A Self-Powered, Skin Adhesive, and Flexible Human–Machine Interface Based on Triboelectric Nanogenerator
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PDMS Film with Microstructure
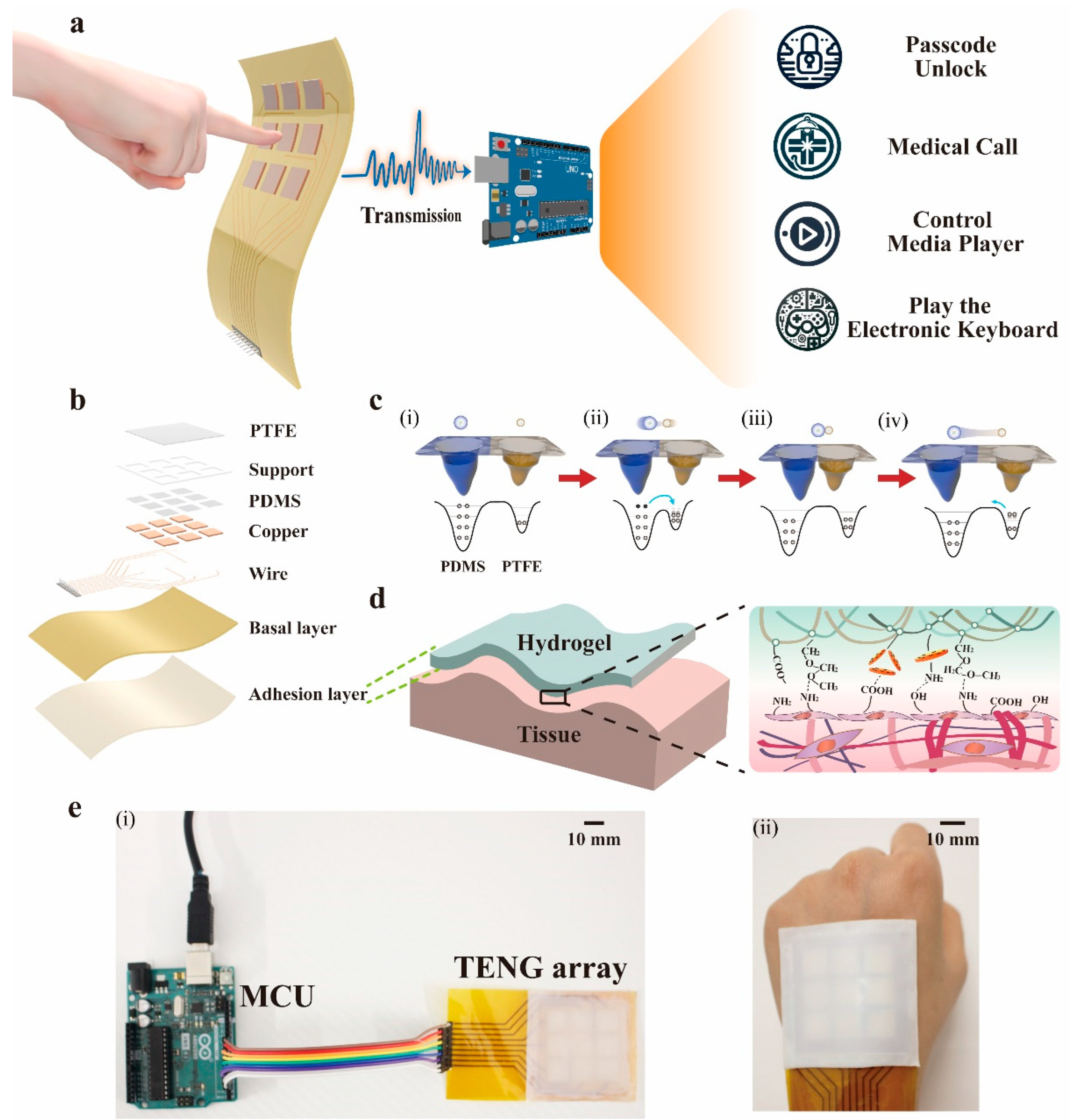
2.2. Fabrication of SSFHMI Based on TENG
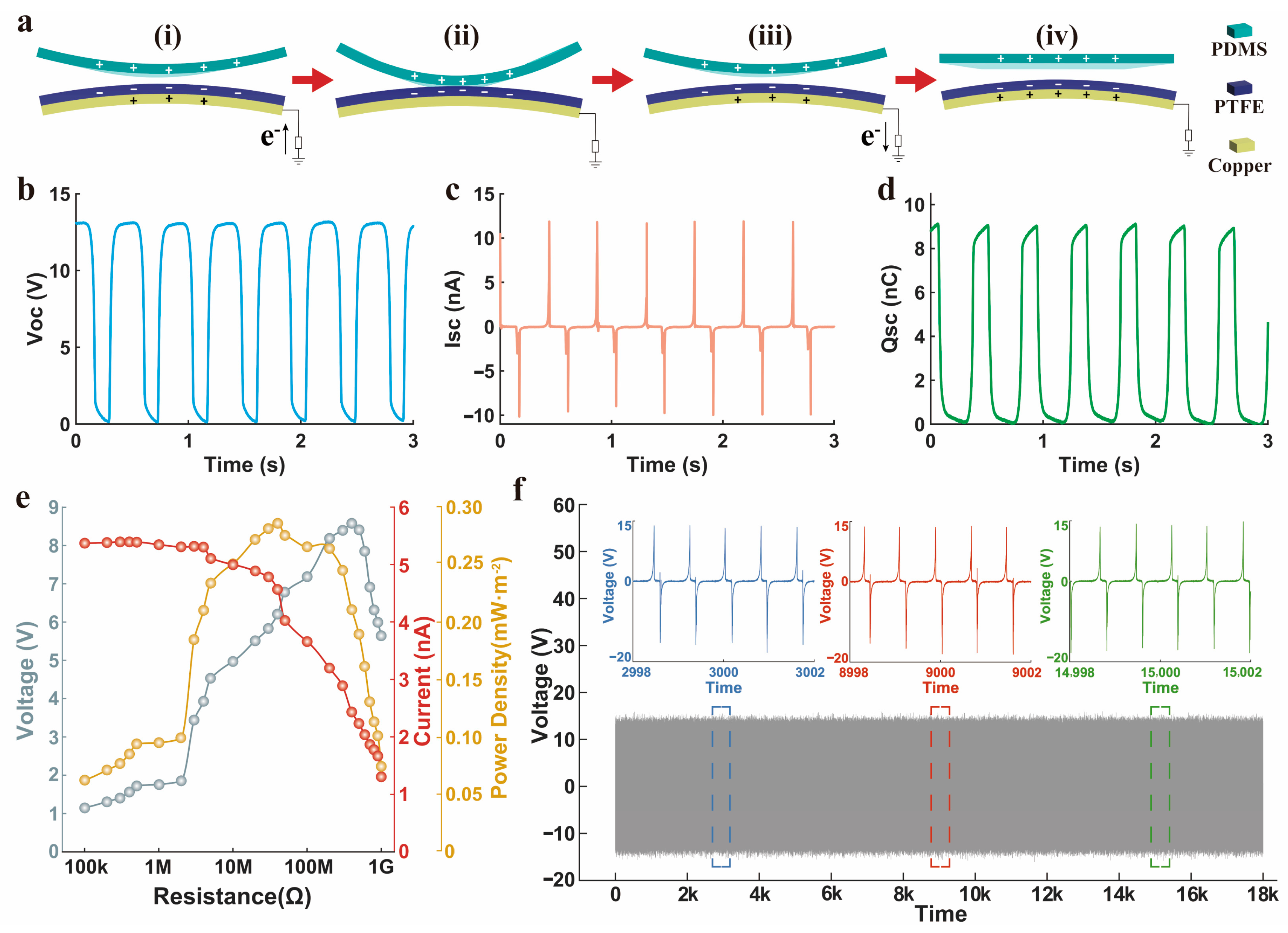
2.3. Characterization of the Electrical Performance of the Device
2.4. Design of the Processing Circuit in Human–Machine Interaction Applications
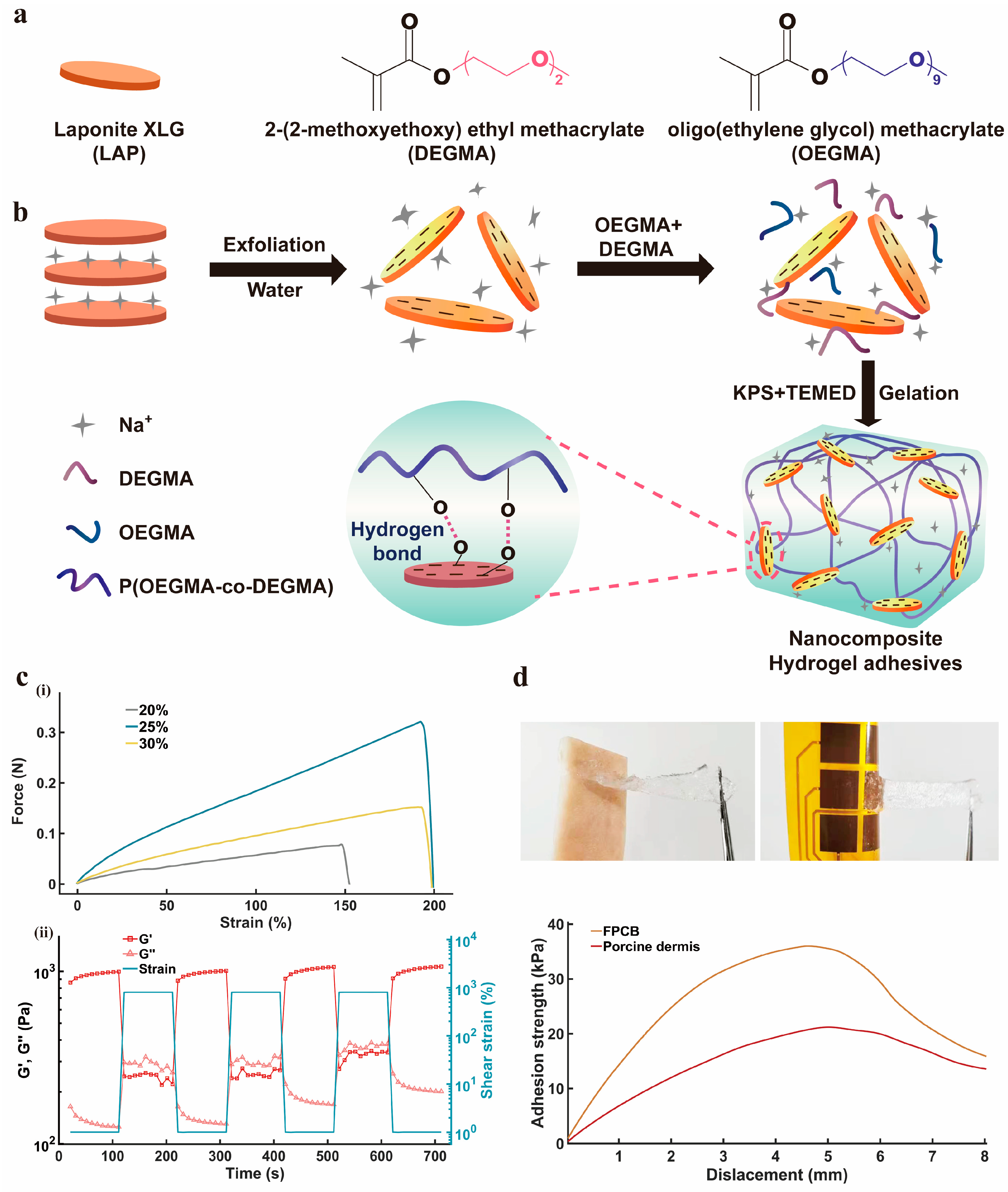
2.5. Synthesis of P(OEGMA-co-DEGMA)/LAP Nanocomposite Hydrogel Adhesives
2.5.1. Materials
2.5.2. Synthesis Process
2.5.3. Adhesive Strength Test and Self-Healing Capability Test of Nanocomposite Hydrogel Adhesive
3. Results and Discussion
3.1. Structure and Working Principle of the SSFHMI
3.2. The Hydrogel-Based Adhesive Layer of SSFHMI
3.3. Output Performance of a Single TENG
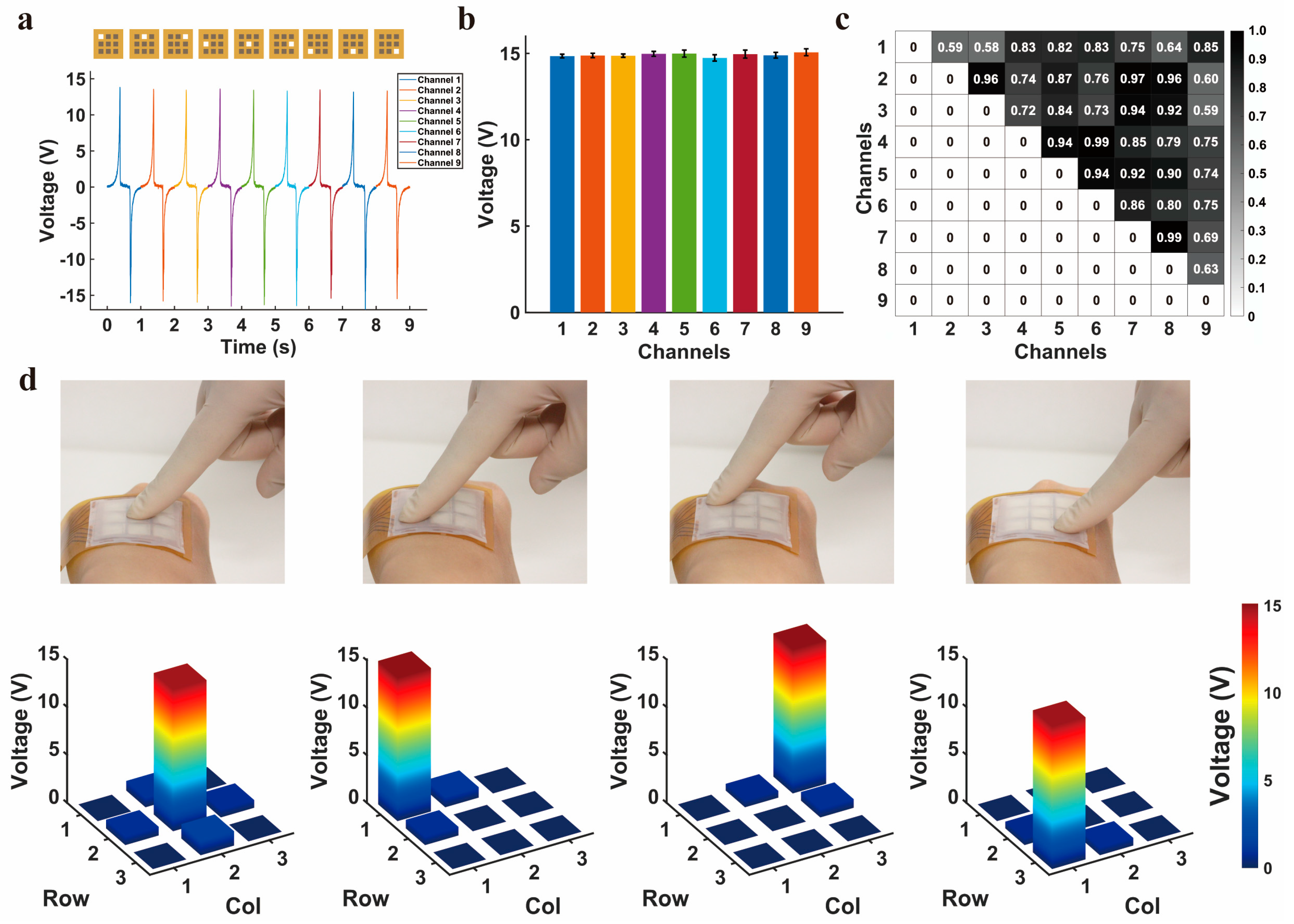
3.4. Output Characterization of the TENG Array
3.5. Signal Coding of the Proposed SSFHMI for Intelligent Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, Z.; Duan, S.; Liu, M.; Dang, C.; Qian, S.; Zhang, L.; Wang, H.; Yan, W.; Zhu, M. Insights into materials, physics, and applications in flexible and wearable acoustic sensing technology. Adv. Mater. 2024, 36, 2306880. [Google Scholar] [CrossRef]
- Luo, Y.; Abidian, M.R.; Ahn, J.-H.; Akinwande, D.; Andrews, A.M.; Antonietti, M.; Bao, Z.; Berggren, M.; Berkey, C.A.; Bettinger, C.J.; et al. Technology Roadmap for Flexible Sensors. ACS Nano 2023, 17, 5211–5295. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Rajagopal, S.; Prieto-Simón, B.; Pogue, B.W. Recent advances in smart wearable sensors for continuous human health monitoring. Talanta 2024, 272, 125817. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Wu, X.; Zhu, S.; Zeng, X.; Zeng, Z.; Zheng, Q. Triboelectric nanogenerators for clinical diagnosis and therapy: A report of recent progress. Med. Nov. Technol. Devices 2022, 16, 100195. [Google Scholar] [CrossRef]
- Wang, H.; Ding, Q.; Luo, Y.; Wu, Z.; Yu, J.; Chen, H.; Zhou, Y.; Zhang, H.; Tao, K.; Chen, X.; et al. High-performance hydrogel sensors enabled multimodal and accurate human–machine interaction system for active rehabilitation. Adv. Mater. 2024, 36, 2309868. [Google Scholar] [CrossRef] [PubMed]
- Heng, W.; Solomon, S.; Gao, W. Flexible electronics and devices as human–machine interfaces for medical robotics. Adv. Mater. 2022, 34, 2107902. [Google Scholar] [CrossRef]
- Shang, Y.; Zhang, B.; Liu, J.; Xia, C.; Yang, X.; Yan, D.; Sun, J. Facile and economical fabrication of superhydrophobic flexible resistive strain sensors for human motion detection. Nanomanufact. Metrol. 2023, 6, 2. [Google Scholar] [CrossRef]
- Cheng, A.J.; Wu, L.; Sha, Z.; Chang, W.; Chu, D.; Wang, C.H.; Peng, S. Recent advances of capacitive sensors: Materials, microstructure designs, applications, and opportunities. Adv. Mater. Technol. 2023, 8, 2201959. [Google Scholar] [CrossRef]
- Fan, F.-R.; Tian, Z.-Q.; Wang, Z.L. Flexible triboelectric generator. Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Jan, A.A.; Kim, S.; Kim, S. A skin-wearable and self-powered laminated pressure sensor based on triboelectric nanogenerator for monitoring human motion. Soft Sci. 2024, 4, 10. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, X.; Zhu, M.; Sun, Z.; Zhang, Z.; He, T.; Lee, C. Triboelectric nanogenerator enabled wearable sensors and electronics for sustainable internet of things integrated green earth. Adv. Energy Mater. 2023, 13, 2203040. [Google Scholar] [CrossRef]
- Niu, Z.; Wang, Q.; Lu, J.; Hu, Y.; Huang, J.; Zhao, W.; Liu, Y.; Long, Y.; Han, G. Electrospun cellulose nanocrystals reinforced flexible sensing paper for triboelectric energy harvesting and dynamic self-powered tactile perception. Small 2024, 20, 2307810. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Yang, J.; Cao, X.; Wang, N. Flexible and highly sensitive triboelectric nanogenerator with magnetic nanocomposites for cultural heritage conservation and human motion monitoring. Nano Energy 2022, 101, 107570. [Google Scholar] [CrossRef]
- Ding, W.; Wang, A.C.; Wu, C.; Guo, H.; Wang, Z.L. Human–machine interfacing enabled by triboelectric nanogenerators and tribotronics. Adv. Mater. Technol. 2019, 4, 1800487. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, P.; Chang, B.; Ning, J.; Yan, T.; Yang, Z.; Lu, H. Hierarchical structure by self-sedimentation of liquid metal for flexible sensor integrating pressure detection and triboelectric nanogenerator. Adv. Funct. Mater. 2024, 34, 2400363. [Google Scholar] [CrossRef]
- Gai, Y.; Jiang, Y.; Li, Z. Advances in health rehabilitation devices based on triboelectric nanogenerators. Nano Energy 2023, 116, 108787. [Google Scholar] [CrossRef]
- Kim, H.; Rana, S.M.S.; Islam, M.R.; Faruk, O.; Shrestha, K.; Pradhan, G.B.; Park, J.Y. A molybdenum-disulfide nanocomposite film-based stretchable triboelectric nanogenerator for wearable biomechanical energy harvesting and self-powered human motion monitoring. Chem. Eng. J. 2024, 491, 151980. [Google Scholar] [CrossRef]
- Mao, J.; Zhou, P.; Wang, X.; Yao, H.; Liang, L.; Zhao, Y.; Zhang, J.; Ban, D.; Zheng, H. A health monitoring system based on flexible triboelectric sensors for intelligence medical internet of things and its applications in virtual reality. Nano Energy 2023, 118, 108984. [Google Scholar] [CrossRef]
- Hou, C.; Geng, J.; Yang, Z.; Tang, T.; Sun, Y.; Wang, F.; Liu, H.; Chen, T.; Sun, L. A delta-parallel-inspired human machine interface by using self-powered triboelectric nanogenerator toward 3D and VR/AR manipulations. Adv. Mater. Technol. 2021, 6, 2000912. [Google Scholar] [CrossRef]
- Shen, S.; Xiao, X.; Yin, J.; Xiao, X.; Chen, J. Self-powered smart gloves based on triboelectric nanogenerators. Small Methods 2022, 6, 2200830. [Google Scholar] [CrossRef]
- Zheng, Q.; Zou, Y.; Zhang, Y.; Liu, Z.; Shi, B.; Wang, X.; Jin, Y.; Ouyang, H.; Li, Z.; Wang, Z.L. Biodegradable triboelectric nanogenerator as a life-time designed implantable power source. Sci. Adv. 2016, 2, e1501478. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.; Aazem, I.; Walden, R.; Bairagi, S.; Mulvihill, D.M.; Pillai, S.C. Electrospun nanofiber based TENGs for wearable electronics and self-powered sensing. Chem. Eng. J. 2023, 452, 139060. [Google Scholar] [CrossRef]
- Wu, C.; McClements, D.J.; Li, L.; He, M.; Li, Y.; Teng, F. Fabrication of composite hydrogels by assembly of okara cellulose nanofibers and gum arabic in ionic liquids: Structure and properties. J. Mol. Liq. 2022, 349, 118132. [Google Scholar] [CrossRef]
- Yi, J.; Dong, K.; Shen, S.; Jiang, Y.; Peng, X.; Ye, C.; Wang, Z.L. Fully fabric-based triboelectric nanogenerators as self-powered human–machine interactive keyboards. Nano-Micro Lett. 2021, 13, 103. [Google Scholar] [CrossRef]
- Shen, S.; Yi, J.; Sun, Z.; Guo, Z.; He, T.; Ma, L.; Li, H.; Fu, J.; Lee, C.; Wang, Z.L. Human machine interface with wearable electronics using biodegradable triboelectric films for calligraphy practice and correction. Nano-Micro Lett. 2022, 14, 225. [Google Scholar] [CrossRef]
- Wang, H.; Han, M.; Song, Y.; Zhang, H. Design, manufacturing and applications of wearable triboelectric nanogenerators. Nano Energy 2021, 81, 105627. [Google Scholar] [CrossRef]
- Wang, H.; Xiang, Z.; Zhao, P.; Wan, J.; Miao, L.; Guo, H.; Xu, C.; Zhao, W.; Han, M.; Zhang, H. Double-sided wearable multifunctional sensing system with anti-interference design for human–ambience interface. ACS Nano 2022, 16, 14679–14692. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Du, J.; Yang, P.; Shi, Y.; Liu, Z.; Yang, D.; Zheng, L.; Chen, X.; Wang, Z.L. Human–machine interaction via dual modes of voice and gesture enabled by triboelectric nanogenerator and machine learning. ACS Appl. Mater. Interfaces 2023, 15, 17009–17018. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Z.; Wang, J.; Xiao, X.; Li, Q.; Ding, W.; Fu, H.Y. Triboelectric bending sensor based smart glove towards intuitive multi-dimensional human-machine interfaces. Nano Energy 2021, 89, 106330. [Google Scholar] [CrossRef]
- Fang, P.; Zhu, M.; Zeng, Z.; Lu, W.; Wang, F.; Zhang, L.; Chen, T.; Sun, L. A multi-module sensing and bi-directional HMI integrating interaction, recognition, and feedback for intelligent robots. Adv. Funct. Mater. 2024, 34, 2310254. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, K.; Chen, S.; Huang, Y.; Askari, H.; Yu, N.; Mo, J.; Xu, N.; Wu, M.; Chen, H.; et al. Triboelectric nanogenerator sensors for intelligent steering wheel aiming at automated driving. Nano Energy 2023, 113, 108575. [Google Scholar] [CrossRef]
- Zheng, Q.; Hou, Y.; Yang, H.; Tan, P.; Shi, H.; Xu, Z.; Ye, Z.; Chen, N.; Qu, X.; Han, X.; et al. Towards a sustainable monitoring: A self-powered smart transportation infrastructure skin. Nano Energy 2022, 98, 107245. [Google Scholar] [CrossRef]
- Ma, P.; Liang, W.; Huang, R.; Zheng, B.; Feng, K.; He, W.; Huang, Z.; Shen, H.; Wang, H.; Wu, D. Super-structured wet-adhesive hydrogel with ultralow swelling, ultrahigh burst pressure tolerance, and anti-postoperative adhesion properties for tissue adhesion. Adv. Mater. 2024, 36, 2305400. [Google Scholar] [CrossRef]
- Kim, H.; Dutta, S.D.; Randhawa, A.; Patil, T.V.; Ganguly, K.; Acharya, R.; Lee, J.; Park, H.; Lim, K.-T. Recent advances and biomedical application of 3D printed nanocellulose-based adhesive hydrogels: A review. Int. J. Biol. Macromol. 2024, 264, 130732. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Chee, P.L.; Sugiarto, S.; Yu, Y.; Shi, C.; Yan, R.; Yao, Z.; Shi, X.; Zhi, J.; Kai, D.; et al. Hydrogel-based flexible electronics. Adv. Mater. 2023, 35, 2205326. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Chen, Y.; Jin, M.; Jia, Y.; Wang, Y.; Ren, L. Weak hydrogen bonds lead to self-healable and bioadhesive hybrid polymeric hydrogels with mineralization-active functions. Biomacromolecules 2018, 19, 1939–1949. [Google Scholar] [CrossRef]
- Yang, J.; An, J.; Sun, Y.; Zhang, J.; Zu, L.; Li, H.; Jiang, T.; Chen, B.; Wang, Z.L. Transparent self-powered triboelectric sensor based on PVA/PA hydrogel for promoting human-machine interaction in nursing and patient safety. Nano Energy 2022, 97, 107199. [Google Scholar] [CrossRef]
- Wei, P.; Chen, T.; Chen, G.; Liu, H.; Mugaanire, I.T.; Hou, K.; Zhu, M. Conductive self-healing nanocomposite hydrogel skin sensors with antifreezing and thermoresponsive properties. ACS Appl. Mater. Interfaces 2020, 12, 3068–3079. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, M.; Sun, Y.; Zuo, B. Self-healing, wet-adhesion silk fibroin conductive hydrogel as a wearable strain sensor for underwater applications. Chem. Eng. J. 2022, 446, 136931. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wang, A.C. On the origin of contact-electrification. Mater. Today 2019, 30, 34–51. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.; Lao, J.; Gao, H.; Yu, J. Hydrogels for flexible electronics. ACS Nano 2023, 17, 9681–9693. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Cross, L.M.; Peak, C.W.; Gold, K.; Carrow, J.K.; Brokesh, A.; Singh, K.A. 2D nanoclay for biomedical applications: Regenerative medicine, therapeutic delivery, and additive manufacturing. Adv. Mater. 2019, 31, 1900332. [Google Scholar] [CrossRef]
- Pertici, V.; Trimaille, T.; Gigmes, D. Inputs of macromolecular engineering in the design of injectable hydrogels based on synthetic thermoresponsive polymers. Macromolecules 2020, 53, 682–692. [Google Scholar] [CrossRef]
- Liu, Y.; Mo, J.; Fu, Q.; Lu, Y.; Zhang, N.; Wang, S.; Nie, S. Enhancement of triboelectric charge density by chemical functionalization. Adv. Funct. Mater. 2020, 30, 2004714. [Google Scholar] [CrossRef]
- Guo, X.; Yang, F.; Sun, X.; Bai, Y.; Liu, G.; Liu, W.; Wang, R.; He, X. Anti-Freezing Self-Adhesive Self-Healing Degradable Touch Panel with Ultra-Stretchable Performance Based on Transparent Triboelectric Nanogenerators. Adv. Funct. Mater. 2022, 32, 2201230. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, L.; Xia, Y.; Qiu, R.; Liu, W.; Wu, M.; Zhu, Y.; Zhu, S.; Jia, C.; Zhu, M.; et al. Flexible high-resolution triboelectric sensor array based on patterned laser-induced graphene for self-powered real-time tactile sensing. Adv. Funct. Mater. 2021, 31, 2100709. [Google Scholar] [CrossRef]
- Gao, G.; Yang, F.; Zhou, F.; He, J.; Lu, W.; Xiao, P.; Yan, H.; Pan, C.; Chen, T.; Wang, Z.L. Bioinspired self-healing human–machine interactive touch pad with pressure-sensitive adhesiveness on targeted substrates. Adv. Mater. 2020, 32, 2004290. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Tang, Q.; Chen, W.; Huang, Z.; Liu, G.; Zeng, Q.; Chen, J.; Guo, H.; Xin, L.; Hu, C. Flexible triboelectric 3D touch pad with unit subdivision structure for effective XY positioning and pressure sensing. Nano Energy 2020, 76, 105047. [Google Scholar] [CrossRef]
- Chen, T.; Shi, Q.; Li, K.; Yang, Z.; Liu, H.; Sun, L.; Dziuban, J.A.; Lee, C. Investigation of Position Sensing and Energy Harvesting of a Flexible Triboelectric Touch Pad. Nanomaterials 2018, 8, 613. [Google Scholar] [CrossRef]
- Li, S.; Peng, W.; Wang, J.; Lin, L.; Zi, Y.; Zhang, G.; Wang, Z.L. All-Elastomer-Based Triboelectric Nanogenerator as a Keyboard Cover to Harvest Typing Energy. ACS Nano 2016, 10, 7973–7981. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, H.; Sun, X.; Diao, N.; Wang, J.; Zhang, B.; Qin, C.; Liang, E.; Mao, Y. Triboelectric touch-free screen sensor for noncontact gesture recognizing. Adv. Funct. Mater. 2020, 30, 1907893. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Z.; Liao, Q.; Xun, X.; Gao, F.; Xu, L.; Kang, Z.; Zhang, Y. Self-powered user-interactive electronic skin for programmable touch operation platform. Sci. Adv. 2020, 6, eaba4294. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Yang, Z.; Dong, Y.; Teng, L.; Li, D.; Han, H.; Zhu, S.; Sun, X.; Zeng, Z.; Zeng, X.; et al. A Self-Powered, Skin Adhesive, and Flexible Human–Machine Interface Based on Triboelectric Nanogenerator. Nanomaterials 2024, 14, 1365. https://doi.org/10.3390/nano14161365
Wu X, Yang Z, Dong Y, Teng L, Li D, Han H, Zhu S, Sun X, Zeng Z, Zeng X, et al. A Self-Powered, Skin Adhesive, and Flexible Human–Machine Interface Based on Triboelectric Nanogenerator. Nanomaterials. 2024; 14(16):1365. https://doi.org/10.3390/nano14161365
Chicago/Turabian StyleWu, Xujie, Ziyi Yang, Yu Dong, Lijing Teng, Dan Li, Hang Han, Simian Zhu, Xiaomin Sun, Zhu Zeng, Xiangyu Zeng, and et al. 2024. "A Self-Powered, Skin Adhesive, and Flexible Human–Machine Interface Based on Triboelectric Nanogenerator" Nanomaterials 14, no. 16: 1365. https://doi.org/10.3390/nano14161365
APA StyleWu, X., Yang, Z., Dong, Y., Teng, L., Li, D., Han, H., Zhu, S., Sun, X., Zeng, Z., Zeng, X., & Zheng, Q. (2024). A Self-Powered, Skin Adhesive, and Flexible Human–Machine Interface Based on Triboelectric Nanogenerator. Nanomaterials, 14(16), 1365. https://doi.org/10.3390/nano14161365






