Nebivolol Polymeric Nanoparticles-Loaded In Situ Gel for Effective Treatment of Glaucoma: Optimization, Physicochemical Characterization, and Pharmacokinetic and Pharmacodynamic Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical and Bioanalytical Methods
2.3. Selection of Excipients and Process Parameters for the Preparation of NEB-PNPs
2.4. Formulation of NEB-PNPs
2.4.1. Optimization by Applying Design of Experiments (DoE) for the Preparation of NEB-PNPs
2.4.2. Optimization and Validation of the Regression Equations
2.5. Evaluation of Various Physical Characteristics of NEB-PNPs
2.5.1. Analysis of Particle Size and Zeta Potential of NEB-PNPs
2.5.2. Analysis of Drug Loading and Entrapment Efficiency of NEB-PNPs
2.6. Scanning Electron Microscopy (SEM) Imaging of the Optimized NEB-PNPs
2.7. Differential Scanning Calorimetry (DSC) of the Optimized NEB-PNPs
2.8. X-ray Diffractometric Analysis of the Optimized NEB-PNPs
2.9. Preparation of NEB-PNPs-Loaded Dual-Sensitive In Situ Gel
2.10. Rheological Evaluation of NEB-PNPs-ISG Formulation
2.11. In Vitro Drug-Release Studies of NEB-PNPs-Susp and NEB-PNPs-ISG Formulations
2.12. In Vivo Evaluation of Optimized NEB-PNPs and NEB-PNPs-ISG Formulations
2.12.1. Ocular Pharmacokinetic Studies
2.12.2. Ocular Pharmacodynamic Studies of NEB-PNP Formulations
3. Results and Discussion
3.1. Preliminary Trials for the Preparation of NEB-PNPs
3.2. Optimization of NEB-PNPs
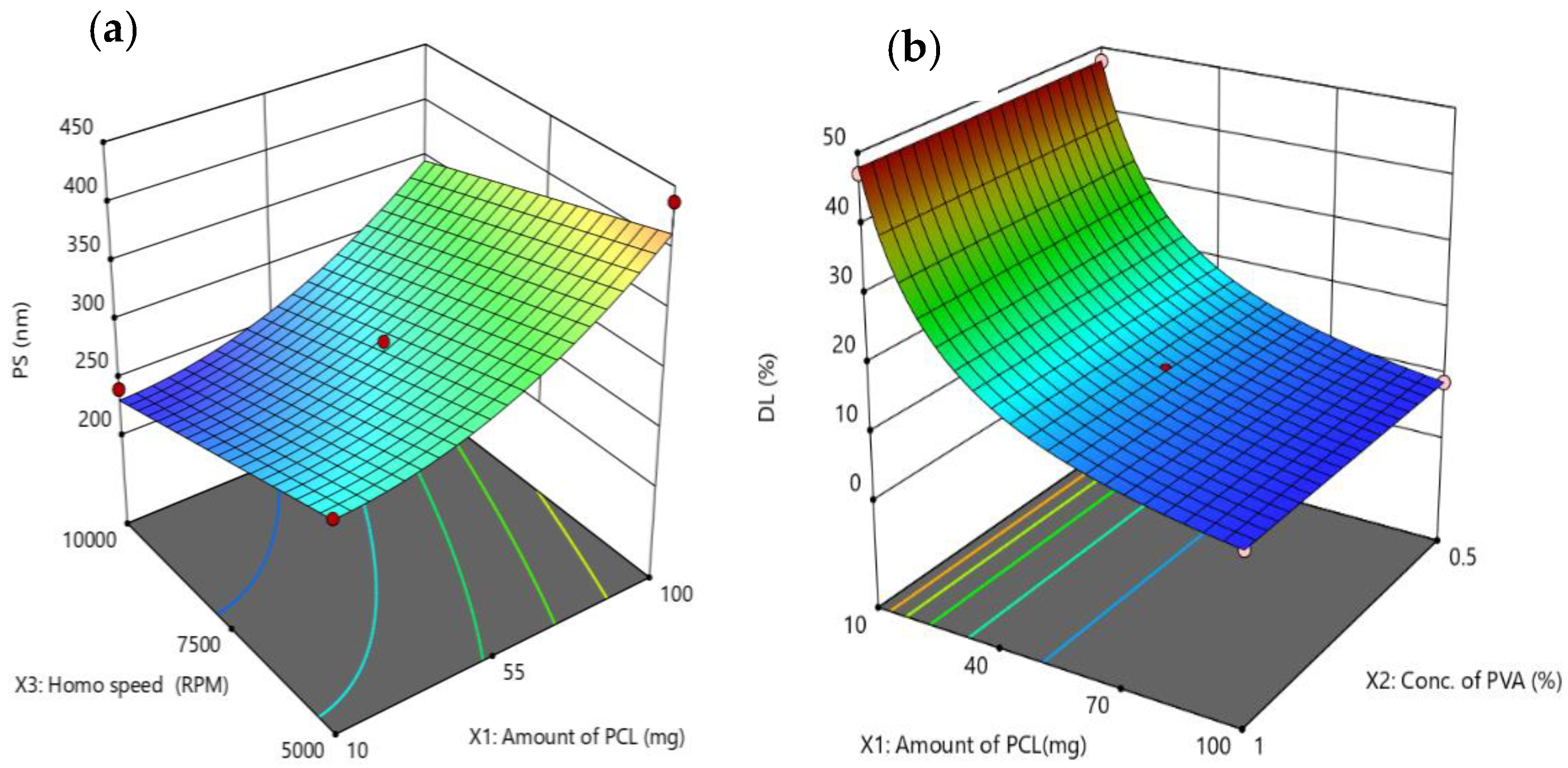
3.2.1. Impact of Critical Factors on Particle Size of NEB-PNPs
3.2.2. Impact of Critical Factors on Drug Loading of NEB-PNPs
3.2.3. Validation of the Regression Equations for Particle Size and Loading Efficiency
3.3. Physical Characterization of the Optimized NEB-PNPs Using Zeta-Sizer, SEM, DSC, and pXRD
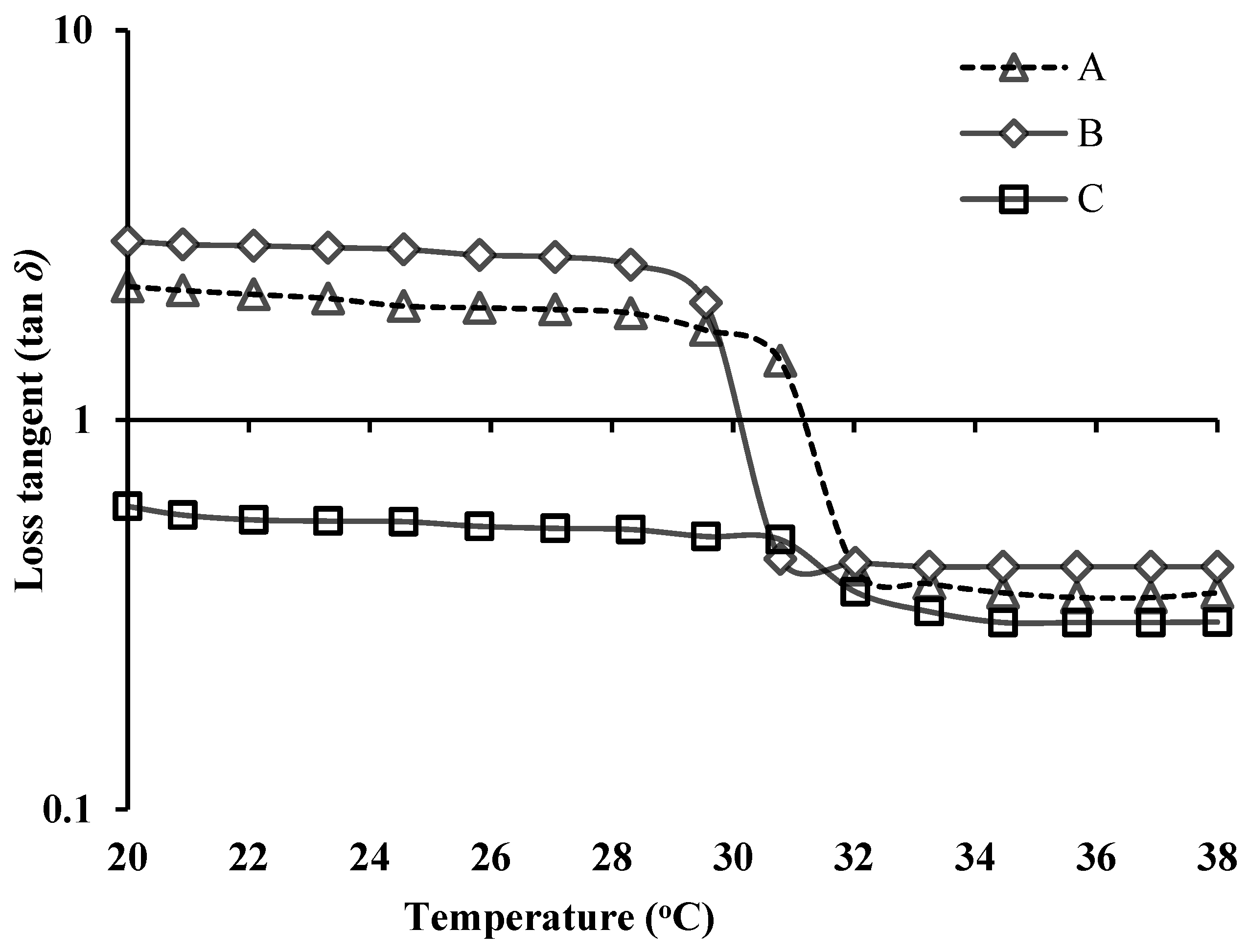
3.4. Rheological Evaluation of NEB-PNPs-ISG Formulation
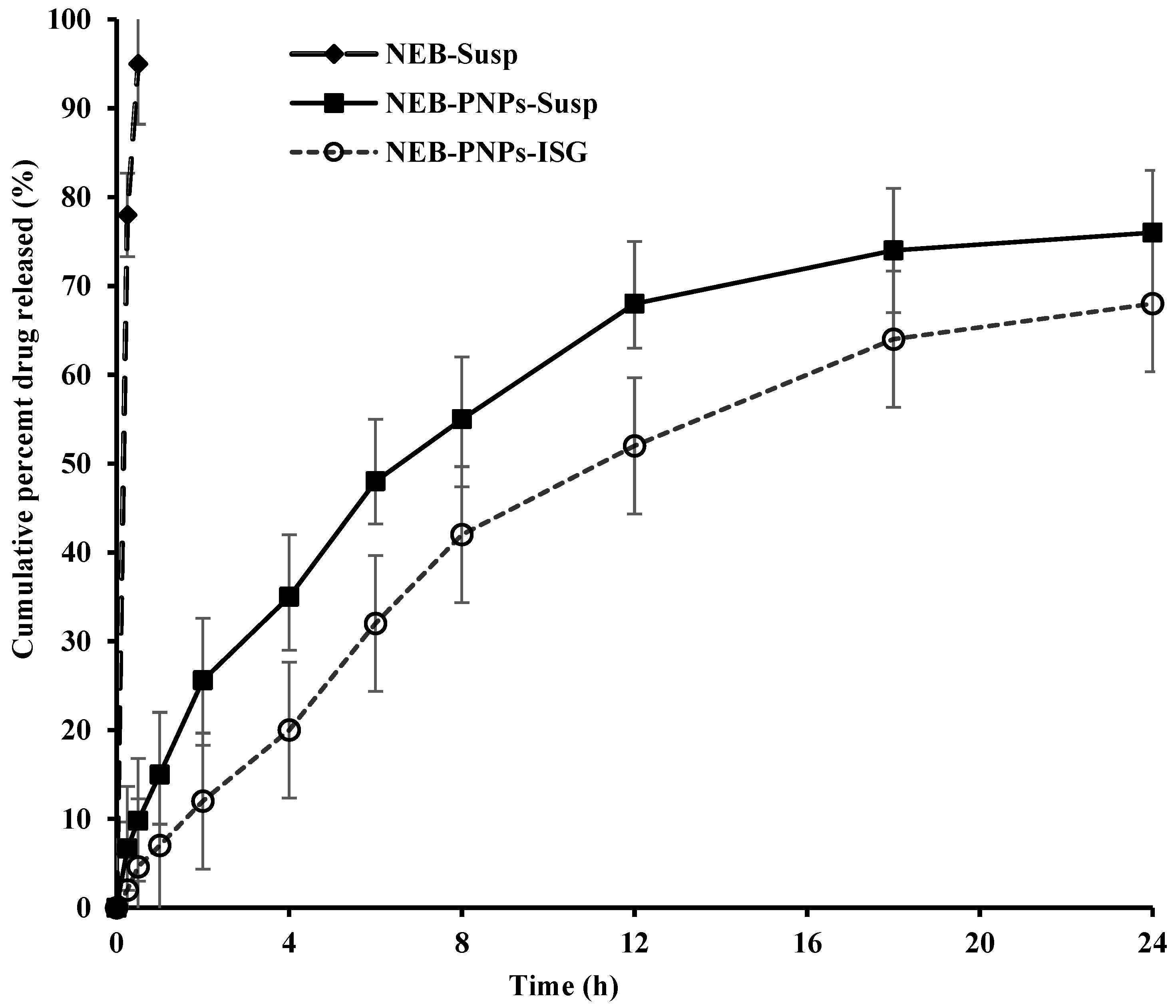
3.5. Drug-Release Studies of NEB-PNPs Formulations
3.6. Stability Studies of PNPs Formulations
3.7. In Vivo Studies of the NEB-PNPs Formulations
3.7.1. Ocular Pharmacokinetic Studies of NEB-PNPs Formulations
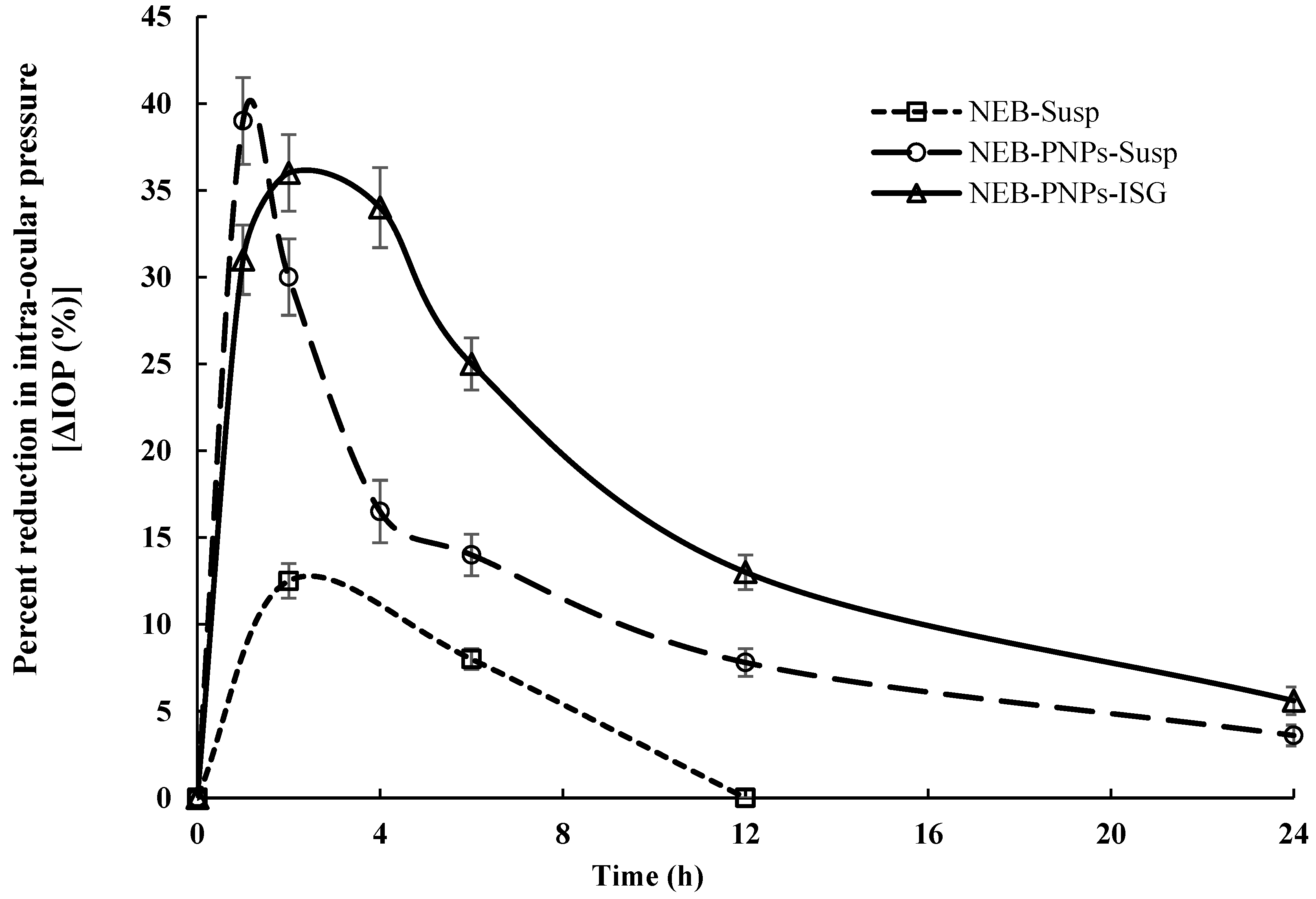
3.7.2. Ocular Pharmacodynamic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Geewoo, N.P.; Hyuck, J.L. Chemical Insights into Topical Agents in Intraocular Pressure Management: From Glaucoma Etiopathology to Therapeutic Approaches. Pharmaceutics 2024, 16, 274. [Google Scholar] [CrossRef] [PubMed]
- Maffei, A.; Lembo, G. Nitric oxide mechanisms of nebivolol. Ther. Adv. Cardiovasc. Dis. 2009, 3, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Pradeep Singh, R.; Punna Rao, R.; Shahid Iqbal, M.; Mohammed Shareef, K.; Himanshu, K.; Prasanna, K.; Upendra, B. Design, Characterization and Pharmacokinetic–Pharmacodynamic Evaluation of Poloxamer and Kappa-Carrageenan-Based Dual-Responsive In Situ Gel of Nebivolol for Treatment of Open-Angle Glaucoma. Pharmaceutics 2023, 15, 405. [Google Scholar] [CrossRef]
- Kolawole, M.O.; Cook, T., M. In situ gelling drug delivery systems for topical drug delivery. Eur. J. Pharm. Biopharm. 2023, 184, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Yumei, W.; Yuanyuan, L.; Xinyue, L.; Dereje, K.; Bing, Z.; Jing, R.; Jun, L.; Jiawei, L.; Shouying, D.; Zhidong, L. Research progress of in-situ gelling ophthalmic drug delivery system. Asian J. Pharm. Sci. 2019, 14, 1–15. [Google Scholar]
- Mohammed Shareef, K.; Punna Rao, R.; Divya Shrikant, D. Design, optimization and pharmacokinetic evaluation of PLGA phosphatidylcholine hybrid nanoparticles of triamcinolone acetonide loaded in situ gel for topical ocular delivery. Int. J. Pharm. 2023, 647, 123530. [Google Scholar]
- Almeida, H.; Amaral, H.M.; Lobão, P.; Silva, C.A.; Sousa Loboa, J.M. Applications of polymeric and lipid nanoparticles in ophthalmic pharmaceutical formulations: Present and future considerations. J. Pharm. Pharm. Sci. 2014, 17, 278–293. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Salama, A.H.; AbouSamra, M.M.; Awad, E.A.G.; Mansy, S.S. Promising bioadhesive ofloxacin-loaded polymeric nanoparticles for the treatment of ocular inflammation: Formulation and in vivo evaluation. Drug Deliv. Transl. Res. 2021, 11, 1943–1957. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Chauhan, M.K. Optimization and evaluation of encapsulated brimonidine tartrate-loaded nanoparticles incorporation in situ gel for efficient intraocular pressure reduction. J. Sol-Gel Sci. Technol. 2020, 95, 190–201. [Google Scholar] [CrossRef]
- Szabo, Z.I.; Szabo, T.; Emoke, R.; Sipos, E. Validated HPLC method for determination of nebivolol in pharmaceutical dosage form and in vitro dissolution studies. Stud. Univ. BabesBolyai. Chem. 2014, 59, 195–203. [Google Scholar]
- Rawat, P.S.; Ravi, P.R.; Kaswan, L.; Raghuvanshi, R.S. Development and validation of a bio-analytical method for simultaneous quantification of nebivolol and labetalol in aqueous humor and plasma using LC-MS/MS and its application to ocular pharmacokinetic studies. J. Chromatogr. B 2020, 1136, 121908. [Google Scholar] [CrossRef] [PubMed]
- Fereig, S.A.; El-Zaafarany, G.M.; Arafa, M.G.; Abdel-Mottaleb, M.M.A. Self-assembled tacrolimus-loaded lecithin-chitosan hybrid nanoparticles for in vivo management of psoriasis. Int. J. Pharm. 2021, 608, 121114. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Saraf, S.; Pradhan, M.; Patel, R.J.; Singhvi, G.; Alexander, A. Design and optimization of curcumin loaded nano lipid carrier system using Box-Behnken design. Biomed. Pharmacother. 2021, 141, 111919. [Google Scholar] [CrossRef]
- Garms, B.C.; Hamish, P.; Darcy, B.; Felicity, Y.H.; Andrew, K.W.; Anitha, A.; Lisbeth, G. Evaluating the effect of synthesis, isolation, and characterisation variables on reported particle size and dispersity of drug loaded PLGA nanoparticles. Mater. Adv. 2021, 2, 5657–5671. [Google Scholar] [CrossRef]
- Dalvi, A.; Ravi, P.R.; Uppuluri, C.T. Rufinamide-loaded chitosan nanoparticles in xyloglucan-based thermoresponsive in situ gel for direct nose to brain delivery. Front. Pharmacol. 2021, 12, 691936. [Google Scholar] [CrossRef] [PubMed]
- Murthy, A.; Ravi, P.R.; Kathuria, H.; Vats, R. Self-assembled lecithin-chitosan nanoparticles improve the oral bioavailability and alter the pharmacokinetics of raloxifene. Int. J. Pharm. 2020, 588, 119731. [Google Scholar] [CrossRef] [PubMed]
- Akbari, B.; Tavandashti, M.P.; Zandrahimi, M. Particle size characterization of nanoparticles–a practical approach. Iran. J. Mater. Sci. Eng. 2011, 8, 48–56. [Google Scholar]
- Ceulemans, J.; Ludwig, A. Optimisation of carbomer viscous eye drops: An in vitro experimental design approach using rheological techniques. Eur. J. Pharm. Biopharm. 2002, 54, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Gratieri, T.; Gelfuso, G.M.; Rocha, E.M.; Sarmento, V.H.; Freitas, D.O.; Lopez, R.F.V. A poloxamer/chitosan in situ forming gel with prolonged retention time for ocular delivery. Eur. J. Pharm. Biopharm. 2010, 75, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Singh, D.; Brar, V. Bioadhesive okra polymer based buccal patches as platform for controlled drug delivery. Int. J. Biol. Macromol. 2014, 70, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Azadi, S.; Ashrafi, H.; Azadi, A. Mathematical modeling of drug release from swellable polymeric nanoparticles. J. Appl. Pharm. Sci. 2017, 7, 125–133. [Google Scholar]
- Singhvi, G.; Singh, M. In-vitro drug release characterization models. Int. J. Pharm. Stud. Res. 2011, 2, 77–84. [Google Scholar]
- Javadzadeh, Y.; Hamedeyazdan, S. Floating Drug Delivery Systems for Eradication of Helicobacter pylori in Treatment of Peptic Ulcer Disease. In Trends in Helicobacter Pylori Infection; IntechOpen Limited: London, UK, 2014; p. 13. [Google Scholar] [CrossRef]
- Diwan, R.; Ravi, P.R.; Agarwal, S.I.; Aggarwal, V. Cilnidipine loaded poly (ε-caprolactone) nanoparticles for enhanced oral delivery: Optimization using DoE, physical characterization, pharmacokinetic, and pharmacodynamic evaluation. Pharm. Dev. Technol. 2021, 26, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Reddy, I.K.; Vaithiyalingam, S.R.; Khan, M.A.; Bodor, N.S. Intraocular pressure-lowering activity and in vivo disposition of dipivalyl terbutalone in rabbits. Drug Dev. Ind. Pharm. 2001, 27, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Genotoxicity and in vitro investigation of Gefitinib-loaded polycaprolactone fabricated nanoparticles for anticancer activity against NCI-H460 cell lines. J. Exp. Nanosci. 2022, 17, 214–246. [Google Scholar] [CrossRef]


| Design used for optimization | Box–Behnken design (BBD): A response surface methodology suitable to optimize and determine the mathematical equations relating three or four independent factors and one or more critical responses. |
| Criteria for selection the design | Though BBD and central composite design (CCD) are the two most popular optimization designs available, BBD was selected for the following reasons:
|
| No. of experimental runs used in the design | In the current work, which involves three critical factors, a total 17 runs were performed for optimization (12 factorial runs + 5 center-point runs) |
| Critical factors | X1—amount of PCL; X2—concentration of stabilizer; X3—homogenization speed |
| Critical responses | Y1—particle size; Y2—drug loading (%) |
| Method to determine optimal solution | Simultaneous optimization of both the critical responses based on the highest overall desirability value. |
| Source | Particle Size (Y1) | Drug Loading (Y2) | ||||||
|---|---|---|---|---|---|---|---|---|
| SS | DF | Fcal | Pcal | SS | DF | Fcal | Pcal | |
| Model | 38,605.75 | 3 | 36.68 | <0.0001 | 0.018 | 1 | 37,300 | <0.0001 |
| X1 | 27,097.92 | 1 | 77.23 | <0.0001 | 0.018 | 1 | 37,300 | <0.0001 |
| X3 | 8508.6 | 1 | 24.25 | 0.0003 | ||||
| X32 | 2999.23 | 1 | 8.55 | 0.0119 | ||||
| Residual | 4561.33 | 13 | 0.00001 | 15 | ||||
| Lack-of-Fit | 3913.55 | 9 | 2.69 | 0.1773 | 0.0001 | 11 | 1.16 | 0.482 |
| Pure Error | 647.78 | 4 | 0.000002 | 4 | ||||
| Total | 43,167.08 | 16 | 0.0177 | 16 | ||||
| Composition of Optimized NEB-PNPs | * Physicochemical Characteristics of Optimized NEB-PNPs | ||||
|---|---|---|---|---|---|
| PS (nm) | PDI | ZP (mV) | EE (%) | DL (%) | |
| Organic Phase: 10 mg of NEB + 25 mg of PCL dissolved in 1 mL of NMP Aqueous Phase: PVA (0.75% w/v in water) | 270.9 ± 6.3 | 0.24 ± 0.03 | −8.2 ± 1.2 | 96.7 ± 0.3 | 28.8 ± 2.4 |
| Biological Matrix | PK Parameters | Units | Treatments | ||
|---|---|---|---|---|---|
| NEB-Susp # | NEB-PNPs-Susp | NEB-PNPs-ISG | |||
| Aqueous humor | Cmax a | ng/mL | 28.2 ± 3.1 | 36.8 ± 3.2 | 30.2 ± 2.1 |
| Tmax b | h | 2.0 | 4.0 | 4.0 | |
| AUC0–t c | ng × h/mL | 189 | 204.4 | 329.2 | |
| MRT0–∞ c | h | 6.1 | 6.4 | 9.7 | |
| Plasma d | Cmax | ng/mL | 1.86 ± 0.1 | 1.15 ± 0.08 | 0.58 ± 0.03 |
| Tmax | h | 1.0 | 2.0 | 4.0 | |
| AUC0–t | ng × h/mL | 20.2 ± 2.7 | 12.1 ± 0.9 | 8.38 ± 0.56 | |
| MRT0–∞ | h | 25.8 ± 1.5 | 10.4 ± 1.1 | 4.6 ± 0.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rawat, P.S.; Ravi, P.R.; Khan, M.S.; Mahajan, R.R.; Szeleszczuk, Ł. Nebivolol Polymeric Nanoparticles-Loaded In Situ Gel for Effective Treatment of Glaucoma: Optimization, Physicochemical Characterization, and Pharmacokinetic and Pharmacodynamic Evaluation. Nanomaterials 2024, 14, 1347. https://doi.org/10.3390/nano14161347
Rawat PS, Ravi PR, Khan MS, Mahajan RR, Szeleszczuk Ł. Nebivolol Polymeric Nanoparticles-Loaded In Situ Gel for Effective Treatment of Glaucoma: Optimization, Physicochemical Characterization, and Pharmacokinetic and Pharmacodynamic Evaluation. Nanomaterials. 2024; 14(16):1347. https://doi.org/10.3390/nano14161347
Chicago/Turabian StyleRawat, Pradeep Singh, Punna Rao Ravi, Mohammed Shareef Khan, Radhika Rajiv Mahajan, and Łukasz Szeleszczuk. 2024. "Nebivolol Polymeric Nanoparticles-Loaded In Situ Gel for Effective Treatment of Glaucoma: Optimization, Physicochemical Characterization, and Pharmacokinetic and Pharmacodynamic Evaluation" Nanomaterials 14, no. 16: 1347. https://doi.org/10.3390/nano14161347






