Sensors Based on Molecularly Imprinted Polymers in the Field of Cancer Biomarker Detection: A Review
Abstract
:1. Introduction
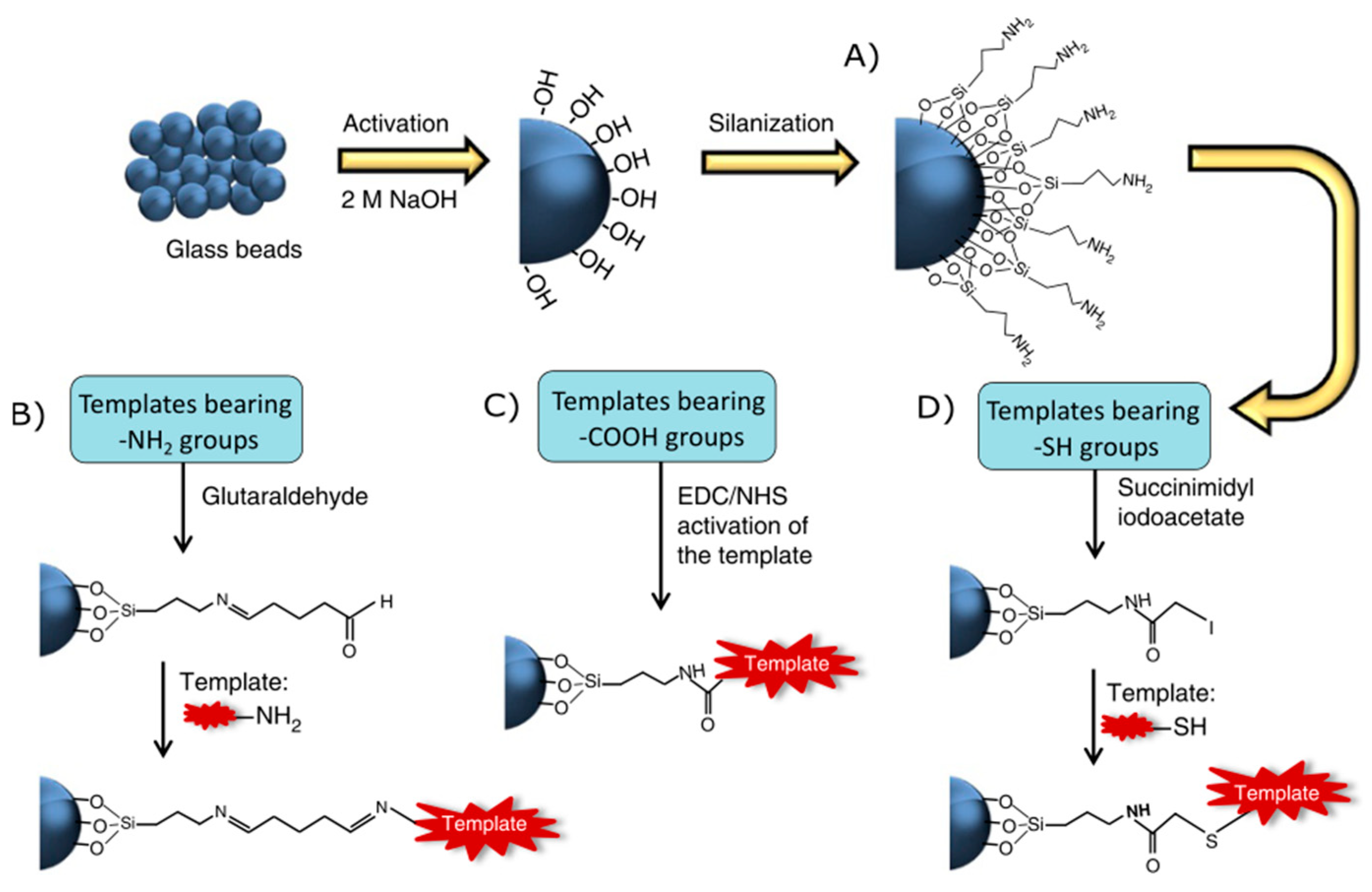
2. Molecularly Imprinted Polymers
- (A)
- The preparation of MIPs requires the interaction of a template (target molecule) that interacts with the functional groups of the monomer and a cross-linking agent.
- (B)
- Polymerization is initiated, and then the template molecule is removed from the acquired polymer, leaving behind the imprinted cavities to bind to the specific target.
- (C)
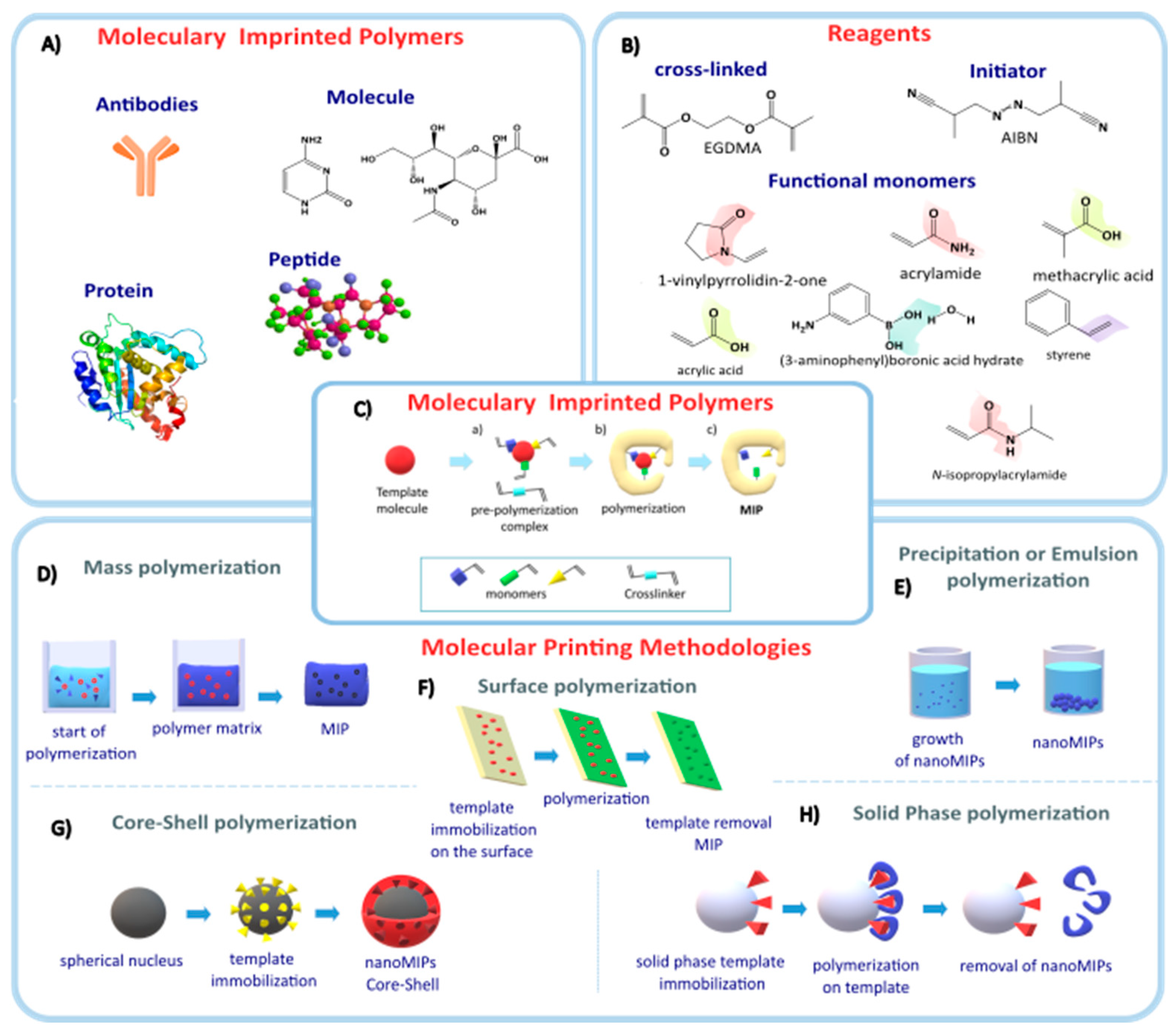
2.1. MIP Synthesis Methods
2.1.1. Bulk Polymerization
2.1.2. Precipitation Polymerization
2.1.3. Emulsion Polymerization
2.1.4. Surface Printing
2.1.5. Core-Shell Molecular Printing
2.1.6. Solid-Phase Printing
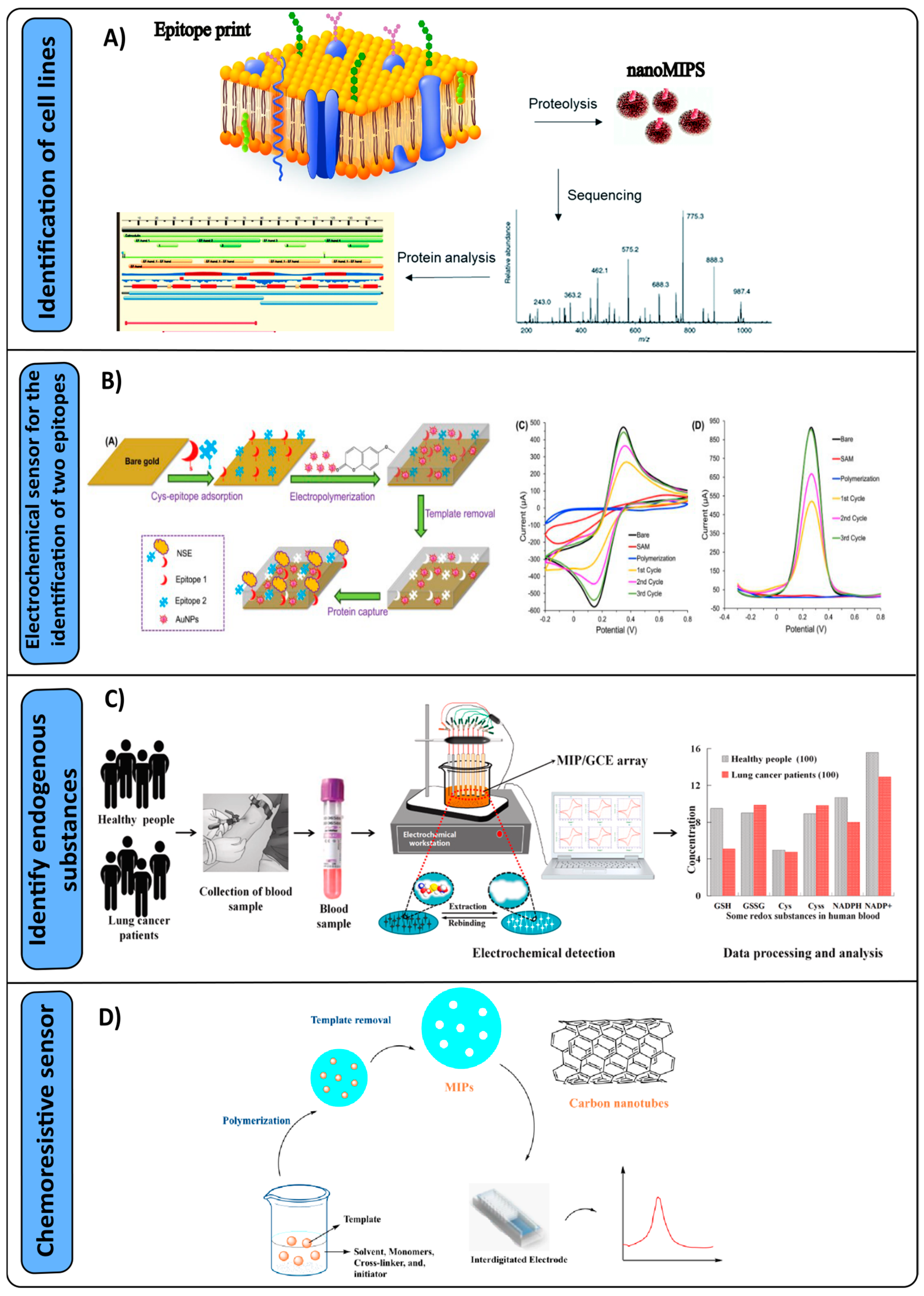
3. Molecular Imprinting Polymers Used in Sensors for Cancers
3.1. Detection of Lung Cancer
3.2. Breast Cancer Detection
3.3. Detection of Cancer of the Digestive System
3.4. Detection of Cancer of the Reproductive System
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhatt, A.N.; Mathur, R.; Farooque, A.; Verma, A.; Dwarakanath, B.S. Cancer biomarkers—Current perspectives. Indian J. Med. Res. 2010, 132, 129–149. [Google Scholar] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Bohunicky, B.; Mousa, S.A. Biosensors: The new wave in cancer diagnosis. Nanotechnol. Sci. Appl. 2010, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, B.; Shende, P.; Augustine, S. Current trends and emerging diagnostic techniques for lung cancer. Biomed. Pharmacother. 2018, 106, 1586–1599. [Google Scholar] [CrossRef] [PubMed]
- Altintas, Z.; Tothill, I. Biomarkers and biosensors for the early diagnosis of lung cancer. Sens. Actuators B Chem. 2013, 188, 988–998. [Google Scholar] [CrossRef]
- Mittal, S.; Kaur, H.; Gautam, N.; Mantha, A.K. Biosensors for breast cancer diagnosis: A review of bioreceptors, biotransducers and signal amplification strategies. Biosens. Bioelectron. 2017, 88, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-M.; Noh, H.-B.; Park, D.S.; Ryu, S.-H.; Koo, J.S.; Shim, Y.-B. Immunosensors for detection of Annexin II and MUC5AC for early diagnosis of lung cancer. Biosens. Bioelectron. 2009, 25, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Sidransky, D. Emerging molecular markers of cancer. Nat. Rev. Cancer 2002, 2, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Jo, S.; Mikos, A.G. Biomimetic materials for tissue engineering. Biomaterials 2003, 24, 4353–4364. [Google Scholar] [CrossRef] [PubMed]
- Adibnia, V.; Mirbagheri, M.; Faivre, J.; Robert, J.; Lee, J.; Matyjaszewski, K.; Lee, D.W.; Banquy, X. Bioinspired polymers for lubrication and wear resistance. Prog. Polym. Sci. 2020, 110, 101298. [Google Scholar] [CrossRef]
- Viveiros, R.; Lopes, M.I.; Heggie, W.; Casimiro, T. Green approach on the development of lock-and-key polymers for API purification. Chem. Eng. J. 2017, 308, 229–239. [Google Scholar] [CrossRef]
- Piletsky, S.S.; Piletska, E.; Poblocka, M.; Macip, S.; Jones, D.J.; Braga, M.; Cao, T.H.; Singh, R.; Spivey, A.C.; Aboagye, E.O. Snapshot imprinting: Rapid identification of cancer cell surface proteins and epitopes using molecularly imprinted polymers. Nano Today 2021, 41, 101304. [Google Scholar] [CrossRef]
- Huang, S.; Xu, J.; Zheng, J.; Zhu, F.; Xie, L.; Ouyang, G. Synthesis and application of magnetic molecularly imprinted polymers in sample preparation. Anal. Bioanal. Chem. 2018, 410, 3991–4014. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.A. Molecular imprinting polymers and their composites: A promising material for diverse applications. Biomater. Sci. 2017, 5, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Otsuka, K. Recent progress in molecularly imprinted media by new preparation concepts and methodological approaches for selective separation of targeting compounds. TrAC Trends Anal. Chem. 2016, 81, 102–109. [Google Scholar] [CrossRef]
- Nurhayati, T.; Royani, I. Synthesis and characterization of MAA-based molecularly-imprinted polymer (MIP) with D-glucose template. J. Phys. Conf. Ser. 2016, 739, 012143. [Google Scholar] [CrossRef]
- Bhawani, S.A.; Sen, T.S.; Ibrahim, M.N.M. Synthesis of molecular imprinting polymers for extraction of gallic acid from urine. Chem. Cent. J. 2018, 12, 19. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.; Chen, W.; Li, Y.; Wei, Y.; Luo, A. Magnetic Fluorescence Molecularly Imprinted Polymer Based on FeOx/ZnS Nanocomposites for Highly Selective Sensing of Bisphenol A. Polymers 2019, 11, 1210. [Google Scholar] [CrossRef] [PubMed]
- Schirhagl, R. Bioapplications for Molecularly Imprinted Polymers. Anal. Chem. 2014, 86, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef]
- Scorrano, S.; Mergola, L.; Del Sole, R.; Vasapollo, G. Synthesis of Molecularly Imprinted Polymers for Amino Acid Derivates by Using Different Functional Monomers. Int. J. Mol. Sci. 2011, 12, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.-R.; Hu, C.-W.; Chen, J.-L. Fluorescent turn-on detection of cysteine using a molecularly imprinted polyacrylate linked to allylthiol-capped CdTe quantum dots. Microchim. Acta 2014, 181, 1085–1091. [Google Scholar] [CrossRef]
- Schnettelker, A.; Lieberzeit, P. A Self-Organisation Synthesis Approach for Bacteria Molecularly Imprinted Polymers. Procedia Eng. 2016, 168, 557–560. [Google Scholar] [CrossRef]
- Birnbaumer, G.M.; Lieberzeit, P.A.; Richter, L.; Schirhagl, R.; Milnera, M.; Dickert, F.L.; Bailey, A.; Ertl, P. Detection of viruses with molecularly imprinted polymers integrated on a microfluidic biochip using contact-less dielectric microsensors. Lab Chip 2009, 9, 3549–3556. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, A.; Raffa, V.; Canfarotta, F.; Signore, G.; Piletsky, S.; MacDonald, M.P.; Cuschieri, A. In Vivo Recognition of Human Vascular Endothelial Growth Factor by Molecularly Imprinted Polymers. Nano Lett. 2017, 17, 2307–2312. [Google Scholar] [CrossRef] [PubMed]
- Canfarotta, F.; Lezina, L.; Guerreiro, A.; Czulak, J.; Petukhov, A.; Daks, A.; Smolinska-Kempisty, K.; Poma, A.; Piletsky, S.A.; Barlev, N.A. Specific Drug Delivery to Cancer Cells with Double-Imprinted Nanoparticles against Epidermal Growth Factor Receptor. Nano Lett. 2018, 18, 4641–4646. [Google Scholar] [CrossRef] [PubMed]
- Bossi, A.M. Plastic antibodies for cancer therapy? Nat. Chem. 2020, 12, 111–112. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Gandhi, S. A concise review on potential cancer biomarkers and advanced manufacturing of smart platform-based biosensors for early-stage cancer diagnostics. Biosens. Bioelectron. X 2022, 11, 100178. [Google Scholar] [CrossRef]
- Henderson, M.C.; Hollingsworth, A.B.; Gordon, K.; Silver, M.; Mulpuri, R.; Letsios, E.; Reese, D.E. Integration of Serum Protein Biomarker and Tumor Associated Autoantibody Expression Data Increases the Ability of a Blood-Based Proteomic Assay to Identify Breast Cancer. PLoS ONE 2016, 11, e0157692. [Google Scholar] [CrossRef]
- Erbes, T.; Hirschfeld, M.; Rücker, G.; Jaeger, M.; Boas, J.; Iborra, S.; Mayer, S.; Gitsch, G.; Stickeler, E. Feasibility of urinary microRNA detection in breast cancer patients and its potential as an innovative non-invasive biomarker. BMC Cancer 2015, 15, 193. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, M.; Rücker, G.; Weiß, D.; Berner, K.; Ritter, A.; Jäger, M.; Erbes, T. Urinary Exosomal MicroRNAs as Potential Non-invasive Biomarkers in Breast Cancer Detection. Mol. Diagn. Ther. 2020, 24, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Gashimova, E.M.; Temerdashev, A.Z.; Porkhanov, V.A.; Polyakov, I.S.; Perunov, D.V. Volatile Organic Compounds in Exhaled Breath as Biomarkers of Lung Cancer: Advances and Potential Problems. J. Anal. Chem. 2022, 77, 785–810. [Google Scholar] [CrossRef]
- Buchwald, P.; Hall, C.; Davidson, C.; Dixon, L.; Dobbs, B.; Robinson, B.; Frizelle, F. Improved survival for rectal cancer compared to colon cancer: The four cohort study. ANZ J. Surg. 2018, 88, E114–E117. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, P.R.; Kramer, B.S.; Srivastava, S. Trends in biomarker research for cancer detection. Lancet Oncol. 2001, 2, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mohan, A.; Guleria, R. Biomarkers in cancer screening, research and detection: Present and future: A review. Biomarkers 2008, 11, 385–405. [Google Scholar] [CrossRef] [PubMed]
- Bensalah, K.; Montorsi, F.; Shariat, S.F. Challenges of Cancer Biomarker Profiling. Eur. Urol. 2007, 52, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Suzaei, F.M.; Batista, A.D.; Mizaikoff, B.; Rahimi, S.; Daryanavard, S.M.; Abdel-Rehim, M. Molecularly imprinted polymers for selective extraction/microextraction of cancer biomarkers: A review. Microchim. Acta 2022, 189, 255. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.O.; Yip, C.-H.; Smith, R.A.; Shyyan, R.; Sener, S.F.; Eniu, A.; Carlson, R.W.; Azavedo, E.; Harford, J. Guideline implementation for breast healthcare in low-income and middle-income countries. Cancer 2008, 113 (Suppl. S8), 2221–2243. [Google Scholar] [CrossRef]
- Zubor, P.; Kubatka, P.; Kajo, K.; Dankova, Z.; Polacek, H.; Bielik, T.; Kudela, E.; Samec, M.; Liskova, A.; Vlcakova, D.; et al. Why the Gold Standard Approach by Mammography Demands Extension by Multiomics? Application of Liquid Biopsy miRNA Profiles to Breast Cancer Disease Management. Int. J. Mol. Sci. 2019, 20, 2878. [Google Scholar] [CrossRef] [PubMed]
- Nassar, F.J.; Nasr, R.; Talhouk, R. MicroRNAs as biomarkers for early breast cancer diagnosis, prognosis and therapy prediction. Pharmacol. Ther. 2017, 172, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Dave, R.; Sanadya, J.; Sharma, P.; Sharma, K. Various types and management of breast cancer: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 109. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, H.; Liu, D.; Ji, N.; Zong, G. Synthesis of polyacrylonitrile using AGET-ATRP in emulsion. Mater. Sci. Eng. C 2013, 33, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Ando, W.; Kikuchi, K.; Uematsu, T.; Yokomori, H.; Takaki, T.; Sogabe, M.; Kohgo, Y.; Otori, K.; Ishikawa, S.; Okazaki, I. Novel breast cancer screening: Combined expression of miR-21 and MMP-1 in urinary exosomes detects 95% of breast cancer without metastasis. Sci. Rep. 2019, 9, 13595. [Google Scholar] [CrossRef] [PubMed]
- Cala, M.; Aldana, J.; Sánchez, J.; Guio, J.; Meesters, R.J. Urinary metabolite and lipid alterations in Colombian Hispanic women with breast cancer: A pilot study. J. Pharm. Biomed. Anal. 2018, 152, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Mangler, M.; Freitag, C.; Lanowska, M.; Staeck, O.; Schneider, A.; Speiser, D. Volatile organic compounds (VOCs) in exhaled breath of patients with breast cancer in a clinical setting. Ginekol. Pol. 2012, 83, 730–736. [Google Scholar] [PubMed]
- Vaihinger, D.; Landfester, K.; Kräuter, I.; Brunner, H.; Tovar, G.E. Molecularly imprinted polymer nanospheres as synthetic affinity receptors obtained by miniemulsion polymerisation. Macromol. Chem. Phys. 2002, 203, 1965–1973. [Google Scholar] [CrossRef]
- Hammerschmidt, S.; Wirtz, H. Lung Cancer. Dtsch. Arztebl. Int. 2009, 106, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Cataneo, R.N.; Cummin, A.R.; Gagliardi, A.J.; Gleeson, K.; Greenberg, J.; Maxfield, R.A.; Rom, W.N. Detection of lung cancer with volatile markers in the breatha. Chest 2003, 123, 2115–2123. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, A.R.; Thunnissen, F.B.J.M. Histological typing of lung and pleural tumours: Third edition. J. Clin. Pathol. 2001, 54, 498–499. [Google Scholar] [CrossRef] [PubMed]
- Walters, S.; Maringe, C.; Coleman, M.P.; Peake, M.D.; Butler, J.; Young, N.; Bergström, S.; Hanna, L.; Jakobsen, E.; Kölbeck, K.; et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: A population-based study, 2004–2007. Thorax 2013, 68, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. New Pickering Emulsions Stabilized by Bacterial Cellulose Nanocrystals. Langmuir 2011, 27, 7471–7479. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.; Chen, Q.; Pan, J.; Zhang, Y.; Huang, Y.; Qi, X. Selective removal of erythromycin by magnetic imprinted polymers synthesized from chitosan-stabilized Pickering emulsion. J. Hazard. Mater. 2015, 289, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Motaharian, A.; Hosseini, M.R.M.; Naseri, K. Determination of psychotropic drug chlorpromazine using screen printed carbon electrodes modified with novel MIP-MWCNTs nano-composite prepared by suspension polymerization method. Sens. Actuators B Chem. 2019, 288, 356–362. [Google Scholar] [CrossRef]
- Duan, F.; Chen, C.; Zhao, X.; Yang, Y.; Liu, X.; Qin, Y. Water-compatible surface molecularly imprinted polymers with synergy of bi-functional monomers for enhanced selective adsorption of bisphenol A from aqueous solution. Environ. Sci. Nano 2016, 3, 213–222. [Google Scholar] [CrossRef]
- Qi, J.; Li, B.; Zhou, N.; Wang, X.; Deng, D.; Luo, L.; Chen, L. The strategy of antibody-free biomarker analysis by in-situ synthesized molecularly imprinted polymers on movable valve paper-based device. Biosens. Bioelectron. 2019, 142, 111533. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Ding, J.; Gao, S.; Qin, W. Mussel-Inspired Surface-Imprinted Sensors for Potentiometric Label-Free Detection of Biological Species. Angew. Chem. Int. Ed. 2017, 56, 6833–6837. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, M.; Huang, Z.; Shao, Y.; Bai, L.; Zhou, L. Well-defined nanostructured core–shell magnetic surface imprinted polymers (Fe3O4@SiO2@MIPs) for effective extraction of trace tetrabromobisphenol A from water. J. Ind. Eng. Chem. 2018, 60, 268–278. [Google Scholar] [CrossRef]
- Zhao, Q.; Ma, C.; Liu, J.; Chen, Z.; Zhao, H.; Li, B.; Yang, X. Synthesis of magnetic covalent organic framework molecularly imprinted polymers at room temperature: A novel imprinted strategy for thermo-sensitive substance. Talanta 2021, 225, 121958. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, Q.; Jin, S.; Song, Y.; Gao, J.; Wang, Y.; Mi, H. Core–shell molecularly imprinted polymer nanoparticles with assistant recognition polymer chains for effective recognition and enrichment of natural low-abundance protein. Acta Biomater. 2014, 10, 769–775. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, T.; Chen, Y. Simultaneous quantification of Cyt c interactions with HSP27 and Bcl-xL using molecularly imprinted polymers (MIPs) coupled with liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based targeted proteomics. J. Proteom. 2019, 192, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, Y.; Wei, X.; Chen, J.; Xu, P.; Ni, R.; Meng, J.; Zhou, Y. Fabrication of magnetic polymers based on deep eutectic solvent for separation of bovine hemoglobin via molecular imprinting technology. Anal. Chim. Acta 2019, 1048, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Doostmohammadi, A.; Youssef, K.; Akhtarian, S.; Tabesh, E.; Kraft, G.; Brar, S.K.; Rezai, P. Molecularly imprinted polymer (MIP) based core-shell microspheres for bacteria isolation. Polymer 2022, 251, 124917. [Google Scholar] [CrossRef]
- Hashemi-Moghaddam, H.; Zavareh, S.; Karimpour, S.; Madanchi, H. Evaluation of molecularly imprinted polymer based on HER2 epitope for targeted drug delivery in ovarian cancer mouse model. React. Funct. Polym. 2017, 121, 82–90. [Google Scholar] [CrossRef]
- Jalilzadeh, M.; Çimen, D.; Denizli, A. Adenosine-imprinted magnetic core-shell polyvinylbutyral microbeads for quantification of adenosine in plasma. J. Chromatogr. B 2020, 1147, 122149. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Okita, E.; Kawai-Uchida, M.; Fukuda, N.; Shoukei, Y.; Soshiroda, K.; Yamada, K.; Kanda, T.; Uchida, S. Anti-parkinsonian activity of the adenosine A2A receptor antagonist/inverse agonist KW-6356 as monotherapy in MPTP-treated common marmosets. Eur. J. Pharmacol. 2023, 950, 175773. [Google Scholar] [CrossRef] [PubMed]
- Çorbacioğlu, K.; Uzunosmanoglu, H.; Karaarslan, F.N.; Dağar, S.; Emektar, E.; Çevik, Y. The effect of patient weight on the success of converting sinus rhythm in patients with PSVT treated with standard dose adenosine. Am. J. Emerg. Med. 2023, 69, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Canfarotta, F.; Poma, A.; Guerreiro, A.; Piletsky, S. Solid-phase synthesis of molecularly imprinted nanoparticles. Nat. Protoc. 2016, 11, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Poma, A.; Turner, A.P.; Piletsky, S.A. Advances in the manufacture of MIP nanoparticles. Trends Biotechnol. 2010, 28, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Poma, A.; Guerreiro, A.; Whitcombe, M.J.; Piletska, E.V.; Turner, A.P.F.; Piletsky, S.A. Solid-Phase Synthesis of Molecularly Imprinted Polymer Nanoparticles with a Reusable Template-“Plastic Antibodies”. Adv. Funct. Mater. 2013, 23, 2821–2827. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.G.; Haq, I.; Cowen, T.; Di Masi, S.; Trivedi, S.; Alanazi, K.; Piletska, E.; Mujahid, A.; Piletsky, S.A. Design and fabrication of a smart sensor using in silico epitope mapping and electro-responsive imprinted polymer nanoparticles for determination of insulin levels in human plasma. Biosens. Bioelectron. 2020, 169, 112536. [Google Scholar] [CrossRef]
- García, Y.; Czulak, J.; Pereira, E.D.; Piletsky, S.A.; Piletska, E. A magnetic molecularly imprinted nanoparticle assay (MINA) for detection of pepsin. React. Funct. Polym. 2022, 170, 105133. [Google Scholar] [CrossRef]
- Canfarotta, F.; Czulak, J.; Guerreiro, A.; Cruz, A.G.; Piletsky, S.; Bergdahl, G.E.; Hedström, M.; Mattiasson, B. A novel capacitive sensor based on molecularly imprinted nanoparticles as recognition elements. Biosens. Bioelectron. 2018, 120, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Strachota, B.; Hodan, J.; Matějka, L. Poly(N-isopropylacrylamide)–clay hydrogels: Control of mechanical properties and structure by the initiating conditions of polymerization. Eur. Polym. J. 2016, 77, 1–15. [Google Scholar] [CrossRef]
- Cowen, T.; Stefanucci, E.; Piletska, E.; Marrazza, G.; Canfarotta, F.; Piletsky, S.A. Synthetic Mechanism of Molecular Imprinting at the Solid Phase. Macromolecules 2020, 53, 1435–1442. [Google Scholar] [CrossRef]
- Tang, S.-P.; Canfarotta, F.; Smolinska-Kempisty, K.; Piletska, E.; Guerreiro, A.; Piletsky, S. A pseudo-ELISA based on molecularly imprinted nanoparticles for detection of gentamicin in real samples. Anal. Methods 2017, 9, 2853–2858. [Google Scholar] [CrossRef]
- Feng, X.; Ashley, J.; Zhou, T.; Halder, A.; Sun, Y. A facile molecularly imprinted polymer-based fluorometric assay for detection of histamine. RSC Adv. 2018, 8, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Canfarotta, F.; Czulak, J.; Betlem, K.; Sachdeva, A.; Eersels, K.; van Grinsven, B.; Cleij, T.J.; Peeters, M. A novel thermal detection method based on molecularly imprinted nanoparticles as recognition elements. Nanoscale 2018, 10, 2081–2089. [Google Scholar] [CrossRef] [PubMed]
- Moczko, E.; Guerreiro, A.; Cáceres, C.; Piletska, E.; Sellergren, B.; Piletsky, S.A. Epitope approach in molecular imprinting of antibodies. J. Chromatogr. B 2019, 1124, 1–6. [Google Scholar] [CrossRef]
- Bagán, H.; Zhou, T.; Eriksson, N.L.; Bülow, L.; Ye, L. Synthesis and characterization of epitope-imprinted polymers for purification of human hemoglobin. RSC Adv. 2017, 7, 41705–41712. [Google Scholar] [CrossRef]
- Gui, R.; Jin, H. Recent advances in synthetic methods and applications of photo-luminescent molecularly imprinted polymers. J. Photochem. Photobiol. C Photochem. Rev. 2019, 41, 100315. [Google Scholar] [CrossRef]
- Mahmoudpour, M.; Torbati, M.; Mousavi, M.-M.; de la Guardia, M.; Dolatabadi, J.E.N. Nanomaterial-based molecularly imprinted polymers for pesticides detection: Recent trends and future prospects. TrAC Trends Anal. Chem. 2020, 129, 115943. [Google Scholar] [CrossRef]
- Ahmad, O.S.; Bedwell, T.S.; Esen, C.; Garcia-Cruz, A.; Piletsky, S.A. Molecularly Imprinted Polymers in Electrochemical and Optical Sensors. Trends Biotechnol. 2019, 37, 294–309. [Google Scholar] [CrossRef]
- Piletsky, S.; Canfarotta, F.; Poma, A.; Bossi, A.M.; Piletsky, S. Molecularly Imprinted Polymers for Cell Recognition. Trends Biotechnol. 2020, 38, 368–387. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.; Subedi, P.; Yilmaz, E.; Marcus, K.; Laurell, T.; Ekström, S. Molecularly imprinted polymers synthesized via template immobilization on fumed silica nanoparticles for the enrichment of phosphopeptides. J. Mol. Recognit. 2018, 31, e2677. [Google Scholar] [CrossRef] [PubMed]
- Cenci, L.; Tatti, R.; Tognato, R.; Ambrosi, E.; Piotto, C.; Bossi, A.M. Synthesis and characterization of peptide-imprinted nanogels of controllable size and affinity. Eur. Polym. J. 2018, 109, 453–459. [Google Scholar] [CrossRef]
- Rossetti, C.; Świtnicka-Plak, M.A.; Halvorsen, T.G.; Cormack, P.A.; Sellergren, B.; Reubsaet, L. Automated Protein Biomarker Analysis: On-line extraction of clinical samples by Molecularly Imprinted Polymers. Sci. Rep. 2017, 7, 44298. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-R.; Oh, I.-J.; Shin, M.-G.; Park, J.-S.; Choi, H.-J.; Ban, H.-J.; Kim, K.-S.; Kim, Y.-C.; Shin, J.-H.; Ryang, D.-W.; et al. Plasma proGRP Concentration is Sensitive and Specific for Discriminating Small Cell Lung Cancer from Nonmalignant Conditions or Non-small Cell Lung Cancer. J. Korean Med. Sci. 2011, 26, 625–630. [Google Scholar] [CrossRef] [PubMed]
- McKitterick, N.; Bicak, T.C.; Switnicka-Plak, M.A.; Cormack, P.A.; Reubsaet, L.; Halvorsen, T.G. On-line duplex molecularly imprinted solid-phase extraction for analysis of low-abundant biomarkers in human serum by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2021, 1655, 462490. [Google Scholar] [CrossRef] [PubMed]
- Piletska, E.; Magumba, K.; Joseph, L.; Cruz, A.G.; Norman, R.; Singh, R.; Tabasso, A.F.S.; Jones, D.J.L.; Macip, S.; Piletsky, S. Molecular imprinting as a tool for determining molecular markers: A lung cancer case. RSC Adv. 2022, 12, 17747–17754. [Google Scholar] [CrossRef]
- Pirzada, M.; Sehit, E.; Altintas, Z. Cancer biomarker detection in human serum samples using nanoparticle decorated epitope-mediated hybrid MIP. Biosens. Bioelectron. 2020, 166, 112464. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Liu, X.; Yuan, Q.; Zhang, Y.; Li, Y. Novel molecularly imprinted polymer (MIP) multiple sensors for endogenous redox couples determination and their applications in lung cancer diagnosis. Talanta 2019, 199, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Janfaza, S.; Nojavani, M.B.; Nikkhah, M.; Alizadeh, T.; Esfandiar, A.; Ganjali, M.R. A selective chemiresistive sensor for the cancer-related volatile organic compound hexanal by using molecularly imprinted polymers and multiwalled carbon nanotubes. Microchim. Acta 2019, 186, 137. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, Z.; Gu, S.; Wang, J. Detection of hexanal in humid circumstances using hydrophobic molecularly imprinted polymers composite. Sens. Actuators B Chem. 2019, 291, 141–147. [Google Scholar] [CrossRef]
- Jahangiri-Manesh, A.; Mousazadeh, M.; Nikkhah, M.; Abbasian, S.; Moshaii, A.; Masroor, M.J.; Norouzi, P. Molecularly imprinted polymer-based chemiresistive sensor for detection of nonanal as a cancer related biomarker. Microchem. J. 2022, 173, 106988. [Google Scholar] [CrossRef]
- Xu, Q.; Zhu, M.; Yang, T.; Xu, F.; Liu, Y.; Chen, Y. Quantitative assessment of human serum transferrin receptor in breast cancer patients pre- and post-chemotherapy using peptide immunoaffinity enrichment coupled with targeted proteomics. Clin. Chim. Acta 2015, 448, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhong, T.; Xu, Q.; Chen, Y. Efficient Molecular Imprinting Strategy for Quantitative Targeted Proteomics of Human Transferrin Receptor in Depleted Human Serum. Anal. Chem. 2015, 87, 10910–10919. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, J.P.G.; Rebelo, P.; Freitas, M.; Nouws, H.P.A.; Delerue-Matos, C. Breast cancer biomarker (HER2-ECD) detection using a molecularly imprinted electrochemical sensor. Sens. Actuators B Chem. 2018, 273, 1008–1014. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, W.; Liu, L.; Chen, Y. Simultaneous detection of site-specific histone methylations and acetylation assisted by single template oriented molecularly imprinted polymers. Anal. 2020, 145, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, J.P.G.; Silva, M.S.V.; Freitas, M.; Nouws, H.P.A.; Delerue-Matos, C. Molecularly imprinted electrochemical sensor for the point-of-care detection of a breast cancer biomarker (CA 15-3). Sens. Actuators B Chem. 2018, 256, 905–912. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ahn, S.W.; Lee, S.S.; Kim, J.M. Lateral migration and focusing of colloidal particles and DNA molecules under viscoelastic flow. Lab Chip 2012, 12, 2807–2814. [Google Scholar] [CrossRef] [PubMed]
- Lahcen, A.A.; Rauf, S.; Aljedaibi, A.; Filho, J.I.d.O.; Beduk, T.; Mani, V.; Alshareef, H.N.; Salama, K.N. Laser-scribed graphene sensor based on gold nanostructures and molecularly imprinted polymers: Application for Her-2 cancer biomarker detection. Sens. Actuators B Chem. 2021, 347, 130556. [Google Scholar] [CrossRef]
- Wei, H.; Wang, Z.; Wang, Y.; Ma, J.; Chen, Y.; Guo, M.; Li, Y.; Du, Y.; Hu, F. Detection of depression marker ASS1 in urine by gold nanoparticles based dual epitope-peptides imprinted sensor. Anal. Chim. Acta 2023, 1273, 341479. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, H.; Tang, X.; Zhao, L. Molecularly imprinted polymers-based novel optical biosensor for the detection of cancer marker lysozyme. Sens. Actuators A Phys. 2022, 334, 113324. [Google Scholar] [CrossRef]
- El-Schich, Z.; Zhang, Y.; Göransson, T.; Dizeyi, N.; Persson, J.L.; Johansson, E.; Caraballo, R.; Elofsson, M.; Shinde, S.; Sellergren, B.; et al. Sialic Acid as a Biomarker Studied in Breast Cancer Cell Lines In Vitro Using Fluorescent Molecularly Imprinted Polymers. Appl. Sci. 2021, 11, 3256. [Google Scholar] [CrossRef]
- Shinde, S.; El-Schich, Z.; Malakpour-Permlid, A.; Wan, W.; Dizeyi, N.; Mohammadi, R.; Rurack, K.; Wingren, A.G.; Sellergren, B. Sialic Acid-Imprinted Fluorescent Core–Shell Particles for Selective Labeling of Cell Surface Glycans. J. Am. Chem. Soc. 2015, 137, 13908–13912. [Google Scholar] [CrossRef] [PubMed]
- Taheri, N.; Khoshsafar, H.; Ghanei, M.; Ghazvini, A.; Bagheri, H. Dual-template rectangular nanotube molecularly imprinted polypyrrole for label-free impedimetric sensing of AFP and CEA as lung cancer biomarkers. Talanta 2022, 239, 123146. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.B.; Jaiswal, S.; Singh, K. Ultra-trace analysis of d-and l-aspartic acid applying one-by-one approach on a dual imprinted electrochemical sensor. Sens. Actuators B Chem. 2017, 240, 631–639. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Y.; Xing, R.; Chen, J.; Liu, J.; Li, W.; Liu, Z. Orthogonal dual molecularly imprinted polymer-based plasmonic immunosandwich assay: A double characteristic recognition strategy for specific detection of glycoproteins. Biosens. Bioelectron. 2019, 145, 111729. [Google Scholar] [CrossRef]
- Piloto, A.M.L.; Ribeiro, D.S.M.; Rodrigues, S.S.M.; Santos, J.L.M.; Sampaio, P.; Sales, M.G.F. Cellulose-based hydrogel on quantum dots with molecularly imprinted polymers for the detection of CA19-9 protein cancer biomarker. Microchim. Acta 2022, 189, 134. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H.; Chen, Y.-C.; Ho, M.-H.; Lin, H.-Y. Optical recognition of salivary proteins by use of molecularly imprinted poly(ethylene-co-vinyl alcohol)/quantum dot composite nanoparticles. Anal. Bioanal. Chem. 2010, 397, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Selvam, S.P.; Cho, S. Silver chalcogenide loaded V2CTx MXene-molecularly imprinted polymer-based novel ratiometric sensor for the early predictive cancer marker: L-Fucose. Chem. Eng. J. 2023, 469, 144016. [Google Scholar] [CrossRef]
- Viswanathan, S.; Rani, C.; Ribeiro, S.; Delerue-Matos, C. Molecular imprinted nanoelectrodes for ultra sensitive detection of ovarian cancer marker. Biosens. Bioelectron. 2012, 33, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, T.S.C.R.; Costa, R.; Brandão, A.T.S.C.; Silva, A.F.; Sales, M.G.F.; Pereira, C.M. Molecularly imprinted polymer SPE sensor for analysis of CA-125 on serum. Anal. Chim. Acta 2019, 1082, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Nguy, T.P.; Van Phi, T.; Tram, D.T.N.; Eersels, K.; Wagner, P.; Lien, T.T.N. Development of an impedimetric sensor for the label-free detection of the amino acid sarcosine with molecularly imprinted polymer receptors. Sens. Actuators B Chem. 2017, 246, 461–470. [Google Scholar] [CrossRef]
- Sheydaei, O.; Khajehsharifi, H.; Rajabi, H.R. Rapid and selective diagnose of Sarcosine in urine samples as prostate cancer biomarker by mesoporous imprinted polymeric nanobeads modified electrode. Sens. Actuators B Chem. 2020, 309, 127559. [Google Scholar] [CrossRef]
- Fernández-Puig, S.; Lazo-Fraga, A.; Korgel, B.A.; Oza, G.; Dutt, A.; Vallejo-Becerra, V.; Valdés-González, A.; Chávez-Ramírez, A. Molecularly imprinted polymer-silica nanocomposite based potentiometric sensor for early prostate cancer detection. Mater. Lett. 2022, 309, 131324. [Google Scholar] [CrossRef]
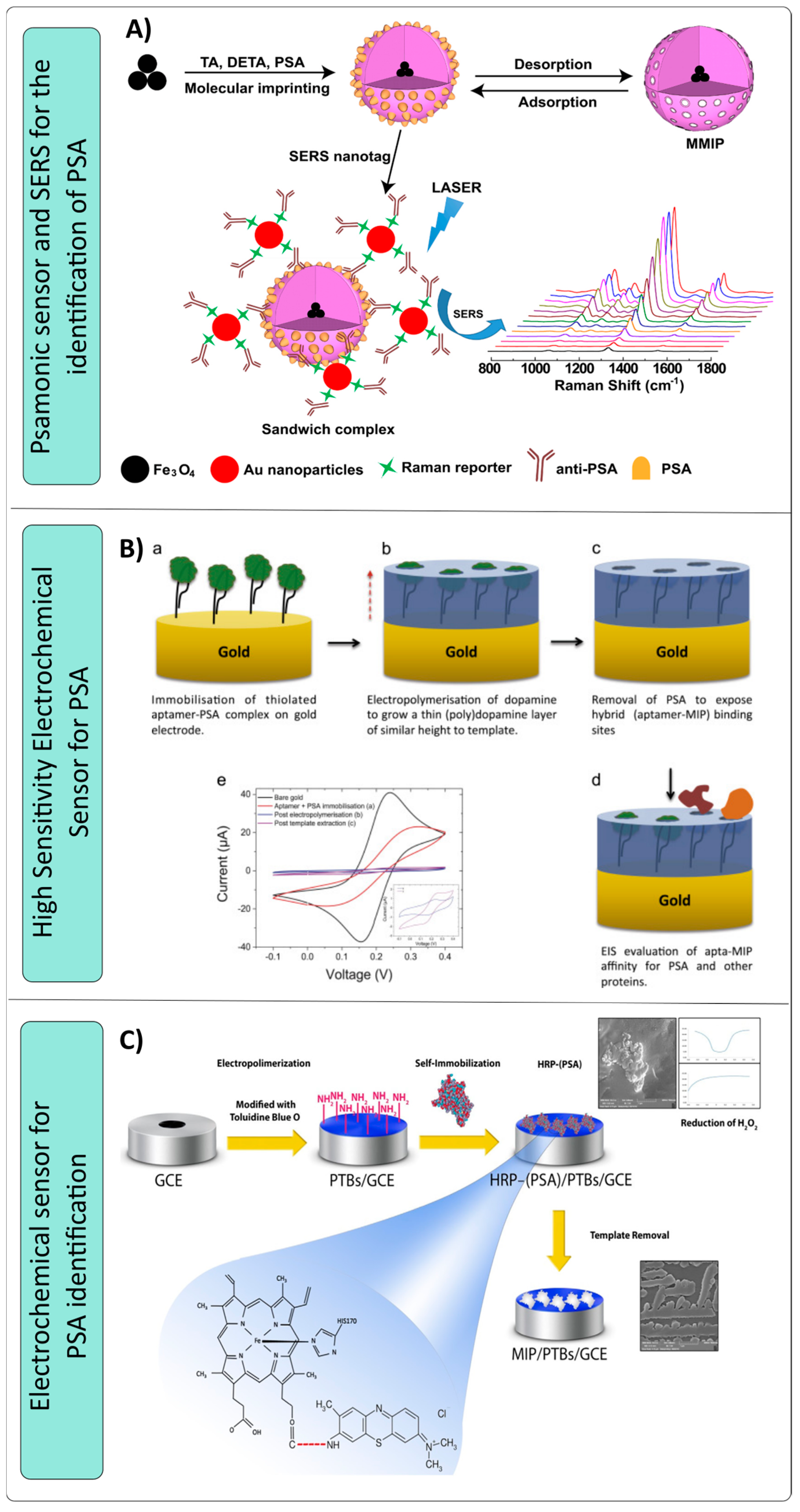
- Turan, E.; Zengin, A.; Suludere, Z.; Kalkan, N.; Tamer, U. Construction of a sensitive and selective plasmonic biosensor for prostate specific antigen by combining magnetic molecularly-imprinted polymer and surface-enhanced Raman spectroscopy. Talanta 2022, 237, 122926. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Sunayama, H.; Kitayama, Y.; Takano, E.; Takeuchi, T. Site-specific post-imprinting modification of molecularly imprinted polymer nanocavities with a modifiable functional monomer for prostate cancer biomarker recognition. Sci. Technol. Adv. Mater. 2019, 20, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Jolly, P.; Tamboli, V.; Harniman, R.L.; Estrela, P.; Allender, C.J.; Bowen, J.L. Aptamer–MIP hybrid receptor for highly sensitive electrochemical detection of prostate specific antigen. Biosens. Bioelectron. 2016, 75, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Sardaremelli, S.; Razmi, H.; Hasanzadeh, M.; Shadjou, N. A novel bioassay for the monitoring of hydrogen peroxide in human plasma samples based on binding of horseradish peroxidase-conjugated prostate specific antigen to poly (toluidine blue) as imprinted polymer receptor. Int. J. Biol. Macromol. 2020, 145, 311–324. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quezada, C.; Samhitha, S.S.; Salas, A.; Ges, A.; Barraza, L.F.; Blanco-López, M.C.; Solís-Pomar, F.; Pérez-Tijerina, E.; Medina, C.; Meléndrez, M. Sensors Based on Molecularly Imprinted Polymers in the Field of Cancer Biomarker Detection: A Review. Nanomaterials 2024, 14, 1361. https://doi.org/10.3390/nano14161361
Quezada C, Samhitha SS, Salas A, Ges A, Barraza LF, Blanco-López MC, Solís-Pomar F, Pérez-Tijerina E, Medina C, Meléndrez M. Sensors Based on Molecularly Imprinted Polymers in the Field of Cancer Biomarker Detection: A Review. Nanomaterials. 2024; 14(16):1361. https://doi.org/10.3390/nano14161361
Chicago/Turabian StyleQuezada, Camila, S. Shiva Samhitha, Alexis Salas, Adrián Ges, Luis F. Barraza, María Carmen Blanco-López, Francisco Solís-Pomar, Eduardo Pérez-Tijerina, Carlos Medina, and Manuel Meléndrez. 2024. "Sensors Based on Molecularly Imprinted Polymers in the Field of Cancer Biomarker Detection: A Review" Nanomaterials 14, no. 16: 1361. https://doi.org/10.3390/nano14161361








