Toughening Mechanism in Nanotwinned Boron Carbide: A Molecular Dynamics Study
Abstract
:1. Introduction
2. Methodology
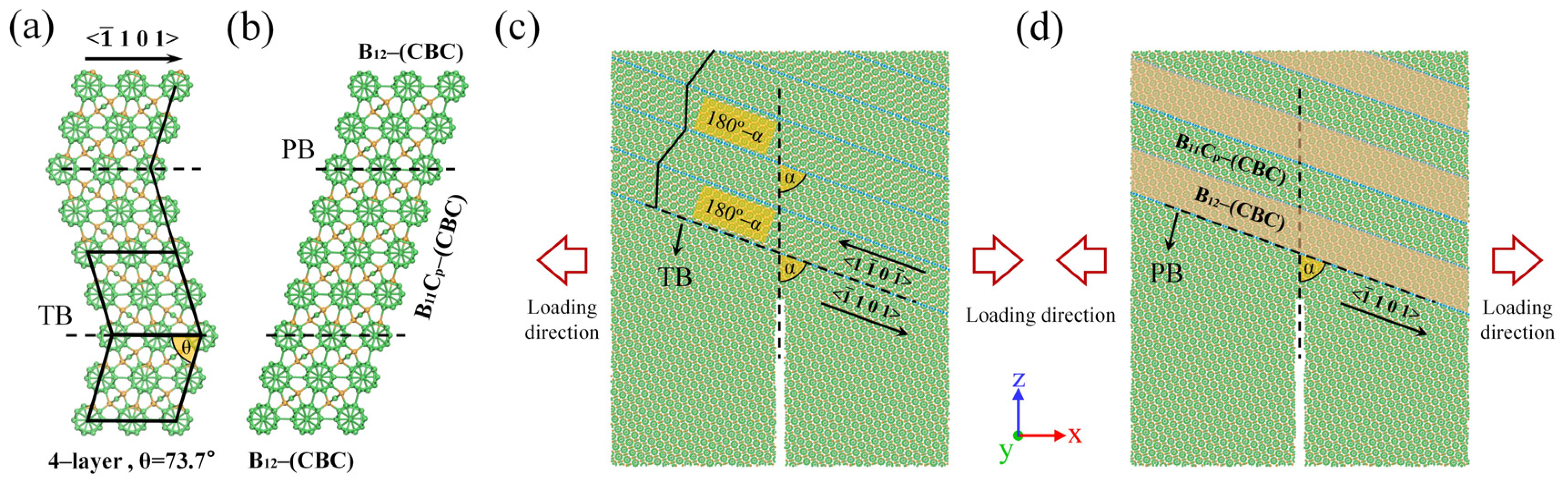
3. Results and Discussion
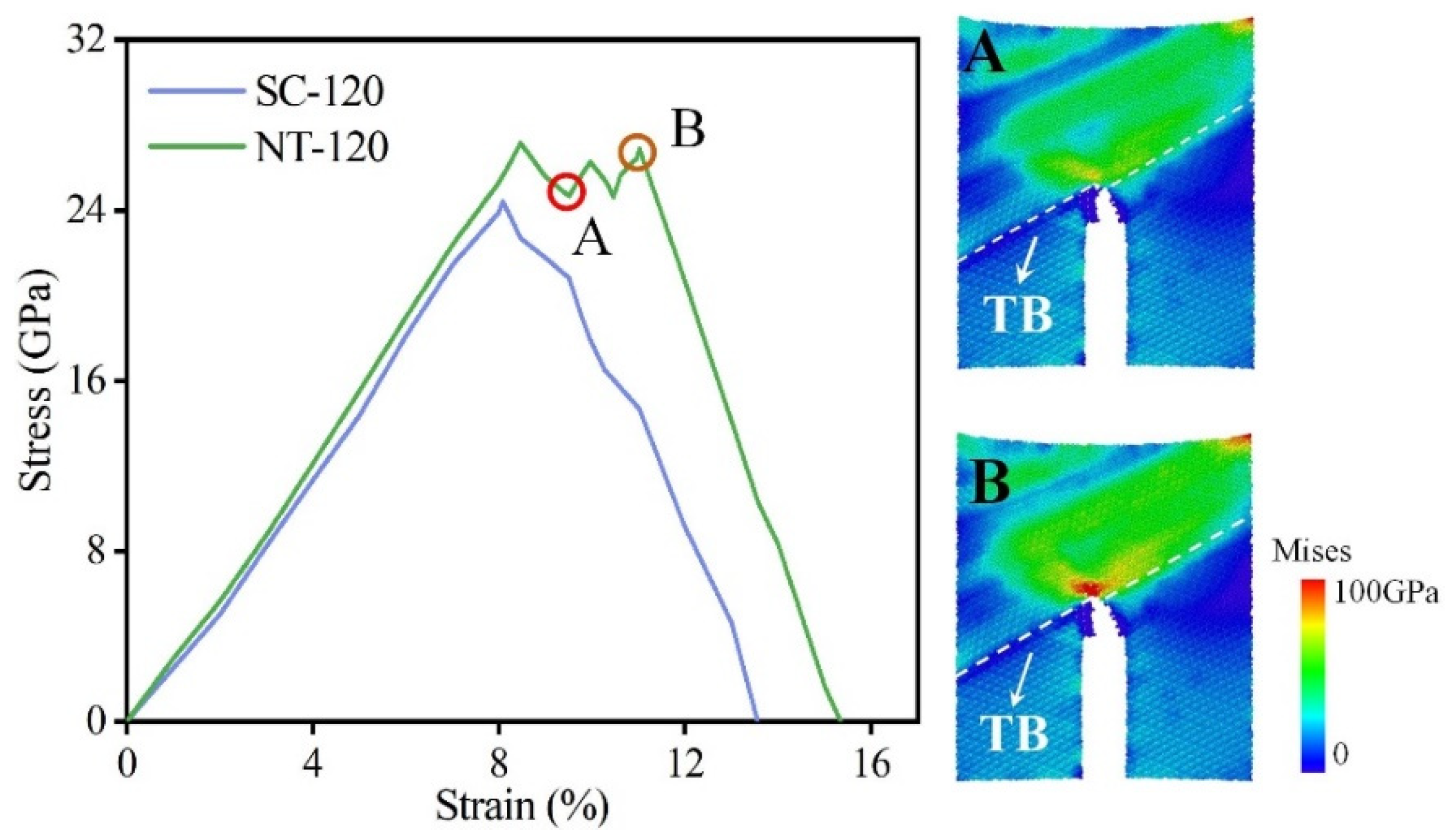
3.1. Effect of TBs on Fracture Toughness
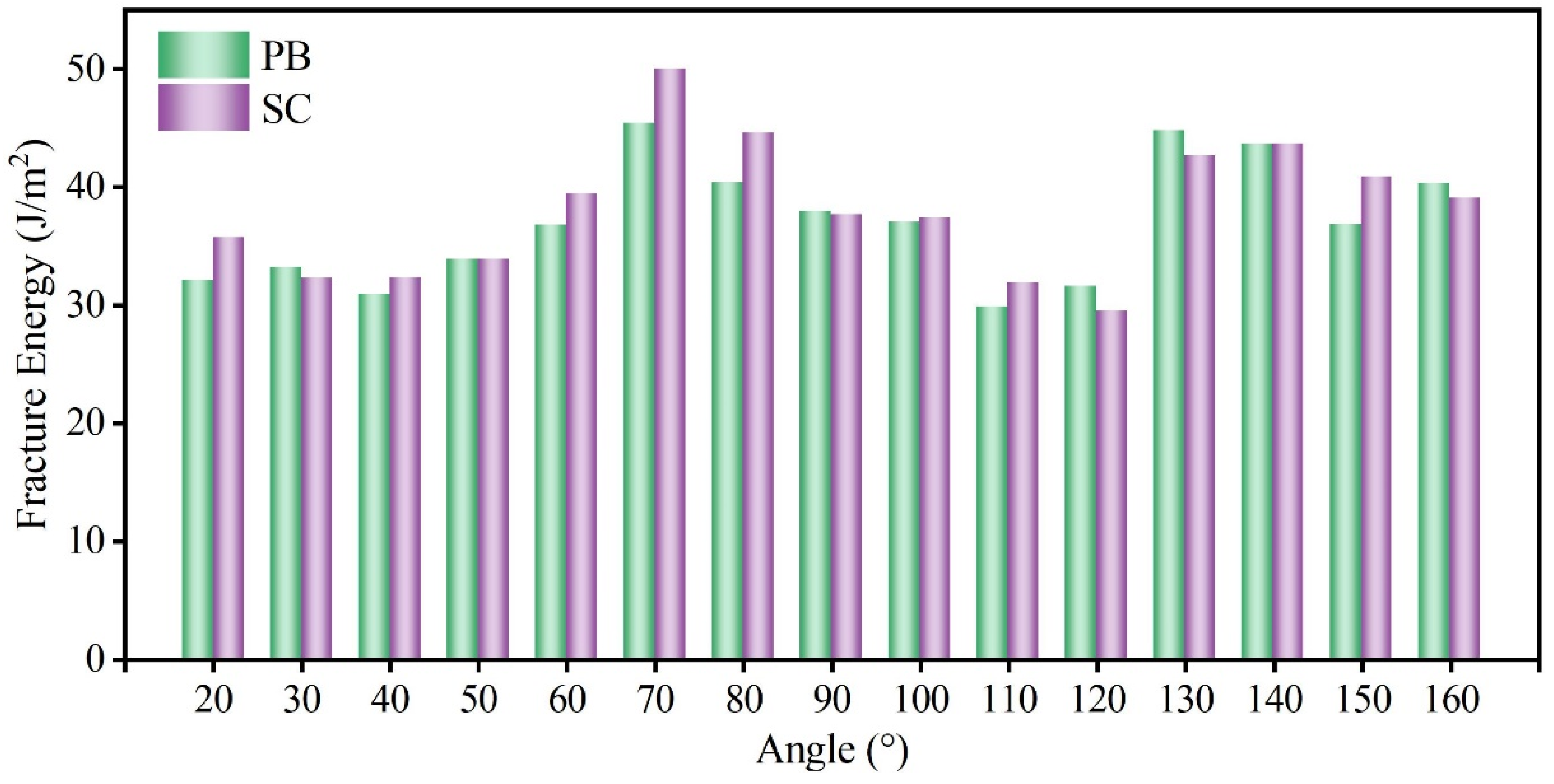
3.2. Effect of PBs on Fracture Toughness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gogotsi, G.; Groushevsky, Y.L.; Dashevskaya, O.; Gogotsi, Y.G.; Lavrenko, V. Complex investigation of hot-pressed boron carbide. J. Less Common Met. 1986, 117, 225–230. [Google Scholar] [CrossRef]
- Thevenot, F. Boron carbide—A comprehensive review. J. Eur. Ceram. Soc. 1990, 6, 205–225. [Google Scholar] [CrossRef]
- Vogler, T.; Reinhart, W.; Chhabildas, L.C. Dynamic behavior of boron carbide. J. Appl. Phys. 2004, 95, 4173–4183. [Google Scholar] [CrossRef]
- Savio, S.; Rao, A.S.; Reddy, P.R.S.; Madhu, V. Microstructure and ballistic performance of hot pressed & reaction bonded boron carbides against an armour piercing projectile. Adv. Appl. Ceram. 2019, 118, 264–273. [Google Scholar]
- Le Godec, Y.; Courac, A.; Solozhenko, V.L. High-pressure synthesis of superhard and ultrahard materials. J. Appl. Phys. 2019, 126, 151102. [Google Scholar] [CrossRef]
- Dresch, A.B.; Venturini, J.; Arcaro, S.; Montedo, O.R.; Bergmann, C.P. Ballistic ceramics and analysis of their mechanical properties for armour applications: A review. Ceram. Int. 2021, 47, 8743–8761. [Google Scholar] [CrossRef]
- Jiménez, I.; Sutherland, D.; Van Buuren, T.; Carlisle, J.A.; Terminello, L.J.; Himpsel, F.J. Photoemission and x-ray-absorption study of boron carbide and its surface thermal stability. Phys. Rev. B 1998, 57, 13167. [Google Scholar] [CrossRef]
- Vast, N.; Sjakste, J.; Betranhandy, E. Boron carbides from first principles. J. Phys. Conf. Ser. 2009, 176, 012002. [Google Scholar]
- Silver, A.; Bray, P. Nuclear magnetic resonance study of boron carbide. J. Chem. Phys. 1959, 31, 247–253. [Google Scholar] [CrossRef]
- Domnich, V.; Reynaud, S.; Haber, R.A.; Chhowalla, M. Boron carbide: Structure, properties, and stability under stress. J. Am. Ceram. Soc. 2011, 94, 3605–3628. [Google Scholar] [CrossRef]
- Xie, K.Y.; Yang, Q.; Marvel, C.J.; He, M.R.; LaSalvia, J.C.; Harmer, M.P.; Hwang, C.; Haber, R.A.; Hemker, K.J. Experimental observations of amorphization in multiple generations of boron carbide. J. Am. Ceram. Soc. 2022, 105, 3008–3029. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Mei, H.; Liu, L.; Xu, S.; Zhang, J. Boron carbide nanopillars under impact loading: Mechanical response and amorphous bands formation mechanism. Comput. Mater. Sci. 2022, 214, 111746. [Google Scholar] [CrossRef]
- Farbaniec, L.; Hogan, J.; Xie, K.; Shaeffer, M.; Hemker, K.; Ramesh, K. Damage evolution of hot-pressed boron carbide under confined dynamic compression. Int. J. Impact Eng. 2017, 99, 75–84. [Google Scholar] [CrossRef]
- Shen, Y.; Li, G.; An, Q. Enhanced fracture toughness of boron carbide from microalloying and nanotwinning. Scr. Mater. 2019, 162, 306–310. [Google Scholar] [CrossRef]
- Chen, M.; McCauley, J.W.; Hemker, K.J. Shock-induced localized amorphization in boron carbide. Science 2003, 299, 1563–1566. [Google Scholar] [CrossRef]
- Bourne, N. Shock–induced brittle failure of boron carbide. Proc. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 2002, 458, 1999–2006. [Google Scholar] [CrossRef]
- Grady, D. Shock-wave strength properties of boron carbide and silicon carbide. Le J. De Phys. IV 1994, 4, C8-385. [Google Scholar] [CrossRef]
- Suri, A.; Subramanian, C.; Sonber, J.; Murthy, T.C. Synthesis and consolidation of boron carbide: A review. Int. Mater. Rev. 2010, 55, 4–40. [Google Scholar] [CrossRef]
- Moshtaghioun, B.M.; Cumbrera-Hernández, F.L.; Gómez-García, D.; de Bernardi-Martín, S.; Domínguez-Rodríguez, A.; Monshi, A.; Abbasi, M.H. Effect of spark plasma sintering parameters on microstructure and room-temperature hardness and toughness of fine-grained boron carbide (B4C). J. Eur. Ceram. Soc. 2013, 33, 361–369. [Google Scholar] [CrossRef]
- Sairam, K.; Sonber, J.; Murthy, T.C.; Subramanian, C.; Fotedar, R.; Nanekar, P.; Hubli, R. Influence of spark plasma sintering parameters on densification and mechanical properties of boron carbide. Int. J. Refract. Met. Hard Mater. 2014, 42, 185–192. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Sun, Y.; Xiang, M.; Wang, G.; Bai, Y.; Mu, J.; Che, H.; Wang, W. Preparation, microstructure and toughening mechanism of superhard ultrafine-grained boron carbide ceramics with outstanding fracture toughness. J. Alloys Compd. 2018, 762, 125–132. [Google Scholar] [CrossRef]
- Shen, Y.; Fuller, J.; An, Q. Mitigating the formation of amorphous shear band in boron carbide. J. Appl. Phys. 2021, 129, 140902. [Google Scholar] [CrossRef]
- Guo, D.; An, Q. Transgranular amorphous shear band formation in polycrystalline boron carbide. Int. J. Plast. 2019, 121, 218–226. [Google Scholar] [CrossRef]
- Behler, K.D.; Marvel, C.J.; LaSalvia, J.C.; Walck, S.D.; Harmer, M.P. Observations of grain boundary chemistry variations in a boron carbide processed with oxide additives. Scr. Mater. 2018, 142, 106–110. [Google Scholar] [CrossRef]
- Ye, F.; Hou, Z.; Zhang, H.; Liu, L. Densification and mechanical properties of spark plasma sintered B4C with Si as a sintering aid. J. Am. Ceram. Soc. 2010, 93, 2956–2959. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, J.-X.; Xu, F.; Zhang, G.-J. Pressureless sintering of titanium carbide doped with boron or boron carbide. J. Eur. Ceram. Soc. 2017, 37, 539–547. [Google Scholar] [CrossRef]
- Dai, J.; Singh, J.; Yamamoto, N. Fabrication and characterization of FAST sintered micro/nano boron carbide composites with enhanced fracture toughness. J. Eur. Ceram. Soc. 2020, 40, 5272–5285. [Google Scholar] [CrossRef]
- Vargas-Gonzalez, L.; Speyer, R.F.; Campbell, J. Flexural strength, fracture toughness, and hardness of silicon carbide and boron carbide armor ceramics. Int. J. Appl. Ceram. Technol. 2010, 7, 643–651. [Google Scholar] [CrossRef]
- Sedlák, R.; Kovalčíková, A.; Múdra, E.; Rutkowski, P.; Dubiel, A.; Girman, V.; Bystrický, R.; Dusza, J. Boron carbide/graphene platelet ceramics with improved fracture toughness and electrical conductivity. J. Eur. Ceram. Soc. 2017, 37, 3773–3780. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, H.; Peng, S. Electrically conductive graphene nanoplatelet/boron carbide composites with high hardness and toughness. Scr. Mater. 2016, 114, 98–102. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Li, X.; Xu, L.; Cao, X.; Wang, Y.; Wang, Z.; Meng, C.; Zhu, W.; Ouyang, X. Enhancing toughness in boron carbide with reduced graphene oxide. J. Am. Ceram. Soc. 2016, 99, 257–264. [Google Scholar] [CrossRef]
- Itoh, H.; Maekawa, I.; Iwahara, H. Microstructure and mechanical properties of B6O-B4C sintered composites prepared under high pressure. J. Mater. Sci. 2000, 35, 693–698. [Google Scholar] [CrossRef]
- Tang, B.; An, Q.; Goddard, W.A., III. Improved ductility of boron carbide by microalloying with boron suboxide. J. Phys. Chem. C 2015, 119, 24649–24656. [Google Scholar] [CrossRef]
- Tang, B.; He, Y.; Goddard, W.A., III; An, Q. First principles predicting enhanced ductility of boride carbide through magnesium microalloying. J. Am. Ceram. Soc. 2019, 102, 5514–5523. [Google Scholar] [CrossRef]
- Xiang, S.; Ma, L.; Yang, B.; Dieudonne, Y.; Pharr, G.M.; Lu, J.; Yadav, D.; Hwang, C.; LaSalvia, J.C.; Haber, R.A. Tuning the deformation mechanisms of boron carbide via silicon doping. Sci. Adv. 2019, 5, eaay0352. [Google Scholar] [CrossRef]
- Christian, J.W.; Mahajan, S. Deformation twinning. Prog. Mater. Sci. 1995, 39, 1–157. [Google Scholar] [CrossRef]
- Huang, Q.; Yu, D.; Xu, B.; Hu, W.; Ma, Y.; Wang, Y.; Zhao, Z.; Wen, B.; He, J.; Liu, Z. Nanotwinned diamond with unprecedented hardness and stability. Nature 2014, 510, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Gao, Y.; Hu, W.; Xu, B.; Wang, J.; Zhang, X.; Zhang, Q.; Wang, Y.; Ge, B.; Yang, Z. Hierarchically structured diamond composite with exceptional toughness. Nature 2020, 582, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, Q.; Wang, Y.; Jiang, J.; Xing, H.; Li, X. Toughening and crack healing mechanisms in nanotwinned diamond composites with various polytypes. Phys. Rev. Lett. 2021, 127, 066101. [Google Scholar] [CrossRef]
- Ma, X.; Shi, L.; Yang, L.; Yi, J.; Wang, B.; Li, M.; Zheng, B.; Hou, C.; Ye, L.; Zhong, Y. Layer-defect toughened hierarchically structured diamond composites. Eng. Fract. Mech. 2023, 279, 109052. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, B.; Yu, D.; Ma, Y.; Wang, Y.; Jiang, Y.; Hu, W.; Tang, C.; Gao, Y.; Luo, K. Ultrahard nanotwinned cubic boron nitride. Nature 2013, 493, 385–388. [Google Scholar] [CrossRef]
- Fujita, T.; Guan, P.; Madhav Reddy, K.; Hirata, A.; Guo, J.; Chen, M. Asymmetric twins in rhombohedral boron carbide. Appl. Phys. Lett. 2014, 104, 021907. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, H.; Ma, X.; Yang, L.; Zhong, Y.; He, X. Superhardness in nanotwinned boron carbide: A molecular dynamics study. Phys. Chem. Chem. Phys. 2023, 25, 19585–19595. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Wang, Z. Twins enhanced mechanical properties of boron carbide. Ceram. Int. 2022, 48, 14499–14506. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- An, Q.; Goddard, W.A., III. Atomistic origin of brittle failure of boron carbide from large-scale reactive dynamics simulations: Suggestions toward improved ductility. Phys. Rev. Lett. 2015, 115, 105501. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Mei, H.; Xu, S.; Li, J.; Zhang, J. The formation mechanisms of amorphous bands of boron carbide nanopillars under uniaxial compressions and their effects on mechanical properties from molecular dynamics simulation. Comput. Mater. Sci. 2021, 199, 110708. [Google Scholar] [CrossRef]
- DeVries, M.; Subhash, G.; Awasthi, A. Shocked ceramics melt: An atomistic analysis of thermodynamic behavior of boron carbide. Phys. Rev. B 2020, 101, 144107. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO–the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2009, 18, 015012. [Google Scholar] [CrossRef]
- Xie, K.Y.; An, Q.; Toksoy, M.F.; McCauley, J.W.; Haber, R.A.; Goddard, W.A., III; Hemker, K.J. Atomic-level understanding of “asymmetric twins” in boron carbide. Phys. Rev. Lett. 2015, 115, 175501. [Google Scholar] [CrossRef]
- An, Q.; Goddard, W.A., III; Cheng, T. Atomistic explanation of shear-induced amorphous band formation in boron carbide. Phys. Rev. Lett. 2014, 113, 095501. [Google Scholar] [CrossRef]
- Saal, J.E.; Shang, S.; Liu, Z.-K. The structural evolution of boron carbide via ab initio calculations. Appl. Phys. Lett. 2007, 91, 231915. [Google Scholar] [CrossRef]
- Powell, M.J.D. Restart procedures for the conjugate gradient method. Math. Program. 1977, 12, 241–254. [Google Scholar] [CrossRef]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695. [Google Scholar] [CrossRef]
- Pittari, J., III; Subhash, G.; Zheng, J.; Halls, V.; Jannotti, P. The rate-dependent fracture toughness of silicon carbide-and boron carbide-based ceramics. J. Eur. Ceram. Soc. 2015, 35, 4411–4422. [Google Scholar] [CrossRef]
- Vast, N.; Lazzari, R.; Besson, J.; Baroni, S.; Dal Corso, A. Atomic structure and vibrational properties of icosahedral α-boron and B4C boron carbide. Comput. Mater. Sci. 2000, 17, 127–132. [Google Scholar] [CrossRef]
- Lazzari, R.; Vast, N.; Besson, J.; Baroni, S.; Dal Corso, A. Atomic structure and vibrational properties of icosahedral B 4 C boron carbide. Phys. Rev. Lett. 1999, 83, 3230. [Google Scholar] [CrossRef]
- Lee, H.; Speyer, R.F. Hardness and fracture toughness of pressureless-sintered boron carbide (B4C). J. Am. Ceram. Soc. 2002, 85, 1291–1293. [Google Scholar] [CrossRef]
- Li, P.; Ma, M.; Wu, Y.; Zhang, X.; Chang, Y.; Zhuge, Z.; Sun, L.; Hu, W.; Yu, D.; Xu, B. Preparation of dense B4C ceramics by spark plasma sintering of high-purity nanoparticles. J. Eur. Ceram. Soc. 2021, 41, 3929–3936. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhong, Y.; Ma, X.; Yang, L.; He, X.; Shi, L. Toughening Mechanism in Nanotwinned Boron Carbide: A Molecular Dynamics Study. Nanomaterials 2024, 14, 1493. https://doi.org/10.3390/nano14181493
Zhang H, Zhong Y, Ma X, Yang L, He X, Shi L. Toughening Mechanism in Nanotwinned Boron Carbide: A Molecular Dynamics Study. Nanomaterials. 2024; 14(18):1493. https://doi.org/10.3390/nano14181493
Chicago/Turabian StyleZhang, Hongchi, Yesheng Zhong, Xiaoliang Ma, Lin Yang, Xiaodong He, and Liping Shi. 2024. "Toughening Mechanism in Nanotwinned Boron Carbide: A Molecular Dynamics Study" Nanomaterials 14, no. 18: 1493. https://doi.org/10.3390/nano14181493





