Laser-Assisted Photo-Thermal Reaction for Ultrafast Synthesis of Single-Walled Carbon Nanotube/Copper Nanoparticles Hybrid Films as Flexible Electrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation of SWCNT/Cu-Complex
2.2. Fabrication and Characterization of the SWCNTs/Cu Electrodes
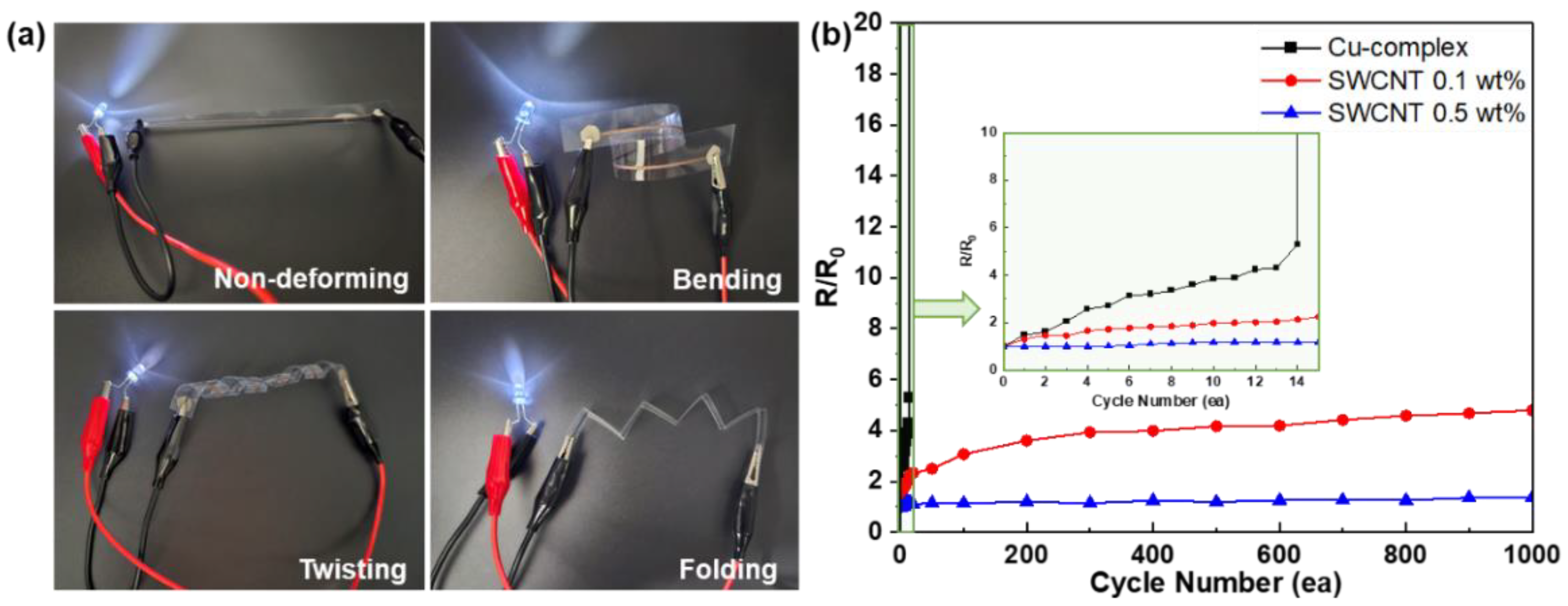
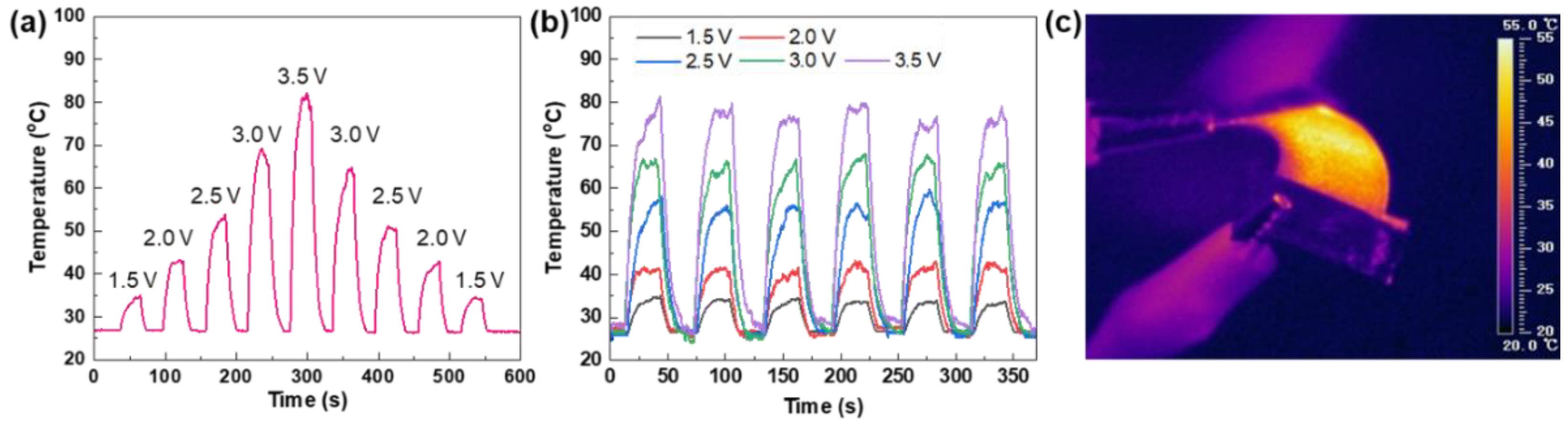
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pang, C.; Lee, G.-Y.; Kim, T.-I.; Kim, S.M.; Kim, H.N.; Ahn, S.-H.; Suh, K.-Y. A flexible and highly sensitive strain-gauge sensor using reversible interlocking of nanofibers. Nat. Mater. 2012, 11, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Woo, K.; Kim, I.; Kim, H.; Ko, P.; Kang, D.; Kwon, S.; Kim, H.; Youn, H.; Moon, J. Defect-Free, Highly Uniform Washable Transparent Electrodes Induced by Selective Light Irradiation. Small 2018, 14, 1800676. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, L.; Chen, D.; Hajagos, T.; Ren, Z.; Chou, S.-Y.; Hu, W.; Pei, Q. Intrinsically stretchable and transparent thin-film transistors based on printable silver nanowires, carbon nanotubes and an elastomeric dielectric. Nat. Commun. 2015, 6, 7647. [Google Scholar] [CrossRef]
- Han, B.; Huang, Y.; Li, R.; Peng, Q.; Luo, J.; Pei, K.; Herczynski, A.; Kempa, K.; Ren, Z.; Gao, J. Bio-inspired networks for optoelectronic applications. Nat. Commun. 2014, 5, 5674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ryu, K.; Liu, X.; Polikarpov, E.; Ly, J.; Tompson, M.E.; Zhou, C. Transparent, Conductive, and Flexible Carbon Nanotube Films and Their Application in Organic Light-Emitting Diodes. Nano Lett. 2006, 6, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Scardaci, V.; Coull, R.; Coleman, J.N. Very thin transparent, conductive carbon nanotube films on flexible substrates. Appl. Phys. Lett. 2010, 97, 023114. [Google Scholar] [CrossRef]
- Geng, H.-Z.; Kim, K.K.; So, K.P.; Lee, Y.S.; Chang, Y.; Lee, Y.H. Effect of Acid Treatment on Carbon Nanotube-Based Flexible Transparent Conducting Films. J. Am. Chem. Soc. 2007, 129, 7758–7759. [Google Scholar] [CrossRef]
- Han, J.T.; Kim, J.S.; Jeong, H.D.; Jeong, H.J.; Jeong, S.Y.; Lee, G.-W. Modulating Conductivity, Environmental Stability of Transparent Conducting Nanotube Films on Flexible Substrates by Interfacial Engineering. ACS Nano 2010, 4, 4551–4558. [Google Scholar] [CrossRef]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Kim, H.R.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef]
- Zhao, J.; Pei, S.; Ren, W.; Gao, L.; Cheng, H.-M. Efficient Preparation of Large-Area Graphene Oxide Sheets for Transparent Conductive Films. ACS Nano 2010, 4, 5245–5252. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Kim, S.H.; Han, J.T.; Jeong, H.J.; Yang, S.; Lee, G.-W. High-Performance Transparent Conductive Films Using Rheologically Derived Reduced Graphene Oxide. ACS Nano 2011, 5, 870–878. [Google Scholar] [CrossRef]
- Chun, K.-Y.; Oh, Y.; Rho, J.; Ahn, J.-H.; Kim, Y.-J.; Choi, H.R.; Baik, S. Highly conductive, printable and stretchable composite films of carbon nanotubes and silver. Nat. Nanotechnol. 2010, 5, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-S.; Lee, K.; Kim, S.-Y.; Lee, H.; Park, J.; Choi, K.-H.; Kim, H.-K.; Kim, D.-G.; Lee, D.-Y.; Nam, S.; et al. High-Performance, Transparent, and Stretchable Electrodes Using Graphene—Metal Nanowire Hybrid Structures. Nano Lett. 2013, 13, 2814–2821. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chang, Q.; Huang, L. Transparent, flexible conducting graphene hybrid films with a subpercolating network of silver nanowires. J. Mater. Chem. C 2013, 1, 2970–2974. [Google Scholar] [CrossRef]
- Kong, B.-S.; Jung, D.-H.; Oh, S.-K.; Han, C.-S.; Jung, H.-T. Single-Walled Carbon Nanotube Gold Nanohybrids: Application in Highly Effective Transparent and Conductive Films. J. Phys. Chem. C 2007, 111, 8377–8382. [Google Scholar] [CrossRef]
- Yun, Y.J.; Ah, C.S.; Hong, W.G.; Kim, H.J.; Shin, J.-H.; Jun, Y. Highly conductive and environmentally stable gold/graphene yarns for flexible and wearable electronics. Nanoscale 2017, 9, 11439–11445. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-Y.; Zhang, K.; Yuen, M.M.F.; Fu, X.-Z.; Sun, R.S.; Wong, C.-P. Facile synthesis of flexible graphene–silver composite papers with promising electrical and thermal conductivity performances. RSC Adv. 2014, 4, 34156–34160. [Google Scholar] [CrossRef]
- Janas, D.; Rdest, M.; Koziol, K.K.K. Free-standing conductive thin films from gold-carbon nanotube composites. Mater. Lett. 2017, 201, 101–104. [Google Scholar] [CrossRef]
- Kim, I.; Woo, K.; Zhong, Z.; Lee, E.; Kang, D.; Jeong, S.; Choi, Y.-M.; Jang, Y.; Kwon, S.; Moon, J. Selective Light-Induced Patterning of Carbon Nanotube/Silver Nanoparticle Composite To Produce Extremely Flexible Conductive Electrodes. ACS Appl. Mater. Interfaces 2017, 9, 6163–6170. [Google Scholar] [CrossRef]
- Kholmanov, I.N.; Domingues, S.H.; Chou, H.; Wang, X.; Tan, C.; Kim, J.-Y.; Li, H.; Piner, R.; Zarbin, A.J.G.; Ruoff, R.S. Reduced Graphene Oxide/Copper Nanowire Hybrid Films as High-Performance Transparent Electrodes. ACS Nano 2013, 7, 1811–1816. [Google Scholar] [CrossRef]
- Subramaniam, C.; Yamada, T.; Kobashi, K.; Sekiguchi, A.; Futaba, D.N.; Yumura, M.; Hata, K. One hundred fold increase in current carrying capacity in a carbon nanotube–copper composite. Nat. Commun. 2013, 4, 2202. [Google Scholar] [CrossRef]
- Suh, Y.D.; Kwon, J.; Lee, J.; Lee, H.; Jeong, S.; Kim, D.; Cho, H.; Yeo, J.; Ko, S.H. Maskless Fabrication of Highly Robust, Flexible Transparent Cu Conductor by Random Crack Network Assisted Cu Nanoparticle Patterning and Laser Sintering. Adv. Electron. Mater. 2016, 2, 1600277. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Chang, W.-C.; Chen, G.-L.; Chung, C.-H.; Liang, J.-X.; Ma, W.-Y.; Yang, T.-N. A Study of the Preparation and Properties of Antioxidative Copper Inks with High Electrical Conductivity. Nanoscale Res. Lett. 2015, 10, 357. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Lee, S.H.; Jo, Y.; Lee, S.S.; Seo, Y.-H.; Ahn, B.W.; Kim, G.; Jang, G.-E.; Park, J.-U.; Ryu, B.-H.; et al. Air-stable, surface-oxide free Cu nanoparticles for highly conductive Cu ink and their application to printed graphene transistors. J. Mater. Chem. C 2013, 1, 2704–2710. [Google Scholar] [CrossRef]
- Huang, K.-M.; Tsukamoto, H.; Yong, Y.; Chiu, H.-L.; Nguyen, M.T.; Yonezawa, T.; Liao, Y.-C. Stabilization of the thermal decomposition process of self-reducible copper ion ink for direct printed conductive patterns. RSC Adv. 2017, 7, 25095–25100. [Google Scholar] [CrossRef]
- Farraj, Y.; Grouchko, M.; Magdassi, S. Self-reduction of a copper complex MOD ink for inkjet printing conductive patterns on plastics. Chem. Commun. 2015, 51, 1587–1590. [Google Scholar] [CrossRef]
- Choi, Y.-H.; Hong, S.-H. Effect of the Amine Concentration on Phase Evolution and Densification in Printed Films Using Cu(II) Complex Ink. Langmuir 2015, 31, 8101–8110. [Google Scholar] [CrossRef]
- Rosen, Y.S.; Yakushenko, A.; Offenhäusser, A.; Magdassi, S. Self-Reducing Copper Precursor Inks and Photonic Additive Yield Conductive Patterns under Intense Pulsed Light. ACS Omega 2017, 2, 573–581. [Google Scholar] [CrossRef]
- Lee, J.-W.; Kwak, J.H.; Kim, J.; Jeong, S.; Park, J.H.; Jeong, S.Y.; Seo, S.H.; Han, J.T.; Lee, G.-W.; Baeg, K.-J.; et al. Minimizing Temperature Gradient in Photonic Sintering for Defect-Free High-Conductivity Cu-Based Printed Patterns by Bidirectional Irradiation. Adv. Mater. Interfaces 2021, 8, 2100769. [Google Scholar] [CrossRef]
- Perelaer, J.; Gans, B.-J.; Schubert, U.S. Ink-jet Printing and Microwave Sintering of Conductive Silver Tracks. Adv. Mater. 2006, 18, 2101–2104. [Google Scholar] [CrossRef]
- Kumpulainen, T.; Pekkanen, J.; Valkama, J.; Laakso, J.; Tuokko, R.; Mantysalo, M. Low temperature nanoparticle sintering with continuous wave and pulse lasers. Opt. Laser Technol. 2011, 43, 570–576. [Google Scholar] [CrossRef]
- Ruia, J.; Kima, K.; Kim, H.-S.; Hahn, H.T.; Lashmore, D. Intense pulsed light induced platinum–gold alloy formation on carbon nanotubes for non-enzymatic glucose detection. Biosens. Bioelectron. 2010, 26, 602–607. [Google Scholar]
- Park, J.H.; Hwang, G.-T.; Kim, S.; Seo, J.; Park, H.-J.; Yu, K.; Kim, T.-S.; Lee, K.J. Flash-Induced Self-Limited Plasmonic Welding of Silver Nanowire Network for Transparent Flexible Energy Harvester. Adv. Mater. 2017, 29, 1603473. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Woo, K.; Kim, I.; Hwang, H.; Kwon, S.; Choi, Y.-M.; Lee, Y.; Lee, T.-M.; Kim, K.; Moon, J. Roll-to-roll-compatible, flexible, transparent electrodes based on self-nanoembedded Cu nanowires using intense pulsed light irradiation. Nanoscale 2016, 8, 8995–9003. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Kim, H.; Kim, J.; Lee, Y.J.; Lee, W.; Hwang, J.Y.; Kim, K.K.; Jeong, H.J. Molecular-level hybridization of single-walled carbon nanotubes and a copper complex with counterbalanced electrostatic interactions. Commun. Mater. 2024, 5, 111. [Google Scholar] [CrossRef]
- Dong, Y.; Lin, Z.; Li, X.; Zhu, Q.; Li, J.-G.; Sun, X. A low temperature and air-sinterable copper–diamine complex-based metal organic decomposition ink for printed electronics. J. Mater. Chem. C 2018, 6, 6406–6415. [Google Scholar] [CrossRef]
- Ahangarkani, M. In-situ formation of tungsten nano-particles by oxidation-reduction mechanism-New approach in sintering activating by means of oxide nanostructures. Mater. Lett. 2020, 261, 127128. [Google Scholar] [CrossRef]
- Sharna, S.; Bahri, M.; Bouillet, C.; Rouchon, V.; Lambert, A.; Gay, A.-S.; Chiche, D.; Ersen, O. In situ STEM study on the morphological evolution of copper-based nanoparticles during high-temperature redox reactions. Nanoscale 2021, 13, 9747–9756. [Google Scholar] [CrossRef]
- Allaf, R.M.; Rivero, I.V.; Spearman, S.S.; Hope-Weeks, L.J. On the preparation of as-produced and purified single-walled carbon nanotube samples for standardized X-ray diffraction characterization. Mater. Charact. 2011, 62, 857–864. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, X.; Jiang, L.; Liang, M.; Zhang, X.; Wu, S.; Wu, J.; Tian, M.; Zhao, Y.; Qu, L. Laser maskless fast patterning for multitype microsupercapacitors. Nat. Commun. 2023, 14, 396. [Google Scholar] [CrossRef]
- An, J.W.; Hyeong, S.-K.; Kim, K.M.; Lee, H.R.; Kim, T.-W.; Bae, S.; Lee, S.-K. Facile synthesis of laser-induced graphene oxide and its humidity sensing properties. Carbon Lett. 2024, 34, 1173–1185. [Google Scholar] [CrossRef]
- Goak, J.C.; Kim, T.Y.; Kim, D.U.; Chang, K.S.; Lee, C.S.; Lee, N. Stable heating performance of carbon nanotube/silver nanowire transparent heaters. Appl. Surf. Sci. 2020, 510, 145445. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-J.; Jeong, H.J. Laser-Assisted Photo-Thermal Reaction for Ultrafast Synthesis of Single-Walled Carbon Nanotube/Copper Nanoparticles Hybrid Films as Flexible Electrodes. Nanomaterials 2024, 14, 1454. https://doi.org/10.3390/nano14171454
Kim M-J, Jeong HJ. Laser-Assisted Photo-Thermal Reaction for Ultrafast Synthesis of Single-Walled Carbon Nanotube/Copper Nanoparticles Hybrid Films as Flexible Electrodes. Nanomaterials. 2024; 14(17):1454. https://doi.org/10.3390/nano14171454
Chicago/Turabian StyleKim, Mi-Jeong, and Hee Jin Jeong. 2024. "Laser-Assisted Photo-Thermal Reaction for Ultrafast Synthesis of Single-Walled Carbon Nanotube/Copper Nanoparticles Hybrid Films as Flexible Electrodes" Nanomaterials 14, no. 17: 1454. https://doi.org/10.3390/nano14171454




