Engineering Moderately Lithiophilic Paper-Based Current Collectors with Variable Solid Electrolyte Interface Films for Anode-Free Lithium Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of LDF @Cu, LDP @ Ag, and LDP @Cu-Ag
2.3. Electrochemical Measurements
3. Results and Discussion
3.1. Characterization of Lithiophilic Current Collectors
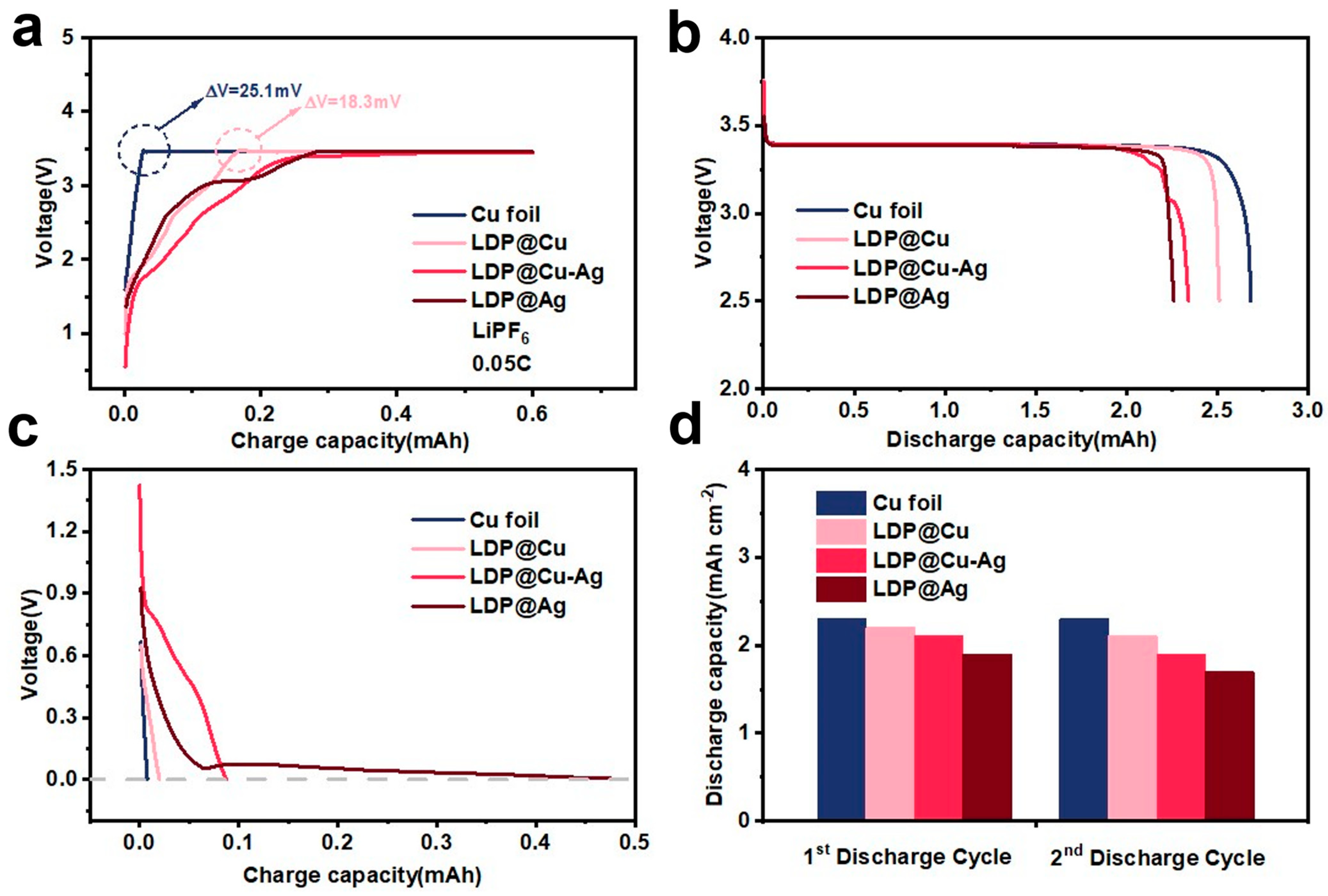
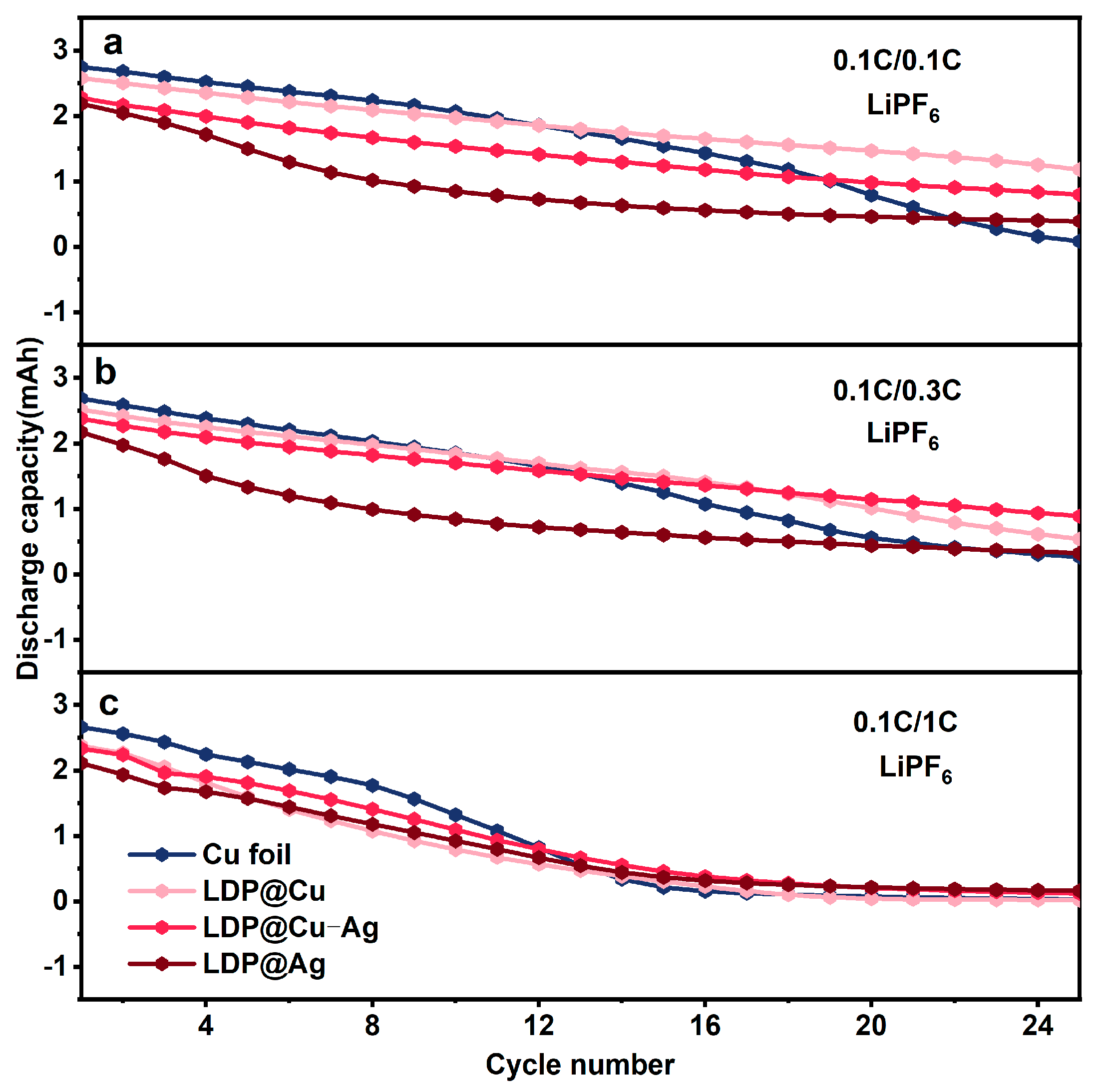
3.2. Electrochemical Performance Analysis
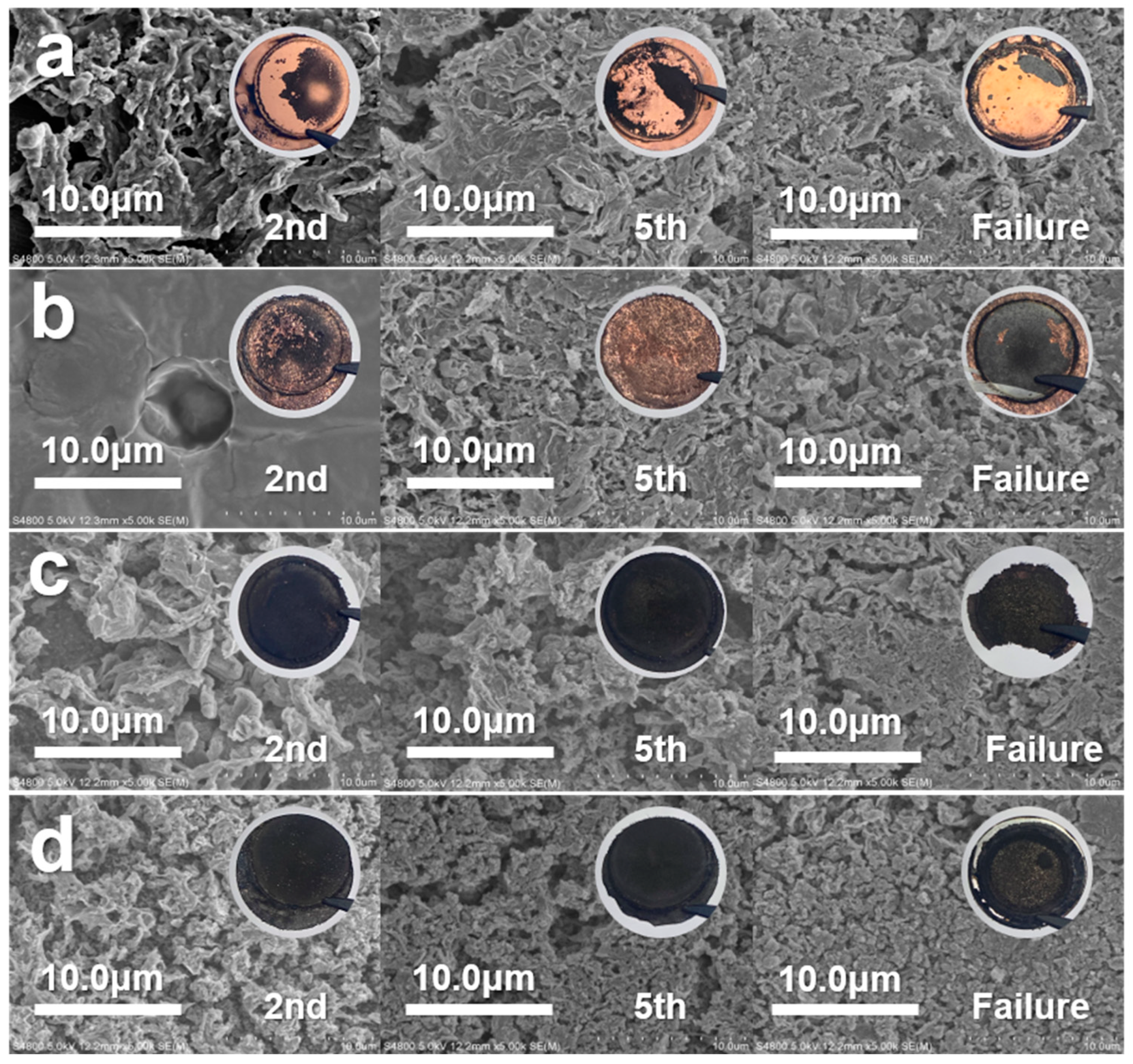
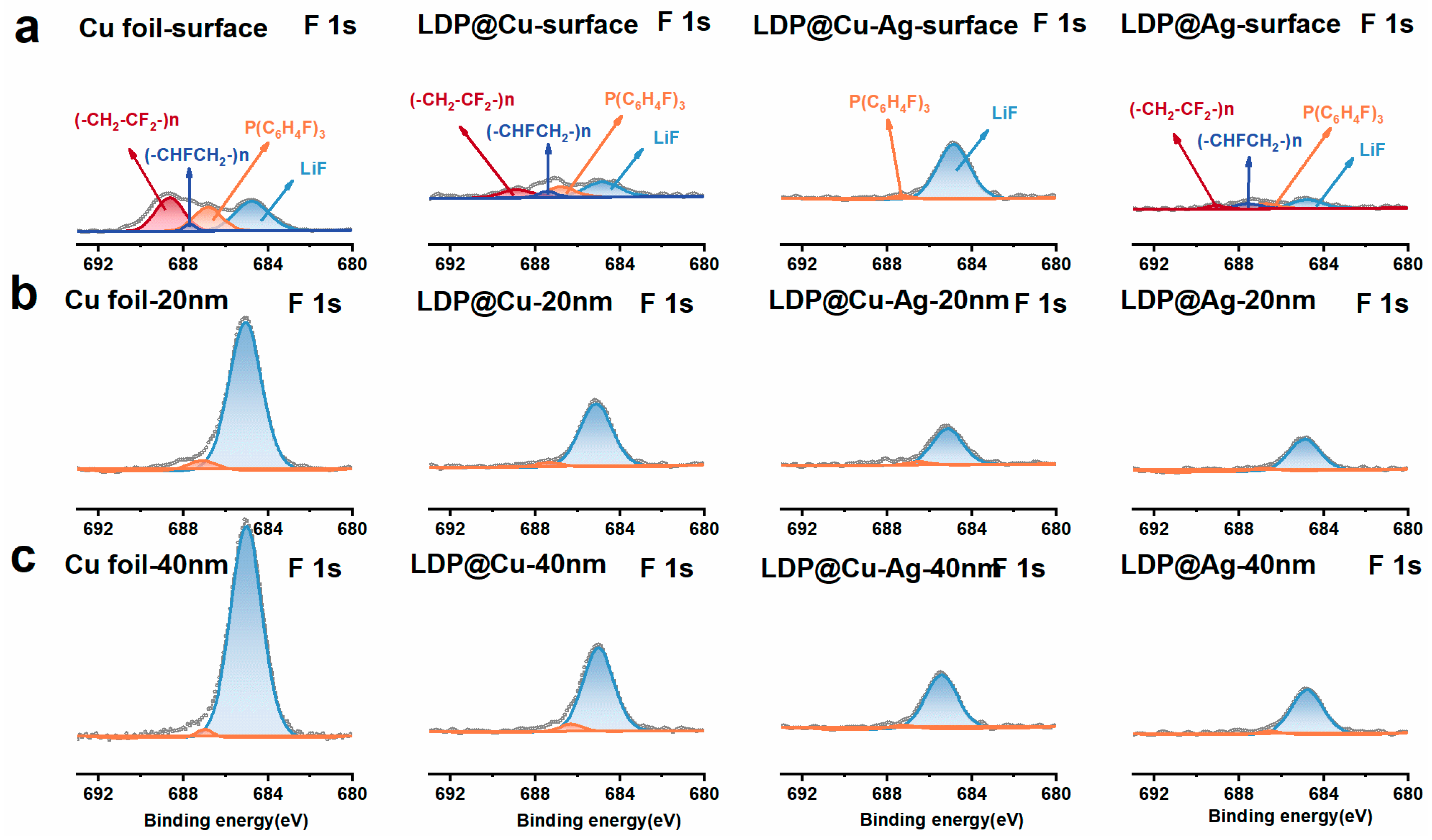
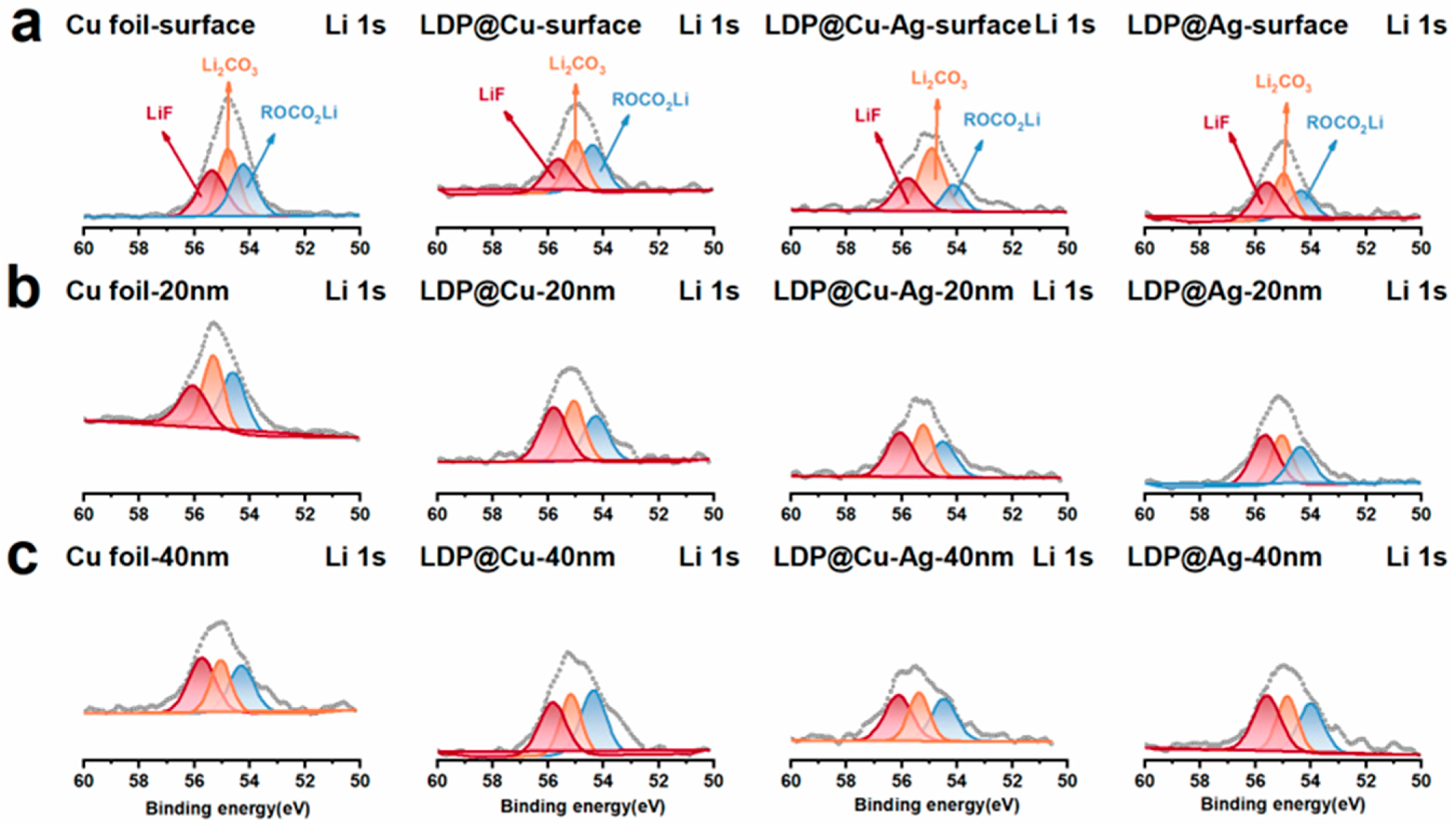
3.3. Lithium Stripping Morphology, Impedance, and XPS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.-G.; Xu, W. Advancing Lithium Metal Batteries. Joule 2018, 2, 833–845. [Google Scholar] [CrossRef]
- Buqa, H.; Goers, D.; Holzapfel, M.; Spahr, M.E.; Novák, P. High Rate Capability of Graphite Negative Electrodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2005, 152, A474. [Google Scholar] [CrossRef]
- Zheng, H.; Han, X.; Guo, W.; Lin, L.; Xie, Q.; Liu, P.; He, W.; Wang, L.; Peng, D.-L. Recent developments and challenges of Li-rich Mn-based cathode materials for high-energy lithium-ion batteries. Mater. Today Energy 2020, 18, 100518. [Google Scholar] [CrossRef]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 2–17. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.-G.; Shen, G. Pursuing two-dimensional nanomaterials for flexible lithium-ion batteries. Nano Today 2016, 11, 82–97. [Google Scholar] [CrossRef]
- Kim, S.; Park, G.; Lee, S.J.; Seo, S.; Ryu, K.; Kim, C.H.; Choi, J.W. Lithium Metal Batteries: From Fundamental Research to Industrialization. Adv. Mater. 2022, 35, 2206625. [Google Scholar] [CrossRef]
- Qian, J.; Adams, B.D.; Zheng, J.; Xu, W.; Henderson, W.A.; Wang, J.; Bowden, M.E.; Xu, S.; Hu, J.; Zhang, J.-G. Anode-free rechargeable lithium metal batteries. Adv. Funct. Mater. 2016, 26, 7094–7102. [Google Scholar] [CrossRef]
- Nanda, S.; Gupta, A.; Manthiram, A. Anode-free full cells: A pathway to high-energy density lithium-metal batteries. Adv. Energy Mater. 2021, 11, 2000804. [Google Scholar] [CrossRef]
- Xie, Z.; Wu, Z.; An, X.; Yue, X.; Wang, J.; Abudula, A.; Guan, G. Anode-free rechargeable lithium metal batteries: Progress and prospects. Energy Storage Mater. 2020, 32, 386–401. [Google Scholar] [CrossRef]
- Molaiyan, P.; Abdollahifar, M.; Boz, B.; Beutl, A.; Krammer, M.; Zhang, N.; Tron, A.; Romio, M.; Ricci, M.; Adelung, R.; et al. Optimizing Current Collector Interfaces for Efficient “Anode-Free” Lithium Metal Batteries. Adv. Funct. Mater. 2024, 34, 2311301. [Google Scholar] [CrossRef]
- Zhang, J.-G. Anode-less. Nat. Energy 2019, 4, 637–638. [Google Scholar] [CrossRef]
- Ponnada, S.; Kiai, M.S.; Krishnapriya, R.; Singhal, R.; Sharma, R.K. Lithium-Free Batteries: Needs and Challenges. Energy Fuels 2022, 36, 6013–6026. [Google Scholar] [CrossRef]
- Beyene, T.T.; Bezabh, H.K.; Weret, M.A.; Hagos, T.M.; Huang, C.-J.; Wang, C.-H.; Su, W.-N.; Dai, H.; Hwang, B.-J. Concentrated dual-salt electrolyte to stabilize Li metal andincrease cycle life of anode free Li-metal batteries. J. Electrochem. Soc. 2019, 166, A1501. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, X.; Zhao, S.; He, P.; Zhou, H. Research Progress of Organic Electrolyte Based Lithium-Air Batteries. Acta Chim. Sin. 2014, 72, 417. [Google Scholar] [CrossRef]
- Li, N.-W.; Shi, Y.; Yin, Y.-X.; Zeng, X.-X.; Li, J.-Y.; Li, C.-J.; Wan, L.-J.; Wen, R.; Guo, Y.-G. A Flexible Solid Electrolyte Interphase Layer for Long-Life Lithium Metal Anodes. Angew. Chem. 2018, 130, 1521–1525. [Google Scholar] [CrossRef]
- Lin, L.; Qin, K.; Hu, Y.; Li, H.; Huang, X.; Suo, L.; Chen, L. A Better Choice to Achieve High Volumetric Energy Density: Anode-Free Lithium-Metal Batteries. Adv. Mater. 2022, 34, 2110323. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xiang, J.; Chen, X.; Yuan, L.; Li, Z.; Huang, Y. Li2S-based anode-free full batteries with modified Cu current collector. Energy Storage Mater. 2020, 30, 179–186. [Google Scholar] [CrossRef]
- Zhou, B.; Bonakdarpour, A.; Stoševski, I.; Fang, B.; Wilkinson, D.P. Modification of Cu current collectors for lithium metal batteries—A review. Prog. Mater. Sci. 2022, 130, 100996. [Google Scholar] [CrossRef]
- An, G.-H.; Cha, S.; Ahn, H.-J. Surface functionalization of the terraced surface-based current collector for a supercapacitor with an improved energy storage performance. Appl. Surf. Sci. 2019, 478, 435–440. [Google Scholar] [CrossRef]
- Li, D.; Hu, H.; Chen, B.; Lai, W. Advanced Current Collector Materials for High-Performance Lithium Metal Anodes. Small 2022, 18, 2200010. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Pathak, R.; Gurung, A.; Reza, K.M.; Ghimire, N.; Baniya, A.; He, W.; Wu, J.J.; Zhou, Y. Copper-clad Lithiophilic Current Collector for Dendrite-Free Lithium Metal Anode. J. Mater. Chem. A 2020, 9, 1911–1919. [Google Scholar] [CrossRef]
- Loghavi, M.M.; Askari, M.; Babaiee, M.; Ghasemi, A. Improvement of the cyclability of Li-ion battery cathode using a chemical-modified current collector. J. Electroanal. Chem. 2019, 841, 107–110. [Google Scholar] [CrossRef]
- Liu, Y.; Xiong, S.; Wang, J.; Jiao, X.; Li, S.; Zhang, C.; Song, Z.; Song, J. Dendrite-free lithium metal anode enabled by separator engineering via uniform loading of lithiophilic nucleation sites. Energy Storage Mater. 2019, 19, 24–30. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Hitz, E.; Luo, W.; Yao, Y.; Li, Y.; Dai, J.; Chen, C.; Wang, Y.; Yang, C.; et al. A carbon-based 3D current collector with surface protection for Li metal anode. Nano Res. 2017, 10, 1356–1365. [Google Scholar] [CrossRef]
- Lin, L.; Suo, L.; Hu, Y.S.; Li, H.; Huang, X.; Chen, L. Epitaxial Induced Plating Current-Collector Lasting Lifespan of Anode-Free Lithium Metal Battery. Adv. Energy Mater. 2021, 11, 2003709. [Google Scholar] [CrossRef]
- Xiong, C.; Zhang, Y.; Zheng, C.; Yin, Y.; Xiong, Q.; Zhao, M.; Wang, B. Farication of metal-organic framework@cellulose nanofibers/reduced graphene oxide-Vitrimer composite electrode materials with shape memory for supercapacitors. Electrochim. Acta 2024, 493, 144373. [Google Scholar] [CrossRef]
- Yan, K.; Lu, Z.; Lee, H.-W.; Xiong, F.; Hsu, P.-C.; Li, Y.; Zhao, J.; Chu, S.; Cui, Y. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat. Energy 2016, 1, 16010. [Google Scholar] [CrossRef]
- Cha, E.; Yun, J.H.; Ponraj, R.; Kim, D.K. A mechanistic review of lithiophilic materials: Resolving lithium dendrites and advancing lithium metal-based batteries. Mater. Chem. Front. 2021, 5, 6294–6314. [Google Scholar] [CrossRef]
- Liang, X.; Pang, Q.; Kochetkov, I.R.; Sempere, M.S.; Huang, H.; Sun, X.; Nazar, L.F. A facile surface chemistry route to a stabilized lithium metal anode. Nat. Energy 2017, 2, 17119. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, S.L.; Wu, Z.P.; Luan, D.; Lou, X.W.D. A highly stable lithium metal anode enabled by Ag nanoparticle–embedded nitrogen-doped carbon macroporous fibers. Sci. Adv. 2021, 7, eabg3626. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Sekine, K.; Takamura, T. Evaluation of the Diffusion Coefficient of Li in Ag Using a Li+ Sensor Electrode Mounted in a Bipolar Cell. Electrochemistry 2006, 74, 303–308. [Google Scholar] [CrossRef]
- Krauskopf, T.; Mogwitz, B.; Rosenbach, C.; Zeier, W.G.; Janek, J. Diffusion Limitation of Lithium Metal and Li–Mg Alloy Anodes on LLZO Type Solid Electrolytes as a Function of Temperature and Pressure. Adv. Energy Mater. 2019, 9, 1902568. [Google Scholar] [CrossRef]
- Lodding, A.; Mundy, J.N.; Ott, A. Isotope Inter-Diffusion and Self-Diffusion in Solid Lithium Metal. Physia. Status Solidi B 1970, 38, 559–569. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Wan, M.; Wang, Z.; Zou, X.; Zhao, Y.; Sun, L. A Facile Method for the Fabrication of Silver Nanoparticles Surface Decorated Polyvinyl Alcohol Electrospun Nanofibers and Controllable Antibacterial Activities. Polymers 2020, 12, 2486. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, L.; Li, J.; Kou, L.; Huang, J.; Feng, Y.; Chen, S. Cu/Cu2O@Ppy Nanowires as a Long-Life and High-Capacity Anode for Lithium Ion Battery. Chem. Eng. J. 2020, 391, 123597. [Google Scholar] [CrossRef]
- Sand HJ, S. On the Concentration at the Electrodes in a Solution, with special reference to the Liberation of Hydrogen by Electrolysis of a Mixture of Copper Sulphate and Sulphuric Acid. Proc. Phys. Soc. Lond. 1899, 17, 496–534. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Naotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Ji, X.; Li, Y.; Shao, Y.; Chen, Z.; Zhong, C.; Amine, K. Design strategies for nonaqueous multivalent-ion and monovalent-ion battery anodes. Nat. Rev. Mater. 2020, 5, 276–294. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Wei, F.; Zhang, J.-G.; Zhang, Q. A Review of Solid Electrolyte Interphases on Lithium Metal Anode. Adv. Sci. 2016, 3, 1500213. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, S.; Li, Y.; Xin, S.; Manthiram, A.; Goodenough, J.B. Plating a Dendrite-Free Lithium Anode with a Polymer/Ceramic/Polymer Sandwich Electrolyte. J. Am. Chem. Soc. 2016, 138, 9385–9388. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, D.; Xiang, H.; Feng, X.; Yu, Y. Research Progress on Copper-Based Current Collector for Lithium Metal Batteries. Energy Fuels 2021, 35, 12921–12937. [Google Scholar] [CrossRef]
- Tan, Y.-H.; Lu, G.-X.; Zheng, J.-H.; Zhou, F.; Chen, M.; Ma, T.; Lu, L.-L.; Song, Y.-H.; Guan, Y.; Wang, J.; et al. Lithium Fluoride in Electrolyte for Stable and Safe Lithium-Metal Batteries. Adv. Mater. 2021, 33, 2102134. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, W.; Chen, Z.; Zhang, C.; Jiang, L.; Yu, F. A lithiophilic hyperbranched polymer-decorated three-dimensional carbon skeleton boosting highly reversible lithium metal anode. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129104. [Google Scholar] [CrossRef]
- Ismail, I.; Noda, A.; Nishimoto, A.; Watanabe, M. XPS study of lithium surface after contact with lithium-salt doped polymer electrolytes. Electrochim. Acta 2001, 46, 1595–1603. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Wei, H.; Wang, H.; Wu, H.; Guo, Y.; Ren, X.; Xiong, C.; Liu, H.; Wu, H. Engineering Moderately Lithiophilic Paper-Based Current Collectors with Variable Solid Electrolyte Interface Films for Anode-Free Lithium Batteries. Nanomaterials 2024, 14, 1461. https://doi.org/10.3390/nano14171461
Yang B, Wei H, Wang H, Wu H, Guo Y, Ren X, Xiong C, Liu H, Wu H. Engineering Moderately Lithiophilic Paper-Based Current Collectors with Variable Solid Electrolyte Interface Films for Anode-Free Lithium Batteries. Nanomaterials. 2024; 14(17):1461. https://doi.org/10.3390/nano14171461
Chicago/Turabian StyleYang, Baohong, Hairu Wei, Huan Wang, Haoteng Wu, Yanbo Guo, Xuan Ren, Chuanyin Xiong, Hanbin Liu, and Haiwei Wu. 2024. "Engineering Moderately Lithiophilic Paper-Based Current Collectors with Variable Solid Electrolyte Interface Films for Anode-Free Lithium Batteries" Nanomaterials 14, no. 17: 1461. https://doi.org/10.3390/nano14171461
APA StyleYang, B., Wei, H., Wang, H., Wu, H., Guo, Y., Ren, X., Xiong, C., Liu, H., & Wu, H. (2024). Engineering Moderately Lithiophilic Paper-Based Current Collectors with Variable Solid Electrolyte Interface Films for Anode-Free Lithium Batteries. Nanomaterials, 14(17), 1461. https://doi.org/10.3390/nano14171461







