A Biocompatible, Highly Sensitive, and Non-Enzymatic Glucose Electrochemical Sensor Based on a Copper-Cysteamine (Cu-Cy)/Chitosan-Modified Electrode
Abstract
:1. Introduction
2. Experimental Details
2.1. Chemicals
2.2. Instruments
2.3. Preparation of Cu-Cy-Modified Electrodes
2.4. Electrochemical Measurements
2.5. Analysis of Real Samples
3. Results and Discussion
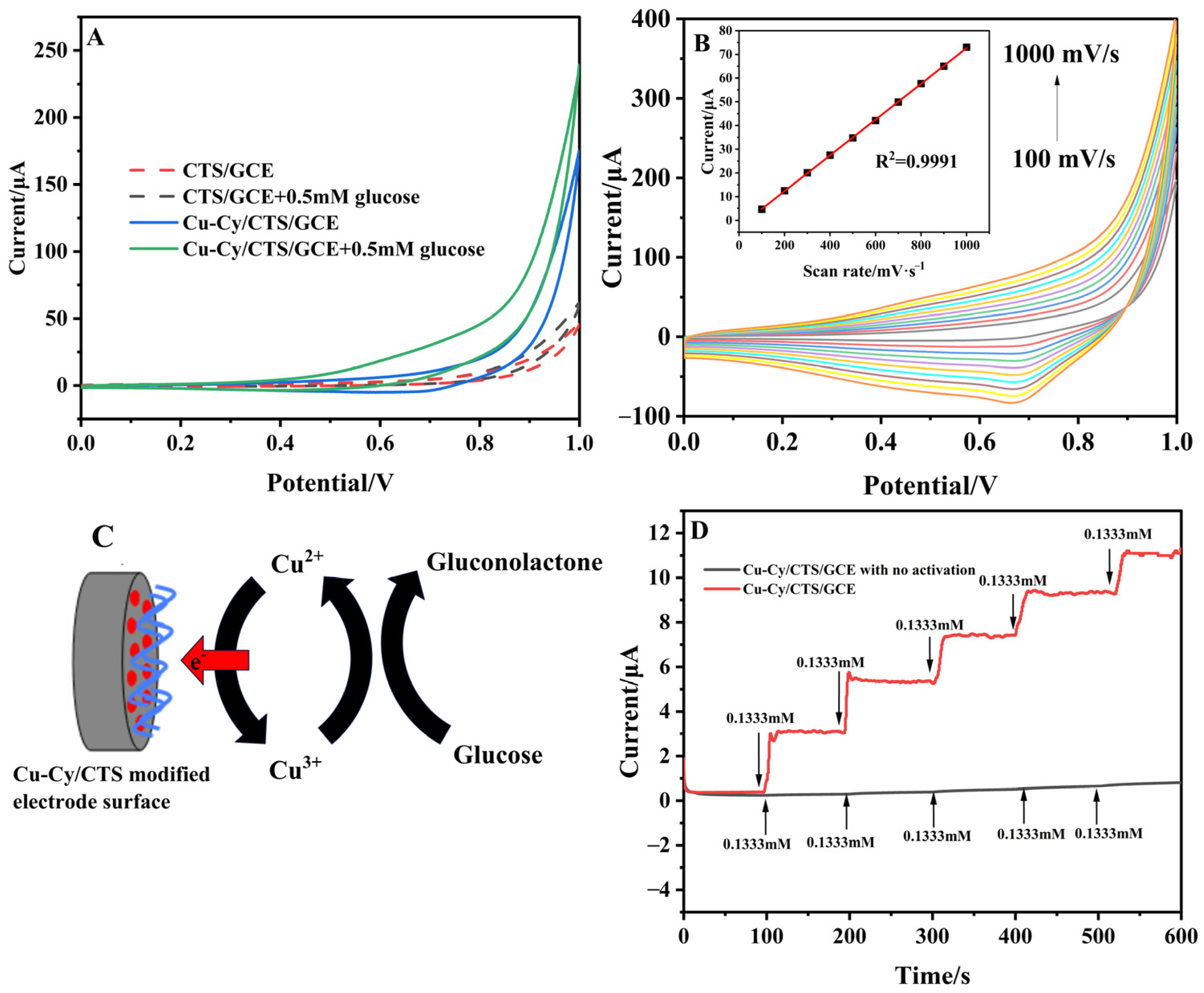
3.1. Characterization and Electrochemical Behaviors of Cu-Cy/CTS/GCE
3.2. Electrochemical Response of Cu-Cy/CTS/GCE to Glucose
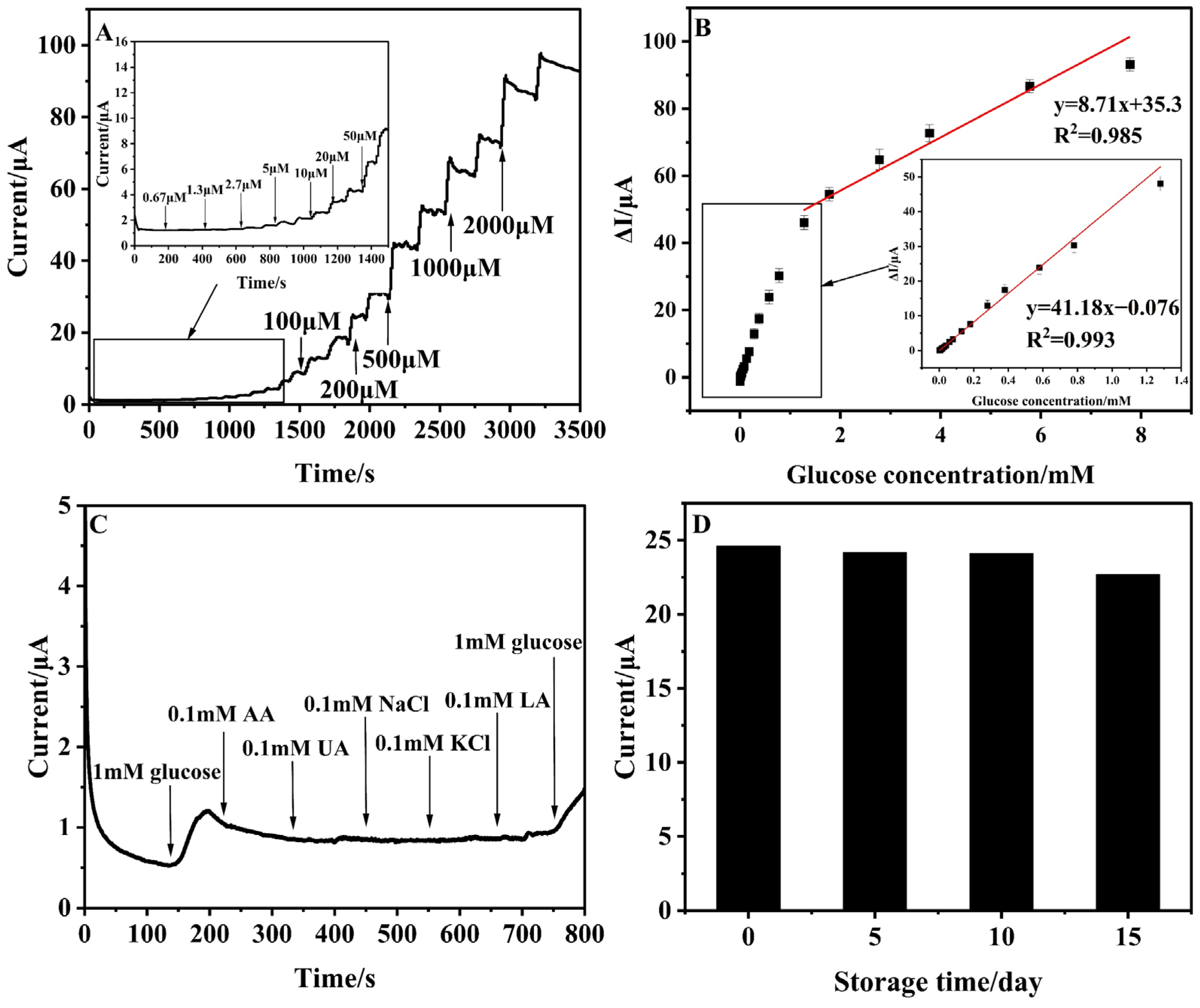
3.3. The Performance of the Amperometirc Glucose Sensors
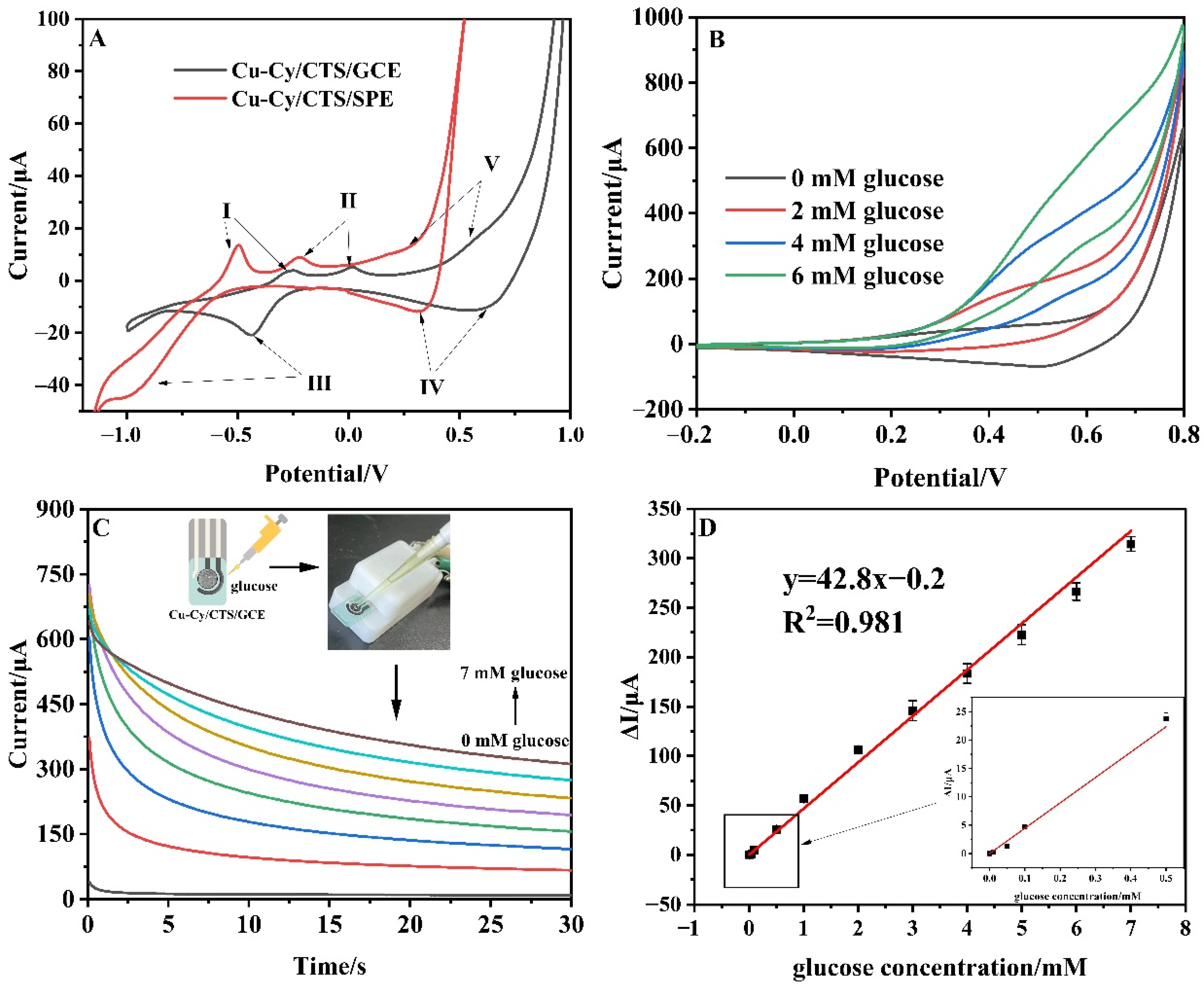
3.4. Analysis of Real-Life Samples
3.5. Portable Direct Detection of Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, C.X.; Xie, M.S.; Hong, F.; Chai, X.Y.; Mi, H.W.; Zhou, X.C.; Fan, L.D.; Zhang, Q.L.; Ngai, T.; Liu, J.H. A Highly Sensitive Glucose Biosensor Based on Gold Nanoparticles/Bovine Serum Albumin/FeO Biocomposite Nanoparticles. Electrochim. Acta 2016, 222, 1709–1715. [Google Scholar] [CrossRef]
- Park, S.; Kang, H.-J.; Jeon, J.-H.; Kim, M.-J.; Lee, I.-K. Recent advances in the pathogenesis of microvascular complications in diabetes. Arch. Pharmacal Res. 2019, 42, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Guo, Q.; Zhao, X.; Zhang, B.; Wang, M.; Dai, Y.; Qi, Y. The dependence of CuO morphology on different surfactants and its application for non-enzymatic glucose detection. Colloid. Surf. B 2021, 208, 112087. [Google Scholar] [CrossRef]
- Hawkes, M.; Conroy, A.L.; Opoka, R.O.; Namasopo, S.; Liles, W.C.; John, C.C.; Kain, K.C. Performance of Point-of-Care Diagnostics for Glucose, Lactate, and Hemoglobin in the Management of Severe Malaria in a Resource-Constrained Hospital in Uganda. Am. J. Trop. Med. Hyg. 2014, 90, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Setter, S.M.; Vue, M.H. Drug-Induced Glucose Alterations Part 2: Drug-Induced Hyperglycemia. Diabetes Spectr. 2011, 24, 234–238. [Google Scholar] [CrossRef]
- Shabnam, L.; Faisal, S.N.; Roy, A.K.; Haque, E.; Minett, A.I.; Gomes, V.G. Doped graphene/Cu nanocomposite: A high sensitivity non-enzymatic glucose sensor for food. Food Chem. 2017, 221, 751–759. [Google Scholar] [CrossRef]
- Mu, L.; Xu, Y.; Li, G.; Dai, S.; Tong, Q.; Liu, B. Determination of glucosamine and galactosamine in food by liquid chromatography with pre-column derivatization. Food Agric. Immunol. 2022, 33, 419–437. [Google Scholar] [CrossRef]
- Chaskar, U.M.; Paralikar, A.; Patil, S.L. Measurement of Blood Glucose Using Near Infrared Spectrophotometry. Int. J. Engg. Res. Indu. Appl. (IJERIA) 2010, 3, 237–246. [Google Scholar]
- Cao, K.; Jiang, X.; Yan, S.; Zhang, L.; Wu, W. Phenylboronic acid modified silver nanoparticles for colorimetric dynamic analysis of glucose. Biosens. Bioelectron. 2014, 52, 188–195. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, X.; Cao, Z.; Chen, S.; Zhu, B. Synthesis of mesoporous CuO microspheres with core-in-hollow-shell structure and its application for non-enzymatic sensing of glucose. J. Appl. Electrochem. 2015, 45, 131–138. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, Y.; Wang, H.; Hou, S. Gold nanoparticles decorated on single layer graphene applied for electrochemical ultrasensitive glucose biosensor. J. Electroanal. Chem. 2019, 855, 113495. [Google Scholar] [CrossRef]
- Zhu, H.; Li, L.; Zhou, W.; Shao, Z.; Chen, X. Advances in non-enzymatic glucose sensors based on metal oxides. J. Mater. Chem. B 2016, 4, 7333–7349. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ling, S.Q.; Lin, Z.H.; Wu, Y.X.; Bao, J.; Liu, X. Low-applied Potential Non-enzymatic Glucose Sensor Based on Ultrathin 2-D CuS Nanowall Arrays. Electroanal 2021, 33, 2204–2215. [Google Scholar] [CrossRef]
- Tian, K.; Baskaran, K.; Tiwari, A. Nonenzymatic glucose sensing using metal oxides—Comparison of CuO, Co3O4, and NiO. Vacuum 2018, 155, 696–701. [Google Scholar] [CrossRef]
- Hao, X.; Jia, J.; Chang, Y.; Jia, M.; Wen, Z. Monodisperse copper selenide nanoparticles for ultrasensitive and selective non-enzymatic glucose biosensor. Electrochim. Acta 2019, 327, 135020. [Google Scholar] [CrossRef]
- Ren, Z.; Mao, H.; Luo, H.; Liu, Y. Glucose sensor based on porous Ni by using a graphene bottom layer combined with a Ni middle layer. Carbon 2019, 149, 609–617. [Google Scholar] [CrossRef]
- Dayakar, T.; Vinodkumar, M.; Bikshalu, K.; Chakradhar, B. Novel synthesis and characterization of Ag@TiO core shell nanostructure for non-enzymatic glucose sensor. Appl. Surf. Sci. 2018, 435, 216–224. [Google Scholar]
- Zhao, Z.; Wang, T.; Li, K.; Long, D.; Zhao, J.; Zhu, F.; Gong, W. A flexible nonenzymatic sweat glucose sensor based on Au nanoflowers coated carbon cloth. Sens. Actuators B Chem. 2023, 388, 133798. [Google Scholar] [CrossRef]
- Luo, J.; Jiang, S.; Zhang, H.; Jiang, J.; Liu, X. A novel non-enzymatic glucose sensor based on Cu nanoparticle modified graphene sheets electrode. Anal. Chim. Acta 2012, 709, 47–53. [Google Scholar] [CrossRef]
- Gupta, P.; Gupta, V.K.; Huseinov, A.; Rahm, C.E.; Gazica, K.; Alvarez, N.T. Highly sensitive non-enzymatic glucose sensor based on carbon nanotube microelectrode set. Sens. Actuators B Chem. 2021, 348, 130688. [Google Scholar] [CrossRef]
- Sajadpour, M.; Siampour, H.; Abbasian, S.; Amiri, M.; Rameshan, R.; Rameshan, C.; Hajian, A.; Bagheri, H.; Moshaii, A. A Non-Enzymatic Glucose Sensor Based on the Hybrid Thin Films of Cu on Acetanilide/ITO. J. Electrochem. Soc. 2019, 166, B1116–B1125. [Google Scholar] [CrossRef]
- Sridara, T.; Upan, J.; Saianand, G.; Tuantranont, A.; Karuwan, C.; Jakmunee, J. Non-Enzymatic Amperometric Glucose Sensor Based on Carbon Nanodots and Copper Oxide Nanocomposites Electrode. Sensors 2020, 20, 808. [Google Scholar] [CrossRef]
- Ayaz, S.; Karakaya, S.; Emir, G.; Dilgin, D.G.; Dilgin, Y. A novel enzyme-free FI-amperometric glucose biosensor at Cu nanoparticles modified graphite pencil electrode. Microchem. J. 2020, 154, 104586. [Google Scholar] [CrossRef]
- Kolská, Z.; Řezníčková, A.; Nagyová, M.; Kasálková, N.S.; Sajdl, P.; Slepička, P.; Švorčík, V. Plasma activated polymers grafted with cysteamine improving surfaces cytocompatibility. Polym. Degrad. Stab. 2014, 101, 1–9. [Google Scholar] [CrossRef]
- Ma, L.; Chen, W.; Schatte, G.; Wang, W.; Joly, A.G.; Huang, Y.; Sammynaiken, R.; Hossu, M. A new Cu–cysteamine complex: Structure and optical properties. J. Mater. Chem. C 2014, 2, 4239–4246. [Google Scholar] [CrossRef]
- Pandey, N.K.; Chudal, L.; Phan, J.; Lin, L.; Johnson, O.; Xing, M.; Liu, J.P.; Li, H.; Huang, X.; Shu, Y.; et al. A facile method for the synthesis of copper–cysteamine nanoparticles and study of ROS production for cancer treatment. J. Mater. Chem. B 2019, 7, 6630–6642. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, X.; Ma, L.; Sahi, S.; Li, L.; Wang, X.; Wang, Q.; Chen, Y.; Chen, W.; Liu, Q. Nanosonosensitization by Using Copper–Cysteamine Nanoparticles Augmented Sonodynamic Cancer Treatment. Part. Part. Syst. Charact. 2018, 35, 1700378. [Google Scholar] [CrossRef]
- Poletti, F.; Favaretto, L.; Kovtun, A.; Treossi, E.; Corticelli, F.; Gazzano, M.; Palermo, V.; Zanardi, C.; Melucci, M. Electrochemical sensing of glucose by chitosan modified graphene oxide. J. Phys. Mater. 2020, 3, 014011. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Chenite, A.; Felt-Baeyens, O.; Mayer, J.; Gurny, R. Pseudo-thermosetting chitosan hydrogels for biomedical application. Int. J. Pharm. 2005, 288, 17–25. [Google Scholar] [CrossRef]
- Bocchetta, P.; Othman, A.; Gupta, M.; Andriani, G.; Martin, P.; Kumar, Y.; Joly, N.; Sacco, P.; Javed, M.S. Chitosan in electrochemical (bio)sensors: Nanostructuring and methods of synthesis. Eur. Polym. J. 2024, 213, 113092. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Bounegru, I. Chitosan-Based Electrochemical Sensors for Pharmaceuticals and Clinical Applications. Polymers 2023, 15, 3539. [Google Scholar] [CrossRef]
- Gu, T.; Xia, H.Q.; Hu, Y.; Jiang, Y. Electrochemical biosensor for polycyclic organic compounds screening based on a methylene nlue-incorporated DNA polyion complex modified electrode. Anal. Sci. 2018, 34, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Guo, H.; Wang, Z.; Long, Y.; Li, W.; Tu, Y. Au@CuO core-shell structure for high sensitive non-enzymatic glucose sensor. Sens. Actuat B-Chem. 2018, 255, 2510–2519. [Google Scholar] [CrossRef]
- Kihal, R.; Fisli, H.; Chelaghmia, M.L.; Drissi, W.; Boukharouba, C.; Abdi, S.; Nacef, M.; Affoune, A.M.; Pontié, M. A novel and ultrasensitive non-enzymatic electrochemical glucose sensor in real human blood samples based on facile one-step electrochemical synthesis of nickel hydroxides nanoparticles onto a three-dimensional Inconel 625 foam. J. Appl. Electrochem. 2023, 53, 315–329. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, H.; Gao, Y.; Huang, L.; Pan, H.; Du, M. Miniaturised Electrochemical Analyser for Glucose Determination Based on Chitosan/GOD/Electroreduced Graphene Oxide Sensor. Int. J. Electrochem. Sci. 2020, 15, 2458–2469. [Google Scholar] [CrossRef]
- Ma, L.; Zou, X.; Chen, W. A New X-Ray Activated Nanoparticle Photosensitizer for Cancer Treatment. J. Biomed. Nanotechnol. 2014, 10, 1501–1508. [Google Scholar] [CrossRef]
- Alam, M.M.; Howlader, M.M. Nonenzymatic electrochemical sensors via Cu native oxides (CuNOx) for sweat glucose monitoring. Sens. Bio-Sens. Res. 2021, 34, 100453. [Google Scholar] [CrossRef]
- Marioli, J.M.; Kuwana, T. Electrochemical characterization of carbohydrate oxidation at copper electrodes. Electrochim. Acta 1992, 37, 1187–1197. [Google Scholar] [CrossRef]
- Anand, V.K.; Bukke, A.; Bhatt, K.; Kumar, S.; Sharma, S.; Goyal, R.; Virdi, G.S. Highly sensitive and reusable Cu+2/polyaniline/reduced graphene oxide nanocomposite ink-based non-enzymatic glucose sensor. Appl. Phys. A 2020, 126, 500. [Google Scholar] [CrossRef]
- Wei, C.H.N.; Zou, X.; Liu, Q.M.; Li, S.X.; Kang, C.X.; Xiang, W. A highly sensitive non-enzymatic glucose sensor based on CuS nanosheets modified CuO/CuO nanowire arrays. Electrochim. Acta 2020, 334, 135630. [Google Scholar] [CrossRef]
- Toghill, K.E.; Compton, R.G. Electrochemical Non-enzymatic Glucose Sensors: A Perspective and an Evaluation. Int. J. Electrochem. Sci. 2010, 5, 1246–1301. [Google Scholar] [CrossRef]
- Heller, A.; Fedman, B. Electrochemical glucose sensors and their application in diabetes management, Applications of Electrochemistry in Medicine. Mod. Asp. Electrochem. 2013, 56, 121–187. [Google Scholar]
- Ghaffarirad, M.A.; Sabahi, A.; Golshani, Z.; Manteghi, F.; Ghaffarinejad, A. Non-enzymatic glucose electrochemical sensor based on nitrogen-doped graphene modified with polyaniline and Fe3O4@MIL-101-NH2 nano framework. Inorg. Chem. Commun. 2024, 159, 111812. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, L.; Xu, R.; Ma, Y.; Ma, L. Facile fabrication of biosensors based on Cu nanoparticles modified as-grown CVD graphene for non-enzymatic glucose sensing. J. Electroanal. Chem. 2019, 853, 113527. [Google Scholar] [CrossRef]
- Wu, L.; Lu, Z.; Ye, J. Enzyme-free glucose sensor based on layer-by-layer electrodeposition of multilayer films of multi-walled carbon nanotubes and Cu-based metal framework modified glassy carbon electrode. Biosens. Bioelectron. 2019, 135, 45–49. [Google Scholar] [CrossRef]
- Xu, G.-R.; Ge, C.; Liu, D.; Jin, L.; Li, Y.-C.; Zhang, T.-H.; Rahman, M.; Li, X.-B.; Kim, W. In-situ electrochemical deposition of dendritic Cu-Cu2S nanocomposites onto glassy carbon electrode for sensitive and non-enzymatic detection of glucose. J. Electroanal. Chem. 2019, 847, 113177. [Google Scholar] [CrossRef]
- Velmurugan, M.; Karikalan, N.; Chen, S.-M. Synthesis and characterizations of biscuit-like copper oxide for the non-enzymatic glucose sensor applications. J. Colloid Interface Sci. 2017, 493, 349–355. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, X.; Li, Z.; Zheng, X.; Zheng, J. Synthesis of CuO on AlOOH/reduced graphene oxide for non-enzymatic amperometric glucose sensing. Anal. Methods 2016, 8, 1527–1531. [Google Scholar] [CrossRef]
- Babu, K.J.; Sheet, S.; Lee, Y.S.; Kumar, G.G. Three-Dimensional Dendrite Cu–Co/Reduced Graphene Oxide Architectures on a Disposable Pencil Graphite Electrode as an Electrochemical Sensor for Nonenzymatic Glucose Detection. ACS Sustain. Chem. Eng. 2018, 6, 1909–1918. [Google Scholar] [CrossRef]
- Hassan, H.B.; Hamid, Z.A. Electrodeposited Cu-CuO Composite Films for Electrochemical Detection of Glucose. Int. J. Electrochem. Sci. 2011, 6, 5741–5758. [Google Scholar] [CrossRef]
- Zheng, Q.-Q.; Lu, Y.-C.; Ye, Z.-Z.; Ping, J.-F.; Wu, J.; Ying, Y.-B. An anti-passivation ink for the preparation of electrodes for use in electrochemical immunoassays. J. Zhejiang Univ.-Sci. B 2018, 19, 726–734. [Google Scholar] [CrossRef] [PubMed]



| Electrode Materials | Sensitivity (μA·mM−1·cm−2) | Linear Range (mM) | LOD (μM) | Ref. |
|---|---|---|---|---|
| Cu nanoparticles modified as-grown CVD graphene | 379.31 | 0.02–2.3 | 1.39 | [44] |
| Cu-MOF/MWCNTs/GCE | 3.878 | 0.50–11.84 | 0.40 | [45] |
| Cu-Cu2S/GCE | 5.02 | 0.10–500 | 0.33 | [46] |
| Cu2O biscuit/SPCE | 309 | 0.0005–4.03 | 0.1 | [47] |
| Cu2O/AlOOH/rGO | 155.1 | 0.005–14.77 | 2.6 | [48] |
| Cu-Co/rGO | 240 | 0.001–4 | 0.15 | [49] |
| Cu-Cy/CTS/GCE | 588.28/124.42 | 0.0027–1.3/ 1.3–7.7 | 0.28 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Gu, T.; Lv, L.; Chen, X.; Lu, Q.; Kotb, A.; Chen, W. A Biocompatible, Highly Sensitive, and Non-Enzymatic Glucose Electrochemical Sensor Based on a Copper-Cysteamine (Cu-Cy)/Chitosan-Modified Electrode. Nanomaterials 2024, 14, 1430. https://doi.org/10.3390/nano14171430
Chen H, Gu T, Lv L, Chen X, Lu Q, Kotb A, Chen W. A Biocompatible, Highly Sensitive, and Non-Enzymatic Glucose Electrochemical Sensor Based on a Copper-Cysteamine (Cu-Cy)/Chitosan-Modified Electrode. Nanomaterials. 2024; 14(17):1430. https://doi.org/10.3390/nano14171430
Chicago/Turabian StyleChen, Huan, Tingting Gu, Longyang Lv, Xing Chen, Qifeng Lu, Amer Kotb, and Wei Chen. 2024. "A Biocompatible, Highly Sensitive, and Non-Enzymatic Glucose Electrochemical Sensor Based on a Copper-Cysteamine (Cu-Cy)/Chitosan-Modified Electrode" Nanomaterials 14, no. 17: 1430. https://doi.org/10.3390/nano14171430
APA StyleChen, H., Gu, T., Lv, L., Chen, X., Lu, Q., Kotb, A., & Chen, W. (2024). A Biocompatible, Highly Sensitive, and Non-Enzymatic Glucose Electrochemical Sensor Based on a Copper-Cysteamine (Cu-Cy)/Chitosan-Modified Electrode. Nanomaterials, 14(17), 1430. https://doi.org/10.3390/nano14171430








