Dual-Mode Sensing of Fe(III) Based on Etching Induced Modulation of Localized Surface Plasmon Resonance and Surface Enhanced Raman Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instruments
- Spectrophotometer: UV-Vis-NIR absorption spectra of colloidal suspensions, in the 300–1800 nm range, were taken with a Varian Cary 6000i spectrophotometer (Agilent, Santa Clara, CA, USA), while for the ones in the 300–1100 nm range, we used a Varian Cary 60 (Agilent, Santa Clara, CA, USA).
- Transmission Electron Microscopy (TEM): TEM images of the samples were collected on a Jeol JEM-1200 EX II instrument (JEOL Italia, Milan, Italy). To prepare the grids, 10 µL of the sample was dropped on nickel grids, 300 mesh, coated with a Parlodion membrane. The samples to be deposited were diluted with double-distilled water in different ways: colloidal GNSs were diluted 1:200; GNS@Ag@MMC were diluted 1:50; GNS@Ag@MMC with different quantities of Fe(III) were also diluted 1:50.
- Raman and SERS measurements were performed on the investigated samples using an automated and integrated confocal micro-Raman spectrometer, XploRA Plus HORIBA Scientific (Horiba, Ltd., Kyoto, Japan), equipped with an Olympus microscope BX43 (Horiba, Ltd., Kyoto, Japan). The microscope set-up consists of four different objectives with 10×, 50×, 50× with a long working distance and 100× magnification. The spectrometer is equipped with a motorized xy stage on which the investigated samples are positioned. The spectral resolution is about 1 cm−1. An Open Electrode CCD camera (Horiba, Ltd., Kyoto, Japan), with a multistage Peltier air-cooling system, is used as a detector. Two different solid-state laser sources have been used emitting light at 532 nm and 638 nm. The maximum power was 100 mW for green light and 90 mW for the red one. Neutral filters with different optical density allow for setting the incident laser power. The spectral resolution is about 1 cm−1; neutral filters with optical density equal to 2, 1, 0.6, 0.3 and 0 can be used to adapt the laser incident power, leading to power density values from 5 × 103 W/cm2 to 5 × 105 W/cm2.
2.3. Synthesis of Gold Nanostars (GNSs)
2.4. Silver Coating of Gold Nanostars (GNS@Ag)
2.5. Preparation of GNS and GNS@Ag Coated with 7-Mercapto-4-methylcoumarine
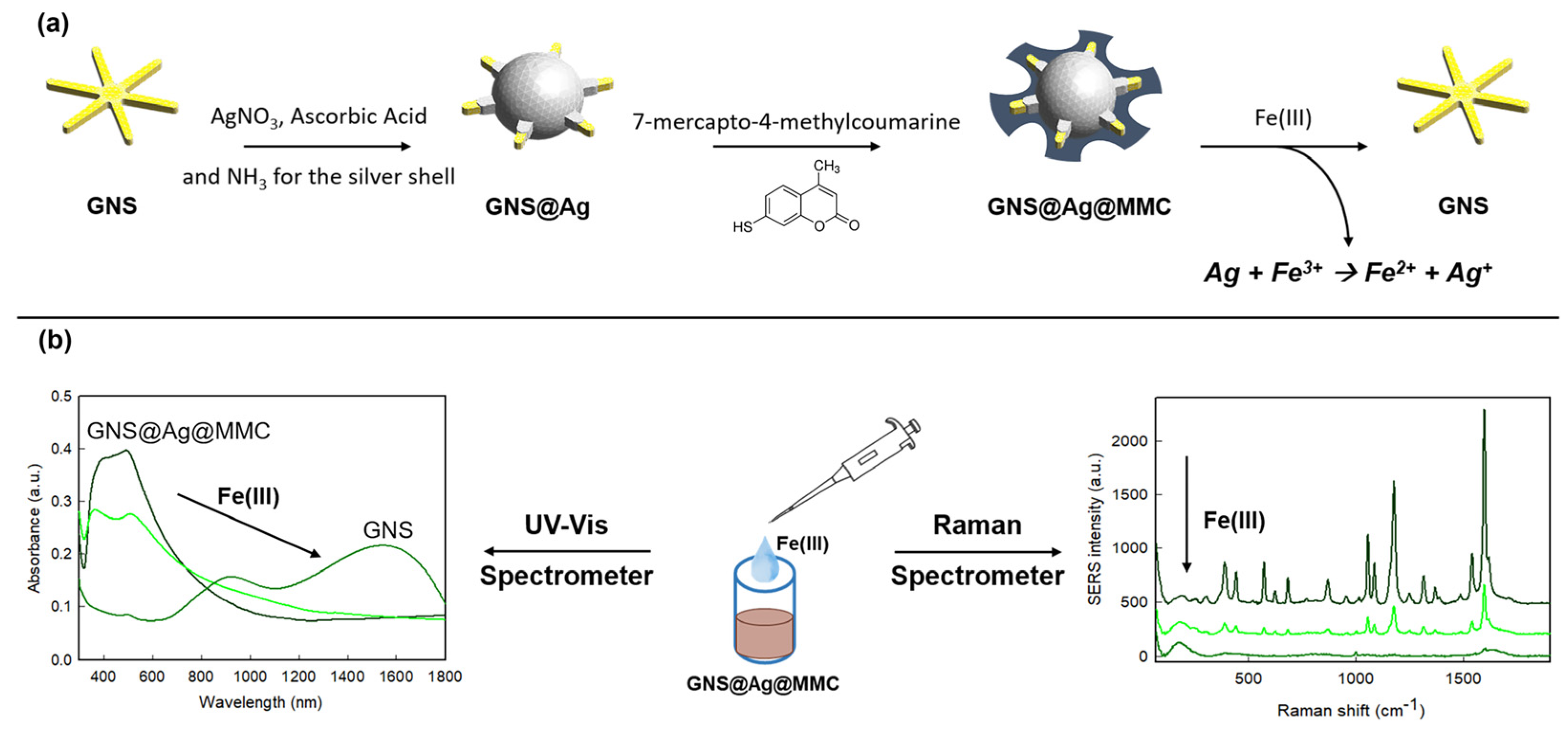
2.6. Detection of Fe(III)
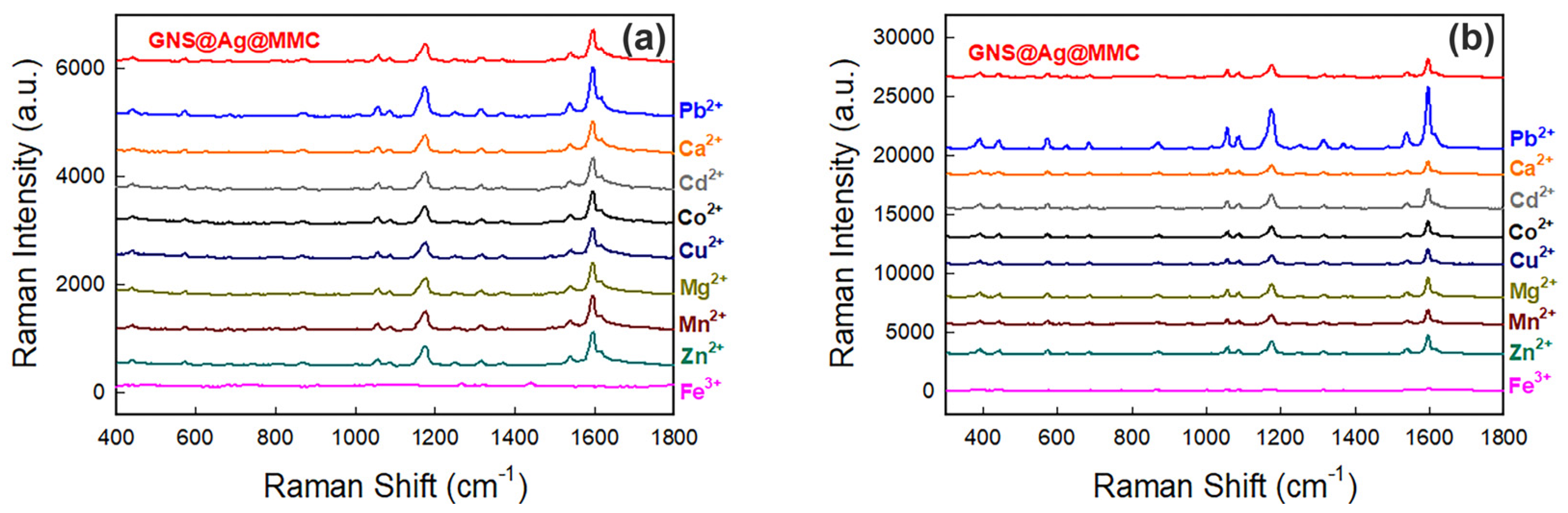
2.7. Detection of Potentially Interfering Cations
3. Results and Discussion
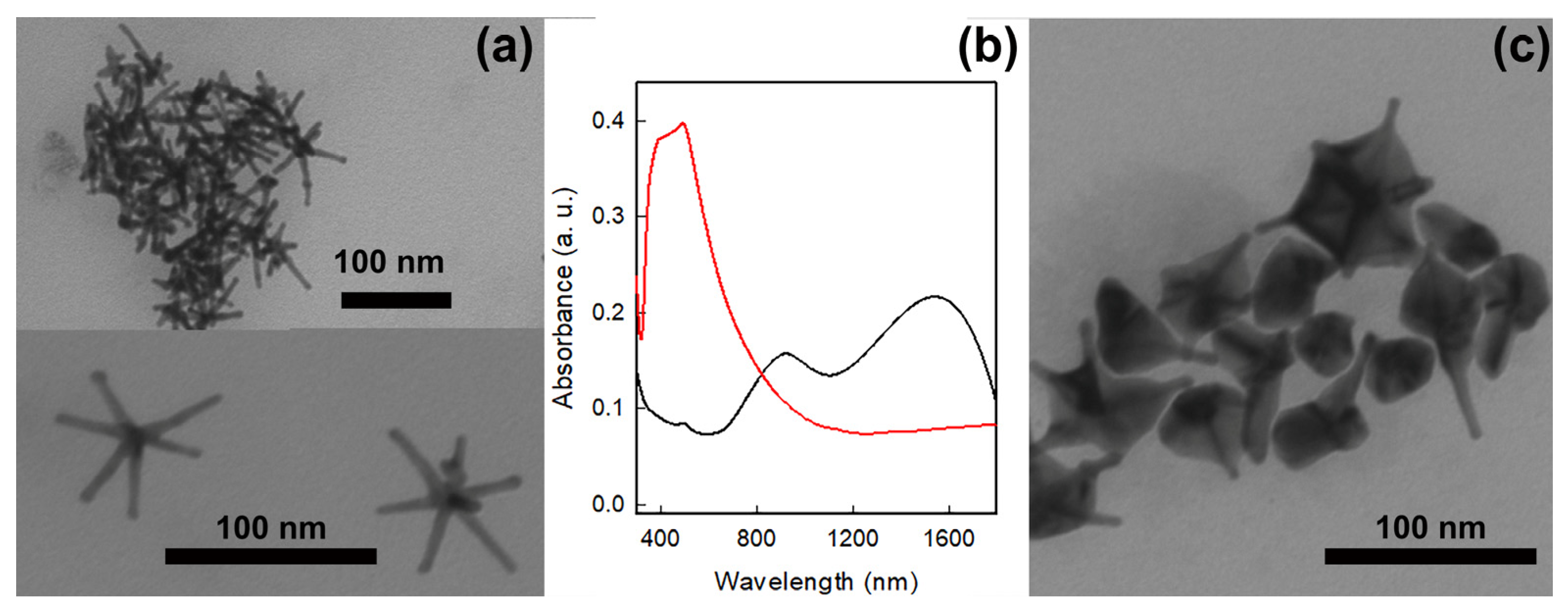
3.1. GNS and GNS@Ag Preparation, Characterization and Coating
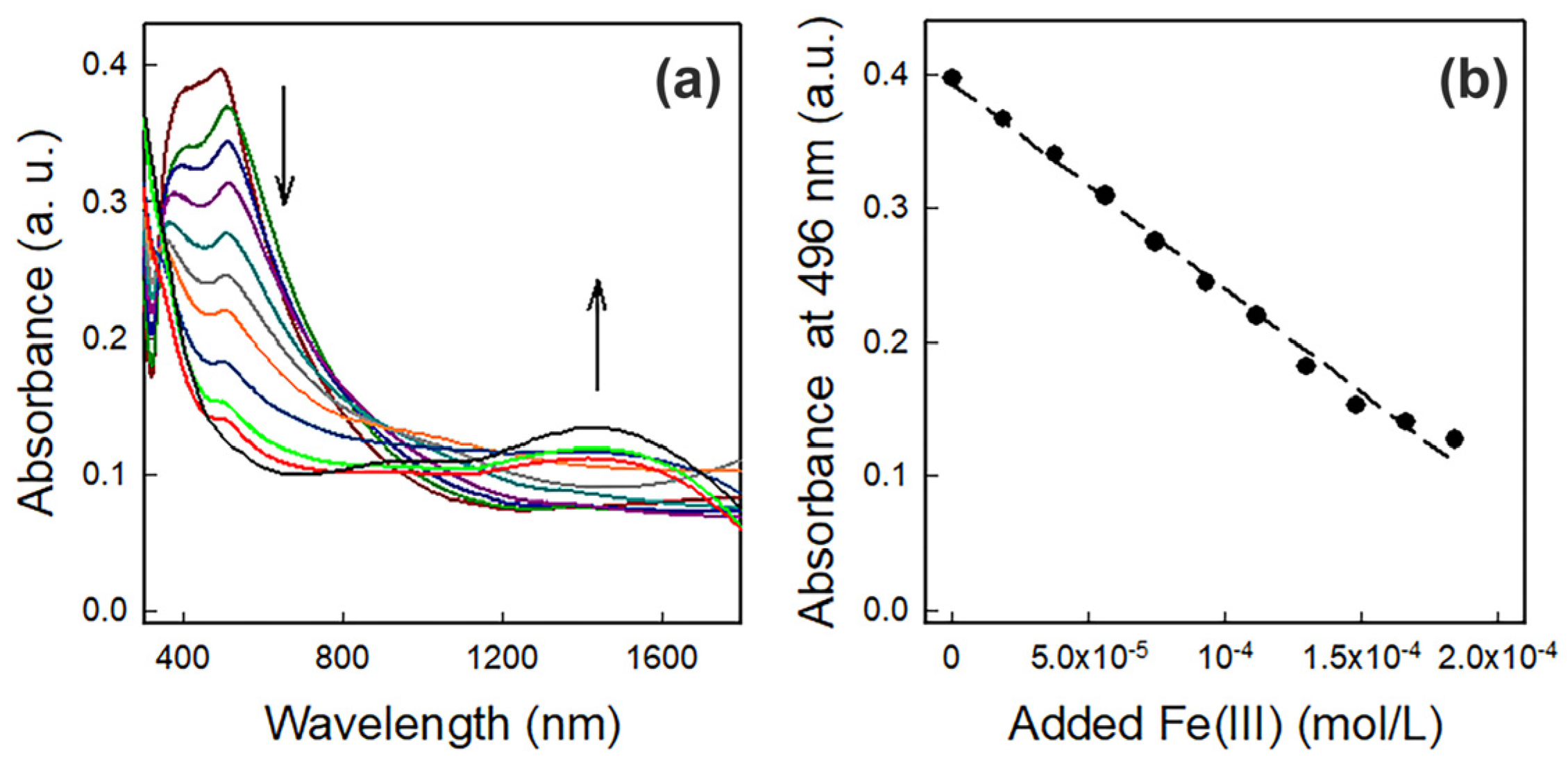
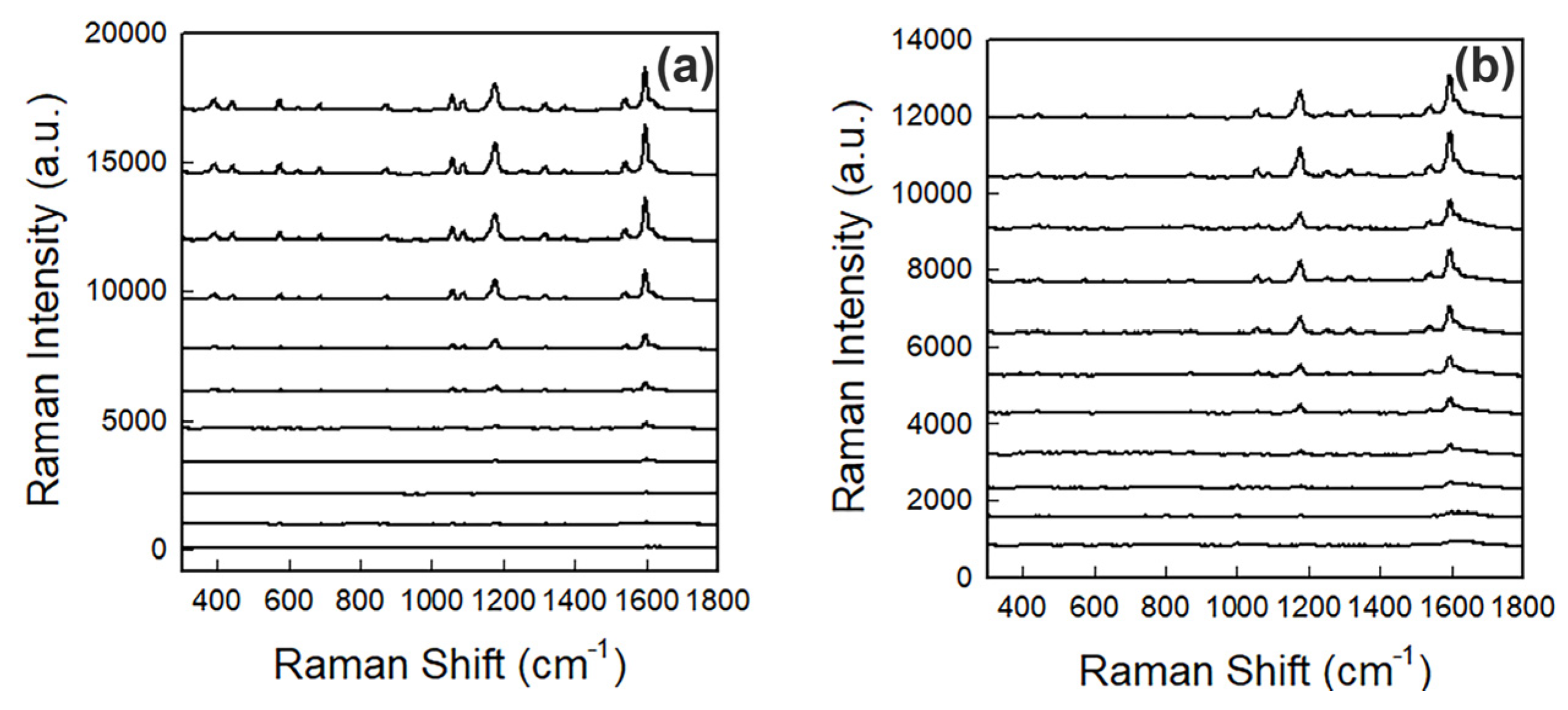
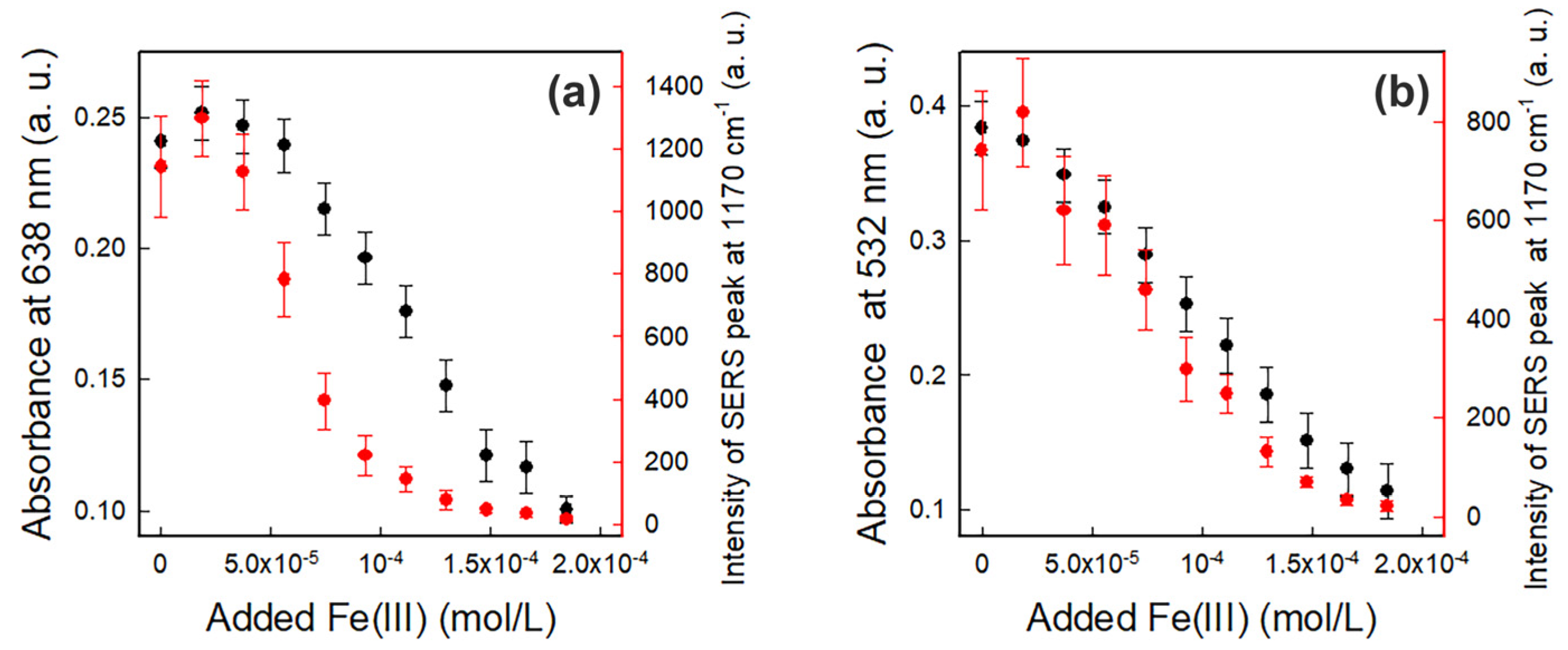
3.2. Fe(III) Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merck Manual. Available online: https://www.merckmanuals.com/professional/multimedia/table/symptoms-and-treatment-of-specific-poisons (accessed on 10 June 2024).
- Chatterjee, S.; Gohil, H.; Paital, A.R. Dual Functions of Selective Ferric Ion Detection and Removal by a Recyclable Fluorescence Active Multifunctional Silica Material and Toxic Dye Removal from Aqueous Solution. ChemistrySelect 2017, 2, 5348–5359. [Google Scholar] [CrossRef]
- Ghosh, D.; Solanki, H.; Purkait, M.K. Removal of Fe(II) from Tap Water by Electrocoagulation Technique. J. Hazard. Mater. 2008, 155, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Hu, L.; You, J. Sensing Materials Developed and Applied for Bio-Active Fe3+ Recognition in Water Environment. Anal. Methods 2016, 8, 5738–5754. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, S.; Huang, Y.; Yuan, D. Redox Speciation Analysis of Dissolved Iron in Estuarine and Coastal Waters with On-Line Solid Phase Extraction and Graphite Furnace Atomic Absorption Spectrometry Detection. Talanta 2015, 137, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Alam, R. Recent Developments in the Creation of a Single Molecular Sensing Tool for Ternary Iron(III), Chromium(III), Aluminium(III) Ionic Species: A Review. Luminescence 2023, 38, 1026–1046. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Sharma, D.; Ber, R.K.; Crisponi, G.; Callan, J.F. Iron(III) Selective Molecular and Supramolecular Fluorescent Probes. Chem. Soc. Rev. 2012, 41, 7195–7227. [Google Scholar] [CrossRef]
- Santonocito, R.; Intravaia, M.; Caruso, I.M.; Pappalardo, A.; Trusso Sfrazzetto, G.; Tuccitto, N. Fluorescence Sensing by Carbon Nanoparticles. Nanoscale Adv. 2022, 4, 1926–1948. [Google Scholar] [CrossRef]
- Bothra, S.; Solanki, J.N.; Sahoo, S.K.; Callan, J.F. Anion-Driven Selective Colorimetric Detection of Hg2+ and Fe3+ Using Functionalized Silver Nanoparticles. RSC Adv. 2014, 4, 1341–1346. [Google Scholar] [CrossRef]
- Abbasi, N.M.; Hameed, M.U.; Nasim, N.; Ahmed, F.; Altaf, F.; Shahida, S.; Fayyaz, S.; Sabir, S.M.; Bocchetta, P. Plasmonic Nano Silver: An Efficient Colorimetric Sensor for the Selective Detection of Hg2+ Ions in Real Samples. Coatings 2022, 12, 763. [Google Scholar] [CrossRef]
- Wu, S.P.; Chen, Y.P.; Sung, Y.M. Colorimetric Detection of Fe3+ Ions Using Pyrophosphate Functionalized Gold Nanoparticles. Analyst 2011, 136, 1887–1891. [Google Scholar] [CrossRef]
- Nguyen, T.H.A.; Nguyen, V.C.; Phan, T.N.H.; Le, V.T.; Vasseghian, Y.; Trubitsyn, M.A.; Nguyen, A.T.; Chau, T.P.; Doan, V.D. Novel Biogenic Silver and Gold Nanoparticles for Multifunctional Applications: Green Synthesis, Catalytic and Antibacterial Activity, and Colorimetric Detection of Fe(III) Ions. Chemosphere 2022, 287, 132271. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Lu, Y.; He, S.; Li, X.; Chen, W. Colorimetric Detection of Iron Ions(III) Based on the Highly Sensitive Plasmonic Response of the N-Acetyl-l-Cysteine-Stabilized Silver Nanoparticles. Anal. Chim. Acta 2015, 879, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.F.; Eisa, W.H.; El-kousy, S.M.; Mleha, W.K.; Kamal, N. Ficus Retusa-Stabilized Gold and Silver Nanoparticles: Controlled Synthesis, Spectroscopic Characterization, and Sensing Properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 214, 496–512. [Google Scholar] [CrossRef] [PubMed]
- Moletti, A.; Coluccini, C.; Pasini, D.; Taglietti, A. A Chiral Probe for the Detection of Cu(II) by UV, CD and Emission Spectroscopies. Dalt. Trans. 2007, 16, 1588–1592. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-Enhanced Spectroscopy. Rev. Mod. Phys. 1985, 57, 783–826. [Google Scholar] [CrossRef]
- Otto, A. Surface-Enhanced Raman Scattering: “Classical” and “Chemical” Origins. In Light Scattering in Solids IV. Topics in Applied Physics; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 3540119426. [Google Scholar]
- Yan, F.; Shrestha, Y.K.; Spurgeon, C.L. Determination of Ferric Ions Using Surface-Enhanced Raman Scattering Based on Desferrioxamine-Functionalized Silver Nanoparticles. Chem. Commun. 2013, 49, 7962–7964. [Google Scholar] [CrossRef]
- Galinetto, P.; Taglietti, A.; Pasotti, L.; Pallavicini, P.; Dacarro, G.; Giulotto, E.; Grandi, M.S. SERS Activity of Silver Nanoparticles Functionalized with A Desferrioxamine B Derived Ligand for FE(III) Binding and Sensing. J. Appl. Spectrosc. 2016, 82, 1052–1059. [Google Scholar] [CrossRef]
- Sreedevi, P.; Nair, J.B.; Preethanuj, P.; Jeeja, B.S.; Suresh, C.H.; Maiti, K.K.; Varma, R.L. Calix[4]arene Based Redox Sensitive Molecular Probe for SERS Guided Recognition of Labile Iron Pool in Tumor Cells. Anal. Chem. 2018, 90, 7148–7153. [Google Scholar] [CrossRef]
- Zou, Q.; Ai, X.; Xue, T. Synthesis of Environment-Friendly and Label-Free SERS Probe for Iron(III) Detection in Integrated Circuit Cleaning Solution Waste. Microchem. J. 2021, 169, 106549. [Google Scholar] [CrossRef]
- Muhammad, P.; Hanif, S.; Yan, J.; Rehman, F.U.; Wang, J.; Khan, M.; Chung, R.; Lee, A.; Zheng, M.; Wang, Y.; et al. SERS-Based Nanostrategy for Rapid Anemia Diagnosis. Nanoscale 2020, 12, 1948–1957. [Google Scholar] [CrossRef]
- Focsan, M.; Craciun, A.M.; Potara, M.; Leordean, C.; Vulpoi, A.; Maniu, D.; Astilean, S. Flexible and Tunable 3D Gold Nanocups Platform as Plasmonic Biosensor for Specific Dual LSPR-SERS Immuno-Detection. Sci. Rep. 2017, 7, 14240. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cong, Y.; Lin, X.; Cao, B.; Dong, D.; Liu, K.; Xiao, Y.; Shang, J.; Bao, Y.; Liu, Y.; et al. Dual LSPR of Au/W18O49 Heterostructures for Upconversion Enhancement and Application of Molecular Detection. J. Mater. Chem. A 2020, 8, 4040–4048. [Google Scholar] [CrossRef]
- Song, C.; Jiang, X.; Yang, Y.; Zhang, J.; Larson, S.; Zhao, Y.; Wang, L. High-Sensitive Assay of Nucleic Acid Using Tetrahedral DNA Probes and DNA Concatamers with a Surface-Enhanced Raman Scattering/Surface Plasmon Resonance Dual-Mode Biosensor Based on a Silver Nanorod-Covered Silver Nanohole Array. ACS Appl. Mater. Interfaces 2020, 12, 31242–31254. [Google Scholar] [CrossRef] [PubMed]
- Hao, E.; Bailey, R.C.; Schatz, G.C.; Hupp, J.T.; Li, S. Synthesis and Optical Properties of “Branched” Gold Nanocrystals. Nano Lett. 2004, 4, 327–330. [Google Scholar] [CrossRef]
- Talamona, F.; Truffi, M.; Caldarone, A.A.; Ricciardi, A.; Corsi, F.; Pellegrini, G.; Morasso, C.; Taglietti, A. Stable and Scalable SERS Tags Conjugated with Neutravidin for the Detection of Fibroblast Activation Protein (FAP) in Primary Fibroblasts. Nanotechnology 2021, 32, 295703. [Google Scholar] [CrossRef] [PubMed]
- Fales, A.M.; Yuan, H.; Vo-Dinh, T. Development of Hybrid Silver-Coated Gold Nanostars for Nonaggregated Surface-Enhanced Raman Scattering. J. Phys. Chem. C 2014, 118, 3708–3715. [Google Scholar] [CrossRef]
- Ran, Y.; Strobbia, P.; Cupil-Garcia, V.; Vo-Dinh, T. Fiber-Optrode SERS Probes Using Plasmonic Silver-Coated Gold Nanostars. Sens. Actuators B Chem. 2019, 287, 95–101. [Google Scholar] [CrossRef]
- Parmigiani, M.; Schifano, V.; Taglietti, A.; Galinetto, P.; Albini, B. Increasing Gold Nanostars SERS Response with Silver Shells: A Surface-Based Seed-Growth Approach. Nanotechnology 2024, 35, 195603. [Google Scholar] [CrossRef]
- Parmigiani, M.; Albini, B.; Pellegrini, G.; Genovesi, M.; De Vita, L.; Pallavicini, P.; Dacarro, G.; Galinetto, P.; Taglietti, A. Surface-Enhanced Raman Spectroscopy Chips Based on Silver Coated Gold Nanostars. Nanomaterials 2022, 12, 3609. [Google Scholar] [CrossRef]
- Pallavicini, P.; Donà, A.; Casu, A.; Chirico, G.; Collini, M.; Dacarro, G.; Falqui, A.; Milanese, C.; Sironi, L.; Taglietti, A. Triton X-100 for Three-Plasmon Gold Nanostars with Two Photothermally Active NIR (near IR) and SWIR (Short-Wavelength IR) Channels. Chem. Commun. 2013, 49, 6265–6267. [Google Scholar] [CrossRef]
- Vogel, E.; Kiefer, W. Investigation of the Metal Adsorbate Interface of the System Silver Coumarin and Silver Hydrocoumarin by Means of Surface Enhanced Raman Spectroscopy. Fresenius. J. Anal. Chem. 1998, 361, 628–630. [Google Scholar] [CrossRef]
- Cara, E.; Mandrile, L.; Ferrarese Lupi, F.; Giovannozzi, A.M.; Dialameh, M.; Portesi, C.; Sparnacci, K.; De Leo, N.; Rossi, A.M.; Boarino, L. Influence of the Long-Range Ordering of Gold-Coated Si Nanowires on SERS. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Sortur, V.; Yenagi, J.; Tonannavar, J.; Jadhav, V.B.; Kulkarni, M.V. Vibrational Assignments for 7-Methyl-4-Bromomethylcoumarin, as Aided by RHF and B3LYP/6-31G* Calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 71, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Taglietti, A.; Arciola, C.R.; D’Agostino, A.; Dacarro, G.; Montanaro, L.; Campoccia, D.; Cucca, L.; Vercellino, M.; Poggi, A.; Pallavicini, P.; et al. Antibiofilm Activity of a Monolayer of Silver Nanoparticles Anchored to an Amino-Silanized Glass Surface. Biomaterials 2014, 35, 1779–1788. [Google Scholar] [CrossRef]
- Bassi, B.; Taglietti, A.; Galinetto, P.; Marchesi, N.; Pascale, A.; Cabrini, E.; Pallavicini, P.; Dacarro, G. Tunable Coating of Gold Nanostars: Tailoring Robust SERS Labels for Cell Imaging. Nanotechnology 2016, 27, 265302. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Galloway, C.; Etchegoin, P.G. On the Connection between Optical Absorption/Extinction and SERS Enhancements. Phys. Chem. Chem. Phys. 2006, 8, 3083–3087. [Google Scholar] [CrossRef]
- Tunaboylu, K.; Schwarzenbach, G. Silbermercaptide Und-mercaptokomplexe. Helv. Chim. Acta 1971, 54, 2166–2185. [Google Scholar] [CrossRef]
- Foti, A.; D’Andrea, C.; Villari, V.; Micali, N.; Donato, M.G.; Fazio, B.; Maragò, O.M.; Gillibert, R.; de la Chapelle, M.L.; Gucciardi, P.G. Optical Aggregation of Gold Nanoparticles for SERS Detection of Proteins and Toxins in Liquid Environment: Towards Ultrasensitive and Selective Detection. Materials 2018, 11, 440. [Google Scholar] [CrossRef]
- Pellacani, P.; Morasso, C.; Picciolini, S.; Gallach, D.; Fornasari, L.; Marabelli, F.; Silvan, M.M. Plasma Fabrication and SERS Functionality of Gold Crowned Silicon Submicrometer Pillars. Materials 2020, 13, 1244. [Google Scholar] [CrossRef]
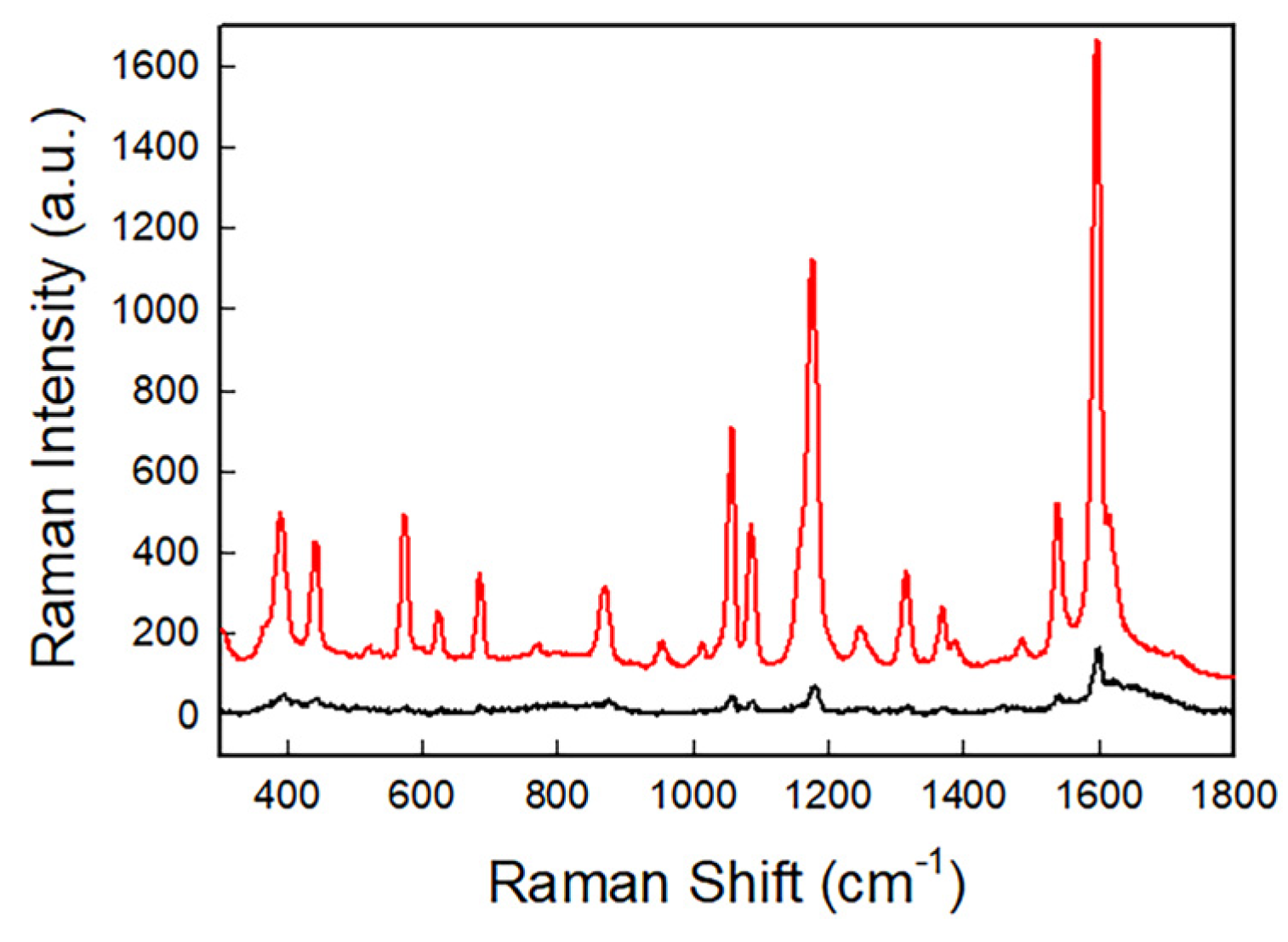


| Peak Position (cm−1) | Assignment |
|---|---|
| 438 | Skeletal vibration |
| 570 | Ring deformation |
| 685 | Skeletal vibration and -C-O deformation |
| 1057 | Characteristic ring vibration |
| 1102 | Characteristic ring vibration |
| 1170 | -C-O- Stretching |
| 1318 | -C-O- Stretching |
| 1366 | Conjugated C=C- asymmetric stretching and -C-H deformation |
| 1384 | -C-CH3 deformation |
| 1544 | Stretching -C-C-, -CH3 deformation and -C-Hring in-plane deformation |
| 1593 | Conjugated -C=C-symmetric stretching |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parmigiani, M.; Albini, B.; Galinetto, P.; Taglietti, A. Dual-Mode Sensing of Fe(III) Based on Etching Induced Modulation of Localized Surface Plasmon Resonance and Surface Enhanced Raman Spectroscopy. Nanomaterials 2024, 14, 1467. https://doi.org/10.3390/nano14181467
Parmigiani M, Albini B, Galinetto P, Taglietti A. Dual-Mode Sensing of Fe(III) Based on Etching Induced Modulation of Localized Surface Plasmon Resonance and Surface Enhanced Raman Spectroscopy. Nanomaterials. 2024; 14(18):1467. https://doi.org/10.3390/nano14181467
Chicago/Turabian StyleParmigiani, Miriam, Benedetta Albini, Pietro Galinetto, and Angelo Taglietti. 2024. "Dual-Mode Sensing of Fe(III) Based on Etching Induced Modulation of Localized Surface Plasmon Resonance and Surface Enhanced Raman Spectroscopy" Nanomaterials 14, no. 18: 1467. https://doi.org/10.3390/nano14181467
APA StyleParmigiani, M., Albini, B., Galinetto, P., & Taglietti, A. (2024). Dual-Mode Sensing of Fe(III) Based on Etching Induced Modulation of Localized Surface Plasmon Resonance and Surface Enhanced Raman Spectroscopy. Nanomaterials, 14(18), 1467. https://doi.org/10.3390/nano14181467








