Functionalization of Polypropylene by TiO2 Photocatalytic Nanoparticles: On the Importance of the Surface Oxygen Plasma Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
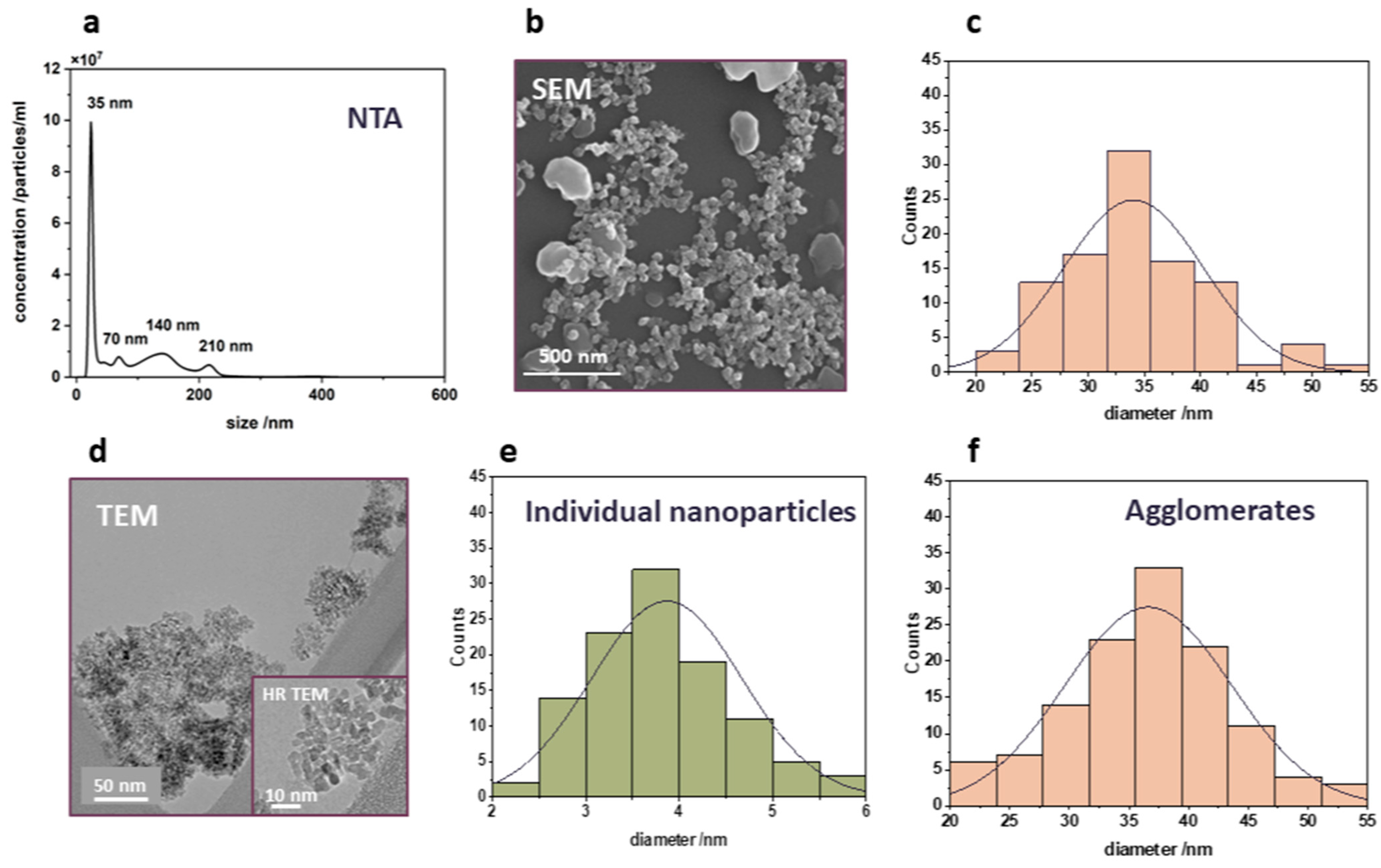
2.2. Nanoparticle Tracking Analysis (NTA)
2.3. Attenuated Total Reflection–Fourier Transformation Infrared Spectroscopy (ATR-FTIR)
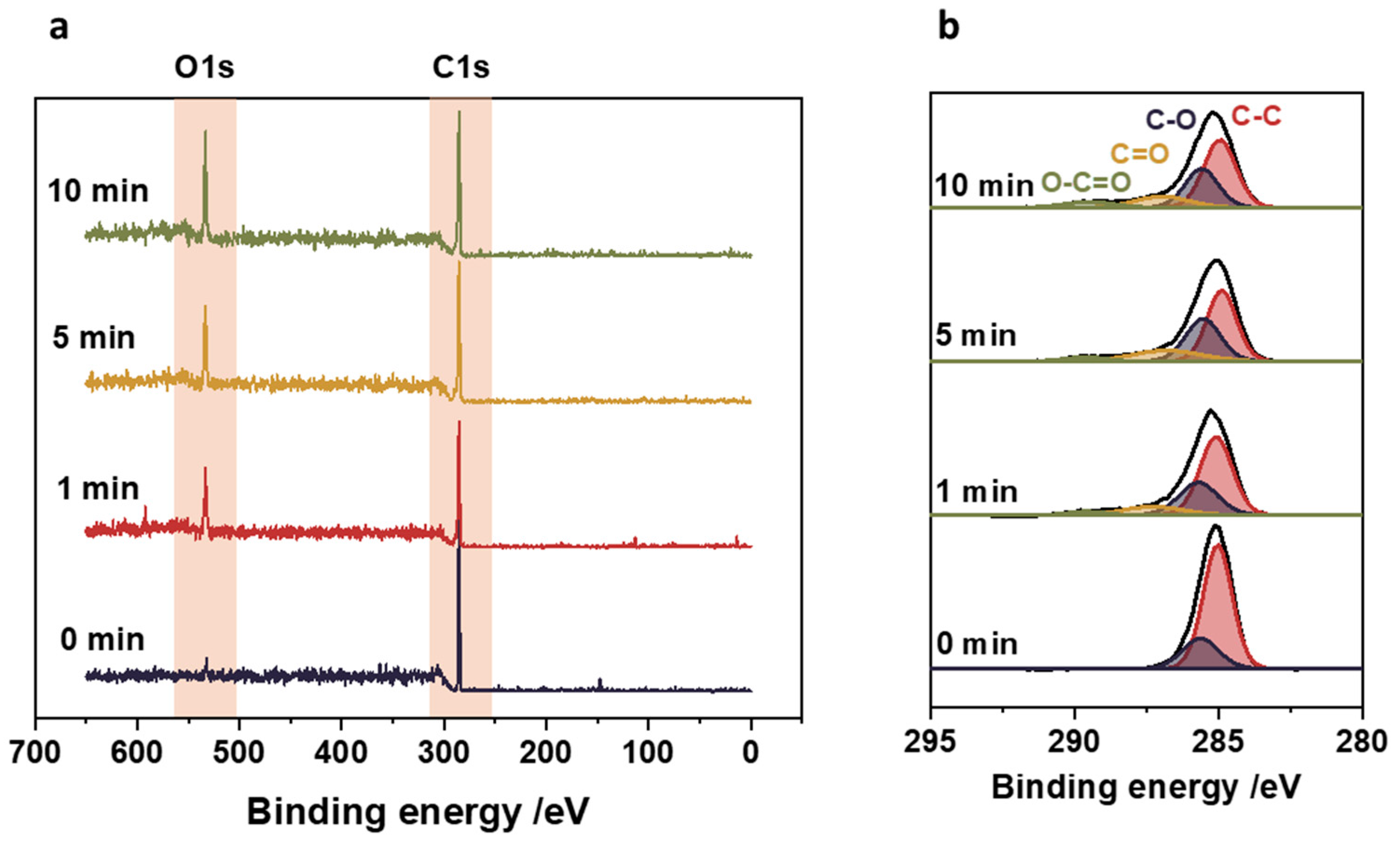
2.4. X-ray Photoelectron Spectroscopy (XPS)
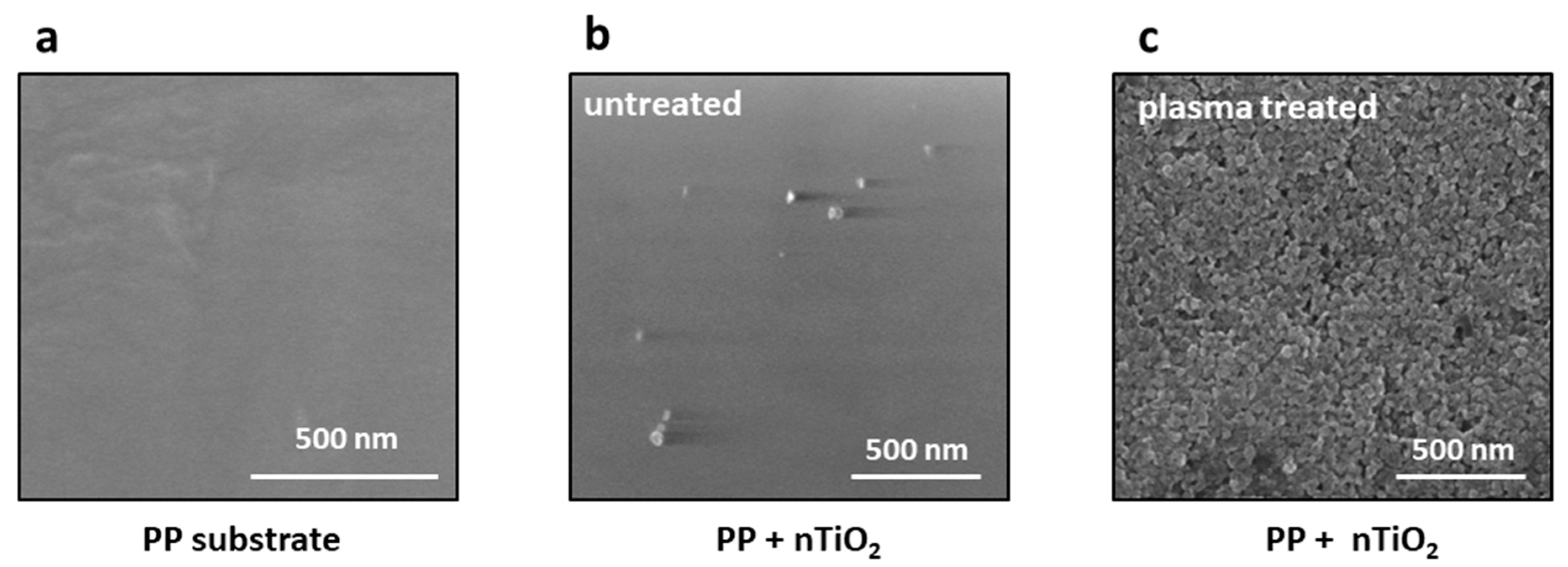
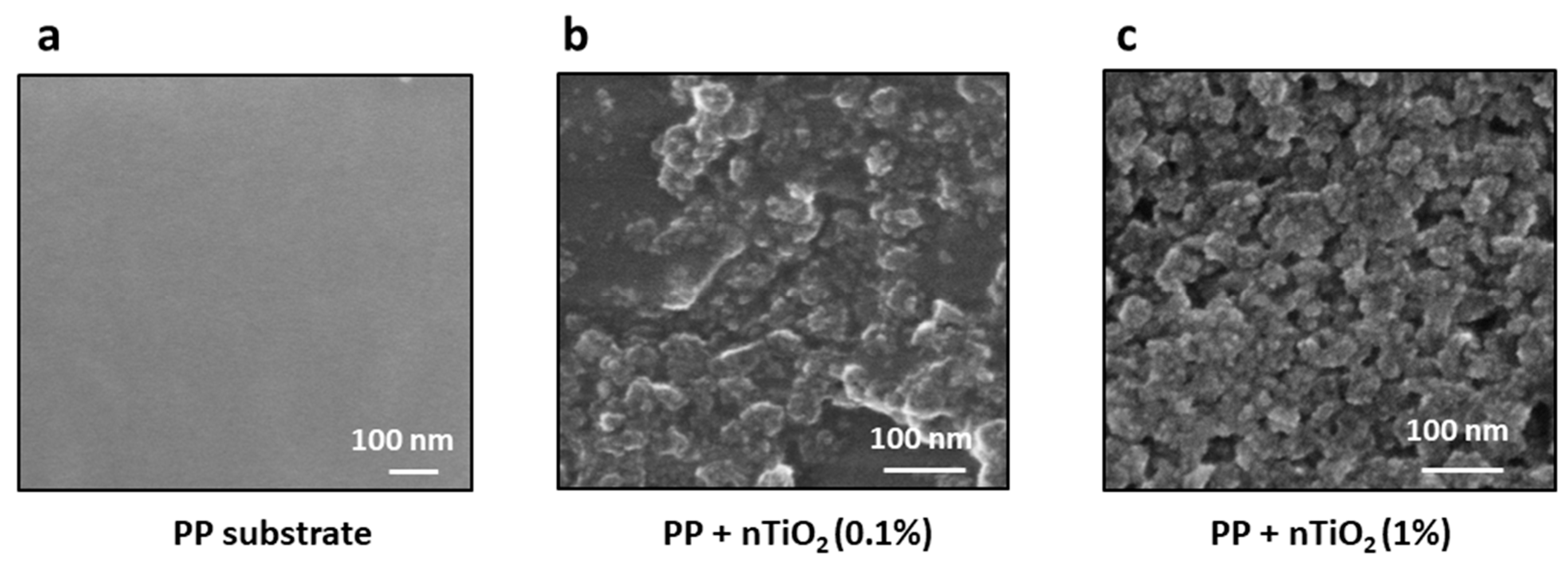
2.5. SEM
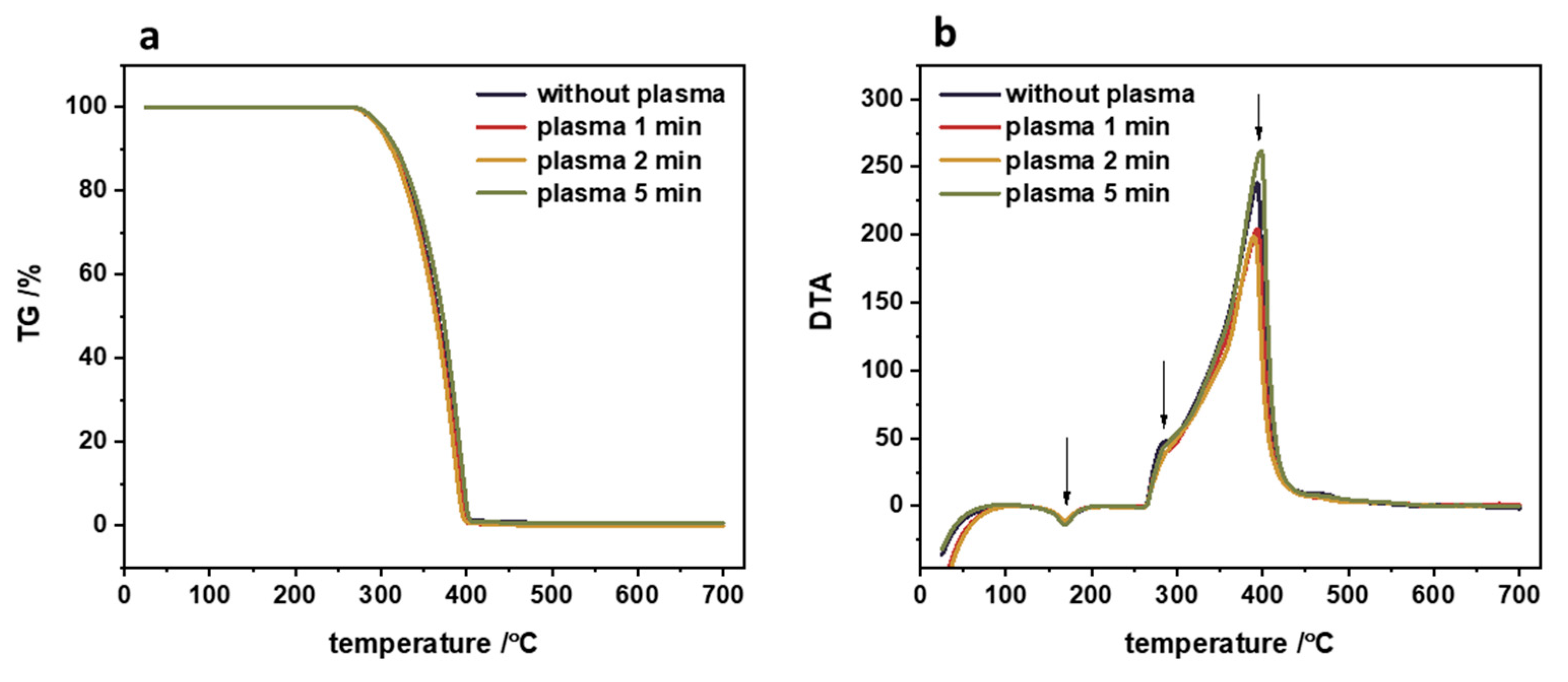
2.6. TG/DTA
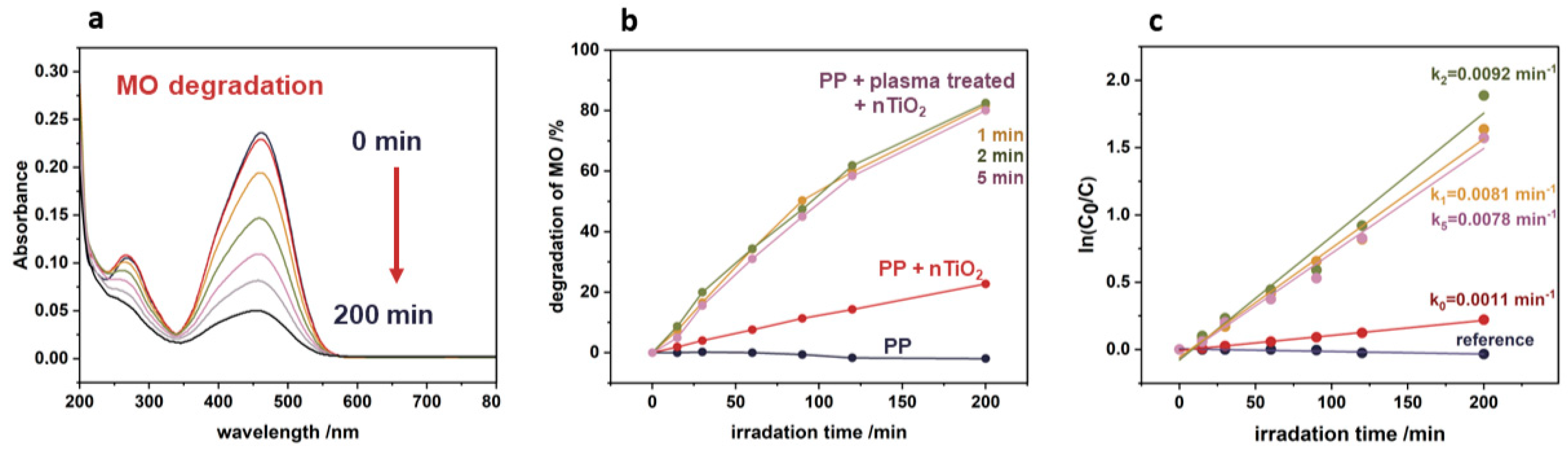
2.7. Photocatalytic Tests
2.8. Diffusion Reflectance Spectroscopy
2.9. XRD Measurements
3. Results
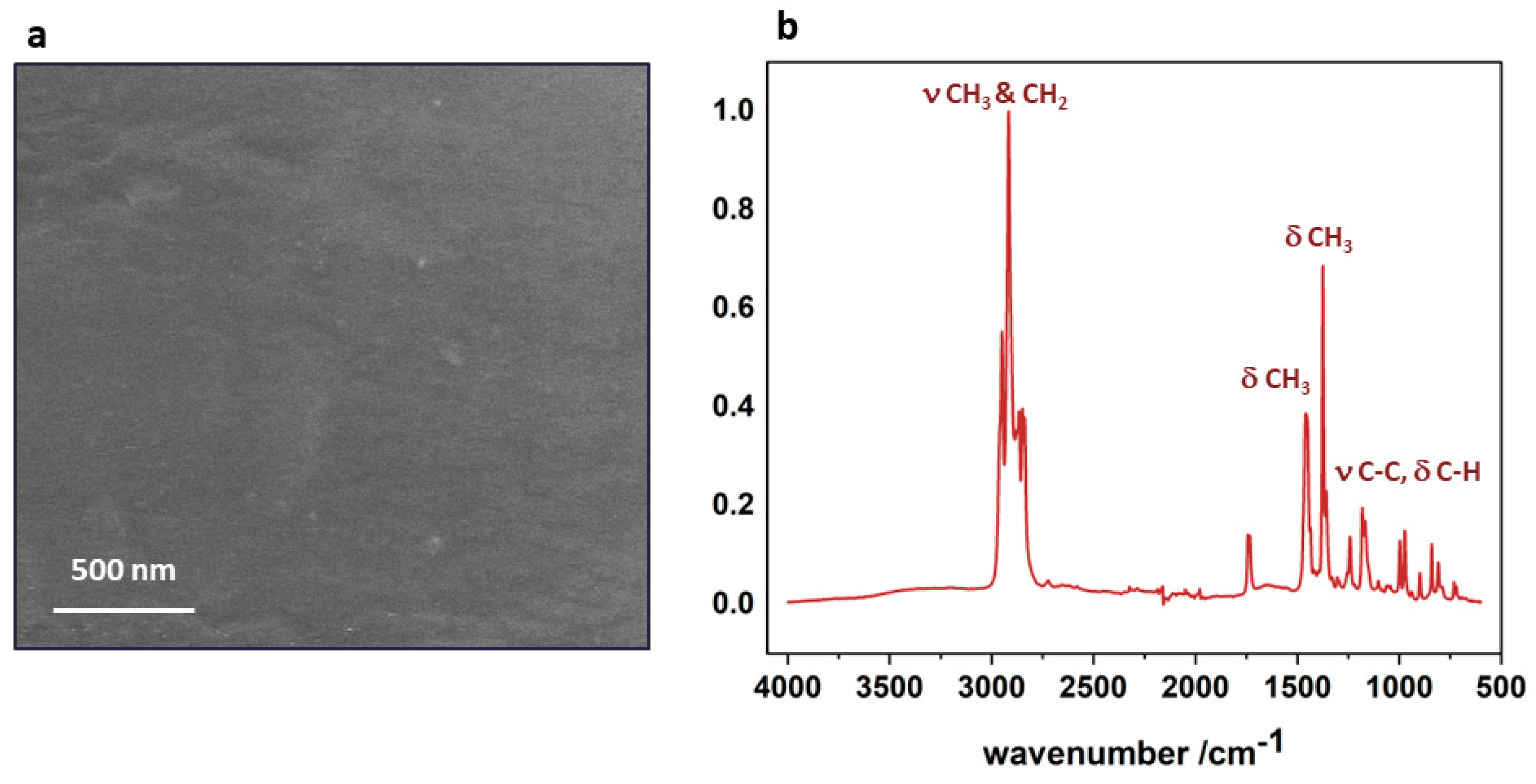
3.1. Polypropylene Substrate
3.2. Characteristics of Titanium Dioxide Nanoparticles
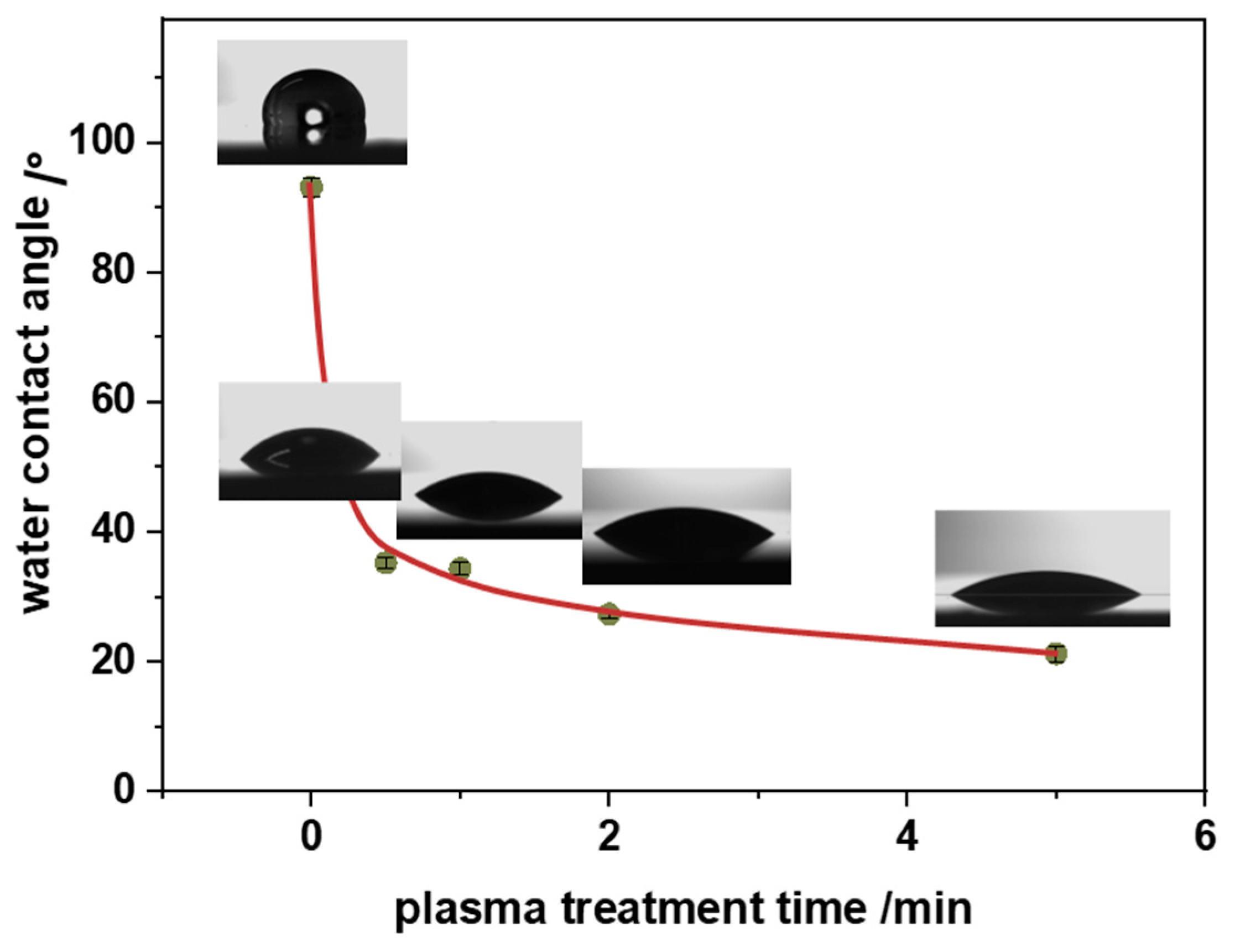
3.3. Surface Functional Groups
3.4. Nanocomposite
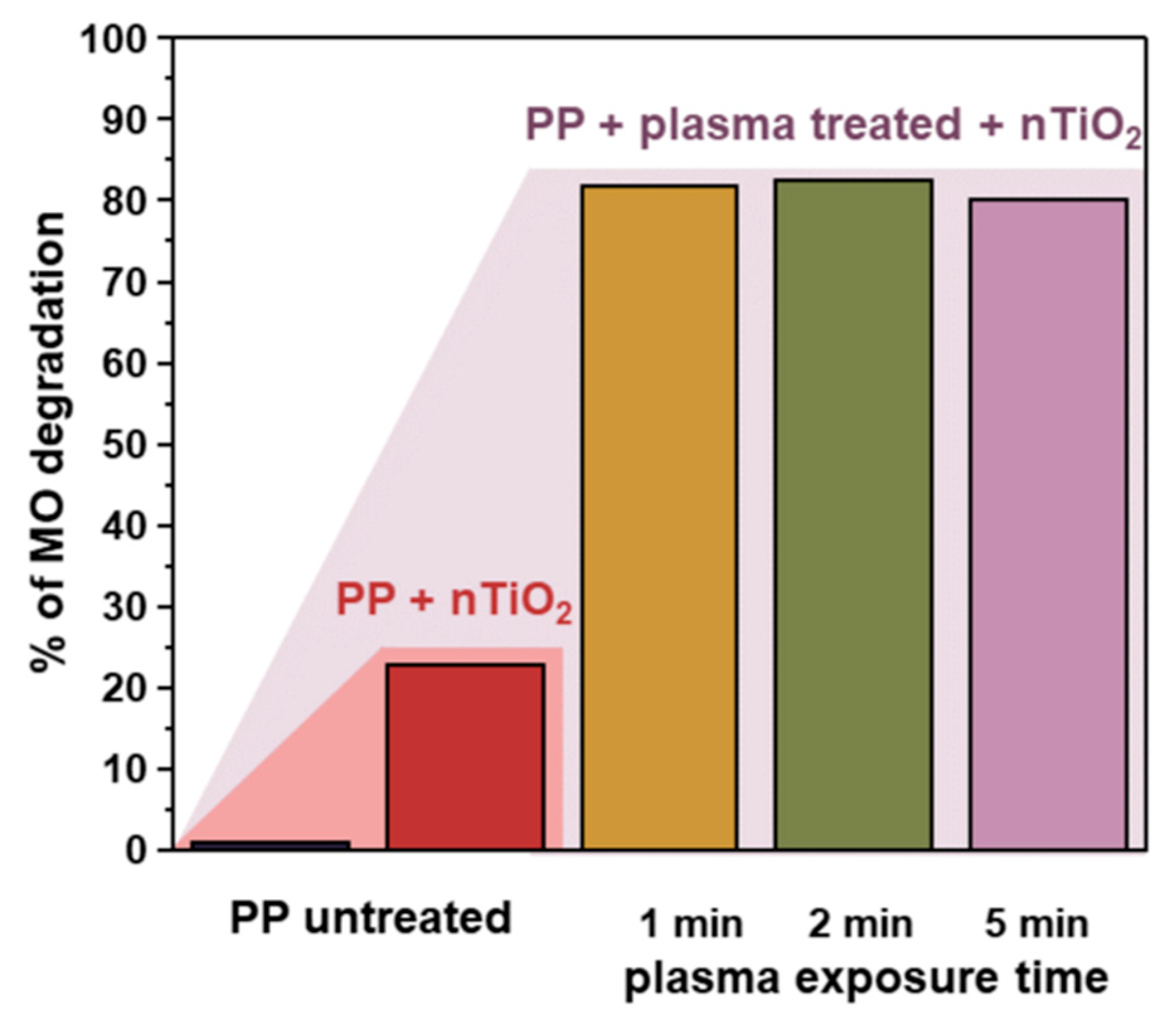
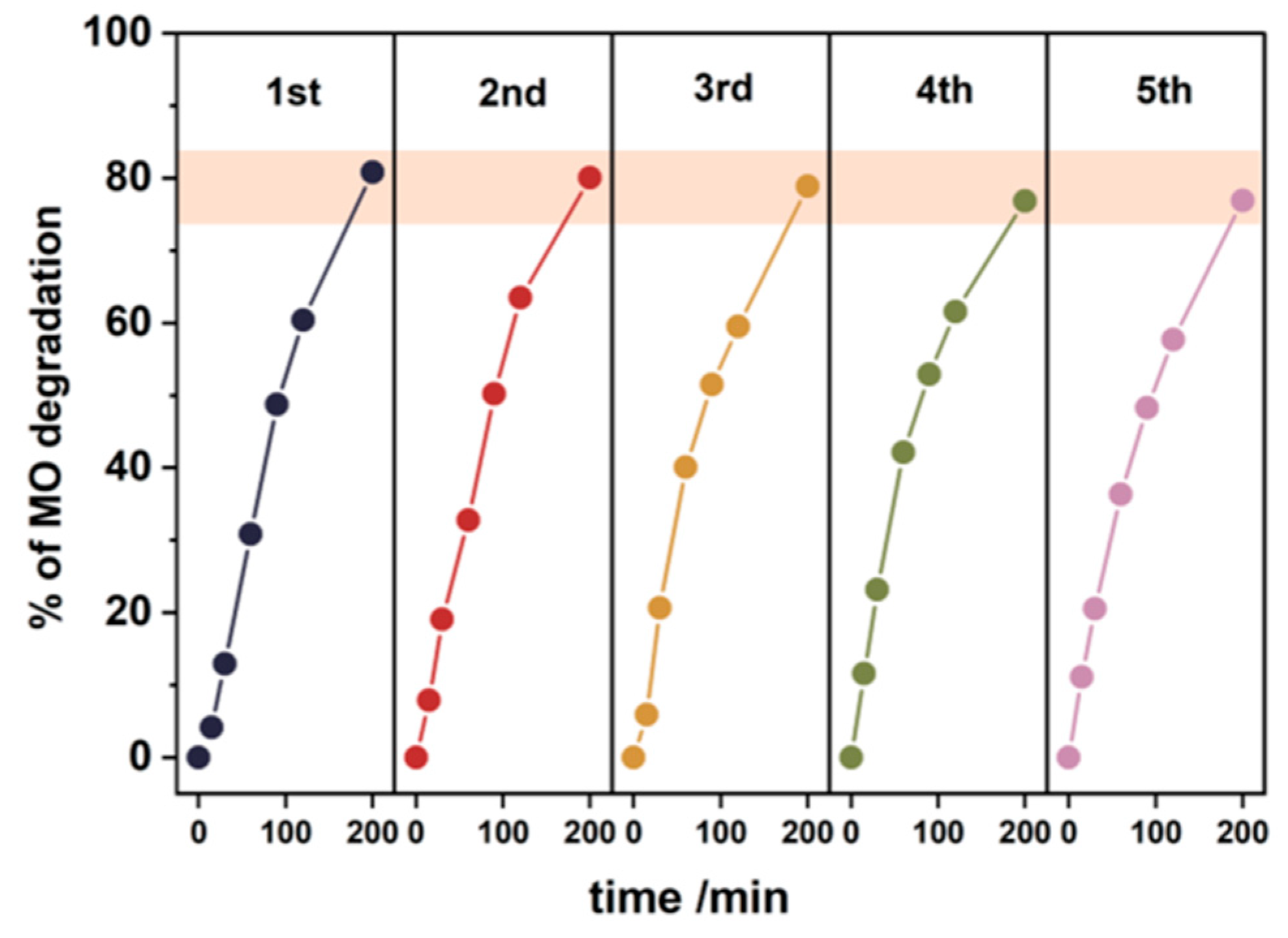
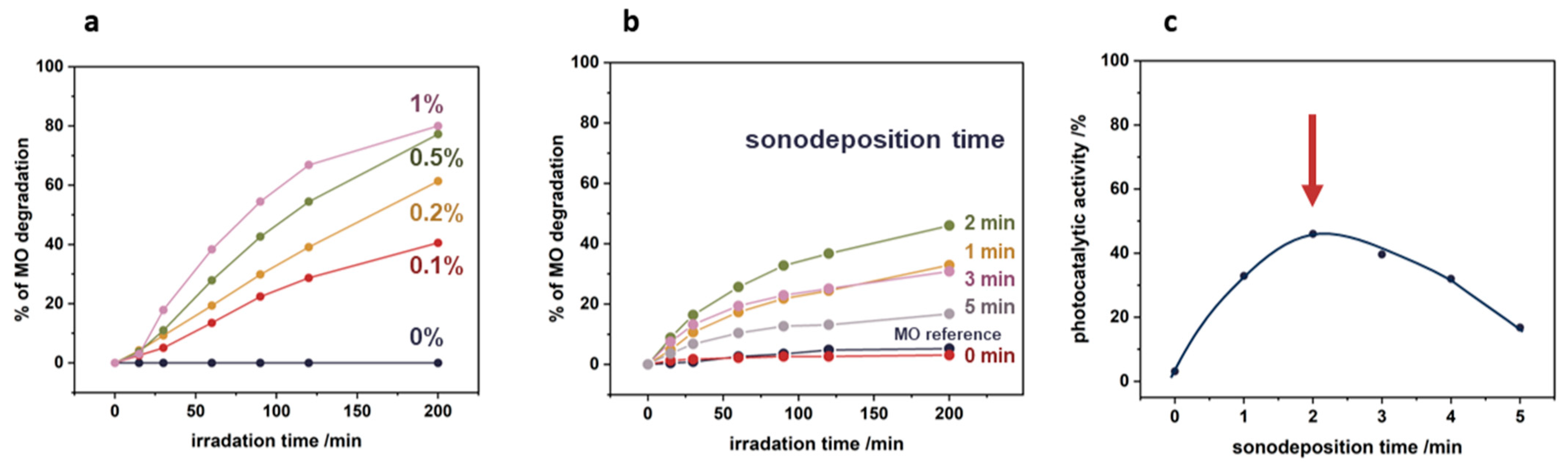
3.5. Photocatalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statista Research Department. Global Production of Plastics since 1950. 2024. Available online: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/ (accessed on 1 July 2024).
- Maddah, H.A. Polypropylene as a Promising Plastic: A Review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar]
- Hossain, M.T.; Shahid, M.A.; Mahmud, N.; Habib, A.; Rana, M.; Khan, S.A.; Hossain, D. Research and application of polypropylene: A review. Discov. Nano 2024, 19, 2. [Google Scholar] [CrossRef]
- Harish, V.; Ansari, M.M.; Tewari, D.; Gaur, M.; Yadav, A.B.; García-Betancourt, M.-L.; Abdel-Haleem, F.M.; Bechelany, M.; Barhoum, A. Nanoparticle and Nanostructure Synthesis and Controlled Growth Methods. Nanomaterials 2022, 12, 3226. [Google Scholar] [CrossRef] [PubMed]
- Ubaldi, F.; Valeriani, F.; Volpini, V.; Lofrano, G.; Romano Spica, V. Antimicrobial Activity of Photocatalytic Coatings on Surfaces: A Systematic Review and Meta Analysis. Coatings 2024, 14, 92. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, Q.; Meng, H.; Zhang, Y.; Cao, C. Recent advances in photocatalytic self-cleaning performances of TiO2-based building materials. RSC Adv. 2023, 13, 20584–20597. [Google Scholar] [PubMed]
- Howard, A. Foster Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. Recent Advances in TiO2 Films Prepared by Sol-Gel Methods for Photocatalytic Degradation of Organic Pollutants and Antibacterial Activities. Coatings 2019, 9, 613. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Iesalnieks, M.; Eglıtis, R.; Juhna, T.; Šmits, K.; Šutka, A. Photocatalytic Activity of TiO2 Coatings Obtained at Room Temperature on a Polymethyl Methacrylate Substrate. Int. J. Mol. Sci. 2022, 23, 12936. [Google Scholar] [CrossRef]
- Sun, L.; Guan, J.; Xu, Q.; Yang, X.; Wang, J.; Hu, X. Synthesis and Applications of Molecularly Imprinted Polymers Modified TiO2 Nanomaterials: A Review. Polymers 2018, 10, 1248. [Google Scholar] [CrossRef]
- Zhang, H.; Han, J.; Yang, B. Structural Fabrication and Functional Modulation of Nanoparticle-Polymer Composites. Adv. Funct. Mater. 2010, 20, 1533–1550. [Google Scholar] [CrossRef]
- Cazan, C.; Enesca, A.; Andronic, L. Synergic Effect of TiO2 Filler on the Mechanical Properties of Polymer Nanocomposites. Polymers 2021, 13, 2017. [Google Scholar] [CrossRef]
- Lai, J.; Sunderland, B.; Xue, J.; Yan, S.; Zhao, W.; Folkard, M.; Michael, B.D.; Wang, Y. Study on hydrophilicity of polymer surfaces improved by plasma treatment. Appl. Surf. Sci. 2006, 252, 3375–3379. [Google Scholar]
- Nemani, S.K.; Annavarapu, R.K.; Mohammadian, B.; Raiyan, A.; Heil, J.; Haque, M.A.; Sojoudi, H. Surface Modification of Polymers: Methods and Application. Adv. Mater. Interfaces 2018, 5, 1801247. [Google Scholar] [CrossRef]
- Sadowski, R.; Wach, A.; Buchalska, M.; Kuśtrowski, P.; Macyk, W. Photosensitized TiO2 films on polymers—Titania-polymer interactions and visible light induced photoactivity. Appl. Surf. Sci. 2019, 475, 710–719. [Google Scholar] [CrossRef]
- Chytrosz-Wróbel, P.; Golda-Cepa, M.; Stodolak-Zych, E.; Rysz, J.; Kotarba, A. Effect of oxygen plasma-treatment on surface functional groups, wettability, and nanotopography features of medically relevant polymers with various crystallinities. Appl. Surf. Sci. Adv. 2023, 18, 100497. [Google Scholar] [CrossRef]
- Ricard, A. The production of active plasma species for surface treatments. J. Phys. D Appl. Phys. 1997, 30, 2261. [Google Scholar] [CrossRef]
- Fleischer, M.; Kielar Tuceková, Z.; Galmiz, O.; Baková, E.; Plšek, T.; Kolárová, T.; Kovácik, D.; Kelar, J. Plasma Treatment of Large-Area Polymer Substrates for the Enhanced Adhesion of UV–Digital Printing. Nanomaterials 2024, 14, 426. [Google Scholar] [CrossRef] [PubMed]
- Gedanken, A. Using sonochemistry for the fabrication of nanomaterials. Ultrason. Sonochemistry 2004, 11, 47–55. [Google Scholar] [CrossRef]
- Ścibik, Ł.; Ochońska, D.; Gołda-Cępa, M.; Kwiecień, K.; Pamuła, E.; Kotarba, A.; Brzychczy-Włoch, M. Sonochemical Deposition of Gentamicin Nanoparticles at the PCV Tracheostomy Tube Surface Limiting Bacterial Biofilm Formation. Materials 2023, 16, 3765. [Google Scholar] [CrossRef]
- Tanaka, Y.; Okawa, H.; Kato, T.; Sugawara, K. Effects of ultrasound irradiation on Au nanoparticles deposition on carbon-coated LiNi0.5Mn1.5O4 and its performance as a cathode material for Li ion batteries. Ultrason. Sonochemistry 2022, 82, 105879. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Leys, C.; Gengembre, L.; Payen, E. Comparison between XPS- and FTIR-analysis of plasma-treated polypropylene film surfaces. Surf. Interface Anal. 2008, 40, 597–600. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zajac, K.; Macyk, J.; Szajna, K.; Krok, F.; Macyk, W.; Kotarba, A. Functionalization of Polypropylene by TiO2 Photocatalytic Nanoparticles: On the Importance of the Surface Oxygen Plasma Treatment. Nanomaterials 2024, 14, 1372. https://doi.org/10.3390/nano14161372
Zajac K, Macyk J, Szajna K, Krok F, Macyk W, Kotarba A. Functionalization of Polypropylene by TiO2 Photocatalytic Nanoparticles: On the Importance of the Surface Oxygen Plasma Treatment. Nanomaterials. 2024; 14(16):1372. https://doi.org/10.3390/nano14161372
Chicago/Turabian StyleZajac, Karolina, Joanna Macyk, Konrad Szajna, Franciszek Krok, Wojciech Macyk, and Andrzej Kotarba. 2024. "Functionalization of Polypropylene by TiO2 Photocatalytic Nanoparticles: On the Importance of the Surface Oxygen Plasma Treatment" Nanomaterials 14, no. 16: 1372. https://doi.org/10.3390/nano14161372






