Introducing Molecular Sieve into Activated Carbon to Achieve High-Effective Adsorption for Ethylene Oxide
Abstract
:1. Introduction
2. Experimental Sections
2.1. Materials
2.2. Preparation of AC/ZSM-5 Composites
2.3. Material Characterization
2.4. Calculation Models and Methods
3. Results and Discussion
3.1. Structural Analysis
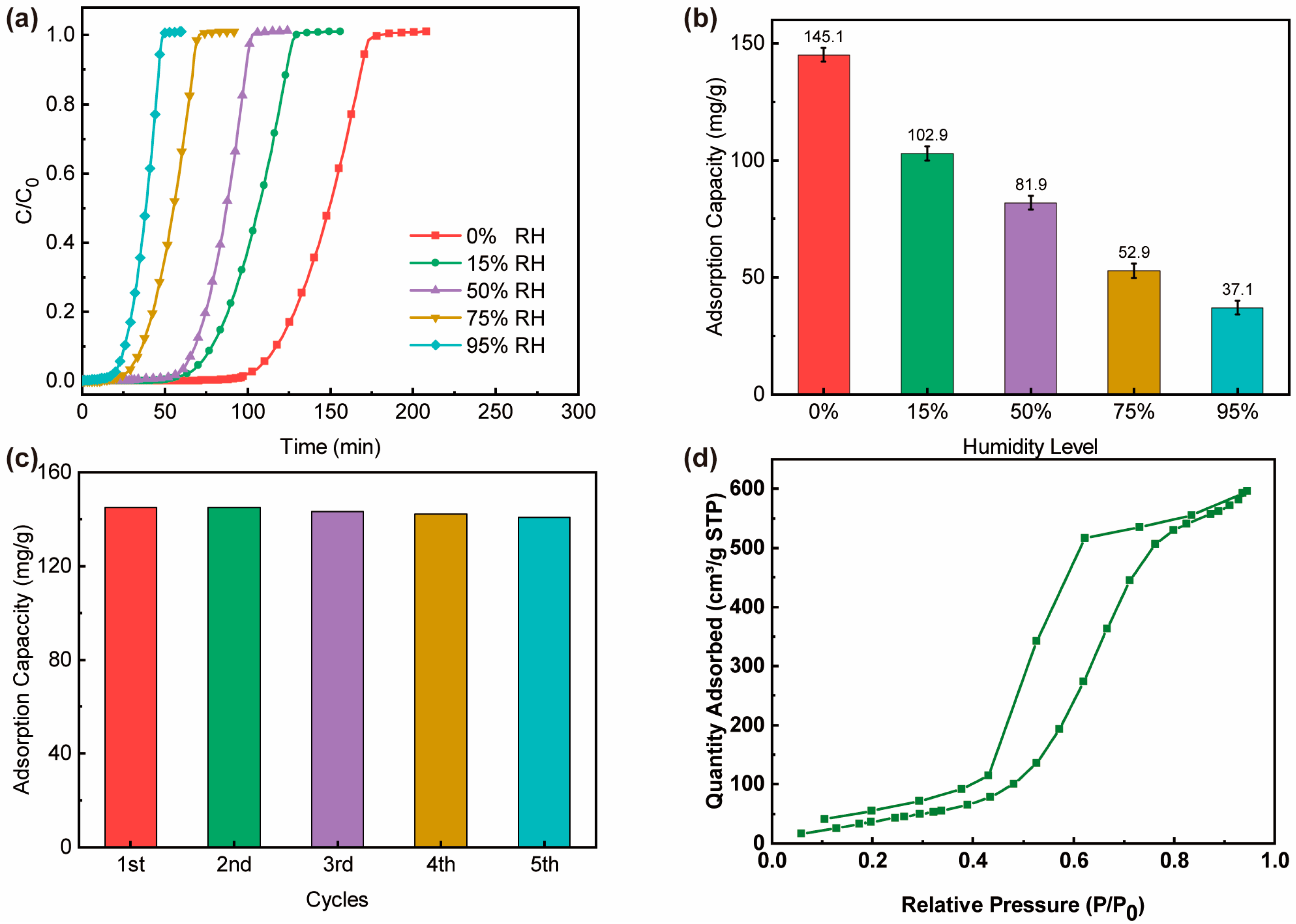
3.2. EtO Adsorption Breakthrough Experiments
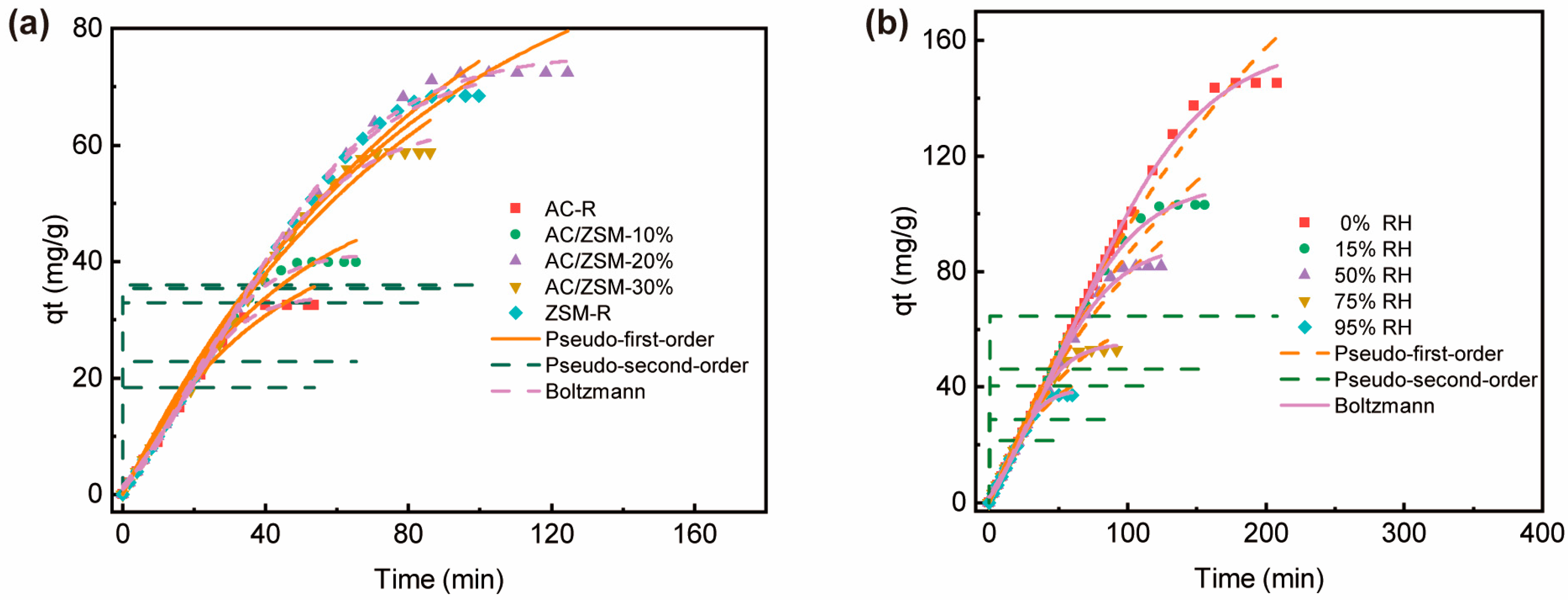
3.3. Adsorption Experiments and Model Fitting
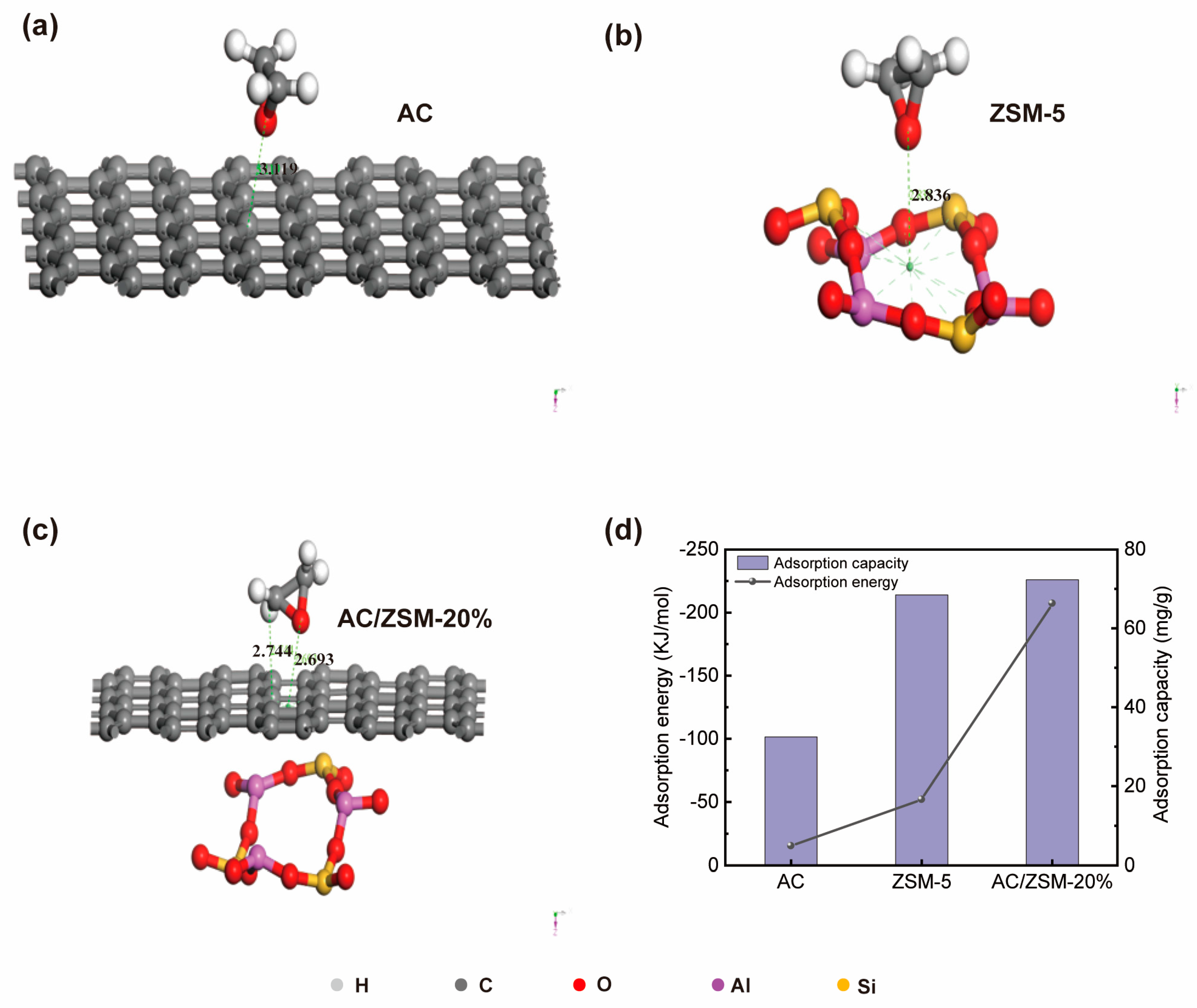
3.4. Molecular Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Liso, B.A.; Palma, V.; Pio, G.; Renda, S.; Salzano, E. Extremely low temperatures for the synthesis of ethylene oxide. Ind. Eng. Chem. Res. 2023, 62, 6943–6952. [Google Scholar] [CrossRef]
- Park, R.M. Associations between exposure to ethylene oxide, job termination, and cause-specific mortality risk. Am. J. Ind. Med. 2020, 63, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Wen, M.; Ma, X.; Zhou, Y.; Zhu, Q.; Lu, H. Rapid absorption of ethylene oxide exhaust by catalytic hydration on acid zeolite catalysts. Environ. Technol. Innov. 2019, 15, 100376–100386. [Google Scholar] [CrossRef]
- Perona, C.; Borrego-Marin, E.; Delgado, P.; Vismara, R.; Maldonado, C.R.; Barea, E.; Bandosz, T.J.; Navarro, J.A.R. Zirconium-metal–organic framework@activated carbon composites for prevention of secondary emission of nerve agents. J. Mater. Chem. A 2024, 12, 1772–1778. [Google Scholar] [CrossRef]
- Chen, R.; Han, N.; Li, L.; Wang, S.; Ma, X.; Wang, C.; Li, H.; Li, H.; Zeng, L. Fundamental understanding of oxygen content in activated carbon on acetone adsorption desorption. Appl. Surf. Sci. 2020, 508, 145211–145231. [Google Scholar] [CrossRef]
- Demiral, H.; Güngör, C. Adsorption of copper(II) from aqueous solutions on activated carbon prepared from grape bagasse. J. Clean. Prod. 2016, 124, 103–113. [Google Scholar] [CrossRef]
- Yao, X.; Liu, Y.; Li, T.; Zhang, T.; Li, H.; Wang, W.; Shen, X.; Qian, F.; Yao, Z. Adsorption behavior of multicomponent volatile organic compounds on a citric acid residue waste-based activated carbon: Experiment and molecular simulation. J. Hazard. Mater. 2020, 392, 122323–122331. [Google Scholar] [CrossRef]
- Ho, B.N.; Pino-Perez, D.; Ghimbeu, C.M.; Diaz, J.; Peredo-Mancilla, D.; Hort, C.; Bessieres, D. Determination of methane, ethane and propane on activated carbons by experimental pressure swing adsorption method. J. Nat. Gas Sci. Eng. 2021, 95, 104124–104134. [Google Scholar] [CrossRef]
- Lu, Z.; Sun, W.; Li, C.; Cao, W.; Jing, Z.; Li, S.; Ao, X.; Chen, C.; Liu, S. Effect of granular activated carbon pore-size distribution on biological activated carbon filter performance. Water Res. 2020, 177, 115768–115781. [Google Scholar] [CrossRef]
- Ma, X.; Yang, L.; Wu, H. Removal of volatile organic compounds from the coal-fired flue gas by adsorption on activated carbon. J. Clean. Prod. 2021, 302, 126925–126934. [Google Scholar] [CrossRef]
- Li, H.; Zheng, F.; Wang, J.; Zhou, J.; Huang, X.; Chen, L.; Hu, P.; Gao, J.-M.; Zhen, Q.; Bashir, S.; et al. Facile preparation of zeolite-activated carbon composite from coal gangue with enhanced adsorption performance. Chem. Eng. J. 2020, 390, 124513–124524. [Google Scholar] [CrossRef]
- Yang, K.; Su, B.; Shi, L.; Wang, H.; Cui, Q. Adsorption mechanism and regeneration performance of 13X for H2S and SO2. Energy Fuels 2018, 32, 12742–12749. [Google Scholar] [CrossRef]
- Lu, J.; Tang, J.; Li, J.; Wang, S. The comparison of adsorption characteristics of CO2/H2O and N2/H2O on activated carbon, activated alumina, zeolite 3A and 13X. Appl. Therm. Eng. 2022, 213, 118746–118758. [Google Scholar] [CrossRef]
- Huang, W.L.; Li, J.; Liu, Z.; Zhou, J.; Ma, C.; Wen, L.-X. Mesoscale distribution of adsorbates in ZSM-5 zeolite. Chem. Eng. Sci. 2019, 198, 253–259. [Google Scholar] [CrossRef]
- Jia, Y.; Shi, Q.; Wang, J.; Ding, C.; Zhang, K. Synthesis, characterization, and catalytic application of hierarchical nano-ZSM-5 zeolite. RSC Adv. 2020, 10, 29618–29626. [Google Scholar] [CrossRef]
- Azmi, N.Z.M.; Buthiyappan, A.; Raman, A.A.A.; Patah, M.F.A.; Sufian, S. Recent advances in biomass based activated carbon for carbon dioxide capture-A review. J. Ind. Eng. Chem. 2022, 116, 1–20. [Google Scholar] [CrossRef]
- Wang, M.; Xie, R.; Chen, Y.; Pu, X.; Jiang, W.; Yao, L. A novel mesoporous zeolite-activated carbon composite as an effective adsorbent for removal of ammonia-nitrogen and methylene blue from aqueous solution. Bioresour. Technol. 2018, 268, 726–732. [Google Scholar] [CrossRef]
- Ndagijimana, P.; Rong, H.; Ndokoye, P.; Mwizerwa, J.P.; Nkinahamira, F.; Luo, S.; Guo, D.; Cui, B. A review on activated carbon/graphene composite-based materials: Synthesis and applications. J. Clean. Prod. 2023, 417, 138006–138023. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.J.; He, P.Y.; Liu, L.C. Synthesis, characterization, and selective CO2 capture performance of a new type of activated carbon-geopolymer composite adsorbent. J. Clean. Prod. 2021, 325, 129271–129282. [Google Scholar] [CrossRef]
- Yagnamurthy, S.; Rakshit, D.; Jain, S.; Rocky, K.A.; Islam, M.A.; Saha, B.B. Adsorption of difluoromethane onto activated carbon based composites: Thermophysical properties and adsorption characterization. Int. J. Heat Mass Transf. 2021, 171, 121112–121124. [Google Scholar] [CrossRef]
- Zhang, W.J.; Rabiei, S.; Bagreev, A.; Zhuang, M.S.; Rasouli, F. Study of NO adsorption on activated carbons. Appl. Catal. B 2008, 83, 63–71. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, K.; Li, H.; Xu, X.; Liu, B.; Li, H.; Zeng, Z.; Ma, W.; Li, L. Pressure swing adsorption properties of activated carbon for methanol, acetone and toluene. Chem. Eng. J. 2021, 413, 127384–127396. [Google Scholar] [CrossRef]
- Awad, R.; Mamaghani, A.H.; Boluk, Y.; Hashisho, Z. Synthesis and characterization of electrospun PAN-based activated carbon nanofibers reinforced with cellulose nanocrystals for adsorption of VOCs. Chem. Eng. J. 2021, 410, 128412–128422. [Google Scholar] [CrossRef]
- Cheng, W.P.; Gao, W.; Cui, X.; Ma, J.H.; Li, R.F. Phenol adsorption equilibrium and kinetics on zeolite X/activated carbon composite. J. Taiwan Inst. Chem. Eng. 2016, 62, 192–198. [Google Scholar] [CrossRef]
- Zhang, D.; Cheng, W.; Ma, J.; Li, R. Influence of activated carbon in zeolite X/activated carbon composites on CH4/N2 adsorption separation ability. Adsorption 2016, 22, 1129–1135. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, F.; Men, Y.; Fang, X.; Zhao, J.; Xiao, P.; Webley, P.A.; Grande, C.A. CO2 capture using a novel hybrid monolith (H-ZSM-5/activated carbon) as adsorbent by combined vacuum and electric swing adsorption (VESA). Chem. Eng. J. 2019, 358, 707–717. [Google Scholar] [CrossRef]
- Li, Z.; Song, G.; Yang, X.; Song, Q. Mechanism of O2-promoted NO adsorption on activated carbon: An experimental and computational study. Chem. Eng. J. 2024, 481, 148391–148403. [Google Scholar] [CrossRef]
- Gu, X.-W.; Pei, J.; Shao, K.; Wen, H.-M.; Li, B.; Qian, G. Chemically stable hafnium-based metal-organic framework for highly efficient C2H6/C2H4 separation under humid onditions. ACS Appl. Mater. Interfaces 2021, 13, 18792–18799. [Google Scholar] [CrossRef]
- Diao, Z.; Zhang, L.; Li, Q.; Gao, X.; Gao, X.; Seliem, M.K.; Dhaoudi, F.; Sellaoui, L.; Deng, S.; Bonilla-Petriciolet, A.; et al. Adsorption of food dyes from aqueous solution on a sweet potato residue-derived carbonaceous adsorbent: Analytical interpretation of adsorption mechanisms via adsorbent characterization and statistical physics modeling. Chem. Eng. J. 2024, 482, 148982–148994. [Google Scholar] [CrossRef]
- Sellaoui, L.; Gómez-Avilés, A.; Dhaouadi, F.; Bedia, J.; Bonilla-Petriciolet, A.; Rtimi, S.; Belver, C. Adsorption of emerging pollutants on lignin-based activated carbon: Analysis of adsorption mechanism via characterization, kinetics and equilibrium studies. Chem. Eng. J. 2023, 452, 139399–139408. [Google Scholar] [CrossRef]
- Xue, H.; Gao, X.; Seliem, M.K.; Mobarak, M.; Dong, R.; Wang, X.; Fu, K.; Li, Q.; Li, Z. Efficient adsorption of anionic azo dyes on porous heterostructured mxene/biomass activated carbon composites: Experiments, characterization, and theoretical analysis via advanced statistical physics models. Chem. Eng. J. 2023, 451, 138735–138748. [Google Scholar] [CrossRef]
- Sun, R.; Fu, B.; Luo, G.; Li, X.; Tian, H.; Yao, H. Theoretical research on mercury-laden halogenated activated carbon adsorbent bonding nature. Chem. Eng. J. 2022, 428, 131076–131084. [Google Scholar] [CrossRef]
- Hong, S.-H.; Jung, M.Y.; Lee, C.-H. Performance and dynamic behavior of H2 layered-bed PSA processes using various activated carbons and zeolite LiX for steam methane reforming gas. Chem. Eng. J. 2023, 473, 144942–144960. [Google Scholar] [CrossRef]
- Edi-Soetaredjo, F.; Slama, M.; Sellaoui, L.; Ghalla, H.; El Hadj Rhouma, M.B.; Ismadji, S.; Ernst, B.; Bonilla-Petriciolet, A.; Lamine, A.B. Highlighting the single and binary adsorption mechanism of amoxicillin and doripenem on copper benzene-1,3,5-tricarboxylate MOF via experiments, characterization, statistical physics modelling and DFT simulation. Chem. Eng. J. 2023, 474, 145633–145646. [Google Scholar] [CrossRef]
- Zhou, K.; Ma, W.; Zeng, Z.; Ma, X.; Xu, X.; Guo, Y.; Li, H.; Li, L. Experimental and DFT study on the adsorption of VOCs on activated carbon/metal oxides composites. Chem. Eng. J. 2019, 372, 1122–1133. [Google Scholar] [CrossRef]
- Guo, Z.; Huang, J.; Xue, Z.; Wang, X. Electrospun graphene oxide/carbon composite nanofibers with well-developed mesoporous structure and their adsorption performance for benzene and butanone. Chem. Eng. J. 2016, 306, 99–106. [Google Scholar] [CrossRef]


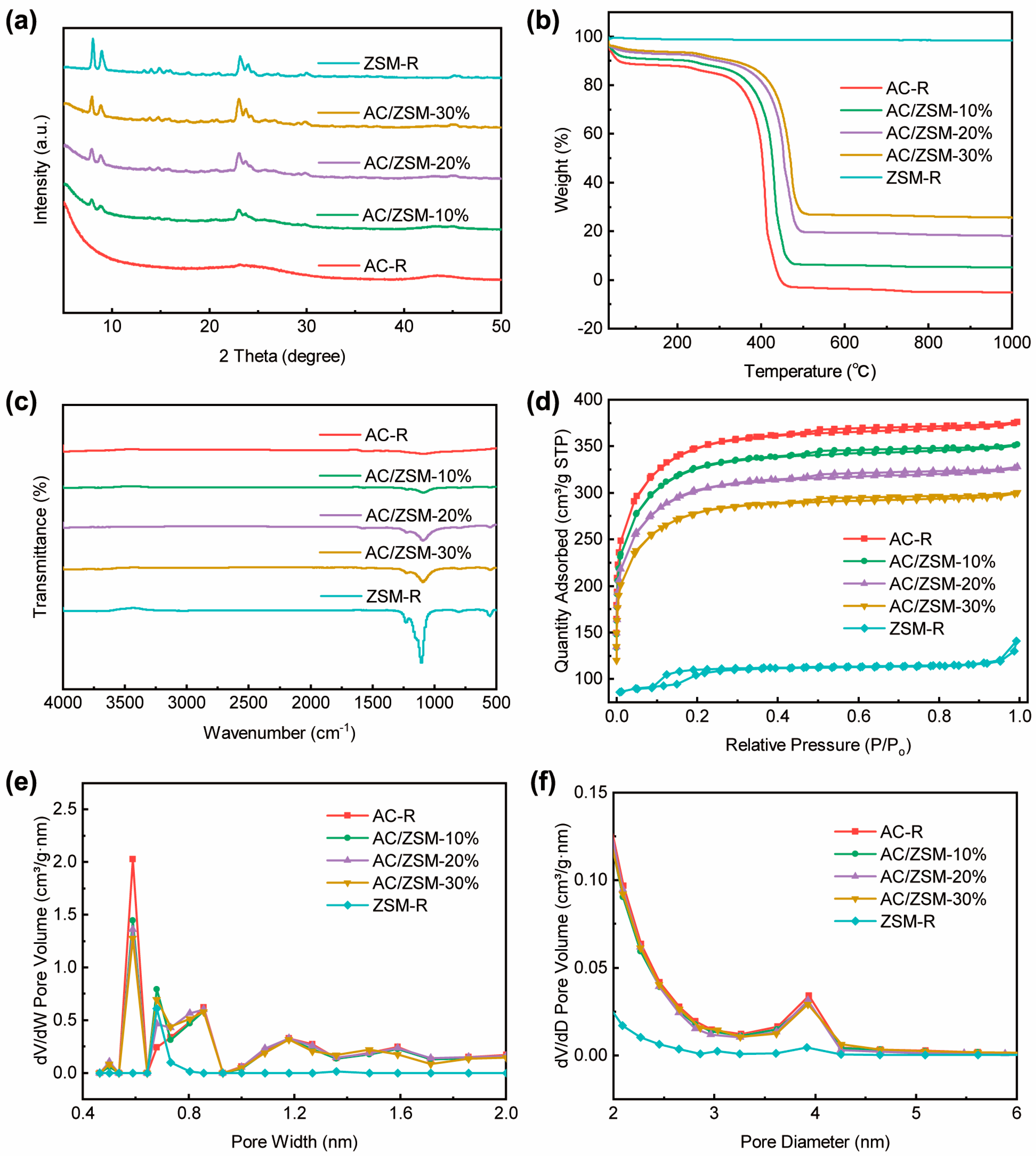


| Samples | SBET (m2/g) | Smic (m2/g) | Vtotal (cm3/g) | Vmic (cm3/g) |
|---|---|---|---|---|
| AC-R | 1375.0 | 767.3 | 0.568 | 0.330 |
| ZSM-R | 364.9 | 123.0 | 0.184 | 0.053 |
| AC/ZSM-10% | 1004.6 | 603.6 | 0.453 | 0.324 |
| AC/ZSM-20% | 931.8 | 607.4 | 0.421 | 0.308 |
| AC/ZSM-30% | 856.1 | 524.8 | 0.383 | 0.281 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Qin, L.; Ye, P.; Yang, B.; Wu, Q.; Li, L.; Dai, Y.; Zhou, C.; Li, S. Introducing Molecular Sieve into Activated Carbon to Achieve High-Effective Adsorption for Ethylene Oxide. Nanomaterials 2024, 14, 1482. https://doi.org/10.3390/nano14181482
Liu F, Qin L, Ye P, Yang B, Wu Q, Li L, Dai Y, Zhou C, Li S. Introducing Molecular Sieve into Activated Carbon to Achieve High-Effective Adsorption for Ethylene Oxide. Nanomaterials. 2024; 14(18):1482. https://doi.org/10.3390/nano14181482
Chicago/Turabian StyleLiu, Feng, Lingyan Qin, Pingwei Ye, Bo Yang, Qiong Wu, Li Li, Yuwei Dai, Chuan Zhou, and Sumin Li. 2024. "Introducing Molecular Sieve into Activated Carbon to Achieve High-Effective Adsorption for Ethylene Oxide" Nanomaterials 14, no. 18: 1482. https://doi.org/10.3390/nano14181482






