Adhesion Strength Enhancement of Butyl Rubber and Aluminum Using Nanoscale Self-Assembled Monolayers of Various Silane Coupling Agents for Vibration Damping Plates
Abstract
1. Introduction
2. Materials and Method
3. Results
3.1. Water Contact Angle
3.2. FT-IR Analysis
3.3. XPS Analysis
3.4. Adhesion Strength Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qin, Y.; Tang, X.; Jia, T.; Duan, Z.; Zhang, J.; Li, Y.; Zheng, L. Noise and vibration suppression in hybrid electric vehicles: State of the art and challenges. Renew. Sustain. Energy Rev. 2020, 124, 109782. [Google Scholar] [CrossRef]
- Maheri, M.; Adams, R.; Hugon, J. Vibration damping in sandwich panels. J. Mater. Sci. 2008, 43, 6604–6618. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, D.; Shao, X.; Zhang, S.; Wang, S. Research and applications of viscoelastic vibration damping materials: A review. Compos. Struct. 2016, 136, 460–480. [Google Scholar] [CrossRef]
- Pavlović, A.; Sintoni, D.; Minak, G.; Fragassa, C. On the modal behaviour of ultralight composite sandwich automotive panels. Compos. Struct. 2020, 248, 112523. [Google Scholar] [CrossRef]
- Rajak, D.K.; Kumaraswamidhas, L.; Das, S. Technical Overview of Aluminum Alloy Foam. Rev. Adv. Mater. Sci. 2017, 49, 68–86. [Google Scholar]
- Maeda, T.; Miyazaki, H.; Iwasaki, R.; Kobayashi, R.; Miyanaga, M. Heatproof aluminum with excellent electric conductivity and thermal conductivity. Sumitomo Electr. Tech. Rev. 2021, 92, 68–72. [Google Scholar]
- Zhang, P.; Li, Y.; Liu, Y.; Zhang, Y.; Liu, J. Analysis of the microhardness, mechanical properties and electrical conductivity of 7055 aluminum alloy. Vacuum 2020, 171, 109005. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J. Advanced lightweight materials for Automobiles: A review. Mater. Des. 2022, 221, 110994. [Google Scholar] [CrossRef]
- Hashimoto, N. Application of Aluminum extrusions to automotive parts. Kobelco Technol. Rev. 2017, 35, 69–75. [Google Scholar]
- Mayer, P.; Dmitruk, A.; Jóskiewicz, M.; Głuch, M. Pull-off strength of fiber-reinforced composite polymer coatings on aluminum substrate. J. Adhes. 2021, 97, 1371–1387. [Google Scholar] [CrossRef]
- Layec, J.; Ansart, F.; Duluard, S.; Turq, V.; Aufray, M.; Labeau, M.-P. Development of new surface treatments for the adhesive bonding of aluminum surfaces. Int. J. Adhes. Adhes. 2022, 117, 103006. [Google Scholar] [CrossRef]
- Qiu, J.; Sakai, E.; Lei, L.; Takarada, Y.; Murakami, S. Improving the shear strength by silane treatments of aluminum for direct joining of phenolic resin. J. Mater. Process. Technol. 2012, 212, 2406–2412. [Google Scholar] [CrossRef]
- Ahangaran, F.; Navarchian, A.H. Recent advances in chemical surface modification of metal oxide nanoparticles with silane coupling agents: A review. Adv. Colloid Interface Sci. 2020, 286, 102298. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, J.; Luo, H.; Wang, J.; Wang, Q. A new organofunctional ethoxysilane self-assembly monolayer for promoting adhesion of rubber to aluminum. Molecules 2009, 14, 4087–4097. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.; Du, P.; Zhou, H.; Zhang, K.; Liu, P. Fabrication and tribological properties of self-assembled monolayer of n-alkyltrimethoxysilane on silicon: Effect of SAM alkyl chain length. Appl. Surf. Sci. 2017, 396, 865–869. [Google Scholar] [CrossRef]
- Lee, S.R.; Man Bae, K.; Baek, J.J.; Kang, M.C.; Lee, T.I. Adhesion enhancement between aluminum and butyl rubber by (3-mercaptopropyl) trimethoxy silane for vibration damping plate. J. Adhes. Sci. Technol. 2021, 35, 1114–1124. [Google Scholar] [CrossRef]
- Sang, J.; Aisawa, S.; Miura, K.; Hirahara, H.; Jan, O.; Jozef, P.; Pavol, M. Adhesion of carbon steel and natural rubber by functionalized silane coupling agents. Int. J. Adhes. Adhes. 2017, 72, 70–74. [Google Scholar] [CrossRef]
- Jayaseelan, S.K.; Van Ooij, W.J. Rubber-to-metal bonding by silanes. J. Adhes. Sci. Technol. 2001, 15, 967–991. [Google Scholar] [CrossRef]
- Joseph, E.; Rajput, S.S.; Patil, S.; Nisal, A. Mechanism of adhesion of natural polymer coatings to chemically modified siloxane polymer. Langmuir 2021, 37, 2974–2984. [Google Scholar] [CrossRef]
- Ressel, B.; Urbančič, J.; Beltrami, M.; Betz-Güttner, E.; Cepek, C.; Conti, M.; Farooq, A.; Melpignano, P. Enhancing optical biosensing: Comparing two physical treatments for GPTES chemical functionalization of Cyclo-Olefin Copolymer foil. Surf. Interfaces 2024, 51, 104663. [Google Scholar] [CrossRef]
- Byun, I.; Coleman, A.W.; Kim, B. Transfer of thin Au films to polydimethylsiloxane (PDMS) with reliable bonding using (3-mercaptopropyl) trimethoxysilane (MPTMS) as a molecular adhesive. J. Micromechan. Microeng. 2013, 23, 085016. [Google Scholar] [CrossRef]
- Sivakumar, P.; Du, S.M.; Selter, M.; Daye, J.; Cho, J. Improved adhesion of polyurethane-based nanocomposite coatings to tin surface through silane coupling agents. Int. J. Adhes. Adhes. 2021, 110, 102948. [Google Scholar] [CrossRef]
- Karade, V.; Sharma, A.; Dhavale, R.; Dhavale, R.; Shingte, S.; Patil, P.; Kim, J.; Zahn, D.; Chougale, A.; Salvan, G. APTES monolayer coverage on self-assembled magnetic nanospheres for controlled release of anticancer drug Nintedanib. Sci. Rep. 2021, 11, 5674. [Google Scholar] [CrossRef]
- Zhang, D.; Hegab, H.E.; Lvov, Y.; Dale Snow, L.; Palmer, J. Immobilization of cellulase on a silica gel substrate modified using a 3-APTES self-assembled monolayer. SpringerPlus 2016, 5, 48. [Google Scholar] [CrossRef]
- Plutino, M.; Colleoni, C.; Donelli, I.; Freddi, G.; Guido, E.; Maschi, O.; Mezzi, A.; Rosace, G. Sol-gel 3-glycidoxypropyltriethoxysilane finishing on different fabrics: The role of precursor concentration and catalyst on the textile performances and cytotoxic activity. J. Colloid Interface Sci. 2017, 506, 504–517. [Google Scholar] [CrossRef]
- Thanganathan, U.; Nogami, M. Synthesis of mixed composite membranes based polymer/HPA: Electrochemical performances on low temperature PEMFCs. J. Membr. Sci. 2012, 411, 109–116. [Google Scholar] [CrossRef]
- Wu, J.; Ling, L.; Xie, J.; Ma, G.; Wang, B. Surface modification of nanosilica with 3-mercaptopropyl trimethoxysilane: Experimental and theoretical study on the surface interaction. Chem. Phys. Lett. 2014, 591, 227–232. [Google Scholar] [CrossRef]
- Kwak, S.-Y.; Kim, N.R.; Lee, K.; Yi, J.; Kim, J.H.; Bae, B.-S. Enhancement of fluorescence and lasing properties of covalent bridged fluorescent dye in organic–inorganic hybrid materials. J. Sol-Gel Sci. Technol. 2011, 60, 137–143. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Wei, Y.; Zhang, X. Robust urethane-bridged silica aerogels available for water-carved aerosculptures. New J. Chem. 2017, 41, 1953–1958. [Google Scholar] [CrossRef]
- Chang, C.-C.; Huang, F.-H.; Lin, Z.-M.; Cheng, L.-P. Thermal analyses of dye Disperse Red 1 grafted onto silica nanoparticles. J. Coat. Technol. Res. 2015, 12, 731–738. [Google Scholar] [CrossRef]
- Jeong, J.; Ayyoob, M.; Kim, J.-H.; Nam, S.W.; Kim, Y.J. In situ formation of PLA-grafted alkoxysilanes for toughening a biodegradable PLA stereocomplex thin film. RSC Adv. 2019, 9, 21748–21759. [Google Scholar] [CrossRef]
- Maaz, M.; Elzein, T.; Dragoe, D.; Bejjani, A.; Jarroux, N.; Poulard, C.; Aubry-Barroca, N.; Nsouli, B.; Roger, P. Poly (4-vinylpyridine)-modified silica for efficient oil/water separation. J. Mater. Sci. 2019, 54, 1184–1196. [Google Scholar] [CrossRef]
- Xiao, S.; Xu, P.; Peng, Q.; Chen, J.; Huang, J.; Wang, F.; Noor, N. Layer-by-layer assembly of polyelectrolyte multilayer onto PET fabric for highly tunable dyeing with water soluble dyestuffs. Polymers 2017, 9, 735. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, L.-Y.; Liang, Y.; Chen, Q.; Li, Z.-S.; Li, C.-H.; Wang, Z.-H.; Li, W. Coconut-based activated carbon fibers for efficient adsorption of various organic dyes. RSC Adv. 2018, 8, 42280–42291. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, S.; Ren, K.; Zhao, S.; Chen, Z.; Li, W.; Guan, J. Facile preparation of graphite particles fully coated with thin Ag shell layers for high performance conducting and electromagnetic shielding composite materials. J. Mater. Chem. C 2016, 4, 2566–2578. [Google Scholar] [CrossRef]
- Ravi, S.; Zhang, S.; Lee, Y.-R.; Kang, K.-K.; Kim, J.-M.; Ahn, J.-W.; Ahn, W.-S. EDTA-functionalized KCC-1 and KIT-6 mesoporous silicas for Nd3+ ion recovery from aqueous solutions. J. Ind. Eng. Chem. 2018, 67, 210–218. [Google Scholar] [CrossRef]
- Shin, J.; Nazarenko, S.; Hoyle, C.E. Enthalpy Relaxation of Photopolymerized Thiol–Ene Networks: Structural Effects. Macromolecules 2008, 41, 6741–6746. [Google Scholar] [CrossRef]
- Roper, T.; Kwee, T.; Lee, T.; Guymon, C.; Hoyle, C. Photopolymerization of pigmented thiol–ene systems. Polymer 2004, 45, 2921–2929. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lee, T.Y.; Roper, T. Thiol–enes: Chemistry of the past with promise for the future. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5301–5338. [Google Scholar] [CrossRef]
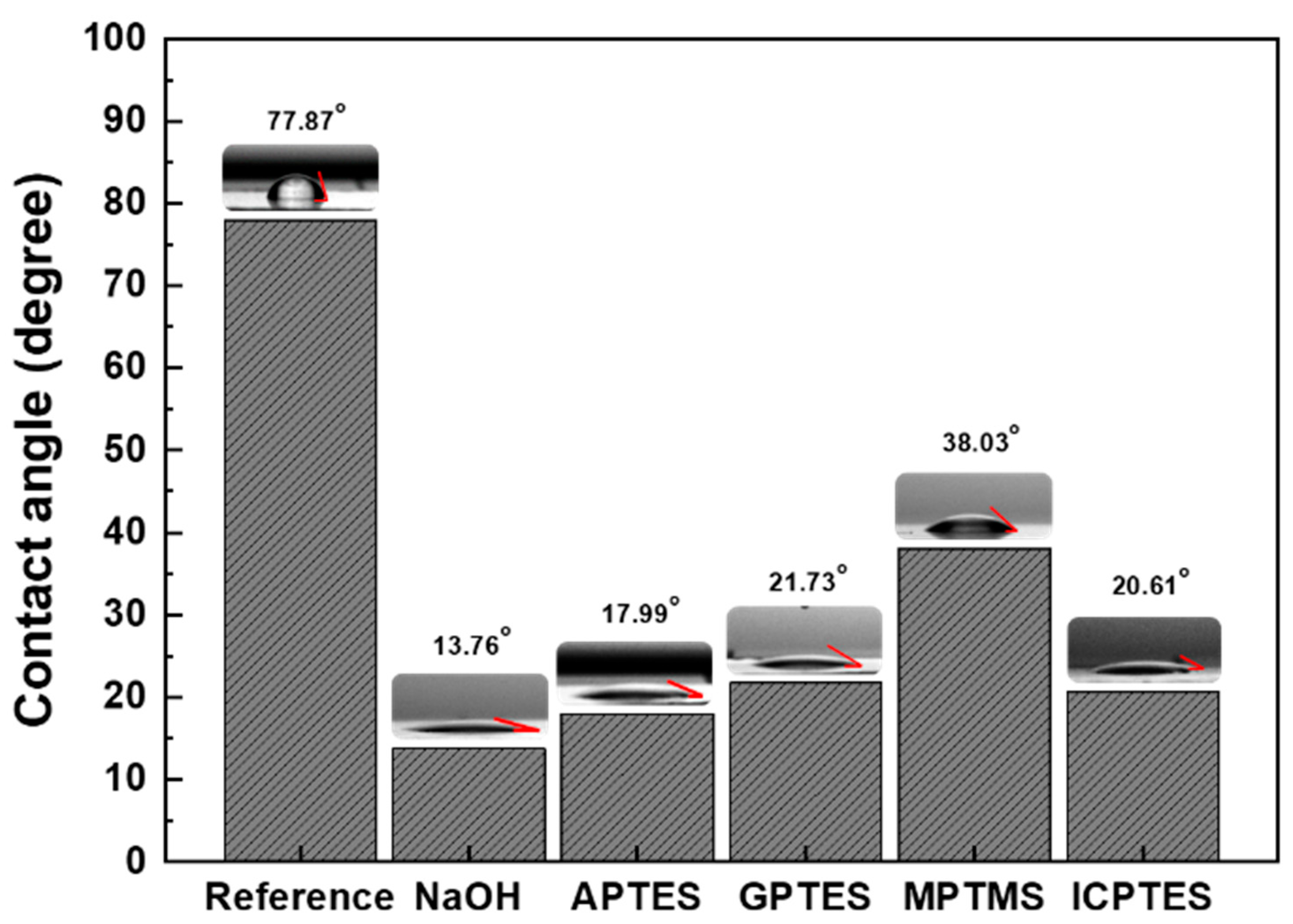
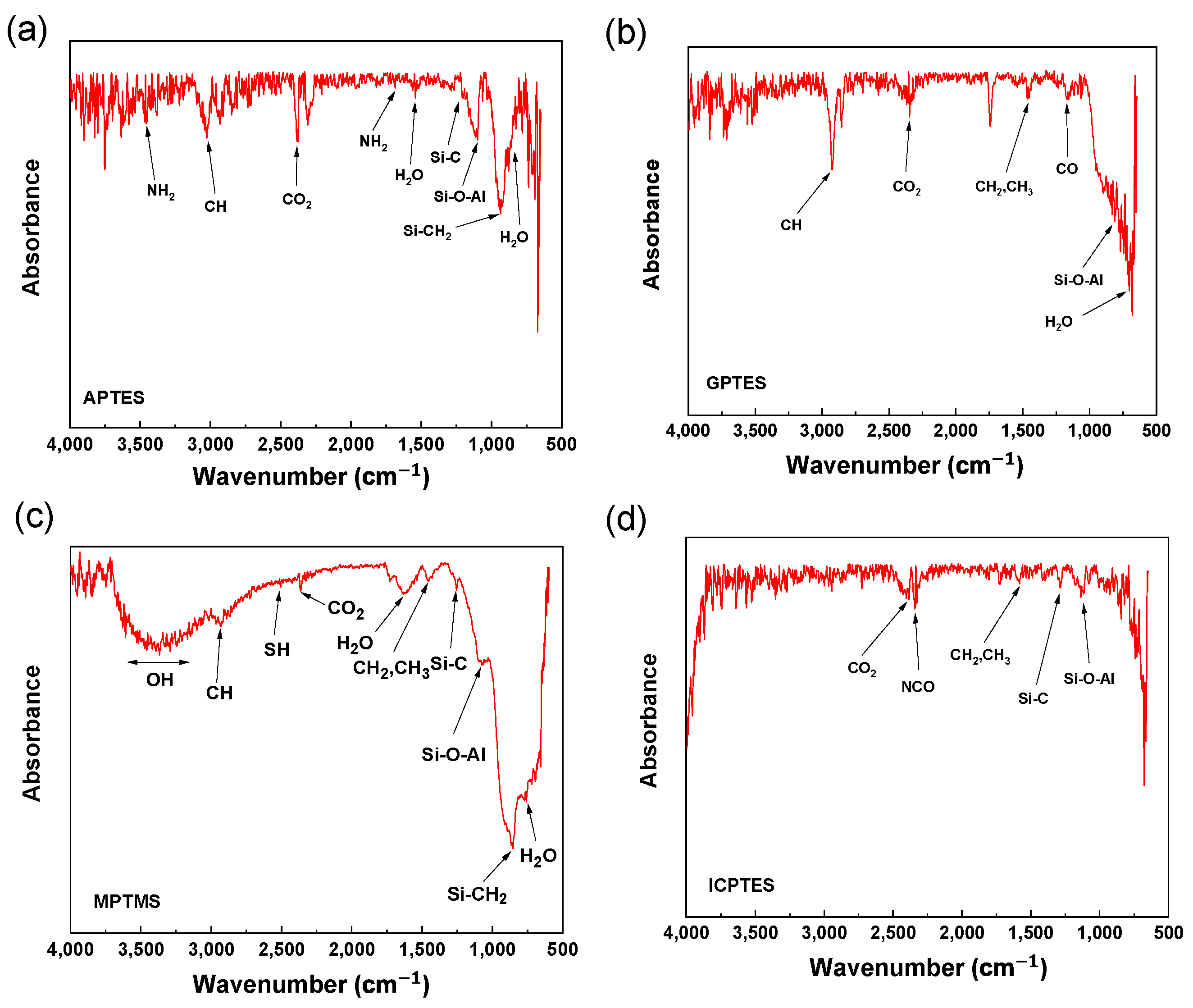
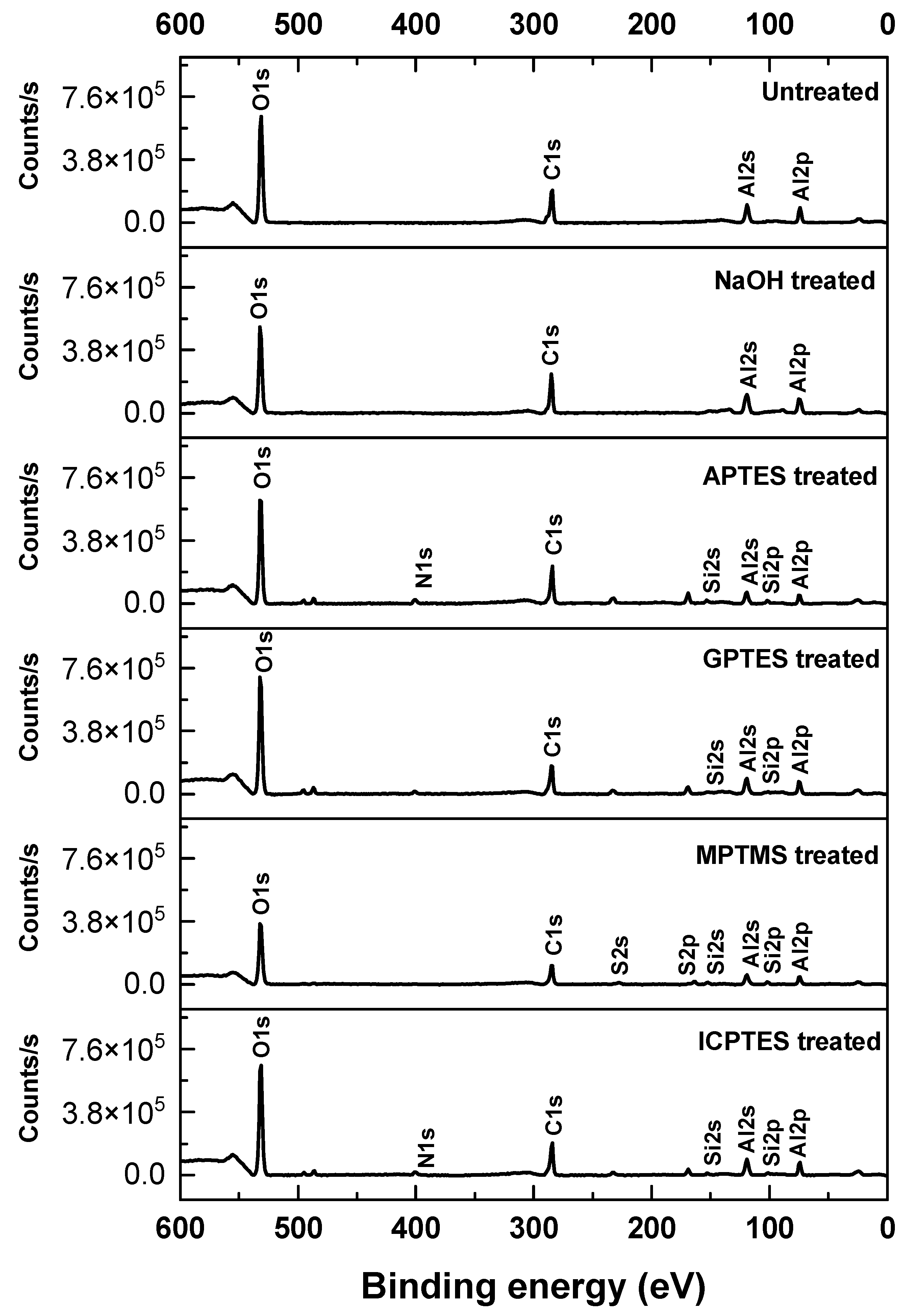


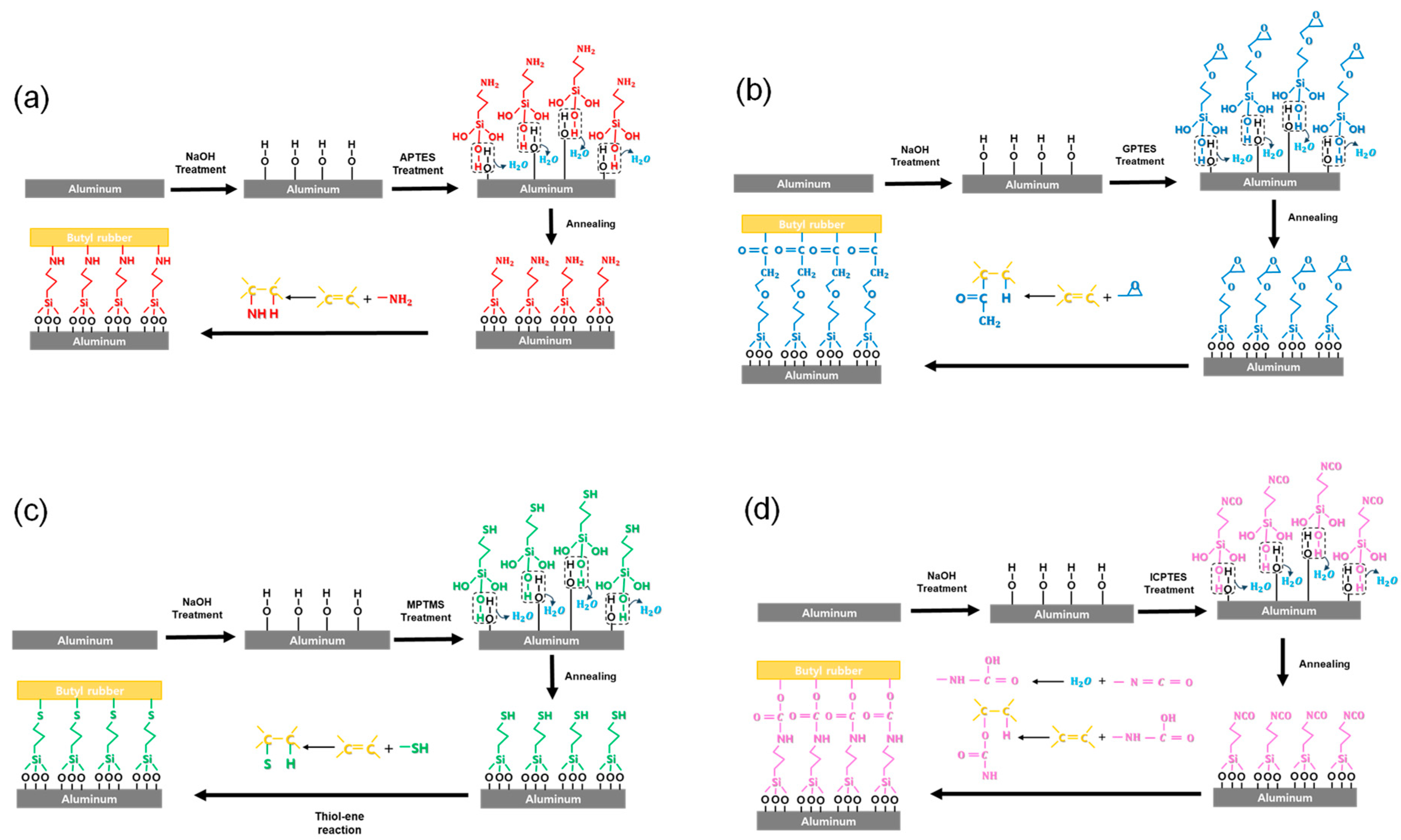
| Elements | C | Si | O | Al | N | S |
|---|---|---|---|---|---|---|
| Untreated | 29.8 | - | 50.0 | 20.2 | - | - |
| NaOH | 30.6 | - | 44.0 | 25.4 | - | - |
| APTES | 33.64 | 2.65 | 46.5 | 14.83 | 2.38 | - |
| GPTES | 29.09 | 1.31 | 49.42 | 20.18 | - | - |
| MPTMS | 29.2 | 3.1 | 45.1 | 17 | - | 5.5 |
| ICPTES | 30.01 | 1.64 | 47.6 | 19.46 | 1.29 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.R.; Nghia, D.X.; Oh, J.Y.; Lee, T.I. Adhesion Strength Enhancement of Butyl Rubber and Aluminum Using Nanoscale Self-Assembled Monolayers of Various Silane Coupling Agents for Vibration Damping Plates. Nanomaterials 2024, 14, 1480. https://doi.org/10.3390/nano14181480
Lee SR, Nghia DX, Oh JY, Lee TI. Adhesion Strength Enhancement of Butyl Rubber and Aluminum Using Nanoscale Self-Assembled Monolayers of Various Silane Coupling Agents for Vibration Damping Plates. Nanomaterials. 2024; 14(18):1480. https://doi.org/10.3390/nano14181480
Chicago/Turabian StyleLee, So Rim, Dang Xuan Nghia, Jin Young Oh, and Tae Il Lee. 2024. "Adhesion Strength Enhancement of Butyl Rubber and Aluminum Using Nanoscale Self-Assembled Monolayers of Various Silane Coupling Agents for Vibration Damping Plates" Nanomaterials 14, no. 18: 1480. https://doi.org/10.3390/nano14181480
APA StyleLee, S. R., Nghia, D. X., Oh, J. Y., & Lee, T. I. (2024). Adhesion Strength Enhancement of Butyl Rubber and Aluminum Using Nanoscale Self-Assembled Monolayers of Various Silane Coupling Agents for Vibration Damping Plates. Nanomaterials, 14(18), 1480. https://doi.org/10.3390/nano14181480







