Ultraviolet Light-Induced Surface Changes of Tungsten Oxide in Air: Combined Scanning Transmission Electron Microscopy and X-ray Photoelectron Spectroscopy Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identical-Location-STEM (IL-STEM) Analysis
2.2. XPS Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, S.G.; Koteswara, K.S.R. Comparison of modification strategies towards enhanced charge carrier separation and photocatalytic degradation activity of metal oxide semiconductors (TiO2, WO3 and ZnO). Appl. Surf. Sci. 2017, 391, 124–148. [Google Scholar] [CrossRef]
- Sun, Y.F.; Liu, S.B.; Meng, F.L.; Liu, J.Y.; Jin, Z.; Kong, L.T.; Liu, J.H. Metal Oxide Nanostructures and Their Gas Sensing Properties: A Review. Sensors 2012, 12, 2610–2631. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Zhang, L.; Jeem, M.; Okamoto, K.; Nakagawa, Y.; Shibayama, T.; Ohnuma, M.; Watanabe, S. Photo- & radio-chromic iron-doped tungstic acids fabricated via submerged photosynthesis. Opt. Mater. 2022, 124, 111966. [Google Scholar] [CrossRef]
- Khan, S.; Je, M.; Kim, D.; Lee, S.; Cho, S.H.; Song, T.; Choi, H. Mapping Point Defects of Brookite TiO2 for Photocatalytic Activity Beyond Anatase and P25. J. Phys. Chem. C 2020, 124, 10376–10384. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Hayashi, Y.; Yang, S.; Shibayama, T. Effects of gas phase ion irradiation and vacuum annealing on the visible light photocatalytic properties of WO3. Vacuum 2022, 206, 111519. [Google Scholar] [CrossRef]
- Chen, S.; Xiao, Y.; Xie, W.; Wang, Y.; Hu, Z.; Zhang, W.; Zhao, H. Facile Strategy for Synthesizing Non-Stoichiometric Monoclinic Structured Tungsten Trioxide (WO3-x) with Plasma Resonance Absorption and Enhanced Photocatalytic Activity. Nanomaterials 2018, 8, 553. [Google Scholar] [CrossRef]
- Gao, D.; Liu, W.; Xu, Y.; Wang, P.; Fan, J.; Yu, H. Core-shell Ag@Ni cocatalyst on the TiO2 photocatalyst: One-step photoinduced deposition and its improved H2-evolution activity. Appl. Catal. B 2020, 260, 118190. [Google Scholar] [CrossRef]
- Tomita, O.; Otsubo, T.; Higashi, M.; Ohtani, B.; Abe, R. Partial Oxidation of Alcohols on Visible-Light-Responsive WO3 Photocatalysts Loaded with Palladium Oxide Cocatalyst. ACS. Catal. 2016, 6, 1134–1144. [Google Scholar] [CrossRef]
- Yu, Z.; Zhu, S.; Zhang, L.; Watanabe, S. Submerged photosynthesis of TiO2-CuO hetero-nanoparticles and their multiple applications for water issues under solar light. J. Alloys Compd. 2024, 992, 174624. [Google Scholar] [CrossRef]
- Li, F.; Liao, B.; Shen, J.; Ke, J.; Zhang, R.; Wang, Y.; Niu, Y. Enhancing Photocatalytic Activities for Sustainable Hydrogen Evolution on Structurally Matched CuInS2/ZnIn2S4 Heterojunctions. Molecules 2024, 29, 2447. [Google Scholar] [CrossRef]
- Zak, A.M. Light-Induced In Situ Transmission Electron Microscopy―Development, Challenges, and Perspectives. Nano Lett. 2022, 22, 9219–9226. [Google Scholar] [CrossRef]
- Yoshida, K.; Yamasaki, J.; Tanaka, N. In situ high-resolution transmission electron microscopy observation of photodecomposition process of poly-hydrocarbons on catalytic TiO2 films. Appl. Phys. Lett. 2004, 84, 2542–2544. [Google Scholar] [CrossRef]
- Cavalca, F.; Laursen, A.B.; Wagner, J.B.; Damsgaard, C.D.; Chorkendorff, L.; Hansen, T.W. Light-Induced Reduction of Cuprous Oxide in an Environmental Transmission Electron Microscope. ChemCatChem 2013, 5, 2667–2672. [Google Scholar] [CrossRef]
- Ishioka, J.; Kogure, K.; Ofuji, K.; Kawaguchi, K.; Jeem, M.; Kato, T.; Shibayama, T.; Watanabe, S. In situ direct observation of photocorrosion in ZnO crystals in ionic liquid using a laser-equipped high-voltage electron microscope. AIP Adv. 2017, 7, 035220. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Miyauchi, M.; Nakajima, A.; Fujishima, A.; Hashimoto, K.; Watanabe, T. Photoinduced Surface Reactions on TiO2 and SrTiO3 Films: Photocatalytic Oxidation and Photoinduced Hydrophilicity. Chem. Mater. 2000, 12, 3–5. [Google Scholar] [CrossRef]
- Sun, R.D.; Nakajima, A.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Photoinduced Surface Wettability Conversion of ZnO and TiO2 Thin Films. J. Phys. Chem. B 2001, 105, 1984–1990. [Google Scholar] [CrossRef]
- Sakai, N.; Wang, R.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Effect of Ultrasonic Treatment on Highly Hydrophilic TiO2 Surfaces. Langmuir 1998, 14, 5918–5920. [Google Scholar] [CrossRef]
- Kamei, M.; Mitsuhashi, T. Hydrophobic drawings on hydrophilic surfaces of single crystalline titanium dioxide: Surface wettability control by mechanochemical treatment. Surf. Sci. 2000, 463, L609–L612. [Google Scholar] [CrossRef]
- Miyauchi, M.; Shibuya, M.; Zhao, Z.G.; Liu, Z. Surface Wetting Behavior of a WO3 Electrode under Light-Irradiated or Potential-Controlled Conditions. J. Phys. Chem. C 2009, 113, 10642–10646. [Google Scholar] [CrossRef]
- Miyauchi, M. Photocatalysis and photoinduced hydrophilicity of WO3 thin films with underlying Pt nanoparticles. Phys. Chem. Chem. Phys. 2008, 10, 6258–6265. [Google Scholar] [CrossRef]
- Murayama, T.; Sato, M.; Nagai, H.; Yasui, E. Visible-light-induced superhydrophilicity of crystallized WO3 thin films fabricated by using a newly isolated W6+ complex salt of citric acid. Nanoscale Adv. 2023, 5, 1990–1998. [Google Scholar] [CrossRef]
- Meng, J.; Lan, Z.; Castelli, I.E.; Zheng, K. Atomic-Scale Observation of Oxygen Vacancy-Induced Step Reconstruction in WO3. J. Phys. Chem. C 2021, 125, 8456–8460. [Google Scholar] [CrossRef]
- Li, T.; He, J.; Pena, B.; Berlinguette, C.P. Exposure of WO3 Photoanodes to Ultraviolet Light Enhances Photoeletrochemical Water Oxidation. ACS Appl. Mater. Interfaces 2016, 8, 25010–25013. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. Reliable determination of chemical state in x-ray photoelectron spectroscopy based on sample-work-function referencing to adventitious carbon: Resolving the myth of apparent constant binding energy of the C 1s peak. Appl. Surf. Sci. 2018, 451, 99–103. [Google Scholar] [CrossRef]
- Halek, G.; Baikie, I.D.; Teterycz, H.; Halek, P.; Suchorska-Wozniak, P.; Wisniewski, K. Work function analysis of gas sensitive WO3 layers with Pt doping. Sens. Actuators B 2013, 187, 379–385. [Google Scholar] [CrossRef]
- Akhter, S.; Allan, K.; Buchanan, D.; Cook, J.A.; Campion, A.; White, J.M. XPS and IR study of X-ray induced degradation of PVA polymer film. Appl. Surf. Sci. 1988, 35, 241–258. [Google Scholar] [CrossRef]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice Jr, C.A.; et al. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Fukushima, K.; Lee, S.Y.; Tanaka, K.; Sasaki, K.; Ishizaki, T. Effect of Surface Modification for Carbon Cathode Materials on Charge-Discharge Performance of Li-Air Batteries. Materials 2022, 15, 3270. [Google Scholar] [CrossRef]
- Grey, L.H.; Nie, H.Y.; Biesinger, M.K. Defining the nature of adventitious carbon and improving its merit as a charge correction reference for XPS. Appl. Surf. Sci. 2024, 653, 159319. [Google Scholar] [CrossRef]
- Ohtsu, N.; Masahashi, N.; Mizukoshi, Y.; Wagatsuma, K. Hydrocarbon Decomposition on a Hydrophilic TiO2 Surface by UV Irradiation: Spectral and Quantitative Analysis Using in-Situ XPS Technique. Langmuir 2009, 25, 11586–11591. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Arai, T.; Yanagida, M.; Konishi, Y.; Iwasaki, Y.; Sugihara, H.; Sayama, K. Efficient Complete Oxidation of Acetaldehyde into CO2 over CuBi2O4/WO3 Composite Photocatalyst under Visible and UV Light Irradiation. J. Phys. Chem. C 2007, 111, 7574–7577. [Google Scholar] [CrossRef]
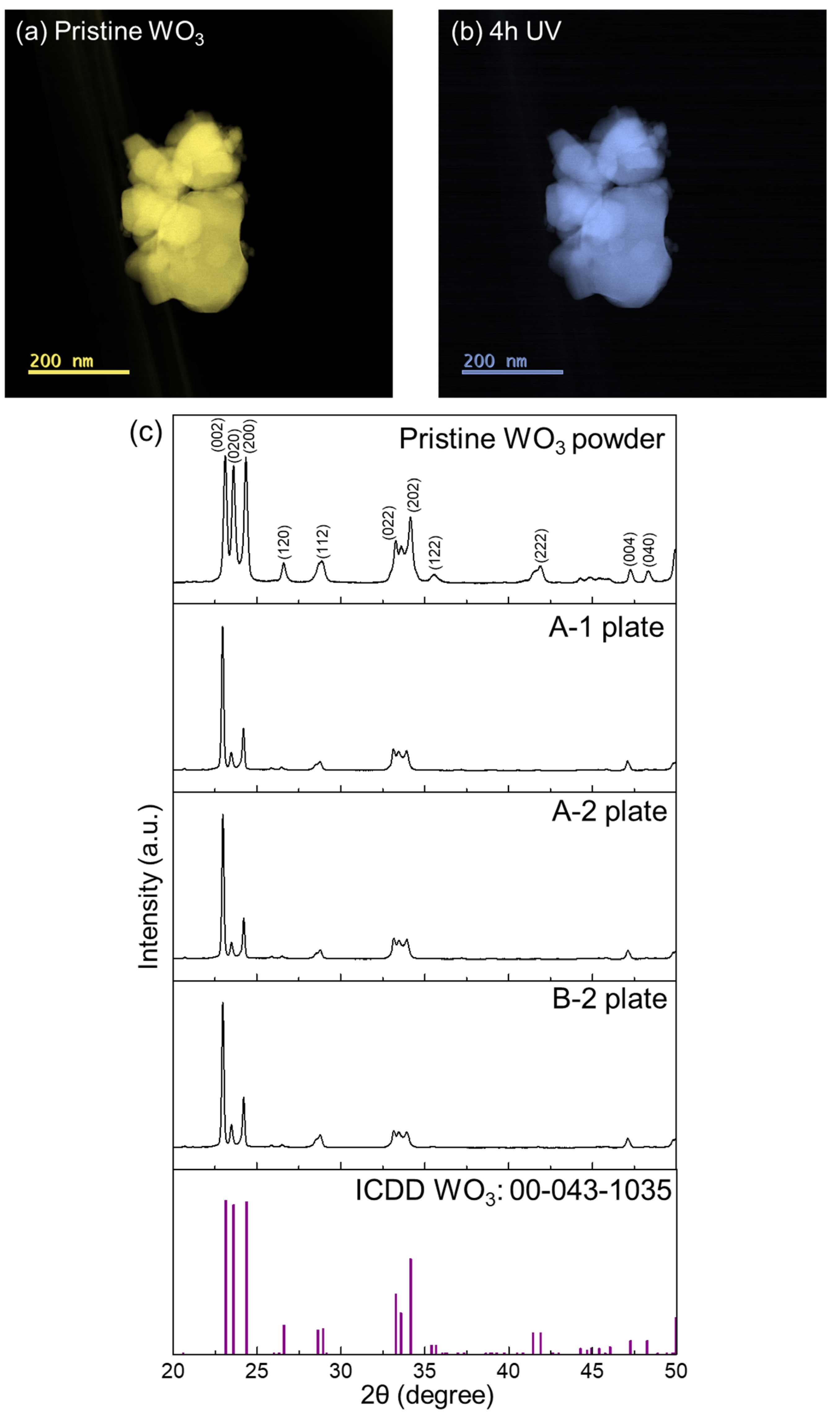

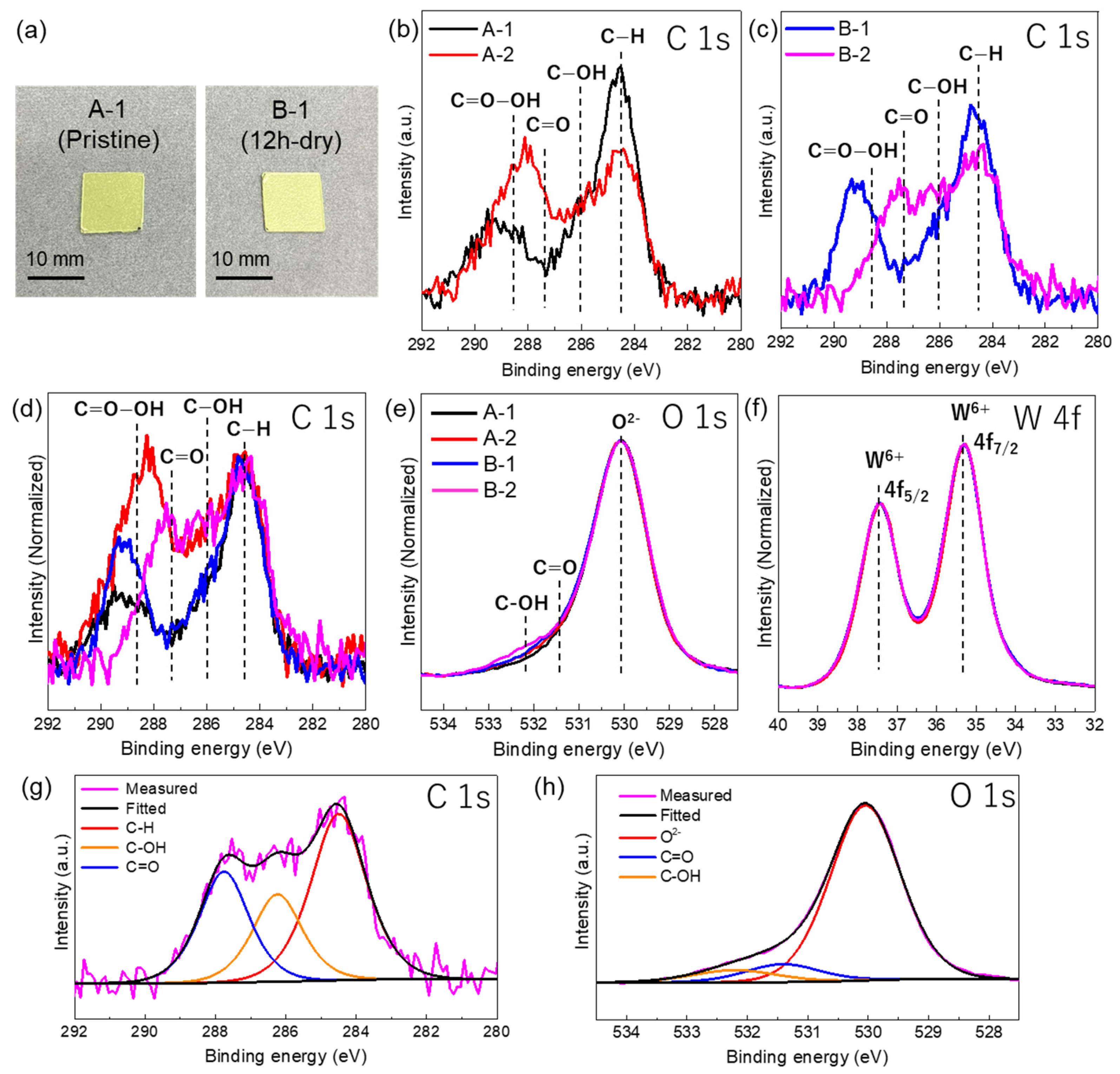
| Sample | Sample Treatment | The Number of Days That Passed between UV Irradiation and the Date of XPS Analysis | Average Humidity (%) | Average Temperature (°C) |
|---|---|---|---|---|
| A-1 | None (pristine) | – | – | – |
| B-1 | 12 h irradiation/dry | 108 | 30 | 17.9 |
| A-2 | A-1 + 4 h irradiation/humid | 1 | 72 | 27.7 |
| B-2 | B-1 + 4 h irradiation-humid | 1 | 72 | 27.7 |
| Sample | Sample Treatment | C (at%) | O (at%) | W (at%) | C/W Ratio | O/W Ratio |
|---|---|---|---|---|---|---|
| A-1 | Pristine | 23.5 | 55.0 | 21.5 | 1.09 | 2.56 |
| B-1 | 12 h irradiation/dry | 22.8 | 55.8 | 21.4 | 1.07 | 2.61 |
| A-2 | A-1 + 4 h irradiation/humid | 23.9 | 54.8 | 21.3 | 1.12 | 2.57 |
| B-2 | B-1 + 4 h irradiation/humid | 19.8 | 58.4 | 21.8 | 0.91 | 2.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakagawa, Y.; Shiratsuchi, Y.; Shibayama, T.; Takeguchi, M. Ultraviolet Light-Induced Surface Changes of Tungsten Oxide in Air: Combined Scanning Transmission Electron Microscopy and X-ray Photoelectron Spectroscopy Analysis. Nanomaterials 2024, 14, 1486. https://doi.org/10.3390/nano14181486
Nakagawa Y, Shiratsuchi Y, Shibayama T, Takeguchi M. Ultraviolet Light-Induced Surface Changes of Tungsten Oxide in Air: Combined Scanning Transmission Electron Microscopy and X-ray Photoelectron Spectroscopy Analysis. Nanomaterials. 2024; 14(18):1486. https://doi.org/10.3390/nano14181486
Chicago/Turabian StyleNakagawa, Yuki, Yasuhiro Shiratsuchi, Tamaki Shibayama, and Masaki Takeguchi. 2024. "Ultraviolet Light-Induced Surface Changes of Tungsten Oxide in Air: Combined Scanning Transmission Electron Microscopy and X-ray Photoelectron Spectroscopy Analysis" Nanomaterials 14, no. 18: 1486. https://doi.org/10.3390/nano14181486







