Comparative Study of Cu Ion Adsorption by Nano-Hydroxyapatite Powder Synthesized from Chemical Reagents and Clam Shell-Derived Calcium Sources
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Chem-HA and Bio-HA Adsorbents
2.2. Preparation of a Cu Ion Solution
2.3. UV-VIS Spectroscopy Analysis
2.4. Structural and Morphological Observation
2.5. Adsorption Kinetics
2.6. Adsorption Isotherms
2.7. Adsorption Thermodynamics
3. Results and Discussion
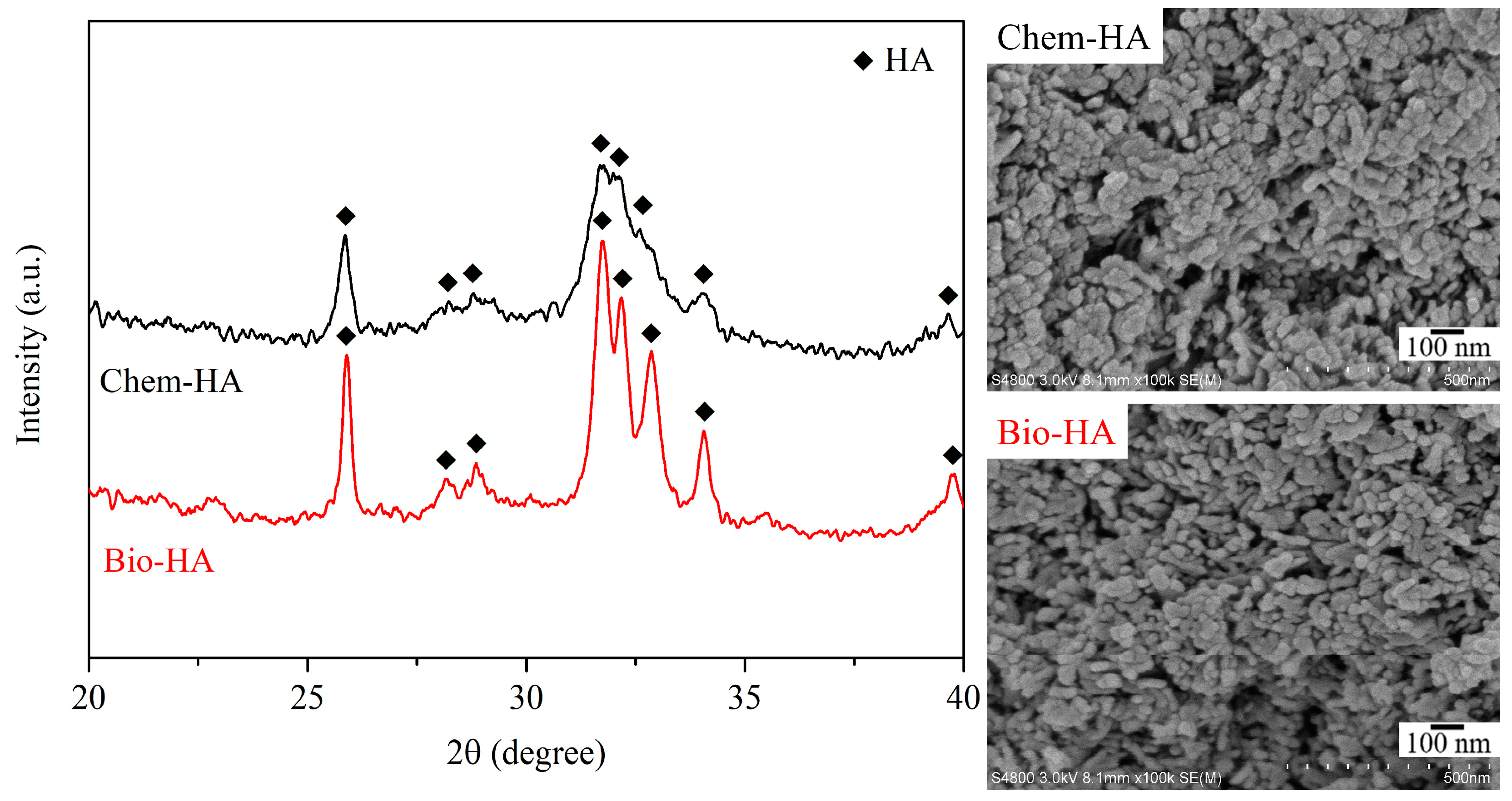
3.1. Structural and Morphological Analysis of Chem-HA and Bio-HA Powders before and after Cu Ion Adsorption
3.2. Adsorption Kinetics Models
3.3. Langmuir and Freundlich Isotherm Models
3.4. Thermodynamic Analysis
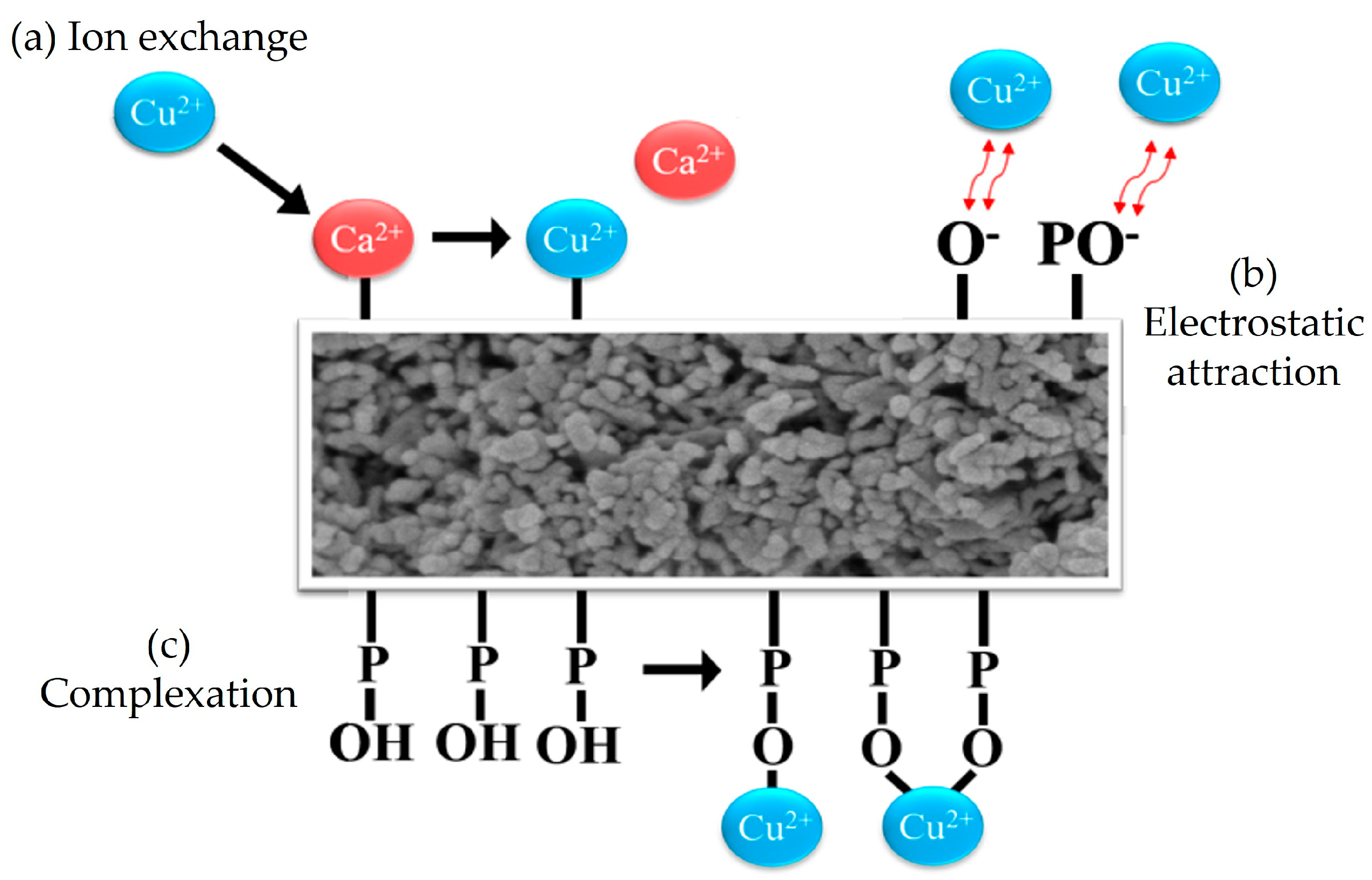
3.5. Adsorption Mechanisms
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayach, J.; El Malti, W.; Duma, L.; Lalevée, J.; Al Ajami, M.; Hamad, H.; Hijazi, A. Comparing Conventional and Advanced Approaches for Heavy Metal Removal in Wastewater Treatment: An In-Depth Review Emphasizing Filter-Based Strategies. Polymers 2024, 16, 1959. [Google Scholar] [CrossRef] [PubMed]
- Al-Amrani, W.A.; Onaizi, S.A. Adsorptive removal of heavy metals from wastewater using emerging nanostructured materials: A state-of-the-art review. Sep. Purif. Technol. 2024, 343, 127018. [Google Scholar] [CrossRef]
- Robledo Ardila, P.A.; Álvarez-Alonso, R.; Árcega-Cabrera, F.; Durán Valsero, J.J.; Morales García, R.; Lamas-Cosío, E.; Oceguera-Vargas, I.; DelValls, A. Assessment and Review of Heavy Metals Pollution in Sediments of the Mediterranean Sea. Appl. Sci. 2024, 14, 1435. [Google Scholar] [CrossRef]
- Bunani, S.; Abbt-Braun, G.; Horn, H. Heavy Metal Removal from Aqueous Solutions Using a Customized Bipolar Membrane Electrodialysis Process. Molecules 2024, 29, 1754. [Google Scholar] [CrossRef]
- Alvizuri-Tintaya, P.A.; d’Abzac, P.; Lo-Iacono-Ferreira, V.G.; Torregrosa-López, J.I.; Lora-García, J. Zinc Recovery from a Water Supply by Reverse Osmosis Operated at Low Pressures: Looking for Sustainability in Water Treatment Advanced Processes. Membranes 2024, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Asiminicesei, D.-M.; Fertu, D.I.; Gavrilescu, M. Impact of Heavy Metal Pollution in the Environment on the Metabolic Profile of Medicinal Plants and Their Therapeutic Potential. Plants 2024, 13, 913. [Google Scholar] [CrossRef]
- Suljkanović, M.; Suljagić, J.; Bjelić, E.; Prkić, A.; Bošković, P. Chemical Characterization of Terpene-Based Hydrophobic Eutectic Solvents and Their Application for Pb(II) Complexation during Solvent Extraction Procedure. Molecules 2024, 29, 2122. [Google Scholar] [CrossRef]
- Mai, N.T.; Van Thanh, D.; Hien, T.N.; Hanh, H.T.H.; Hoa, L.T.T.; Khai, N.M.; Bich, D.D.; Nguyen, D.D.; Tan, C.M.; Van Hao, P. Highly adsorptive removal of heavy metal, dye, and antibiotic pollutants using functionalized graphene nanosheets sono-electrochemically derived from graphitic waste. J. Environ. Chem. Eng. 2024, 12, 113020. [Google Scholar] [CrossRef]
- Rostami, M.S.; Khodaei, M.M. Recent Advances in Chitosan-Based Nanocomposites for Adsorption and Removal of Heavy Metal Ions. Int. J. Biol. Macromol. 2024, 270, 132386. [Google Scholar] [CrossRef] [PubMed]
- Ofiera, L.M.; Bose, P.; Kazner, C. Removal of Heavy Metals and Bulk Organics towards Application in Modified Constructed Wetlands Using Activated Carbon and Zeolites. Water 2024, 16, 511. [Google Scholar] [CrossRef]
- Huang, T.; Cao, Z.; Jin, J.; Zhou, L.; Zhang, S.; Liu, L. Hydroxyapatite nanoparticle functionalized activated carbon particle electrode that removes strontium from spiked soils in a unipolar three-dimensional electrokinetic system. J. Environ. Manag. 2021, 280, 111697. [Google Scholar] [CrossRef] [PubMed]
- Bazargan-Lari, R.; Zafarani, H.R.; Bahrololoom, M.E.; Nemati, A. Removal of Cu(II)ions from aqueous solutions by low-cost natural hydroxyapatite/chitosan composite: Equilibrium, kinetic and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 2014, 45, 1642–1648. [Google Scholar] [CrossRef]
- Cruz-Briano, S.A.; Medellin-Castillo, N.A.; Delgado-Sanchez, P.; Castro-Larragoitia, G.J.; Leyva-Ramos, R.; Cortina-Rangel, M.A.; Labrada-Delgado, G.J.; Villela-Martinez, D.E.; Flores-Rojas, A.I.; Gonzalez-Fernandez, L.A.; et al. Binary fluoride and As(V) adsorption in water using pleco fish bone chars. Environ. Sci. Pollut. Res. 2024, 31, 40156–40173. [Google Scholar] [CrossRef] [PubMed]
- Viotti, P.; Marzeddu, S.; Antonucci, A.; Décima, M.A.; Lovascio, P.; Tatti, F.; Boni, M.R. Biochar as Alternative Material for Heavy Metal Adsorption from Groundwaters: Lab-Scale (Column) Experiment Review. Materials 2024, 17, 809. [Google Scholar] [CrossRef]
- Hsieh, K.-H.; Hsu, H.-C.; Kao, Y.-L.; Wu, S.-C.; Yang, T.-Y.; Ho, W.-F. Nanohydroxyapatite/Peptide Composite Coatings on Pure Titanium Surfaces with Nanonetwork Structures Using Oyster Shells. Nanomaterials 2024, 14, 577. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wang, W.; Yang, H.; Ye, B.C. Hydrothermal synthesis of hierarchical hollow hydroxyapatite microspheres with excellent fluoride adsorption property. Micropor. Mesopor. Mater. 2019, 289, 109620. [Google Scholar] [CrossRef]
- El-Maghrabi, H.; Younes, A.A.; Salem, A.; Rabie, K.; El-Shereafy, E.-S. Magnetically modified hydroxyapatite nanoparticles for the removal of uranium (VI): Preparation, characterization and adsorption optimization. J. Hazard. Mater. 2019, 378, 120703. [Google Scholar] [CrossRef]
- Wang, T.; Cao, W.; Dong, K.; Li, H.; Wang, D.; Xu, Y. Hydroxyapatite and its composite in heavy metal decontamination: Adsorption mechanisms, challenges, and future perspective. Chemosphere 2024, 352, 141367. [Google Scholar] [CrossRef]
- Pereira, M.B.B.; França, D.B.; Araújo, R.C.; Silva Filho, E.C.; Rigaud, B.; Fonseca, M.G.; Jaber, M. Amino hydroxyapatite/chitosan hybrids reticulated with glutaraldehyde at different pH values and their use for diclofenac removal. Carbohydr. Polym. 2020, 236, 116036. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Cocoletzi, H.; Salinas, R.A.; Águila-Almanza, E.; Rubio-Rosas, E.; Chai, W.S.; Chew, K.W.; Mariscal-Hernández, C.; Show, P.L. Natural hydroxyapatite from fishbone waste for the rapid adsorption of heavy metals of aqueous effluent. Environ. Technol. Innov. 2020, 20, 101109. [Google Scholar] [CrossRef]
- Kekes, T.; Tzia, C. Adsorption of indigo carmine on functional chitosan and β-cyclodextrin/chitosan beads: Equilibrium, kinetics and mechanism studies. J. Environ. Manag. 2020, 262, 110372. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, M.; Jin, Z.; Wang, G.; Li, R.; Zhang, X.; Wang, H. Characterization of biochars derived from various spent mushroom substrates and evaluation of their adsorption performance of Cu (II) ions from aqueous solution. Environ. Res. 2021, 196, 110323. [Google Scholar] [CrossRef]
- Yang, L.; Zhong, W.; Cui, J.; Wei, Z.; Wei, W. Enhanced removal of Cu (II) ions from aqueous solution by poorly crystalline hydroxyapatite nanoparticles. J. Dispers. Sci. Technol. 2016, 37, 956–968. [Google Scholar] [CrossRef]
- Ayodele, O.; Olusegun, S.J.; Oluwasina, O.O.; Okoronkwo, E.A.; Olanipekun, E.O.; Mohallem, N.D.S.; Guimarães, W.G.; Gomes, B.L.F.M.; Souza, G.O.; Duarte, H.A. Experimental and theoretical studies of the adsorption of Cu and Ni ions from wastewater by hydroxyapatite derived from eggshells. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100439. [Google Scholar] [CrossRef]
- Yang, L.; Wei, Z.; Zhong, W.; Cui, J.; Wei, W. Modifying hydroxyapatite nanoparticles with humic acid for highly efficient removal of Cu (II) from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 9–21. [Google Scholar] [CrossRef]
- Wu, S.-C.; Hsu, H.-C.; Wu, W.-H.; Ho, W.-F. Enhancing Bioactivity and Mechanical Properties of Nano-Hydroxyapatite Derived from Oyster Shells through Hydrothermal Synthesis. Nanomaterials 2024, 14, 1281. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-C.; Hsu, H.-C.; Wang, H.-F.; Liou, S.-P.; Ho, W.-F. Synthesis and Characterization of Nano-Hydroxyapatite Obtained from Eggshell via the Hydrothermal Process and the Precipitation Method. Molecules 2023, 28, 4926. [Google Scholar] [CrossRef]
- Wu, S.C.; Hsu, H.C.; Liu, M.Y.; Ho, W.F. Characterization of nanosized hydroxyapatite prepared by an aqueous precipitation method using eggshells and mulberry leaf extract. J. Korean Ceram. Soc. 2021, 58, 116–122. [Google Scholar] [CrossRef]
- Wu, S.-C.; Hsu, H.-C.; Yu, H.C.; Shen, C.E.; Ho, W.-F. Preparation and evaluation of osteoinductive porous biphasic calcium phosphate granules obtained from eggshell for bone tissue engineering. Adv. Powder Technol. 2023, 34, 103909. [Google Scholar] [CrossRef]
- Wu, S.-C.; Kao, Y.L.; Lu, Y.C.; Hsu, H.-C.; Ho, W.-F. Preparation and characterization of microrod hydroxyapatite bundles obtained from oyster shells through microwave irradiation. J. Aust. Ceram. Soc. 2021, 57, 1541–1551. [Google Scholar] [CrossRef]
- Jiang, J.; Long, Y.; Hu, X.; Hu, J.; Zhu, M.; Zhou, S. A facile microwave-assisted synthesis of mesoporous hydroxyapatite as an efficient adsorbent for Pb2+ adsorption. J. Solid State Chem. 2020, 289, 121491. [Google Scholar] [CrossRef]
- Zengin, H.B.; Gürkan, R. Application of a novel poly(SMAm)-Tris-Fe3O4 nanocomposite for selective extraction and enrichment of Cu(I)/Cu(II) from beer, soft drinks and wine samples, and speciation analysis by micro-volume UV–Vis spectrophotometry. Talanta 2021, 224, 121789. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.H.; Deng, R.R.; Gao, Y.K.; Qin, Y.; Liu, X.L. Measuring absorption coefficient spectrum (400–900 nm) of copper ions in water. J. Remote Sens. 2016, 20, 27–34. [Google Scholar] [CrossRef]
- Mobasherpour, I.; Salahi, E.; Pazouki, M. Comparative of the removal of Pb2+, Cd2+ and Ni2+ by nano crystallite hydroxyapatite from aqueous solutions: Adsorption isotherm study. Arab. J. Chem. 2012, 5, 439–446. [Google Scholar] [CrossRef]
- Pooladi, A.; Bazargan-Lari, R. Simultaneous removal of copper and zinc ions by Chitosan/Hydroxyapatite/nano-Magnetite composite. J. Mater. Res. Technol. 2020, 9, 14841–14852. [Google Scholar] [CrossRef]
- Othmani, M.; Bachoua, H.; Ghandour, Y.; Aissa, A.; Debbabi, M. Synthesis, characterization and catalytic properties of copper-substituted hydroxyapatite nanocrystals. Mater. Res. Bull. 2018, 97, 560–566. [Google Scholar] [CrossRef]
- Guo, J.; Han, Y.; Mao, Y.; Wickramaratne, M.N. Influence of alginate fixation on the adsorption capacity of hydroxyapatite nanocrystals to Cu2+ ions. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 801–807. [Google Scholar] [CrossRef]
- Wang, Y.J.; Chen, J.H.; Cui, Y.X.; Wang, S.Q.; Zhou, D.M. Effects of low-molecular-weight organic acids on Cu (II) adsorption onto hydroxyapatite nanoparticles. J. Hazard. Mater. 2009, 162, 1135–1140. [Google Scholar] [CrossRef]
- Su, Y.P.; Wang, J.; Li, S.; Zhu, J.H.; Liu, W.S.; Zhang, Z.T. Self-templated microwave-assisted hydrothermal synthesis of two-dimensional holey hydroxyapatite nanosheets for efficient heavy metal removal. Environ. Sci. Pollut. Res. 2019, 26, 30076–30086. [Google Scholar] [CrossRef]
- Rosskopfová, O.; Galamboš, M.; Ometáková, J.; Čaplovičová, M.; Rajec, P. Study of sorption processes of copper on synthetic hydroxyapatite. J. Radioanal. Nucl. Chem. 2012, 293, 641–647. [Google Scholar] [CrossRef]
- Wang, H.; Yan, K.; Xing, H.; Chen, J.; Lu, R. Effective removal of Cu2+ from aqueous solution by synthetic abalone shell hydroxyapatite microspheres adsorbent. Environ. Technol. Innov. 2021, 23, 101663. [Google Scholar] [CrossRef]
- Núñez, D.; Serrano, J.A.; Mancisidor, A.; Elgueta, E.; Varaprasad, K.; Oyarzún, P.; Cáceres, R.; Idea, W.; Rivas, B. Heavy metal removal from aqueous systems using hydroxyapatite nanocrystals derived from clam shells. RSC Adv. 2019, 9, 22883–22890. [Google Scholar] [CrossRef] [PubMed]
- Ngueagni, P.T.; Woumfo, E.D.; Kumar, P.S.; Siéwé, M.; Vieillard, J.; Brun, N.; Nkuigue, P.F. Adsorption of Cu (II) ions by modified horn core: Effect of temperature on adsorbent preparation and extended application in river water. J. Mol. Liq. 2020, 298, 112023. [Google Scholar] [CrossRef]
- Trang, S.; Nguyen, C.; Hoang, N.; Pham, T.; Pham, A.; Pham, V.; Nguyen, V. Valorization of fish and shrimp wastes to nano-hydroxyapatite/chitosan biocomposite for wastewater treatment. J. Sci. Adv. Mater. Device 2022, 7, 100485. [Google Scholar] [CrossRef]
- Jung, K.W.; Lee, S.Y.; Choi, J.W.; Lee, Y.J. A facile one-pot hydrothermal synthesis of hydroxyapatite/biochar nanocomposites: Adsorption behavior and mechanisms for the removal of copper (II) from aqueous media. Chem. Eng. J. 2019, 369, 529–541. [Google Scholar] [CrossRef]
- Arokiasamy, P.; Abdullah, M.M.A.B.; Abd Rahim, S.Z.; Sandu, A.V.; Fedrigo, A.F.; Ediati, R.E.; Ishak, S.; Kaus, N.H.M. Hydroxyapatite incorporated metakaolin/sludge based geopolymer adsorbent for copper ions and ciprofloxacin removal: Synthesis, characterization and mechanisms. Arab. J. Chem. 2024, 17, 105745. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Saber-Samandari, S.; Gazi, M. Cellulose-graft-polyacrylamide/hydroxyapatite composite hydrogel with possible application in removal of Cu (II) ions. React. Funct. Polym. 2013, 73, 1523–1530. [Google Scholar] [CrossRef]
- Thanh, D.N.; Novák, P.; Vejpravova, J.; Vu, H.N.; Lederer, J.; Munshi, T. Removal of copper and nickel from water using nanocomposite of magnetic hydroxyapatite nanorods. J. Magn. Magn. Mater. 2018, 456, 451–460. [Google Scholar] [CrossRef]

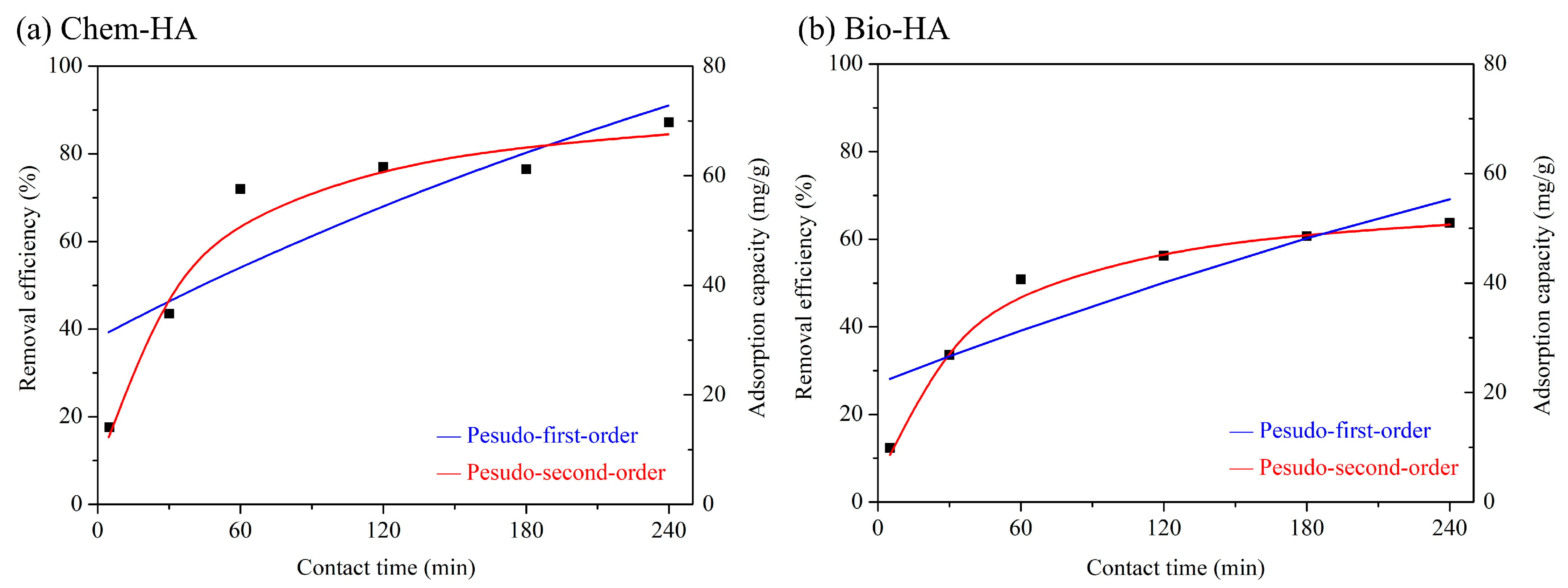
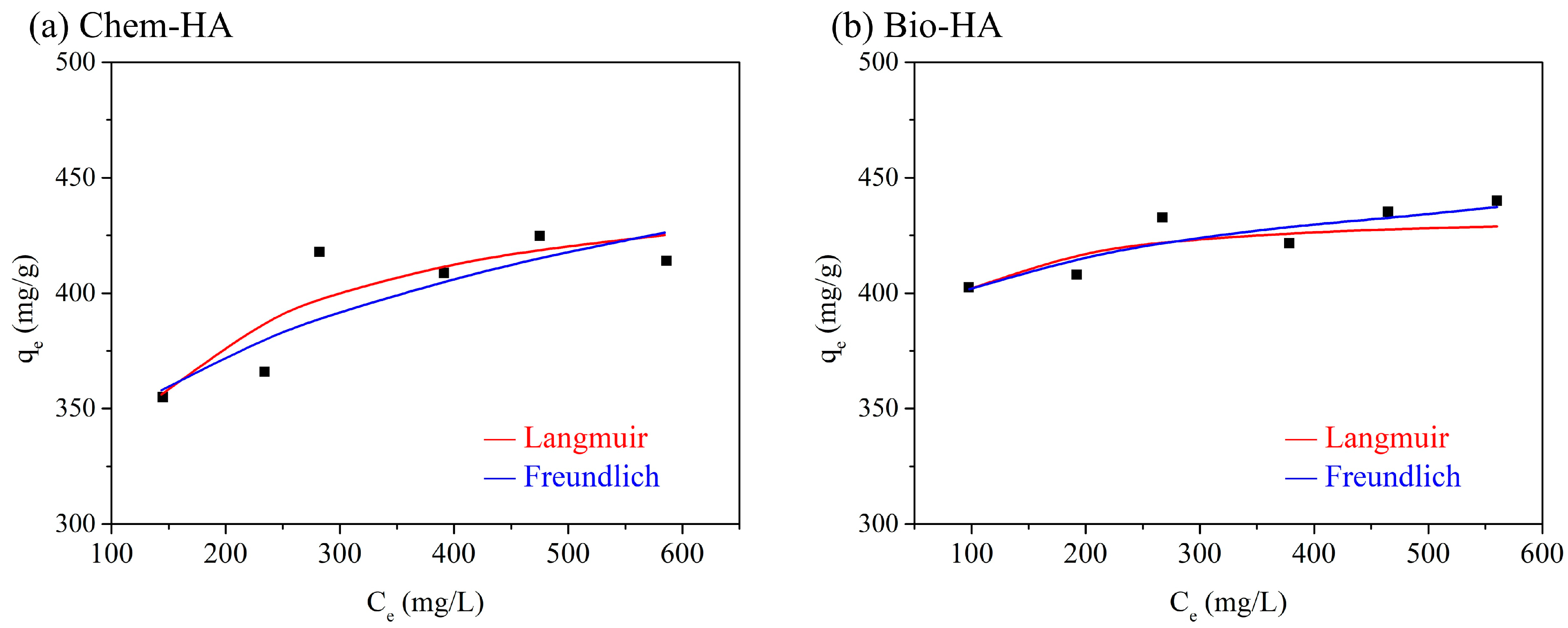
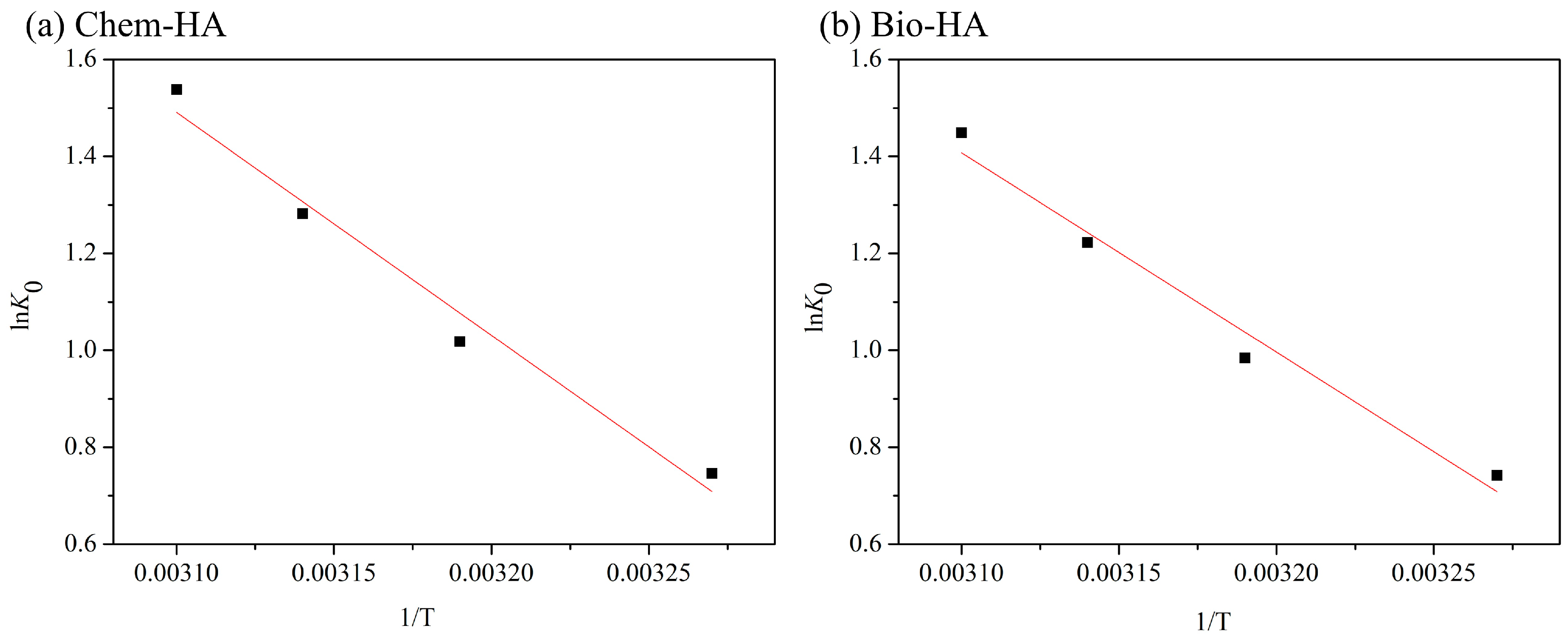
| Sample | Pesudo-First-Order Kinetic Model | Pesudo-Second-Order Kinetic Model | ||
|---|---|---|---|---|
| k1 (1/min) | R2 | k2 (g/mg·min) | R2 | |
| Chem-HA | 2.41 × 10−3 | 0.756 | 5.16 × 10−4 | 0.989 |
| Bio-HA | 1.60 × 10−3 | 0.779 | 6.39 × 10−4 | 0.998 |
| Sample | Langmuir Isotherm Model | Freundlich Isotherm Model | ||||
|---|---|---|---|---|---|---|
| qe (mg/g) | KL (L/mg) | R2 | KF L1/n·mg(1−1/n)·g−1 | 1/n | R2 | |
| Chem-HA | 426.6 | 0.0251 | 0.9961 | 192.4 | 0.125 | 0.7037 |
| Bio-HA | 428.5 | 0.1229 | 0.9989 | 317.9 | 0.0507 | 0.8083 |
| Adsorbent | qm (mg/g) | References |
|---|---|---|
| Chem-HA | 426.7 | This study |
| Bio-HA | 436.8 | This study |
| Synthetic HA | 31.6 | [39] |
| Synthetic HA | 29.2 | [40] |
| Synthetic HA | 517.1 | [41] |
| Synthetic HA | 55.9 | [42] |
| Natural HA | 100.0 | [43] |
| Natural HA | 10.6 | [24] |
| Nano-HA/chitosan biocomposites | 237.0 | [44] |
| HA/biochar nanocomposites | 99.0 | [45] |
| HA/geopolymer | 13.7 | [46] |
| HA/cellulose-g-poly(acrylamide) | 175.0 | [47] |
| HA-bound Fe3O4 magnetic nanoparticles | 48.8 | [48] |
| T (K) | ∆G (kJ/mol) | ∆H° (kJ/mol) | ∆S° (J/mol·K) |
|---|---|---|---|
| Chem-HA | |||
| 308 | –1.91 | 43.77 | 148.30 |
| 313 | –2.65 | ||
| 318 | –3.39 | ||
| 323 | –4.13 | ||
| Bio-HA | |||
| 308 | –1.90 | 39.00 | 132.80 |
| 313 | –2.56 | ||
| 318 | –3.23 | ||
| 323 | –3.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.-C.; Hsu, H.-C.; Ji, H.-Y.; Ho, W.-F. Comparative Study of Cu Ion Adsorption by Nano-Hydroxyapatite Powder Synthesized from Chemical Reagents and Clam Shell-Derived Calcium Sources. Nanomaterials 2024, 14, 1431. https://doi.org/10.3390/nano14171431
Wu S-C, Hsu H-C, Ji H-Y, Ho W-F. Comparative Study of Cu Ion Adsorption by Nano-Hydroxyapatite Powder Synthesized from Chemical Reagents and Clam Shell-Derived Calcium Sources. Nanomaterials. 2024; 14(17):1431. https://doi.org/10.3390/nano14171431
Chicago/Turabian StyleWu, Shih-Ching, Hsueh-Chuan Hsu, Hong-Yi Ji, and Wen-Fu Ho. 2024. "Comparative Study of Cu Ion Adsorption by Nano-Hydroxyapatite Powder Synthesized from Chemical Reagents and Clam Shell-Derived Calcium Sources" Nanomaterials 14, no. 17: 1431. https://doi.org/10.3390/nano14171431






