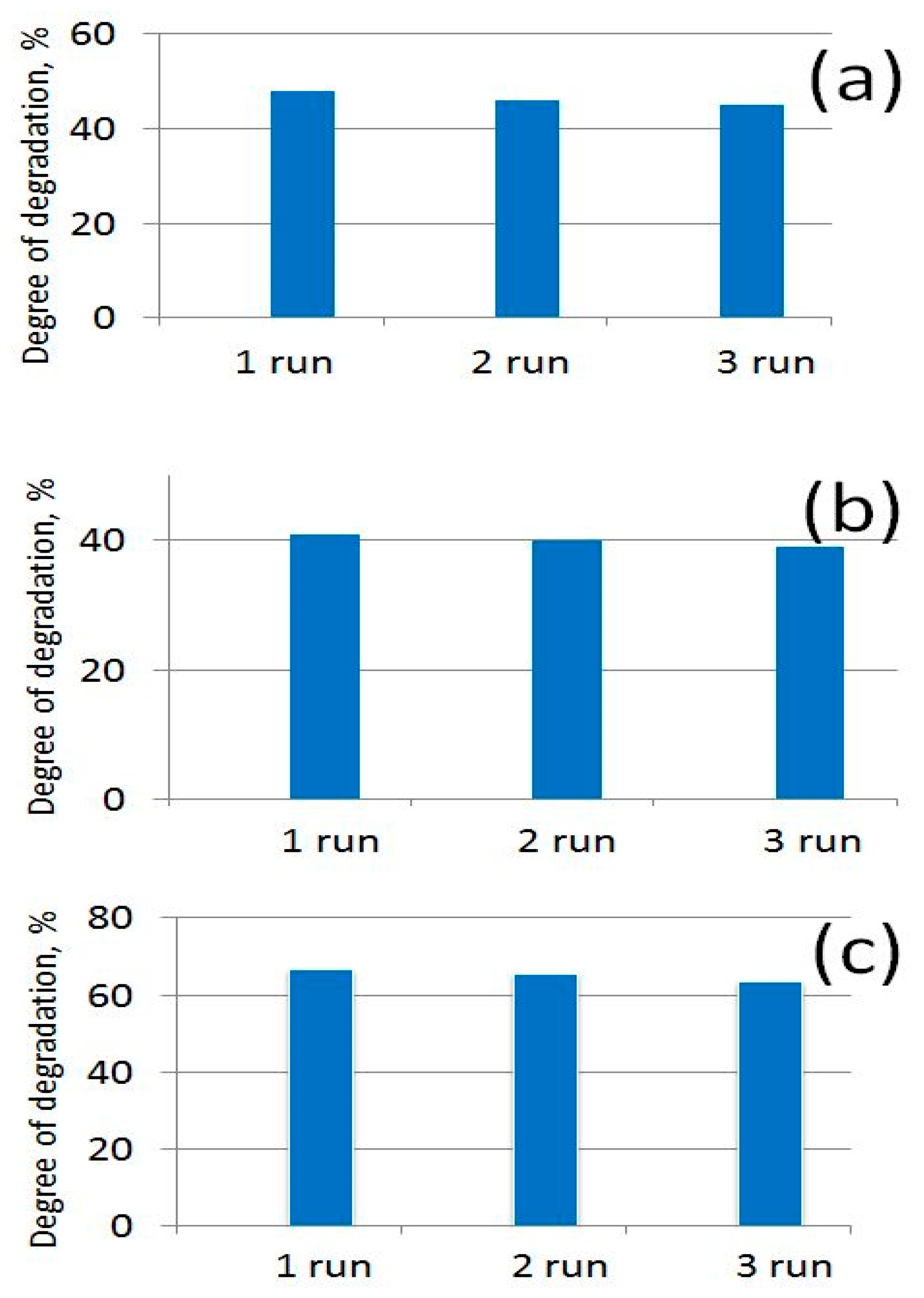
3.3. Photocatalytic Study of Prepared PBI-Stabilized Carbon Nanotubes/GREEN Synthesized ZnO Composites
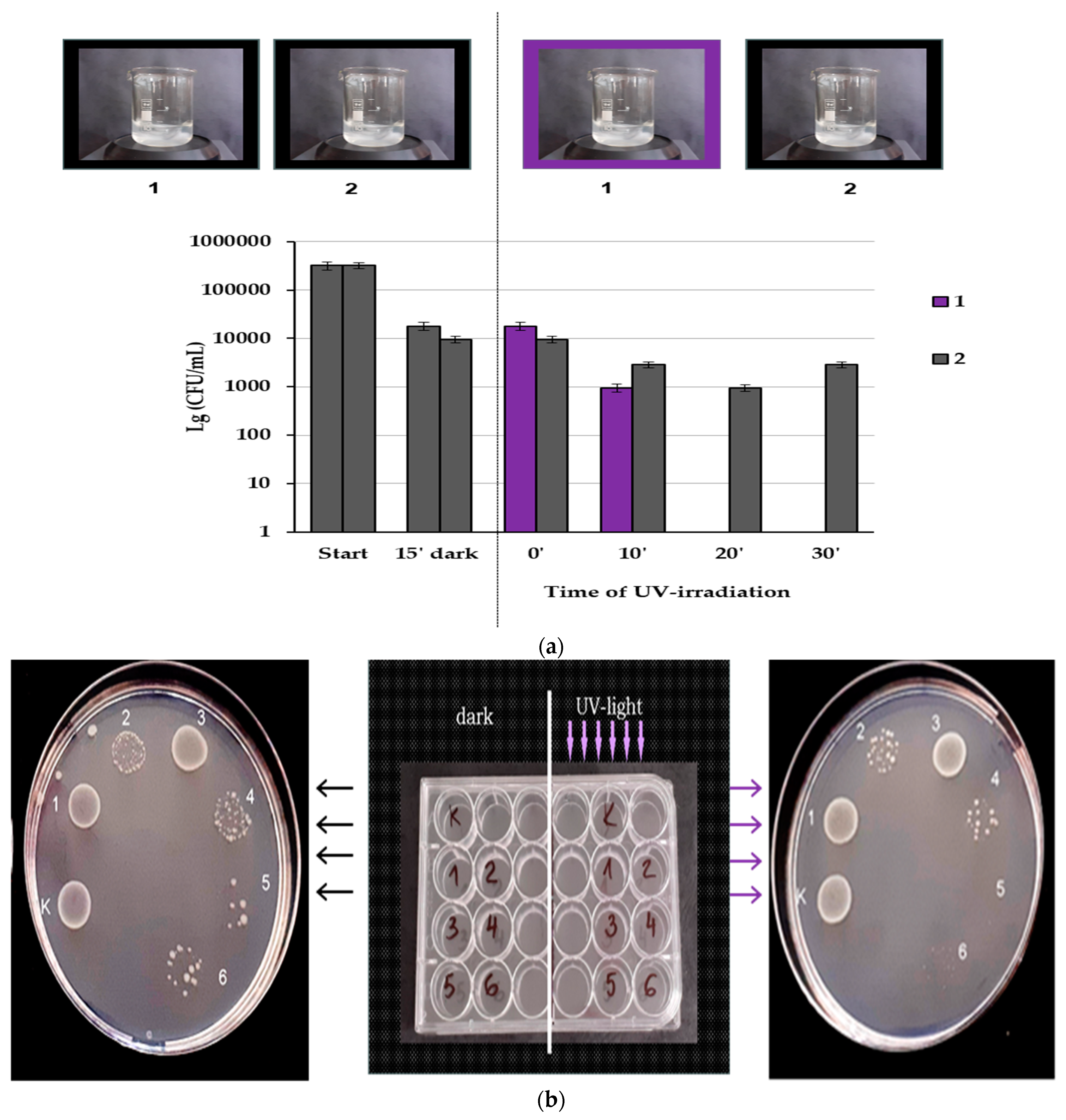
3.4. Antibacterial Activity of Prepared PBI-Stabilized Carbon Nanotubes/Green Synthesized ZnO Composites
Photocatalytic Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mary Rajaitha, P.; Shamsa, K.; Sheebha, I.; Vidhya, B.; Maheskumar, V.; Rajesh, S. Influence of the Positioning of the Incorporated Carbon Nanostructures on the Morphology and Photocatalytic Activity of Microwave Synthesized ZnO Nanorods. J. Nanosci. Nanotechnol. 2019, 19, 5303–5309. [Google Scholar] [CrossRef]
- Malekkiani, M.; Jannat Magham, A.H.; Ravari, F.; Dadmehr, M. Facile fabrication of ternary MWCNTs/ZnO/Chitosan nanocomposite for enhanced photocatalytic degradation of methylene blue and antibacterial activity. Sci. Rep. 2022, 12, 5927. [Google Scholar] [CrossRef] [PubMed]
- Dindar, B.; Içli, S. Unusual photoreactivity of zinc oxide irradiated by concentrated sunlight. J. Photochem. Photobiol. A Chem. 2001, 140, 263–268. [Google Scholar] [CrossRef]
- Shinde, D.; Tambade, P.; Chaskar, M.; Gadave, K. Photocatalytic degradation of dyes in water by analytical reagent grades ZnO, TiO2 and SnO2: A comparative study. Drink. Water Eng. Sci. Discuss. 2017, 10, 109–117. [Google Scholar] [CrossRef]
- Mohd Adnan, M.; Muhd Julkapli, N.; Amir, M.; Maamor, A. Effect on different TiO2 photocatalyst supports on photodecolorization of synthetic dyes: A review. Int. J. Environ. Sci. Technol. 2018, 16, 547–566. [Google Scholar] [CrossRef]
- Han, C.; Yang, M.; Weng, B.; Xu, Y. Improving the photocatalytic activity and antiphotocorrosion of semiconductor ZnO by coupling with versatile carbon. Phys. Chem. Chem. Phys. 2014, 16, 16891. [Google Scholar] [CrossRef] [PubMed]
- Kusiak-Nejman, E.; Wojnarowicz, J.; Morawski, A.W.; Narkiewicz, U.; Sobczak, K.; Gierlotka, S.; Lojkowski, W. Size-dependent effects of ZnO nanoparticles on the photocatalytic degradation of phenol in a water solution. Appl. Surf. Sci. 2021, 541, 148416. [Google Scholar] [CrossRef]
- Krishnamoorthy, A.; Varghese, S. Role of surfactants on the stability of nano-zinc oxide dispersions. Particul. Sci. Technol. 2015, 35, 67–70. [Google Scholar]
- Hosseini Largani, S.; Akbarzadeh Pasha, M. The effect of concentration ratio and type of functional group on synthesis of CNT–ZnO hybrid nanomaterial by an in situ sol–gel process. Int. Nano Lett. 2017, 7, 25–33. [Google Scholar] [CrossRef]
- Sui, J.; Li, J.; Li, Z.; Cai, W. Synthesis and characterization of one-dimensional magnetic photocatalytic CNTs/Fe3O4–ZnO nanohybrids. Mater. Chem. Phys. 2012, 134, 229–234. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, M.; Zhang, S.; Dai, M.; Zhang, P.; Wang, S.; He, Z. Recent Progress on Carbon-Nanotube-Based Materials for Photocatalytic Applications: A Review. RRL Sol. 2022, 6, 2200243. [Google Scholar] [CrossRef]
- Byrne, M.T.; Gun’ko, Y.K. Recent Advances in Research on Carbon Nanotube–Polymer Composites. Adv. Mater. 2010, 22, 1672–1688. [Google Scholar] [CrossRef]
- Chen, C.S.; Liu, T.G.; Lin, L.W.; Xie, X.D.; Chen, X.H.; Liu, Q.C.; Liang, B.; Yu, W.W.; Qiu, C.Y. Multi-walled carbon nanotube-supported metal-doped ZnO nanoparticles and their photocatalytic property. J. Nanopart. Res. 2013, 15, 1295. [Google Scholar] [CrossRef] [PubMed]
- Araújo, E.S.; Pereira, M.F.G.; da Silva, G.M.G.; Tavares, G.F.; Oliveira, C.Y.B.; Faia, P.M. A Review on the Use of Metal Oxide-Based Nanocomposites for the Remediation of Organics-Contaminated Water via Photocatalysis: Fundamentals, Bibliometric Study and Recent Advances. Toxics 2023, 11, 658. [Google Scholar] [CrossRef]
- Mallakpour, S.; Khadem, E. Carbon nanotube–metal oxide nanocomposites: Fabrication, properties and applications. Chem. Eng. J. 2016, 302, 344–367. [Google Scholar] [CrossRef]
- Madhusudhana Reddy, M.; Ramanjaneya Reddy, G.; Chennakesavulu, K.; Sundaravadive, E.; Prasath, S.S.; Rabel, A.M.; Sreeramulu, J. Synthesis of zinc oxide and carbon nanotube composites by CVD method: Photocatalytic studies. J. Porous Mater. 2017, 24, 149–156. [Google Scholar] [CrossRef]
- Powers, E.J.; Serad, G.A. History and development of polybenzimidazoles. In High Performance Polymers: Their Origin and Development; Seymour, R.B., Kirshenbaum, G.S., Eds.; Elsevier: New York, NY, USA, 1986; pp. 355–373. [Google Scholar]
- Kausar, A. Polybenzimidazole-based nanocomposite: Current status and emerging developments. Polym. Plast. Technol. Mater. 2019, 58, 1979–1992. [Google Scholar] [CrossRef]
- Wu, J.-F.; Lo, C.-F.; Li, L.-Y.; Li, H.-Y.; Chang, C.-M.; Liao, K.-S.; Hu, C.-C.; Liu, Y.-L.; Lue, S.J. Thermally stable polybenzimidazole/carbon nano-tube composites for alkaline direct methanol fuel cell applications. J. Power Sources 2014, 246, 39–48. [Google Scholar] [CrossRef]
- Sudhaik, A.; Raizada, P.; Ahamad, T.; Alshehri, S.M.; Nguyen, V.-H.; Van Le, Q.; Thakur, S.; Kumar Thakur, V.; Selvasembian, R.; Singh, P. Recent advances in cellulose supported photocatalysis for pollutant mitigation: A review. Int. J. Biol. Macromol. 2023, 226, 1284–1308. [Google Scholar] [CrossRef]
- Gupta, K.; Sharma, B.; Garg, V.; Neelratan, P.P.; Kumar, V.; Kumar, D.; Sharma, S.K. Enhanced photocatalytic degradation of methylene blue and methyl orange using biogenic ZnO NPs synthesized via Vachellia nilotica (Babool) leaves extract. Hybrid Adv. 2024, 5, 100160. [Google Scholar] [CrossRef]
- Olaitan Ogunyemi, S.; Abdallah, Y.; Zhang, M.; Fouad, H.; Hong, X.; Ibrahim, E.; Islam Masum, M.M.; Hossain, A.; Mo, J.; Li, B. Green synthesis of zinc oxide nanoparticles using different plant extracts and their antibacterial activity against Xanthomonas oryzae pv. Oryzae. Artif. Cells Nanomed. Biotechnol. 2019, 47, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kumar, S.; Alok, A.; Kumar Upadhyay, S.; Rawat, M.; Tsang, D.C.W.; Bolan, N.; Kim, K.-H. The potential of green synthesized zinc oxide nanoparticles as nutrient source for plant growth. J. Clean. Prod. 2019, 214, 1061–1070. [Google Scholar] [CrossRef]
- Verma, R.; Pathak, S.; Srivastava, A.K.; Prawer, S.; Tomljenovic-Hanic, S. ZnO nanomaterials: Green synthesis, toxicity evaluation and new insights in biomedical applications. J. Alloys Compd. 2021, 876, 160175. [Google Scholar] [CrossRef]
- Mostafa, A.M.; Mwafy, E.A.; Toghan, A. ZnO nanoparticles decorated carbon nanotubes via pulsed laser ablation method for degradation of methylene blue dyes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127204. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Ghanem, M.A.; Khairy, M.; Naguib, E.; Alotaibi, N.H. Zinc oxide incorporated carbon nanotubes or graphene oxide nanohybrids for enhanced sonophotocatalytic degradation of methylene blue dye. Appl. Surf. Sci. 2019, 487, 539–549. [Google Scholar] [CrossRef]
- Phin, H.-Y.; Ong, Y.-T.; Sin, J.-C. Effect of carbon nanotubes loading on the photocatalytic activity of zinc oxide/carbon nanotubes photocatalyst synthesized via a modified sol-gel method. J. Environ. Chem. Eng. 2020, 8, 103222. [Google Scholar] [CrossRef]
- Hanif, M.A.; Kim, Y.-S.; Akter, J.; Kim, H.G.; Kwac, L.K. Fabrication of Robust and Stable N-Doped ZnO/Single-Walled Carbon Nanotubes: Characterization, Photocatalytic Application, Kinetics, Degradation Products, and Toxicity Analysis. ACS Omega 2023, 8, 16174–16185. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.A.; Akter, J.; Lee, I.; Islam, M.A.; Sapkota, K.P.; Abbas, H.G.; Hahn, J.R. Formation of chemical heterojunctions between ZnO nanoparticles and single-walled carbon nanotubes for synergistic enhancement of photocatalytic activity. J. Photochem. Photobiol. A Chem. 2021, 413, 113260. [Google Scholar] [CrossRef]
- Aljeboree, A.M.; Hussein, S.A.; Jawad, M.A.; Alkaim, A.F. Hydrothermal synthesis of eco-friendly ZnO/CNT nanocomposite and efficient removal of Brilliant Green cationic dye. Results Chem. 2024, 7, 101364. [Google Scholar] [CrossRef]
- Oda, A.M.; Khudheyer, F.Y.; Obaid, E.K. Photocatalytic Decolorization of Crystal Violate Dye Solution by ZnO/MWCNT Nanocomposite. Iraqi J. Sci. 2023, 64, 3748–3762. [Google Scholar] [CrossRef]
- Pang, Z.; Sun, X.; Wu, X.; Nie, Y.; Liu, Z.; Yue, L. Fabrication and application of carbon nanotubes/cellulose composite paper. Vacuum 2015, 122 Pt A, 135–142. [Google Scholar] [CrossRef]
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 184–192. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of Nanoparticle-Induced Oxidative Stress and Toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef]
- Mammari, N.; Lamouroux, E.; Boudier, A.; Duval, R.E. Current Knowledge on the Oxidative-Stress-Mediated Antimicrobial Properties of Metal-Based Nanoparticles. Microorganisms 2022, 10, 437. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Mohd Bakhori, S.K.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.D.M.R.; Lopes, P.R.M.; Bueno de Moraes, P.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef]
- Penchev, H.; Ublekov, F.; Budurova, D.; Sinigersky, V. Novel electrospun polybenzimidazole fibers and yarns from ethanol/potassium hydroxide solution. Mater. Lett. 2017, 187, 89–93. [Google Scholar] [CrossRef]
- Kraus, W.; Nolze, G. PowderCell for Windows; Federal Institute for Materials Research and Testing: Berlin, Germany, 2000. [Google Scholar]
- Williams, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- ASTM E2149-10; Standard Test Method for Determining the Antimicrobial Activity of Immobilized Antimicrobial Agents Under Dynamic Contact Conditions. ASTM: West Conshohocken, PA, USA, 2013.
- Marra, A.; Silvestre, C.; Duraccio, D.; Cimmino, S. Polylactic acid/zinc oxide biocomposite films for food packaging application. Int. J. Biol. Macromol. 2016, 88, 254–262. [Google Scholar] [CrossRef]
- Kórösi, L.; Pertics, B.; Schneider, G.; Bognár, B.; Kovács, J.; Meynen, V.; Scarpellini, A.; Pasquale, L.; Prato, M. Photocatalytic Inactivation of Plant Pathogenic Bacteria Using TiO2 Nanoparticles Prepared Hydrothermally. Nanomaterials 2020, 10, 1730. [Google Scholar] [CrossRef]
- Kang, J.-Y.; Eo, S.-M.; Jeon, I.-Y.; Choi, Y.S.; Tan, L.-S.; Baek, J.-B. Multifunctional Poly(2,5-benzimidazole)/Carbon Nanotube. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1067–1078. [Google Scholar] [CrossRef]
- Eren, E.O.; Ozkan, N.; Devrim, Y. Polybenzimidazole-modified carbon nanotubes as a support material for platinum-based hightemperature proton exchange membrane fuel cell electrocatalysts Composite Films. Int. J. Hydrogen Energy 2021, 46, 29556–29567. [Google Scholar] [CrossRef]
- Jung, W.R.; Choi, J.H.; Lee, N.; Shin, K.; Moon, J.-H.; Seo, Y.-S. Reduced damage to carbon nanotubes during ultrasound-assisted dispersion as a result of supercritical-fluid treatment. Carbon 2012, 50, 633–636. [Google Scholar] [CrossRef]
- Rennhofer, H.; Zanghellini, B. Dispersion State and Damage of Carbon Nanotubes and Carbon Nanofibers by Ultrasonic Dispersion: A Review. Nanomaterials 2021, 11, 1469. [Google Scholar] [CrossRef]
- Penchev, H.; Zaharieva, K.; Milenova, K.; Ublekov, F.; Dimova, S.; Budurova, D.; Staneva, M.; Stambolova, I.; Sinigersky, V.; Blaskov, V. Novel meta-and AB-polybenzimidazole/zinc oxide polymer hybrid nanomaterials for photocatalytic degradation of organic dyes. Mater. Lett. 2018, 230, 187–190. [Google Scholar] [CrossRef]
- Badaire, S.; Poulin, P.; Maugey, M.; Zakri, C. In situ measurements of nanotube dimensions in suspensions by depolarized dynamic light scattering. Langmuir 2004, 20, 10367–10370. [Google Scholar] [CrossRef]
- Malvern Instruments Limited. Whitepaper, Characterization of Single Wall Carbon Nanotubes (SWNTs) by Combining Dynamic Light Scattering and Raman Spectroscopy; Malvern Instruments Limited: Malvern, UK, 2015; pp. 1–10. [Google Scholar]
- Kuzmenko, V.; Wang, N.; Haque, M.; Naboka, O.; Flygare, M.; Svensson, K.; Gatenholm, P.; Liu, J.; Enoksson, P. Cellulose-derived carbon nanofibers/grapheme composite electrodes for powerful compact supercapacitors. RSC Adv. 2017, 7, 45968. [Google Scholar] [CrossRef]
- Xin, L.; Yang, F.; Qiu, Y.; Uzunoglu, A.; Rockward, T.; Borup, R.L.; Stanciu, L.A.; Li, W.; Xie, J. Polybenzimidazole (PBI) Functionalized Nanographene as Highly Stable Catalyst Support for Polymer Electrolyte Membrane Fuel Cells (PEMFCs). J. Electrochem. Soc. 2016, 163, F1228–F1236. [Google Scholar] [CrossRef]
- Moraes, R.A.; Matos, C.F.; Castro, E.G.; Schreiner, W.H.; Oliveira, M.M.; Zarbin, A.J.G. The Effect of Different Chemical Treatments on the Structure and Stability of Aqueous Dispersion of Iron- and Iron Oxide-Filled Multi-Walled Carbon Nanotubes. J. Braz. Chem. Soc. 2011, 22, 2191–2201. [Google Scholar] [CrossRef]
- Bagabas, A.; Alshammari, A.; Aboud, M.F.; Kosslick, H. Room-temperature synthesis of zinc oxide nanoparticles in different media and their application in cyanide photodegradation. Nanoscale Res. Lett. 2013, 8, 516. [Google Scholar] [CrossRef]
- Bagheri, M.; Rostami Najafabadi, N.; Borna, E. Removal of reactive blue 203 dye photocatalytic using ZnO nanoparticles stabilized on functionalized MWCNTs. J. King Saud Univ. Sci. 2020, 32, 799–804. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, E.; Hong, Z.L.; Ahmed, W.; Elhissi, A.; Khalid, N.R. Photocatalytic, sonocatalytic and sonophotocatalytic degradation of Rhodamine B using ZnO/CNTs composites photocatalysts. Ultrason. Sonochemistry 2014, 21, 761–773. [Google Scholar] [CrossRef]
- Amenu, B.; Taddesse, A.M.; Kebede, T.; Mengesha, E.T.; Bezu, Z. Polyaniline-supported MWCNTs/ZnO/Ag2CO3 composite with enhanced photocatalytic and antimicrobial applications. Environ. Nanotechnol. Monit. Manag. 2024, 21, 100926. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Abd Mutalib, M.; Mohd Hir, Z.A.; Zain, M.F.M.; Mohamad, A.B.; Minggu, L.J.; Awang, N.A.; Salleh, W.N.W. An overview on cellulose-based material in tailoring bio-hybrid nanostructured photocatalysts for water treatment and renewable energy applications. Int. J. Biol. Macromol. 2017, 103, 1232–1256. [Google Scholar] [CrossRef]
- Bu, I.Y.Y. Optoelectronic properties of solution synthesis of carbon nanotube/ZnO:Al:N nanocomposite and its potential as a photocatalyst. Mater. Sci. Semicond. Process. 2014, 22, 76–82. [Google Scholar] [CrossRef]
- Dostanić, J.M.; Lončarević, D.R.; Banković, P.T.; Cvetković, O.G.; Jovanović, D.M.; Mijin, D.Ž. Influence of process parameters on the photodegradation of synthesized azo pyridone dye in TiO2 water suspension under simulated sunlight. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2011, 46, 70–79. [Google Scholar]
- Klink, S.; Keller, A.B.; Wild, A.J.; Baumert, V.L.; Gube, M.; Lehndorff, E.; Meyer, N.; Mueller, C.W.; Phillips, R.P.; Pausch, J. Stable isotopes reveal that fungal residues contribute more to mineral-associated organic matter pools than plant residues. Soil Biol. Biochem. 2022, 168, 108634. [Google Scholar] [CrossRef]
- Kamaruzzaman, N.F.; Tan, L.P.; Hamdan, R.H.; Choong, S.S.; Wong, W.K.; Gibson, A.J.; Chivu, A.; de Fatima Pina, M. Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics? Int. J. Mol. Sci. 2019, 20, 2747. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef] [PubMed]
- Alekish, M.; Ismail, Z.B.; Albiss, B.; Nawasrah, S. In vitro antibacterial effects of zinc oxide nanoparticles on multiple drug-resistant strains of Staphylococcus aureus and Escherichia coli: An alternative approach for antibacterial therapy of mastitis in sheep. Vet. World 2018, 11, 1428–1432. [Google Scholar] [CrossRef]
- Maurer, E.I.; Comfort, K.K.; Hussain, S.M.; Schlager, J.J.; Mukhopadhyay, S.M. Novel platform development using an assembly of carbon nanotube, nanogold and immobilized RNA capture element towards rapid, selective sensing of bacteria. Sensors 2012, 12, 8135–8144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]



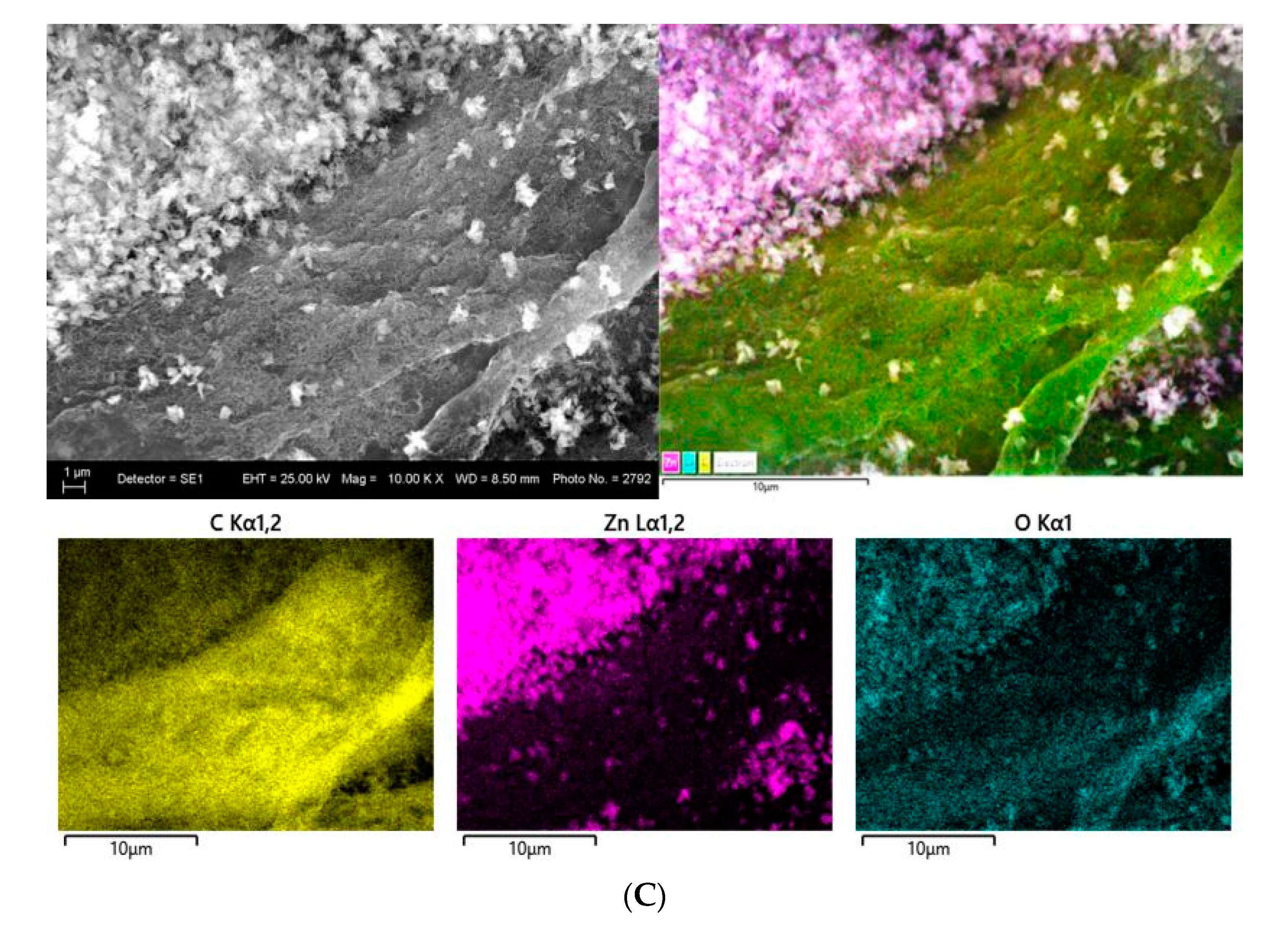
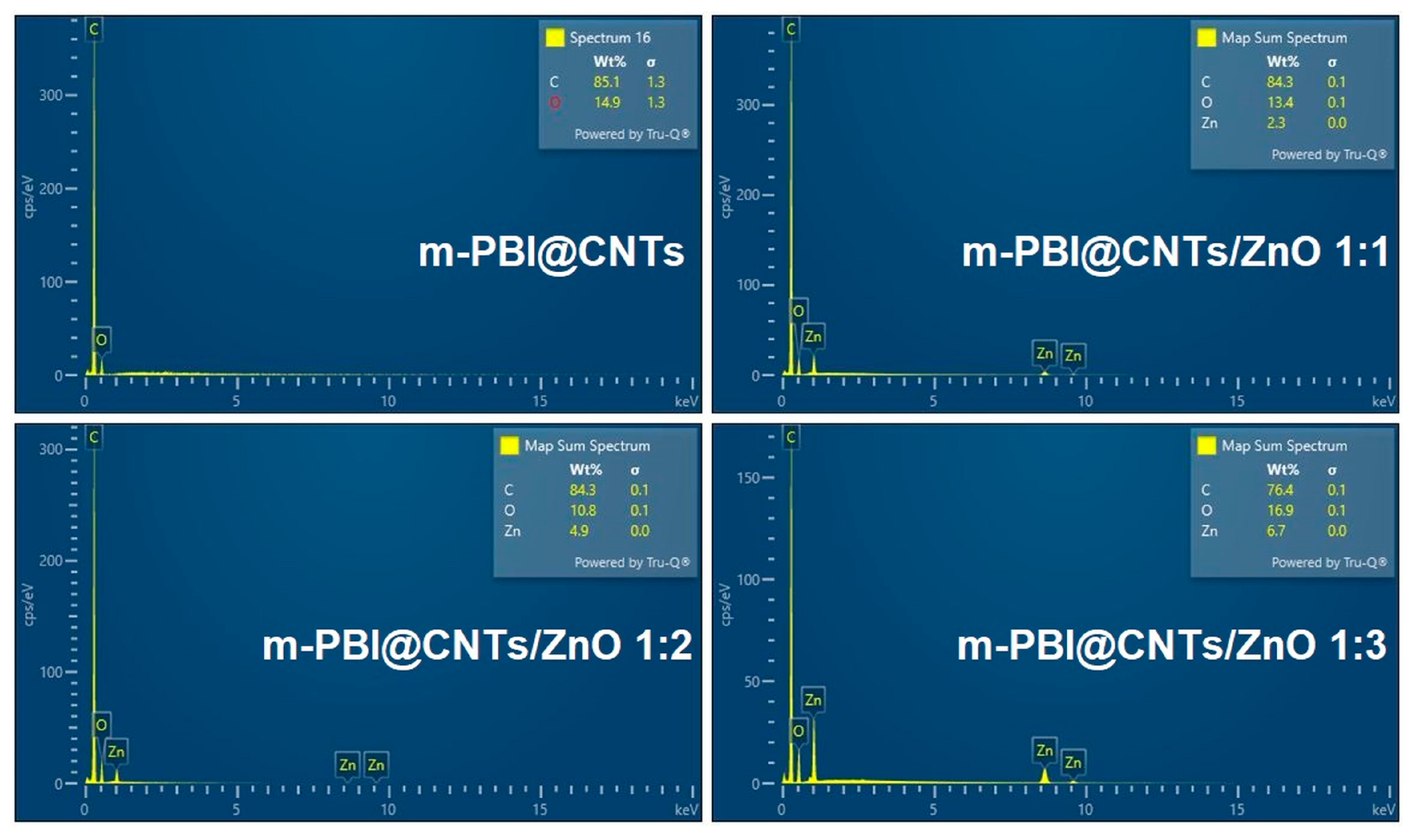
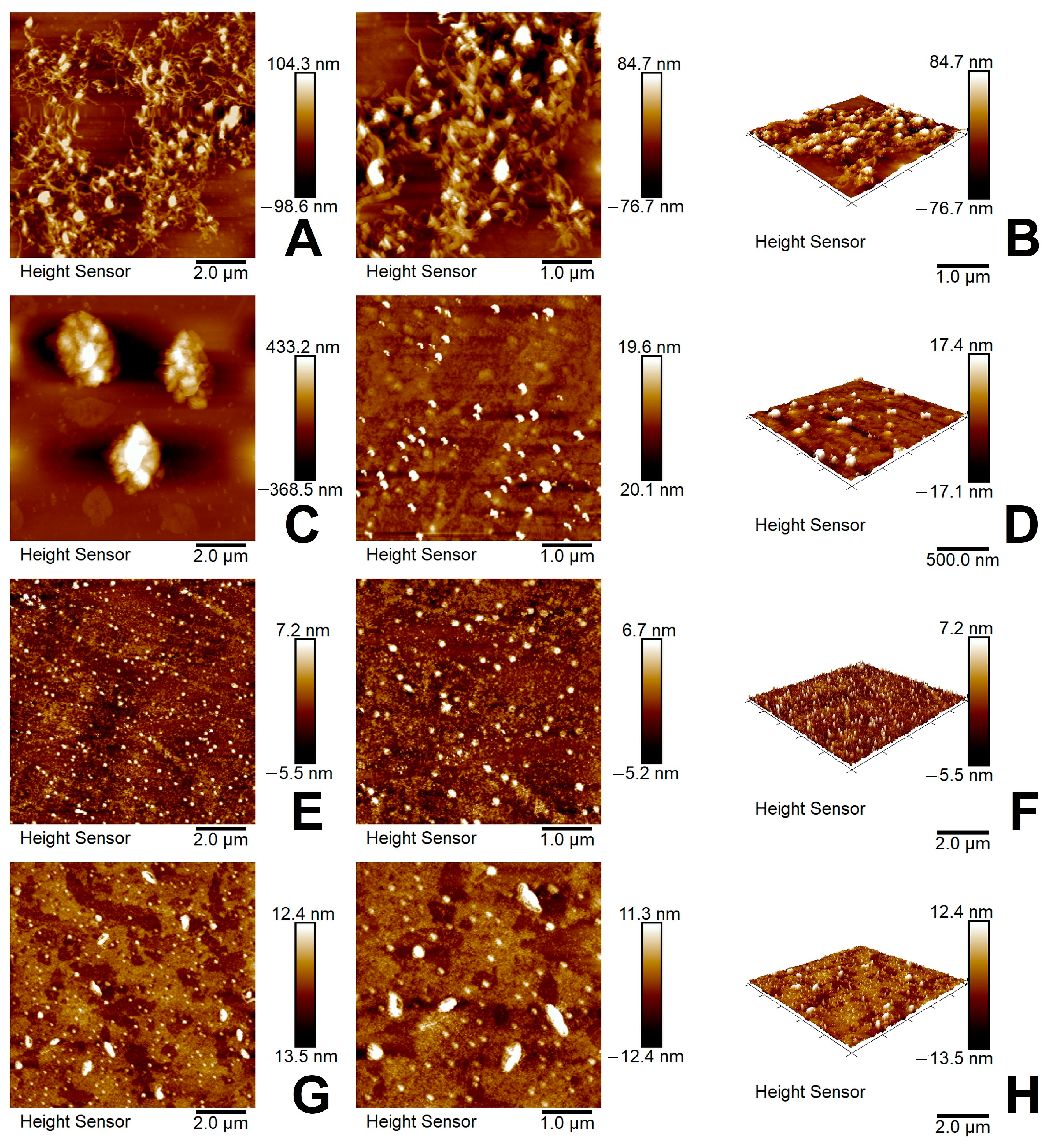
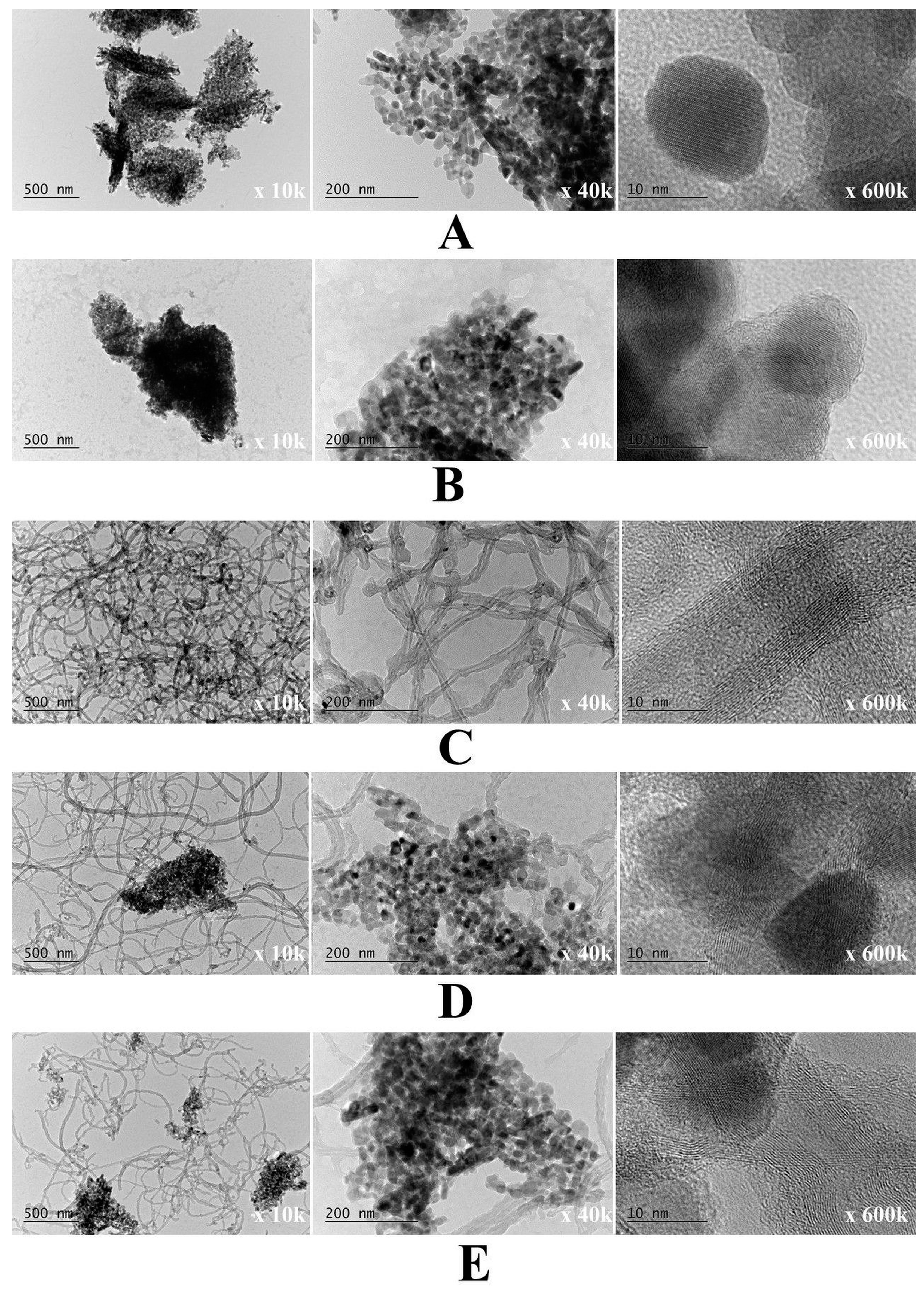




| Samples | C, at.% | O, at.% | N, at.% | Zn, at.% |
|---|---|---|---|---|
| Cellulose | 57.3 | 42.3 | 0.4 | - |
| Pure ZnO | 57.5 | 42.0 | 0.4 | 0.1 |
| m-PBI/CNTs | 59.9 | 33.1 | 3.5 | 3.5 |
| m-PBI/ZnO | 89.8 | 4.6 | 5.6 | - |
| m-PBI@CNTs/ZnO 1:1 | 65.1 | 31.6 | 3.0 | 0.3 |
| m-PBI@CNTs/ZnO 1:3 | 66.2 | 29.4 | 3.9 | 0.5 |
| Binding Energy, eV | Chemical Bonding | Concentration, % | ||
|---|---|---|---|---|
| Cellulose and Pure ZnO | Cellulose | Pure ZnO | ||
| 284.5 | C=C | 7.9 | 6.2 | |
| 286.2 | C-O/C=N | 70.2 | 52.7 | |
| 287.5 | C=O | 21.9 | 33.5 | |
| 288.6 | O-C=O | - | 7.6 | |
| ZnO-CNT-PBI | ZnO-CNT-PBI 3:1 | ZnO-CNT-PBI 1:1 | ZnO-PBI | |
| 284.4 | C=C sp2 | 23.4 | 18.6 | 11.9 |
| 285.3 | C-C sp3 | 17.7 | 17.1 | 16.3 |
| 286.7 | C-O/C=N | 42.0 | 46.6 | 40.7 |
| 287.8 | C=O | 14.1 | 14.9 | 24.9 |
| 289.2 | O-C=O | 2.8 | 2.7 | 6.21 |
| CNT-PBI | CNT-PBI | |||
| 284.4 | C=C sp2 | 81.9 | ||
| 285.3 | C-C sp3 | 12.6 | ||
| 286.7 | C-O/C=N | 5.5 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penchev, H.; Zaharieva, K.; Dimova, S.; Grancharov, G.; Petrov, P.D.; Shipochka, M.; Dimitrov, O.; Lazarkevich, I.; Engibarov, S.; Eneva, R. Hybrid Cellulosic Substrates Impregnated with Meta-PBI-Stabilized Carbon Nanotubes/Plant Extract-Synthesized Zinc Oxide—Antibacterial and Photocatalytic Dye Degradation Study. Nanomaterials 2024, 14, 1346. https://doi.org/10.3390/nano14161346
Penchev H, Zaharieva K, Dimova S, Grancharov G, Petrov PD, Shipochka M, Dimitrov O, Lazarkevich I, Engibarov S, Eneva R. Hybrid Cellulosic Substrates Impregnated with Meta-PBI-Stabilized Carbon Nanotubes/Plant Extract-Synthesized Zinc Oxide—Antibacterial and Photocatalytic Dye Degradation Study. Nanomaterials. 2024; 14(16):1346. https://doi.org/10.3390/nano14161346
Chicago/Turabian StylePenchev, Hristo, Katerina Zaharieva, Silvia Dimova, Georgy Grancharov, Petar D. Petrov, Maria Shipochka, Ognian Dimitrov, Irina Lazarkevich, Stephan Engibarov, and Rumyana Eneva. 2024. "Hybrid Cellulosic Substrates Impregnated with Meta-PBI-Stabilized Carbon Nanotubes/Plant Extract-Synthesized Zinc Oxide—Antibacterial and Photocatalytic Dye Degradation Study" Nanomaterials 14, no. 16: 1346. https://doi.org/10.3390/nano14161346






