Influence of the Structure of Hydrothermal-Synthesized TiO2 Nanowires Formed by Annealing on the Photocatalytic Reduction of CO2 in H2O Vapor
Abstract
:1. Introduction
2. Materials and Methods
3. Results
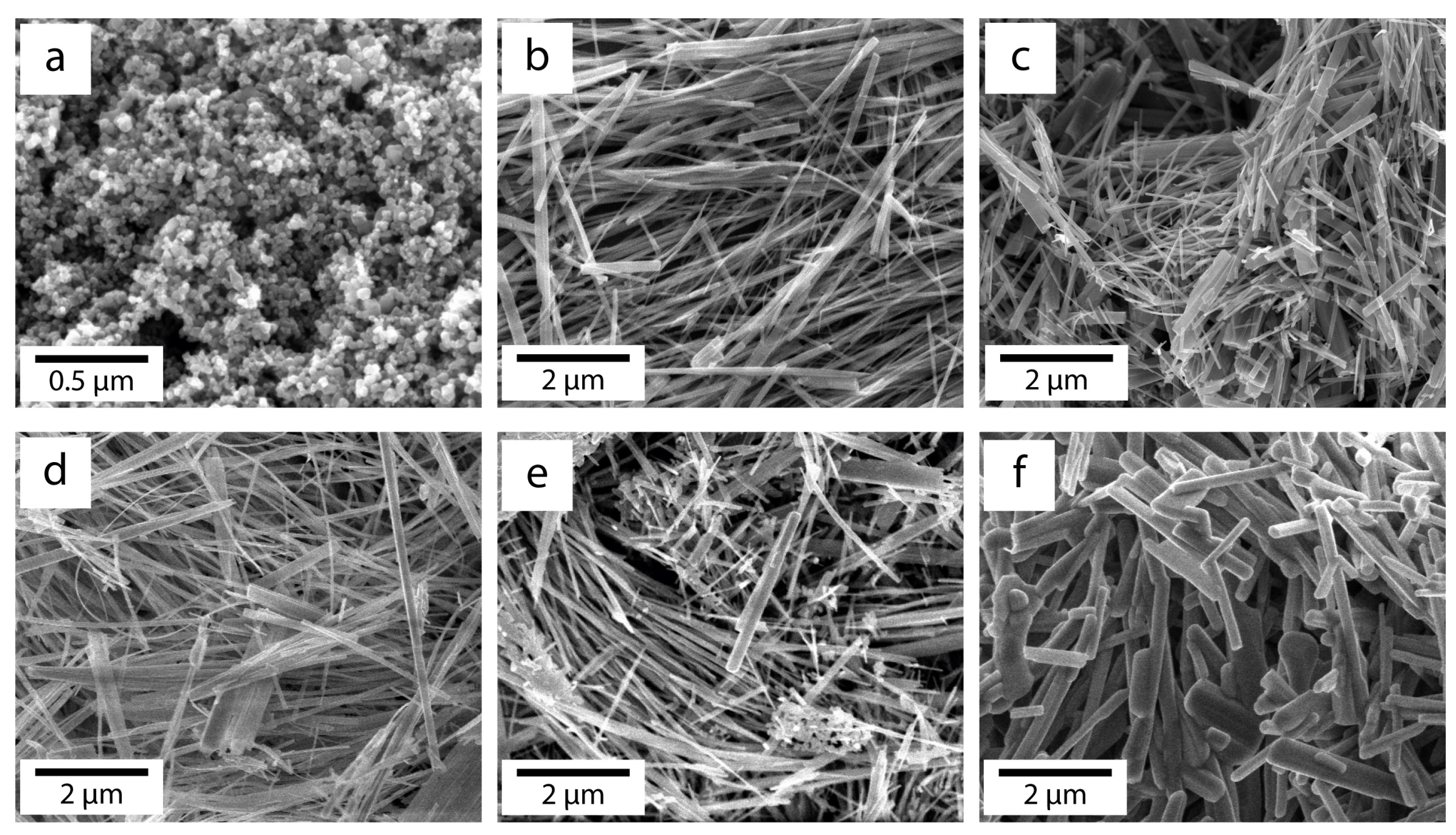
3.1. SEM Study of Morphology of Experimental Samples
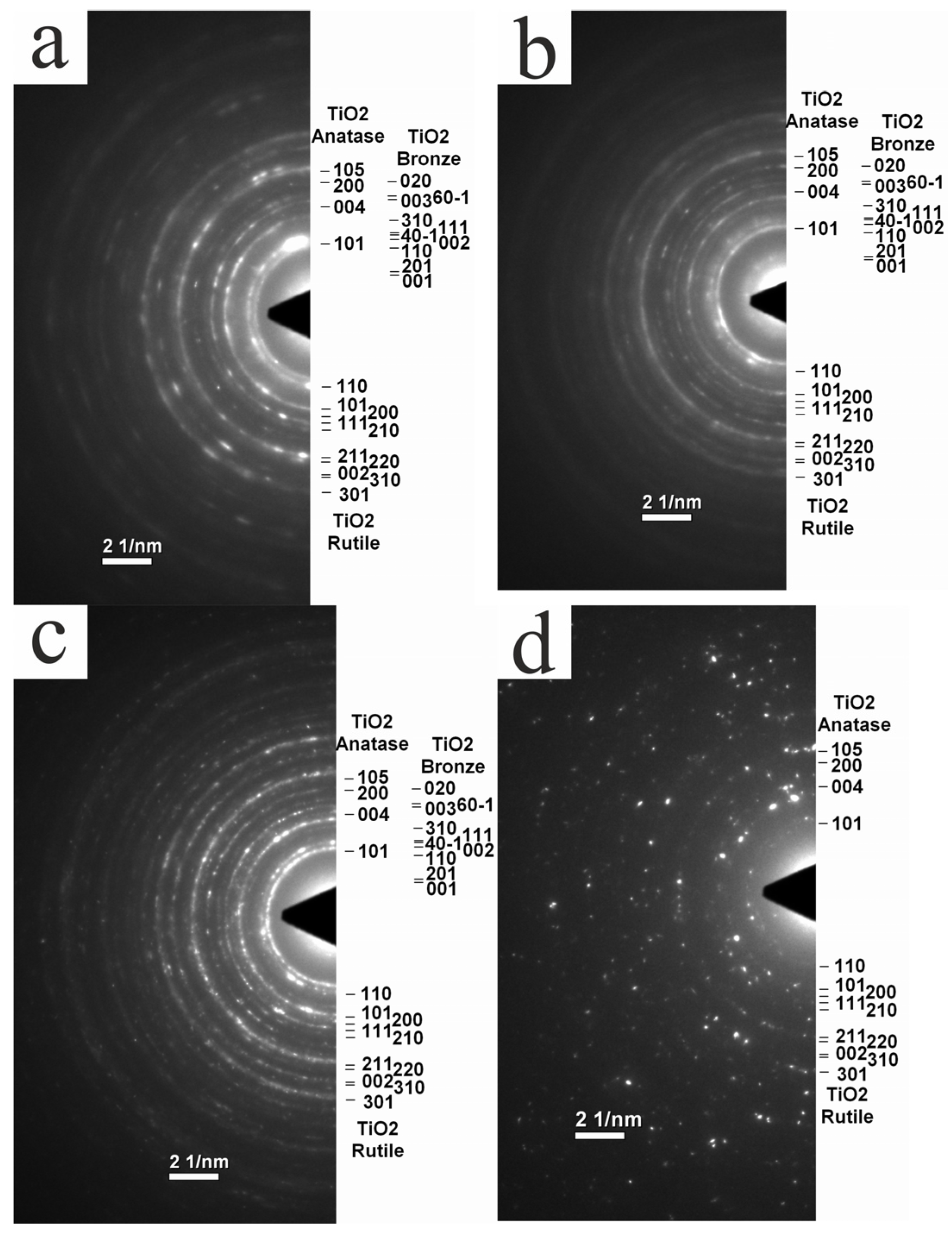
3.2. XRD Studies of Phase Composition of Experimental Samples
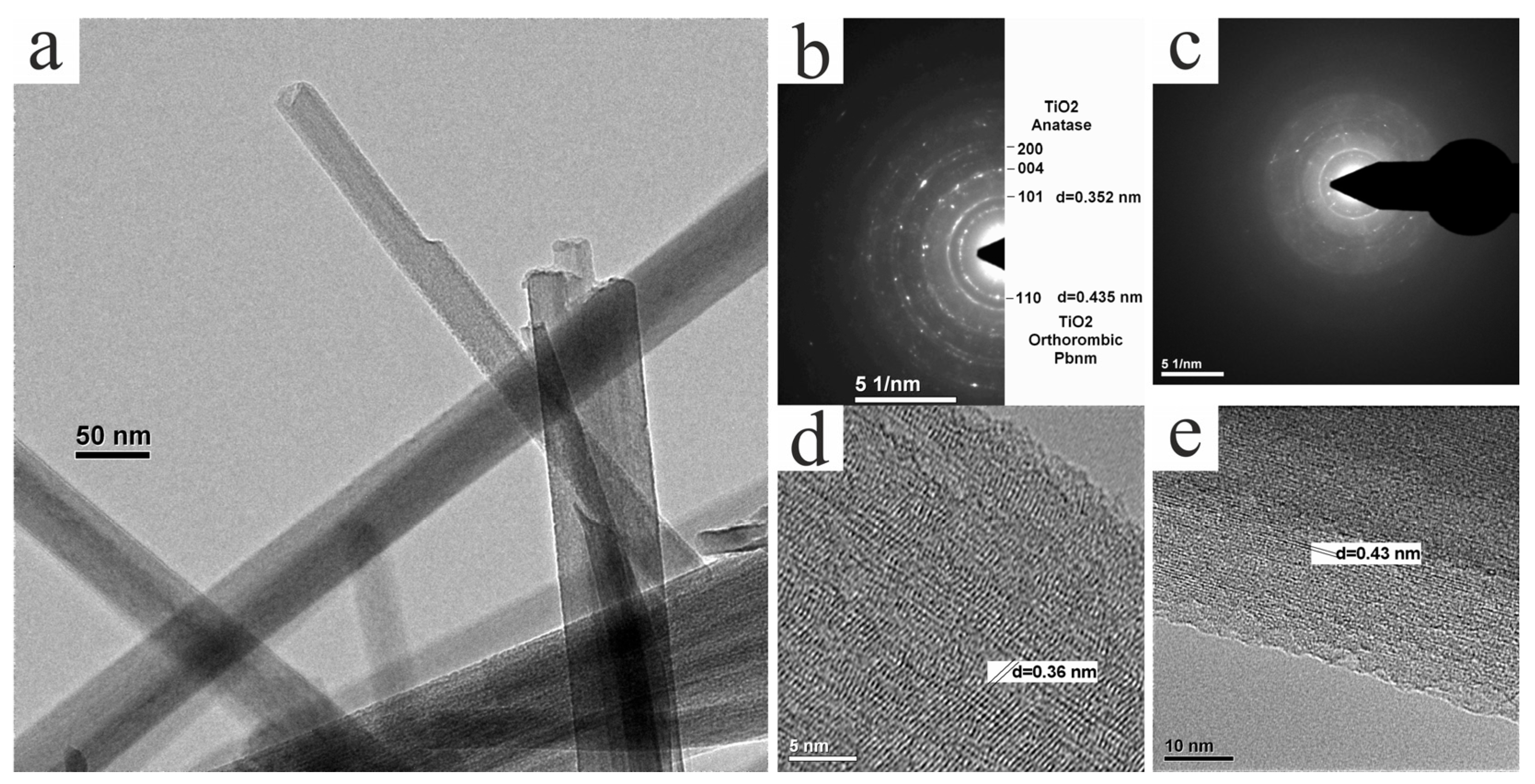
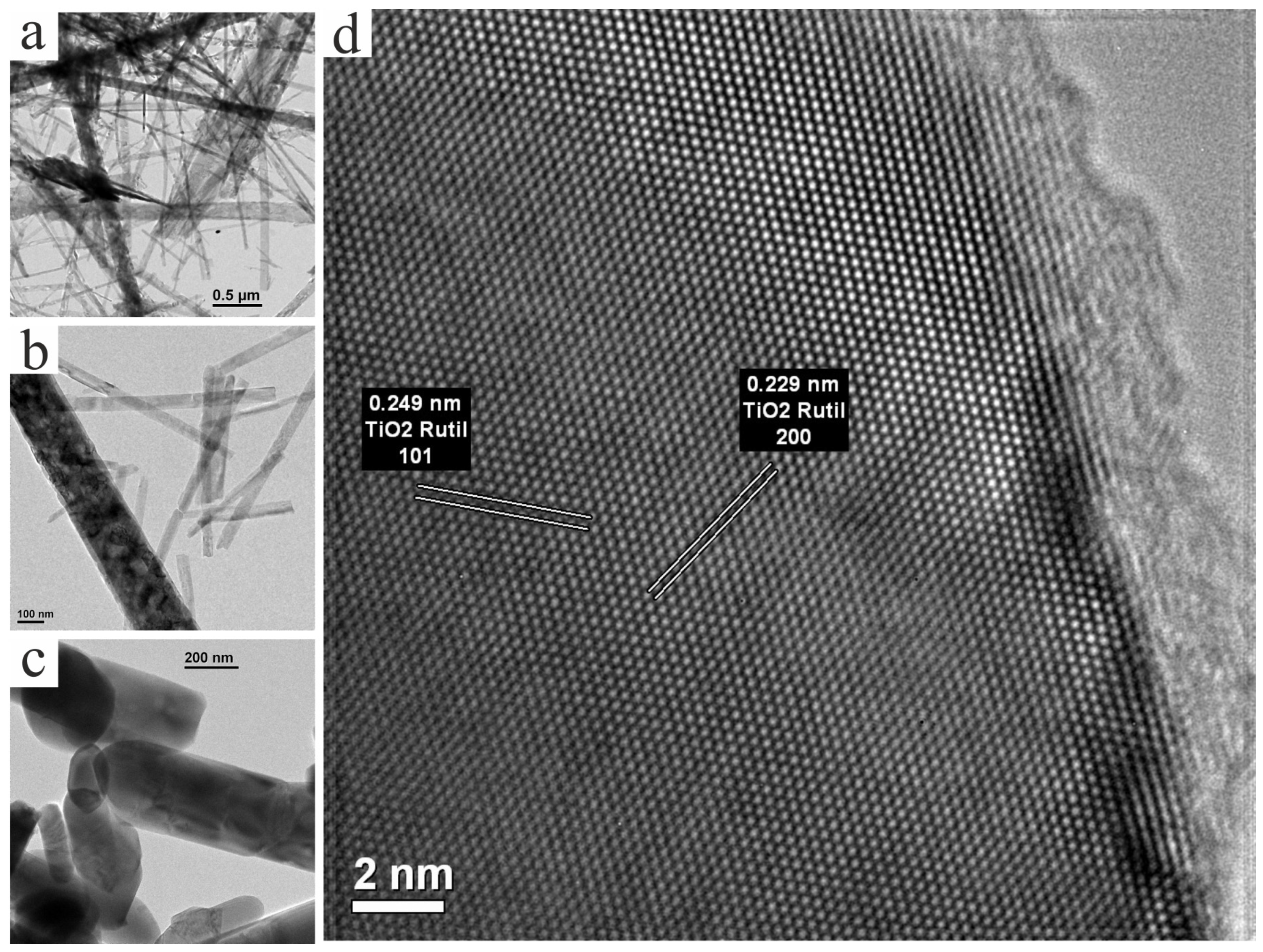
3.3. TEM Study of Structure and Phase Composition of Experimental Samples
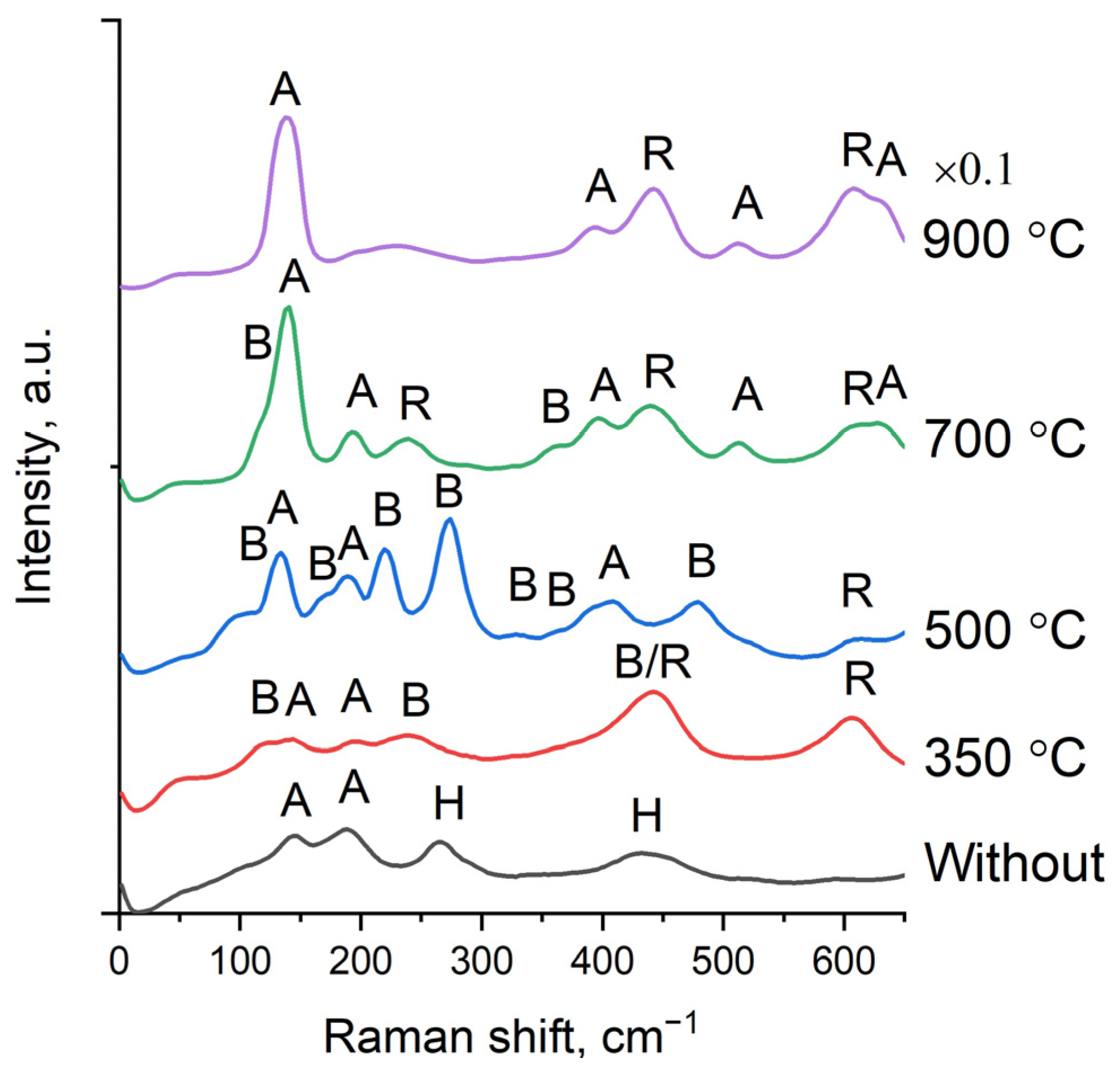
3.4. Raman Studies of the Phase Composition of Experimental Samples
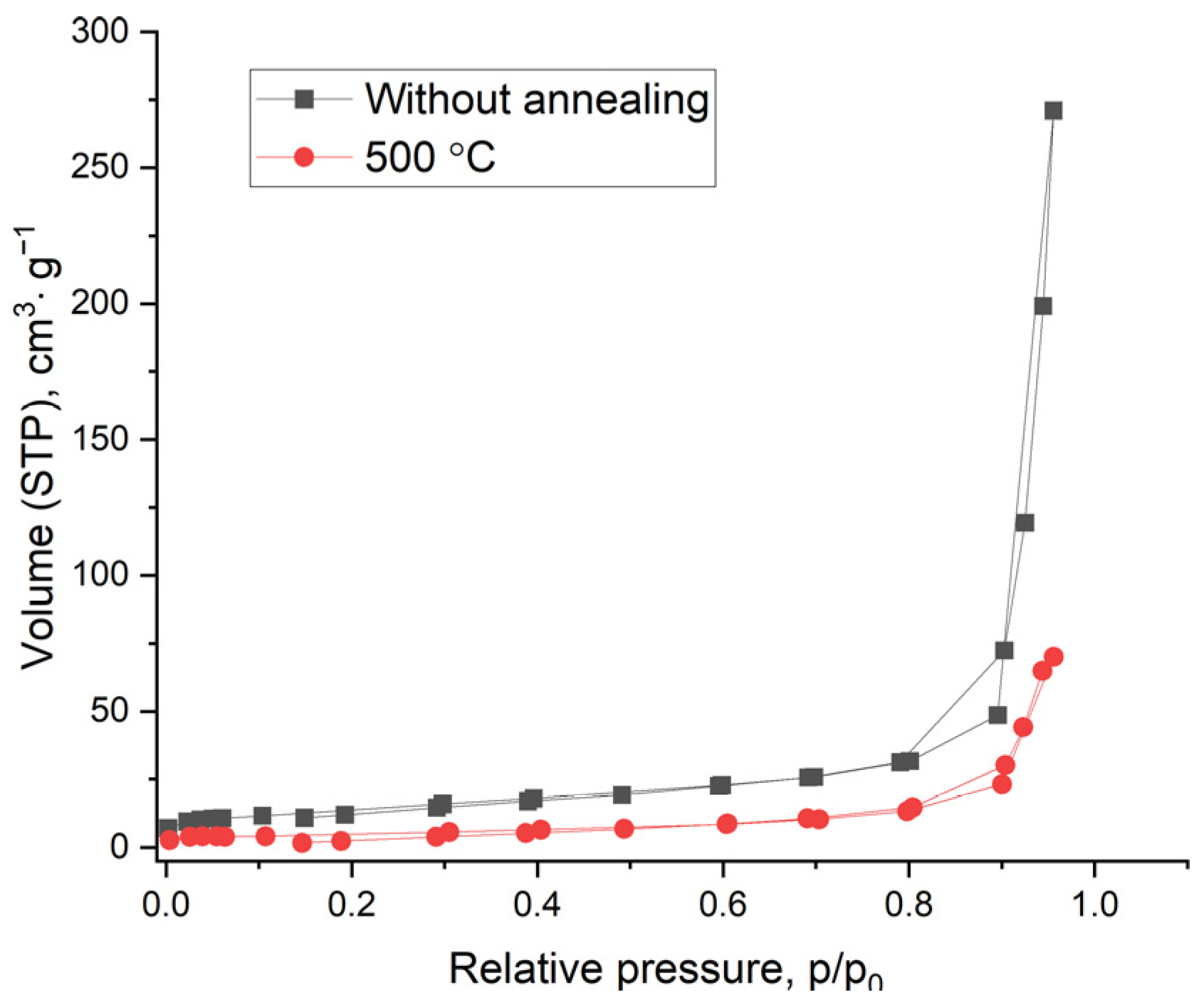
3.5. BET Studies of the Specific Surface Area of Experimental Samples
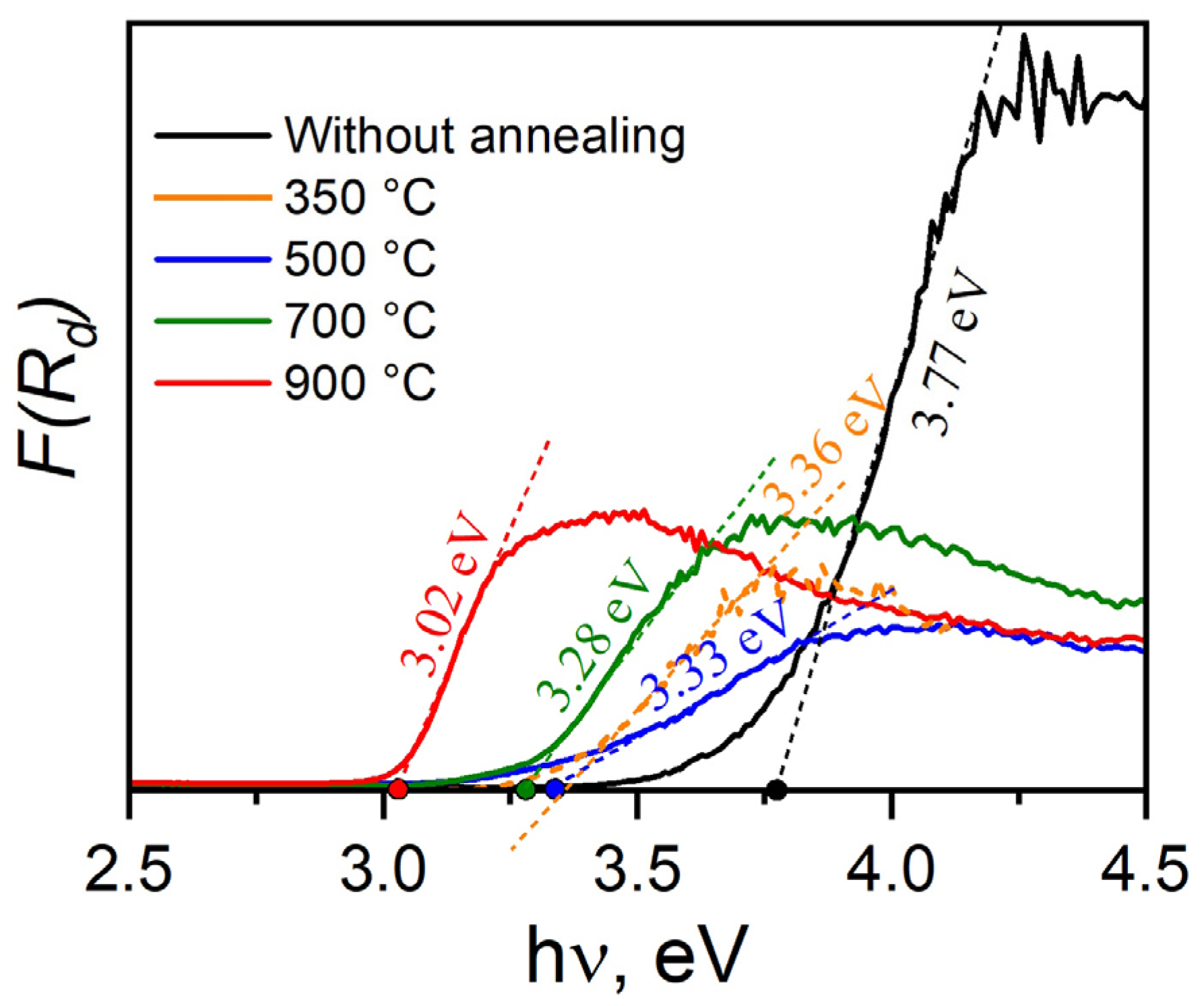
3.6. Optical Properties
3.7. Analysis of Structural Characterization of TiO2 Nanowire Samples
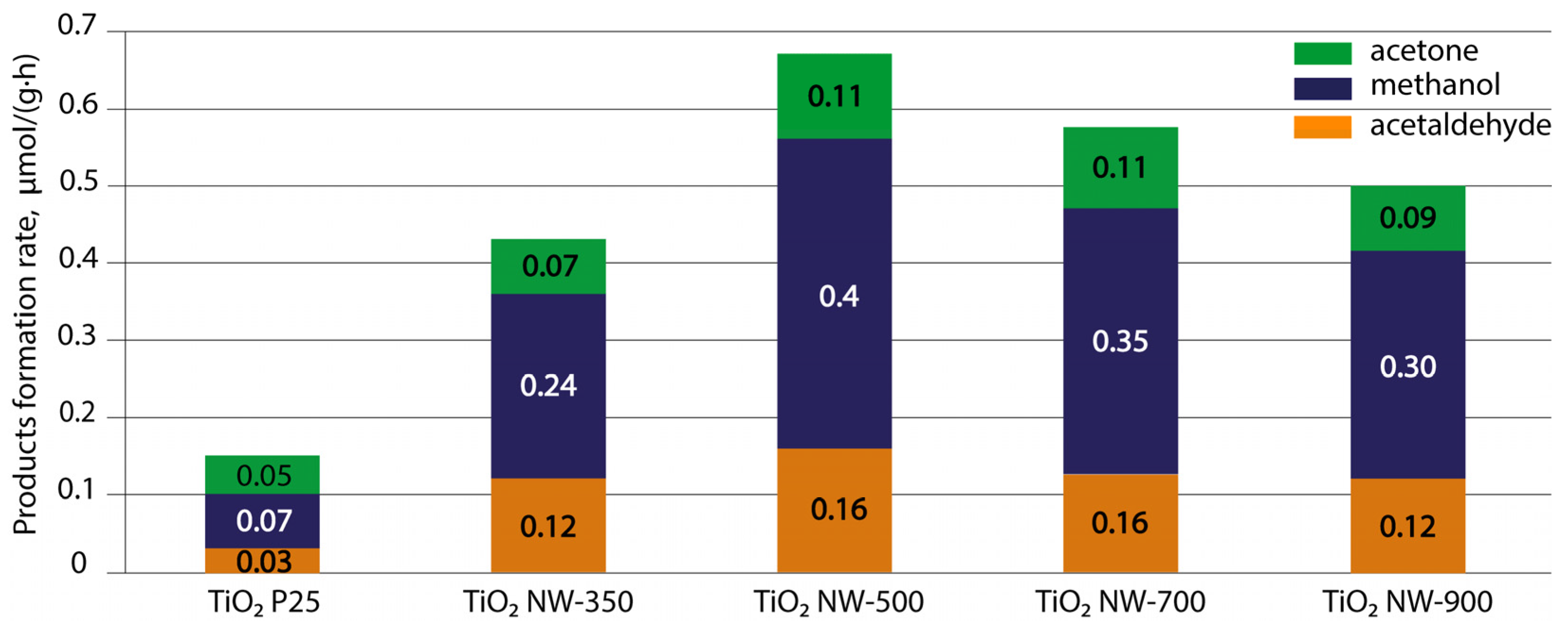
3.8. Photocatalytic Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium Dioxide Photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Kumar, A.; Saxena, V.K.; Thangavel, R.; Nandi, B.K. A Dual Effect of Surface Roughness and Photocatalysis of Crystalline TiO2-Thin Film for Self-Cleaning Application on a Photovoltaic Covering Glass. Mater. Chem. Phys. 2022, 289, 126427. [Google Scholar] [CrossRef]
- Bai, X.; Yang, S.; Tan, C.; Jia, T.; Guo, L.; Song, W.; Jian, M.; Zhang, X.; Zhang, Z.; Wu, L.; et al. Synthesis of TiO2 Based Superhydrophobic Coatings for Efficient Anti-Corrosion and Self-Cleaning on Stone Building Surface. J. Clean. Prod. 2022, 380, 134975. [Google Scholar] [CrossRef]
- Perović, K.; Dela Rosa, F.M.; Kovačić, M.; Kušić, H.; Štangar, U.L.; Fresno, F.; Dionysiou, D.D.; Loncaric Bozic, A. Recent Achievements in Development of TiO2-Based Composite Photocatalytic Materials for Solar Driven Water Purification and Water Splitting. Materials 2020, 13, 1338. [Google Scholar] [CrossRef]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Titanium Dioxide as the Most Used Photocatalyst for Water Purification: An Overview. Catalysts 2022, 13, 26. [Google Scholar] [CrossRef]
- Chen, X.-F.; Jiao, C.-J. A Photocatalytic Mortar Prepared by Tourmaline and TiO2 Treated Recycled Aggregates and Its Air-Purifying Performance. Case Stud. Constr. Mater. 2022, 16, e01073. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Role of Titanium Dioxide (TiO2) Structural Design/Morphology in Photocatalytic Air Purification. Appl. Catal. B Environ. 2020, 269, 118735. [Google Scholar] [CrossRef]
- Liu, R.; Fang, S.-Y.; Dong, C.-D.; Tsai, K.-C.; Yang, W.-D. Enhancing Hydrogen Evolution of Water Splitting under Solar Spectra Using Au/TiO2 Heterojunction Photocatalysts. Int. J. Hydrogen Energy 2021, 46, 28462–28473. [Google Scholar] [CrossRef]
- Li, H.; Wu, S.; Hood, Z.D.; Sun, J.; Hu, B.; Liang, C.; Yang, S.; Xu, Y.; Jiang, B. Atomic Defects in Ultra-Thin Mesoporous TiO2 Enhance Photocatalytic Hydrogen Evolution from Water Splitting. Appl. Surf. Sci. 2020, 513, 145723. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic Degradation of Organic Pollutants Using TiO2-Based Photocatalysts: A Review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Kreft, S.; Wei, D.; Junge, H.; Beller, M. Recent Advances on TiO2-Based Photocatalytic CO2 Reduction. EnergyChem 2020, 2, 100044. [Google Scholar] [CrossRef]
- Zhang, T.; Han, X.; Nguyen, N.T.; Yang, L.; Zhou, X. TiO2-Based Photocatalysts for CO2 Reduction and Solar Fuel Generation. Chin. J. Catal. 2022, 43, 2500–2529. [Google Scholar] [CrossRef]
- Thakur, N.; Thakur, N.; Kumar, K. Phytochemically and PVP Stabilized TiO2 Nano spheres for Enhanced Photocatalytic and Antioxidant Efficiency. Mater. Today Commun. 2023, 35, 105587. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, L.; Huang, B.; Li, X. Enhanced Sensing Performance of Au-Decorated TiO2 Nanospheres with Hollow Structure for Formaldehyde Detection at Room Temperature. Sens. Actuators B Chem. 2022, 358, 131465. [Google Scholar] [CrossRef]
- Hoang, S.; Guo, Y.; Binder, A.J.; Tang, W.; Wang, S.; Liu, J.; Tran, H.; Lu, X.; Wang, Y.; Ding, Y.; et al. Activating Low-Temperature Diesel Oxidation by Single-Atom Pt on TiO2 Nanowire Array. Nat. Commun. 2020, 11, 1062. [Google Scholar] [CrossRef]
- Tshabalala, Z.P.; Mokoena, T.P.; Jozela, M.; Tshilongo, J.; Hillie, T.K.; Swart, H.C.; Motaung, D.E. TiO2 Nanowires for Humidity-Stable Gas Sensors for Toluene and Xylene. ACS Appl. Nano Mater. 2021, 4, 702–716. [Google Scholar] [CrossRef]
- Ikreedeegh, R.R.; Hossen, M.A.; Tahir, M.; Aziz, A.A. A Comprehensive Review on Anodic TiO2 Nanotube Arrays (TNTAs) and Their Composite Photocatalysts for Environmental and Energy Applications: Fundamentals, Recent Advances and Applications. Coord. Chem. Rev. 2024, 499, 215495. [Google Scholar] [CrossRef]
- Feng, Y.; Rijnaarts, H.H.M.; Yntema, D.; Gong, Z.; Dionysiou, D.D.; Cao, Z.; Miao, S.; Chen, Y.; Ye, Y.; Wang, Y. Applications of Anodized TiO2 Nanotube Arrays on the Removal of Aqueous Contaminants of Emerging Concern: A Review. Water Res. 2020, 186, 116327. [Google Scholar] [CrossRef]
- Han, Q.; Wu, C.; Jiao, H.; Xu, R.; Wang, Y.; Xie, J.; Guo, Q.; Tang, J. Rational Design of High-Concentration Ti3+ in Porous Carbon-Doped TiO2 Nanosheets for Efficient Photocatalytic Ammonia Synthesis. Adv. Mater. 2021, 33, 2008180. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zhu, X.; Liu, Y.; Wu, Z. Fluorine-Induced Oxygen Vacancies on TiO2 Nanosheets for Photocatalytic Indoor VOCs Degradation. Appl. Catal. B Environ. 2022, 316, 121610. [Google Scholar] [CrossRef]
- Cao, S.; Tao, F.F.; Tang, Y.; Li, Y.; Yu, J. Size-and Shape-Dependent Catalytic Performances of Oxidation and Reduction Reactions on Nanocatalysts. Chem. Soc. Rev. 2016, 45, 4747–4765. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Yang, S.; Lee, H. Surface Analysis of N-Doped TiO2 Nanorods and Their Enhanced Photocatalytic Oxidation Activity. Appl. Catal. B Environ. 2017, 204, 209–215. [Google Scholar] [CrossRef]
- Tian, J.; Zhao, Z.; Kumar, A.; Boughton, R.I.; Liu, H. Recent Progress in Design, Synthesis, and Applications of One-Dimensional TiO2 Nanostructured Surface Heterostructures: A Review. Chem. Soc. Rev. 2014, 43, 6920–6937. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Gatto, S.; Pirola, C.; Naldoni, A.; Di Michele, A.; Cerrato, G.; Crocellà, V.; Capucci, V. Photocatalytic Degradation of Acetone, Acetaldehyde and Toluene in Gas-Phase: Comparison between Nano and Micro-Sized TiO2. Appl. Catal. B Environ. 2014, 146, 123–130. [Google Scholar] [CrossRef]
- Tichapondwa, S.M.; Newman, J.P.; Kubheka, O. Effect of TiO2 Phase on the Photocatalytic Degradation of Methylene Blue Dye. Phys. Chem. Earth Parts A/B/C 2020, 118–119, 102900. [Google Scholar] [CrossRef]
- Luís, A.M.; Neves, M.C.; Mendonça, M.H.; Monteiro, O.C. Influence of Calcination Parameters on the TiO2 Photocatalytic Properties. Mater. Chem. Phys. 2011, 125, 20–25. [Google Scholar] [CrossRef]
- Qian, R.; Zong, H.; Schneider, J.; Zhou, G.; Zhao, T.; Li, Y.; Yang, J.; Bahnemann, D.W.; Pan, J.H. Charge Carrier Trapping, Recombination and Transfer during TiO2 Photocatalysis: An Overview. Catal. Today 2019, 335, 78–90. [Google Scholar] [CrossRef]
- Ozawa, K.; Yamamoto, S.; Mase, K.; Matsuda, I. A Surface Science Approach to Unveiling the TiO2 Photocatalytic Mechanism: Correlation between Photocatalytic Activity and Carrier Lifetime. E-J. Surf. Sci. Nanotechnol. 2019, 17, 130–147. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Chen, Z.; Zhang, K.-Q.; Al-Deyab, S.S.; Lai, Y. A Review of One-Dimensional TiO2 Nanostructured Materials for Environmental and Energy Applications. J. Mater. Chem. A 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Adachi, M.; Murata, Y.; Takao, J.; Jiu, J.; Sakamoto, M.; Wang, F. Highly Efficient Dye-Sensitized Solar Cells with a Titania Thin-Film Electrode Composed of a Network Structure of Single-Crystal-like TiO2 Nanowires Made by the “Oriented Attachment” Mechanism. J. Am. Chem. Soc. 2004, 126, 14943–14949. [Google Scholar] [CrossRef]
- Huang, S.; Kilin, D.S. Anatase TiO2 Nanowires, Thin Films, and Surfaces: Ab Initio Studies of Electronic Properties and Non-Adiabatic Excited State Dynamics. MRS Proc. 2014, 1659, 129–134. [Google Scholar] [CrossRef]
- Choi, S.K.; Kim, S.; Lim, S.K.; Park, H. Photocatalytic Comparison of TiO2 Nanoparticles and Electrospun TiO2 Nanofibers: Effects of Mesoporosity and Interparticle Charge Transfer. J. Phys. Chem. C 2010, 114, 16475–16480. [Google Scholar] [CrossRef]
- Wu, H.B.; Hng, H.H.; Lou, X.W.D. Direct Synthesis of Anatase TiO2 Nanowires with Enhanced Photocatalytic Activity. Adv. Mater. 2012, 24, 2567–2571. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Suzuki, Y.; Yoshikawa, S. Syntheses of TiO2(B) Nanowires and TiO2 Anatase Nanowires by Hydrothermal and Post-Heat Treatments. J. Solid State Chem. 2005, 178, 2179–2185. [Google Scholar] [CrossRef]
- Akimoto, J.; Gotoh, Y.; Oosawa, Y.; Nonose, N.; Kumagai, T.; Aoki, K.; Takei, H. Topotactic Oxidation of Ramsdellite-Type Li0.5TiO2, a New Polymorph of Titanium Dioxide: TiO2(R). J. Solid State Chem. 1994, 113, 27–36. [Google Scholar] [CrossRef]
- Sun, J.; Wu, J.-M. A Comparative Study on Photocatalytic Activity of Titania Nanowires Subjected to High-Temperature Calcination and Low-Temperature HCl Treatment. Sci. Adv. Mat. 2013, 5, 549–556. [Google Scholar] [CrossRef]
- Hardcastle, F.D.; Ishihara, H.; Sharma, R.; Biris, A.S. Photoelectroactivity and Raman Spectroscopy of Anodized Titania (TiO2) Photoactive Water-Splitting Catalysts as a Function of Oxygen-Annealing Temperature. J. Mater. Chem. 2011, 21, 6337–6345. [Google Scholar] [CrossRef]
- Makal, P.; Das, D. Self-Doped TiO2 Nanowires in TiO2-B Single Phase, TiO2-B/Anatase and TiO2-Anatase/Rutile Heterojunctions Demonstrating Individual Superiority in Photocatalytic Activity under Visible and UV Light. Applied Surface Science 2018, 455, 1106–1115. [Google Scholar] [CrossRef]
- Simmons, E.L. Diffuse Reflectance Spectroscopy: A Comparison of the Theories. Appl. Opt. 1975, 14, 1380–1386. [Google Scholar] [CrossRef]
- Elsayed, W.; Abdalla, S.; Seriani, N. Quasiparticle and Optical Properties of Hydrogen Titanate and Its Defective Systems: An Investigation by Density Functional Theory with Hubbard Correction, Many-Body Perturbation Theory, and Bethe–Salpeter Equation. Phys. Status Solidi (b) 2020, 257, 1900054. [Google Scholar] [CrossRef]
- Makal, P.; Das, D. Superior Photocatalytic Dye Degradation under Visible Light by Reduced Graphene Oxide Laminated TiO2-B Nanowire Composite. J. Environ. Chem. Eng. 2019, 7, 103358. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the Anatase to Rutile Phase Transformation. J. Mater. Sci 2011, 46, 855–874. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-Gap Energy Estimation from Diffuse Reflectance Measurements on Sol–Gel and Commercial TiO2: A Comparative Study. J. Sol-Gel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Nguyen, D.L.T.; Nguyen, V.-H.; Le, T.-H.; Vo, D.-V.N.; Trinh, Q.T.; Bae, S.-R.; Chae, S.Y.; Kim, S.Y.; Le, Q.V. Recent Advances in TiO2-Based Photocatalysts for Reduction of CO2 to Fuels. Nanomaterials 2020, 10, 337. [Google Scholar] [CrossRef]
- Wu, N.; Wang, J.; Tafen, D.N.; Wang, H.; Zheng, J.-G.; Lewis, J.P.; Liu, X.; Leonard, S.S.; Manivannan, A. Shape-Enhanced Photocatalytic Activity of Single-Crystalline Anatase TiO2 (101) Nanobelts. J. Am. Chem. Soc. 2010, 132, 6679–6685. [Google Scholar] [CrossRef]
- Zaw, Y.Y.; Channei, D.A.D.; Threrujirapapong, T.; Khanitchaidecha, W.; Nakaruk, A. Effect of Anatase/Rutile Phase Ratio on the Photodegradation of Methylene Blue under UV Irradiation. MSF 2020, 998, 78–83. [Google Scholar] [CrossRef]
- Su, R.; Bechstein, R.; Sø, L.; Vang, R.T.; Sillassen, M.; Esbjörnsson, B.; Palmqvist, A.; Besenbacher, F. How the Anatase-to-Rutile Ratio Influences the Photoreactivity of TiO2. J. Phys. Chem. C 2011, 115, 24287–24292. [Google Scholar] [CrossRef]
- Tan, S.S.; Zou, L.; Hu, E. Kinetic Modelling for Photosynthesis of Hydrogen and Methane through Catalytic Reduction of Carbon Dioxide with Water Vapour. Catal. Today 2008, 131, 125–129. [Google Scholar] [CrossRef]
- Zhang, Q.-H.; Han, W.-D.; Hong, Y.-J.; Yu, J.-G. Photocatalytic Reduction of CO2 with H2O on Pt-Loaded TiO2 Catalyst. Catal. Today 2009, 148, 335–340. [Google Scholar] [CrossRef]
- Ali, S.; Flores, M.C.; Razzaq, A.; Sorcar, S.; Hiragond, C.B.; Kim, H.R.; Park, Y.H.; Hwang, Y.; Kim, H.S.; Kim, H.; et al. Gas Phase Photocatalytic CO2 Reduction, “A Brief Overview for Benchmarking”. Catalysts 2019, 9, 727. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, C.; Tang, X.; Qin, X.; Liu, X.; Peng, M.; Xu, Y.; Song, C.; Zhang, J.; Liang, X.; et al. CO-Tolerant RuNi/TiO2 Catalyst for the Storage and Purification of Crude Hydrogen. Nat. Commun. 2022, 13, 4404. [Google Scholar] [CrossRef] [PubMed]
- Vaiano, V.; Sannino, D.; Ciambelli, P. Steam Reduction of CO2 on Pd/TiO2 Catalysts: A Comparison between Thermal and Photocatalytic Reactions. Photochem. Photobiol. Sci. 2015, 14, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Hsu, Y.-H. Effects of Reaction Temperature on the Photocatalytic Activity of TiO2 with Pd and Cu Cocatalysts. Catalysts 2021, 11, 966. [Google Scholar] [CrossRef]





| Annealing Mode | P25 | No Annealing | 350 °C | 500 °C | 700 °C | 900 °C |
|---|---|---|---|---|---|---|
| S, m2/g | 57.0 | 49.0 | 39.0 | 17.3 | 12.3 | 3.2 |
| Annealing | Sample Designation | Surface Area, m2/g | Optical Band Gap, eV | Crystallite Size, nm | Phases |
|---|---|---|---|---|---|
| No annealing | As-synthesized TiO2 NW | 49.0 | 3.77 | ~12 | Titanate, amorphous, anatase |
| 350 °C | TiO2 NW-350 | 39.0 | 3.36 | ~13 | Amorphous, anatase, bronze, rutile |
| 500 °C | TiO2 NW-500 | 17.3 | 3.33 | ~16 | Amorphous, anatase, bronze, rutile |
| 700 °C | TiO2 NW-700 | 12.3 | 3.28 | ~26 | Amorphous, anatase, bronze, rutile |
| 900 °C | TiO2 NW-900 | 3.2 | 3.02 | ~63 | Anatase, rutile |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarasov, A.M.; Sorokina, L.I.; Dronova, D.A.; Volovlikova, O.; Trifonov, A.Y.; Itskov, S.S.; Tregubov, A.V.; Shabaeva, E.N.; Zhurina, E.S.; Dubkov, S.V.; et al. Influence of the Structure of Hydrothermal-Synthesized TiO2 Nanowires Formed by Annealing on the Photocatalytic Reduction of CO2 in H2O Vapor. Nanomaterials 2024, 14, 1370. https://doi.org/10.3390/nano14161370
Tarasov AM, Sorokina LI, Dronova DA, Volovlikova O, Trifonov AY, Itskov SS, Tregubov AV, Shabaeva EN, Zhurina ES, Dubkov SV, et al. Influence of the Structure of Hydrothermal-Synthesized TiO2 Nanowires Formed by Annealing on the Photocatalytic Reduction of CO2 in H2O Vapor. Nanomaterials. 2024; 14(16):1370. https://doi.org/10.3390/nano14161370
Chicago/Turabian StyleTarasov, Andrey M., Larisa I. Sorokina, Daria A. Dronova, Olga Volovlikova, Alexey Yu. Trifonov, Sergey S. Itskov, Aleksey V. Tregubov, Elena N. Shabaeva, Ekaterina S. Zhurina, Sergey V. Dubkov, and et al. 2024. "Influence of the Structure of Hydrothermal-Synthesized TiO2 Nanowires Formed by Annealing on the Photocatalytic Reduction of CO2 in H2O Vapor" Nanomaterials 14, no. 16: 1370. https://doi.org/10.3390/nano14161370





