Comprehensive Utilization of Formation Water Scale to Prepare Controllable Size CaCO3 Nanoparticles: A New Method to Improve Oil Recovery
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Instruments
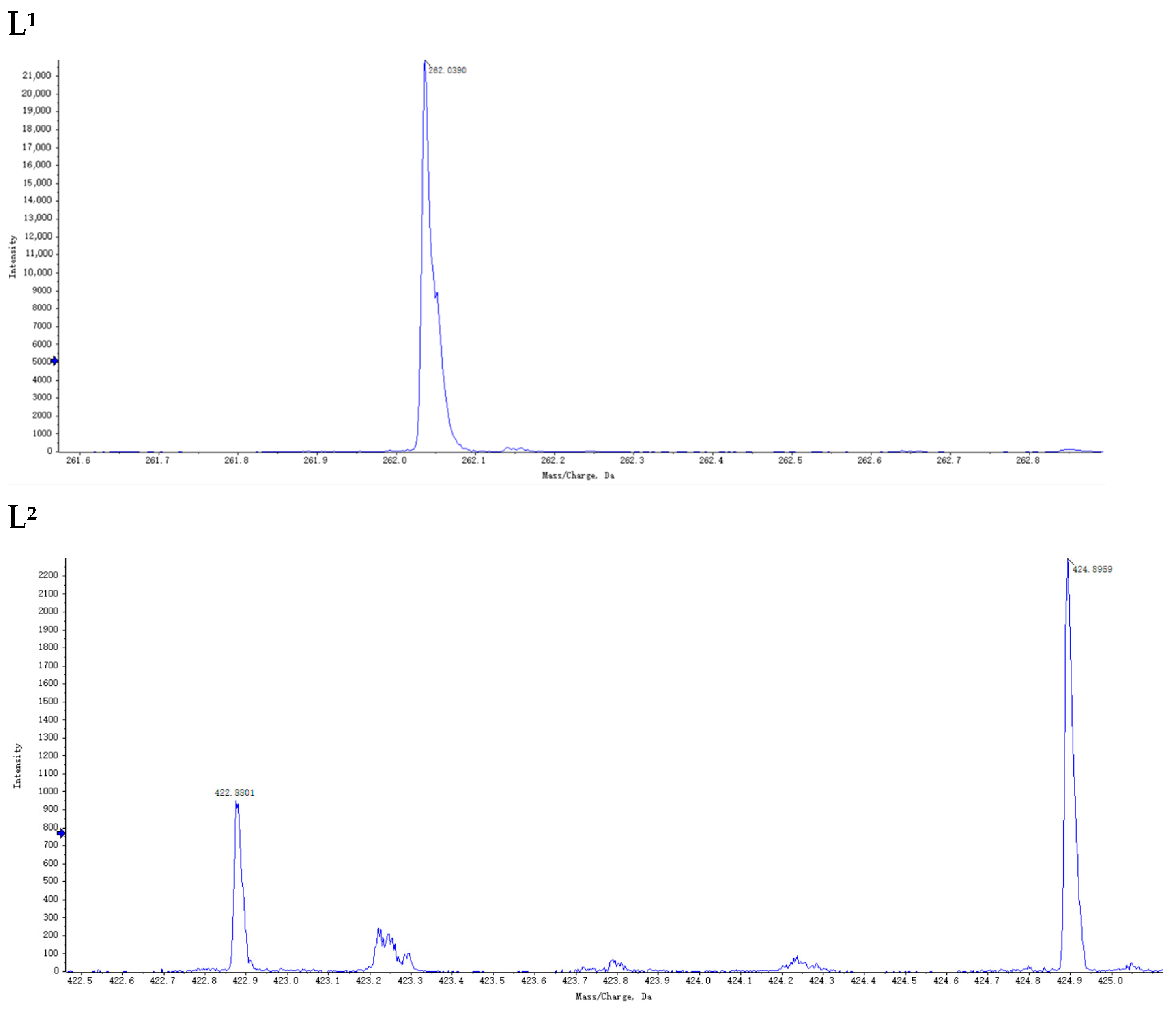
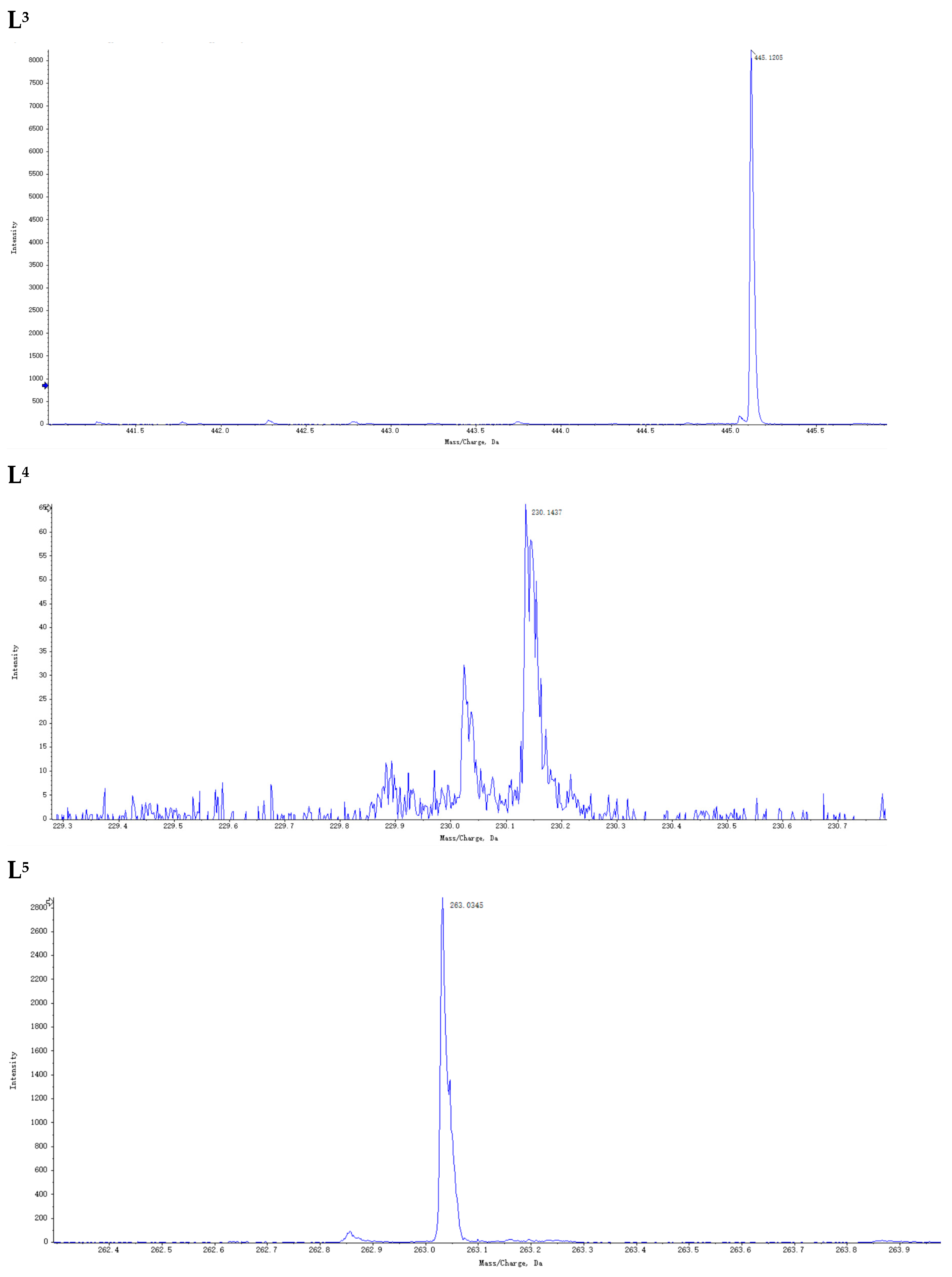
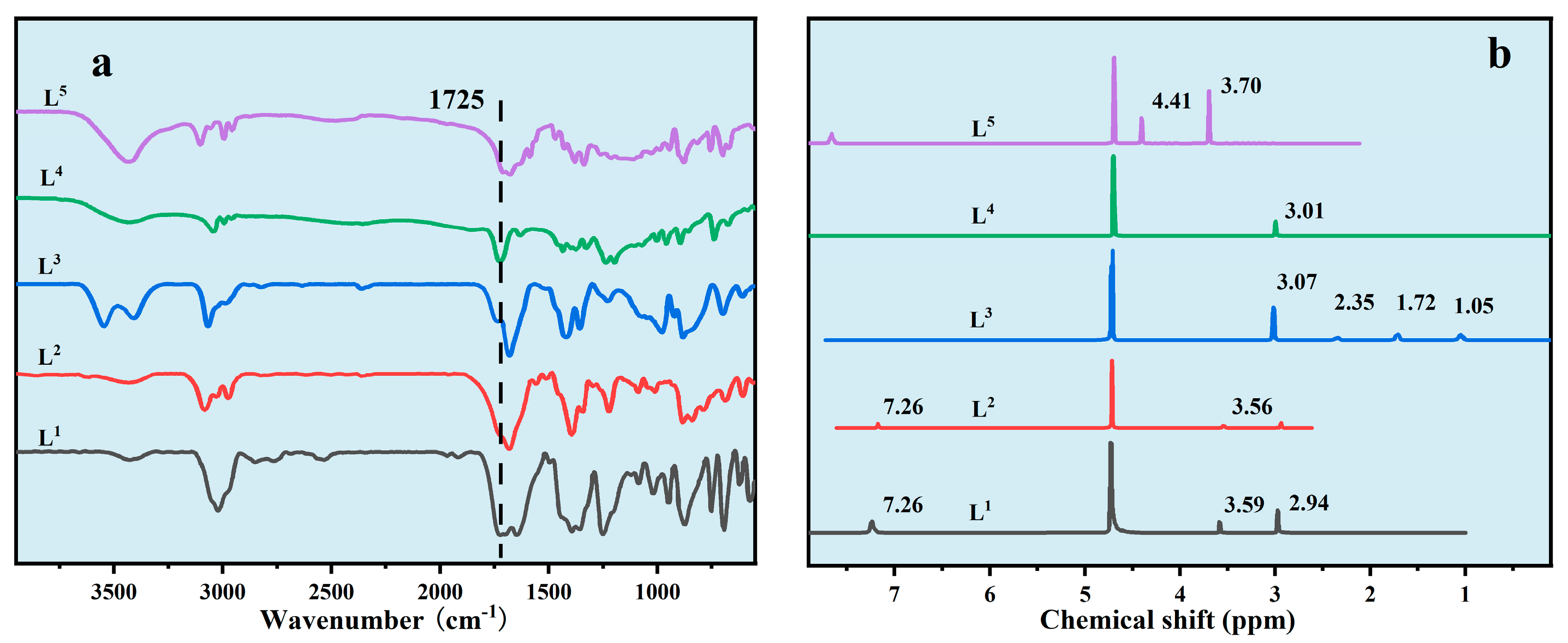
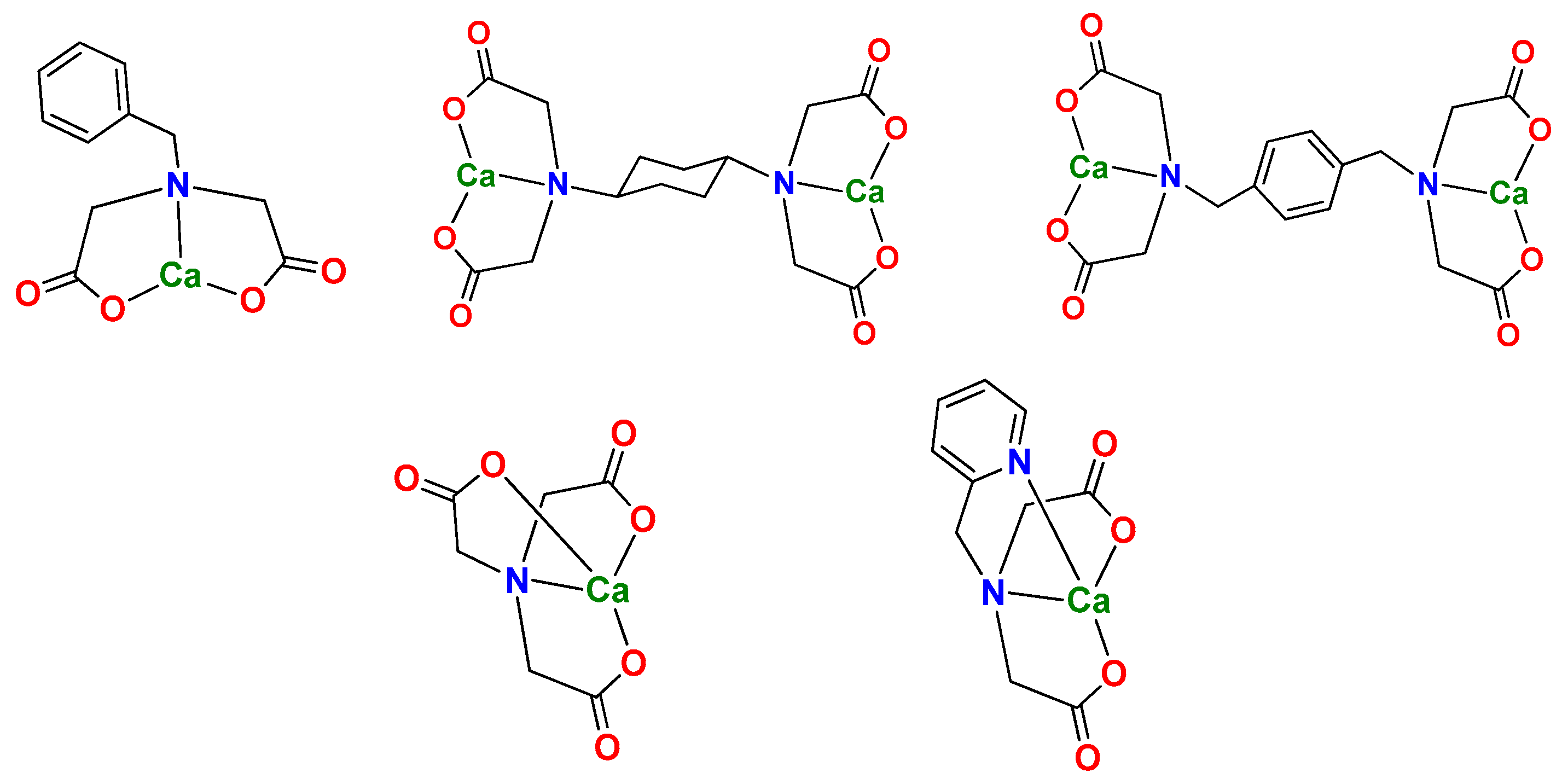
2.3. Preparation of Ion Regulators
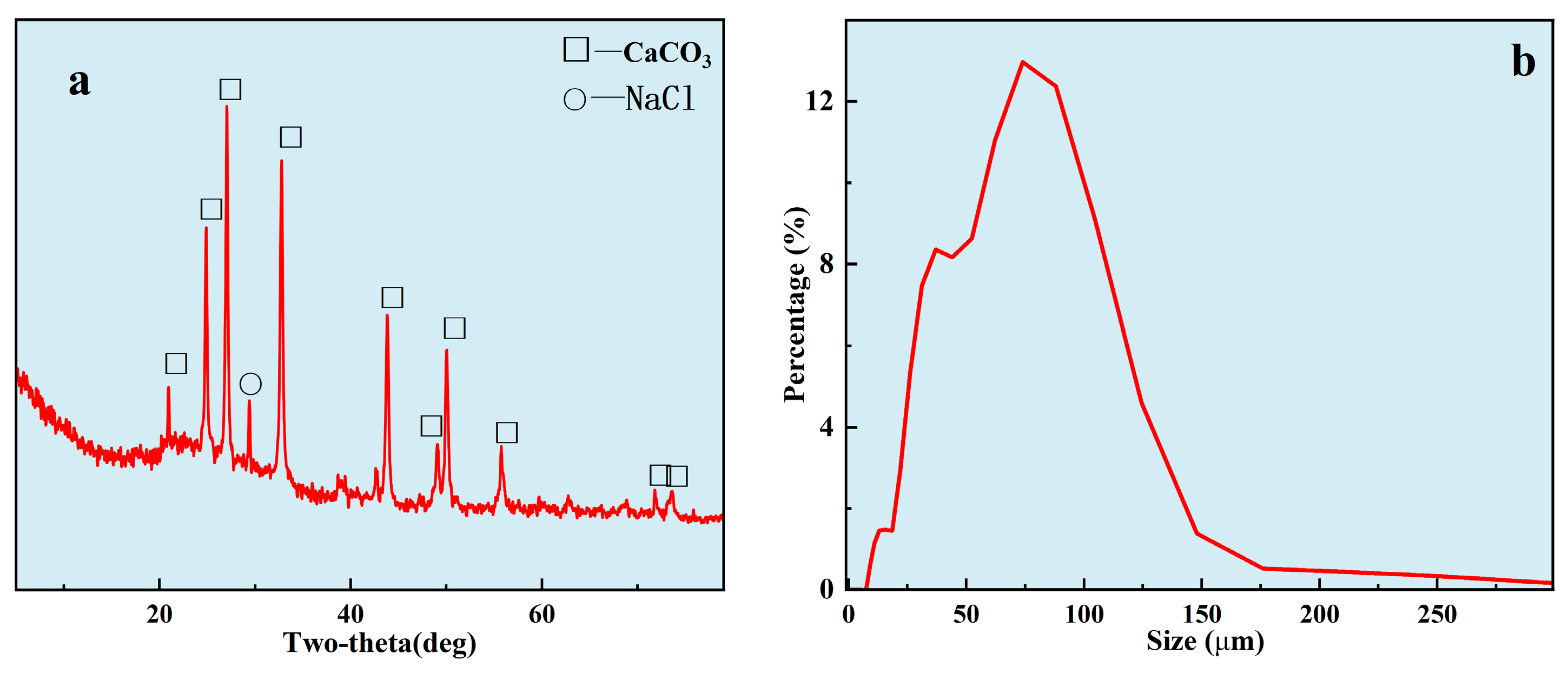
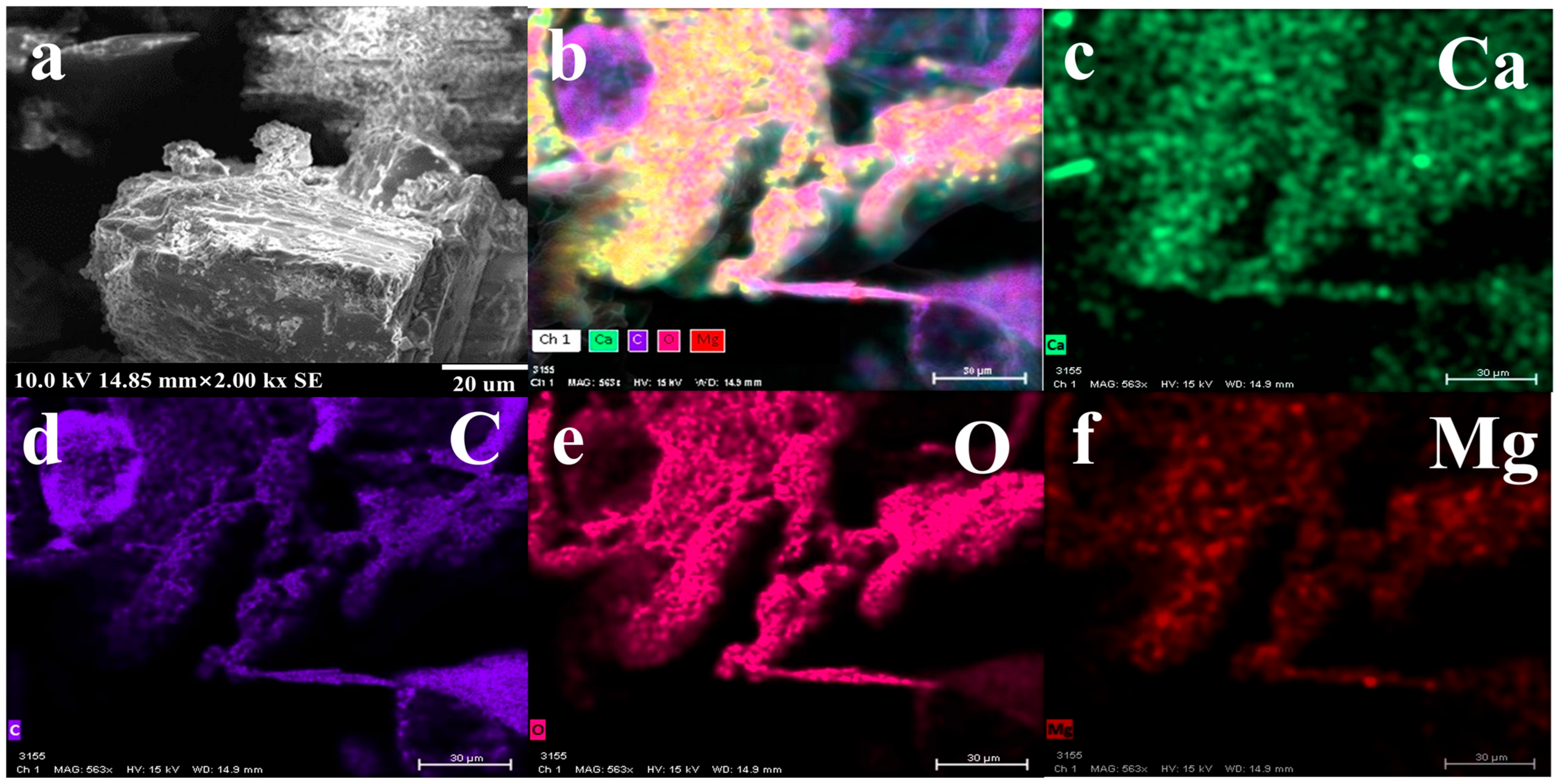
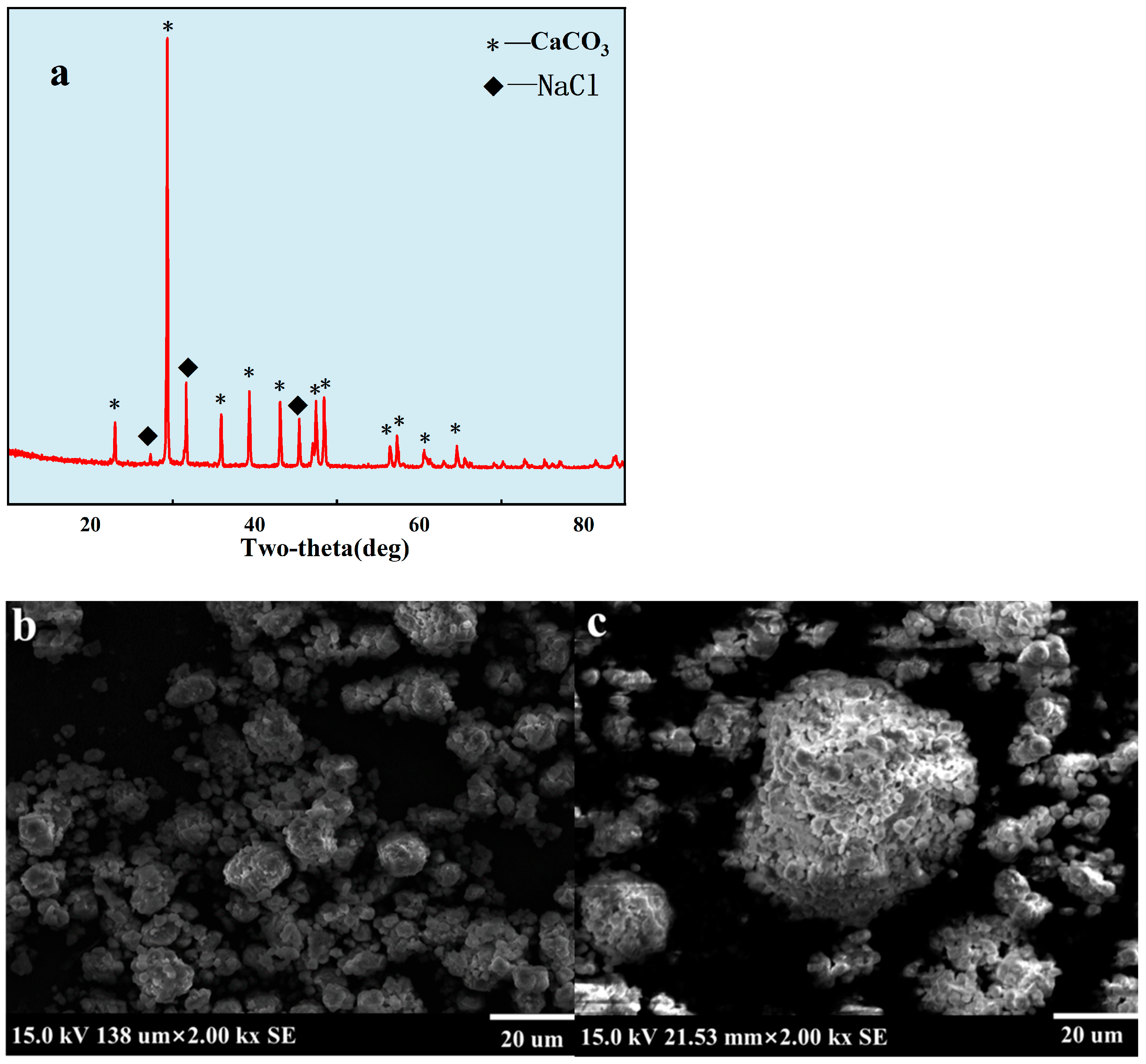
2.4. Properties of Scale under Uncontrolled Condition
2.5. The Capturing of Ca2+ by L1–L5
2.6. CaCO3 Particle Formation by Na2CO3 or NaHCO3
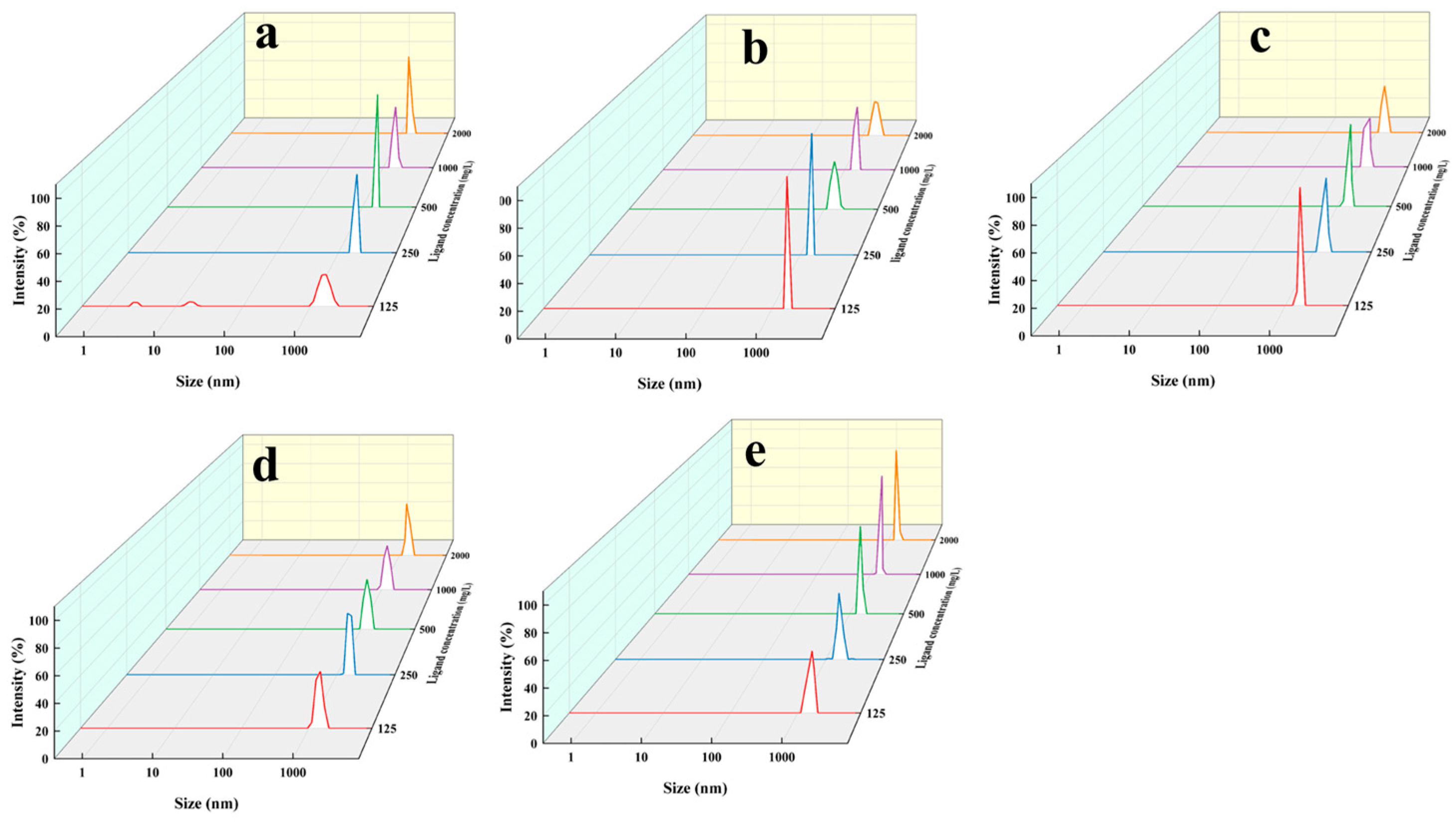
2.7. CaCO3 Nanoparticles Formation and Capturing Yield via L1–L5
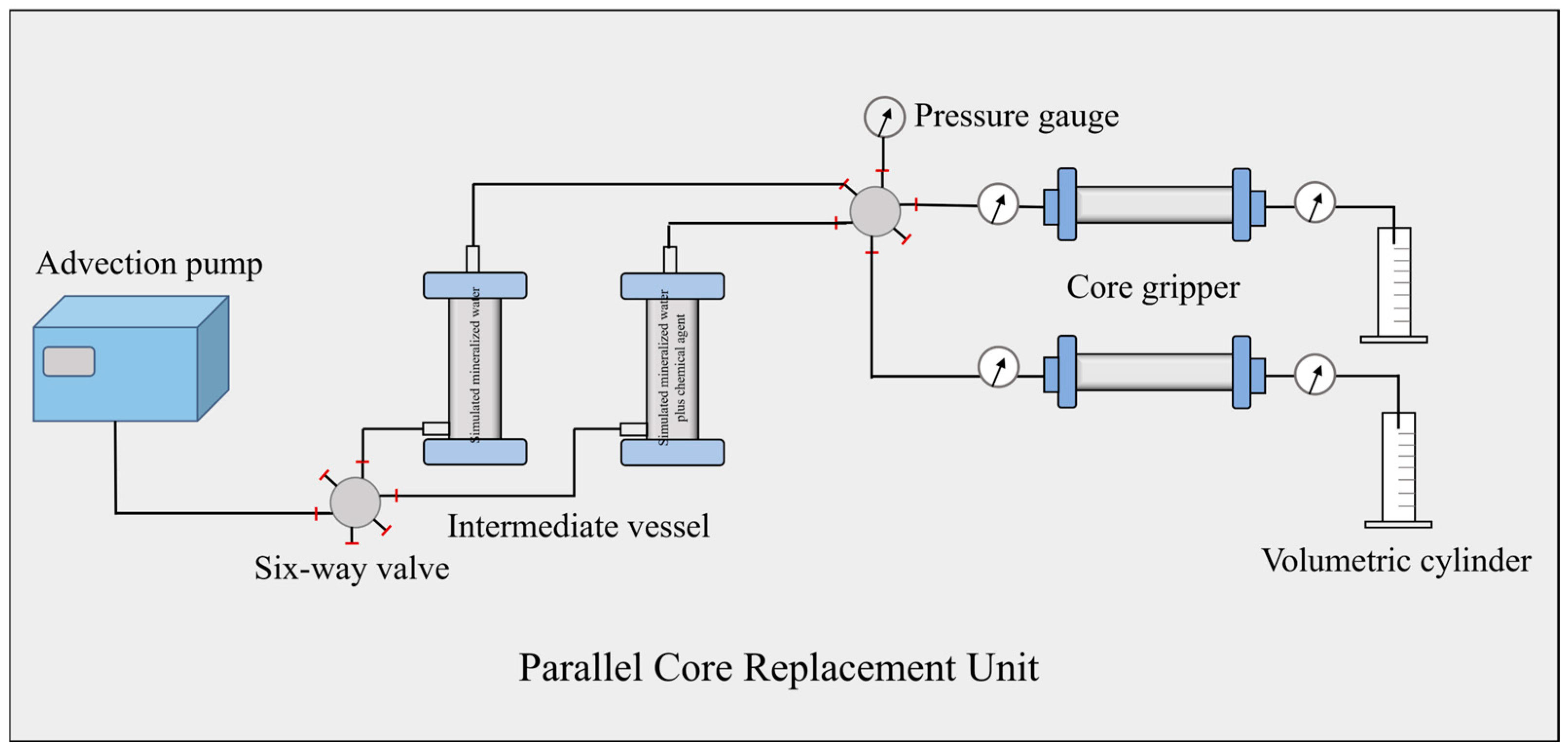
2.8. The Flooding Experiment
- Firstly, simulated formation water was placed in an intermediate container and injected into the core at a flow rate of 1 mL/min to establish the original water saturation. Core dry weight m1 and core wet weight m2 after water injection were recorded, respectively.
- Simulated oil was then injected into the core to establish the original oil saturation. The simulated formation water was then injected into the core at a flow rate of 1 mL/min until no oil was produced. The injection volume and differential pressure were recorded, respectively, and the permeability was calculated via Darcy’s equation to get the recovery rate of the water flooding.
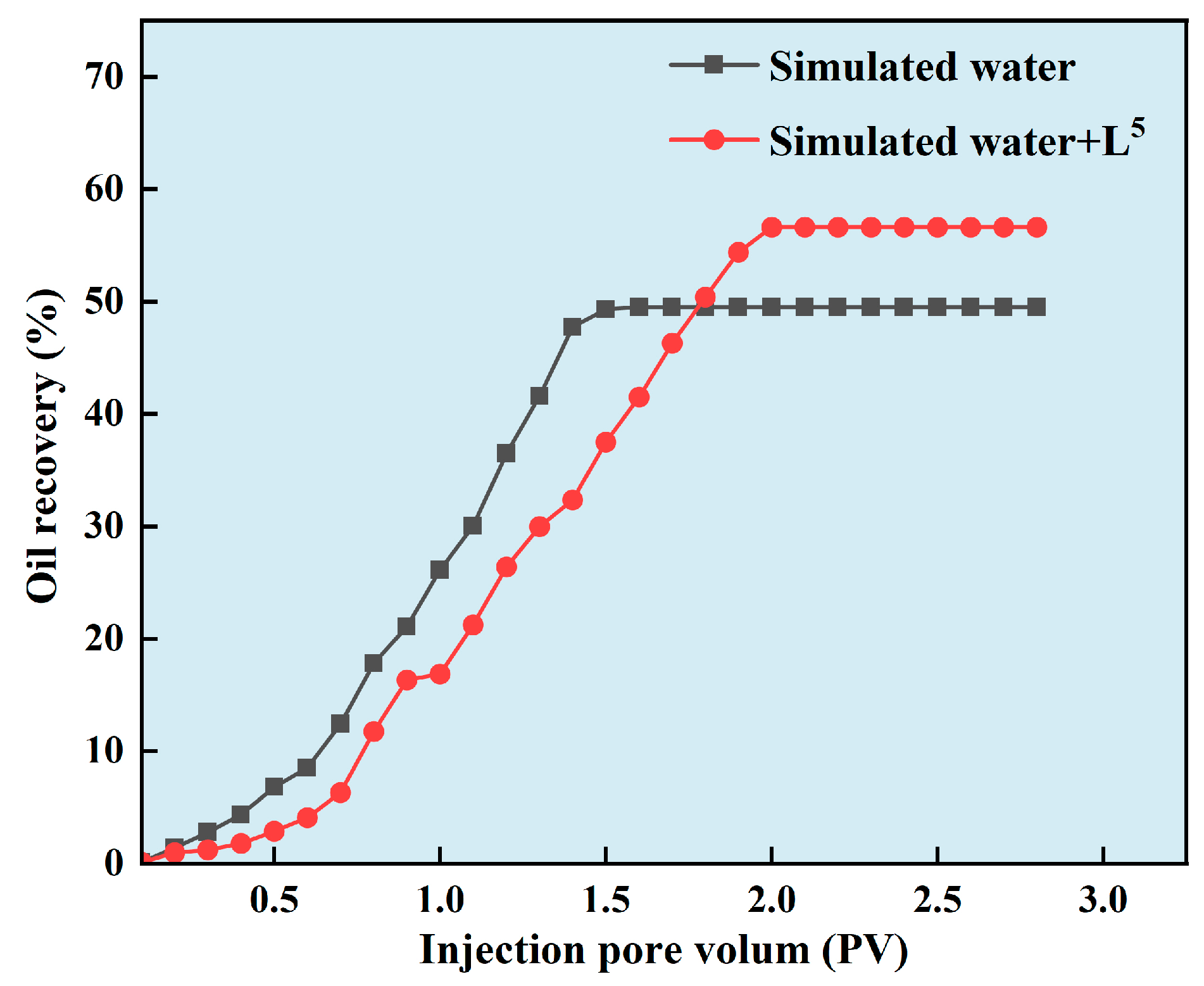
- The simulated formation water with and without additive were placed in an intermediate container, respectively. The water drive recovery rate was calculated by injecting the core at a flow rate of 1 mL/min when the pressure was stable and no oil was produced. The experiment was repeated for each solution to avoid error. The detailed information can be seen in Figure 2.
3. Results and Discussion
3.1. Preparation of Ion Regulator
3.2. Properties of Scale under Uncontrolled Condition
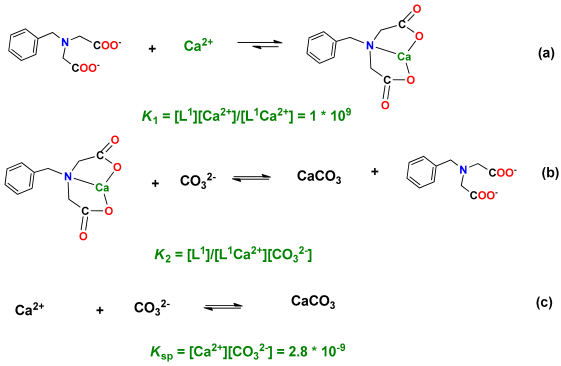
3.3. The Capturing of Ca2+ by L1–L5
3.4. CaCO3 Particle Formation by Na2CO3 or NaHCO3
3.5. CaCO3 Nanoparticles Formation and Capturing Yield via L1–L5
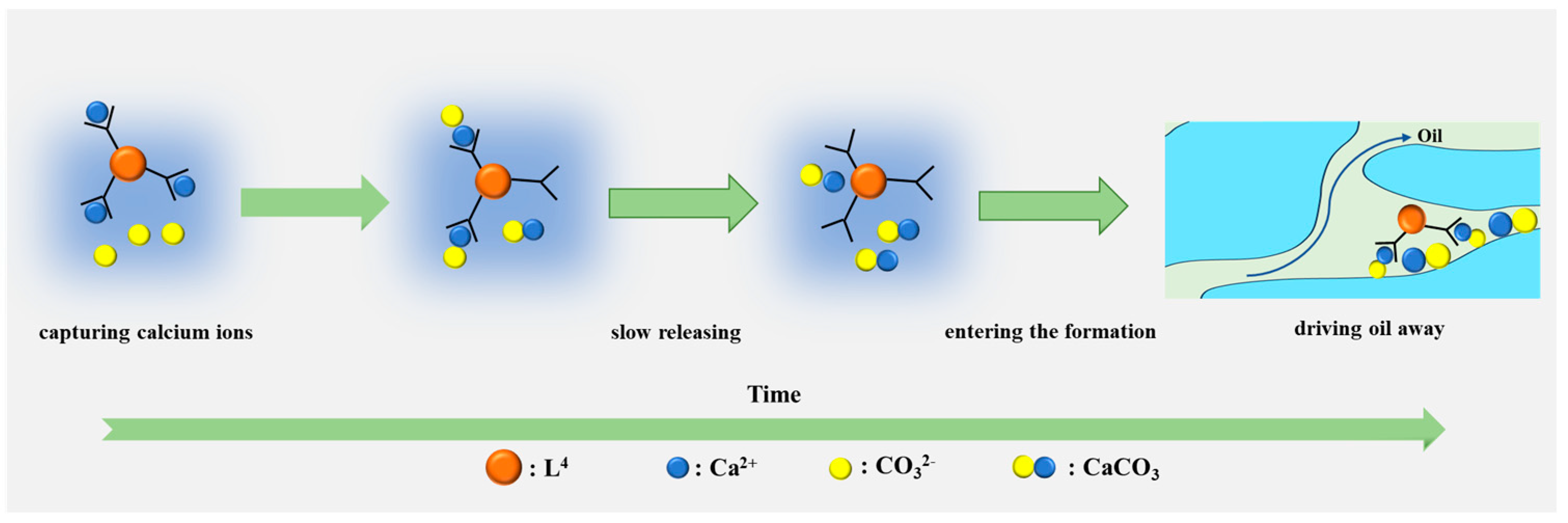
3.6. Controllable CaCO3 Particles Improved Oil Recovery
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Suleimanov, B.A.; Abbasov, H.F.; Ismayilov, R.H. Enhanced oil recovery with nanofluid injection. Pet. Sci. Technol. 2023, 41, 1734–1751. [Google Scholar] [CrossRef]
- Liang, T.; Hou, J.-R.; Qu, M.; Xi, J.-X.; Raj, I. Application of nanomaterial for enhanced oil recovery. Pet. Sci. 2022, 19, 882–899. [Google Scholar] [CrossRef]
- Pourabdollah, K. Matrix acidizing: A fouling mitigation process in oil and gas wells. Rev. Chem. Eng. 2020, 36, 311–331. [Google Scholar] [CrossRef]
- Mahmoodi, L.; Malayeri, M.R.; Tabrizi, F.F. Abatement of scale precipitation in oilfields using green scale inhibitors. J. Pet. Sci. Eng. 2022, 208, 109237. [Google Scholar] [CrossRef]
- Sun, H.; Liu, Y.; Liu, D.; Li, S.; Li, X.; Chen, H.; Han, Z.; Li, L.; Li, X. Enhanced removal of scaling cations from oilfield produced water by carrier mineral floatation. Water Sci. Technol. 2021, 84, 3629–3640. [Google Scholar] [CrossRef]
- Ituen, E.; Singh, A.; Li, R.; Yuanhua, L.; Guo, C. Nanostructure, surface and anticorrosion properties of phyto-fabricated copper nanocomposite in simulated oilfield descaling fluid. Surf. Interfaces 2020, 19, 100514. [Google Scholar] [CrossRef]
- Kamal, M.S.; Hussein, I.; Mahmoud, M.; Sultan, A.S.; Saad, M.A.S. Oilfield scale formation and chemical removal: A review. J. Pet. Sci. Eng. 2018, 171, 127–139. [Google Scholar] [CrossRef]
- Shafer-Peltier, K.; Kenner, C.; Albertson, E.; Chen, M.; Randtke, S.; Peltier, E. Removing scale-forming cations from produced waters. Environ. Sci. 2020, 6, 132–143. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, X.; Zhang, J.; He, X.; Kang, S.; Pu, J.; Fan, S.; Li, X. Experimental and statistical study on wellbore scaling mechanisms and characteristics for Huanjiang oilfield. Geofluids 2022, 2022, 9068440. [Google Scholar] [CrossRef]
- Wang, H.; Dong, H.; Liu, X.; Zhang, P. Preparation and laboratory testing of polymeric scale Inhibitor colloidal materials for oilfield mineral scale control. Polymers 2022, 14, 4240. [Google Scholar] [CrossRef]
- Samimi, A.; Bagheri, A.; Dokhani, S. Solousion for corrosion reducing gas pipe line with inspection for preventing fouling in oil exchangers. Int. J. Basic Appl. Sci. 2013, 2, 291–301. [Google Scholar]
- Shen, C.; Zhao, Y.; Li, W.; Yang, Y.; Liu, R.; Morgen, D. Global profile of heavy metals and semimetals adsorption using drinking water treatment residual. Chem. Eng. J. 2019, 372, 1019–1027. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Xu, Y.; Wu, B. A deep desalination and anti-scaling electrodeionization (EDI) process for high purity water preparation. Desalination 2019, 468, 114075. [Google Scholar] [CrossRef]
- Liu, Q.; Ren, J.; Lu, Y.; Zhang, X.; Roddick, F.A.; Fan, L.; Wang, Y.; Yu, H.; Yao, P. A review of the current in-situ fouling control strategies in MBR: Biological versus physicochemical. J. Ind. Eng. Chem. 2021, 98, 42–59. [Google Scholar] [CrossRef]
- Malaeb, L.; Le-Clech, P.; Vrouwenvelder, J.S.; Ayoub, G.M.; Saikaly, P.E. Do biological-based strategies hold promise to biofouling control in MBRs? Water Res. 2013, 47, 5447–5463. [Google Scholar] [CrossRef]
- Belattar, M.; Hadfi, A.; Ben-Aazza, S.; Karmal, I.; Mohareb, S.; El Housse, M.; Hafid, N.; Driouiche, A. Efficiency of one scale inhibitor on calcium carbonate precipitation from hot water sanitary: Effect of temperature and concentration. Heliyon 2021, 7, e06152. [Google Scholar] [CrossRef]
- Zhang, P.; Kan, A.T.; Tomson, M.B. Oil Field Mineral Scale Control. In Mineral Scales and Deposits; Elsevier: Amsterdam, The Netherlands, 2015; pp. 603–617. [Google Scholar] [CrossRef]
- Demadis, K.D.; Stavgianoudaki, N.; Grossmann, G.; Gruner, M.; Schwartz, J.L. Calcium-Phosphonate Interactions: Solution Behavior and Ca2+ Binding by 2-Hydroxyethylimino- bis (methylenephosphonate) Studied by Multinuclear NMR Spectroscopy. Inorg. Chem. 2009, 48, 4154–4164. [Google Scholar] [CrossRef] [PubMed]
- Mady, M.F.; Rehman, A.; Kelland, M.A. Synthesis and Antiscaling Evaluation of Novel Hydroxybisphosphonates for Oilfield Applications. ACS Omega 2021, 6, 6488–6497. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.M.; Williams, H.; Linares-Samaniego, S.; Frigo, D.M. New Insights on the Impact of High Temperature Conditions (176 °C) on Carbonate and Sulphate Scale Dissolver Performance. In Proceedings of the SPE International Oilfield Scale Conference and Exhibition, Aberdeen, UK, 14–15 May 2014. [Google Scholar] [CrossRef]
- Zhou, Y.; Luo, Z.; Xu, M.; Zhao, T.; Ma, X.; Zhou, S.; Wen, B.; Yang, D. Preparation and Properties of Temperature-Sensitive P(NIPAM-AM) Nano–Microspheres in Enhanced Oil Recovery. Energy Fuels 2023, 37, 204–213. [Google Scholar] [CrossRef]
- Wang, S.; Tang, Z.; Qu, J.; Wu, T.; Liu, Y.; Wang, J.; Liu, X.; Ju, Y.; Liu, F. Research on the mechanisms of polyacrylamide nanospheres with different size distributions in enhanced oil recovery. RSC Adv. 2021, 11, 5763–5772. [Google Scholar] [CrossRef]
- Shagymgereyeva, S.; Sarsenbekuly, B.; Kang, W.; Yang, H.; Turtabayev, S. Advances of polymer microspheres and its applications for enhanced oil recovery. Colloids. Surf. B 2024, 233, 113622. [Google Scholar] [CrossRef]
- Bu, X.; Jiang, K.; Wang, X.; Liu, X.; Tan, X.; Kong, Y.; Wang, L. Analysis of calcium carbonate scaling and antiscaling field experiment. Geothermics 2022, 104, 102433. [Google Scholar] [CrossRef]
- Kumar, T.; Vishwanatham, S.; Kundu, S.S. A laboratory study on pteroyl-l-glutamic acid as a scale prevention inhibitor of calcium carbonate in aqueous solution of synthetic produced water. J. Pet. Sci. Eng. 2010, 71, 1–7. [Google Scholar] [CrossRef]
- Duong, H.C.; Duke, M.; Gray, S.; Cooper, P.; Nghiem, L.D. Membrane scaling and prevention techniques during seawater desalination by air gap membrane distillation. Desalination 2016, 397, 92–100. [Google Scholar] [CrossRef]
- Chen, T.; Neville, A.; Yuan, M. Assessing the effect of on scale formation–bulk precipitation and surface deposition. J. Cryst. Growth 2005, 275, e1341–e1347. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, J.; Han, H.; Sun, W.; Zhang, X. The enhanced flotation separation of magnesite and dolomite by introducing chelating reagent EDTA. Colloids. Surf. A 2024, 682, 132969. [Google Scholar] [CrossRef]
- Mahmoud, M.; Attia, M.; Al-Hashim, H. EDTA chelating agent/seawater solution as enhanced oil recovery fluid for sandstone reservoirs. J. Pet. Sci. Eng. 2017, 152, 275–283. [Google Scholar] [CrossRef]
- Sengupta, A.; Seitz, A.; Merz, K.M. Simulating the Chelate Effect. J. Am. Chem. Soc. 2018, 140, 15166–15169. [Google Scholar] [CrossRef]
- Larsen, T.; Randhol, P.; Lioliou, M.; Jøsang, L.O.; Østvold, T. Kinetics of CaCO3 Scale Formation During Core Flooding. In Proceedings of the SPE International Oilfield Scale Conference and Exhibition, Aberdeen, UK, 28–29 May 2008. [Google Scholar] [CrossRef]
- Gower, L.B.; Odom, D.J. Deposition of calcium carbonate films by a polymer-induced liquid-precursor (PILP) process. J. Cryst. Growth 2000, 210, 719–734. [Google Scholar] [CrossRef]
- Feng, S.J.; Zhao, J.F.; Gao, Y.L.; Yun, J.X. Polydopamine-CaCO3 modified superhydrophilic nanocomposite membrane used for highly efficient separation of oil-in-water emulsions. Colloids Surf. A 2022, 639, 128355. [Google Scholar] [CrossRef]
- Tian, K.P.; Pu, W.F.; Wang, Q.L.; Xie, M.K.; Wang, D.D.; Wang, M.M.; Liu, S. A Feasibility study on enhanced oil recovery of modified Janus nano calcium carbonate-assisted alkyl polyglycoside to form nanofluids in emulsification flooding. Langmuir 2024, 40, 4174–4185. [Google Scholar] [CrossRef] [PubMed]

| Na+ | K+ | Ca2+ | Mg2+ | Cl | SO42 | HCO3− | Total Mineralization |
|---|---|---|---|---|---|---|---|
| 11,977 | 133 | 695 | 269 | 21,939 | 15 | 370 | 35,397 |
| Ligands | c(L) (mg/L) | nCa2+/nL | c(Ca2+) (mg/L) | Capturing Yield (%) | Particle Size (nm) |
|---|---|---|---|---|---|
| L1 | 125 | 38.4:1 | 150 | 67.4 | 1652 |
| 250 | 19.2:1 | 144 | 68.7 | 1896 | |
| 500 | 9.6:1 | 142 | 69.0 | 1841 | |
| 1000 | 4.8:1 | 158 | 65.6 | 1653 | |
| 2000 | 2.4:1 | 165 | 64.1 | 1451 | |
| L2 | 125 | 31.8:1 | 152 | 67.0 | 1668 |
| 250 | 15.9:1 | 150 | 67.4 | 1539 | |
| 500 | 7.95:1 | 143 | 68.9 | 1519 | |
| 1000 | 3.98:1 | 163 | 64.5 | 1698 | |
| 2000 | 1.99:1 | 177 | 61.4 | 1843 | |
| L3 | 125 | 60:1 | 163 | 64.5 | 1651 |
| 250 | 30:1 | 161 | 65.0 | 1614 | |
| 500 | 15:1 | 157 | 65.8 | 1756 | |
| 1000 | 7.5:1 | 156 | 66.0 | 1687 | |
| 2000 | 3.74:1 | 165 | 64.1 | 1563 | |
| L4 | 125 | 16.5:1 | 124 | 73.0 | 1362 |
| 250 | 8.22:1 | 60 | 87.0 | 1467 | |
| 500 | 4.12:1 | 80 | 82.6 | 1324 | |
| 1000 | 2.06:1 | 74 | 83.8 | 1266 | |
| 2000 | 1.03:1 | 80 | 82.5 | 1385 | |
| L5 | 125 | 38.7:1 | 157 | 65.8 | 1652 |
| 250 | 19.4:1 | 150 | 67.4 | 1632 | |
| 500 | 9.7:1 | 143 | 69.0 | 1534 | |
| 1000 | 4.84:1 | 155 | 66.2 | 1654 | |
| 2000 | 2.42:1 | 160 | 65.4 | 1469 |
| Entry | nCa2+/nL4 | nCa2+/nNaHCO3 | Capturing Yield (%) |
|---|---|---|---|
| 1 | 8:1 | 0.726:1 | 72.3 |
| 2 | 8:1 | 0.363:1 | 87.0 |
| 3 | 8:1 | 0.181:1 | 81.2 |
| 4 | 8:1 | 0.09:1 | 78.4 |
| 5 | 8:1 | 0.045:1 | 61.2 |
| c(L4) mg/L | c(PASP) mg/L | nCa2+/n | nCa2+/nNaHCO3 | Capturing Yield (%) | Particle Size (nm) without PASP | Particle Size (nm) with (PASP) |
|---|---|---|---|---|---|---|
| 250 | 125 | 8:1 | 0.363:1 | 83.2 | 1362 | 997.6 |
| 250 | 250 | 8:1 | 0.363:1 | 84.0 | 1467 | 929.6 |
| 250 | 500 | 8:1 | 0.363:1 | 85.1 | 1324 | 764.8 |
| 250 | 1000 | 8:1 | 0.363:1 | 84.6 | 1266 | 1011 |
| 250 | 2000 | 8:1 | 0.363:1 | 85.2 | 1385 | 943.2 |
| Core Flooding Setup | Core Length/cm | Core Diameter/cm | Effective Porosity/% | Permeability /mD | Porosity/% | Oil Recovery/% |
|---|---|---|---|---|---|---|
| Simulated water | 10 | 2.5 | 20.4 | 19.52 | 20.5 | 54.6 |
| 10 | 2.5 | 20.6 | 19.69 | 21.6 | 55.2 | |
| Simulated Water + L4 | 10 | 2.5 | 20.9 | 19.89 | 21.3 | 60.3 |
| 10 | 2.5 | 21.5 | 20.01 | 22.5 | 61.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, B.; Hou, S.; Hua, Z.; Zhang, J.; Yang, H.; Zhu, Y.; Tang, Y.; Wang, B. Comprehensive Utilization of Formation Water Scale to Prepare Controllable Size CaCO3 Nanoparticles: A New Method to Improve Oil Recovery. Nanomaterials 2024, 14, 1452. https://doi.org/10.3390/nano14171452
Huang B, Hou S, Hua Z, Zhang J, Yang H, Zhu Y, Tang Y, Wang B. Comprehensive Utilization of Formation Water Scale to Prepare Controllable Size CaCO3 Nanoparticles: A New Method to Improve Oil Recovery. Nanomaterials. 2024; 14(17):1452. https://doi.org/10.3390/nano14171452
Chicago/Turabian StyleHuang, Bo, Shengzhen Hou, Zhao Hua, Jian Zhang, Huan Yang, Yuejun Zhu, Yumiao Tang, and Benru Wang. 2024. "Comprehensive Utilization of Formation Water Scale to Prepare Controllable Size CaCO3 Nanoparticles: A New Method to Improve Oil Recovery" Nanomaterials 14, no. 17: 1452. https://doi.org/10.3390/nano14171452
APA StyleHuang, B., Hou, S., Hua, Z., Zhang, J., Yang, H., Zhu, Y., Tang, Y., & Wang, B. (2024). Comprehensive Utilization of Formation Water Scale to Prepare Controllable Size CaCO3 Nanoparticles: A New Method to Improve Oil Recovery. Nanomaterials, 14(17), 1452. https://doi.org/10.3390/nano14171452





