Bandgap Engineering via Doping Strategies for Narrowing the Bandgap below 1.2 eV in Sn/Pb Binary Perovskites: Unveiling the Role of Bi3+ Incorporation on Different A-Site Compositions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Perovskite Precursors
2.3. Fabrication of Tin-Lead Perovskite Thin Films and Devices
2.4. Characterizations
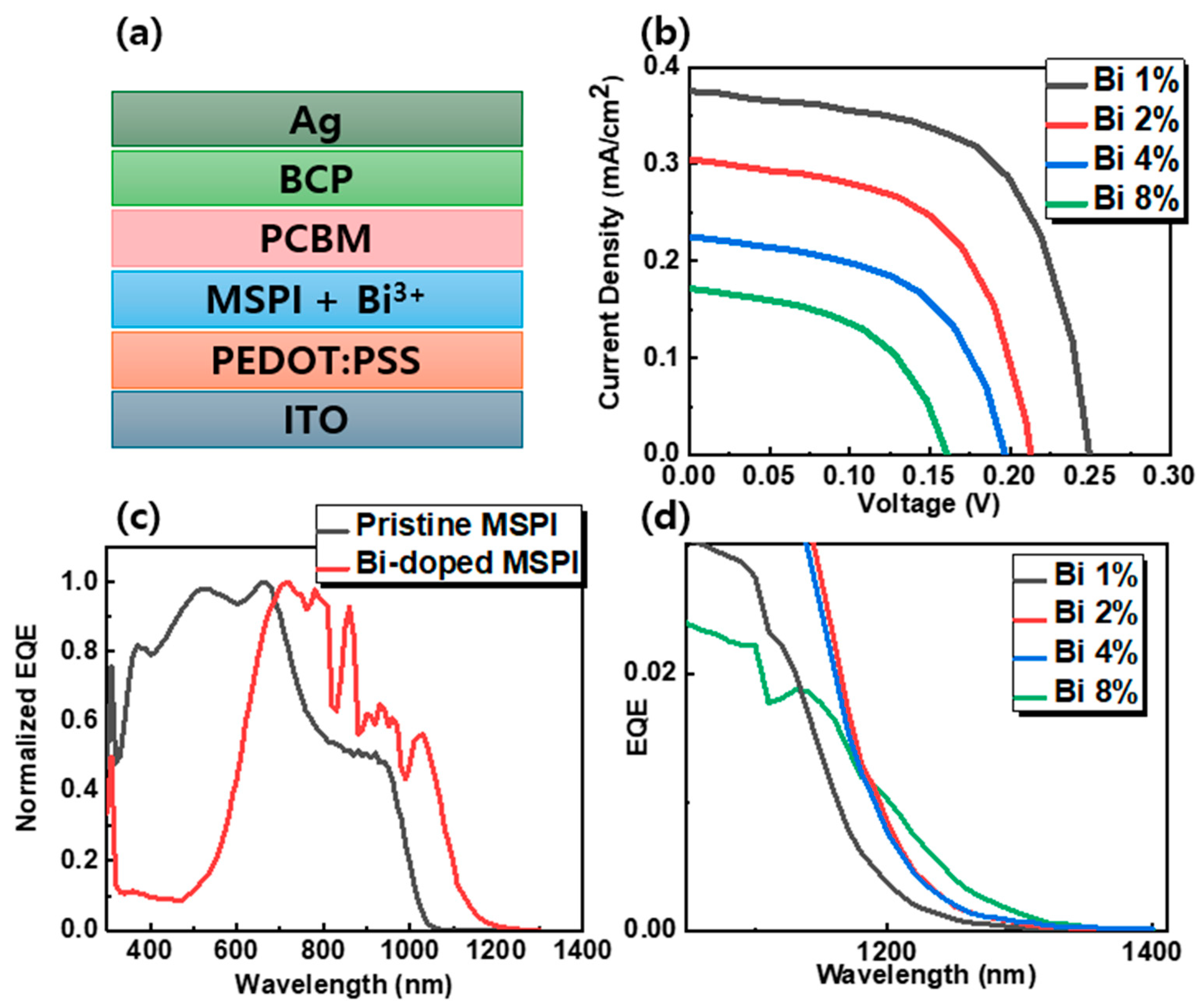
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, J.; Yun, H.-S.; Paik, M.J.; Noh, E.; Mun, H.J.; Kim, M.G.; Shin, T.J.; Seok, S.I. Controlled growth of perovskite layers with volatile alkylammonium chlorides. Nature 2023, 616, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Burschka, J.; Pellet, N.; Moon, S.-J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Tsai, H.; Asadpour, R.; Blancon, J.-C.; Neukirch, A.J.; Gupta, G.; Crochet, J.J.; Chhowalla, M.; Tretiak, S.; Alam, M.A. High-efficiency solution-processed perovskite solar cells with millimeter-scale grains. Science 2015, 347, 522–525. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef]
- Wehrenfennig, C.; Eperon, G.E.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. High charge carrier mobilities and lifetimes in organolead trihalide perovskites. Adv. Mater. 2014, 26, 1584. [Google Scholar] [CrossRef]
- Baranowski, M.; Plochocka, P. Excitons in metal-halide perovskites. Adv. Energy Mater. 2020, 10, 1903659. [Google Scholar] [CrossRef]
- Lim, J.; Kober-Czerny, M.; Lin, Y.-H.; Ball, J.M.; Sakai, N.; Duijnstee, E.A.; Hong, M.J.; Labram, J.G.; Wenger, B.; Snaith, H.J. Long-range charge carrier mobility in metal halide perovskite thin-films and single crystals via transient photo-conductivity. Nat. Commun. 2022, 13, 4201. [Google Scholar] [CrossRef]
- Xu, J.; Maxwell, A.; Wei, M.; Wang, Z.; Chen, B.; Zhu, T.; Sargent, E.H. Defect tolerance of mixed B-site organic–inorganic halide perovskites. ACS Energy Lett. 2021, 6, 4220–4227. [Google Scholar] [CrossRef]
- Zhang, W.; Saliba, M.; Moore, D.T.; Pathak, S.K.; Hörantner, M.T.; Stergiopoulos, T.; Stranks, S.D.; Eperon, G.E.; Alexander-Webber, J.A.; Abate, A. Ultrasmooth organic–inorganic perovskite thin-film formation and crystallization for efficient planar heterojunction solar cells. Nat. Commun. 2015, 6, 6142. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Huang, F.; Huang, W.; Dkhissi, Y.; Zhu, Y.; Etheridge, J.; Gray-Weale, A.; Bach, U.; Cheng, Y.B.; Spiccia, L. A fast deposition-crystallization procedure for highly efficient lead iodide perovskite thin-film solar cells. Angew. Chem. Int. Ed. 2014, 53, 9898–9903. [Google Scholar] [CrossRef] [PubMed]
- Pellet, N.; Gao, P.; Gregori, G.; Yang, T.Y.; Nazeeruddin, M.K.; Maier, J.; Grätzel, M. Mixed-organic-cation Perovskite photovoltaics for enhanced solar-light harvesting. Angew. Chem. Int. Ed. 2014, 53, 3151–3157. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.C.; Hoke, E.T.; Solis-Ibarra, D.; McGehee, M.D.; Karunadasa, H.I. A layered hybrid perovskite solar-cell absorber with enhanced moisture stability. Angew. Chem. Int. Ed. 2014, 53, 11232–11235. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Z.; Zhou, Y.; Pang, S.; Wang, D.; Xu, H.; Liu, Z.; Padture, N.P.; Cui, G. Methylamine-gas-induced defect-healing behavior of CH3NH3PbI3 thin films for perovskite solar cells. Angew. Chem. 2015, 127, 9841–9845. [Google Scholar] [CrossRef]
- Yang, T.Y.; Gregori, G.; Pellet, N.; Grätzel, M.; Maier, J. The significance of ion conduction in a hybrid organic–inorganic lead-iodide-based perovskite photosensitizer. Angew. Chem. 2015, 127, 8016–8021. [Google Scholar] [CrossRef]
- Zhang, H.; Pfeifer, L.; Zakeeruddin, S.M.; Chu, J.; Grätzel, M. Tailoring passivators for highly efficient and stable perovskite solar cells. Nat. Rev. Chem. 2023, 7, 632–652. [Google Scholar] [CrossRef]
- Jaffe, A.; Lin, Y.; Mao, W.L.; Karunadasa, H.I. Pressure-induced metallization of the halide perovskite (CH3NH3) PbI3. J. Am. Chem. Soc. 2017, 139, 4330–4333. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Pandey, P.; Lee, S.; Shen, Q.; Kang, D.-W. Super narrow bandgap (<1.2 eV) halide double perovskites: Recent advancements and future perspectives. Chem. Eng. J. 2024, 491, 152026. [Google Scholar] [CrossRef]
- Xiao, G.; Cao, Y.; Qi, G.; Wang, L.; Liu, C.; Ma, Z.; Yang, X.; Sui, Y.; Zheng, W.; Zou, B. Pressure effects on structure and optical properties in cesium lead bromide perovskite nanocrystals. J. Am. Chem. Soc. 2017, 139, 10087–10094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Pei, L.; Li, J.; Wang, K.; Zeng, Q.; Yu, H. Achieving band gap reduction and carrier lifetime enhancement in metal halide perovskites via mechanical stretching. J. Phys. Chem. Lett. 2021, 12, 7207–7212. [Google Scholar] [CrossRef] [PubMed]
- Linaburg, M.R.; McClure, E.T.; Majher, J.D.; Woodward, P.M. Cs1−xRbxPbCl3 and Cs1−xRbxPbBr3 solid solutions: Understanding octahedral tilting in lead halide perovskites. Chem. Mater. 2017, 29, 3507–3514. [Google Scholar] [CrossRef]
- Xiang, W.; Wang, Z.; Kubicki, D.J.; Wang, X.; Tress, W.; Luo, J.; Zhang, J.; Hofstetter, A.; Zhang, L.; Emsley, L. Ba-induced phase segregation and band gap reduction in mixed-halide inorganic perovskite solar cells. Nat. Commun. 2019, 10, 4686. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ryu, J.; Park, S.S.; Yoon, S.; Lee, D.-G.; Moon, J.; Kim, Y.J.; Kang, D.-W. A self-assembled hierarchical structure to keep the 3D crystal dimensionality in n-butylammonium cation-capped Pb–Sn perovskites. J. Mater. Chem. A 2021, 9, 27541–27550. [Google Scholar] [CrossRef]
- Tang, Z.; Bessho, T.; Awai, F.; Kinoshita, T.; Maitani, M.M.; Jono, R.; Murakami, T.N.; Wang, H.; Kubo, T.; Uchida, S. Hysteresis-free perovskite solar cells made of potassium-doped organometal halide perovskite. Sci. Rep. 2017, 7, 12183. [Google Scholar] [CrossRef]
- Lee, S.; Kang, D.-W. Highly efficient and stable Sn-rich perovskite solar cells by introducing bromine. ACS Appl. Mater. Interfaces 2017, 9, 22432–22439. [Google Scholar] [CrossRef]
- Li, C.; Wei, J.; Sato, M.; Koike, H.; Xie, Z.-Z.; Li, Y.-Q.; Kanai, K.; Kera, S.; Ueno, N.; Tang, J.-X. Halide-substituted electronic properties of organometal halide perovskite films: Direct and inverse photoemission studies. ACS Appl. Mater. Interfaces 2016, 8, 11526–11531. [Google Scholar] [CrossRef]
- Zhao, B.; Abdi-Jalebi, M.; Tabachnyk, M.; Glass, H.; Kamboj, V.S.; Nie, W.; Pearson, A.J.; Puttisong, Y.; Gödel, K.C.; Beere, H.E. High open-circuit voltages in tin-rich low-bandgap perovskite-based planar heterojunction photovoltaics. Adv. Mater. 2017, 29, 1604744. [Google Scholar] [CrossRef]
- Lee, S.; Moon, J.; Ryu, J.; Parida, B.; Yoon, S.; Lee, D.-G.; Cho, J.S.; Hayase, S.; Kang, D.-W. Inorganic narrow bandgap CsPb0. 4Sn0. 6I2. 4Br0. 6 perovskite solar cells with exceptional efficiency. Nano Energy 2020, 77, 105309. [Google Scholar] [CrossRef]
- Lau, C.F.J.; Zhang, M.; Deng, X.; Zheng, J.; Bing, J.; Ma, Q.; Kim, J.; Hu, L.; Green, M.A.; Huang, S. Strontium-doped low-temperature-processed CsPbI2Br perovskite solar cells. ACS Energy Lett. 2017, 2, 2319–2325. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Meggiolaro, D.; Dang, Z.; De Angelis, F.; Manna, L. Fluorescent alloy CsPbxMn1−xI3 perovskite nanocrystals with high structural and optical stability. ACS Energy Lett. 2017, 2, 2183–2186. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Liu, Y.; Li, J.; Liu, C.; Feng, R.; Jiang, F.; Li, Y.; Song, J.; Zeng, H.; Hong, M. Stabilizing cesium lead halide perovskite lattice through Mn (II) substitution for air-stable light-emitting diodes. J. Am. Chem. Soc. 2017, 139, 11443–11450. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Xu, L.; Wu, P.; Sun, L.; Xie, G.; Hu, B. Bismuth doping–induced stable seebeck effect based on MAPbI3 polycrystalline thin films. Adv. Funct. Mater. 2019, 29, 1900615. [Google Scholar] [CrossRef]
- Hu, Y.; Qiu, T.; Bai, F.; Miao, X.; Zhang, S. Enhancing moisture-tolerance and photovoltaic performances of FAPbI3 by bismuth incorporation. J. Mater. Chem. A 2017, 5, 25258–25265. [Google Scholar] [CrossRef]
- Yavari, M.; Ebadi, F.; Meloni, S.; Wang, Z.S.; Yang, T.C.-J.; Sun, S.; Schwartz, H.; Wang, Z.; Niesen, B.; Durantini, J. How far does the defect tolerance of lead-halide perovskites range? The example of Bi impurities introducing efficient recombination centers. J. Mater. Chem. A 2019, 7, 23838–23853. [Google Scholar] [CrossRef]
- Kundu, S.; Zhang, D.; Askar, A.M.; Moloney, E.G.; Adachi, M.M.; Nadeem, A.; Moradi, S.; Yeddu, V.; Abdelhady, A.L.; Voznyy, O. Bismuth stabilizes the α-phase of formamidinium lead iodide perovskite single crystals. ACS Mater. Lett. 2022, 4, 707–712. [Google Scholar] [CrossRef]
- Hu, Y.; Bai, F.; Liu, X.; Ji, Q.; Miao, X.; Qiu, T.; Zhang, S. Bismuth incorporation stabilized α-CsPbI3 for fully inorganic perovskite solar cells. ACS Energy Lett. 2017, 2, 2219–2227. [Google Scholar] [CrossRef]
- Kajal, S.; Kim, J.; Shin, Y.S.; Singh, A.N.; Myung, C.W.; Kim, J.Y.; Kim, K.S. Unfolding the influence of metal doping on properties of CsPbI3 perovskite. Small Methods 2020, 4, 2000296. [Google Scholar] [CrossRef]
- Wang, G.; Cheng, L.; Bi, J.; Chang, J.; Meng, F. B-site doping with bismuth ion enhances the efficiency and stability of inorganic CsSnI3 perovskite solar cell. Mater. Lett. 2024, 354, 135394. [Google Scholar] [CrossRef]
- Lee, M.; Yoo, B.; Im, J.; Hyeon, T.; Chung, I. Electronic band engineering via MI3 (M = Sb, Bi) doping remarkably enhances the air stability of perovskite CsSnI3. ACS Appl. Energy Mater. 2020, 3, 10477–10484. [Google Scholar] [CrossRef]
- Tauc, J. Amorphous and Liquid Semiconductors; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Lehner, A.J.; Fabini, D.H.; Evans, H.A.; Hébert, C.-A.; Smock, S.R.; Hu, J.; Wang, H.; Zwanziger, J.W.; Chabinyc, M.L.; Seshadri, R. Crystal and electronic structures of complex bismuth iodides A3Bi2I9 (A = K, Rb, Cs) related to perovskite: Aiding the rational design of photovoltaics. Chem. Mater. 2015, 27, 7137–7148. [Google Scholar] [CrossRef]
- Park, B.-W.; Philippe, B.; Zhang, X.; Rensmo, H.; Boschloo, G.; Johansson, E. Bismuth Based Hybrid Perovskites A3Bi2I9 (A: Methylammonium or Cesium) for Solar Cell Application. Adv. Mater. 2015, 27, 6806–6813. [Google Scholar] [CrossRef] [PubMed]
- Saparov, B.; Hong, F.; Sun, J.-P.; Duan, H.-S.; Meng, W.; Cameron, S.; Hill, I.G.; Yan, Y.; Mitzi, D.B. Thin-film preparation and characterization of Cs3Sb2I9: A lead-free layered perovskite semiconductor. Chem. Mater. 2015, 27, 5622–5632. [Google Scholar] [CrossRef]
- Wang, L.; Chen, P.; Kuttipillai, P.S.; King, I.; Staples, R.; Sun, K.; Lunt, R.R. Epitaxial stabilization of tetragonal cesium tin iodide. ACS Appl. Mater. Interfaces 2019, 11, 32076–32083. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, C.; Wang, L.; Li, Y.; Ren, Y.; Shum, K. Energy barrier at the N719-dye/CsSnI3 interface for photogenerated holes in dye-sensitized solar cells. Sci. Rep. 2014, 4, 6954. [Google Scholar] [CrossRef]
- Lee, B.; Stoumpos, C.C.; Zhou, N.; Hao, F.; Malliakas, C.; Yeh, C.-Y.; Marks, T.J.; Kanatzidis, M.G.; Chang, R.P. Air-stable molecular semiconducting iodosalts for solar cell applications: Cs2SnI6 as a hole conductor. J. Am. Chem. Soc. 2014, 136, 15379–15385. [Google Scholar] [CrossRef]
- Saparov, B.; Sun, J.-P.; Meng, W.; Xiao, Z.; Duan, H.-S.; Gunawan, O.; Shin, D.; Hill, I.G.; Yan, Y.; Mitzi, D.B. Thin-film deposition and characterization of a Sn-deficient perovskite derivative Cs2SnI6. Chem. Mater. 2016, 28, 2315–2322. [Google Scholar] [CrossRef]
- Qiu, X.; Cao, B.; Yuan, S.; Chen, X.; Qiu, Z.; Jiang, Y.; Ye, Q.; Wang, H.; Zeng, H.; Liu, J. From unstable CsSnI3 to air-stable Cs2SnI6: A lead-free perovskite solar cell light absorber with bandgap of 1.48 eV and high absorption coefficient. Sol. Energy Mater. Sol. Cells 2017, 159, 227–234. [Google Scholar] [CrossRef]
- Zhang, Z.; Ren, L.; Yan, H.; Guo, S.; Wang, S.; Wang, M.; Jin, K. Bandgap narrowing in Bi-doped CH3NH3PbCl3 perovskite single crystals and thin films. J. Phys. Chem. C 2017, 121, 17436–17441. [Google Scholar] [CrossRef]
- Abdelhady, A.L.; Saidaminov, M.I.; Murali, B.; Adinolfi, V.; Voznyy, O.; Katsiev, K.; Alarousu, E.; Comin, R.; Dursun, I.; Sinatra, L. Heterovalent dopant incorporation for bandgap and type engineering of perovskite crystals. J. Phys. Chem. Lett. 2016, 7, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, X.; He, J.; Ma, C.; Xu, L.; Sheng, P.; Huang, F. Bi3+-doped CH3NH3PbI3: Red-shifting absorption edge and longer charge carrier lifetime. J. Alloys Compd. 2017, 695, 555–560. [Google Scholar] [CrossRef]
- Ouyang, R. Exploiting ionic radii for rational design of halide perovskites. Chem. Mater. 2019, 32, 595–604. [Google Scholar] [CrossRef]
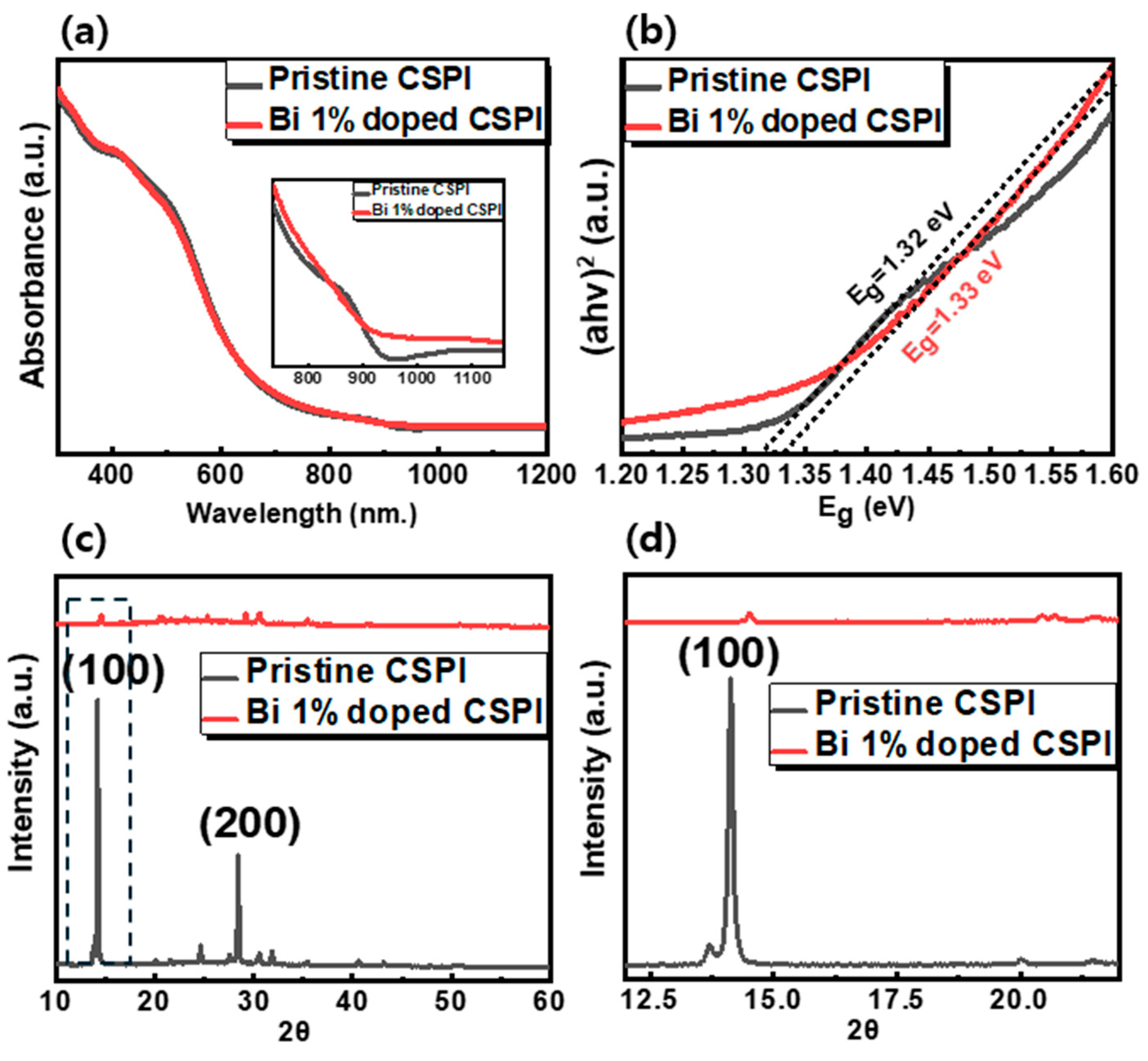
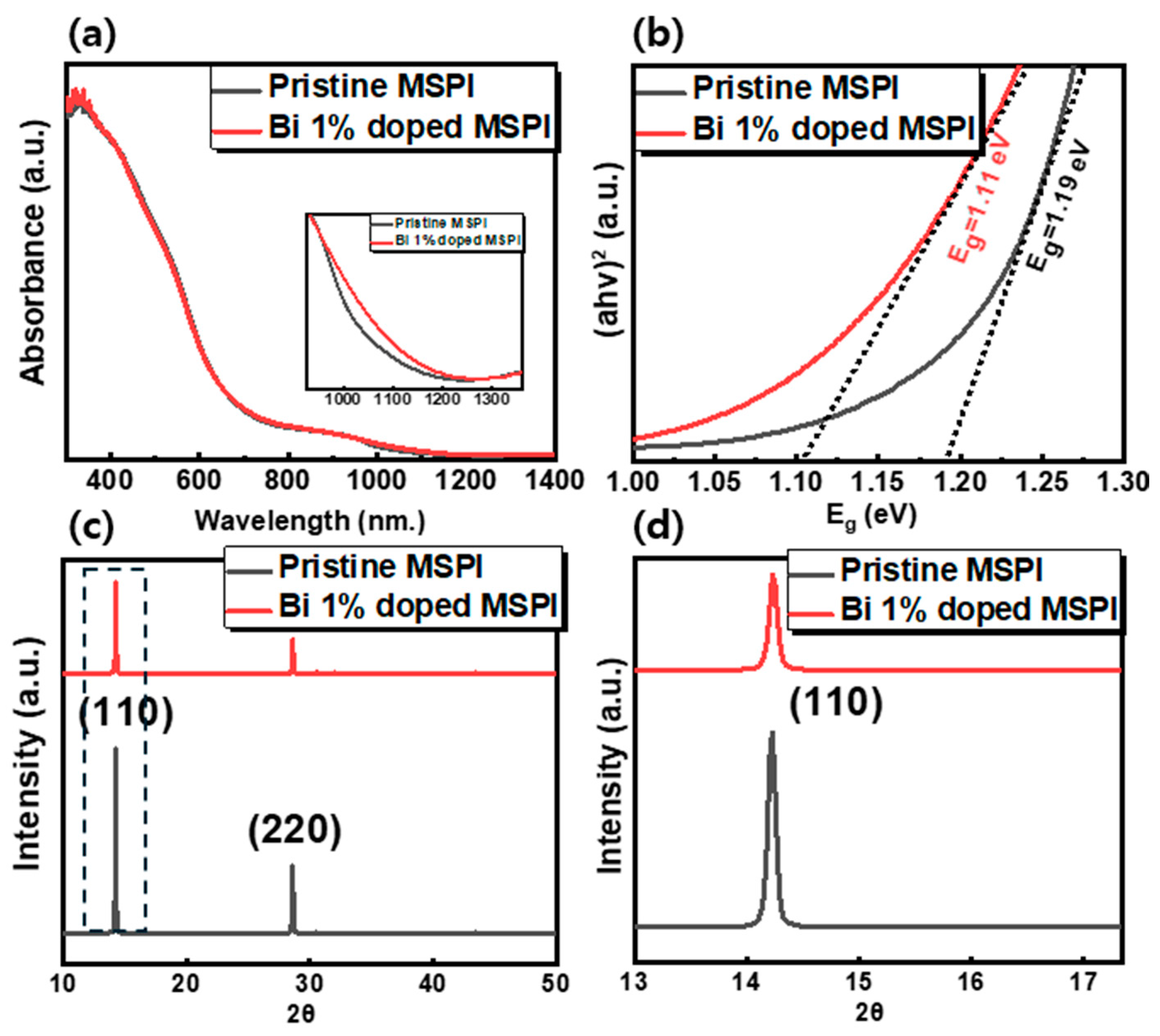

| Bi3+ Concentration [%] | Band Gap [eV] | Lattice Parameter [Å] | Crystallite Size [nm] | |
|---|---|---|---|---|
| CSPI | 0 | 1.32 | 6.27 | 78.34 |
| 1 | 1.33 | 6.10 | 66.38 | |
| MSPI | 0 | 1.19 | 6.21 | 86.08 |
| 1 | 1.11 | 6.22 | 77.13 |
| Bi Concentration [%] | VOC [V] | JSC [mA cm−2] | FF [%] | PCE [%] |
|---|---|---|---|---|
| 0 | 0.656 | 24.73 | 73.58 | 11.95 |
| 1 | 0.2501 | 0.375 | 62.0258 | 0.0582 |
| 2 | 0.2122 | 0.3 | 60.07 | 0.0383 |
| 4 | 0.197 | 0.225 | 57.24 | 0.0254 |
| 8 | 0.155 | 0.175 | 50.69 | 0.0138 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-Y.; Lee, S.; Ryu, J.; Kang, D.-W. Bandgap Engineering via Doping Strategies for Narrowing the Bandgap below 1.2 eV in Sn/Pb Binary Perovskites: Unveiling the Role of Bi3+ Incorporation on Different A-Site Compositions. Nanomaterials 2024, 14, 1554. https://doi.org/10.3390/nano14191554
Lee J-Y, Lee S, Ryu J, Kang D-W. Bandgap Engineering via Doping Strategies for Narrowing the Bandgap below 1.2 eV in Sn/Pb Binary Perovskites: Unveiling the Role of Bi3+ Incorporation on Different A-Site Compositions. Nanomaterials. 2024; 14(19):1554. https://doi.org/10.3390/nano14191554
Chicago/Turabian StyleLee, Jeong-Yeon, Seojun Lee, Jun Ryu, and Dong-Won Kang. 2024. "Bandgap Engineering via Doping Strategies for Narrowing the Bandgap below 1.2 eV in Sn/Pb Binary Perovskites: Unveiling the Role of Bi3+ Incorporation on Different A-Site Compositions" Nanomaterials 14, no. 19: 1554. https://doi.org/10.3390/nano14191554
APA StyleLee, J.-Y., Lee, S., Ryu, J., & Kang, D.-W. (2024). Bandgap Engineering via Doping Strategies for Narrowing the Bandgap below 1.2 eV in Sn/Pb Binary Perovskites: Unveiling the Role of Bi3+ Incorporation on Different A-Site Compositions. Nanomaterials, 14(19), 1554. https://doi.org/10.3390/nano14191554







