Silver Nanoparticles: A Comprehensive Review of Synthesis Methods and Chemical and Physical Properties
Abstract
1. Introduction
2. Properties of Silver Nanoparticles
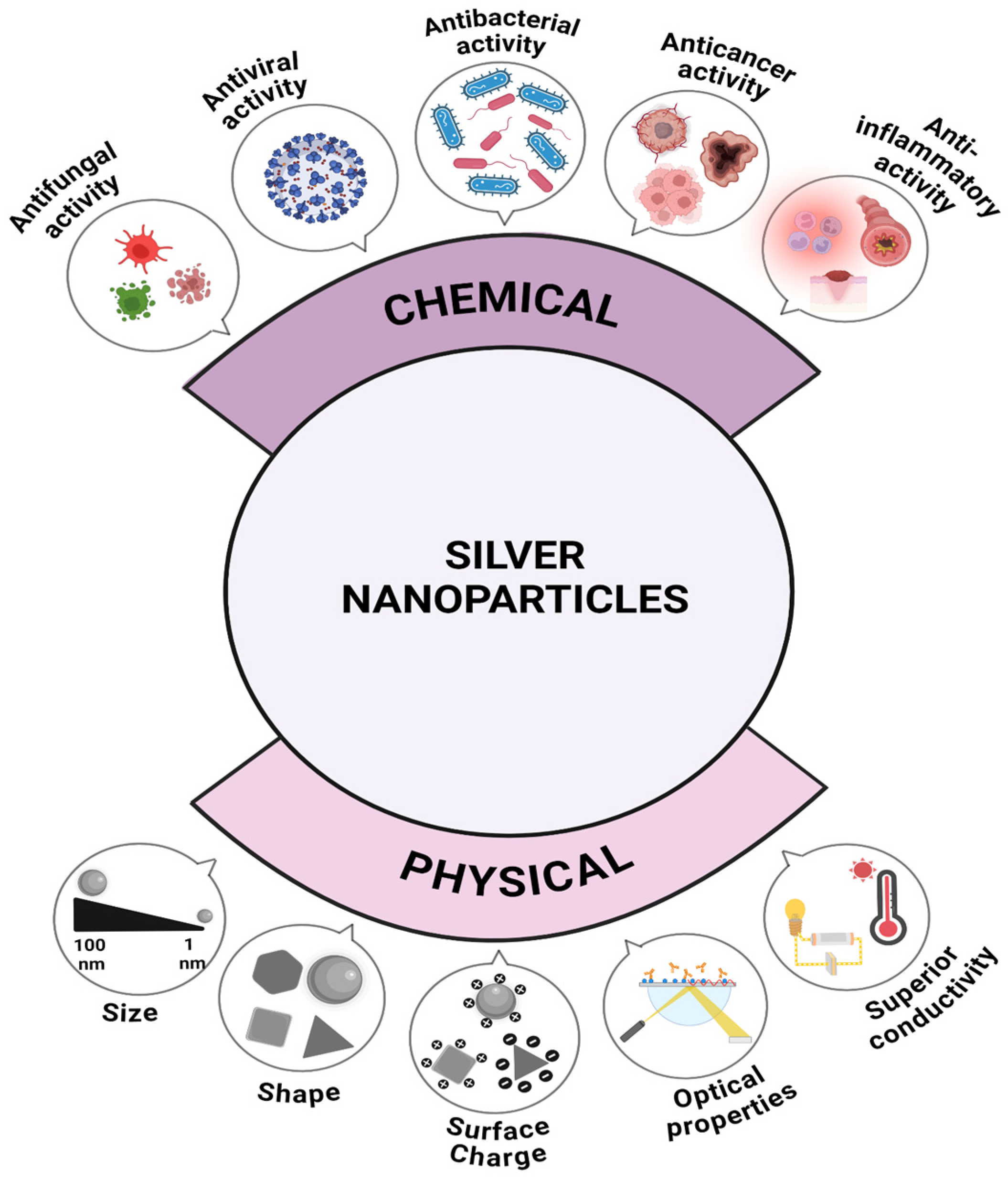
2.1. Size
2.2. Shape
2.3. Surface Charge
2.4. Electrical Conductivity and Melting Point
2.5. Thermal Conductivity
2.6. Optical Properties
2.7. Antibacterial Activity
| Highlighted Property | Result | Reference |
|---|---|---|
| Antibacterial activity | Silver NPs synthesized with Prosopis fracta extract exhibit concentration-dependent antibacterial activity against S. mutans. MIC values are determined as 6.25 µg/mL, 12.5 µg/mL, and 100 µg/mL for NP concentrations of 1 mM, 3 mM, and 5 mM, respectively. | [79] |
| Antibacterial activity | Silver NPs with a size of 15 nm are utilized to sterilize bacteria present in water. Demonstrating a dose-dependent antibacterial effect, these NPs achieve 99.72% of bacterial inhibition. Optimum conditions are determined as pH 6 and 20 min of contact time at a concentration of 0.01 mg/mL. | [80] |
| Antibacterial activity | Antibacterial activity of the silver NPs synthesized from Carduus crispus are tested on both Gram-positive (Micrococcus luteus) and Gram-negative (E. coli) bacteria. Results reveal a size-dependent antibacterial activity. Thirteen nm of silver NPs demonstrat inhibition zones of 7.5 ± 0.3 mm against M. luteus and 6.5 ± 0.3 mm against E. coli in comparison to their larger counterparts. | [81] |
| Antibacterial activity | Spherical silver NPs with an average size of 20 nm are synthesized using Cestrum nocturnum. Bactericidal activity is evaluated on Citrobacter, Salmonella typhi, Enterococcus faecalis, E. coli, Proteus vulgaris, and Vibrio choleraecitrobacter. Maximum zone of inhibition observed is 41 mm against V. cholera, while the minimum is 15 mm against E. faecalis. MIC values are 16 μg/mL for Citrobacter, S. typhi, and V. cholerae; 8 μg/mL for E. coli and P. vulgaris; and 4 μg/mL for E. faecalis These results highlight silver NPs as promising alternatives to overcome antibiotic resistance and develop new antibiotic products. | [82] |
| Antibacterial activity | Spherical silver NPs, with sizes varying from 15 to 25 nm, are synthesized from cell-free beef extracts. NPs exhibit potent antibacterial activity against multidrug-resistant strains of E. coli and S. aureus (with a MIC of 40 µg/mL). Upon exposure to 50 µg/mL silver NPs, 97.5% reduction in colony-forming unit (CFU) values for E. coli and 96.7% reduction in S. aureus are observed. This novel approach represents a high potential for surface decontamination, and it is expected to significantly advance the development of disinfectants, surface treatment products, and nanomedicines containing silver NPs. | [83] |
| Antibacterial activity | Spherical silver NPs are synthesized using Solanum nigrum and Indigofera tinctoria extracts. Antibacterial activity of these NPs is evaluated using various concentrations (50 µL, 100 µL, 150 µL). All tested concentrations effectively inhibit the growth of bacterial pathogens including Pseudomonas sp., S. aureus, and S. mutans. Given these results, silver NPs are highlighted as effective coating materials for the development of surgical sutures, which possess minimal risk to humans and the environment. | [84] |

2.7.1. Effect of Size on Antibacterial Activity
2.7.2. Effect of Shape on Antibacterial Activity
2.7.3. Effect of Surface Charge on Antibacterial Activity
2.7.4. Effect of Surface Functionalization on Antibacterial Activity
2.8. Antifungal Activity
| Highlighted Property | Result | Reference |
|---|---|---|
| Antifungal activity | Spherical silver NPs, with sizes varying from 3 to 13 nm, are synthesized using Nigrospora oryzae. Efficiency of different concentrations (50, 100, 150, and 200 ppm) of silver NPs are evaluated on Fusarium spp. All concentrations are able to inhibit fungal growth, with higher concentrations resulting in greater inhibition. Findings highlight silver NPs as potent antifungal agents in the field of agriculture since they are capable of replacing synthetic chemicals used to control fungal pathogens. | [99] |
| Antifungal activity | Silver NPs are synthesized using different concentrations of SDS (25 and 50 mg) as a reducing agent. NPs containing 50 mg SDS (Silver NP-50) show greater antifungal activity against Candida parapsilosisi Further, an antifungal cream is developed incorporating the silver NP-0.50 formulation with miconazole, a fungicidal agent, to combine its effects and enhance therapeutic efficiency against fungal infections. | [100] |
| Antifungal activity | Ageratum conyzoides leaf extracts are used to synthesize silver NPs. Silver NPs are incorporated into fabrics, which are then tested for their antifungal capability against Aspergillus sp. Findings highlight the fungicidal effectiveness of silver NPs on the development of antifungal textiles as demonstrated by their maintained efficiency even after five washing cycles. | [101] |
| Antifungal activity | Cellulose-based films containing different concentrations of silver NPs (0.10%, 0.25%, 0.50%) are produced. Addition of silver NPs into films causes enhanced antifungal activity, with 0.25% silver NPs showing effective fungicidal properties against Colletotrichum gloeosporioides. Results lead to the development of effective fruit coatings, which prevent fungal growth after 14 days of storage while preserving the fruit’s quality. | [102] |
2.9. Antiviral Activity
| Highlighted Property | Result | Reference |
|---|---|---|
| Antiviral Activity | Silver NPs are synthesized using collagen to evaluate their virucidal activity against SARS-CoV-2. In vitro studies demonstrate silver NPs’ dose-dependent inhibitory effect on SARS-CoV-2, which leads to development of mouthwash and nasal rinse formulations containing silver NPs. These formulations’ efficiency is tested in a clinical trial that results in a significantly lower SARS-CoV-2 infection rate (1.8%) in the experimental group compared to (28.2%) the control group. | [109] |
| Antiviral activity | Silver NPs are synthesized via the reactive blade coating (RC) method to assess their antiviral properties against HCoV-229E. Then, an RC-silver NP coating is applied to personal protective equipment (PPE), including glass, face masks, and cotton textiles, to test its efficiency. Findings reveal that the RC-silver NP coating enhances the virucidal properties of PPE, achieving up to 99.9% reduction in viral activity after 30 min of exposure. | [110] |
| Antiviral activity | Spherical NPs are produced from different silver nitrate (AgNO3) solutions, 50, 100, 150, and 200 mM, to develop an active packaging material, a paper, coated with silver NPs. NPs’ antiviral properties are then tested on Dengue virus serotype 3 (DENV-3) strain P12/08. The paper coated with silver NPs (prepared from the 150 mM AgNO3 solution) demonstrates complete inactivation (100%) of DENV-3 within one minute of exposure. | [111] |
| Antiviral activity | Antiviral paint is developed to reduce the hazards from contaminated high-touch surfaces. Saccharum officinarum leaf extract is utilized to synthesize silver NPs with an average size of 11.7 ± 2.8 nm. Further, synthesized NPs are incorporated into architectural paints and subsequently evaluated for their virucidal efficiency against human coronavirus NL63. Paint containing 80 ppm silver NPs exhibits significant antiviral activity, achieving over a 90% reduction in comparison to the untreated control. | [112] |
2.10. Anticancer Activity
| Highlighted Property | Result | Reference |
|---|---|---|
| Anticancer Activity | Researchers develop bovine serum albumin (BSA) coated spherical silver NPs as effective photothermal therapy (PTT) agents in treating skin cancer. BSA-coated silver NPs effectively convert laser light into heat, depending on the NP concentration and laser power, which further leads to significant reduction in B16F10 melanoma cells. These indicate the importance of developing silver NP-integrated PTT formulations for cancer treatment. | [118] |
| Anticancer activity | Oval PG-Silver-PPa nanoconjugates (NCs) with an average diameter of 61.9 mm are synthesized for enhanced photodynamic therapy (PDT) in treating cancer. NCs’ effectiveness in PDT is tested on Eca-109 cancer cells. Results reveal NCs’ superior performance, evidenced by increased cellular uptake and higher singlet oxygen generation compared to precursor drug PPa alone. These findings suggest that PG-Silver-PPa NCs have the capability of serving as potential alternative PDT agents. | [119] |
| Anticancer activity | Spherical silver NPs, with sizes around 13 ± 1 nm, are synthesized using a chemical solution method. Effectiveness of silver NPs, with varying concentrations (2, 5, 10, 25, 50, 100, and 200 µg/mL) are examined on HepG2 and MCF-7 cancer cell lines. All concentrations of silver NPs induce cytotoxic effects, with higher concentrations resulting in greater cell death. Findings underscore the silver NPs’ potential as effective nanodrugs in emerging cancer research. | [120] |
| Anticancer activity | Dictyota ciliolata extract is used to synthesize spherical silver NPs with an average particle size of 100 nm. Activity of silver NPs are tested at different concentrations (10, 20, 30, and 40 µg/mL) on A549 lung adenocarcinoma cells. NPs are successful in inhibiting the cancer cell proliferation as well as reducing tertiary capillary formation. These underline silver NPs’ antiangiogenic properties and highlight their potential as promising agents in the treatment of lung cancer. | [121] |
2.11. Anti-Inflammatory Activity
| Highlighted Property | Result | Reference |
|---|---|---|
| Anti-inflammatory activity | Spherical silver NPs with an average size of 25.92 nm are incorporated into riclin-based hydrogels. Anti-inflammatory activity of nanocomposite hydrogels are assessed by analyzing the expression of pro-inflammatory cytokines (IL-1α, IL-6, and TNF-α). Wounds treated with riclin-silver NP composite have considerably lower levels of IL-1α, IL-6, and TNF-α compared to controls, indicating reduced inflammation. These suggest that silver NPs have promising potential to be utilized in wound dressings. | [122] |
| Anti-inflammatory activity | Collagen-based hybrid biomaterials containing silver NPs, with sizes 30 to 50 nm, are synthesized. Anti-inflammatory efficiency of the biomaterials is evaluated measuring the secretion of pro-inflammatory cytokines, IL-6, IL-1β, and TNF-α. A significant reduction in the secretion of these cytokines are observed, which is attributed to the presence of silver NPs. Results indicate these hybrid scaffolds are strong anti-inflammatory agents, with potential applicability in periodontal disease treatment. | [123] |
| Anti-inflammatory activity | Silver NPs synthesized from different extracts of Ehretia cymosa (methanol, n-hexane, and ethyl acetate) are included in cream formulations. Anti-inflammatory activity of these creams is measured by carrageenan-induced rat paw edema method on albino rats. Creams demonstrate a significant reduction in inflammation, specifically those containing the NPs synthesized from ethyl acetate, which achieves a 100% anti-inflammatory effect within 4 h. | [124] |
| Anti-inflammatory activity | Spherical silver NPs, with sizes ranging from 30.99 to 68.20 nm, are synthesized using aqueous curcumin extract. Anti-inflammatory effects of silver NPs are tested in a rat model of adjuvant arthritis at a concentration of 100 mg/kg. Silver NPs reduces the levels of inflammatory markers (IL-6 and hs-CRP) and paw edema in arthritic rats. These findings position silver NPs as highly effective candidates for developing anti-inflammatory drugs. | [125] |
3. Synthesis of Silver Nanoparticles
3.1. Physical Methods
3.1.1. Ball Milling Method
3.1.2. Laser Ablation Method
3.1.3. Vapor Condensation Method
3.1.4. Electrical Arc-Discharge Method
3.2. Chemical Methods
3.2.1. Chemical Reduction
3.2.2. Electrochemical Synthetic Method
3.2.3. Microwave-Assisted Synthesis
3.2.4. Photoinduced Reduction
3.2.5. Microemulsion Techniques
3.3. Bio-Based (Green-Synthesized) Methods
3.3.1. Plants
3.3.2. Algae
3.3.3. Fungi
3.3.4. Bacteria
| Types of Methods | Features | Limitations | Studies |
|---|---|---|---|
| Ball milling [85] | -Cost effective -Utilize at ambient temperature | -Energy-intensive process -Potential of agglomeration -Challenging for uniform NP size distribution -Less suitable for large-scale production | -The size of the silver NPs, according to the particle sizing system measurement results, is roughly 100 nm, which is consistent with the findings from the transmission electron microscopy (TEM) and scanning electron microscopy (SEM) [204]. -According to the experimental findings, silver NPs with a limited size distribution (4–8 nm) can be produced [205]. |
| Laser ablation method [206] | -High purity -Small and uniform NP morphology -Low agglomeration rate | -Energy-intensive process -Low production yield -Complex setup and maintenance -Less suitable for large-scale production -Complex equipment and setup | -A shorter average particle size is found by TEM investigation when the laser is used at a high power of 570 mW for 40 min and a short laser wavelength of 532 nm [207]. -Without the requirement for reducing and stabilizing agents in pure acetonitrile and N,N-dimethylformamide, stable colloidal solutions of free silver NPs (4–10 nm) are produced by laser ablation of the bulk metal [208]. |
| Vapor condensation method [128] | -Appropriate for long-term experiment conditions -Suitable for large-scale synthesis | -Low production yield -Energy-intensive process -Low production yield -Less suitable for large-scale production -Complex equipment and setup | -Helium is flowing within the process chamber when silver NPs are generated utilizing an inert gas condensation technique. Depending on the growth circumstances, the particle size varies between 9 and 32 nm. Particles with a spherical shape and less agglomeration form at lower evaporation temperatures and inert gas pressures are produced [209]. |
| Electrical Arc discharge [142,143] | -High purity -Simple equipment -Simple processing -High synthesis rate | -Simple equipment -Requires significant electrical energy to maintain the arc | -Two identical metallic electrodes placed one millimeter apart in a 100-mL liquid produce the plasma arc-discharge. For silver NPs, the size distributions computed from TEM images show mean particle sizes of 73 nm [210]. -Innovative and simple technique for creating silver NPs (20–30 nm) with a predetermined nanosize and spherical shape that makes use of the arc-discharge method [131]. |
| Chemical reduction [211] | -Simple -Cost effective -Good production rate | -Toxic and hazardous chemicals | By reducing AgNO3 with a combination of two chemical agents—sodium citrate and tannic acid—monodisperse silver NPs are produced. Tannic acid and sodium citrate are combined to produce NPs that are uniform in size (approximately 30 nm) and shape [212]. As a reducing agent, 1% trisodium citrate is used for the production of silver NPs. Without utilizing any outside stabilizers or surfactants, the silver NPs with a size of around 103 nm and good dispersion are produced [213]. |
| Electrochemical synthetic method [214] | -Simple reaction control -Less pollutant -Moderate synthesis conditions | -Less suitable for large-scale production | -Two to seven nm silver NPs are produced via an electrochemical process that involves dissolving a metallic anode in an aprotic liquid [154]. -A technique known as electrochemical oxidation/complexation, which is followed by UV irradiation reduction, is used to create distributed chitosan-silver NP. The development of surface plasmon absorbance at about 420 nm indicates the formation of the NPs [215]. |
| Microwave-assisted synthesis [216] | -Time-saving -Efficacy of energy conversion at a high level | -Complex and expensive equipment -Less suitable for scale-up | -Using a microwave combustion process, silver doped lanthanum chromites are synthesized. Through TEM, nanosized particles as tiny as ~7–8 nm and bigger ones ~20–26 nm are seen [217]. |
| Photoinduced reduction [159] | -Utilize at ambient temperature -Safe Chemicals | -Time consuming -Expensive equipment | -Silver nanoprisms (40–220) are produced utilizing a photoinduced process with just three chemical ingredients. The ideal conditions for colloids stability are found using Zeta Potential measurements [218]. -Using several proteins as templates, silver NPs (approximately 8.6 nm) with unique LSPR absorption spectra can be produced upon light irradiation [219]. |
| Microemulsion technique [220] | -Low input of mechanical force | -Susceptible to change -Extensive formulation effort -Low yield | -Using the recovered biosurfactant, silver NPs are synthesized by the microemulsion process, and their properties are assessed through UV-vis spectroscopy, powder-XRD, TEM, and zeta potential. The characteristic UV-vis absorption peak at 440 nm is present in the generated silver NP. The average particle size of the NP is found to be 17.89 ± 8.74 nm using Powder-XRD and TEM investigation, along with its cubic structure [221]. |
| Irradiation method [128] | -Maintenance of synthesis conditions -High purity -Uniform size distribution -Suitable for large-scale production | -Limited reaction flexibility -Potential of agglomeration -Radiation concerns | -Silver NPs, measuring 21.3 ± 7.3 nm on average, are produced by synthesizing 10 mg of chloramine T. Chloramine T concentrations below produce smaller, less stable NPs. The addition of PVP facilitates the formation of larger NPs with diverse shapes, including rods, spheres, and cubes [222]. -The γ-irradiation method creates silver NPs inside the montmorillonite (MMT) interlamellar space without the need for a reducing agent or heat treatment. TEM and X-ray diffraction investigations reveal the creation of face-centered cubic silver NPs with a mean diameter of roughly 21.57–30.63 nm [223]. |
| Plants [136,224,225] | -Simple processing -Wide-ranging applications -Use of safe and non-toxic reagents | -Unknown mechanisms that affect synthesis | -The resulting silver NPs containing L. acapulcensis are spherical or quasi-spherical in shape, with an average diameter of 5 nm. Their diameters vary from 1.2 to 62 nm [226]. -Silver NPs are synthesized from the fruit bodies of the plant Tribulus terrestris L. Upon observation, it is discovered that the spherical-shaped silver NPs range in size from 16 to 28 nm [227]. |
| Algae [178,179] | -Simple processing -Cost effective -Small and uniform NPs morphology -Use of safe and non-toxic reagents -Eco-friendliness | -Slow synthesis rate -Unknown mechanisms that affect synthesis | -Caulerpa racemosa, a marine algae, is used for producing silver NPs. A TEM image reveals the development of silver NPs that range in size from 5 to 25 nm [228]. -Silver NPs mediated by Sargassum coreanum (marine algae) are successfully produced. The interlayer distance (d-spacing value) of about 0.24 nm is found, and the generated silver NPs’ deformed spherical form and mean particle size of 19 nm are validated by the high-resolution transmission electron microscopy (HRTEM) pictures [229]. |
| Fungi [192,230] | -Eco-friendliness -Simple processing -Less non-pathogenic behavior -High intracellular uptake | -Unknown mechanisms that affect synthesis -Pathogenic behavior -Process longevity | -A very stable silver hydrosol is produced when aqueous silver ions are exposed to the fungus Fusarium oxysporum, causing the ions to disappear in solution. Proteins released by the fungus stabilize the silver NPs, which have a diameter of 5 to 15 nm, in solution [191]. -Duddingtonia flagrans (AC001), a nematophagous fungus, is used to produce extremely stable silver NPs. TEM and dynamic light scattering reveal roughly 11, 38 nm monodisperse and quasispherical silver NPs [231] |
| Bacteria [198,224] | -Simple processing -Eco-friendliness | -Unknown mechanisms that affect synthesis -Slow synthesis rate -Pathogenic behavior -Large size distribution | -The results indicate that Lactobacillus bulgaricus has a great deal of potential for producing silver NPs with a size range of 30.65–100 nm [203]. -Rhodococcus, Brevundimonas, and Bacillus—recently identified from a consortium associated with the Antarctic marine ciliate Euplotes focardii—are used as reducing and capping agents in the production of silver NPs. Despite not being in contact with one another, the NPs are grouped together and have a spherical to rod-shaped shape. Their diameters range from 20 to 50 nm [201]. |
3.4. Factors Affecting Silver NP Synthesis and Their Stability
3.4.1. Factors Affecting Silver NP Synthesis
3.4.2. Factors Affecting Silver NP Stability
4. Toxicity
5. Conclusions and Future Trends
Author Contributions
Funding
Conflicts of Interest
References
- Eker, F.; Duman, H.; Akdaşçi, E.; Bolat, E.; Sarıtaş, S.; Karav, S.; Witkowska, A.M. A Comprehensive Review of Nanoparticles: From Classification to Application and Toxicity. Molecules 2024, 29, 3482. [Google Scholar] [CrossRef] [PubMed]
- Bamal, D.; Singh, A.; Chaudhary, G.; Kumar, M.; Singh, M.; Rani, N.; Mundlia, P.; Sehrawat, A.R. Silver Nanoparticles Biosynthesis, Characterization, Antimicrobial Activities, Applications, Cytotoxicity and Safety Issues: An Updated Review. Nanomaterials 2021, 11, 2086. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Paladini, F.; Pollini, M. Antimicrobial Silver Nanoparticles for Wound Healing Application: Progress and Future Trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef]
- Terra, A.L.M.; Kosinski, R.D.C.; Moreira, J.B.; Costa, J.A.V.; Morais, M.G.D. Microalgae Biosynthesis of Silver Nanoparticles for Application in the Control of Agricultural Pathogens. J. Environ. Sci. Health Part B 2019, 54, 709–716. [Google Scholar] [CrossRef]
- Bapat, R.A.; Chaubal, T.V.; Joshi, C.P.; Bapat, P.R.; Choudhury, H.; Pandey, M.; Gorain, B.; Kesharwani, P. An Overview of Application of Silver Nanoparticles for Biomaterials in Dentistry. Mater. Sci. Eng. C 2018, 91, 881–898. [Google Scholar] [CrossRef]
- Haque, S.; Norbert, C.C.; Acharyya, R.; Mukherjee, S.; Kathirvel, M.; Patra, C.R. Biosynthesized Silver Nanoparticles for Cancer Therapy and In Vivo Bioimaging. Cancers 2021, 13, 6114. [Google Scholar] [CrossRef]
- Chen, D.; Qiao, X.; Qiu, X.; Chen, J. Synthesis and Electrical Properties of Uniform Silver Nanoparticles for Electronic Applications. J. Mater. Sci. 2009, 44, 1076–1081. [Google Scholar] [CrossRef]
- Kalidasan, B.; Pandey, A.K.; Saidur, R.; Tyagi, V.V. Energizing Organic Phase Change Materials Using Silver Nanoparticles for Thermal Energy Storage. J. Energy Storage 2023, 58, 106361. [Google Scholar] [CrossRef]
- Farooq, S.; Dias Nunes, F.; de Araujo, R.E. Optical Properties of Silver Nanoplates and Perspectives for Biomedical Applications. Photonics Nanostruct. 2018, 31, 160–167. [Google Scholar] [CrossRef]
- Ravindran, A.; Chandran, P.; Khan, S.S. Biofunctionalized Silver Nanoparticles: Advances and Prospects. Colloids Surf. B Biointerfaces 2013, 105, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Hoang, V.T.; Mai, M.; Thi Tam, L.; Vu, N.P.; Tien Khi, N.; Dinh Tam, P.; Quang Huy, T.; Le, A.T.; Xuan Dinh, N.; Tran, V.H. Functionalized-AgNPs for Long-Term Stability and Its Applicability in the Detection of Manganese Ions. Adv. Polym. Technol. 2020, 2020, 9437108. [Google Scholar] [CrossRef]
- Dhaka, A.; Chand Mali, S.; Sharma, S.; Trivedi, R. A Review on Biological Synthesis of Silver Nanoparticles and Their Potential Applications. Results Chem. 2023, 6, 101108. [Google Scholar] [CrossRef]
- Mustafa, G.; Hasan, M.; Yamaguchi, H.; Hitachi, K.; Tsuchida, K.; Komatsu, S. A Comparative Proteomic Analysis of Engineered and Bio Synthesized Silver Nanoparticles on Soybean Seedlings. J. Proteom. 2020, 224, 103833. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, P.; Preece, J.A.; Mendes, P.M. Nanotechnology: The “Top-Down” and “Bottom-Up” Approaches. In Supramolecular Chemistry: From Molecules to Nanomaterials; John Wiley & Sons, Ltd.: Chichester, UK, 2012. [Google Scholar] [CrossRef]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of Nanomaterials Using Various Top-down and Bottom-up Approaches, Influencing Factors, Advantages, and Disadvantages: A Review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef]
- Document Search—Web of Science Core Collection. Available online: https://www.webofscience.com/wos/woscc/basic-search (accessed on 28 August 2024).
- Google Patents. Available online: https://patents.google.com/ (accessed on 28 August 2024).
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Kanwar, R.; Fatima, R.; Kanwar, R.; Javid, M.T.; Muhammad, U.W.; Ashraf, Z.; Khalid, A.; Tariq Javid, M.; Biological, A.K. Biological, Physical and Chemical Synthesis of Silver Nanoparticles and Their Non-Toxic Bio-Chemical Application: A Brief Review. Pure Appl. Biol. 2022, 11, 421–438. [Google Scholar] [CrossRef]
- Burdușel, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Jaswal, T.; Gupta, J. A Review on the Toxicity of Silver Nanoparticles on Human Health. Mater. Today Proc. 2023, 81, 859–863. [Google Scholar] [CrossRef]
- Galatage, S.T.; Hebalkar, A.S.; Dhobale, S.V.; Mali, O.R.; Kumbhar, P.S.; Nikade, S.V.; Killedar, S.G.; Galatage, S.T.; Hebalkar, A.S.; Dhobale, S.V.; et al. Silver Nanoparticles: Properties, Synthesis, Characterization, Applications and Future Trends. In Silver Micro-Nanoparticles—Properties, Synthesis, Characterization, and Applications; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Almatroudi, A. Silver Nanoparticles: Synthesis, Characterisation and Biomedical Applications. Open Life Sci. 2020, 15, 819–839. [Google Scholar] [CrossRef]
- Natsuki, J.; Natsuki, T.; Hashimoto, Y. A Review of Silver Nanoparticles: Synthesis Methods, Properties and Applications. Int. J. Mater. Sci. Appl. 2015, 4, 325–332. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, M.; Park, H.S.; Shin, U.S.; Gong, M.S.; Kim, H.W. Size-Dependent Cellular Toxicity of Silver Nanoparticles. J. Biomed. Mater. Res. Part A 2012, 100, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Shenashen, M.A.; El-Safty, S.A.; Elshehy, E.A. Synthesis, Morphological Control, and Properties of Silver Nanoparticles in Potential Applications. Part. Part. Syst. Charact. 2014, 31, 293–316. [Google Scholar] [CrossRef]
- Sironmani, A.; Daniel, K.; Sironmani, A.; Daniel, K. Silver Nanoparticles—Universal Multifunctional Nanoparticles for Bio Sensing, Imaging for Diagnostics and Targeted Drug Delivery for Therapeutic Applications. In Drug Discovery and Development—Present and Future; IntechOpen: Rijeka, Croatia, 2011; pp. 463–484. [Google Scholar] [CrossRef]
- Cho, Y.M.; Mizuta, Y.; Akagi, J.I.; Toyoda, T.; Sone, M.; Ogawa, K. Size-Dependent Acute Toxicity of Silver Nanoparticles in Mice. J. Toxicol. Pathol. 2018, 31, 73–80. [Google Scholar] [CrossRef]
- Zhang, J.; Ahmadi, M.; Fargas, G.; Perinka, N.; Reguera, J.; Lanceros-Méndez, S.; Llanes, L.; Jiménez-Piqué, E. Silver Nanoparticles for Conductive Inks: From Synthesis and Ink Formulation to Their Use in Printing Technologies. Metals 2022, 12, 234. [Google Scholar] [CrossRef]
- Taheri, S.; Cavallaro, A.; Christo, S.N.; Smith, L.E.; Majewski, P.; Barton, M.; Hayball, J.D.; Vasilev, K. Substrate Independent Silver Nanoparticle Based Antibacterial Coatings. Biomaterials 2014, 35, 4601–4609. [Google Scholar] [CrossRef]
- Todorova, M.; Milusheva, M.; Kaynarova, L.; Georgieva, D.; Delchev, V.; Simeonova, S.; Pilicheva, B.; Nikolova, S. Drug-Loaded Silver Nanoparticles—A Tool for Delivery of a Mebeverine Precursor in Inflammatory Bowel Diseases Treatment. Biomedicines 2023, 11, 1593. [Google Scholar] [CrossRef]
- Cheon, J.Y.; Kim, S.J.; Rhee, Y.H.; Kwon, O.H.; Park, W.H. Shape-Dependent Antimicrobial Activities of Silver Nanoparticles. Int. J. Nanomed. 2019, 14, 2773–2780. [Google Scholar] [CrossRef]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size- and Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef]
- Bansal, V.; Li, V.; O’Mullane, A.P.; Bhargava, S.K. Shape Dependent Electrocatalytic Behaviour of Silver Nanoparticles. CrystEngComm 2010, 12, 4280–4286. [Google Scholar] [CrossRef]
- Tak, Y.K.; Pal, S.; Naoghare, P.K.; Rangasamy, S.; Song, J.M. Shape-Dependent Skin Penetration of Silver Nanoparticles: Does It Really Matter? Sci. Rep. 2015, 5, 16908. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia Coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wang, D.; Zhang, J.; Li, Y. Shape-Dependent Catalytic Activity of Silver Nanoparticles for the Oxidation of Styrene. Chem. Asian J. 2006, 1, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Amirjani, A.; Koochak, N.N.; Haghshenas, D.F. Investigating the Shape and Size-Dependent Optical Properties of Silver Nanostructures Using UV-Vis Spectroscopy. J. Chem. Educ. 2019, 96, 2584–2589. Available online: https://pubs.acs.org/doi/10.1021/acs.jchemed.9b00559 (accessed on 28 August 2024). [CrossRef]
- Fröhlich, E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Qiao, Z.; Yao, Y.; Song, S.; Yin, M.; Luo, J. Silver Nanoparticles with PH Induced Surface Charge Switchable Properties for Antibacterial and Antibiofilm Applications. J. Mater. Chem. B 2019, 7, 830–840. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Zu, Y.; Fu, Y.; Li, N.; Guo, N.; Liu, R.; Zhang, Y. Synthesis of Polyethylenimine (PEI) Functionalized Silver Nanoparticles by a Hydrothermal Method and Their Antibacterial Activity Study. Mater. Sci. Eng. C 2014, 42, 31–37. [Google Scholar] [CrossRef]
- Kumar-Krishnan, S.; Prokhorov, E.; Hernández-Iturriaga, M.; Mota-Morales, J.D.; Vázquez-Lepe, M.; Kovalenko, Y.; Sanchez, I.C.; Luna-Bárcenas, G. Chitosan/Silver Nanocomposites: Synergistic Antibacterial Action of Silver Nanoparticles and Silver Ions. Eur. Polym. J. 2015, 67, 242–251. [Google Scholar] [CrossRef]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The Effect of Charge at the Surface of Silver Nanoparticles on Antimicrobial Activity against Gram-Positive and Gram-Negative Bacteria: A Preliminary Study. J. Nanomater. 2015, 2015, 720654. [Google Scholar] [CrossRef]
- El Badawy, A.M.; Silva, R.G.; Morris, B.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Surface Charge-Dependent Toxicity of Silver Nanoparticles. Environ. Sci. Technol. 2011, 45, 283–287. [Google Scholar] [CrossRef]
- Kockert, M.; Kojda, D.; Mitdank, R.; Mogilatenko, A.; Wang, Z.; Ruhhammer, J.; Kroener, M.; Woias, P.; Fischer, S.F. Nanometrology: Absolute Seebeck Coefficient of Individual Silver Nanowires. Sci. Rep. 2019, 9, 20265. [Google Scholar] [CrossRef] [PubMed]
- Kittel, C. Introduction to Solid State Physics; John Wiley & Sons, Ltd.: Chichester, UK, 2005; p. 69. [Google Scholar]
- Varga, M.; Prokeš, J.; Bober, P.; Stejskal, J. Electrical Conductivity of Polyaniline-Silver Nanocomposites. In Proceedings of the 21st Annual Conference of Doctoral Students, Prague, The Czech Republic, 29 May–1 June 2012. [Google Scholar]
- Bogle, K.A.; Dhole, S.D.; Bhoraskar, V.N. Silver Nanoparticles: Synthesis and Size Control by Electron Irradiation. Nanotechnology 2006, 17, 3204. [Google Scholar] [CrossRef]
- Prathna, T.C.; Chandrasekaran, N.; Mukherjee, A. Studies on Aggregation Behaviour of Silver Nanoparticles in Aqueous Matrices: Effect of Surface Functionalization and Matrix Composition. Colloids Surf. A Physicochem. Eng. Asp. 2011, 390, 216–224. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Salmiati; Salim, M.R.; Beng Hong Kueh, A.; Hadibarata, T.; Nur, H. A Review of Silver Nanoparticles: Research Trends, Global Consumption, Synthesis, Properties, and Future Challenges. J. Chin. Chem. Soc. 2017, 64, 732–756. [Google Scholar] [CrossRef]
- Asoro, M.A.; Damiano, J.; Ferreira, P.J. Size Effects on the Melting Temperature of Silver Nanoparticles: In-Situ TEM Observations. Microsc. Microanal. 2009, 15, 706–707. [Google Scholar] [CrossRef]
- Luo, W.; Hu, W.; Xiao, S. Size Effect on the Thermodynamic Properties of Silver Nanoparticles. J. Phys. Chem. C 2008, 112, 2359–2369. [Google Scholar] [CrossRef]
- Balantrapu, K.; Goia, D.V. Silver Nanoparticles for Printable Electronics and Biological Applications. J. Mater. Res. 2009, 24, 2828–2836. [Google Scholar] [CrossRef]
- Allen, M.L.; Aronniemi, M.; Mattila, T.; Alastalo, A.; Ojanperä, K.; Suhonen, M.; Seppä, H. Electrical Sintering of Nanoparticle Structures. Nanotechnology 2008, 19, 175201. [Google Scholar] [CrossRef]
- Alshehri, A.H.; Jakubowska, M.; Młozìniak, A.; Horaczek, M.; Rudka, D.; Free, C.; Carey, J.D. Enhanced Electrical Conductivity of Silver Nanoparticles for High Frequency Electronic Applications. ACS Appl. Mater. Interfaces 2012, 4, 7007–7010. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhuang, X.; Wu, F.; Liu, F. High-Performance Thermal Management Nanocomposites: Silver Functionalized Graphene Nanosheets and Multiwalled Carbon Nanotube. Crystals 2018, 8, 398. [Google Scholar] [CrossRef]
- Iyahraja, S.; Rajadurai, J.S. Study of Thermal Conductivity Enhancement of Aqueous Suspensions Containing Silver Nanoparticles. AIP Adv. 2015, 5, 57103. [Google Scholar] [CrossRef]
- Wu, H.; Carrete, J.; Zhang, Z.; Qu, Y.; Shen, X.; Wang, Z.; Zhao, L.D.; He, J.Q. Strong Enhancement of Phonon Scattering through Nanoscale Grains in Lead Sulfide Thermoelectrics. NPG Asia Mater. 2014, 6, e108. [Google Scholar] [CrossRef]
- Sun, Z.; Li, J.; Yu, M.; Kathaperumal, M.; Wong, C.P. A Review of the Thermal Conductivity of Silver-Epoxy Nanocomposites as Encapsulation Material for Packaging Applications. Chem. Eng. J. 2022, 446, 137319. [Google Scholar] [CrossRef]
- Li, J.; Cheng, R.; Cheng, Z.; Duan, C.; Wang, B.; Zeng, J.; Xu, J.; Tian, X.; Chen, H.; Gao, W.; et al. Silver-Nanoparticle-Embedded Hybrid Nanopaper with Significant Thermal Conductivity Enhancement. ACS Appl. Mater. Interfaces 2021, 13, 36171–36181. [Google Scholar] [CrossRef] [PubMed]
- Nosheen, E.; Shah, A.; Iftikhar, F.J.; Aftab, S.; Bakirhan, N.K.; Ozkan, S.A. Optical Nanosensors for Pharmaceutical Detection. In New Developments in Nanosensors for Pharmaceutical Analysis; Academic Press: Cambridge, MA, USA, 2019; pp. 119–140. [Google Scholar] [CrossRef]
- Cobley, C.M.; Skrabalak, S.E.; Campbell, D.J.; Xia, Y. Shape-Controlled Synthesis of Silver Nanoparticles for Plasmonic and Sensing Applications. Plasmonics 2009, 4, 171–179. [Google Scholar] [CrossRef]
- Juma, M.W.; Birech, Z.; Mwenze, N.M.; Ondieki, A.M.; Maaza, M.; Mokhotjwa, S.D. Localized Surface Plasmon Resonance Sensing of Trenbolone Acetate Dopant Using Silver Nanoparticles. Sci. Rep. 2024, 14, 5721. [Google Scholar] [CrossRef] [PubMed]
- McFarland, A.D.; Van Duyne, R.P. Single Silver Nanoparticles as Real-Time Optical Sensors with Zeptomole Sensitivity. Nano Lett. 2003, 3, 1057–1062. [Google Scholar] [CrossRef]
- Martinsson, E.; Otte, M.A.; Shahjamali, M.M.; Sepulveda, B.; Aili, D. Substrate Effect on the Refractive Index Sensitivity of Silver Nanoparticles. J. Phys. Chem. C 2014, 118, 24680–24687. [Google Scholar] [CrossRef]
- Lee, K.S.; El-Sayed, M.A. Gold and Silver Nanoparticles in Sensing and Imaging: Sensitivity of Plasmon Response to Size, Shape, and Metal Composition. J. Phys. Chem. B 2006, 110, 19220–19225. [Google Scholar] [CrossRef]
- Fan, M.; Brolo, A.G. Silver Nanoparticles Self Assembly as SERS Substrates with near Single Molecule Detection Limit. Phys. Chem. Chem. Phys. 2009, 11, 7381–7389. [Google Scholar] [CrossRef]
- Krishna, R.; Unsworth, T.J.; Edge, R. Raman Spectroscopy and Microscopy. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Mlalila, N.G.; Swai, H.S.; Hilonga, A.; Kadam, D.M. Antimicrobial Dependence of Silver Nanoparticles on Surface Plasmon Resonance Bands against Escherichia coli. Nanotechnol. Sci. Appl. 2016, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, Y.; Li, C.; Ma, G.; Shang, Y.; Sun, Y. A Comparative Study of the Antibacterial Mechanisms of Silver Ion and Silver Nanoparticles by Fourier Transform Infrared Spectroscopy. Vib. Spectrosc. 2016, 85, 112–121. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, J.Y.; Kim, J.; Lee, J.H.; Hahn, J.S.; Gu, M.B.; Yoon, J. Silver-Ion-Mediated Reactive Oxygen Species Generation Affecting Bactericidal Activity. Water Res. 2009, 43, 1027–1032. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- More, P.R.; Pandit, S.; Filippis, A.D.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef]
- Nie, P.; Zhao, Y.; Xu, H. Synthesis, Applications, Toxicity and Toxicity Mechanisms of Silver Nanoparticles: A Review. Ecotoxicol. Environ. Saf. 2023, 253, 114636. [Google Scholar] [CrossRef]
- Istiqola, A.; Syafiuddin, A. A Review of Silver Nanoparticles in Food Packaging Technologies: Regulation, Methods, Properties, Migration, and Future Challenges. J. Chin. Chem. Soc. 2020, 67, 1942–1956. [Google Scholar] [CrossRef]
- Saliminasab, M.; Jabbari, H.; Farahmand, H.; Asadi, M.; Soleimani, M.; Fathi, A. Study of Antibacterial Performance of Synthesized Silver Nanoparticles on Streptococcus Mutans Bacteria. Nanomed. Res. J. 2022, 7, 391–396. [Google Scholar] [CrossRef]
- Rangaraju, M.; Abewaa, M.; Hailemariam, E.; Abay, Y.; Prabhu, S.V.; Abdu, J.; Mengistu, A. Bacterial Growth Inhibition in Spring Water Utilizing Silver Nanoparticles: Optimization Using Central Composite Design. Results Eng. 2024, 23, 102562. [Google Scholar] [CrossRef]
- Urnukhsaikhan, E.; Bold, B.-E.; Gunbileg, A.; Sukhbaatar, N.; Mishig-Ochir, T. Antibacterial Activity and Characteristics of Silver Nanoparticles Biosynthesized from Carduus Crispus. Sci. Rep. 2021, 11, 21047. [Google Scholar] [CrossRef] [PubMed]
- Keshari, A.K.; Srivastava, R.; Singh, P.; Yadav, V.B.; Nath, G. Antioxidant and Antibacterial Activity of Silver Nanoparticles Synthesized by Cestrum Nocturnum. J. Ayurveda Integr. Med. 2020, 11, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Ghodake, G.; Kim, M.; Sung, J.S.; Shinde, S.; Yang, J.; Hwang, K.; Kim, D.Y. Extracellular Synthesis and Characterization of Silver Nanoparticles—Antibacterial Activity against Multidrug-Resistant Bacterial Strains. Nanomaterials 2020, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Sabarathinam, J. Citation: Jembulingam Sabarathinam, Madhulaxmi, Rajeshkumar. Development Of Anti Inflammatory and Antimicrobial Silver Nanoparticles Coated Suture Materials. Int. J. Dent. Oral Sci. 2021, 8, 2006–2013. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Oves, M.; Khan, M.S.; Zaidi, A.; Ahmed, A.S.; Ahmed, F.; Ahmad, E.; Sherwani, A.; Owais, M.; Azam, A. Antibacterial and Cytotoxic Efficacy of Extracellular Silver Nanoparticles Biofabricated from Chromium Reducing Novel OS4 Strain of Stenotrophomonas Maltophilia. PLoS ONE 2013, 8, e59140. [Google Scholar] [CrossRef]
- Onodera, A.; Nishiumi, F.; Kakiguchi, K.; Tanaka, A.; Tanabe, N.; Honma, A.; Yayama, K.; Yoshioka, Y.; Nakahira, K.; Yonemura, S.; et al. Short-Term Changes in Intracellular ROS Localisation after the Silver Nanoparticles Exposure Depending on Particle Size. Toxicol. Rep. 2015, 2, 574–579. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.J.; Singh, H.; Wang, C.; Hwang, K.H.; Farh, M.E.A.; Yang, D.C. Biosynthesis, Characterization, and Antimicrobial Applications of Silver Nanoparticles. Int. J. Nanomed. 2015, 10, 2567–2577. [Google Scholar] [CrossRef]
- Van Dong, P.; Ha, C.H.; Binh, L.T.; Kasbohm, J. Chemical Synthesis and Antibacterial Activity of Novel-Shaped Silver Nanoparticles. Int. Nano Lett. 2012, 2, 9. [Google Scholar] [CrossRef]
- Romero-Urbina, D.G.; Lara, H.H.; Velázquez-Salazar, J.; Arellano-Jiménez, M.J.; Larios, E.; Srinivasan, A.; Lopez-Ribot, J.L.; Yacamán, M.J. Ultrastructural Changes in Methicillin-Resistant Staphylococcus Aureus Induced by Positively Charged Silver Nanoparticles. Beilstein J. Nanotechnol. 2015, 6, 2396–2405. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 231711. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.; Nawaz, M.; Hussain, R.; Price, G.J.; Warsi, M.F.; Waseem, M. Enhanced Antibacterial Activity of Size-Controlled Silver and Polyethylene Glycol Functionalized Silver Nanoparticles. Chem. Pap. 2021, 75, 743–752. [Google Scholar] [CrossRef]
- Mumtaz, S.; Ali, S.; Mumtaz, S.; Mughal, T.A.; Tahir, H.M.; Shakir, H.A. Chitosan Conjugated Silver Nanoparticles: The Versatile Antibacterial Agents. Polym. Bull. 2023, 80, 4719–4736. [Google Scholar] [CrossRef]
- Żarowska, B.; Koźlecki, T.; Piegza, M.; Jaros-Koźlecka, K.; Robak, M. New Look on Antifungal Activity of Silver Nanoparticles (AgNPs). Pol. J. Microbiol. 2019, 68, 515–525. [Google Scholar] [CrossRef]
- Li, L.; Pan, H.; Deng, L.; Qian, G.; Wang, Z.; Li, W.; Zhong, C. The Antifungal Activity and Mechanism of Silver Nanoparticles against Four Pathogens Causing Kiwifruit Post-Harvest Rot. Front. Microbiol. 2022, 13, 988633. [Google Scholar] [CrossRef]
- Xue, B.; He, D.; Gao, S.; Wang, D.; Yokoyama, K.; Wang, L. Biosynthesis of Silver Nanoparticles by the Fungus Arthroderma fulvum and Its Antifungal Activity against Genera of Candida, Aspergillus and Fusarium. Int. J. Nanomed. 2016, 11, 1899–1906. [Google Scholar] [CrossRef]
- Panáček, A.; Kolář, M.; Večeřová, R.; Prucek, R.; Soukupová, J.; Kryštof, V.; Hamal, P.; Zbořil, R.; Kvítek, L. Antifungal Activity of Silver Nanoparticles against Candida Spp. Biomaterials 2009, 30, 6333–6340. [Google Scholar] [CrossRef]
- Matras, E.; Gorczyca, A.; Przemieniecki, S.W.; Oćwieja, M. Surface Properties-Dependent Antifungal Activity of Silver Nanoparticles. Sci. Rep. 2022, 12, 18046. [Google Scholar] [CrossRef]
- Dawoud, T.M.; Yassin, M.A.; El-Samawaty, A.R.M.; Elgorban, A.M. Silver Nanoparticles Synthesized by Nigrospora Oryzae Showed Antifungal Activity. Saudi J. Biol. Sci. 2021, 28, 1847–1852. [Google Scholar] [CrossRef]
- Maciel, A.A.M.; Cunha, F.A.; Freire, T.M.; de Menezes, F.L.; Fechine, L.M.U.D.; Rocha, J.S.; de Cássia Carvalho Barbosa, R.; Martins, R.T.; da Conceição dos Santos Oliveira Cunha, M.; Santos-Oliveira, R.; et al. Development and Evaluation of an Anti-Candida Cream Based on Silver Nanoparticles. 3 Biotech 2023, 13, 352. [Google Scholar] [CrossRef] [PubMed]
- Ratnasari, A.; Endarko, E.; Syafiuddin, A. A Green Method for the Enhancement of Antifungal Properties of Various Textiles Functionalized with Silver Nanoparticles. Biointerface Res. Appl. Chem. 2020, 10, 7284–7294. [Google Scholar] [CrossRef]
- Vieira, A.C.F.; de Matos Fonseca, J.; Menezes, N.M.C.; Monteiro, A.R.; Valencia, G.A. Active Coatings Based on Hydroxypropyl Methylcellulose and Silver Nanoparticles to Extend the Papaya (Carica papaya L.) Shelf Life. Int. J. Biol. Macromol. 2020, 164, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Naumenko, K.; Zahorodnia, S.; Pop, C.V.; Rizun, N. Antiviral Activity of Silver Nanoparticles against the Influenza A Virus. J. Virus Erad. 2023, 9, 100330. [Google Scholar] [CrossRef] [PubMed]
- Ratan, Z.A.; Mashrur, F.R.; Chhoan, A.P.; Shahriar, S.M.; Haidere, M.F.; Runa, N.J.; Kim, S.; Kweon, D.H.; Hosseinzadeh, H.; Cho, J.Y. Silver Nanoparticles as Potential Antiviral Agents. Pharmaceutics 2021, 13, 2034. [Google Scholar] [CrossRef]
- Jeremiah, S.S.; Miyakawa, K.; Morita, T.; Yamaoka, Y.; Ryo, A. Potent Antiviral Effect of Silver Nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020, 533, 195–200. [Google Scholar] [CrossRef]
- Luceri, A.; Francese, R.; Lembo, D.; Ferraris, M.; Balagna, C. Silver Nanoparticles: Review of Antiviral Properties, Mechanism of Action and Applications. Microorganisms 2023, 11, 629. [Google Scholar] [CrossRef]
- Elechiguerra, J.L.; Burt, J.L.; Morones, J.R.; Camacho-Bragado, A.; Gao, X.; Lara, H.H.; Yacaman, M.J. Interaction of Silver Nanoparticles with HIV-1. J. Nanobiotechnol. 2005, 3, 6. [Google Scholar] [CrossRef]
- He, Q.; Lu, J.; Liu, N.; Lu, W.; Li, Y.; Shang, C.; Li, X.; Hu, L.; Jiang, G. Antiviral Properties of Silver Nanoparticles against SARS-CoV-2: Effects of Surface Coating and Particle Size. Nanomaterials 2022, 12, 990. [Google Scholar] [CrossRef]
- Almanza-Reyes, H.; Moreno, S.; Plascencia-López, I.; Alvarado-Vera, M.; Patrón-Romero, L.; Borrego, B.; Reyes-Escamilla, A.; Valencia-Manzo, D.; Brun, A.; Pestryakov, A.; et al. Evaluation of Silver Nanoparticles for the Prevention of SARS-CoV-2 Infection in Health Workers: In Vitro and In Vivo. PLoS ONE 2021, 16, e0256401. [Google Scholar] [CrossRef]
- Abulikemu, M.; Tabrizi, B.E.A.; Ghobadloo, S.M.; Mofarah, H.M.; Jabbour, G.E. Silver Nanoparticle-Decorated Personal Protective Equipment for Inhibiting Human Coronavirus Infectivity. ACS Appl. Nano Mater. 2022, 5, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Srikhao, N.; Ounkaew, A.; Srichiangsa, N.; Phanthanawiboon, S.; Boonmars, T.; Artchayasawat, A.; Theerakulpisut, S.; Okhawilai, M.; Kasemsiri, P. Green-Synthesized Silver Nanoparticle Coating on Paper for Antibacterial and Antiviral Applications. Polym. Bull. 2023, 80, 9651–9668. [Google Scholar] [CrossRef] [PubMed]
- Noppradit, B.; Chaiyosburana, S.; Khupsathianwong, N.; Tapachai, W.A.; Wattanakanjana, Y.; Phengdaam, A. Green Synthesis of Silver Nanoparticles Using Saccharum Officinarum Leaf Extract for Antiviral Paint. Green Process. Synth. 2023, 12, 20230172. [Google Scholar] [CrossRef]
- Abass Sofi, M.; Sunitha, S.; Ashaq Sofi, M.; Khadheer Pasha, S.K.; Choi, D. An Overview of Antimicrobial and Anticancer Potential of Silver Nanoparticles. J. King Saud Univ.-Sci. 2022, 34, 101791. [Google Scholar] [CrossRef]
- Farah, M.A.; Ali, M.A.; Chen, S.M.; Li, Y.; Al-Hemaid, F.M.; Abou-Tarboush, F.M.; Al-Anazi, K.M.; Lee, J. Silver Nanoparticles Synthesized from Adenium Obesum Leaf Extract Induced DNA Damage, Apoptosis and Autophagy via Generation of Reactive Oxygen Species. Colloids Surf. B Biointerfaces 2016, 141, 158–169. [Google Scholar] [CrossRef]
- Piao, M.J.; Kang, K.A.; Lee, I.K.; Kim, H.S.; Kim, S.; Choi, J.Y.; Choi, J.; Hyun, J.W. Silver Nanoparticles Induce Oxidative Cell Damage in Human Liver Cells through Inhibition of Reduced Glutathione and Induction of Mitochondria-Involved Apoptosis. Toxicol. Lett. 2011, 201, 92–100. [Google Scholar] [CrossRef]
- Takáč, P.; Michalková, R.; Čižmáriková, M.; Bedlovičová, Z.; Balážová, Ľ.; Takáčová, G. The Role of Silver Nanoparticles in the Diagnosis and Treatment of Cancer: Are There Any Perspectives for the Future? Life 2023, 13, 466. [Google Scholar] [CrossRef]
- Mao, B.H.; Tsai, J.C.; Chen, C.W.; Yan, S.J.; Wang, Y.J. Mechanisms of Silver Nanoparticle-Induced Toxicity and Important Role of Autophagy. Nanotoxicology 2016, 10, 1021–1040. [Google Scholar] [CrossRef]
- Kim, D.; Amatya, R.; Hwang, S.; Lee, S.; Min, K.A.; Shin, M.C. BSA-Silver Nanoparticles: A Potential Multimodal Therapeutics for Conventional and Photothermal Treatment of Skin Cancer. Pharmaceutics 2021, 13, 575. [Google Scholar] [CrossRef]
- Namulinda, T.; Song, Z.B.; Yan, Y.J.; Zhang, M.; Meerovich, G.A.; Margetic, D.; Chen, Z.L. Enhanced Biosafety, Anticancer and Antibacterial Photodynamic Activities Using Silver-Pyropheophorbide-a Nanoconjugates. Nanomedicine 2024, 19, 1643–1658. [Google Scholar] [CrossRef]
- Al-Khedhairy, A.A.; Wahab, R. Silver Nanoparticles: An Instantaneous Solution for Anticancer Activity against Human Liver (HepG2) and Breast (MCF-7) Cancer Cells. Metals 2022, 12, 148. [Google Scholar] [CrossRef]
- Pavan, S.R.; Venkatesan, J.; Prabhu, A. Anticancer Activity of Silver Nanoparticles from the Aqueous Extract of Dictyota Ciliolata on Non-Small Cell Lung Cancer Cells. J. Drug Deliv. Sci. Technol. 2022, 74, 103525. [Google Scholar] [CrossRef]
- Kong, C.; Chen, S.; Ge, W.; Zhao, Y.; Xu, X.; Wang, S.; Zhang, J. Riclin-Capped Silver Nanoparticles as an Antibacterial and Anti-Inflammatory Wound Dressing. Int. J. Nanomed. 2022, 17, 2629–2641. [Google Scholar] [CrossRef] [PubMed]
- Craciunescu, O.; Seciu, A.M.; Zarnescu, O. In Vitro and in Vivo Evaluation of a Biomimetic Scaffold Embedding Silver Nanoparticles for Improved Treatment of Oral Lesions. Mater. Sci. Eng. C 2021, 123, 112015. [Google Scholar] [CrossRef] [PubMed]
- Adeleye, O.A.; Badru, A.O.; Oyinloye, O.E.; Fagbohun, A.B.; Bakre, L.G.; Bamiro, O.A.; Babalola, C.O.; Lateef, A. Green Synthesized Silver Nanoparticles for Cream Formulation: Its Anti-Inflammatory and Healing Activities. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Online, 18 December 2020; Volume 805, p. 012016. [Google Scholar] [CrossRef]
- Khashan, A.A.; Dawood, Y.; Khalaf, Y.H. Green Chemistry and Anti-Inflammatory Activity of Silver Nanoparticles Using an Aqueous Curcumin Extract. Results Chem. 2023, 5, 100913. [Google Scholar] [CrossRef]
- Barman, K.; Chowdhury, D.; Baruah, P.K. Bio-Synthesized Silver Nanoparticles Using Zingiber Officinale Rhizome Extract as Efficient Catalyst for the Degradation of Environmental Pollutants. Inorg. Nano-Met. Chem. 2020, 50, 57–65. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Li, C.; Nabeel, F.; Khalid, M.; Iqbal, H.M.N. Catalytic Potential of Bio-Synthesized Silver Nanoparticles Using Convolvulus Arvensis Extract for the Degradation of Environmental Pollutants. J. Photochem. Photobiol. B 2018, 181, 44–52. [Google Scholar] [CrossRef]
- Ivanov, I.; Manolov, S.; Phuong, N.; Nguyen, U.; Dang, N.T.; Doan, L.; Thu, T.; Nguyen, H. Synthesis of Silver Nanoparticles: From Conventional to ‘Modern’ Methods—A Review. Processes 2023, 11, 2617. [Google Scholar] [CrossRef]
- Stagon, S.P.; Huang, H. Syntheses and Applications of Small Metallic Nanorods from Solution and Physical Vapor Deposition. Nanotechnol. Rev. 2013, 2, 259–267. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Laser Ablation Synthesis in Solution and Size Manipulation of Noble Metal Nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 3805–3821. [Google Scholar] [CrossRef]
- Tien, D.-C.; Liao, C.-Y.; Huang, J.-C.; Tseng, K.-H.; Lung, J.-K.; Tsung, T.-T.; Kao, W.-S.; Tsai, T.-H.; Cheng, T.-W.; Yu, B.-S.; et al. Novel Technique for Preparing a Nano-Silver Water Suspension by the Arc-Discharge Method. Rev. Adv. Mater. Sci. 2008, 18, 752–758. [Google Scholar]
- Khayati, G.R.; Janghorban, K. The Nanostructure Evolution of Ag Powder Synthesized by High Energy Ball Milling. Adv. Powder Technol. 2012, 23, 393–397. [Google Scholar] [CrossRef]
- Xing, T.; Sunarso, J.; Yang, W.; Yin, Y.; Glushenkov, A.M.; Li, L.H.; Howlett, P.C.; Chen, Y. Ball Milling: A Green Mechanochemical Approach for Synthesis of Nitrogen Doped Carbon Nanoparticles. Nanoscale 2013, 5, 7970–7976. [Google Scholar] [CrossRef] [PubMed]
- Khayati, G.R.; Janghorban, K. An Investigation on the Application of Process Control Agents in the Preparation and Consolidation Behavior of Nanocrystalline Silver by Mechanochemical Method. Adv. Powder Technol. 2012, 23, 808–813. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Raghavendra, G.M.; Varaprasad, K.; Reddy, G.V.S.; Reddy, A.B.; Sudhakar, K.; Sadiku, E.R. Preparation and Characterization of Poly(Ethylene Glycol) Stabilized Nano Silver Particles by a Mechanochemical Assisted Ball Mill Process. J. Appl. Polym. Sci. 2016, 133, 43027. [Google Scholar] [CrossRef]
- Khan, M.; Shaik, M.R.; Adil, S.F.; Khan, S.T.; Al-Warthan, A.; Siddiqui, M.R.H.; Tahir, M.N.; Tremel, W. Plant Extracts as Green Reductants for the Synthesis of Silver Nanoparticles: Lessons from Chemical Synthesis. Dalton Trans. 2018, 47, 11988–12010. [Google Scholar] [CrossRef]
- Sadrolhosseini, A.R.; Mahdi, M.A.; Alizadeh, F.; Rashid, S.A. Laser Ablation Technique for Synthesis of Metal Nanoparticle in Liquid. Laser Technol. Its Appl. 2018, 63–83. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Comesaña, R.; Lusquiños, F.; Riveiro, A.; Del Val, J.; Pou, J. Production of Silver Nanoparticles by Laser Ablation in Open Air. Appl. Surf. Sci. 2015, 336, 108–111. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Izzi, M.; Volpe, A.; Clemente, M.; Picca, R.A.; Ancona, A.; Lugarà, P.M.; Palazzo, G.; Cioffi, N. The Pros and Cons of the Use of Laser Ablation Synthesis for the Production of Silver Nano-Antimicrobials. Antibiotics 2018, 7, 67. [Google Scholar] [CrossRef]
- Wang, Z.X.; Chen, C.Y.; Wang, Y.; Li, F.X.Z.; Huang, J.; Luo, Z.W.; Rao, S.S.; Tan, Y.J.; Liu, Y.W.; Yin, H.; et al. Ångstrom-Scale Silver Particles as a Promising Agent for Low-Toxicity Broad-Spectrum Potent Anticancer Therapy. Adv. Funct. Mater. 2019, 29, 1808556. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, G.; Liu, L.; Tong, H.; Li, Y.; Bai, H.; Wu, A. Synthesis of Silver Nanoparticles Using Large-Area Arc Discharge and Its Application in Electronic Packaging. J. Mater. Sci. 2017, 52, 3375–3387. [Google Scholar] [CrossRef]
- Ashkarran, A.A. A Novel Method for Synthesis of Colloidal Silver Nanoparticles by Arc Discharge in Liquid. Curr. Appl. Phys. 2010, 10, 1442–1447. [Google Scholar] [CrossRef]
- Tseng, K.H.; Chou, C.J.; Liu, T.C.; Tien, D.C.; Chang, C.Y.; Stobinski, L. Relationship between Ag Nanoparticles and Ag Ions Prepared by Arc Discharge Method. Nanotechnol. Rev. 2018, 7, 3240959. [Google Scholar] [CrossRef]
- Li, Z.; Dong, H.; Wu, Z.; Shen, J.; Xu, D.; He, R.; Wan, L.; Zhang, S. Novel P-n Type Porous Ag2O/Bi5O7I Heterojunction for Uv–Vis-NIR Activated High Efficient Photocatalytic Degradation of Bisphenol A: Photoelectric Properties and Degradation Mechanism. Appl. Surf. Sci. 2020, 529, 147162. [Google Scholar] [CrossRef]
- Mallick, K.; Witcomb, M.J.; Scurrell, M.S. Polymer Stabilized Silver Nanoparticles: A Photochemical Synthesis Route. J. Mater. Sci. 2004, 39, 4459–4463. [Google Scholar] [CrossRef]
- Do Kim, K.; Han, D.N.; Kim, H.T. Optimization of Experimental Conditions Based on the Taguchi Robust Design for the Formation of Nano-Sized Silver Particles by Chemical Reduction Method. Chem. Eng. J. 2004, 104, 55–61. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of Silver Nanoparticles: Chemical, Physical and Biological Methods. Res. Pharm. Sci. 2014, 9, 385. [Google Scholar]
- Gudikandula, K.; Charya Maringanti, S. Synthesis of Silver Nanoparticles by Chemical and Biological Methods and Their Antimicrobial Properties. J. Exp. Nanosci. 2016, 11, 714–721. [Google Scholar] [CrossRef]
- Laghrib, F.; Farahi, A.; Bakasse, M.; Lahrich, S.; El Mhammedi, M.A. Chemical Synthesis of Nanosilver on Chitosan and Electroanalysis Activity against the P-Nitroaniline Reduction. J. Electroanal. Chem. 2019, 845, 111–118. [Google Scholar] [CrossRef]
- Dondi, R.; Su, W.; Griffith, G.A.; Clark, G.; Burley, G.A. Highly Size- and Shape-Controlled Synthesis of Silver Nanoparticles via a Templated Tollens Reaction. Small 2012, 8, 770–776. [Google Scholar] [CrossRef]
- Li, X.; Odoom-Wubah, T.; Chen, H.; Jing, X.; Zheng, B.; Huang, J. Biosynthesis of Silver Nanoparticles through Tandem Hydrolysis of Silver Sulfate and Cellulose under Hydrothermal Conditions. J. Chem. Technol. Biotechnol. 2014, 89, 1817–1824. [Google Scholar] [CrossRef]
- Zielińska, A.; Skwarek, E.; Zaleska, A.; Gazda, M.; Hupka, J. Preparation of Silver Nanoparticles with Controlled Particle Size. Procedia Chem. 2009, 1, 1560–1566. [Google Scholar] [CrossRef]
- Ahmad, N.; Ang, B.C.; Amalina, M.A.; Bong, C.W. Influence of Precursor Concentration and Temperature on the Formation of Nanosilver in Chemical Reduction Method. Sains Malays. 2018, 47, 157–168. [Google Scholar] [CrossRef]
- Khaydarov, R.A.; Khaydarov, R.R.; Gapurova, O.; Estrin, Y.; Scheper, T. Electrochemical Method for the Synthesis of Silver Nanoparticles. J. Nanopart. Res. 2009, 11, 1193–1200. [Google Scholar] [CrossRef]
- Jovanović, Ž.; Stojkovska, J.; Obradović, B.; Miskovic-Stankovic, V. Alginate Hydrogel Microbeads Incorporated with Ag Nanoparticles Obtained by Electrochemical Method. Mater. Chem. Phys. 2012, 133, 182–189. [Google Scholar] [CrossRef]
- Naganthran, A.; Verasoundarapandian, G.; Khalid, F.E.; Masarudin, M.J.; Zulkharnain, A.; Nawawi, N.M.; Karim, M.; Abdullah, C.A.C.; Ahmad, S.A. Synthesis, Characterization and Biomedical Application of Silver Nanoparticles. Materials 2022, 15, 427. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Speth, T.F.; Varma, R.S. Microwave-Assisted Green Synthesis of Silver Nanostructures. Acc. Chem. Res. 2011, 44, 469–478. [Google Scholar] [CrossRef]
- David, S.C.; Kim, H.; Ko, J.; Lee, J.; Hwang, B.; Chang, S.; Kim, B.; Chung, S.J. Microwave Synthesis of Silver Nanoparticles Using Different Pentose Carbohydrates as Reducing Agents. J. Chem. Chem. Eng. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Jara, N.; Milán, N.S.; Rahman, A.; Mouheb, L.; Boffito, D.C.; Jeffryes, C.; Dahoumane, S.A. Photochemical Synthesis of Gold and Silver Nanoparticles—A Review. Molecules 2021, 26, 4585. [Google Scholar] [CrossRef]
- Malik, M.A.; Wani, M.Y.; Hashim, M.A. Microemulsion Method: A Novel Route to Synthesize Organic and Inorganic Nanomaterials: 1st Nano Update. Arab. J. Chem. 2012, 5, 397–417. [Google Scholar] [CrossRef]
- Abou El-Nour, K.M.M.; Eftaiha, A.; Al-Warthan, A.; Ammar, R.A.A. Synthesis and Applications of Silver Nanoparticles. Arab. J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef]
- Miyazawa, T.; Itaya, M.; Burdeos, G.C.; Nakagawa, K.; Miyazawa, T. A Critical Review of the Use of Surfactant-Coated Nanoparticles in Nanomedicine and Food Nanotechnology. Int. J. Nanomed. 2021, 16, 3937–3999. [Google Scholar] [CrossRef] [PubMed]
- Klaus, T.; Joerger, R.; Olsson, E.; Granqvist, C.G. Silver-Based Crystalline Nanoparticles, Microbially Fabricated. Proc. Natl. Acad. Sci. USA 1999, 96, 13611–13614. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, S.; Brunetto, P.S.; Gagnon, J.; Priebe, M.; Giese, B.; Fromm, K.M. Nanobio Silver: Its Interactions with Peptides and Bacteria, and Its Uses in Medicine. Chem. Rev. 2013, 113, 4708–4754. [Google Scholar] [CrossRef]
- Bouafia, A.; Laouini, S.E.; Ahmed, A.S.A.; Soldatov, A.V.; Algarni, H.; Chong, K.F.; Ali, G.A.M. The Recent Progress on Silver Nanoparticles: Synthesis and Electronic Applications. Nanomaterials 2021, 11, 2318. [Google Scholar] [CrossRef]
- Mustapha, T.; Misni, N.; Ithnin, N.R.; Daskum, A.M.; Unyah, N.Z. A Review on Plants and Microorganisms Mediated Synthesis of Silver Nanoparticles, Role of Plants Metabolites and Applications. Int. J. Environ. Res. Public Health 2022, 19, 674. [Google Scholar] [CrossRef]
- Castillo-Henríquez, L.; Alfaro-Aguilar, K.; Ugalde-álvarez, J.; Vega-Fernández, L.; de Oca-Vásquez, G.M.; Vega-Baudrit, J.R. Green Synthesis of Gold and Silver Nanoparticles from Plant Extracts and Their Possible Applications as Antimicrobial Agents in the Agricultural Area. Nanomaterials 2020, 10, 1763. [Google Scholar] [CrossRef]
- Biosynthesis, A.; Liotta, L.; La Parola, V.; Zuhrotun, A.; Jihan Oktaviani, D.; Nur Hasanah, A. Biosynthesis of Gold and Silver Nanoparticles Using Phytochemical Compounds. Molecules 2023, 28, 3240. [Google Scholar] [CrossRef]
- Jain, S.; Mehata, M.S. Medicinal Plant Leaf Extract and Pure Flavonoid Mediated Green Synthesis of Silver Nanoparticles and Their Enhanced Antibacterial Property. Sci. Rep. 2017, 7, 15867. [Google Scholar] [CrossRef]
- Küünal, S.; Visnapuu, M.; Volubujeva, O.; Soares Rosario, M.; Rauwel, P.; Rauwel, E. Optimisation of Plant Mediated Synthesis of Silver Nanoparticles by Common Weed Plantago Major and Their Antimicrobial Properties. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Online, 21–22 November 2019; Volume 613, p. 012003. [Google Scholar] [CrossRef]
- Sreelekha, E.; George, B.; Shyam, A.; Sajina, N.; Mathew, B. A Comparative Study on the Synthesis, Characterization, and Antioxidant Activity of Green and Chemically Synthesized Silver Nanoparticles. Bionanoscience 2021, 11, 489–496. [Google Scholar] [CrossRef]
- Naveed, M.; Batool, H.; Rehman, S.U.; Javed, A.; Makhdoom, S.I.; Aziz, T.; Mohamed, A.A.; Sameeh, M.Y.; Alruways, M.W.; Dablool, A.S.; et al. Characterization and Evaluation of the Antioxidant, Antidiabetic, Anti-Inflammatory, and Cytotoxic Activities of Silver Nanoparticles Synthesized Using Brachychiton Populneus Leaf Extract. Processes 2022, 10, 1521. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Mechouet, M.; Wijesekera, K.; Filipe, C.D.M.; Sicard, C.; Bazylinski, D.A.; Jeffryes, C. Algae-Mediated Biosynthesis of Inorganic Nanomaterials as a Promising Route in Nanobiotechnology—A Review. Green Chem. 2017, 19, 552–587. [Google Scholar] [CrossRef]
- da Silva Ferreira, V.; ConzFerreira, M.E.; Lima, L.M.T.R.; Frasés, S.; de Souza, W.; Sant’Anna, C. Green Production of Microalgae-Based Silver Chloride Nanoparticles with Antimicrobial Activity against Pathogenic Bacteria. Enzym. Microb. Technol. 2017, 97, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Chojnacka, K. Algae as Production Systems of Bioactive Compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Khanna, P.; Kaur, A.; Goyal, D. Algae-Based Metallic Nanoparticles: Synthesis, Characterization and Applications. J. Microbiol. Methods 2019, 163, 105656. [Google Scholar] [CrossRef]
- Aziz, N.; Faraz, M.; Pandey, R.; Shakir, M.; Fatma, T.; Varma, A.; Barman, I.; Prasad, R. Facile Algae-Derived Route to Biogenic Silver Nanoparticles: Synthesis, Antibacterial, and Photocatalytic Properties. Langmuir 2015, 31, 11605–11612. [Google Scholar] [CrossRef]
- Sathishkumar, R.S.; Sundaramanickam, A.; Srinath, R.; Ramesh, T.; Saranya, K.; Meena, M.; Surya, P. Green Synthesis of Silver Nanoparticles by Bloom Forming Marine Microalgae Trichodesmium Erythraeum and Its Applications in Antioxidant, Drug-Resistant Bacteria, and Cytotoxicity Activity. J. Saudi Chem. Soc. 2019, 23, 1180–1191. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Wujcik, E.K.; Jeffryes, C. Noble Metal, Oxide and Chalcogenide-Based Nanomaterials from Scalable Phototrophic Culture Systems. Enzym. Microb. Technol. 2016, 95, 13–27. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Malarkodi, C.; Paulkumar, K.; Vanaja, M.; Gnanajobitha, G.; Annadurai, G. Algae Mediated Green Fabrication of Silver Nanoparticles and Examination of Its Antifungal Activity against Clinical Pathogens. Int. J. Met. 2014, 2014, 692643. [Google Scholar] [CrossRef]
- Amar Dahoumane, S.; Mechouet, M.; Alvarez, F.J.; Agathos, S.N.; Jeffryes, C. Microalgae: An Outstanding Tool in Nanotechnology Microalgas: Una Excelente Herramienta en Nanotecnología. Bionatura 2016, 1, 196–201. [Google Scholar] [CrossRef]
- Monteiro, C.M.; Castro, P.M.L.; Malcata, F.X. Metal Uptake by Microalgae: Underlying Mechanisms and Practical Applications. Biotechnol. Prog. 2012, 28, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Kumar, S.; Bafana, A.; Dahoumane, S.A.; Jeffryes, C. Biosynthetic Conversion of Ag+ to Highly Stable Ag0 Nanoparticles by Wild Type and Cell Wall Deficient Strains of Chlamydomonas Reinhardtii. Molecules 2018, 24, 98. [Google Scholar] [CrossRef] [PubMed]
- Barwal, I.; Ranjan, P.; Kateriya, S.; Yadav, S.C. Cellular Oxido-Reductive Proteins of Chlamydomonas Reinhardtii Control the Biosynthesis of Silver Nanoparticles. J. Nanobiotechnol. 2011, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Dahoumane, S.A.; Yéprémian, C.; Djédiat, C.; Couté, A.; Fiévet, F.; Coradin, T.; Brayner, R. A Global Approach of the Mechanism Involved in the Biosynthesis of Gold Colloids Using Micro-Algae. J. Nanopart. Res. 2014, 16, 2607. [Google Scholar] [CrossRef]
- Azizi, S.; Namvar, F.; Mahdavi, M.; Ahmad, M.B.; Mohamad, R. Biosynthesis of Silver Nanoparticles Using Brown Marine Macroalga, Sargassum Muticum Aqueous Extract. Materials 2013, 6, 5942–5950. [Google Scholar] [CrossRef]
- Deepak, P.; Amutha, V.; Birundha, R.; Sowmiya, R.; Kamaraj, C.; Balasubramanian, V.; Balasubramani, G.; Aiswarya, D.; Arul, D.; Perumal, P. Facile Green Synthesis of Nanoparticles from Brown Seaweed Sargassum Wightii and Its Biological Application Potential. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 035019. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Hamzah, H.; Maaroof, M. Analyzing Formation of Silver Nanoparticles from the Filamentous Fungus Fusarium Oxysporum and Their Antimicrobial Activity. Turk. J. Biol. 2018, 42, 54–62. [Google Scholar] [CrossRef]
- Singhal, A.; Singhal, N.; Bhattacharya, A.; Gupta, A. Synthesis of Silver Nanoparticles (AgNPs) Using Ficus Retusa Leaf Extract for Potential Application as Antibacterial and Dye Decolourising Agents. Inorg. Nano-Met. Chem. 2017, 47, 1520–1529. [Google Scholar] [CrossRef]
- Guilger-Casagrande, M.; Lima, R. de Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019, 7, 486092. [Google Scholar] [CrossRef]
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular Biosynthesis of Silver Nanoparticles Using the Fungus Fusarium Oxysporum. Colloids Surf. B Biointerfaces 2003, 28, 313–318. [Google Scholar] [CrossRef]
- Korbekandi, H.; Mohseni, S.; Jouneghani, R.M.; Pourhossein, M.; Iravani, S. Biosynthesis of Silver Nanoparticles Using Saccharomyces Cerevisiae. Artif. Cells Nanomed. Biotechnol. 2016, 44, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.Z.H.; Kiran, U.; Ali, M.I.; Jamal, A.; Hameed, A.; Ahmed, S.; Ali, N. Combined Efficacy of Biologically Synthesized Silver Nanoparticles and Different Antibiotics against Multidrug-Resistant Bacteria. Int. J. Nanomed. 2013, 8, 3187–3195. [Google Scholar] [CrossRef] [PubMed]
- Balaji, D.S.; Basavaraja, S.; Deshpande, R.; Mahesh, D.B.; Prabhakar, B.K.; Venkataraman, A. Extracellular Biosynthesis of Functionalized Silver Nanoparticles by Strains of Cladosporium Cladosporioides Fungus. Colloids Surf. B Biointerfaces 2009, 68, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, H.; Chen, Z.S.; Chen, G. Biosynthesis of Nanoparticles by Microorganisms and Their Applications. J. Nanomater. 2011, 2011, 270974. [Google Scholar] [CrossRef]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Ajaykumar, P.V.; Alam, M.; Kumar, R.; et al. Fungus-Mediated Synthesis of Silver Nanoparticles and Their Immobilization in the Mycelial Matrix: A Novel Biological Approach to Nanoparticle Synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Salvadori, M.R.; Rafatullah, M.; Siddiqui, M.R.; Khan, M.A.; Alshareef, S.A. Exploration of Microbial Factories for Synthesis of Nanoparticles—A Sustainable Approach for Bioremediation of Environmental Contaminants. Front. Microbiol. 2021, 12, 658294. [Google Scholar] [CrossRef]
- Shivaji, S.; Madhu, S.; Singh, S. Extracellular Synthesis of Antibacterial Silver Nanoparticles Using Psychrophilic Bacteria. Process Biochem. 2011, 46, 1800–1807. [Google Scholar] [CrossRef]
- Nanda, A.; Saravanan, M. Biosynthesis of Silver Nanoparticles from Staphylococcus Aureus and Its Antimicrobial Activity against MRSA and MRSE. Nanomedicine 2009, 5, 452–456. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Suresh Babu, R.; Venkataraman, D.; Bilal, M.; Gurunathan, S. Biosynthesis of Silver Nanocrystals by Bacillus Licheniformis. Colloids Surf. B Biointerfaces 2008, 65, 150–153. [Google Scholar] [CrossRef]
- John, M.S.; Nagoth, J.A.; Ramasamy, K.P.; Mancini, A.; Giuli, G.; Miceli, C.; Pucciarelli, S. Synthesis of Bioactive Silver Nanoparticles Using New Bacterial Strains from an Antarctic Consortium. Mar. Drugs 2022, 20, 558. [Google Scholar] [CrossRef]
- Yuan, Q.; Bomma, M.; Xiao, Z. Enhanced Silver Nanoparticle Synthesis by Escherichia Coli Transformed with Candida Albicans Metallothionein Gene. Materials 2019, 12, 4180. [Google Scholar] [CrossRef] [PubMed]
- Naseer, Q.A.; Xue, X.; Wang, X.; Dang, S.; Din, S.U.; Kalsoom; Jamil, J. Synthesis of Silver Nanoparticles Using Lactobacillus Bulgaricus and Assessment of Their Antibacterial Potential. Braz. J. Biol. 2021, 82, e232434. [Google Scholar] [CrossRef] [PubMed]
- Munkhbayar, B.; Tanshen, M.R.; Jeoun, J.; Chung, H.; Jeong, H. Surfactant-Free Dispersion of Silver Nanoparticles into MWCNT-Aqueous Nanofluids Prepared by One-Step Technique and Their Thermal Characteristics. Ceram. Int. 2013, 39, 6415–6425. [Google Scholar] [CrossRef]
- Kumar, N.; Biswas, K.; Gupta, R.K. Green Synthesis of Ag Nanoparticles in Large Quantity by Cryomilling. RSC Adv. 2016, 6, 111380–111388. [Google Scholar] [CrossRef]
- Kim, M.; Osone, S.; Kim, T.; Higashi, H.; Seto, T. Synthesis of Nanoparticles by Laser Ablation: A Review. KONA Powder Part. J. 2017, 2017, 80–90. [Google Scholar] [CrossRef]
- Abd El-kader, F.H.; Hakeem, N.A.; Elashmawi, I.S.; Menazea, A.A. Synthesis and Characterization of PVK/AgNPs Nanocomposites Prepared by Laser Ablation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 138, 331–339. [Google Scholar] [CrossRef]
- Amendola, V.; Polizzi, S.; Meneghetti, M. Free Silver Nanoparticles Synthesized by Laser Ablation in Organic Solvents and Their Easy Functionalization. Langmuir 2007, 23, 6766–6770. [Google Scholar] [CrossRef]
- Raffi, M.; Rumaiz, A.K.; Hasan, M.M.; Shah, S.I. Studies of the Growth Parameters for Silver Nanoparticle Synthesis by Inert Gas Condensation. J. Mater. Res. 2007, 22, 3378–3384. [Google Scholar] [CrossRef]
- Wongrat, E.; Wongkrajang, S.; Chuejetton, A.; Bhoomanee, C.; Choopun, S. Rapid Synthesis of Au, Ag and Cu Nanoparticles by DC Arc-Discharge for Efficiency Enhancement in Polymer Solar Cells. Mater. Res. Innov. 2019, 23, 66–72. [Google Scholar] [CrossRef]
- Reverberi, A.P.; Kuznetsov, N.T.; Meshalkin, V.P.; Salerno, M.; Fabiano, B. Systematical Analysis of Chemical Methods in Metal Nanoparticles Synthesis. Theor. Found. Chem. Eng. 2016, 50, 59–66. [Google Scholar] [CrossRef]
- Ranoszek-Soliwoda, K.; Tomaszewska, E.; Socha, E.; Krzyczmonik, P.; Ignaczak, A.; Orlowski, P.; Krzyzowska, M.; Celichowski, G.; Grobelny, J. The Role of Tannic Acid and Sodium Citrate in the Synthesis of Silver Nanoparticles. J. Nanopart. Res. 2017, 19, 273. [Google Scholar] [CrossRef] [PubMed]
- Kedar, K.; Nayak, S.; Bhaskar, V.; Dist Thane, M. Synthesis of Silver Nanoparticles by Chemical Reduction Method Introduction. Int. J. Pharma Prof. Res. 2022, 25, 364–376. [Google Scholar]
- Kuntyi, O.I.; Kytsya, R.; Bondarenko, A.B.; Mazur, S.; Mertsalo, I.P.; Bazylyak, L.I. Microplasma Synthesis of Silver Nanoparticles in PVP Solutions Using Sacrificial Silver Anodes. Colloid Polym. Sci. 2021, 299, 855–863. [Google Scholar] [CrossRef]
- Reicha, F.M.; Sarhan, A.; Abdel-Hamid, M.I.; El-Sherbiny, I.M. Preparation of Silver Nanoparticles in the Presence of Chitosan by Electrochemical Method. Carbohydr. Polym. 2012, 89, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Crnjak Orel, Z. Microwave-Assisted Non-Aqueous Synthesis of ZnO Nanoparticles. Mater. Technol. 2011, 45, 173–177. [Google Scholar]
- Athawale, A.A.; Desai, P.A. Silver Doped Lanthanum Chromites by Microwave Combustion Method. Ceram. Int. 2011, 37, 3037–3043. [Google Scholar] [CrossRef]
- Saade, J.; De Araújo, C.B. Synthesis of Silver Nanoprisms: A Photochemical Approach Using Light Emission Diodes. Mater. Chem. Phys. 2014, 148, 1184–1193. [Google Scholar] [CrossRef]
- Pu, F.; Ran, X.; Guan, M.; Huang, Y.; Ren, J.; Qu, X. Biomolecule-Templated Photochemical Synthesis of Silver Nanoparticles: Multiple Readouts of Localized Surface Plasmon Resonance for Pattern Recognition. Nano Res. 2018, 11, 3213–3221. [Google Scholar] [CrossRef]
- Charan Teja, V.R. A Glimpse on Solid Lipid Nanoparticles as Drug Delivery Systems. J. Glob. Trends Pharm. Sci. 2014, 5, 1649–1657. [Google Scholar]
- Das, M.; Patowary, K.; Vidya, R.; Malipeddi, H. Microemulsion Synthesis of Silver Nanoparticles Using Biosurfactant Extracted from Pseudomonas Aeruginosa MKVIT3 Strain and Comparison of Their Antimicrobial and Cytotoxic Activities. IET Nanobiotechnol. 2016, 10, 411–418. [Google Scholar] [CrossRef]
- Radoń, A.; Łukowiec, D. Silver Nanoparticles Synthesized by UV-Irradiation Method Using Chloramine T as Modifier: Structure, Formation Mechanism and Catalytic Activity. CrystEngComm 2018, 20, 7130–7136. [Google Scholar] [CrossRef]
- Shameli, K.; Ahmad, M.B.; Yunus, W.M.Z.W.; Ibrahim, N.A.; Gharayebi, Y.; Sedaghat, S. Synthesis of Silver/Montmorillonite Nanocomposites Using γ-Irradiation. Int. J. Nanomed. 2010, 5, 1067–1077. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A Review on Green Synthesis of Silver Nanoparticles and Their Applications. Artif Cells Nanomed Biotechnol 2017, 45, 1272–1291. [Google Scholar] [CrossRef] [PubMed]
- Behravan, M.; Hossein Panahi, A.; Naghizadeh, A.; Ziaee, M.; Mahdavi, R.; Mirzapour, A. Facile Green Synthesis of Silver Nanoparticles Using Berberis Vulgaris Leaf and Root Aqueous Extract and Its Antibacterial Activity. Int. J. Biol. Macromol. 2019, 124, 148–154. [Google Scholar] [CrossRef]
- Garibo, D.; Borbón-Nuñez, H.A.; de León, J.N.D.; García Mendoza, E.; Estrada, I.; Toledano-Magaña, Y.; Tiznado, H.; Ovalle-Marroquin, M.; Soto-Ramos, A.G.; Blanco, A.; et al. Green Synthesis of Silver Nanoparticles Using Lysiloma Acapulcensis Exhibit High-Antimicrobial Activity. Sci. Rep. 2020, 10, 12805. [Google Scholar] [CrossRef]
- Gopinath, V.; MubarakAli, D.; Priyadarshini, S.; Priyadharsshini, N.M.; Thajuddin, N.; Velusamy, P. Biosynthesis of Silver Nanoparticles from Tribulus Terrestris and Its Antimicrobial Activity: A Novel Biological Approach. Colloids Surf. B Biointerfaces 2012, 96, 69–74. [Google Scholar] [CrossRef]
- Kathiraven, T.; Sundaramanickam, A.; Shanmugam, N.; Balasubramanian, T. Green Synthesis of Silver Nanoparticles Using Marine Algae Caulerpa Racemosa and Their Antibacterial Activity against Some Human Pathogens. Appl. Nanosci. 2015, 5, 499–504. [Google Scholar] [CrossRef]
- Somasundaram, C.K.; Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Vinodh, R.; Sundramoorthy, A.K.; Babu, R.S.; Alagan, M.; Lee, Y.R. Sustainable Synthesis of Silver Nanoparticles Using Marine Algae for Catalytic Degradation of Methylene Blue. Catalysts 2021, 11, 1377. [Google Scholar] [CrossRef]
- Fernández, J.G.; Fernández-Baldo, M.A.; Berni, E.; Camí, G.; Durán, N.; Raba, J.; Sanz, M.I. Production of Silver Nanoparticles Using Yeasts and Evaluation of Their Antifungal Activity against Phytopathogenic Fungi. Process Biochem. 2016, 51, 1306–1313. [Google Scholar] [CrossRef]
- Costa Silva, L.P.; Pinto Oliveira, J.; Keijok, W.J.; da Silva, A.R.; Aguiar, A.R.; Guimarães, M.C.C.; Ferraz, C.M.; Araújo, J.V.; Tobias, F.L.; Braga, F.R. Extracellular Biosynthesis of Silver Nanoparticles Using the Cell-Free Filtrate of Nematophagous Fungus Duddingtonia flagrans. Int. J. Nanomed. 2017, 12, 6373–6381. [Google Scholar] [CrossRef]
- Htwe, Y.Z.N.; Chow, W.S.; Suda, Y.; Mariatti, M. Effect of Silver Nitrate Concentration on the Production of Silver Nanoparticles by Green Method. Mater. Today Proc. 2019, 17, 568–573. [Google Scholar] [CrossRef]
- Ondari Nyakundi, E.; Padmanabhan, M.N. Green Chemistry Focus on Optimization of Silver Nanoparticles Using Response Surface Methodology (RSM) and Mosquitocidal Activity: Anopheles Stephensi (Diptera: Culicidae). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 149, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Mahmudin, L.; Suharyadi, E.; Utomo, A.B.S.; Abraha, K. Influence of Stabilizing Agent and Synthesis Temperature on the Optical Properties of Silver Nanoparticles as Active Materials in Surface Plasmon Resonance (SPR) Biosensor. AIP Conf. Proc. 2016, 1725, 20041. [Google Scholar] [CrossRef]
- Stevenson, A.P.Z.; Bea, D.B.; Civit, S.; Contera, S.A.; Cerveto, A.I.; Trigueros, S. Three Strategies to Stabilise Nearly Monodispersed Silver Nanoparticles in Aqueous Solution. Nanoscale Res. Lett. 2012, 7, 151. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Yang, T.H.; Yang, W.S.; Cheng, T.W.; Chiu, W.Y.; Don, T.M. Improved Stability and Film Formability of Oil-Based Silver Nanoparticle Suspensions by Addition of Polystyrene. Mater. Chem. Phys. 2022, 282, 125930. [Google Scholar] [CrossRef]
- Salazar-Bryam, A.M.; Yoshimura, I.; Santos, L.P.; Moura, C.C.; Santos, C.C.; Silva, V.L.; Lovaglio, R.B.; Costa Marques, R.F.; Jafelicci Junior, M.; Contiero, J. Silver Nanoparticles Stabilized by Ramnolipids: Effect of PH. Colloids Surf. B Biointerfaces 2021, 205, 111883. [Google Scholar] [CrossRef]
- Akhtar, N.; Mohammed, H.A.; Yusuf, M.; Al-Subaiyel, A.; Sulaiman, G.M.; Khan, R.A. SPIONs Conjugate Supported Anticancer Drug Doxorubicin’s Delivery: Current Status, Challenges, and Prospects. Nanomaterials 2022, 12, 3686. [Google Scholar] [CrossRef]
- Tortella, G.R.; Rubilar, O.; Durán, N.; Diez, M.C.; Martínez, M.; Parada, J.; Seabra, A.B. Silver Nanoparticles: Toxicity in Model Organisms as an Overview of Its Hazard for Human Health and the Environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar] [CrossRef]
- Patlolla, A.K.; Hackett, D.; Tchounwou, P.B. Silver Nanoparticle-Induced Oxidative Stress-Dependent Toxicity in Sprague-Dawley Rats. Mol. Cell. Biochem. 2015, 399, 257–268. [Google Scholar] [CrossRef]
- Awashra, M.; Młynarz, P. The Toxicity of Nanoparticles and Their Interaction with Cells: An in Vitro Metabolomic Perspective. Nanoscale Adv. 2023, 5, 2674–2723. [Google Scholar] [CrossRef]
- Lee, Y.H.; Cheng, F.Y.; Chiu, H.W.; Tsai, J.C.; Fang, C.Y.; Chen, C.W.; Wang, Y.J. Cytotoxicity, Oxidative Stress, Apoptosis and the Autophagic Effects of Silver Nanoparticles in Mouse Embryonic Fibroblasts. Biomaterials 2014, 35, 4706–4715. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; Kim, S.; Ahn, J.H.; Youn, P.; Kang, J.S.; Park, K.; Yi, J.; Ryu, D.Y. Induction of Oxidative Stress and Apoptosis by Silver Nanoparticles in the Liver of Adult Zebrafish. Aquat. Toxicol. 2010, 100, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Alanazi, A.M.; Alsaif, N.; Al-anazi, M.; Sayed, A.Y.A.; Bhat, M.A. Potential Cytotoxicity of Silver Nanoparticles: Stimulation of Autophagy and Mitochondrial Dysfunction in Cardiac Cells. Saudi J. Biol. Sci. 2021, 28, 2762–2771. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, J.S.; Simões, A.M.; Duarte, F.V.; Rolo, A.P.; Murdoch, R.C.; Hussain, S.M.; Palmeira, C.M. Assessment of the Toxicity of Silver Nanoparticles in Vitro: A Mitochondrial Perspective. Toxicol. Vitr. 2011, 25, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.C.; Pazin, M.; Franco-Bernardes, M.F.; da Cunha Martins, A.; Barcelos, G.R.M.; Pereira, M.C.; Mesquita, J.P.; Rodrigues, J.L.; Barbosa, F.; Dorta, D.J. A Perspective of Mitochondrial Dysfunction in Rats Treated with Silver and Titanium Nanoparticles (AgNPs and TiNPs). J. Trace Elem. Med. Biol. 2018, 47, 63–69. [Google Scholar] [CrossRef]
- Perde-Schrepler, M.; Florea, A.; Brie, I.; Virag, P.; Fischer-Fodor, E.; Vâlcan, A.; Gurzǎu, E.; Lisencu, C.; Maniu, A. Size-Dependent Cytotoxicity and Genotoxicity of Silver Nanoparticles in Cochlear Cells In Vitro. J. Nanomater. 2019, 2019, 6090259. [Google Scholar] [CrossRef]
- Park, E.J.; Bae, E.; Yi, J.; Kim, Y.; Choi, K.; Lee, S.H.; Yoon, J.; Lee, B.C.; Park, K. Repeated-Dose Toxicity and Inflammatory Responses in Mice by Oral Administration of Silver Nanoparticles. Environ. Toxicol. Pharmacol. 2010, 30, 162–168. [Google Scholar] [CrossRef]
- Al Gurabi, M.A.; Ali, D.; Alkahtani, S.; Alarifi, S. In Vivo DNA Damaging and Apoptotic Potential of Silver Nanoparticles in Swiss Albino Mice. OncoTargets Ther. 2015, 8, 295–302. [Google Scholar] [CrossRef]
- Bin-Jumah, M.; Al-Abdan, M.; Albasher, G.; Alarifi, S. Effects of Green Silver Nanoparticles on Apoptosis and Oxidative Stress in Normal and Cancerous Human Hepatic Cells In Vitro. Int. J. Nanomed. 2020, 15, 1537–1548. [Google Scholar] [CrossRef]
- Makama, S.; Kloet, S.K.; Piella, J.; van den Berg, H.; de Ruijter, N.C.A.; Puntes, V.F.; Rietjens, I.M.C.M.; van den Brink, N.W. Effects of Systematic Variation in Size and Surface Coating of Silver Nanoparticles on Their in Vitro Toxicity to Macrophage Raw 264.7 Cells. Toxicol. Sci. 2018, 162, 79–88. [Google Scholar] [CrossRef]
- Löfdahl, A.; Jern, A.; Flyman, S.; Kåredal, M.; Karlsson, H.L.; Larsson-Callerfelt, A.K. Silver Nanoparticles Alter Cell Viability Ex Vivo and in Vitro and Induce Proinflammatory Effects in Human Lung Fibroblasts. Nanomaterials 2020, 10, 1868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lee, P.; Lui, V.C.H.; Chen, Y.; Liu, X.; Lok, C.N.; To, M.; Yeung, K.W.K.; Wong, K.K.Y. Silver Nanoparticles Promote Osteogenesis of Mesenchymal Stem Cells and Improve Bone Fracture Healing in Osteogenesis Mechanism Mouse Model. Nanomedicine 2015, 11, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Ambrožová, N.; Zálešák, B.; Ulrichová, J.; Čížková, K.; Galandáková, A. Low Concentrations of Silver Nanoparticles Have a Beneficial Effect on Wound Healing In Vitro. J. Nanopart. Res. 2017, 19, 1–15. [Google Scholar] [CrossRef]
- Khoshnamvand, M.; Hao, Z.; Fadare, O.O.; Hanachi, P.; Chen, Y.; Liu, J. Toxicity of Biosynthesized Silver Nanoparticles to Aquatic Organisms of Different Trophic Levels. Chemosphere 2020, 258, 127346. [Google Scholar] [CrossRef]
- Lekamge, S.; Miranda, A.F.; Abraham, A.; Li, V.; Shukla, R.; Bansal, V.; Nugegoda, D. The Toxicity of Silver Nanoparticles (AgNPs) to Three Freshwater Invertebrates with Different Life Strategies: Hydra Vulgaris, Daphnia Carinata, and Paratya Australiensis. Front. Environ. Sci. 2018, 6, 152. [Google Scholar] [CrossRef]
- Fabrega, J.; Luoma, S.N.; Tyler, C.R.; Galloway, T.S.; Lead, J.R. Silver Nanoparticles: Behaviour and Effects in the Aquatic Environment. Environ. Int. 2011, 37, 517–531. [Google Scholar] [CrossRef]
- Liu, Z.; Malinowski, C.R.; Sepúlveda, M.S. Emerging Trends in Nanoparticle Toxicity and the Significance of Using Daphnia as a Model Organism. Chemosphere 2022, 291, 132941. [Google Scholar] [CrossRef]
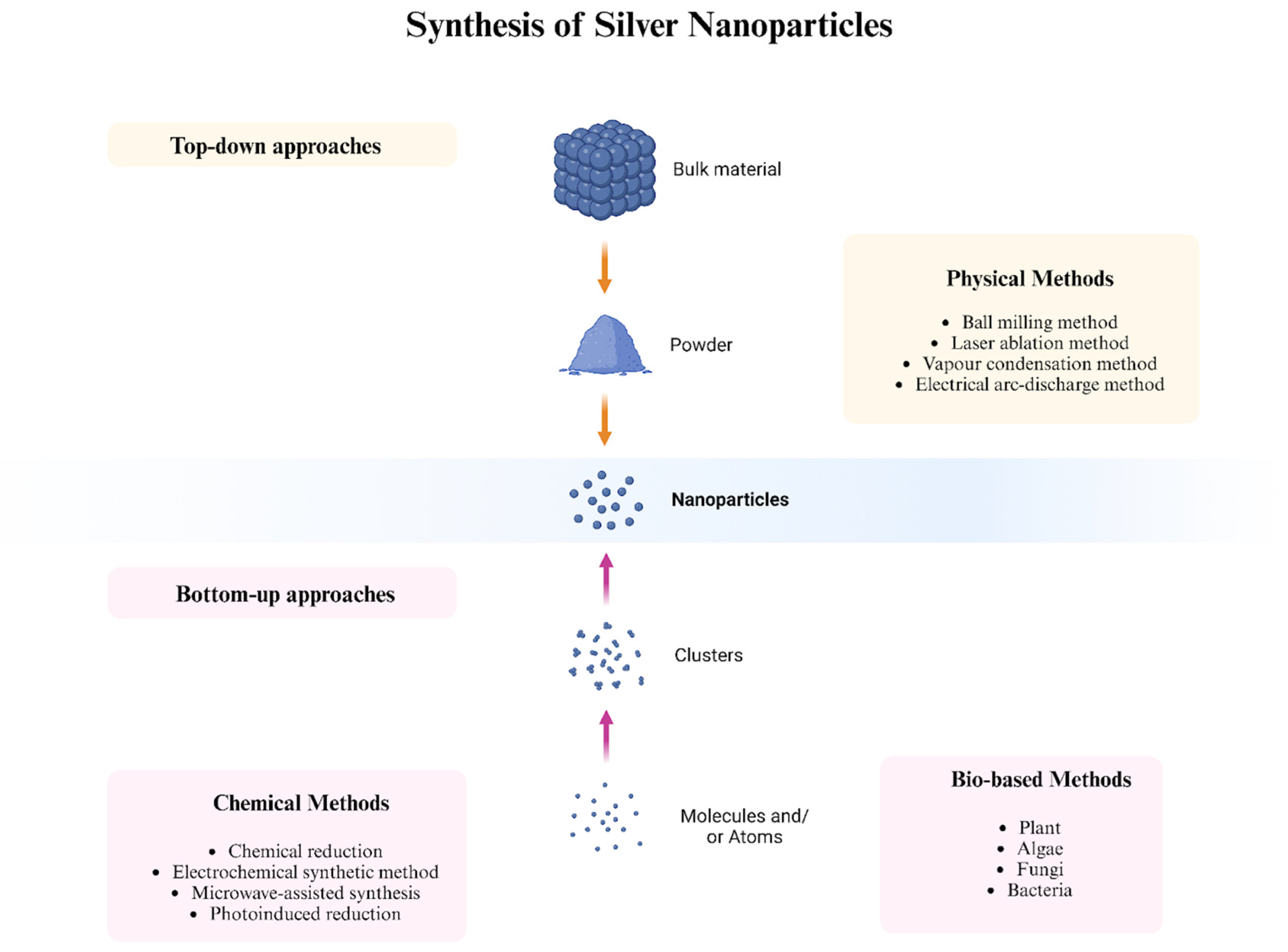
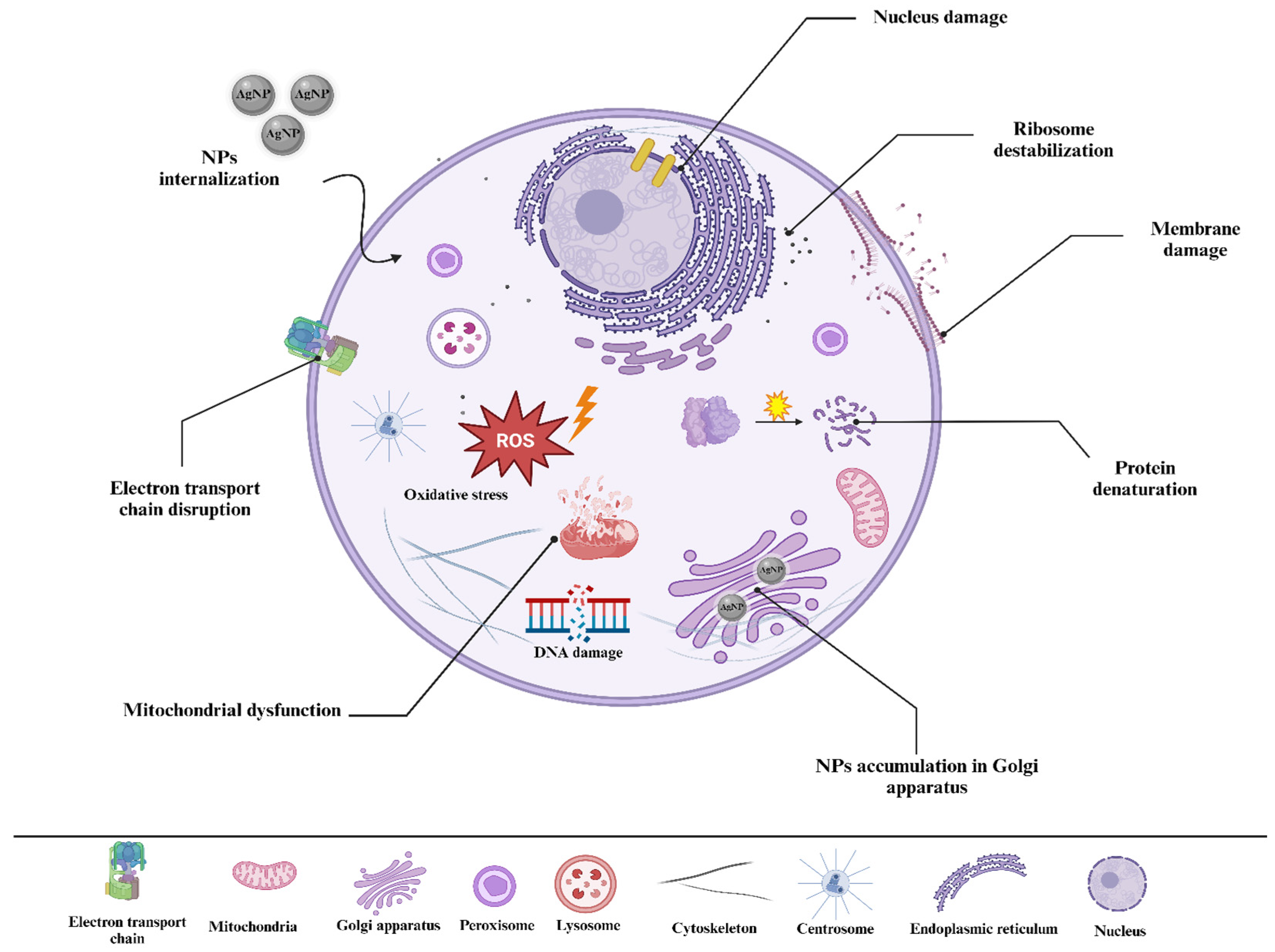
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duman, H.; Eker, F.; Akdaşçi, E.; Witkowska, A.M.; Bechelany, M.; Karav, S. Silver Nanoparticles: A Comprehensive Review of Synthesis Methods and Chemical and Physical Properties. Nanomaterials 2024, 14, 1527. https://doi.org/10.3390/nano14181527
Duman H, Eker F, Akdaşçi E, Witkowska AM, Bechelany M, Karav S. Silver Nanoparticles: A Comprehensive Review of Synthesis Methods and Chemical and Physical Properties. Nanomaterials. 2024; 14(18):1527. https://doi.org/10.3390/nano14181527
Chicago/Turabian StyleDuman, Hatice, Furkan Eker, Emir Akdaşçi, Anna Maria Witkowska, Mikhael Bechelany, and Sercan Karav. 2024. "Silver Nanoparticles: A Comprehensive Review of Synthesis Methods and Chemical and Physical Properties" Nanomaterials 14, no. 18: 1527. https://doi.org/10.3390/nano14181527
APA StyleDuman, H., Eker, F., Akdaşçi, E., Witkowska, A. M., Bechelany, M., & Karav, S. (2024). Silver Nanoparticles: A Comprehensive Review of Synthesis Methods and Chemical and Physical Properties. Nanomaterials, 14(18), 1527. https://doi.org/10.3390/nano14181527










