Producing Freestanding Single-Crystal BaTiO3 Films through Full-Solution Deposition
Abstract
:1. Introduction
2. Materials and Methods
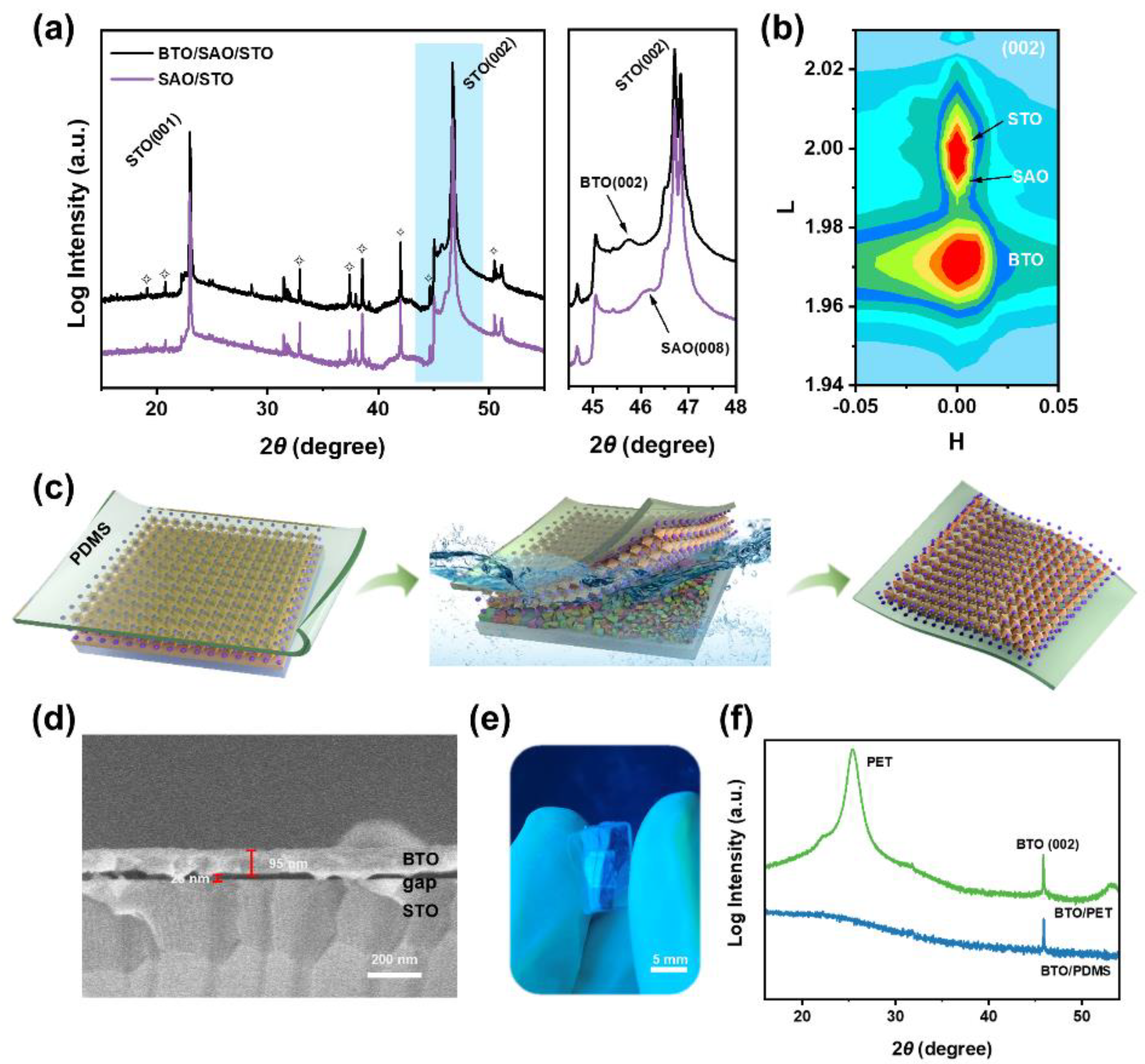
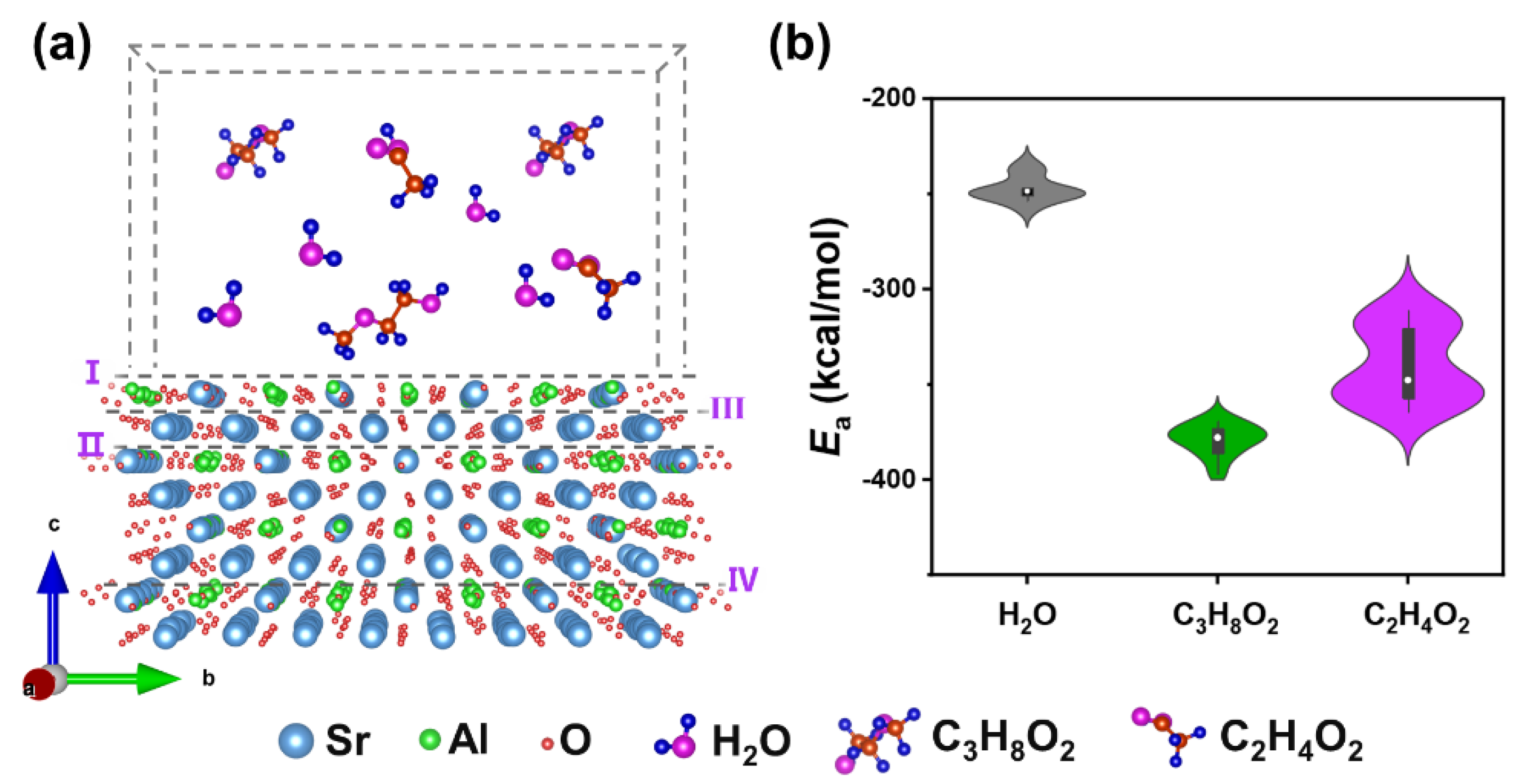
3. Results and Discussion
4. Conclusions
Supplementary Materials
 ,
,  represent +10 V and −10 V, respectively). The domain walls can still be observed in the phase and amplitude images after 10 min, indicating that the film is ferroelectric. There is some attenuation of the signal, probably due to incomplete polarization switching in the film by the low polarization voltage (The maximum polarization voltage for PFM instruments is ±10 V). And the presence of a built-in electric field in the transferred film also leads to the depolarization phenomenon and produces a negative effect.
represent +10 V and −10 V, respectively). The domain walls can still be observed in the phase and amplitude images after 10 min, indicating that the film is ferroelectric. There is some attenuation of the signal, probably due to incomplete polarization switching in the film by the low polarization voltage (The maximum polarization voltage for PFM instruments is ±10 V). And the presence of a built-in electric field in the transferred film also leads to the depolarization phenomenon and produces a negative effect.Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meier, D.; Selbach, S.M. Ferroelectric domain walls for nanotechnology. Nat. Rev. Mater. 2022, 7, 157–173. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Ma, J.; Li, S.; Pan, H.; Nan, C.-W.; Lin, Y.-H. Controllable electrical, magnetoelectric and optical properties of BiFeO3 via domain engineering. Prog. Mater. Sci. 2022, 127, 100943. [Google Scholar] [CrossRef]
- Cui, B.; Fan, Z.; Li, W.; Chen, Y.; Dong, S.; Tan, Z.; Cheng, S.; Tian, B.; Tao, R.; Tian, G.; et al. Ferroelectric photosensor network: An advanced hardware solution to real-time machine vision. Nat. Commun. 2022, 13, 1707. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, J.; Li, W.; Qian, Y.; Shen, X.; Wang, X.; Shen, X.; Hu, Z.; Dong, C.; Huang, Q.; et al. A combinatory ferroelectric compound bridging simple ABO3 and A-site-ordered quadruple perovskite. Nat. Commun. 2021, 12, 747. [Google Scholar] [CrossRef]
- Yang, M.; Li, S.; Wang, Y.; Qi, H.; Lin, K.; Li, Q.; Xing, X. Enhanced ferroelectricity in NaNbO3–LaCoO3:Mn epitaxial thin film. Inorg. Chem. Front. 2021, 8, 5124–5129. [Google Scholar] [CrossRef]
- Dong, Z.; Chen, H.; Qi, M.; Shen, J.; Liu, W.; Guo, E.-J.; Li, D.; Zhang, Y.; Wu, Z. Enhanced Upconversion Photoluminescence Assisted by Flexoelectric Field in Oxide Nanomembranes. Laser Photonics Rev. 2022, 16, 2100454. [Google Scholar] [CrossRef]
- Tu, J.; Ding, J.; Xi, G.; Li, H.; Yang, Q.; Tian, J.; Zhang, L. Controllable chemical composition in double-perovskite Bi0.5Sm0.5FeO3 epitaxial thin films for ferroelectric, photovoltaic, and ferromagnetic properties. Chem. Eng. J. 2023, 453, 139726. [Google Scholar]
- Sun, H.Y.; Zhang, C.C.; Song, J.M.; Gu, J.H.; Zhang, T.W.; Zang, Y.P.; Li, Y.F.; Gu, Z.B.; Wang, P.; Nie, Y.F. Epitaxial optimization of atomically smooth Sr3Al2O6 for freestanding perovskite films by molecular beam epitaxy. Thin Solid Film. 2020, 697, 137815. [Google Scholar] [CrossRef]
- Xi, G.; Pan, Z.; Fang, Y.-W.; Tu, J.; Li, H.; Yang, Q.; Liu, C.; Luo, H.; Ding, J.; Xu, S.; et al. Anion-induced robust ferroelectricity in sulfurized pseudo-rhombohedral epitaxial BiFeO3 thin films via polarization rotation. Mater. Horiz. 2023, 10, 4389–4397. [Google Scholar] [CrossRef]
- Wang, J.; Neaton, J.B.; Zheng, H.; Nagarajan, V.; Ogale, S.B.; Liu, B.; Viehland, D.; Vaithyanathan, V.; Schlom, D.G.; Waghmare, U.V.; et al. Epitaxial BiFeO3 multiferroic thin film heterostructures. Science 2003, 299, 1719–1722. [Google Scholar] [CrossRef]
- You, L.; Zheng, F.; Fang, L.; Zhou, Y.; Tan, L.Z.; Zhang, Z.; Ma, G.; Schmidt, D.; Rusydi, A.; Wang, L.; et al. Enhancing ferroelectric photovoltaic effect by polar order engineering. Sci. Adv. 2018, 4, eaat3438. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Fan, L.; Diéguez, O.; Cao, J.; Pan, Z.; Wang, Y.; Wang, J.; Kim, M.; Deng, S.; et al. Giant polarization in super-tetragonal thin films through interphase strain. Science 2018, 361, 494–497. [Google Scholar] [CrossRef]
- Lu, D.; Baek, D.J.; Hong, S.S.; Kourkoutis, L.F.; Hikita, Y.; Hwang, H.Y. Synthesis of freestanding single-crystal perovskite films and heterostructures by etching of sacrificial water-soluble layers. Nat. Mater. 2016, 15, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, J.; Chen, P.; Jin, K.; Ma, C.; Wu, S.; Guo, E.; Ge, C.; Wang, C.; Xu, X.; et al. Magnetoelectric coupling in multiferroics probed by optical second harmonic generation. Nat. Commun. 2023, 14, 2274. [Google Scholar] [CrossRef]
- Peng, H.; Lu, N.; Yang, S.; Lyu, Y.; Liu, Z.; Bu, Y.; Shen, S.; Li, M.; Li, Z.; Gao, L.; et al. A Generic Sacrificial Layer for Wide-Range Freestanding Oxides with Modulated Magnetic Anisotropy. Adv. Funct. Mater. 2022, 32, 2111907. [Google Scholar] [CrossRef]
- Park, K.-I.; Xu, S.; Liu, Y.; Hwang, G.-T.; Kang, S.-J.L.; Wang, Z.L.; Lee, K.J. Piezoelectric BaTiO3 Thin Film Nanogenerator on Plastic Substrates. Nano Lett. 2010, 10, 4939–4943. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.K.; Cho, S.B.; Han, J.H.; Park, D.Y.; Yang, S.; Park, K.-I.; Ryu, J.; Sohn, H.; Chung, Y.-C.; Lee, K.J. Flexible highly-effective energy harvester via crystallographic and computational control of nanointerfacial morphotropic piezoelectric thin film. Nano Res. 2017, 10, 437–455. [Google Scholar] [CrossRef]
- Shen, L.; Wu, L.; Sheng, Q.; Ma, C.; Zhang, Y.; Lu, L.; Ma, J.; Ma, J.; Bian, J.; Yang, Y.; et al. Epitaxial Lift-Off of Centimeter-Scaled Spinel Ferrite Oxide Thin Films for Flexible Electronics. Adv. Mater. 2017, 29, 1702411. [Google Scholar] [CrossRef]
- Takahashi, R.; Lippmaa, M. Sacrificial Water-Soluble BaO Layer for Fabricating Free-Standing Piezoelectric Membranes. ACS Appl. Mater. Interfaces 2020, 12, 25042–25049. [Google Scholar] [CrossRef]
- Bourlier, Y.; Bérini, B.; Frégnaux, M.; Fouchet, A.; Aureau, D.; Dumont, Y. Transfer of Epitaxial SrTiO3 Nanothick Layers Using Water-Soluble Sacrificial Perovskite Oxides. ACS Appl. Mater. Interfaces 2020, 12, 8466–8474. [Google Scholar] [CrossRef]
- Dong, G.; Li, S.; Yao, M.; Zhou, Z.; Zhang, Y.-Q.; Han, X.; Luo, Z.; Yao, J.; Peng, B.; Hu, Z.; et al. Super-elastic ferroelectric single-crystal membrane with continuous electric dipole rotation. Science 2019, 366, 475–479. [Google Scholar] [CrossRef]
- Huang, J.-K.; Wan, Y.; Shi, J.; Zhang, J.; Wang, Z.; Wang, W.; Yang, N.; Liu, Y.; Lin, C.-H.; Guan, X.; et al. High-κ perovskite membranes as insulators for two-dimensional transistors. Nature 2022, 605, 262–267. [Google Scholar] [CrossRef]
- Han, L.; Addiego, C.; Prokhorenko, S.; Wang, M.; Fu, H.; Nahas, Y.; Yan, X.; Cai, S.; Wei, T.; Fang, Y.; et al. High-density switchable skyrmion-like polar nanodomains integrated on silicon. Nature 2022, 603, 63–67. [Google Scholar] [CrossRef]
- Hong, S.S.; Yu, J.H.; Lu, D.; Marshall, A.F.; Hikita, Y.; Cui, Y.; Hwang, H.Y. Two-dimensional limit of crystalline order in perovskite membrane films. Sci. Adv. 2017, 3, eaao5173. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Cai, S.; Paudel, T.R.; Sun, H.; Zhang, C.; Han, L.; Wei, Y.; Zang, Y.; Gu, M.; Zhang, Y.; et al. Freestanding crystalline oxide perovskites down to the monolayer limit. Nature 2019, 570, 87–90. [Google Scholar] [CrossRef]
- Salles, P.; Guzmán, R.; Zanders, D.; Quintana, A.; Fina, I.; Sánchez, F.; Zhou, W.; Devi, A.; Coll, M. Bendable Polycrystalline and Magnetic CoFe2O4 Membranes by Chemical Methods. ACS Appl. Mater. Interfaces 2022, 14, 12845–12854. [Google Scholar] [CrossRef] [PubMed]
- Salles, P.; Machado, P.; Yu, P.; Coll, M. Chemical synthesis of complex oxide thin films and freestanding membranes. Chem. Commun. 2023, 59, 13820–13830. [Google Scholar] [CrossRef] [PubMed]
- Saikia, N.; Kato, S.; Kojima, T. Influence of Sn on the hydration of tricalcium aluminate, Ca3Al2O6. J. Therm. Anal. Calorim. 2012, 109, 273–286. [Google Scholar] [CrossRef]
- Pai, Y.-Y.; Tylan-Tyler, A.; Irvin, P.; Levy, J. Physics of SrTiO3-based heterostructures and nanostructures: A review. Rep. Prog. Phys. 2018, 81, 036503. [Google Scholar] [CrossRef]
- Huijben, M.; Brinkman, A.; Koster, G.; Rijnders, G.; Hilgenkamp, H.; Blank, D.H.A. Structure–Property Relation of SrTiO3/LaAlO3 Interfaces. Adv. Mater. 2009, 21, 1665–1677. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, Y.; Dong, G.; Peng, B.; Zhou, Z.; Liu, M. Flexible Multiferroic Heterostructure Based on Freestanding Single-Crystalline BaTiO3 Membranes for Spintronic Devices. Adv. Electron. Mater. 2022, 8, 2100923. [Google Scholar] [CrossRef]
- Zhong, G.; An, F.; Qu, K.; Dong, Y.; Yang, Z.; Dai, L.; Xie, S.; Huang, R.; Luo, Z.; Li, J. Highly Flexible Freestanding BaTiO3-CoFe2O4 Heteroepitaxial Nanostructure Self-Assembled with Room-Temperature Multiferroicity. Small 2022, 18, 2104213. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Fang, Y.; Zhao, Y.; Zang, Y.; Gu, Z.; Nie, Y.; Pan, X. Giant Uniaxial Strain Ferroelectric Domain Tuning in Freestanding PbTiO3 Films. Adv. Mater. Interfaces 2020, 7, 1901604. [Google Scholar] [CrossRef]
- Choi, K.J.; Biegalski, M.; Li, Y.L.; Sharan, A.; Schubert, J.; Uecker, R.; Reiche, P.; Chen, Y.B.; Pan, X.Q.; Gopalan, V.; et al. Enhancement of Ferroelectricity in Strained BaTiO3 Thin Films. Science 2004, 306, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-H.; Zhou, Z.; Li, J.; Shen, B.; Zhu, T.; Gao, X.; Tao, R.; Guo, X.; Hu, X.; Shi, Y.; et al. Coupling Enhanced Performance of Triboelectric–Piezoelectric Hybrid Nanogenerator Based on Nanoporous Film of Poly(vinylidene fluoride)/BaTiO3 Composite Electrospun Fibers. ACS Mater. Lett. 2022, 4, 847–852. [Google Scholar] [CrossRef]
- Dong, G.; Li, S.; Li, T.; Wu, H.; Nan, T.; Wang, X.; Liu, H.; Cheng, Y.; Zhou, Y.; Qu, W.; et al. Periodic Wrinkle-Patterned Single-Crystalline Ferroelectric Oxide Membranes with Enhanced Piezoelectricity. Adv. Mater. 2020, 32, e2004477. [Google Scholar] [CrossRef]
- Düren, T.; Bae, Y.-S.; Snurr, R.Q. Using molecular simulation to characterise metal–organic frameworks for adsorption applications. Chem. Soc. Rev. 2009, 38, 1237–1247. [Google Scholar] [CrossRef]
- Anderson, B.J.; Tester, J.W.; Borghi, G.P.; Trout, B.L. Properties of Inhibitors of Methane Hydrate Formation via Molecular Dynamics Simulations. J. Am. Chem. Soc. 2005, 127, 17852–17862. [Google Scholar] [CrossRef]
- Bellucci, M.A.; Walsh, M.R.; Trout, B.L. Molecular Dynamics Analysis of Anti-Agglomerant Surface Adsorption in Natural Gas Hydrates. J. Phys. Chem. C 2018, 122, 2673–2683. [Google Scholar] [CrossRef]
- Kishani, S.; Benselfelt, T.; Wågberg, L.; Wohlert, J. Entropy drives the adsorption of xyloglucan to cellulose surfaces-A molecular dynamics study. J. Colloid Interface Sci. 2021, 588, 485–493. [Google Scholar] [CrossRef]
- Vazdar, M.; Pluhařová, E.; Mason, P.E.; Vácha, R.; Jungwirth, P. Ions at Hydrophobic Aqueous Interfaces: Molecular Dynamics with Effective Polarization. J. Phys. Chem. Lett. 2012, 3, 2087–2091. [Google Scholar] [CrossRef]
- Xi, G.; Ding, J.; Guo, R.; Tian, J.; Zhang, L. Enhanced switchable ferroelectric photovoltaic in BiFeO3 based films through chemical-strain-tuned polarization. Ceram. Int. 2022, 48, 15414–15421. [Google Scholar] [CrossRef]
- Cheng, X.; Xi, G.; Fang, Y.-W.; Ding, J.; Tian, J.; Zhang, L. Chemical and interfacial design in the visible-light-absorbing ferroelectric thin films. J. Eur. Ceram. Soc. 2023, 43, 3275–3288. [Google Scholar] [CrossRef]
- Guo, L.; Kaya, S.; Obot, I.B.; Zheng, X.; Qiang, Y. Toward understanding the anticorrosive mechanism of some thiourea derivatives for carbon steel corrosion: A combined DFT and molecular dynamics investigation. J. Colloid Interface Sci. 2017, 506, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Beixing, L.; Xiuji, F. Quantum chemistry studies on the hydration activity of Ca3Al2O6 and doped Ca3Al2O6. Adv. Cem. Res. 1999, 11, 103–109. [Google Scholar] [CrossRef]

 and
and  represent +10 V and −10 V, respectively). Local PFM amplitude (red) and phase (black)
hysteresis curves were acquired on BaTiO3 films before (g) and after the release (h).
represent +10 V and −10 V, respectively). Local PFM amplitude (red) and phase (black)
hysteresis curves were acquired on BaTiO3 films before (g) and after the release (h).
 and
and  represent +10 V and −10 V, respectively). Local PFM amplitude (red) and phase (black)
hysteresis curves were acquired on BaTiO3 films before (g) and after the release (h).
represent +10 V and −10 V, respectively). Local PFM amplitude (red) and phase (black)
hysteresis curves were acquired on BaTiO3 films before (g) and after the release (h).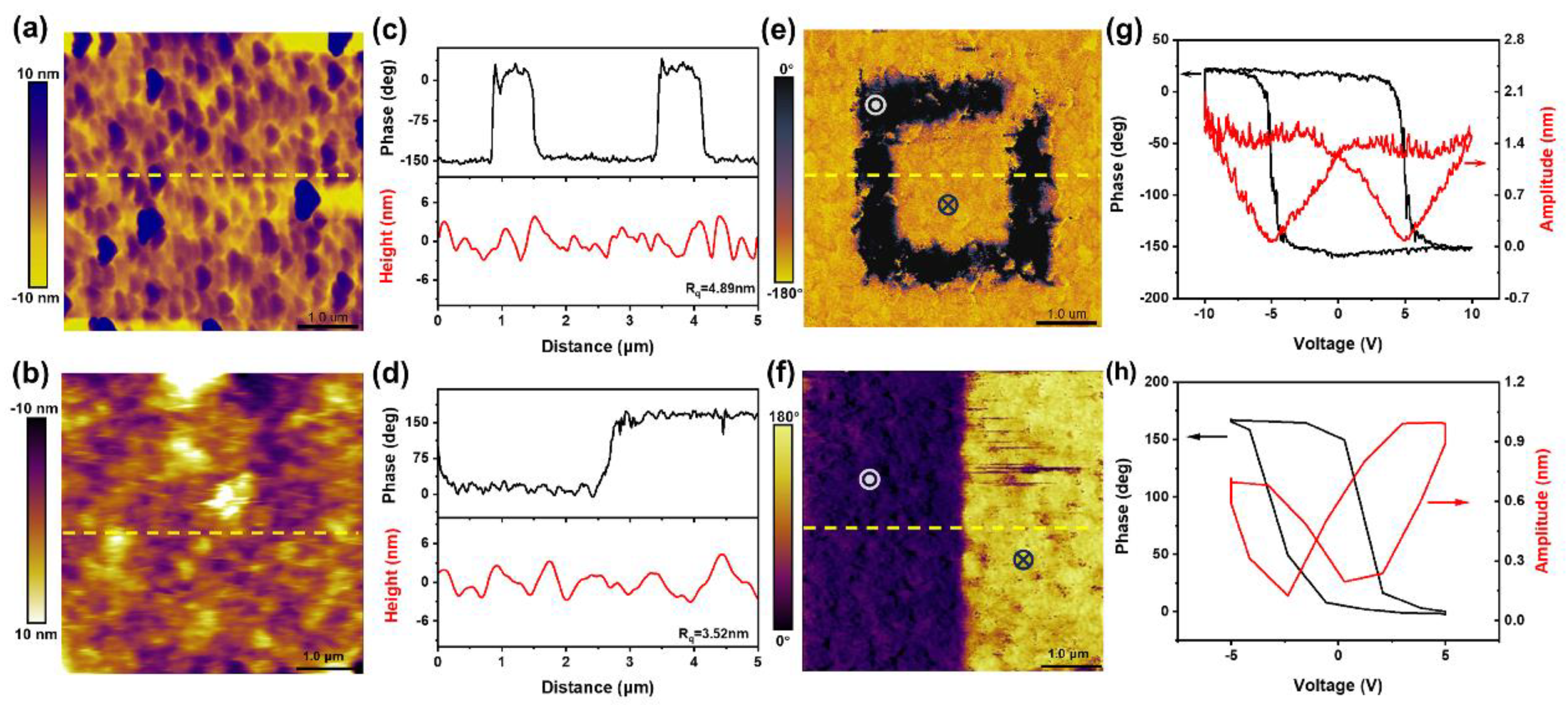
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, G.; Li, H.; Lu, D.; Liu, X.; Liu, X.; Tu, J.; Yang, Q.; Tian, J.; Zhang, L. Producing Freestanding Single-Crystal BaTiO3 Films through Full-Solution Deposition. Nanomaterials 2024, 14, 1456. https://doi.org/10.3390/nano14171456
Xi G, Li H, Lu D, Liu X, Liu X, Tu J, Yang Q, Tian J, Zhang L. Producing Freestanding Single-Crystal BaTiO3 Films through Full-Solution Deposition. Nanomaterials. 2024; 14(17):1456. https://doi.org/10.3390/nano14171456
Chicago/Turabian StyleXi, Guoqiang, Hangren Li, Dongfei Lu, Xudong Liu, Xiuqiao Liu, Jie Tu, Qianqian Yang, Jianjun Tian, and Linxing Zhang. 2024. "Producing Freestanding Single-Crystal BaTiO3 Films through Full-Solution Deposition" Nanomaterials 14, no. 17: 1456. https://doi.org/10.3390/nano14171456
APA StyleXi, G., Li, H., Lu, D., Liu, X., Liu, X., Tu, J., Yang, Q., Tian, J., & Zhang, L. (2024). Producing Freestanding Single-Crystal BaTiO3 Films through Full-Solution Deposition. Nanomaterials, 14(17), 1456. https://doi.org/10.3390/nano14171456






