Applicability of Quantum Dots in Breast Cancer Diagnostic and Therapeutic Modalities—A State-of-the-Art Review
Abstract
:1. Introduction
2. Breast Cancers: Molecular Features, Histopathological Characteristics, and Clinical Management
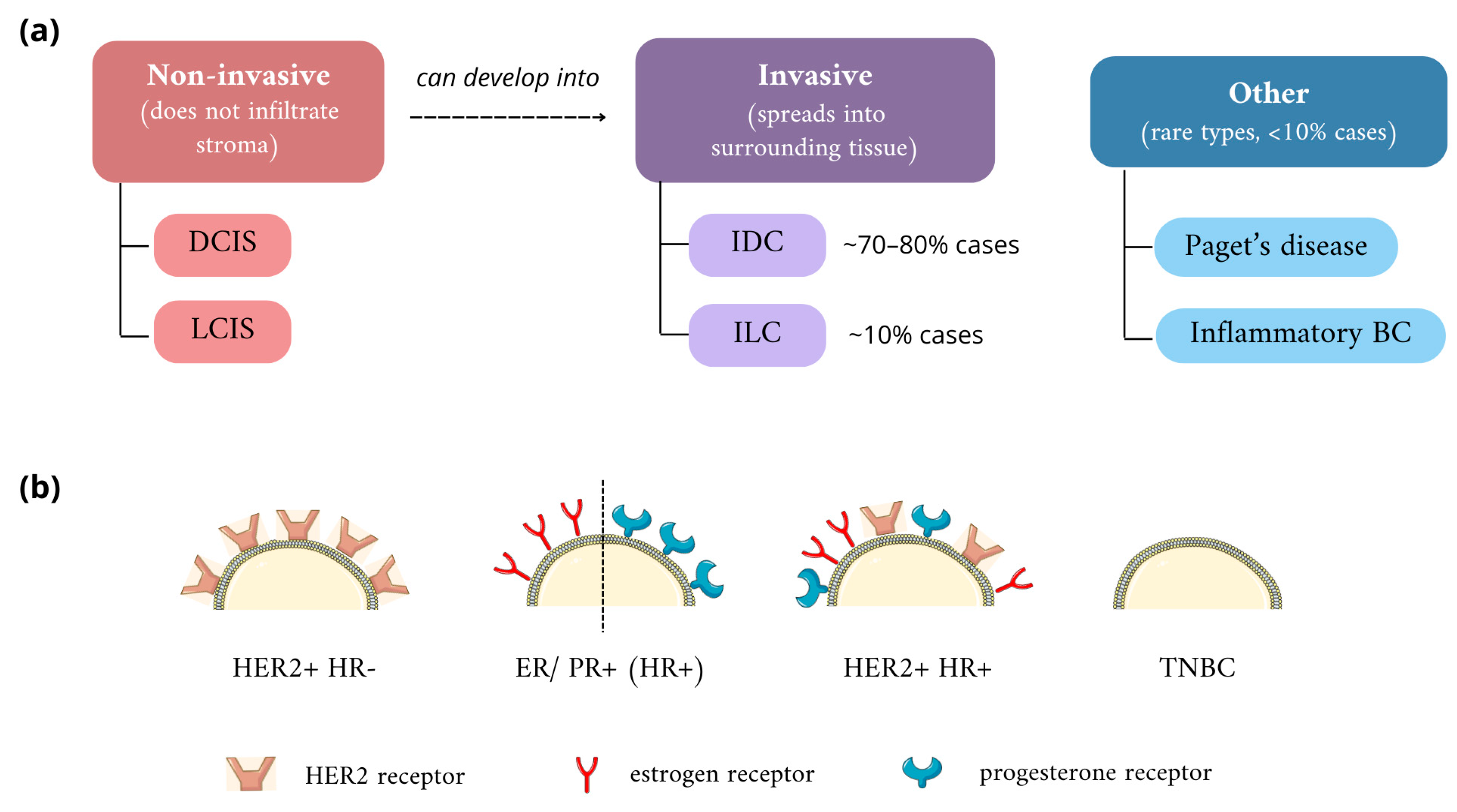
2.1. Classification of Breast Cancers
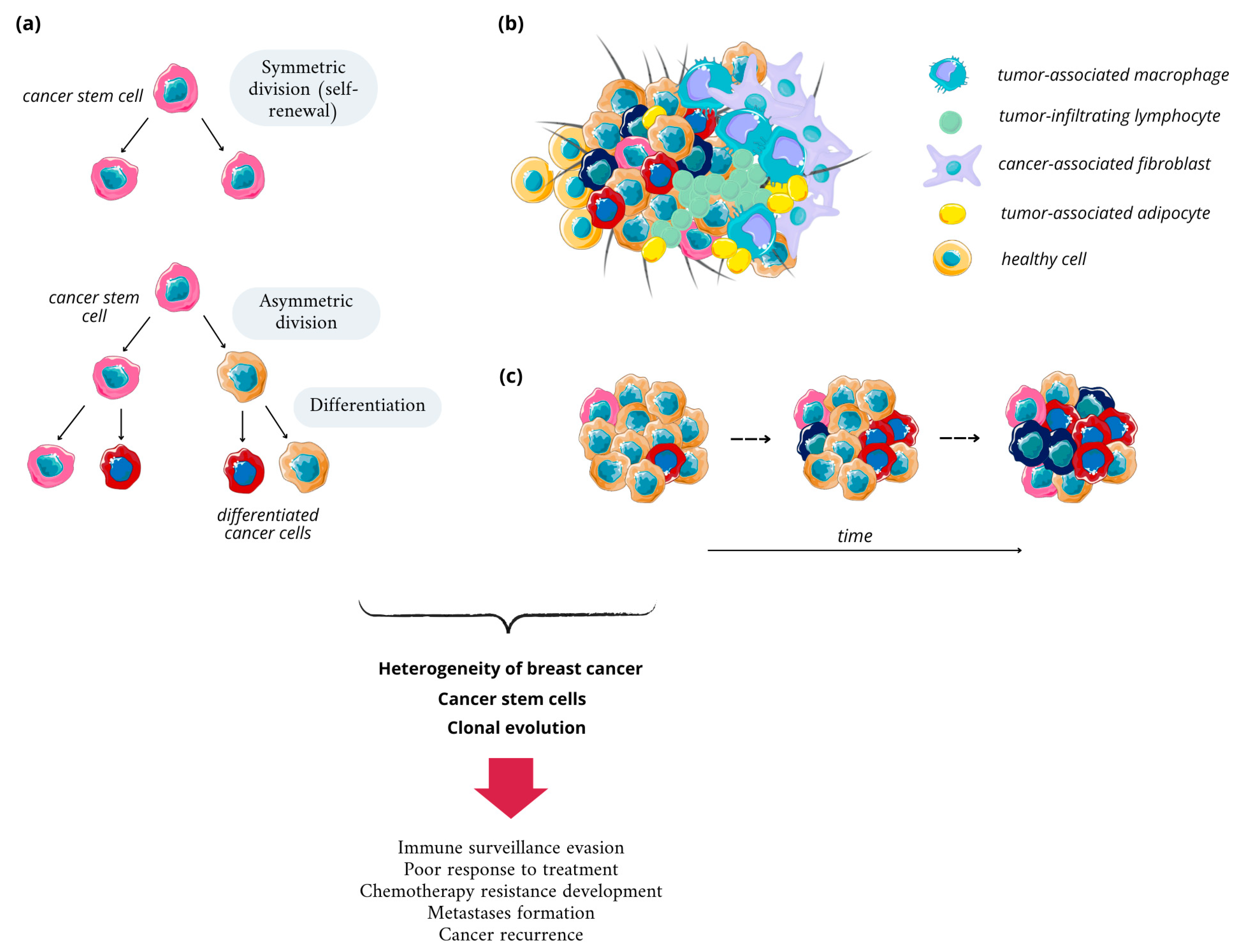
2.2. Breast Cancer Heterogeneity and Its Basis
2.3. Breast Cancer Stem Cells
2.4. Standard Diagnostic and Therapeutic Strategies
2.4.1. Breast Cancer Diagnostic Methods
2.4.2. Standard Therapies in Breast Cancer
3. Structure and Properties of Quantum Dots
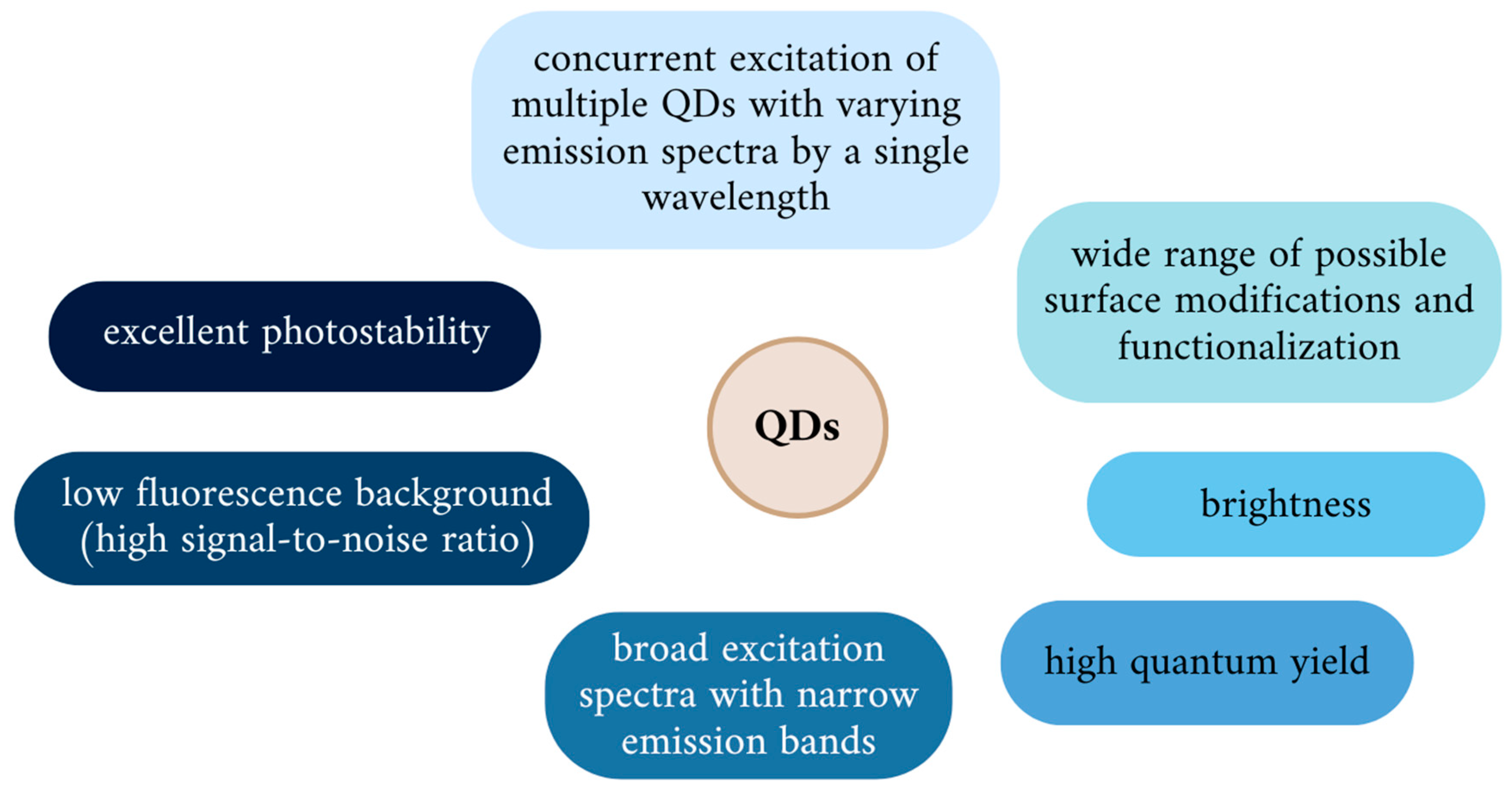
3.1. Physicochemical Properties of QDs
3.2. Types of QDs
3.3. In Vivo Issues: Uptake, Biodistribution, and Clearance of Quantum Dots
3.3.1. Cellular Uptake of Quantum Dots
3.3.2. Distribution of QDs
3.3.3. Clearance of QDs
3.4. QDs-Associated Toxicity
4. Quantum Dots in Breast Cancer Diagnostics
4.1. Antibody Conjugated QD Nanoprobes
4.1.1. HER2
4.1.2. CA 15-3
4.1.3. Ki67
4.2. QD–Aptamer Conjugates
4.3. Non-Coding RNA Detection
4.4. QD-Based Probes in TNBC Detection
4.5. QD-Based Multiplexed BC Biomarker Imaging
5. Advances in QD-Based In Vivo Imaging in Breast Cancer Studies
5.1. BC Xenografts Imaging
5.2. Mapping of Lymphatic Nodes
5.3. Detection of Metastases and Micrometastases
6. Potential of Quantum Dots as a Therapeutic Modality in Breast Cancer
6.1. Phototherapy
6.2. Targeted Drug Delivery by QDs
6.2.1. Targeting Cells of Destination
6.2.2. Targeted Release
6.2.3. QD-Based Targeted Drug Delivery in Breast Cancer
6.3. Non-Coding RNA Delivery
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Kong, D.; Liu, J.; Zhan, L.; Luo, L.; Zheng, W.; Zheng, Q.; Chen, C.; Sun, S. Breast Cancer Heterogeneity and Its Implication in Personalized Precision Therapy. Exp. Hematol. Oncol. 2023, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Salehiniya, H. Epidemiological Characteristics of and Risk Factors for Breast Cancer in the World. Breast Cancer Targets Ther. 2019, 11, 151–164. [Google Scholar] [CrossRef]
- Chan, D.S.M.; Abar, L.; Cariolou, M.; Nanu, N.; Greenwood, D.C.; Bandera, E.V.; McTiernan, A.; Norat, T. World Cancer Research Fund International: Continuous Update Project—Systematic Literature Review and Meta-Analysis of Observational Cohort Studies on Physical Activity, Sedentary Behavior, Adiposity, and Weight Change and Breast Cancer Risk. Cancer Causes Control 2019, 30, 1183–1200. [Google Scholar] [CrossRef]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and Future Burden of Breast Cancer: Global Statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Alipour, B.; Mortezazadeh, T.; Abdulsahib, W.K.; Arzhang, A.; Malekzadeh, R.; Farhood, B. A Systematic Review of Multimodal Application of Quantum Dots in Breast Cancer Diagnosis: Effective Parameters, Status and Future Perspectives. J. Drug Deliv. Sci. Technol. 2023, 86, 104682. [Google Scholar] [CrossRef]
- Oeffinger, K.C.; Fontham, E.T.H.; Etzioni, R.; Herzig, A.; Michaelson, J.S.; Shih, Y.-C.T.; Walter, L.C.; Church, T.R.; Flowers, C.R.; LaMonte, S.J.; et al. Breast Cancer Screening for Women at Average Risk. JAMA 2015, 314, 1599. [Google Scholar] [CrossRef]
- Abdelrahman, L.; Al Ghamdi, M.; Collado-Mesa, F.; Abdel-Mottaleb, M. Convolutional Neural Networks for Breast Cancer Detection in Mammography: A Survey. Comput. Biol. Med. 2021, 131, 104248. [Google Scholar] [CrossRef]
- Prieto-Callejero, B.; Rivera, F.; Fagundo-Rivera, J.; Romero, A.; Romero-Martín, M.; Gómez-Salgado, J.; Ruiz-Frutos, C. Relationship between Chemotherapy-Induced Adverse Reactions and Health-Related Quality of Life in Patients with Breast Cancer. Medicine 2020, 99, e21695. [Google Scholar] [CrossRef]
- Ramos, A.P.; Cruz, M.A.E.; Tovani, C.B.; Ciancaglini, P. Biomedical Applications of Nanotechnology. Biophys. Rev. 2017, 9, 79–89. [Google Scholar] [CrossRef]
- Zottel, A.; Videtič Paska, A.; Jovčevska, I. Nanotechnology Meets Oncology: Nanomaterials in Brain Cancer Research, Diagnosis and Therapy. Materials 2019, 12, 1588. [Google Scholar] [CrossRef]
- Gharpure, K.M.; Wu, S.Y.; Li, C.; Lopez-Berestein, G.; Sood, A.K. Nanotechnology: Future of Oncotherapy. Clin. Cancer Res. 2015, 21, 3121–3130. [Google Scholar] [CrossRef] [PubMed]
- Gidwani, B.; Sahu, V.; Shukla, S.S.; Pandey, R.; Joshi, V.; Jain, V.K.; Vyas, A. Quantum Dots: Prospectives, Toxicity, Advances and Applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102308. [Google Scholar] [CrossRef]
- Bera, D.; Qian, L.; Tseng, T.-K.; Holloway, P.H. Quantum Dots and Their Multimodal Applications: A Review. Materials 2010, 3, 2260–2345. [Google Scholar] [CrossRef]
- Tandale, P.; Choudhary, N.; Singh, J.; Sharma, A.; Shukla, A.; Sriram, P.; Soni, U.; Singla, N.; Barnwal, R.P.; Singh, G.; et al. Fluorescent Quantum Dots: An Insight on Synthesis and Potential Biological Application as Drug Carrier in Cancer. Biochem. Biophys. Rep. 2021, 26, 100962. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, R.; Naderi-Manesh, H.; Farzin, L.; Vaezi, Z.; Ayarri, N.; Samandari, L.; Shamsipur, M. Fluorescence Sensing and Imaging with Carbon-Based Quantum Dots for Early Diagnosis of Cancer: A Review. J. Pharm. Biomed. Anal. 2022, 212, 114628. [Google Scholar] [CrossRef]
- Cowell, C.F.; Weigelt, B.; Sakr, R.A.; Ng, C.K.Y.; Hicks, J.; King, T.A.; Reis-Filho, J.S. Progression from Ductal Carcinoma in Situ to Invasive Breast Cancer: Revisited. Mol. Oncol. 2013, 7, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Solin, L.J. Management of Ductal Carcinoma In Situ (DCIS) of the Breast: Present Approaches and Future Directions. Curr. Oncol. Rep. 2019, 21, 33. [Google Scholar] [CrossRef] [PubMed]
- Groen, E.J.; Elshof, L.E.; Visser, L.L.; Rutgers, E.J.T.; Winter-Warnars, H.A.O.; Lips, E.H.; Wesseling, J. Finding the Balance between Over- and under-Treatment of Ductal Carcinoma in Situ (DCIS). Breast 2017, 31, 274–283. [Google Scholar] [CrossRef]
- Makki, J. Diversity of Breast Carcinoma: Histological Subtypes and Clinical Relevance. Clin. Med. Insights Pathol. 2015, 8, 23–31. [Google Scholar] [CrossRef]
- Cutuli, B.; De Lafontan, B.; Kirova, Y.; Auvray, H.; Tallet, A.; Avigdor, S.; Brunaud, C.; Delva, C. Lobular Carcinoma in Situ (LCIS) of the Breast: Is Long-Term Outcome Similar to Ductal Carcinoma in Situ (DCIS)? Analysis of 200 Cases. Radiat. Oncol. 2015, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.Y.; Brogi, E. Lobular Carcinoma In Situ. Surg. Pathol. Clin. 2018, 11, 123–145. [Google Scholar] [CrossRef]
- Joglekar-Javadekar, M.; Van Laere, S.; Bourne, M.; Moalwi, M.; Finetti, P.; Vermeulen, P.B.; Birnbaum, D.; Dirix, L.Y.; Ueno, N.; Carter, M.; et al. Characterization and Targeting of Platelet-Derived Growth Factor Receptor Alpha (PDGFRA) in Inflammatory Breast Cancer (IBC). Neoplasia 2017, 19, 564–573. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Metzger-Filho, O. Differences between Invasive Lobular and Invasive Ductal Carcinoma of the Breast: Results and Therapeutic Implications. Ther. Adv. Med. Oncol. 2016, 8, 261–266. [Google Scholar] [CrossRef]
- McCart Reed, A.E.; Kalinowski, L.; Simpson, P.T.; Lakhani, S.R. Invasive Lobular Carcinoma of the Breast: The Increasing Importance of This Special Subtype. Breast Cancer Res. 2021, 23, 6. [Google Scholar] [CrossRef] [PubMed]
- Cserni, G. Histological Type and Typing of Breast Carcinomas and the WHO Classification Changes over Time. Pathologica 2020, 112, 25–41. [Google Scholar] [CrossRef]
- Shea, E.K.H.; Koh, V.C.Y.; Tan, P.H. Invasive Breast Cancer: Current Perspectives and Emerging Views. Pathol. Int. 2020, 70, 242–252. [Google Scholar] [CrossRef]
- Matsumoto, A.; Jinno, H.; Ando, T.; Fujii, T.; Nakamura, T.; Saito, J.; Takahashi, M.; Hayashida, T.; Kitagawa, Y. Biological Markers of Invasive Breast Cancer. Jpn. J. Clin. Oncol. 2015, 46, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Kim, H.J.; Kim, M.; Chung, Y.R.; Kang, E.; Kim, E.-K.; Kim, S.H.; Kim, Y.J.; Kim, J.H.; Kim, I.A.; et al. Negative Conversion of Progesterone Receptor Status after Primary Systemic Therapy Is Associated with Poor Clinical Outcome in Patients with Breast Cancer. Cancer Res. Treat. 2018, 50, 1418–1432. [Google Scholar] [CrossRef]
- Schultz, N. Comprehensive Molecular Portraits of Human Breast Tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Polyak, K. Breast Cancer: Origins and Evolution. J. Clin. Investig. 2007, 117, 3155–3163. [Google Scholar] [CrossRef]
- Rivenbark, A.G.; O’Connor, S.M.; Coleman, W.B. Molecular and Cellular Heterogeneity in Breast Cancer. Am. J. Pathol. 2013, 183, 1113–1124. [Google Scholar] [CrossRef]
- Mierke, C.T. Phenotypic Heterogeneity, Bidirectionality, Universal Cues, Plasticity, Mechanics, and the Tumor Microenvironment Drive Cancer Metastasis. Biomolecules 2024, 14, 184. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Jiang, L.; Ding, X. Advancing Breast Cancer Heterogeneity Analysis: Insights from Genomics, Transcriptomics and Proteomics at Bulk and Single-Cell Levels. Cancers 2023, 15, 4164. [Google Scholar] [CrossRef] [PubMed]
- Januškevičienė, I.; Petrikaitė, V. Heterogeneity of Breast Cancer: The Importance of Interaction between Different Tumor Cell Populations. Life Sci. 2019, 239, 117009. [Google Scholar] [CrossRef] [PubMed]
- Turashvili, G.; Brogi, E. Tumor Heterogeneity in Breast Cancer. Front. Med. 2017, 4, 227. [Google Scholar] [CrossRef]
- Andrade de Oliveira, K.; Sengupta, S.; Yadav, A.K.; Clarke, R. The Complex Nature of Heterogeneity and Its Roles in Breast Cancer Biology and Therapeutic Responsiveness. Front. Endocrinol. 2023, 14, 1083048. [Google Scholar] [CrossRef]
- Razi, S.; Haghparast, A.; Chodari Khameneh, S.; Ebrahimi Sadrabadi, A.; Aziziyan, F.; Bakhtiyari, M.; Nabi-Afjadi, M.; Tarhriz, V.; Jalili, A.; Zalpoor, H. The Role of Tumor Microenvironment on Cancer Stem Cell Fate in Solid Tumors. Cell Commun. Signal. 2023, 21, 143. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, C.; Yao, J.; Wan, H.; Wan, G.; Li, Y.; Chen, N. Breast Cancer Stem Cells, Heterogeneity, Targeting Therapies and Therapeutic Implications. Pharmacol. Res. 2021, 163, 105320. [Google Scholar] [CrossRef]
- Nimmakayala, R.K.; Batra, S.K.; Ponnusamy, M.P. Unraveling the Journey of Cancer Stem Cells from Origin to Metastasis. Biochim. Biophys. Acta-Rev. Cancer 2019, 1871, 50–63. [Google Scholar] [CrossRef]
- Hong, D.; Fritz, A.J.; Zaidi, S.K.; van Wijnen, A.J.; Nickerson, J.A.; Imbalzano, A.N.; Lian, J.B.; Stein, J.L.; Stein, G.S. Epithelial-to-mesenchymal Transition and Cancer Stem Cells Contribute to Breast Cancer Heterogeneity. J. Cell. Physiol. 2018, 233, 9136–9144. [Google Scholar] [CrossRef]
- Scioli, M.G.; Storti, G.; D’Amico, F.; Gentile, P.; Fabbri, G.; Cervelli, V.; Orlandi, A. The Role of Breast Cancer Stem Cells as a Prognostic Marker and a Target to Improve the Efficacy of Breast Cancer Therapy. Cancers 2019, 11, 1021. [Google Scholar] [CrossRef] [PubMed]
- Kerneur, C.; Cano, C.E.; Olive, D. Major Pathways Involved in Macrophage Polarization in Cancer. Front. Immunol. 2022, 13, 1026954. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, H.; Wang, K.; Wang, X.; Wang, M.; Zhao, W.; Xi, X.; Li, Y.; Cai, M.; Zhao, W.; et al. Role, Molecular Mechanism and the Potential Target of Breast Cancer Stem Cells in Breast Cancer Development. Biomed. Pharmacother. 2022, 147, 112616. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, A.B. Redefining the Sensitivity of Screening Mammography: A Review. Am. J. Surg. 2019, 218, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Houssami, N. Digital Breast Tomosynthesis (3D-Mammography) Screening: Data and Implications for Population Screening. Expert Rev. Med. Devices 2015, 12, 377–379. [Google Scholar] [CrossRef]
- Guo, R.; Lu, G.; Qin, B.; Fei, B. Ultrasound Imaging Technologies for Breast Cancer Detection and Management: A Review. Ultrasound Med. Biol. 2018, 44, 37–70. [Google Scholar] [CrossRef]
- Wang, L. Early Diagnosis of Breast Cancer. Sensors 2017, 17, 1572. [Google Scholar] [CrossRef]
- Mann, R.M.; Cho, N.; Moy, L. Breast MRI: State of the Art. Radiology 2019, 292, 520–536. [Google Scholar] [CrossRef]
- Zubair, M.; Wang, S.; Ali, N. Advanced Approaches to Breast Cancer Classification and Diagnosis. Front. Pharmacol. 2021, 11, 632079. [Google Scholar] [CrossRef]
- Holmes, D.R. Reducing the Risk of Needle Tract Seeding or Tumor Cell Dissemination during Needle Biopsy Procedures. Cancers 2024, 16, 317. [Google Scholar] [CrossRef]
- Triantafillidou, E.S. Enhancing the Critical Role of Core Needle Biopsy in Breast Cancer. Hell. J. Surg. 2020, 92, 76–84. [Google Scholar] [CrossRef]
- Cho, N. Molecular Subtypes and Imaging Phenotypes of Breast Cancer. Ultrasonography 2016, 35, 281–288. [Google Scholar] [CrossRef]
- Bhatt, A.A.; Whaley, D.H.; Lee, C.U. Ultrasound-Guided Breast Biopsies. J. Ultrasound Med. 2021, 40, 1427–1443. [Google Scholar] [CrossRef]
- Quintana, L.M.; Collins, L.C. Diagnostic Pitfalls in Breast Cancer Pathology With an Emphasis on Core Needle Biopsy Specimens. Arch. Pathol. Lab. Med. 2023, 147, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Quinn, C.; Maguire, A.; Rakha, E. Pitfalls in Breast Pathology. Histopathology 2023, 82, 140–161. [Google Scholar] [CrossRef]
- Chevrier, M.-C.; David, J.; El Khoury, M.; Lalonde, L.; Labelle, M.; Trop, I. Breast Biopsies Under Magnetic Resonance Imaging Guidance: Challenges of an Essential but Imperfect Technique. Curr. Probl. Diagn. Radiol. 2016, 45, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Pulumati, A.; Pulumati, A.; Dwarakanath, B.S.; Verma, A.; Papineni, R.V.L. Technological Advancements in Cancer Diagnostics: Improvements and Limitations. Cancer Rep. 2023, 6, e1764. [Google Scholar] [CrossRef] [PubMed]
- Smith, I. Goals of Treatment for Patients With Metastatic Breast Cancer. Semin. Oncol. 2006, 33, 2–5. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment. JAMA 2019, 321, 288. [Google Scholar] [CrossRef]
- Shah, R. Pathogenesis, Prevention, Diagnosis and Treatment of Breast Cancer. World J. Clin. Oncol. 2014, 5, 283. [Google Scholar] [CrossRef] [PubMed]
- Burguin, A.; Diorio, C.; Durocher, F. Breast Cancer Treatments: Updates and New Challenges. J. Pers. Med. 2021, 11, 808. [Google Scholar] [CrossRef] [PubMed]
- Haussmann, J.; Corradini, S.; Nestle-Kraemling, C.; Bölke, E.; Njanang, F.J.D.; Tamaskovics, B.; Orth, K.; Ruckhaeberle, E.; Fehm, T.; Mohrmann, S.; et al. Recent Advances in Radiotherapy of Breast Cancer. Radiat. Oncol. 2020, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Yang, X.; Tai, H.; Zhong, X.; Luo, T.; Zheng, H. HER2-Targeted Therapies in Cancer: A Systematic Review. Biomark. Res. 2024, 12, 16. [Google Scholar] [CrossRef]
- Shah, A.N.; Gradishar, W.J. Adjuvant Anthracyclines in Breast Cancer: What Is Their Role? Oncologist 2018, 23, 1153–1161. [Google Scholar] [CrossRef]
- Hassan, M.S.U. Hassan Chemotherapy for Breast Cancer (Review). Oncol. Rep. 2010, 24, 1121–1131. [Google Scholar] [CrossRef]
- Semiglazov, V.; Tseluiko, A.; Kudaybergenova, A.; Artemyeva, A.; Krivorotko, P.; Donskih, R. Immunology and Immunotherapy in Breast Cancer. Cancer Biol. Med. 2022, 19, 609–618. [Google Scholar] [CrossRef]
- Tokumaru, Y.; Joyce, D.; Takabe, K. Current Status and Limitations of Immunotherapy for Breast Cancer. Surgery 2020, 167, 628–630. [Google Scholar] [CrossRef]
- Khan, S.U.; Fatima, K.; Aisha, S.; Malik, F. Unveiling the Mechanisms and Challenges of Cancer Drug Resistance. Cell Commun. Signal. 2024, 22, 109. [Google Scholar] [CrossRef]
- An, J.; Peng, C.; Tang, H.; Liu, X.; Peng, F. New Advances in the Research of Resistance to Neoadjuvant Chemotherapy in Breast Cancer. Int. J. Mol. Sci. 2021, 22, 9644. [Google Scholar] [CrossRef]
- Khan, M.M.; Yalamarty, S.S.K.; Rajmalani, B.A.; Filipczak, N.; Torchilin, V.P. Recent Strategies to Overcome Breast Cancer Resistance. Crit. Rev. Oncol. Hematol. 2024, 197, 104351. [Google Scholar] [CrossRef] [PubMed]
- Plaks, V.; Kong, N.; Werb, Z. The Cancer Stem Cell Niche: How Essential Is the Niche in Regulating Stemness of Tumor Cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.-D.; Wei, X.; Xu, T.; Li, T.-P.; Liu, K.-S. Research Progress in Breast Cancer Stem Cells: Characterization and Future Perspectives. Am. J. Cancer Res. 2022, 12, 3208–3222. [Google Scholar] [PubMed]
- Chen, J.; Liu, S.; Su, Y.; Zhang, X. ALDH1+ Stem Cells Demonstrate More Stem Cell-like Characteristics than CD44+/CD24–/Low Stem Cells in Different Molecular Subtypes of Breast Cancer. Transl. Cancer Res. 2020, 9, 1652–1659. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Z.; Yang, Y.; Teng, Q.; Li, Y.; Lei, Z.; Jani, K.A.; Kaushal, N.; Chen, Z. ATP-binding Cassette (ABC) Transporters in Cancer: A Review of Recent Updates. J. Evid. Based. Med. 2021, 14, 232–256. [Google Scholar] [CrossRef]
- Ordaz-Ramos, A.; Tellez-Jimenez, O.; Vazquez-Santillan, K. Signaling Pathways Governing the Maintenance of Breast Cancer Stem Cells and Their Therapeutic Implications. Front. Cell Dev. Biol. 2023, 11, 1221175. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, M.; Zhou, F.; Zhang, L.; Meng, X. The Breast Cancer Stem Cells Traits and Drug Resistance. Front. Pharmacol. 2021, 11, 599965. [Google Scholar] [CrossRef]
- Brus, L.E. A Simple Model for the Ionization Potential, Electron Affinity, and Aqueous Redox Potentials of Small Semiconductor Crystallites. J. Chem. Phys. 1983, 79, 5566–5571. [Google Scholar] [CrossRef]
- Zaini, M.S.; Liew, J.Y.C.; Ahmad, S.A.A.; Mohmad, A.R.; Kamarudin, M.A. Quantum Confinement Effect and Photoenhancement of Photoluminescence of PbS and PbS/MnS Quantum Dots. Appl. Sci. 2020, 10, 6282. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, Z.; Gao, S.; Goh, B.L.; Bin Samsudin, I.; Lwe, K.W.; Wu, Y.; Wu, C.; Su, X. Recent Advances in Non-Toxic Quantum Dots and Their Biomedical Applications. Prog. Nat. Sci. Mater. Int. 2019, 29, 628–640. [Google Scholar] [CrossRef]
- Nikazar, S.; Sivasankarapillai, V.S.; Rahdar, A.; Gasmi, S.; Anumol, P.S.; Shanavas, M.S. Revisiting the Cytotoxicity of Quantum Dots: An in-Depth Overview. Biophys. Rev. 2020, 12, 703–718. [Google Scholar] [CrossRef]
- Zhang, L.J.; Xia, L.; Xie, H.Y.; Zhang, Z.L.; Pang, D.W. Quantum Dot Based Biotracking and Biodetection. Anal. Chem. 2019, 91, 532–547. [Google Scholar] [CrossRef] [PubMed]
- Chinnathambi, S.; Chen, S.; Ganesan, S.; Hanagata, N. Silicon Quantum Dots for Biological Applications. Adv. Healthc. Mater. 2014, 3, 10–29. [Google Scholar] [CrossRef]
- Biju, V.; Itoh, T.; Ishikawa, M. Delivering quantum dots to cells: Bioconjugated quantum dots for targeted and nonspecific extracellular and intracellular imaging. Chem. Soc. Rev. 2010, 39, 3031–3056. [Google Scholar] [CrossRef] [PubMed]
- Farzin, M.A.; Abdoos, H. A Critical Review on Quantum Dots: From Synthesis toward Applications in Electrochemical Biosensors for Determination of Disease-Related Biomolecules. Talanta 2021, 224, 121828. [Google Scholar] [CrossRef]
- Wang, L.; Xu, D.; Gao, J.; Chen, X.; Duo, Y.; Zhang, H. Semiconducting Quantum Dots: Modification and Applications in Biomedical Science. Sci. China Mater. 2020, 63, 1631–1650. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, C.; Zeng, G.; Chen, G.; Wan, J.; Guo, Z.; Wu, H.; Yu, Z.; Zhou, Y.; Liu, J. Metal-Based Quantum Dots: Synthesis, Surface Modification, Transport and Fate in Aquatic Environments and Toxicity to Microorganisms. RSC Adv. 2016, 6, 78595–78610. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Meng, H.; Wang, X.; Lin, S.; Ji, Z.; Zhang, H. Nanomaterial Toxicity Testing in the 21st Century: Use of a Predictive Toxicological Approach and High-Throughput Screening. Acc. Chem. Res. 2013, 46, 607–621. [Google Scholar] [CrossRef]
- Soenen, S.J.; Parak, W.J.; Rejman, J.; Manshian, B. (Intra)Cellular Stability of Inorganic Nanoparticles: Effects on Cytotoxicity, Particle Functionality, and Biomedical Applications. Chem. Rev. 2015, 115, 2109–2135. [Google Scholar] [CrossRef]
- Cao, X.; Ding, C.; Zhang, C.; Gu, W.; Yan, Y.; Shi, X.; Xian, Y. Transition Metal Dichalcogenide Quantum Dots: Synthesis, Photoluminescence and Biological Applications. J. Mater. Chem. B 2018, 6, 8011–8036. [Google Scholar] [CrossRef]
- Girma, W.M.; Fahmi, M.Z.; Permadi, A.; Abate, M.A.; Chang, J.Y. Synthetic Strategies and Biomedical Applications of I-III-VI Ternary Quantum Dots. J. Mater. Chem. B 2017, 5, 6193–6216. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Sedighi, O.; Tabatabaei Rezaei, N.; Abedini, A.A.; Malek Khachatourian, A.; Toprak, M.S.; Seifalian, A. Recent Advances in the Modification of Carbon-Based Quantum Dots for Biomedical Applications. Mater. Sci. Eng. C 2021, 120, 111756. [Google Scholar] [CrossRef]
- Landry, M.L.; Morrell, T.E.; Karagounis, T.K.; Hsia, C.-H.; Wang, C.-Y. Simple Syntheses of CdSe Quantum Dots. J. Chem. Educ. 2014, 91, 274–279. [Google Scholar] [CrossRef]
- Zhou, D.; Lin, M.; Chen, Z.; Sun, H.; Zhang, H.; Sun, H.; Yang, B. Simple Synthesis of Highly Luminescent Water-Soluble CdTe Quantum Dots with Controllable Surface Functionality. Chem. Mater. 2011, 23, 4857–4862. [Google Scholar] [CrossRef]
- Shen, G.; Guyot-Sionnest, P. HgS and HgS/CdS Colloidal Quantum Dots with Infrared Intraband Transitions and Emergence of a Surface Plasmon. J. Phys. Chem. C 2016, 120, 11744–11753. [Google Scholar] [CrossRef]
- Dager, A.; Uchida, T.; Maekawa, T.; Tachibana, M. Synthesis and Characterization of Mono-Disperse Carbon Quantum Dots from Fennel Seeds: Photoluminescence Analysis Using Machine Learning. Sci. Rep. 2019, 9, 14004. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.K.; Javaid, S.; Afzal, H.; Zafar, I.; Fayyaz, K.; Ain, Q.; Rather, M.A.; Hossain, M.J.; Rashid, S.; Khan, K.A.; et al. Exploring the Multifunctional Roles of Quantum Dots for Unlocking the Future of Biology and Medicine. Environ. Res. 2023, 232, 116290. [Google Scholar] [CrossRef]
- García de Arquer, F.P.; Talapin, D.V.; Klimov, V.I.; Arakawa, Y.; Bayer, M.; Sargent, E.H. Semiconductor Quantum Dots: Technological Progress and Future Challenges. Science 2021, 373, eaaz8541. [Google Scholar] [CrossRef]
- Agarwal, K.; Rai, H.; Mondal, S. Quantum Dots: An Overview of Synthesis, Properties, and Applications. Mater. Res. Express 2023, 10, 062001. [Google Scholar] [CrossRef]
- Tambe, V.; Maheshwari, R.; Chourasiya, Y.; Choudhury, H.; Gorain, B.; Tekade, R.K. Clinical Aspects and Regulatory Requirements for Nanomedicines. In Basic Fundamentals of Drug Delivery; Academic Press: Cambridge, MA, USA, 2018; pp. 733–752. [Google Scholar] [CrossRef]
- Gaur, N.; Sharma, N.; Dahiya, A.; Yadav, P.; Ojha, H.; Goyal, R.K.; Sharma, R.K. Toxicity and Regulatory Concerns for Nanoformulations in Medicine. In The ELSI Handbook of Nanotechnology: Risk, Safety, ELSI and Commercialization; Wiley: New York, NY, USA, 2020; pp. 333–357. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Primavera, R.; Wilson, R.J.; Thakor, A.S.; Kevadiya, B.D. Cellular Uptake and Retention of Nanoparticles: Insights on Particle Properties and Interaction with Cellular Components. Mater. Today Commun. 2020, 25, 101692. [Google Scholar] [CrossRef]
- Souza, S.O.; Lira, R.B.; Cunha, C.R.A.; Santos, B.S.; Fontes, A.; Pereira, G. Methods for Intracellular Delivery of Quantum Dots. Top. Curr. Chem. 2021, 379, 1. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Forry, S.P.; Gao, X.; Holbrook, R.D.; Telford, W.G.; Tona, A. Dynamics and Mechanisms of Quantum Dot Nanoparticle Cellular Uptake. J. Nanobiotechnology 2010, 8, 13. [Google Scholar] [CrossRef]
- Díaz-González, M.; de la Escosura-Muñiz, A.; Fernandez-Argüelles, M.T.; Alonso, F.J.G.; Costa-Fernandez, J.M. Quantum Dot Bioconjugates for Diagnostic Applications. In Surface-Modified Nanobiomaterials for Electrochemical and Biomedicine Applications. Topics in Current Chemistry Collections; Springer: Cham, Switzerland, 2020; pp. 133–176. [Google Scholar] [CrossRef]
- Sousa De Almeida, M.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding Nanoparticle Endocytosis to Improve Targeting Strategies in Nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Chang, Q.; Sun, Z.X.; Liu, J.; Deng, X.; Liu, Y.; Cao, A.; Wang, H. Fate of CdSe/ZnS Quantum Dots in Cells: Endocytosis, Translocation and Exocytosis. Colloids Surf. B Biointerfaces 2021, 208, 112140. [Google Scholar] [CrossRef] [PubMed]
- Anas, A.; Okuda, T.; Kawashima, N.; Nakayama, K.; Itoh, T.; Ishikawa, M.; Biju, V. Clathrin-Mediated Endocytosis of Quantum Dot-Peptide Conjugates in Living Cells. ACS Nano 2009, 3, 2419–2429. [Google Scholar] [CrossRef]
- Asadian, Z.; Zare, H.; Aghaei, M.; Panjehpour, M. Caveolae-Dependent Endocytosis Mediates the Cellular Uptake of CdTe Quantum Dots in Ovarian Cancer Cell Lines. Res. Pharm. Sci. 2022, 17, 527–539. [Google Scholar] [CrossRef]
- Zhang, L.W.; Monteiro-Riviere, N.A. Mechanisms of Quantum Dot Nanoparticle Cellular Uptake. Toxicol. Sci. 2009, 110, 138–155. [Google Scholar] [CrossRef]
- Ashree, J.; Wang, Q.; Chao, Y. Glyco-functionalised quantum dots and their progress in cancer diagnosis and treatment. Front. Chem. Sci. Eng. 2020, 14, 365–377. [Google Scholar] [CrossRef]
- Dalal, C.; Jana, N.R. Galactose Multivalency Effect on the Cell Uptake Mechanism of Bioconjugated Nanoparticles. J. Phys. Chem. C 2018, 122, 25651–25660. [Google Scholar] [CrossRef]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Perng, W.; Palui, G.; Wang, W.; Mattoussi, H. Elucidating the Role of Surface Coating in the Promotion or Prevention of Protein Corona around Quantum Dots. Bioconjug. Chem. 2019, 30, 2469–2480. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Shah, K.; Kansara, K.; Kumar, A.; Rawal, R.; Bhatia, D. Tissue-Derived Primary Cell Type Dictates the Endocytic Uptake Route of Carbon Quantum Dots and In Vivo Uptake. ACS Appl. Bio Mater. 2023, 6, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.H.; Song, Z.M.; Liu, Y.Y.; Su, Q.; Liang, W.; Cao, A.; Sun, Y.P.; Wang, H. Effects of Carbon Dots Surface Functionalities on Cellular Behaviors–Mechanistic Exploration for Opportunities in Manipulating Uptake and Translocation. Colloids Surf. B Biointerfaces 2019, 181, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, C.; Han, T.; Zhou, X.; Guo, S.; Zhang, J. Insight into the Cellular Internalization and Cytotoxicity of Graphene Quantum Dots. Adv. Healthc. Mater. 2013, 2, 1613–1619. [Google Scholar] [CrossRef]
- Tomić, S.; Janjetović, K.; Mihajlović, D.; Milenković, M.; Kravić-Stevović, T.; Marković, Z.; Todorović-Marković, B.; Spitalsky, Z.; Micusik, M.; Vučević, D.; et al. Graphene Quantum Dots Suppress Proinflammatory T Cell Responses via Autophagy-Dependent Induction of Tolerogenic Dendritic Cells. Biomaterials 2017, 146, 13–28. [Google Scholar] [CrossRef]
- Saulite, L.; Pleiko, K.; Popena, I.; Dapkute, D.; Rotomskis, R.; Riekstina, U. Nanoparticle Delivery to Metastatic Breast Cancer Cells by Nanoengineered Mesenchymal Stem Cells. Beilstein J. Nanotechnol. 2018, 9, 321–332. [Google Scholar] [CrossRef]
- Breger, J.; Delehanty, J.B.; Medintz, I.L. Continuing progress toward controlled intracellular delivery of semiconductor quantum dots. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 131–151. [Google Scholar] [CrossRef]
- Salykina, Y.F.; Zherdeva, V.V.; Dezhurov, S.V.; Wakstein, M.S.; Shirmanova, M.V.; Zagaynova, E.V.; Martyanov, A.A.; Savitsky, A.P. Biodistribution and Clearance of Quantum Dots in Small Animals. In Proceedings of the Saratov Fall Meeting 2010: Optical Technologies in Biophysics and Medicine XII, Saratov, Russia, 5–8 October 2010; Volume 7999, p. 799908. [Google Scholar] [CrossRef]
- Soo Choi, H.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty Ipe, B.; Bawendi, M.G.; Frangioni, J.V. Renal Clearance of Quantum Dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef]
- Maysinger, D.; Lovrić, J.; Eisenberg, A.; Savić, R. Fate of Micelles and Quantum Dots in Cells. Eur. J. Pharm. Biopharm. 2007, 65, 270–281. [Google Scholar] [CrossRef]
- Lovrić, J.; Bazzi, H.S.; Cuie, Y.; Fortin, G.R.A.; Winnik, F.M.; Maysinger, D. Differences in Subcellular Distribution and Toxicity of Green and Red Emitting CdTe Quantum Dots. J. Mol. Med. 2005, 83, 377–385. [Google Scholar] [CrossRef]
- Le, N.; Zhang, M.; Kim, K. Quantum Dots and Their Interaction with Biological Systems. Int. J. Mol. Sci. 2022, 23, 10763. [Google Scholar] [CrossRef]
- Ekdawi, S.N.; Stewart, J.M.P.; Dunne, M.; Stapleton, S.; Mitsakakis, N.; Dou, Y.N.; Jaffray, D.A.; Allen, C. Spatial and Temporal Mapping of Heterogeneity in Liposome Uptake and Microvascular Distribution in an Orthotopic Tumor Xenograft Model. J. Control. Release 2015, 207, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Meng, K.; Liu, Y.; Pan, Y.; Qu, W.; Chen, D.; Xie, S. Absorption, Distribution, Metabolism, and Excretion of Nanocarriers in Vivo and Their Influences. Adv. Colloid Interface Sci. 2020, 284, 102261. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, Y.; Xu, G.; Liu, D.; Yang, Z.; Chen, T.; Wang, X.; Jiang, W.; Xue, D.; Lin, G. In Vivo Comparison of the Biodistribution and Toxicity of InP/ZnS Quantum Dots with Different Surface Modifications. Int. J. Nanomed. 2020, 15, 1951–1965. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Li, L.; Wu, J.; Ma, M.; Lin, G.; Wang, X.; Xu, G. Evaluation for Adverse Effects of InP/ZnS Quantum Dots on the in Vitro Cultured Oocytes of Mice. ACS Appl. Bio Mater. 2019, 2, 4193–4201. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C.W. Nanoparticle-liver interactions: Cellular uptake and hepatobiliary elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef]
- Lin, X.; Chen, T. A Review of in vivo Toxicity of Quantum Dots in Animal Models. Int. J. Nanomedicine 2024, 18, 8143–8168. [Google Scholar] [CrossRef]
- Liu, N.; Tang, M. Toxicity of Different Types of Quantum Dots to Mammalian Cells in Vitro: An Update Review. J. Hazard. Mater. 2020, 399, 122606. [Google Scholar] [CrossRef]
- Su, Y.; He, Y.; Lu, H.; Sai, L.; Li, Q.; Li, W.; Wang, L.; Shen, P.; Huang, Q.; Fan, C. The Cytotoxicity of Cadmium Based, Aqueous Phase-Synthesized, Quantum Dots and Its Modulation by Surface Coating. Biomaterials 2009, 30, 19–25. [Google Scholar] [CrossRef]
- Fard, J.K.; Jafari, S.; Eghbal, M.A. A Review of Molecular Mechanisms Involved in Toxicity of Nanoparticles. Adv. Pharm. Bull. 2015, 5, 447–454. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, X.; Tang, D.; Wu, S.; Hou, X.; Liu, J.; Wu, P. Phosphorescent Carbon Dots for Highly Efficient Oxygen Photosensitization and as Photo-Oxidative Nanozymes. ACS Appl. Mater. Interfaces 2018, 10, 40808–40814. [Google Scholar] [CrossRef]
- Wang, S.; Cole, I.S.; Li, Q. The Toxicity of Graphene Quantum Dots. RSC Adv. 2016, 6, 89867–89878. [Google Scholar] [CrossRef]
- Sun, H.; Gao, N.; Dong, K.; Ren, J.; Qu, X. Graphene Quantum Dots-Band-Aids Used for Wound Disinfection. ACS Nano 2014, 8, 6202–6210. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tian, F.; Wang, W.; Chen, J.; Wu, M.; Zhao, J.X. Fabrication of Highly Fluorescent Graphene Quantum Dots Using L-Glutamic Acid for in Vitro/in Vivo Imaging and Sensing. J. Mater. Chem. C 2013, 1, 4676–4684. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, M.; Bhandari, B.; Yang, C. Recent Development of Carbon Quantum Dots: Biological Toxicity, Antibacterial Properties and Application in Foods. Food Rev. Int. 2022, 38, 1513–1532. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, H.; Zhang, P.; Gao, N.; Lin, Y.; Luo, Z.; Li, P.; Wang, C.; Liu, L.; Pang, D.; et al. Functionalized quantum dots induce proinflammatory responses in vitro: The role of terminal functional group-associated endocytic pathways. Nanoscale 2013, 5, 5919–5929. [Google Scholar] [CrossRef]
- Zhang, L.W.; Bäumer, W.; Monteiro-Riviere, N.A. Cellular Uptake Mechanisms and Toxicity of Quantum Dots in Dendritic Cells. Nanomedicine 2011, 6, 777–791. [Google Scholar] [CrossRef]
- Nagy, A.; Steinbrück, A.; Gao, J.; Doggett, N.; Hollingsworth, J.A.; Iyer, R. Comprehensive Analysis of the Effects of CdSe Quantum Dot Size, Surface Charge, and Functionalization on Primary Human Lung Cells. ACS Nano 2012, 6, 4748–4762. [Google Scholar] [CrossRef]
- Hu, L.; Zhong, H.; He, Z. Toxicity Evaluation of Cadmium-Containing Quantum Dots: A Review of Optimizing Physicochemical Properties to Diminish Toxicity. Colloids Surf. B Biointerfaces 2021, 200, 111609. [Google Scholar] [CrossRef]
- Manshian, B.B.; Soenen, S.J.; Brown, A.; Hondow, N.; Wills, J.; Jenkins, G.J.S.; Doak, S.H. Genotoxic Capacity of Cd/Se Semiconductor Quantum Dots with Differing Surface Chemistries. Mutagenesis 2016, 31, 97–106. [Google Scholar] [CrossRef]
- Manshian, B.B.; Martens, T.F.; Kantner, K.; Braeckmans, K.; De Smedt, S.C.; Demeester, J.; Jenkins, G.J.S.; Parak, W.J.; Pelaz, B.; Doak, S.H.; et al. The Role of Intracellular Trafficking of CdSe/ZnS QDs on Their Consequent Toxicity Profile. J. Nanobiotechnology 2017, 15, 45. [Google Scholar] [CrossRef]
- Havrdova, M.; Hola, K.; Skopalik, J.; Tomankova, K.; Petr, M.; Cepe, K.; Polakova, K.; Tucek, J.; Bourlinos, A.B.; Zboril, R. Toxicity of Carbon Dots-Effect of Surface Functionalization on the Cell Viability, Reactive Oxygen Species Generation and Cell Cycle. Carbon 2016, 99, 238–248. [Google Scholar] [CrossRef]
- Xie, Y.; Wan, B.; Yang, Y.; Cui, X.; Xin, Y.; Guo, L.H. Cytotoxicity and Autophagy Induction by Graphene Quantum Dots with Different Functional Groups. J. Environ. Sci. 2019, 77, 198–209. [Google Scholar] [CrossRef]
- Bottrill, M.; Green, M. Some Aspects of Quantum Dot Toxicity. Chem. Commun. 2011, 47, 7039–7050. [Google Scholar] [CrossRef]
- Manshian, B.B.; Abdelmonem, A.M.; Kantner, K.; Pelaz, B.; Klapper, M.; Nardi Tironi, C.; Parak, W.J.; Himmelreich, U.; Soenen, S.J. Evaluation of Quantum Dot Cytotoxicity: Interpretation of Nanoparticle Concentrations versus Intracellular Nanoparticle Numbers. Nanotoxicology 2016, 10, 1318–1328. [Google Scholar] [CrossRef]
- Manshian, B.B.; Soenen, S.J.; Al-Ali, A.; Brown, A.; Hondow, N.; Wills, J.; Jenkins, G.J.S.; Doak, S.H. Cell Type-Dependent Changes in Cdse/ZnS Quantum Dot Uptake and Toxic Endpoints. Toxicol. Sci. 2015, 144, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Hoseini, S.J.; Milan, P.B.; Hooshmand, S.; Kim, H.W.; Mozafari, M. Quantum Dots: A Review from Concept to Clinic. Biotechnol. J. 2020, 15, e2000117. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, V.; Chibli, H.; Fiammengo, R.; Galeone, A.; Malvindi, M.A.; Vecchio, G.; Cingolani, R. InP/ZnS as a Safer Alternative to CdSe/ZnS Core/Shell Quantum Dots: In Vitro and in Vivo Toxicity Assessment. Nanoscale 2014, 5, 307–317. [Google Scholar] [CrossRef]
- Hu, X.; Gao, X. Silica-Polymer Dual Layer-Encapsulated Quantum Dots with Remarkable Stability. ACS Nano 2010, 4, 6080–6086. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Ji, C.; Zhou, Y.; Zhao, T.; Leblanc, R.M. Polyethylene Glycol (PEG) Derived Carbon Dots: Preparation and Applications. Appl. Mater. Today 2020, 20, 100677. [Google Scholar] [CrossRef]
- Wagner, A.M.; Knipe, J.M.; Orive, G.; Peppas, N.A. Quantum Dots in Biomedical Applications. Acta Biomater. 2019, 94, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Huy, B.T.; Sakthivel, K.; Choi, H.J.; Joo, W.H.; Shin, S.K.; Lee, M.J.; Lee, Y.I. Highly Fluorescent CdTe Quantum Dots with Reduced Cytotoxicity-A Robust Biomarker. Sens. Bio-Sens. Res. 2015, 3, 46–52. [Google Scholar] [CrossRef]
- Wu, P.; Yan, X.P. Doped Quantum Dots for Chemo/Biosensing and Bioimaging. Chem. Soc. Rev. 2013, 42, 5489–5521. [Google Scholar] [CrossRef] [PubMed]
- Stavitskaya, A.V.; Novikov, A.A.; Kotelev, M.S.; Kopitsyn, D.S.; Rozhina, E.V.; Ishmukhametov, I.R.; Fakhrullin, R.F.; Ivanov, E.V.; Lvov, Y.M.; Vinokurov, V.A. Fluorescence and Cytotoxicity of Cadmium Sulfide Quantum Dots Stabilized on Clay Nanotubes. Nanomaterials 2018, 8, 391. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, H.; Wang, F.; Ren, J.; Qu, X. How Functional Groups Influence the ROS Generation and Cytotoxicity of Graphene Quantum Dots. Chem. Commun. 2017, 53, 10588–10591. [Google Scholar] [CrossRef]
- Fatima, I.; Rahdar, A.; Sargazi, S.; Barani, M.; Hassanisaadi, M.; Thakur, V.K. Quantum Dots: Synthesis, Antibody Conjugation, and Her2-Receptor Targeting for Breast Cancer Therapy. J. Funct. Biomater. 2021, 12, 75. [Google Scholar] [CrossRef]
- Chen, C.; Peng, J.; Xia, H.S.; Yang, G.F.; Wu, Q.S.; Chen, L.D.; Zeng, L.B.; Zhang, Z.L.; Pang, D.W.; Li, Y. Quantum Dots-Based Immunofluorescence Technology for the Quantitative Determination of HER2 Expression in Breast Cancer. Biomaterials 2009, 30, 2912–2918. [Google Scholar] [CrossRef]
- Rakovich, T.Y.; Mahfoud, O.K.; Mohamed, B.M.; Prina-Mello, A.; Crosbie-Staunton, K.; Van Den Broeck, T.; De Kimpe, L.; Sukhanova, A.; Baty, D.; Rakovich, A.; et al. Highly Sensitive Single Domain Antibody-Quantum Dot Conjugates for Detection of HER2 Biomarker in Lung and Breast Cancer Cells. ACS Nano 2014, 8, 5682–5695. [Google Scholar] [CrossRef]
- Rizvi, S.B.; Rouhi, S.; Taniguchi, S.; Yu Yang, S.; Green, M.; Keshtgar, M.; Seifalian, A.M. Near-Infrared Quantum Dots for HER2 Localization and Imaging of Cancer Cells. Int. J. Nanomdicine 2014, 9, 1323–1337. [Google Scholar] [CrossRef]
- Miyashita, M.; Gonda, K.; Tada, H.; Watanabe, M.; Kitamura, N.; Kamei, T.; Sasano, H.; Ishida, T.; Ohuchi, N. Quantitative Diagnosis of HER 2 Protein Expressing Breast Cancer by Single-particle Quantum Dot Imaging. Cancer Med. 2016, 5, 2813–2824. [Google Scholar] [CrossRef]
- Weinberg, F.; Peckys, D.B.; de Jonge, N. Egfr Expression in Her2-Driven Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9008. [Google Scholar] [CrossRef]
- Liu, X.L.; Peng, C.W.; Chen, C.; Yang, X.Q.; Hu, M.B.; Xia, H.S.; Liu, S.P.; Pang, D.W.; Li, Y. Quantum Dots-Based Double-Color Imaging of HER2 Positive Breast Cancer Invasion. Biochem. Biophys. Res. Commun. 2011, 409, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Gomes, F.; Bode, J.; Sukhanova, A.; Bozrova, S.V.; Saccomano, M.; Mitkovski, M.; Krueger, J.E.; Wege, A.K.; Stuehmer, W.; Samokhvalov, P.S.; et al. Single- and Two-Photon Imaging of Human Micrometastases and Disseminated Tumour Cells with Conjugates of Nanobodies and Quantum Dots. Sci. Rep. 2018, 8, 4595. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.; Neves, M.M.P.S.; Nouws, H.P.A.; Delerue-Matos, C. Quantum Dots as Nanolabels for Breast Cancer Biomarker HER2-ECD Analysis in Human Serum. Talanta 2020, 208, 120430. [Google Scholar] [CrossRef] [PubMed]
- Elakkiya, V.; Menon, M.P.; Nataraj, D.; Biji, P.; Selvakumar, R. Optical Detection of CA 15.3 Breast Cancer Antigen Using CdS Quantum Dot. Iet Nanobiotechnology 2016, 11, 268–276. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Tagi, S.; Solhi, E.; Mokhtarzadeh, A.; Shadjou, N.; Eftekhari, A.; Mahboob, S. An Innovative Immunosensor for Ultrasensitive Detection of Breast Cancer Specific Carbohydrate (CA 15-3) in Unprocessed Human Plasma and MCF-7 Breast Cancer Cell Lysates Using Gold Nanospear Electrochemically Assembled onto Thiolated Graphene Quantum Dots. Int. J. Biol. Macromol. 2018, 114, 1008–1017. [Google Scholar] [CrossRef]
- Yuan, J.P.; Wang, L.W.; Qu, A.P.; Chen, J.M.; Xiang, Q.M.; Chen, C.; Sun, S.R.; Pang, D.W.; Liu, J.; Li, Y. Quantum Dots-Based Quantitative and in Situ Multiple Imaging on Ki67 and Cytokeratin to Improve Ki67 Assessment in Breast Cancer. PLoS ONE 2015, 10, e0122734. [Google Scholar] [CrossRef]
- Xiang, Q.M.; Wang, L.W.; Yuan, J.P.; Chen, J.M.; Yang, F.; Li, Y. Quantum Dot-Based Multispectral Fluorescent Imaging to Quantitatively Study Co-Expressions of Ki67 and HER2 in Breast Cancer. Exp. Mol. Pathol. 2015, 99, 133–138. [Google Scholar] [CrossRef]
- Sun, G.; Xing, W.; Xing, R.; Cong, L.; Tong, S.; Yu, S. Targeting Breast Cancer Cells with a Cuins2/ZnS Quantum Dot-Labeled Ki-67 Bioprobe. Oncol. Lett. 2018, 15, 2471–2476. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, D.; Zeng, Z.; Huang, L.; Lin, X.; Hong, S. Aptamer-Based Probes for Cancer Diagnostics and Treatment. Life 2022, 12, 1937. [Google Scholar] [CrossRef]
- Hua, X.; Zhou, Z.; Yuan, L.; Liu, S. Selective Collection and Detection of MCF-7 Breast Cancer Cells Using Aptamer-Functionalized Magnetic Beads and Quantum Dots Based Nano-Bio-Probes. Anal. Chim. Acta 2013, 788, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Mohammadinejad, A.; Taghdisi, S.M.; Es’haghi, Z.; Abnous, K.; Mohajeri, S.A. Targeted Imaging of Breast Cancer Cells Using Two Different Kinds of Aptamers -Functionalized Nanoparticles. Eur. J. Pharm. Sci. 2019, 134, 60–68. [Google Scholar] [CrossRef]
- Tran, H.L.; Dang, V.D.; Dega, N.K.; Lu, S.M.; Huang, Y.F.; Doong, R. Ultrasensitive Detection of Breast Cancer Cells with a Lectin-Based Electrochemical Sensor Using N-Doped Graphene Quantum Dots as the Sensing Probe. Sens. Actuators B Chem. 2022, 368, 132233. [Google Scholar] [CrossRef]
- Borghei, Y.S.; Hosseini, M.; Ganjali, M.R.; Hosseinkhani, S. A Novel Dual-Mode and Label-Free Aptasensor Based Methodology for Breast Cancer Tissue Marker Targeting. Sens. Actuators B Chem. 2020, 315, 128084. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, W.; Chen, Q.; Chen, M. Non-Coding RNAs and Their Integrated Networks. J. Integr. Bioinform. 2019, 16, 20190027. [Google Scholar] [CrossRef] [PubMed]
- Budakoti, M.; Panwar, A.S.; Molpa, D.; Singh, R.K.; Büsselberg, D.; Mishra, A.P.; Coutinho, H.D.M.; Nigam, M. Micro-RNA: The Darkhorse of Cancer. Cell. Signal. 2021, 83, 109995. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.K.W.; Chow, M.Y.T.; Zhang, Y.; Leung, S.W.S. SiRNA versus MiRNA as Therapeutics for Gene Silencing. Mol. Ther. Nucleic Acids 2015, 4, e252. [Google Scholar] [CrossRef]
- MacFarlane, L.-A.; Murphy, P.R. MicroRNA: Biogenesis, Function and Role in Cancer. Curr. Genom. 2010, 11, 537–561. [Google Scholar] [CrossRef]
- Arun, R.P.; Cahill, H.F.; Marcato, P. Breast Cancer Subtype-Specific MiRNAs: Networks, Impacts, and the Potential for Intervention. Biomedicines 2022, 10, 651. [Google Scholar] [CrossRef]
- Hu, O.; Li, Z.; Tong, Y.; Wang, Q.; Chen, Z. DNA Functionalized Double Quantum Dots-Based Fluorescence Biosensor for One-Step Simultaneous Detection of Multiple MicroRNAs. Talanta 2021, 235, 122763. [Google Scholar] [CrossRef]
- Mohammadi, S.; Mohammadi, S.; Salimi, A. A 3D Hydrogel Based on Chitosan and Carbon Dots for Sensitive Fluorescence Detection of MicroRNA-21 in Breast Cancer Cells. Talanta 2021, 224, 121895. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Hu, L.; Zhang, F.; Wang, M.; Xia, Z.; Wei, W. MoS2 Quantum Dots Modified with a Labeled Molecular Beacon as a Ratiometric Fluorescent Gene Probe for FRET Based Detection and Imaging of MicroRNA. Microchim. Acta 2018, 185, 239. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Howes, P.D.; Kim, E.; Spicer, C.D.; Thomas, M.R.; Lin, Y.; Crowder, S.W.; Pence, I.J.; Stevens, M.M. Duplex-Specific Nuclease-Amplified Detection of MicroRNA Using Compact Quantum Dot–DNA Conjugates. ACS Appl. Mater. Interfaces 2018, 10, 28290–28300. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Yang, M.; Hou, C.; Huo, D. An Ultrasensitive Electrochemical Biosensor for Simultaneously Detect MicroRNA-21 and MicroRNA-155 Based on Specific Interaction of Antimonide Quantum Dot with RNA. Microchem. J. 2023, 185, 108173. [Google Scholar] [CrossRef]
- Pothipor, C.; Jakmunee, J.; Bamrungsap, S.; Ounnunkad, K. An Electrochemical Biosensor for Simultaneous Detection of Breast Cancer Clinically Related MicroRNAs Based on a Gold Nanoparticles/Graphene Quantum Dots/Graphene Oxide Film. Analyst 2021, 146, 4000–4009. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shi, W.; Tang, T.; Wang, Y.; Yin, X.; Chen, Y.; Zhang, Y.; Xing, Y.; Shen, Y.; Xia, T.; et al. MiR-29a Contributes to Breast Cancer Cells Epithelial–Mesenchymal Transition, Migration, and Invasion via down-Regulating Histone H4K20 Trimethylation through Directly Targeting SUV420H2. Cell Death Dis. 2019, 10, 176. [Google Scholar] [CrossRef]
- Kuntip, N.; Japrung, D.; Pongprayoon, P. What Happens When a Complementary DNA Meets MiR-29a Cancer Biomarker in Complex with a Graphene Quantum Dot. ACS Appl. Bio Mater. 2021, 4, 8368–8376. [Google Scholar] [CrossRef]
- da Silva, J.L.; Cardoso Nunes, N.C.; Izetti, P.; de Mesquita, G.G.; de Melo, A.C. Triple Negative Breast Cancer: A Thorough Review of Biomarkers. Crit. Rev. Oncol. Hematol. 2020, 145, 102855. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Chen, G.; Li, Y.; Xu, W.; Gong, S. Quantum-Dot-Based Theranostic Micelles Conjugated with an Anti-EGFR Nanobody for Triple-Negative Breast Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 30297–30305. [Google Scholar] [CrossRef]
- Nasrollahi, F.; Koh, Y.R.; Chen, P.; Varshosaz, J.; Khodadadi, A.A.; Lim, S. Targeting Graphene Quantum Dots to Epidermal Growth Factor Receptor for Delivery of Cisplatin and Cellular Imaging. Mater. Sci. Eng. C 2019, 94, 247–257. [Google Scholar] [CrossRef]
- Zheng, H.M.; Chen, C.; Wu, X.H.; Chen, J.; Sun, S.; Sun, J.Z.; Wang, M.W.; Sun, S.R. Quantum Dot-Based in Situ Simultaneous Molecular Imaging and Quantitative Analysis of EGFR and Collagen IV and Identification of Their Prognostic Value in Triple-Negative Breast Cancer. Tumor Biol. 2016, 37, 2509–2518. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ghosal, K.; Mohammad, S.A.; Sarkar, K. Dendrimer Functionalized Carbon Quantum Dot for Selective Detection of Breast Cancer and Gene Therapy. Chem. Eng. J. 2019, 373, 468–484. [Google Scholar] [CrossRef]
- Díaz-García, D.; Díaz-Sánchez, M.; Álvarez-Conde, J.; Gómez-Ruiz, S. Emergence of Quantum Dots as Innovative Tools for Early Diagnosis and Advanced Treatment of Breast Cancer. ChemMedChem 2024, 19, e202400172. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, J.; Hasanzadeh, M.; Somi, M.H.; Ozkan, S.A.; Jouyban, A. Targeting and Sensing of Some Cancer Cells Using Folate Bioreceptor Functionalized Nitrogen-Doped Graphene Quantum Dots. Int. J. Biol. Macromol. 2018, 118, 1021–1034. [Google Scholar] [CrossRef]
- Gadeval, A.; Maheshwari, R.; Raval, N.; Kalyane, D.; Kalia, K.; Tekade, R.K. Green Graphene Nanoplates for Combined Photo-Chemo-Thermal Therapy of Triple-Negative Breast Cancer. Nanomedicine 2020, 15, 581–601. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, D.; Wang, Y.; Lin, S.; Jiang, Y. A Novel 3D Breast-Cancer-on-Chip Platform for Therapeutic Evaluation of Drug Delivery Systems. Anal. Chim. Acta 2018, 1036, 97–106. [Google Scholar] [CrossRef]
- Wang, L.W.; Peng, C.W.; Chen, C.; Li, Y. Quantum Dots-Based Tissue and in Vivo Imaging in Breast Cancer Researches: Current Status and Future Perspectives. Breast Cancer Res. Treat. 2015, 151, 7–17. [Google Scholar] [CrossRef]
- Radenkovic, D.; Kobayashi, H.; Remsey-Semmelweis, E.; Seifalian, A.M. Quantum Dot Nanoparticle for Optimization of Breast Cancer Diagnostics and Therapy in a Clinical Setting. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1581–1592. [Google Scholar] [CrossRef]
- Sokolov, P.; Nifontova, G.; Samokhvalov, P.; Karaulov, A.; Sukhanova, A.; Nabiev, I. Nontoxic Fluorescent Nanoprobes for Multiplexed Detection and 3D Imaging of Tumor Markers in Breast Cancer. Pharmaceutics 2023, 15, 946. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Z.; Lan, X. Quantum Dot-Based Simultaneous Multicolor Imaging. Mol. Imaging Biol. 2020, 22, 820–831. [Google Scholar] [CrossRef]
- Chen, C.; Peng, J.; Xia, H.; Wu, Q.; Zeng, L.; Xu, H.; Tang, H.; Zhang, Z.; Zhu, X.; Pang, D.; et al. Quantum-Dot-Based Immunofluorescent Imaging of HER2 and ER Provides New Insights into Breast Cancer Heterogeneity. Nanotechnology 2010, 21, 095101. [Google Scholar] [CrossRef] [PubMed]
- Au, G.H.T.; Mejias, L.; Swami, V.K.; Brooks, A.D.; Shih, W.Y.; Shih, W.H. Quantitative Assessment of Tn Antigen in Breast Tissue Micro-Arrays Using CdSe Aqueous Quantum Dots. Biomaterials 2014, 35, 2971–2980. [Google Scholar] [CrossRef]
- Yezhelyev, M.V.; Al-Hajj, A.; Morris, C.; Marcus, A.I.; Liu, T.; Lewis, M.; Cohen, C.; Zrazhevskiy, P.; Simons, J.W.; Rogatko, A.; et al. In Situ Molecular Profiling of Breast Cancer Biomarkers with Multicolor Quantum Dots. Adv. Mater. 2007, 19, 3146–3151. [Google Scholar] [CrossRef]
- Sharaf, S.S.; Lekshmi, A.; Aswathy, S.; Anurup, K.G.; SP, A.J.; Chandrasekharan, A.; Somanathan, T.; Kumar, T.S.; Sujathan, K. A Multiplex Immunoprofiling Approach for Detecting the Co-Localization of Breast Cancer Biomarkers Using a Combination of Alexafluor-Quantum Dot Conjugates and a Panel of Chromogenic Dyes. Pathol. Res. Pract. 2024, 253, 155033. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Wang, Z.G.; Liu, S.L.; Wang, H.; Zhu, J.; He, R.; Yang, X.; Liu, X.; Wu, Q.; Wu, J.K. Purified Fluorescent Nanohybrids Based on Quantum Dot–HER2–Antibody for Breast Tumor Target Imaging. Talanta 2023, 260, 124560. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Braun, G.B.; Zhong, H.; Hall, D.J.; Han, W.; Qin, M.; Zhao, C.; Wang, M.; She, Z.G.; Cao, C.; et al. Tumor-Targeted Multimodal Optical Imaging with Versatile Cadmium-Free Quantum Dots. Adv Funct Mater. 2015, 26, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Robinson, J.T.; Zhang, Y.; Diao, S.; Antaris, A.L.; Wang, Q.; Dai, H. In Vivo Fluorescence Imaging with Ag2S Quantum Dots in the Second Near-Infrared Region. Angew. Chem. Int. Ed. 2012, 51, 9818–9821. [Google Scholar] [CrossRef]
- Smith, A.M.; Duan, H.; Mohs, A.M.; Nie, S. Bioconjugated Quantum Dots for in Vivo Molecular and Cellular Imaging. Adv. Drug Deliv. Rev. 2008, 60, 1226–1240. [Google Scholar] [CrossRef]
- Hama, Y.; Koyama, Y.; Urano, Y.; Choyke, P.L.; Kobayashi, H. Simultaneous two-color spectral fluorescence lymphangiography with near infrared quantum dots to map two lymphatic flows from the breast and the upper extremity. Breast Cancer Res. Treat. 2007, 103, 23–28. [Google Scholar] [CrossRef]
- Kosaka, N.; Ogawa, M.; Sato, N.; Choyke, P.L.; Kobayashi, H. In Vivo Real-Time, Multicolor, Quantum Dot Lymphatic Imaging. J. Investig. Dermatol. 2009, 129, 2818–2822. [Google Scholar] [CrossRef]
- Helle, M.; Cassette, E.; Bezdetnaya, L.; Pons, T.; Leroux, A.; Plénat, F.; Guillemin, F.; Dubertret, B.; Marchal, F. Visualisation of Sentinel Lymph Node with Indium-Based near Infrared Emitting Quantum Dots in a Murine Metastatic Breast Cancer Model. PLoS ONE 2012, 7, e44433. [Google Scholar] [CrossRef]
- Sun, X.; Shi, M.; Zhang, C.; Yuan, J.; Yin, M.; Du, S.; Yu, S.; Ouyang, B.; Xue, F.; Yang, S.T. Fluorescent Ag-In-S/ZnS Quantum Dots for Tumor Drainage Lymph Node Imaging in Vivo. ACS Appl. Nano Mater. 2021, 4, 1029–1037. [Google Scholar] [CrossRef]
- Kamkaew, A.; Sun, H.; England, C.G.; Cheng, L.; Liu, Z.; Cai, W. Quantum Dot-NanoLuc Bioluminescence Resonance Energy Transfer Enables Tumor Imaging and Lymph Node Mapping: In Vivo. Chem. Commun. 2016, 52, 6997–7000. [Google Scholar] [CrossRef]
- Tian, R.; Ma, H.; Zhu, S.; Lau, J.; Ma, R.; Liu, Y.; Lin, L.; Chandra, S.; Wang, S.; Zhu, X.; et al. Multiplexed NIR-II Probes for Lymph Node-invaded Cancer Detection and Imaging-guided Surgery. Adv. Mater. 2020, 32, e1907365. [Google Scholar] [CrossRef]
- Gonda, K.; Watanabe, T.M.; Ohuchi, N.; Higuchi, H. In Vivo Nano-Imaging of Membrane Dynamics in Metastatic Tumor Cells Using Quantum Dots. J. Biol. Chem. 2010, 285, 2750–2757. [Google Scholar] [CrossRef]
- Ojima, T.; Kinami, S.; Nakamura, K.; Oyama, K.; Inokuchi, M.; Fujita, H.; Ninomiya, I.; Fushida, S.; Fujimura, T.; Kitamura, S.; et al. Advantages of the Rapid Double-Staining Method for Intraoperative Detection of Micrometastasis in Sentinel Lymph Nodes. Oncol. Rep. 2013, 30, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Bernardo, M.; Mitsunaga, M.; Choyke, P.L.; Kobayashi, H. Optical Imaging of Early Micrometastases in Lymph Nodes: Triple Labeling with Nano-sized Agents Yielding Distinct Signals. Contrast Media Mol. Imaging. 2012, 7, 247–253. [Google Scholar] [CrossRef]
- Sorrin, A.J.; Kemal Ruhi, M.; Ferlic, N.A.; Karimnia, V.; Polacheck, W.J.; Celli, J.P.; Huang, H.C.; Rizvi, I. Photodynamic Therapy and the Biophysics of the Tumor Microenvironment. Photochem. Photobiol. 2020, 96, 232–259. [Google Scholar] [CrossRef]
- Overchuk, M.; Weersink, R.A.; Wilson, B.C.; Zheng, G. Photodynamic and Photothermal Therapies: Synergy Opportunities for Nanomedicine. ACS Nano 2023, 17, 7979–8003. [Google Scholar] [CrossRef]
- Kong, C.; Chen, X. Combined Photodynamic and Photothermal Therapy and Immunotherapy for Cancer Treatment: A Review. Int. J. Nanomed. 2022, 17, 6427–6446. [Google Scholar] [CrossRef] [PubMed]
- Ostańska, E.; Aebisher, D.; Bartusik-Aebisher, D. The Potential of Photodynamic Therapy in Current Breast Cancer Treatment Methodologies. Biomed. Pharmacother. 2021, 137, 111302. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, Z.; Li, X.; Yang, M.; Lv, J.; Li, H.; Yuan, Z. Chemiluminescence in Combination with Organic Photosensitizers: Beyond the Light Penetration Depth Limit of Photodynamic Therapy. Int. J. Mol. Sci. 2022, 23, 12556. [Google Scholar] [CrossRef]
- Pattani, V.P.; Shah, J.; Atalis, A.; Sharma, A.; Tunnell, J.W. Role of Apoptosis and Necrosis in Cell Death Induced by Nanoparticle-Mediated Photothermal Therapy. J. Nanoparticle Res. 2015, 17, 20. [Google Scholar] [CrossRef]
- Wilson, B.C.; Weersink, R.A. The Yin and Yang of PDT and PTT. Photochem Photobiol. 2019, 96, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Kadkhoda, J.; Tarighatnia, A.; Tohidkia, M.R.; Nader, N.D.; Aghanejad, A. Photothermal Therapy-Mediated Autophagy in Breast Cancer Treatment: Progress and Trends. Life Sci. 2022, 298, 120499. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, H.; Xu, W.; Jiang, G.Q. Recent Advances in Photothermal Therapy-Based Multifunctional Nanoplatforms for Breast Cancer. Front. Chem. 2022, 10, 1024177. [Google Scholar] [CrossRef]
- Olszowy, M.; Nowak-Perlak, M.; Woźniak, M. Current Strategies in Photodynamic Therapy (PDT) and Photodynamic Diagnostics (PDD) and the Future Potential of Nanotechnology in Cancer Treatment. Pharmaceutics 2023, 15, 1712. [Google Scholar] [CrossRef]
- Soumya, K.; More, N.; Choppadandi, M.; Aishwarya, D.A.; Singh, G.; Kapusetti, G. A Comprehensive Review on Carbon Quantum Dots as an Effective Photosensitizer and Drug Delivery System for Cancer Treatment. Biomed. Technol. 2023, 4, 11–20. [Google Scholar] [CrossRef]
- Samia, A.C.S.; Dayal, S.; Burda, C. Quantum Dot-based Energy Transfer: Perspectives and Potential for Applications in Photodynamic Therapy. Photochem. Photobiol. 2006, 82, 617–625. [Google Scholar] [CrossRef]
- Yan, Y.; Tian, J.; Hu, F.; Wang, X.; Shen, Z. A near IR Photosensitizer Based on Self-Assembled CdSe Quantum Dot-Aza-BODIPY Conjugate Coated with Poly(Ethylene Glycol) and Folic Acid for Concurrent Fluorescence Imaging and Photodynamic Therapy. RSC Adv. 2016, 6, 113991–113996. [Google Scholar] [CrossRef]
- Montaseri, H.; Kruger, C.A.; Abrahamse, H. Inorganic Nanoparticles Applied for Active Targeted Photodynamic Therapy of Breast Cancer. Pharmaceutics 2021, 13, 296. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ding, H.; Li, Z.; Tedesco, A.C.; Bi, H. Carbon Dots Derived from Tea Polyphenols as Photosensitizers for Photodynamic Therapy. Molecules 2022, 27, 8627. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Lan, M.; Zhou, B.; Liu, W.; Guo, L.; Wang, H.; Jia, Q.; Niu, G.; Huang, X.; Zhou, H.; et al. A Graphene Quantum Dot Photodynamic Therapy Agent with High Singlet Oxygen Generation. Nat. Commun. 2014, 5, 4596. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.; Khor, B.K.; Lee, C.Y.; Doong, R.A.; Oon, C.E.; Thanh, N.T.K.; Lee, H.L. N-Doped Graphene Quantum Dots/Titanium Dioxide Nanocomposites: A Study of ROS-Forming Mechanisms, Cytotoxicity and Photodynamic Therapy. Biomedicines 2022, 10, 421. [Google Scholar] [CrossRef]
- Pandey, S.; Gedda, G.R.; Thakur, M.; Bhaisare, M.L.; Talib, A.; Khan, M.S.; Wu, S.M.; Wu, H.F. Theranostic Carbon Dots ‘Clathrate-like’ Nanostructures for Targeted Photo-Chemotherapy and Bioimaging of Cancer. J. Ind. Eng. Chem. 2017, 56, 62–73. [Google Scholar] [CrossRef]
- Charron, G.; Stuchinskaya, T.; Edwards, D.R.; Russell, D.A.; Nann, T. Insights into the Mechanism of Quantum Dot-Sensitized Singlet Oxygen Production for Photodynamic Therapy. J. Phys. Chem. C 2012, 116, 9334–9342. [Google Scholar] [CrossRef]
- Javani Jouni, F.; Rastegar-Pouyani, N.; Najjar, N.; Nasirpour, M.; Payez, A.; Kashi, G.; Zafari, J. Evaluation of the effects of photodynamic therapy consisted of the blue laser and zinc oxide QDs on MDA-MB-231 cancer cells by inhibiting cancer markers and inducing apoptosis. Lasers Med. Sci. 2024, 39, 28. [Google Scholar] [CrossRef]
- Roshini, A.; Jagadeesan, S.; Cho, Y.J.; Lim, J.H.; Choi, K.H. Synthesis and Evaluation of the Cytotoxic and Anti-Proliferative Properties of ZnO Quantum Dots against MCF-7 and MDA-MB-231 Human Breast Cancer Cells. Mater. Sci. Eng. C 2017, 81, 551–560. [Google Scholar] [CrossRef]
- Czarnecka-Czapczyńska, M.; Aebisher, D.; Oleś, P.; Sosna, B.; Krupka-Olek, M.; Dynarowicz, K.; Latos, W.; Cieślar, G.; Kawczyk-Krupka, A. The Role of Photodynamic Therapy in Breast Cancer–A Review of in Vitro Research. Biomed. Pharmacother. 2021, 144, 112342. [Google Scholar] [CrossRef]
- Hamidu, A.; Pitt, W.G.; Husseini, G.A. Recent Breakthroughs in Using Quantum Dots for Cancer Imaging and Drug Delivery Purposes. Nanomaterials 2023, 13, 2566. [Google Scholar] [CrossRef]
- Nazir, S.; Hussain, T.; Ayub, A.; Rashid, U.; MacRobert, A.J. Nanomaterials in Combating Cancer: Therapeutic Applications and Developments. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Pardo, J.; Peng, Z.; Leblanc, R.M. Cancer Targeting and Drug Delivery Using Carbon-Based Quantum Dots and Nanotubes. Molecules 2018, 23, 378. [Google Scholar] [CrossRef] [PubMed]
- Mahani, M.; Pourrahmani-Sarbanani, M.; Yoosefian, M.; Divsar, F.; Mousavi, S.M.; Nomani, A. Doxorubicin Delivery to Breast Cancer Cells with Transferrin-Targeted Carbon Quantum Dots: An in Vitro and in Silico Study. J. Drug Deliv. Sci. Technol. 2021, 62, 102342. [Google Scholar] [CrossRef]
- Zhao, P.; Xu, Y.; Ji, W.; Zhou, S.; Li, L.; Qiu, L.; Qian, Z.; Wang, X.; Zhang, H. Biomimetic Black Phosphorus Quantum Dots-Based Photothermal Therapy Combined with Anti-PD-L1 Treatment Inhibits Recurrence and Metastasis in Triple-Negative Breast Cancer. J. Nanobiotechnology 2021, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Garg, A.; Mustafa, G.; Ahmad, M.Z.; Aslam, M.; Mishra, A. Hybrid Quantum Dot as Promising Tools for Theranostic Application in Cancer. Electron. 2023, 12, 972. [Google Scholar] [CrossRef]
- Boopathy, L.K.; Gopal, T.; Roy, A.; Kalari Kandy, R.R.; Arumugam, M.K. Recent Trends in Macromolecule-Conjugated Hybrid Quantum Dots for Cancer Theranostic Applications. RSC Adv. 2023, 13, 18760–18774. [Google Scholar] [CrossRef]
- Field, L.D.; Walper, S.A.; Susumu, K.; Lasarte-Aragones, G.; Oh, E.; Medintz, I.L.; Delehanty, J.B. A Quantum Dot-Protein Bioconjugate That Provides for Extracellular Control of Intracellular Drug Release. Bioconjug. Chem. 2018, 29, 2455–2467. [Google Scholar] [CrossRef]
- Bogdanov, A.; Bogdanov, A.; Chubenko, V.; Volkov, N.; Moiseenko, F.; Moiseyenko, V. Tumor Acidity: From Hallmark of Cancer to Target of Treatment. Front. Oncol. 2022, 12, 979154. [Google Scholar] [CrossRef]
- Moudgil, A.; Shende, R.A.; Pawar, A.T.; Gajbhiye, K.R.; Gajbhiye, V.; Chaudhari, B.P. Quantum Dots Based Vehicles for Controlled Drug Release in Conjunction with Bio-Imaging. In Stimuli-Responsive Nanocarriers; Academic Press: Cambridge, MA, USA, 2022; pp. 197–236. [Google Scholar] [CrossRef]
- Sheng, Y.; Dai, W.; Gao, J.; Li, H.; Tan, W.; Wang, J.; Deng, L.; Kong, Y. PH-Sensitive Drug Delivery Based on Chitosan Wrapped Graphene Quantum Dots with Enhanced Fluorescent Stability. Mater. Sci. Eng. C 2020, 112, 110888. [Google Scholar] [CrossRef]
- Javadian, S.; Najafi, K.; Sadrpoor, S.M.; Ektefa, F.; Dalir, N.; Nikkhah, M. Graphene Quantum Dots Based Magnetic Nanoparticles as a Promising Delivery System for Controlled Doxorubicin Release. J. Mol. Liq. 2021, 331, 115746. [Google Scholar] [CrossRef]
- Ziaee, N.; Farhadian, N.; Abnous, K.; Matin, M.M.; Khoshnood, A.; Yaghoobi, E. Dual Targeting of Mg/N Doped-Carbon Quantum Dots with Folic and Hyaluronic Acid for Targeted Drug Delivery and Cell Imaging. Biomed. Pharmacother. 2023, 164, 114971. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Shao, D.; He, X.; Ren, Z.; Ji, W.; Shan, C.; Qu, S.; Li, J.; Chen, L.; Li, Q. Carbon Dots as a Trackable Drug Delivery Carrier for Localized Cancer Therapy: In Vivo. J. Mater. Chem. B 2016, 4, 5119–5126. [Google Scholar] [CrossRef]
- Kong, T.; Hao, L.; Wei, Y.; Cai, X.; Zhu, B. Doxorubicin Conjugated Carbon Dots as a Drug Delivery System for Human Breast Cancer Therapy. Cell Prolif. 2018, 51, e12488. [Google Scholar] [CrossRef]
- Sarkar, P.; Ghosh, S.; Sarkar, K. Folic Acid Based Carbon Dot Functionalized Stearic Acid-g-Polyethyleneimine Amphiphilic Nanomicelle: Targeted Drug Delivery and Imaging for Triple Negative Breast Cancer. Colloids Surf. B Biointerfaces 2021, 197, 111382. [Google Scholar] [CrossRef]
- Sawy, A.M.; Barhoum, A.; Abdel Gaber, S.A.; El-Hallouty, S.M.; Shousha, W.G.; Maarouf, A.A.; Khalil, A.S.G. Insights of Doxorubicin Loaded Graphene Quantum Dots: Synthesis, DFT Drug Interactions, and Cytotoxicity. Mater. Sci. Eng. C 2021, 122, 111921. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, C.; Zhou, X.; Han, T.; Xin, X.; Wu, J.; Zhang, J.; Guo, S. Enhancing Cell Nucleus Accumulation and DNA Cleavage Activity of Anti-Cancer Drug via Graphene Quantum Dots. Sci. Rep. 2013, 3, 2852. [Google Scholar] [CrossRef] [PubMed]
- Mirzababaei, M.; Larijani, K.; Hashemi-Moghaddam, H.; Mirjafary, Z.; Madanchi, H. Graphene Quantum Dots Coated Cationic Polymer for Targeted Drug Delivery and Imaging of Breast Cancer. J. Polym. Res. 2023, 30, 268. [Google Scholar] [CrossRef]
- Feng, T.; Ai, X.; Ong, H.; Zhao, Y. Dual-Responsive Carbon Dots for Tumor Extracellular Microenvironment Triggered Targeting and Enhanced Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 18732–18740. [Google Scholar] [CrossRef]
- Sui, X.; Luo, C.; Wang, C.; Zhang, F.; Zhang, J.; Guo, S. Graphene Quantum Dots Enhance Anticancer Activity of Cisplatin via Increasing Its Cellular and Nuclear Uptake. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1997–2006. [Google Scholar] [CrossRef]
- Ghanbari, N.; Fam, A.M.; Salehi, Z.; Khodadadi, A.A.; Safarian, S. Synergistic Effects of Graphene Quantum Dots Nanocarriers and Folic Acid Targeting Agent on Enhanced Killing of Breast Cancer Cells by Tamoxifen. Can. J. Chem. Eng. 2024, 102, 3029–3038. [Google Scholar] [CrossRef]
- Amraee, N.; Torbati, M.B.; Majd, A.; Shaabanzadeh, M. Efficient and Traceable Tamoxifen Delivery to Breast Cancer Cells Using Folic Acid-Decorated and PEGylated Graphene Quantum Dots. ChemistrySelect 2023, 8, e202302465. [Google Scholar] [CrossRef]
- Samimi, S.; Ardestani, M.S.; Dorkoosh, F.A. Preparation of Carbon Quantum Dots- Quinic Acid for Drug Delivery of Gemcitabine to Breast Cancer Cells. J. Drug Deliv. Sci. Technol. 2021, 61, 102287. [Google Scholar] [CrossRef]
- Yunus, U.; Zulfiqar, M.A.; Ajmal, M.; Bhatti, M.H.; Chaudhry, G.E.S.; Muhammad, T.S.T.; Sung, Y.Y. Targeted Drug Delivery Systems: Synthesis and in Vitro Bioactivity and Apoptosis Studies of Gemcitabine-Carbon Dot Conjugates. Biomed. Mater. 2020, 15, 065004. [Google Scholar] [CrossRef]
- Hussen, B.M.; Hidayat, H.J.; Salihi, A.; Sabir, D.K.; Taheri, M.; Ghafouri-Fard, S. MicroRNA: A Signature for Cancer Progression. Biomed. Pharmacother. 2021, 92, 307–328. [Google Scholar] [CrossRef]
- Ashique, S.; Almohaywi, B.; Haider, N.; Yasmin, S.; Hussain, A.; Mishra, N.; Garg, A. SiRNA-Based Nanocarriers for Targeted Drug Delivery to Control Breast Cancer. Adv. Cancer Biol.-Metastasis 2022, 4, 100047. [Google Scholar] [CrossRef]
- Mirzaei, S.; Paskeh, M.D.A.; Entezari, M.; Bidooki, S.H.; Ghaleh, V.J.; Rezaei, S.; Hejazi, E.S.; Kakavand, A.; Behroozaghdam, M.; Movafagh, A.; et al. SiRNA and Targeted Delivery Systems in Breast Cancer Therapy. Clin. Transl. Oncol. 2023, 25, 1167–1188. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.B.; Jiang, S.; Zhang, Y. Quantum-Dot Based Nanoparticles for Targeted Silencing of HER2/Neu Gene via RNA Interference. Biomaterials 2007, 28, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.; Gao, F.; Yu, C.; Zeng, M.; He, G.; Wu, Y.; Su, Y.; Hu, N.; Zhou, Z.; Yang, Z.; et al. Dual-Targeted Therapy in HER2-Positive Breast Cancer Cells with the Combination of Carbon Dots/HER3 SiRNA and Trastuzumab. Nanotechnology 2020, 31, 335102. [Google Scholar] [CrossRef] [PubMed]
- Yue, R.; Chen, M.; Ma, N. Dual MicroRNA-Triggered Drug Release System for Combined Chemotherapy and Gene Therapy with Logic Operation. ACS Appl. Mater. Interfaces 2020, 12, 32493–32502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Yu, Y.; Yu, R.N.; Wan, M.; Zhang, R.Y.; Zhao, Y.D. Tracking the Down-Regulation of Folate Receptor-α in Cancer Cells through Target Specific Delivery of Quantum Dots Coupled with Antisense Oligonucleotide and Targeted Peptide. Small 2013, 9, 4183–4193. [Google Scholar] [CrossRef]
- Kim, M.W.; Jeong, H.Y.; Kang, S.J.; Jeong, I.H.; Choi, M.J.; You, Y.M.; Im, C.S.; Song, I.H.; Lee, T.S.; Lee, J.S.; et al. Anti-EGF Receptor Aptamer-Guided Co-Delivery of Anti-Cancer SiRNAs and Quantum Dots for Theranostics of Triple-Negative Breast Cancer. Theranostics 2019, 9, 837–852. [Google Scholar] [CrossRef]
- Kim, S.; Choi, Y.; Park, G.; Won, C.; Park, Y.J.; Lee, Y.; Kim, B.S.; Min, D.H. Highly Efficient Gene Silencing and Bioimaging Based on Fluorescent Carbon Dots in Vitro and in Vivo. Nano Res. 2017, 10, 503–519. [Google Scholar] [CrossRef]

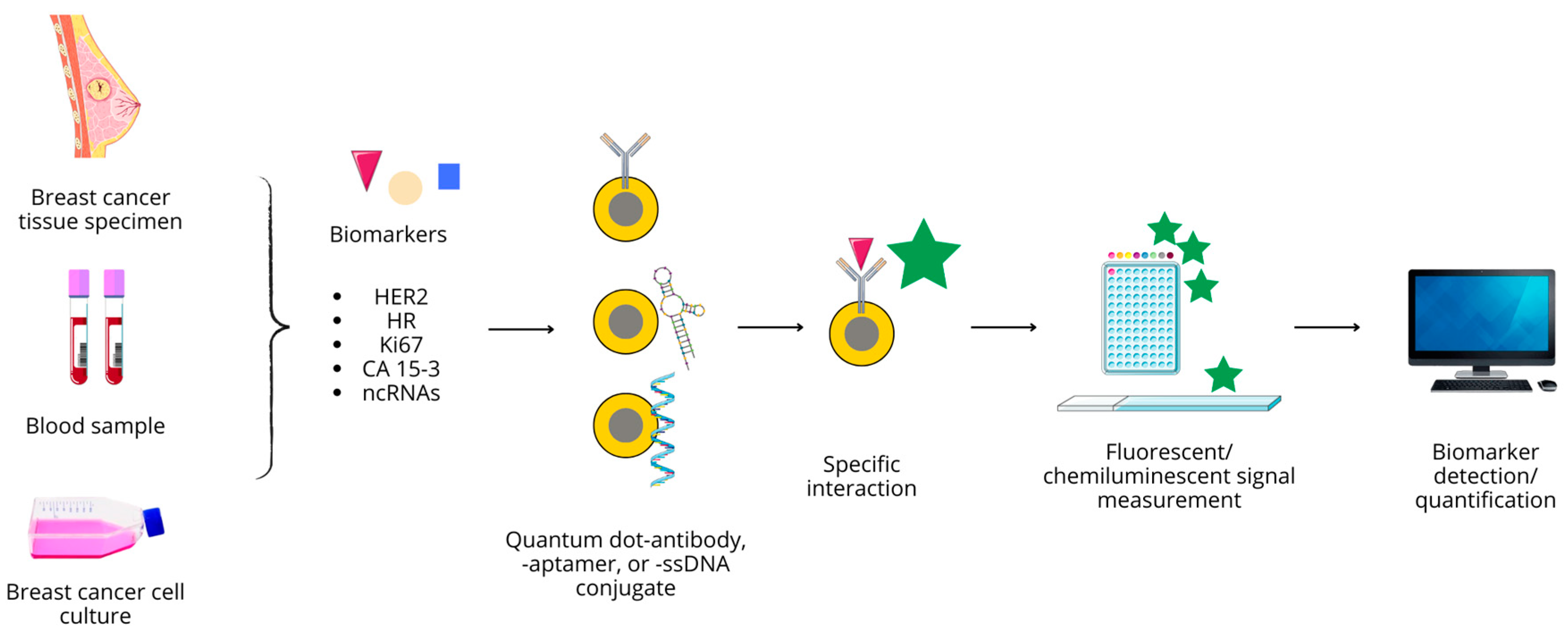
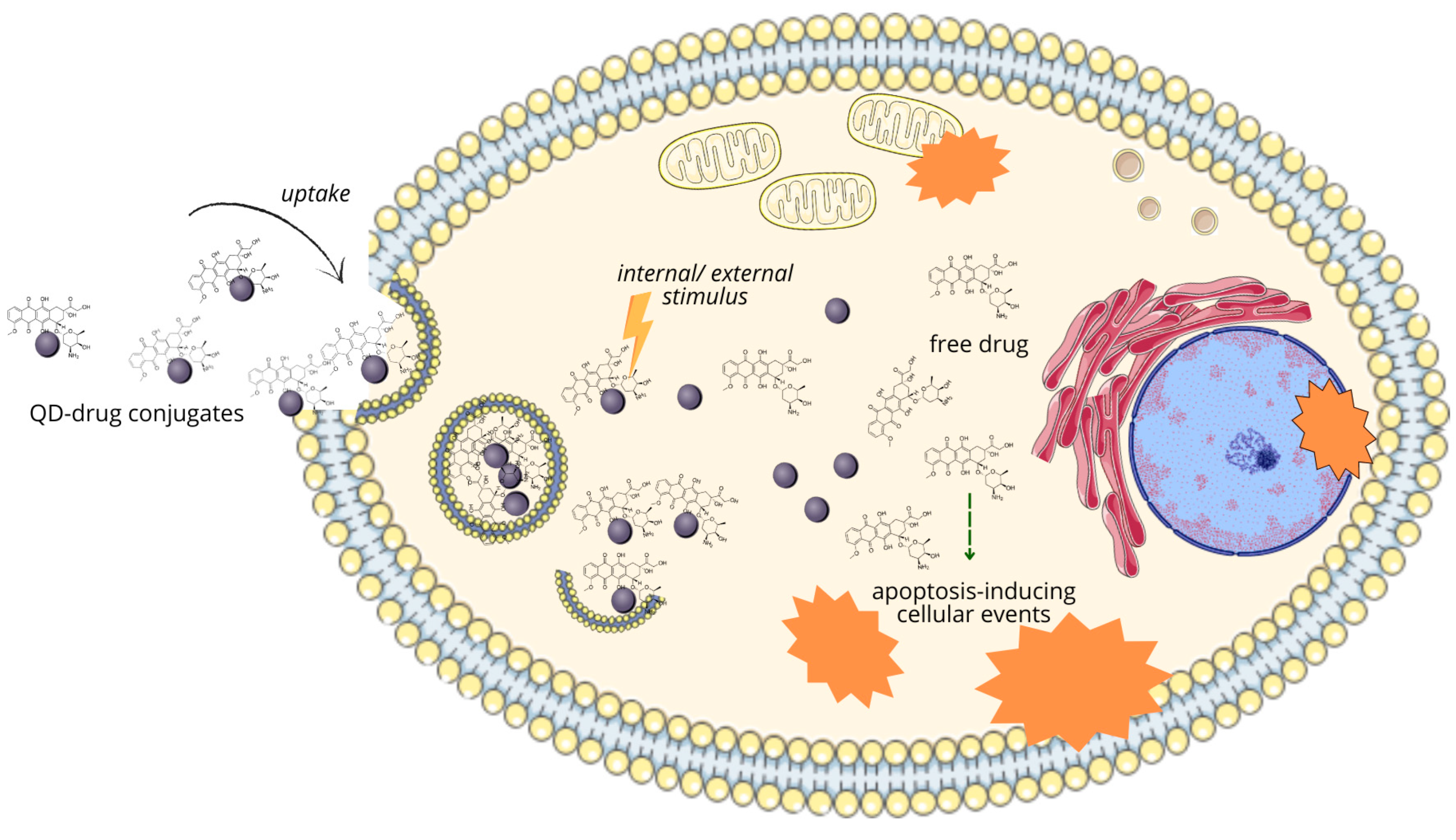
| Diagnostic Modality | Type of QDs | Modification/Conjugated Molecule | Size | Targeted Moiety/Ligand/Other | Targeted Cell Line/Organ/Other | Ref. |
|---|---|---|---|---|---|---|
| Antibody-conjugated QD probes | CdSe/ZnS QDs | biotinylated detection antibody | n/a | HER2-ECD | human serum | [164] |
| CdS QDs, cysteamine-capped | anti-CA 15-3 antibody | ~3 nm | CA 15-3 | human serum | [165] | |
| CuInS2/ZnS QDs | anti-Ki-67 monoclonal antibody | n/a | Ki67 | MDA-MB-231 and HMMECs | [169] | |
| InP/ZnS QDs | anti-EGFR antibody | 3–5 nm | EGFR | mouse breast cancer xenografts | [189] | |
| GQDs | single chain variable fragment of anti-EGFR antibody | <5 nm | EGFR | MDA-MB-231 | [190] | |
| QD–aptamer/QD–ssDNA conjugates and other miRNA probes | CdTe QDs | ATP-aptamer, mucin-1 aptamer-conjugated gold NPs | 2.8 nm | mucin 1 (MUC1) | MCF-7 | [172] |
| CQDs | complementary single-strand DNA (ssDNA) molecules | 3–8 nm | miR-21 | MCF-7 | [181] | |
| CdSe/ZnS QDs | 9 nm | miR-148, miR-21 | MCF-7, MDA-MB-231 | [183] | ||
| Antimonide QDs | n/a | n/a | miR-21, miR-155 | human serum | [184] | |
| AuNPs/GQDs/GO | miRNA capture probes | n/a | miR-21, miR-155, miR-210 | human serum | [185] | |
| Other | N-doped GQDs | phytohemagglutinin-L (PHA-L) | 3–12 nm | PHA-L | MCF-7 | [173] |
| N-doped GQDs | folic acid capping | GQDs: 10 nm, conjugate: 15 nm | folate receptor (FR) | MKN-45, HT-29, MCF-7 | [194] |
| Therapeutic Modality | Type of QDs Used | Conjugated Molecule | Size | Cell Line/Model | Role of QDs | Ref. |
|---|---|---|---|---|---|---|
| Photothermal therapy | tea polyphenol-derived CQDs | - | 1.3–3.7 nm | mouse xenografts of 4T1 cells | type I PS | [232] |
| polythiophene-derived GQDs | - | 2–6 nm | mouse breast cancer xenografts | type II PS | [233] | |
| N-doped GQDs/TiO2 NCs | - | 9.16 ± 2.4 nm | MDA-MB-231 and HS27 | type I/II PS | [234] | |
| ZnO QDs | - | n/a | MDA-MB-231 | type I PS | [237] | |
| Photodynamic therapy | CQD clathrates | MTX | 20–30 nm | HMLER (breast cancer stem cells) | PS and drug carrier | [235] |
| InP/ZnS QDs | chlorin e6 | 2–3 nm | MDA-MB-231 | carrier of a PS, enhancer of energy transfer | [236] | |
| Targeted drug delivery | Mg/N-modified CQDs, FA/HA-coated | epirubicin | 6–7 nm | 4T1, MCF-7 | delivery vehicles of chemotherapeutics to the tumor site, increasing their efficacy * | [252] |
| CQDs | DOX | 2–6 nm | MCF-7 | [253] | ||
| CQDs | DOX | CQDs: 0.5 nm, conjugates: 20 nm | MCF-7 | [254] | ||
| Tf-CQDs | DOX | 1.5 nm | MCF-7 | [243] | ||
| FA-coated CQDs | DOX | 1.5–8 nm | MDA-MB-231 | [255] | ||
| GQDs | DOX | 5 nm | MCF-7 | [256] | ||
| GQDs-cationic polymer | DOX | conjugates: <55 nm | mouse xenografts of 4T1 cells | [258] | ||
| CQDs | cisplatin | 5–8 nm | MDA-MB-231 | [259] | ||
| GQDs | cisplatin, anti-EGFR antibody | <5 nm | MDA-MB-231 | [194] | ||
| GQDs | cisplatin | 40 nm | MCF-7 | [260] | ||
| FA-coated, PEGylated GQDs | tamoxifen | 50–210 nm | MCF-7 | [262] | ||
| quinic acid-coated N-doped GQDs | gemcitabine | n/a | MCF-7 | [263] | ||
| Non-coding RNA/aptamer delivery | CdSe/ZnS QDs | HER2 siRNA, anti-HER2 antibodies | conjugates: 60–80 nm | SK-BR-3, MCF-7 | siRNA/drug delivery vehicles | [268] |
| CQDs | HER3 siRNA, trastuzumab | 4 nm | BT-474 | [269] | ||
| CdTe QDs | DOX, Bcl-2 siRNA | QDs: 26 nm, conjugates: 90 nm | MDA-MB-231 | [270] | ||
| CdSe/ZnS QDs | folate receptor siRNA | ~15 nm | MCF-7 | [271] | ||
| n/a | Bcl-2 and PKC siRNAs | n/a | MDA-MB-231, MDA-MB-453 | fluorescence generator for tracking purposes | [272] | |
| PEI-CQDs | VEGF siRNA | 3–7 nm | MDA-MB-231, mouse xenografts | siRNA delivery vehicles, protection from RNAses | [273] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunachowicz, D.; Kłosowska, K.; Sobczak, N.; Kepinska, M. Applicability of Quantum Dots in Breast Cancer Diagnostic and Therapeutic Modalities—A State-of-the-Art Review. Nanomaterials 2024, 14, 1424. https://doi.org/10.3390/nano14171424
Kunachowicz D, Kłosowska K, Sobczak N, Kepinska M. Applicability of Quantum Dots in Breast Cancer Diagnostic and Therapeutic Modalities—A State-of-the-Art Review. Nanomaterials. 2024; 14(17):1424. https://doi.org/10.3390/nano14171424
Chicago/Turabian StyleKunachowicz, Dominika, Karolina Kłosowska, Natalia Sobczak, and Marta Kepinska. 2024. "Applicability of Quantum Dots in Breast Cancer Diagnostic and Therapeutic Modalities—A State-of-the-Art Review" Nanomaterials 14, no. 17: 1424. https://doi.org/10.3390/nano14171424
APA StyleKunachowicz, D., Kłosowska, K., Sobczak, N., & Kepinska, M. (2024). Applicability of Quantum Dots in Breast Cancer Diagnostic and Therapeutic Modalities—A State-of-the-Art Review. Nanomaterials, 14(17), 1424. https://doi.org/10.3390/nano14171424






