Recent Progress on Layered Sn and Pb-Based Mono Chalcogenides: Synthesis, Structure, Optical, and Thermoelectric Properties and Related Applications
Abstract
1. Introduction
2. Materials and Methods
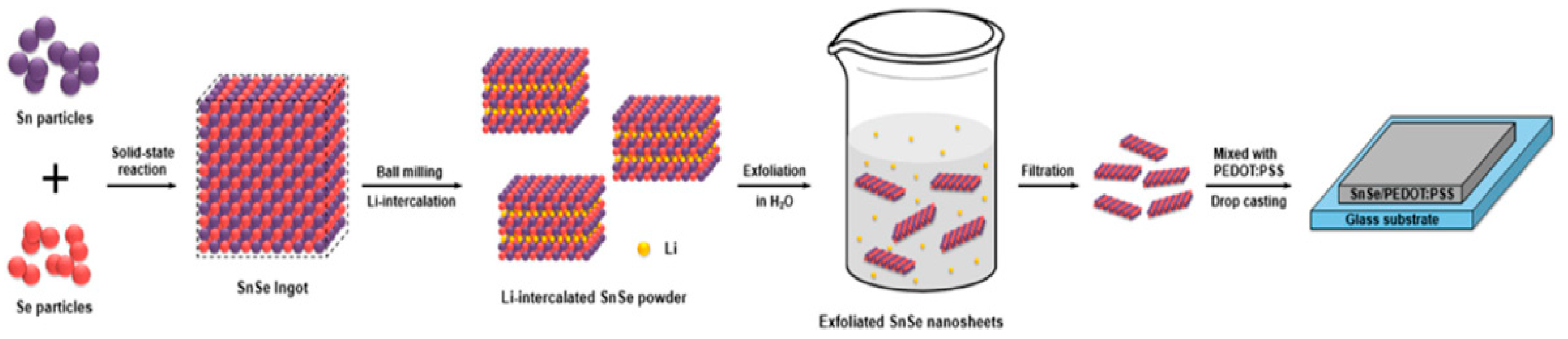
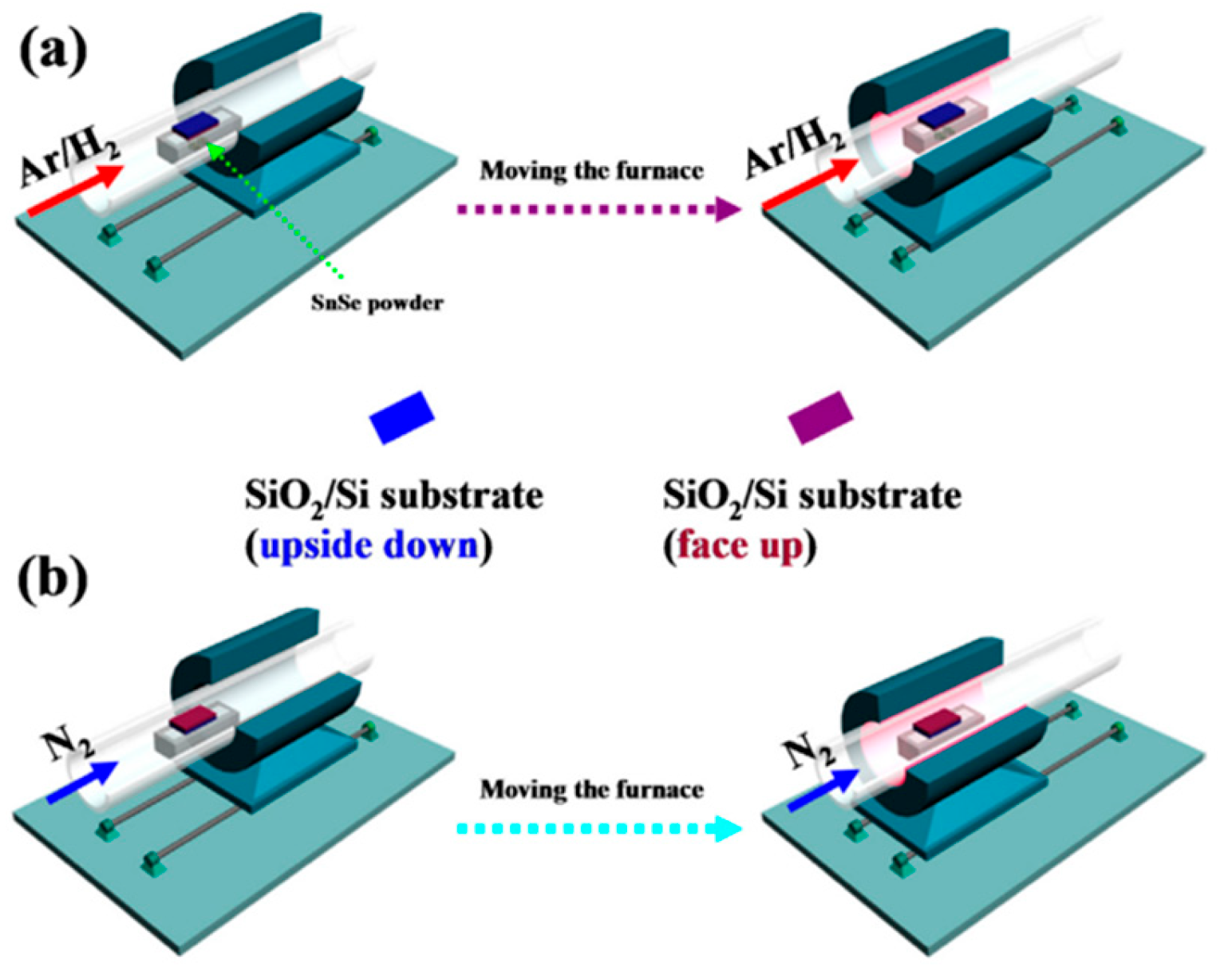
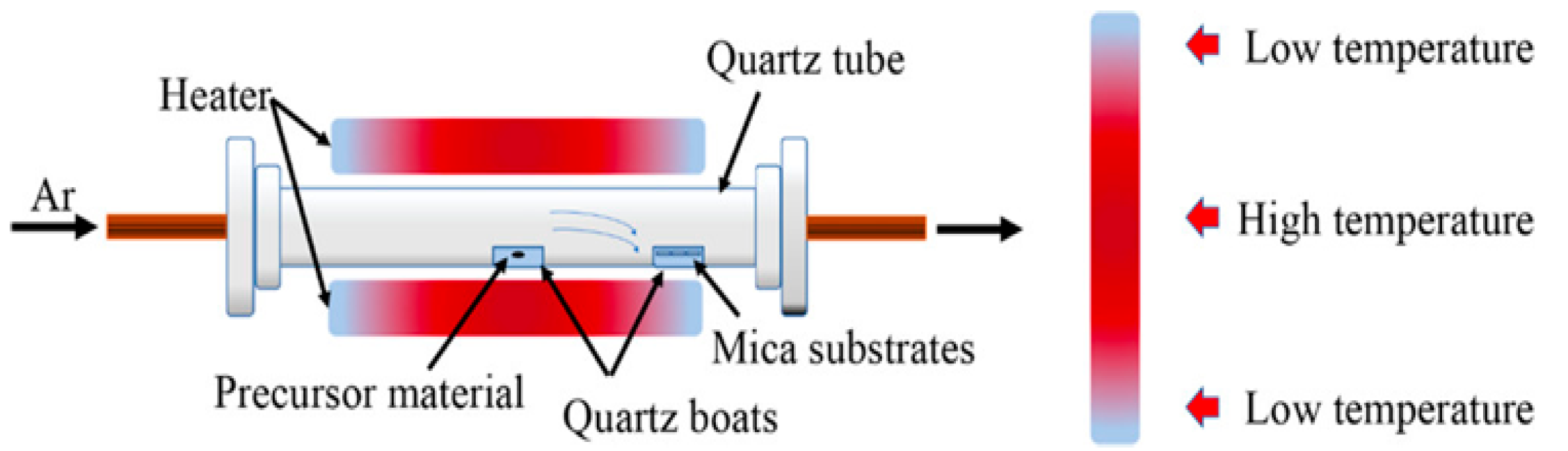
2.1. Synthesis of 2D SnSe
2.2. Synthesis of 2D SnS
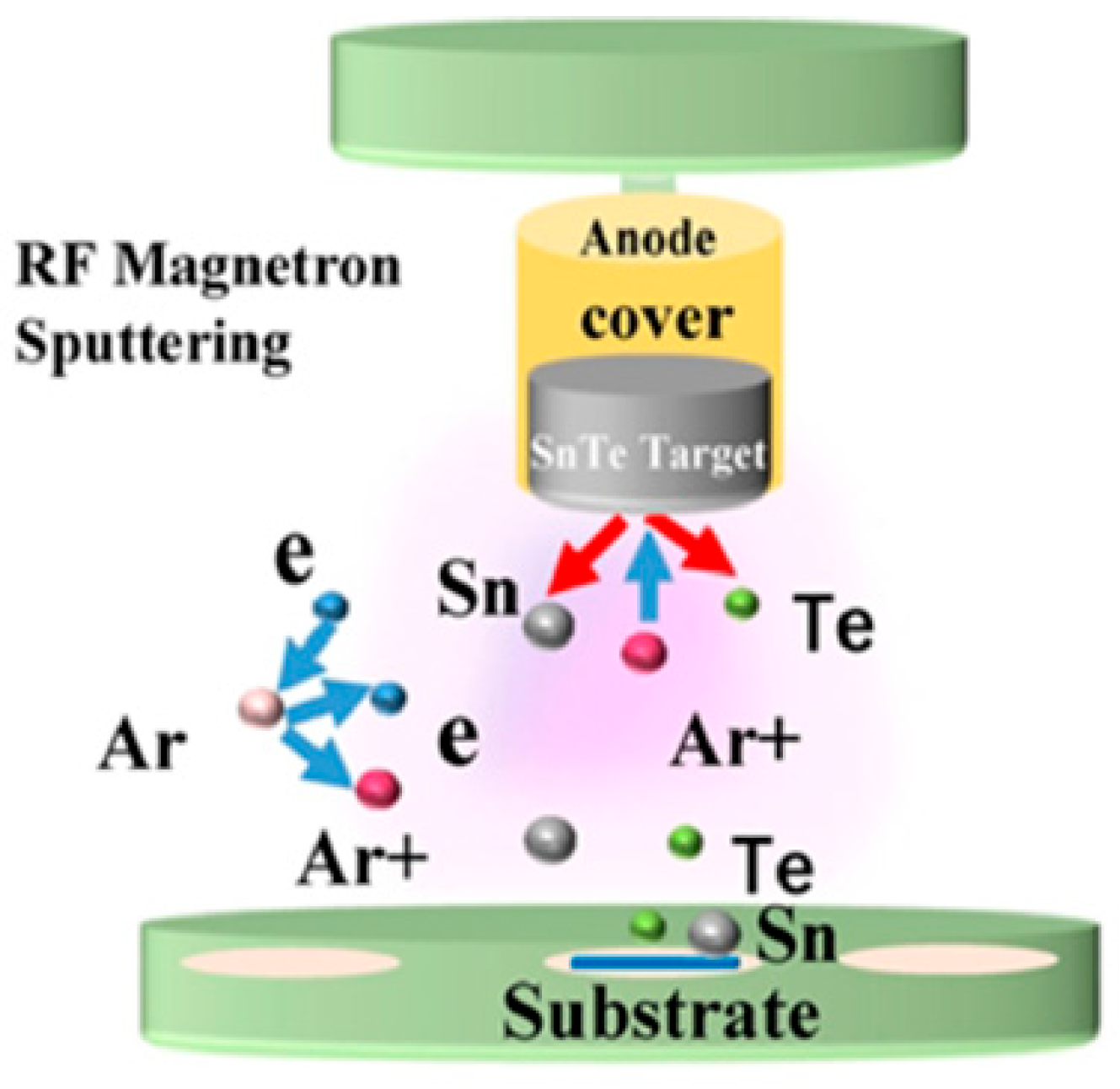
2.3. Synthesis of 2D SnTe
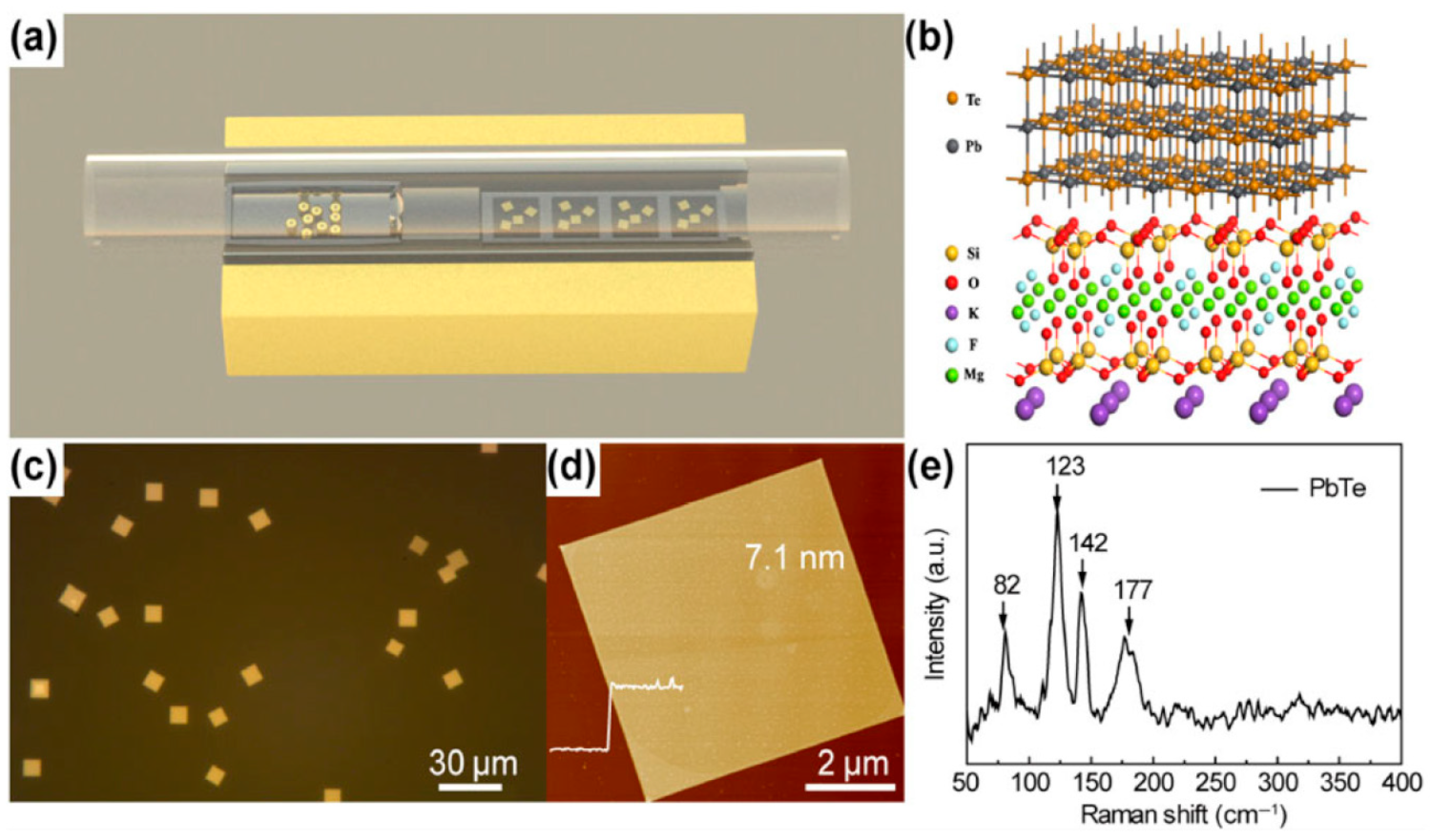
2.4. Synthesis of 2D PbTe
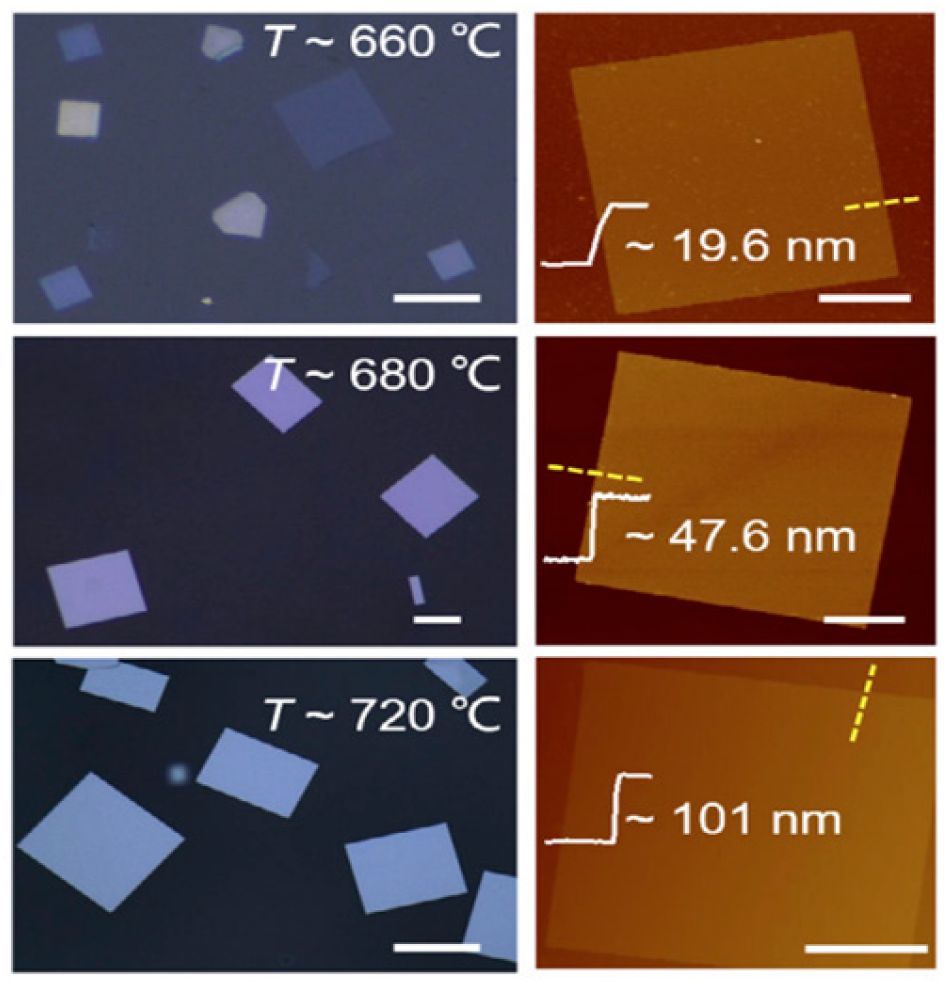
2.5. Synthesis of 2D PbSe
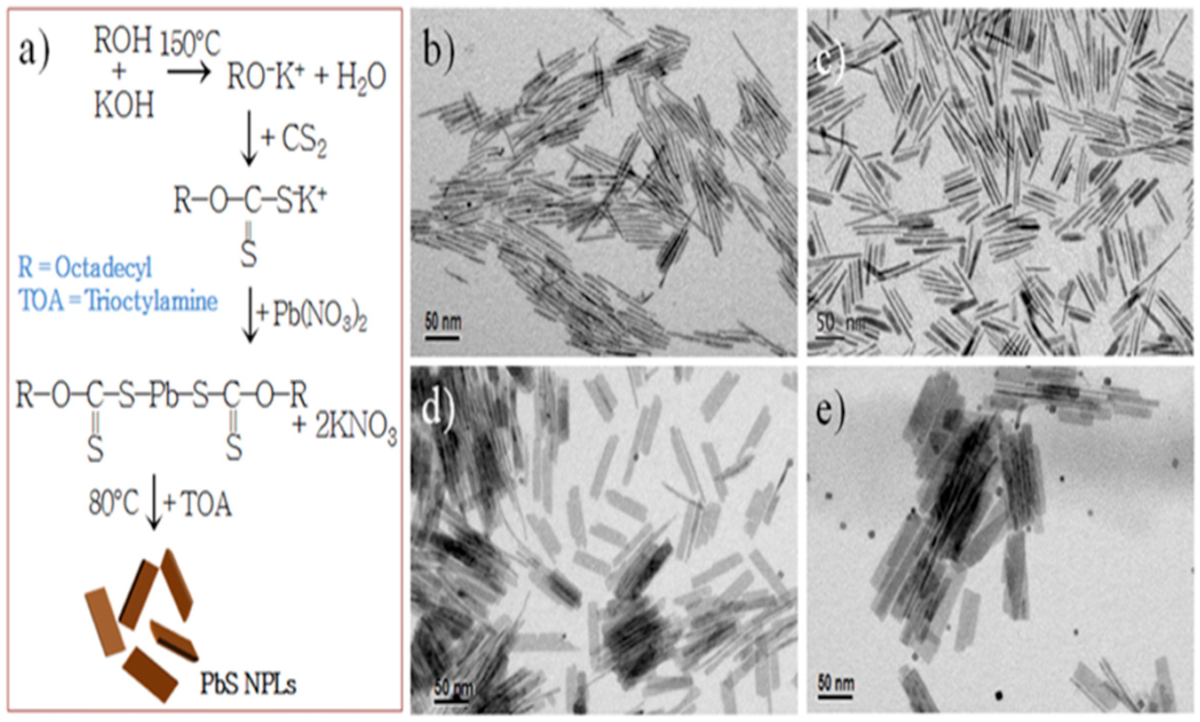
2.6. Synthesis of 2D PbS
3. Structural Properties
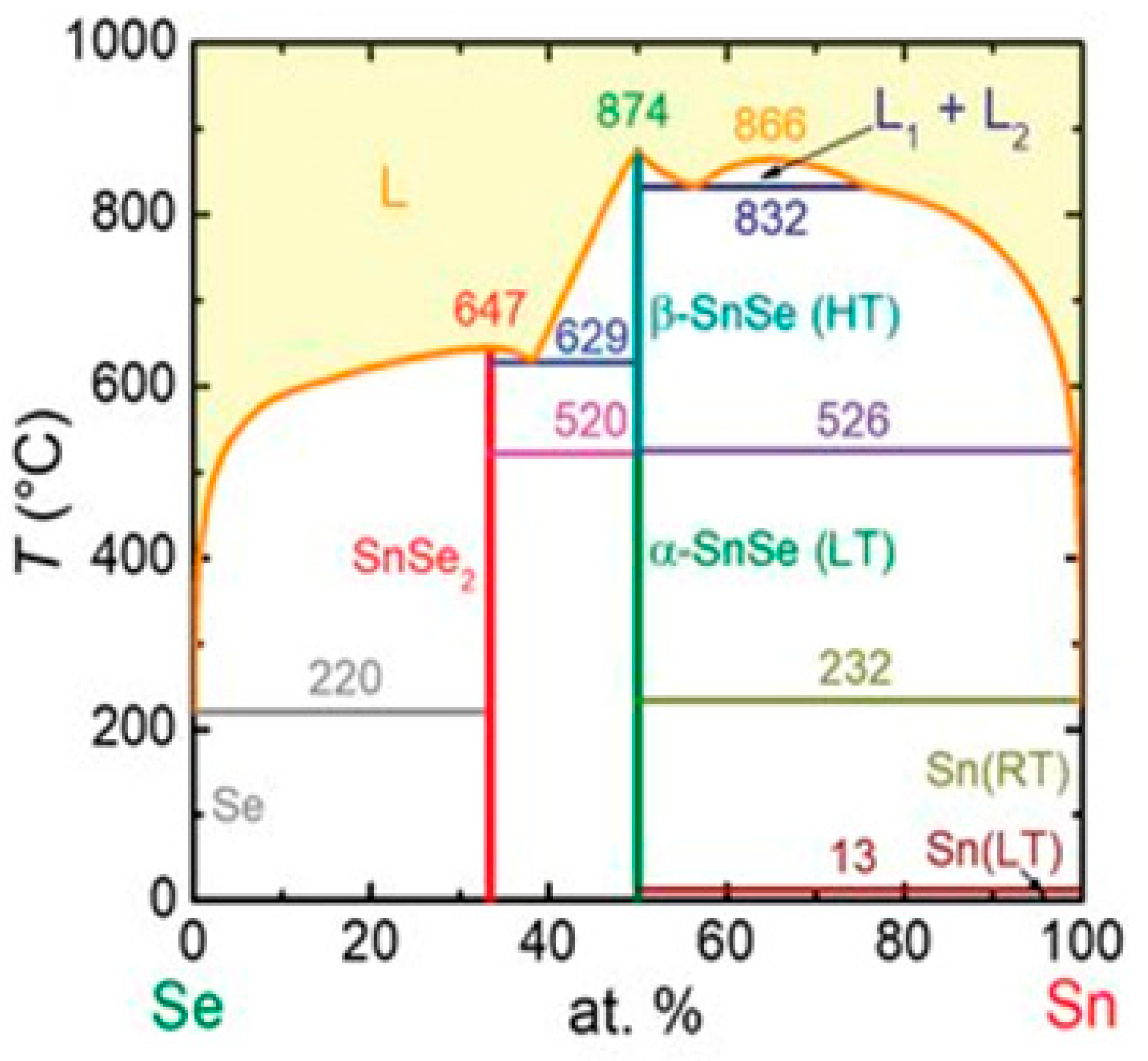
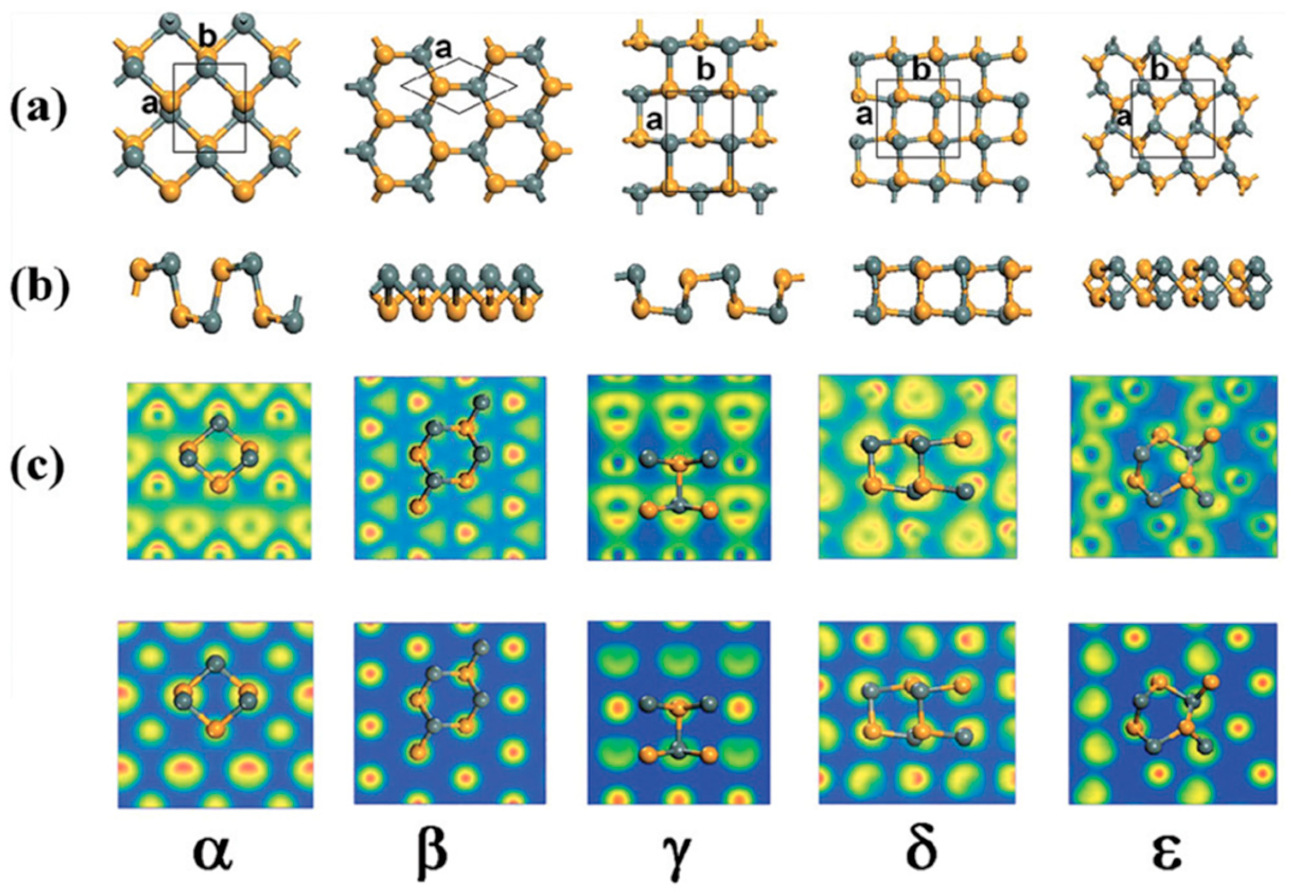
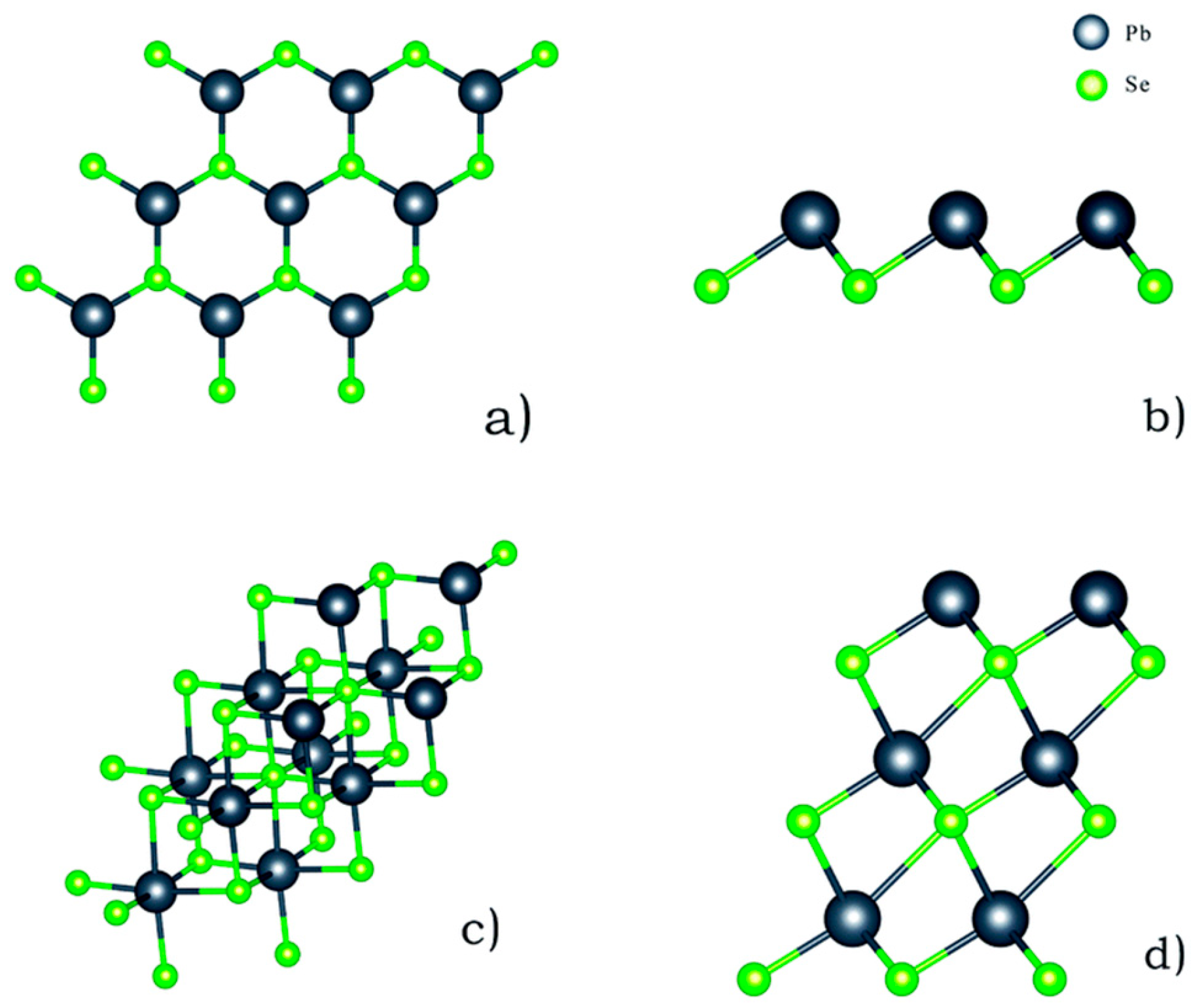
3.1. SnSe Structure
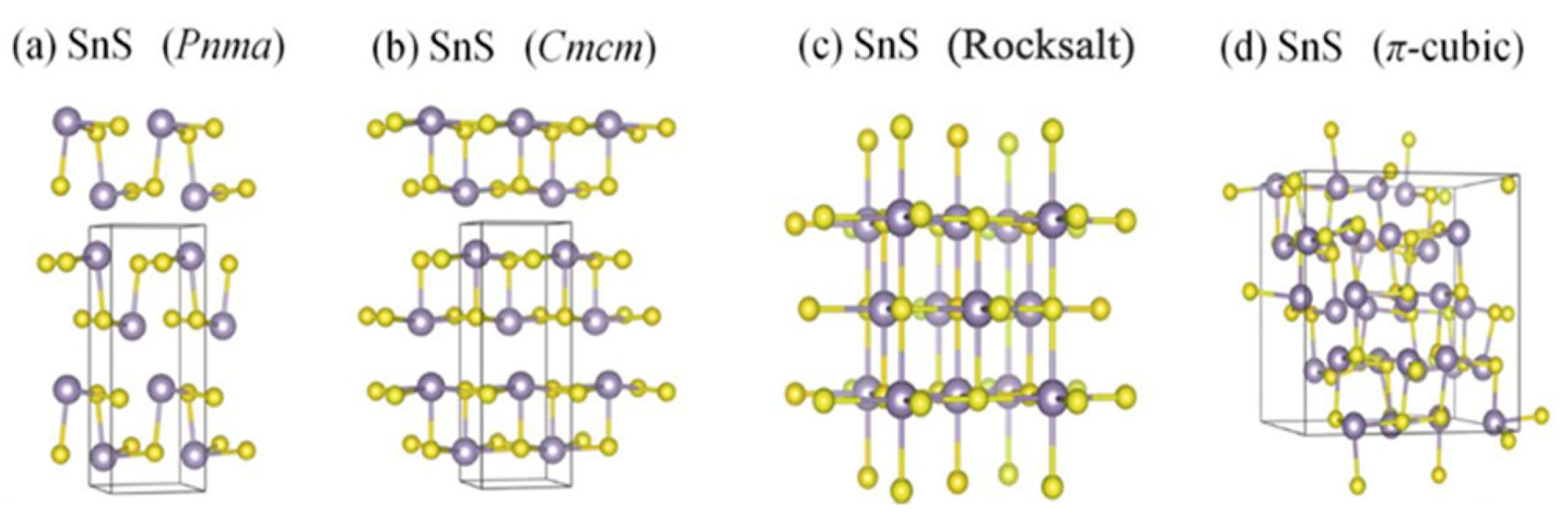
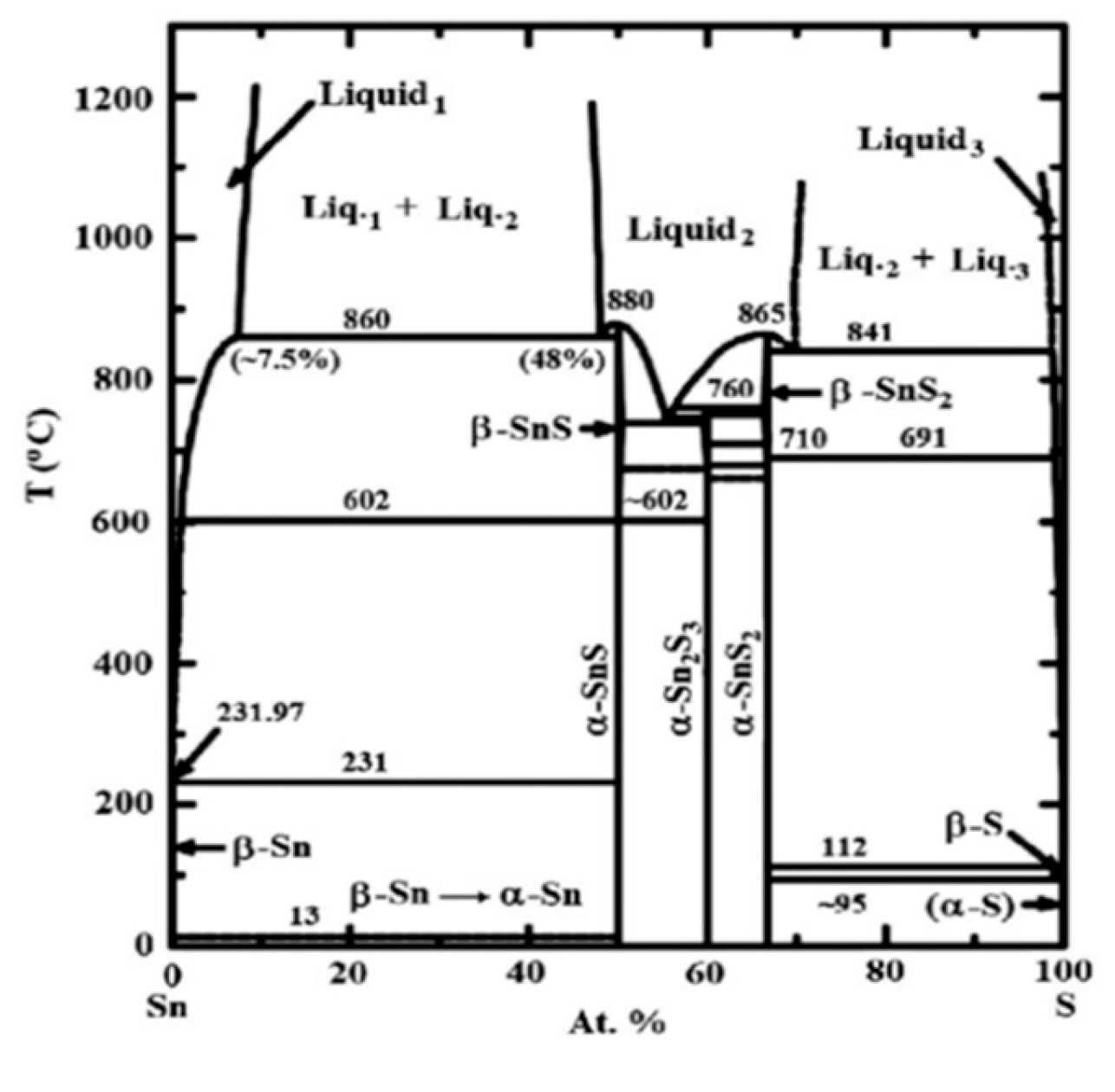
3.2. SnS Structure
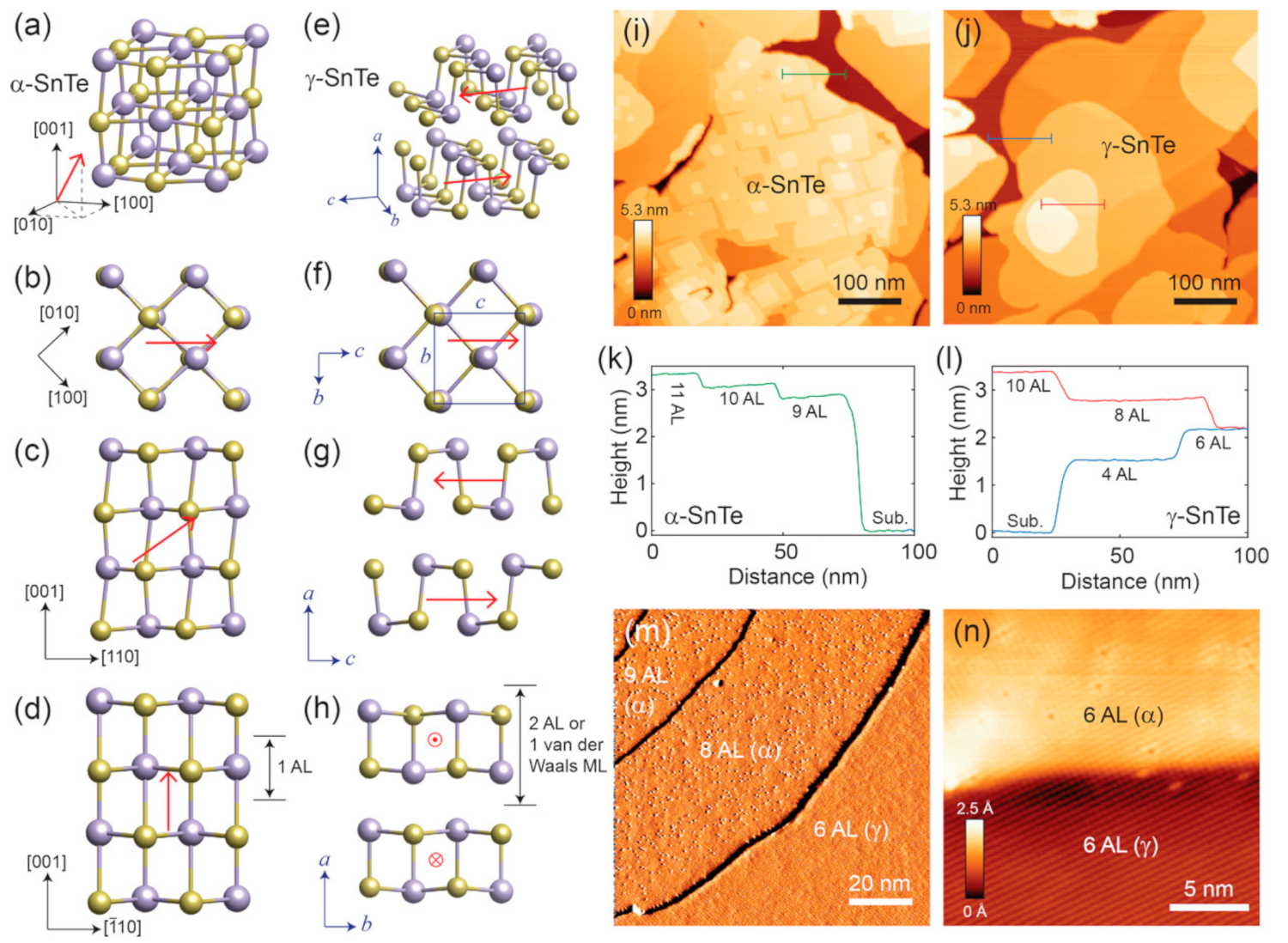
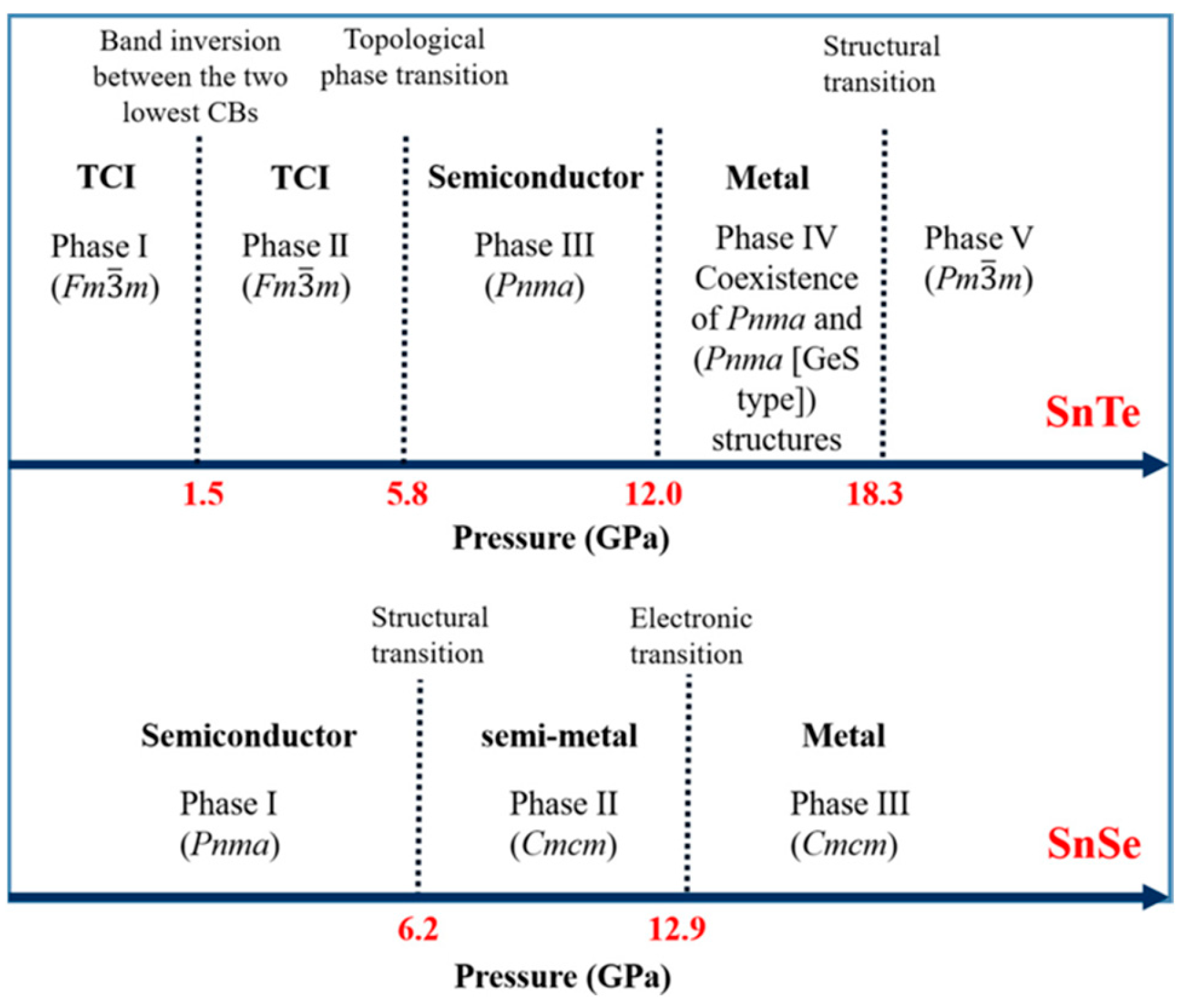
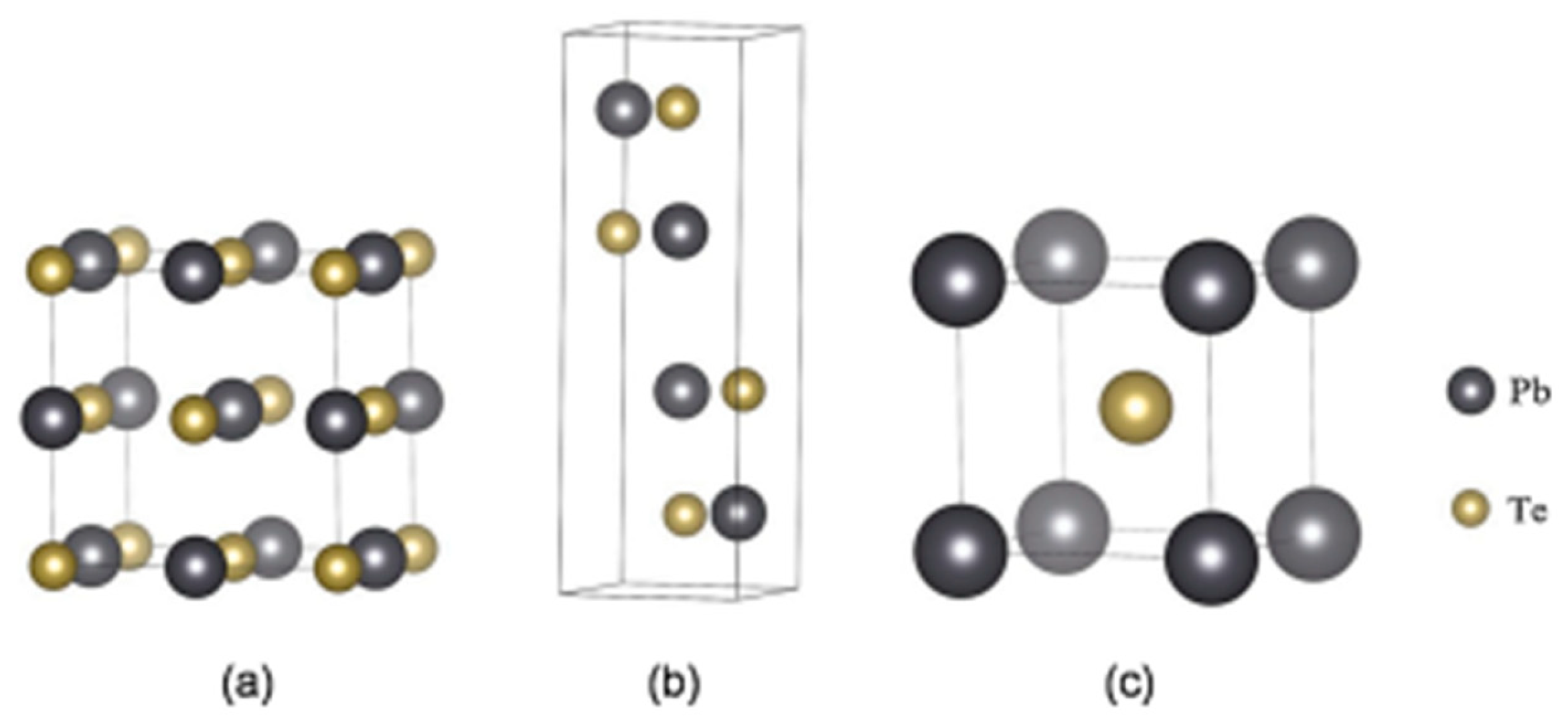
3.3. SnTe Structure
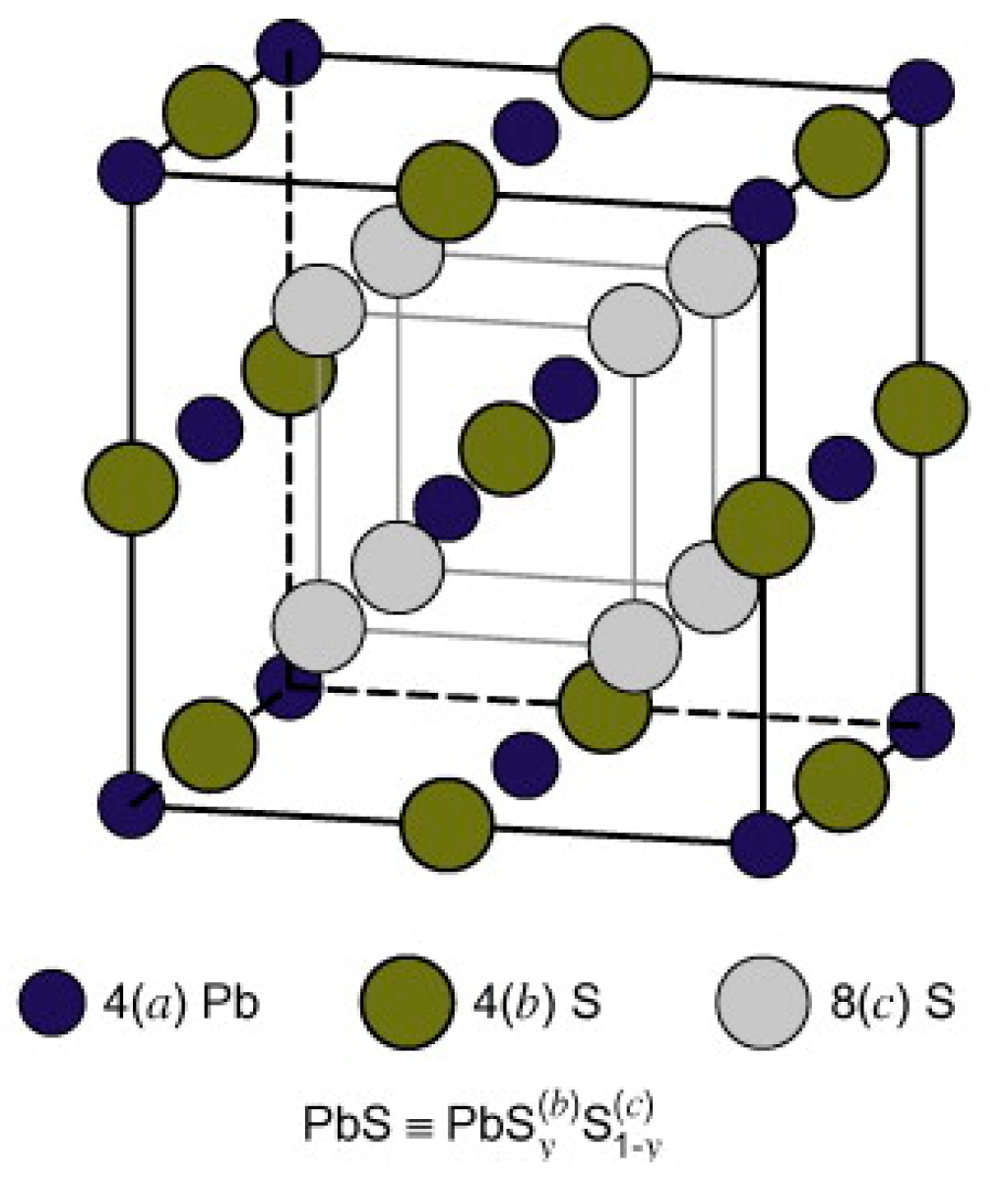
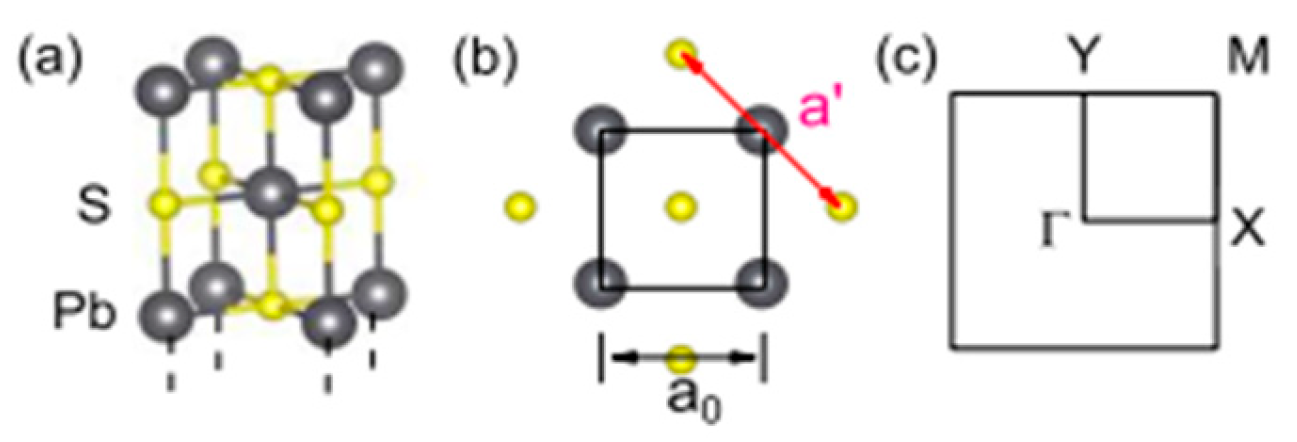
3.4. PbS Structure
3.5. PbSe Structure
3.6. PbTe Structure
4. TE Properties
4.1. SnS TE Properties
4.2. SnTe TE Properties
4.3. SnSe TE Properties
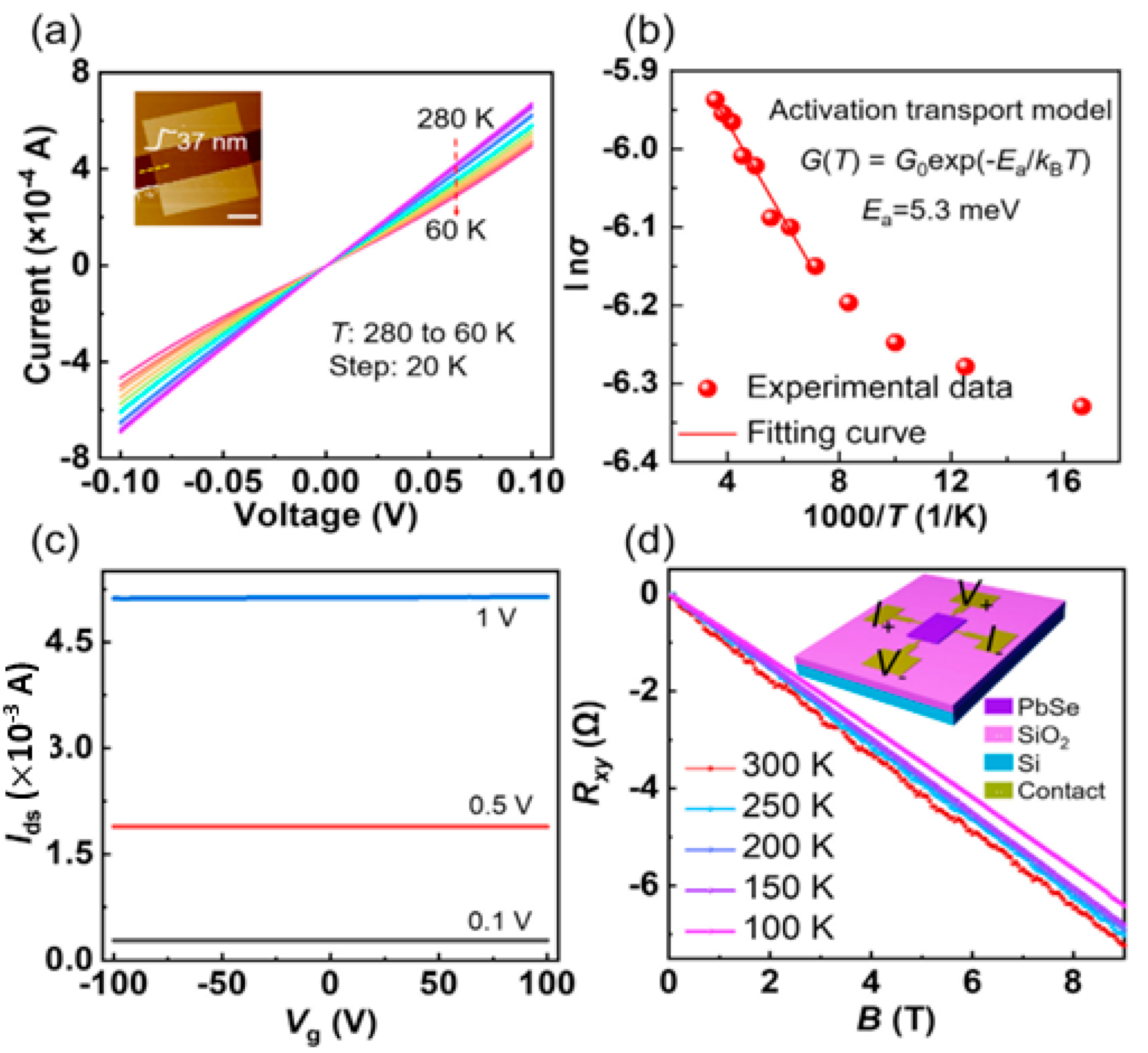
4.4. PbS TE Properties
4.5. PbSe TE Properties
4.6. PbTe TE Properties
5. Optical Properties
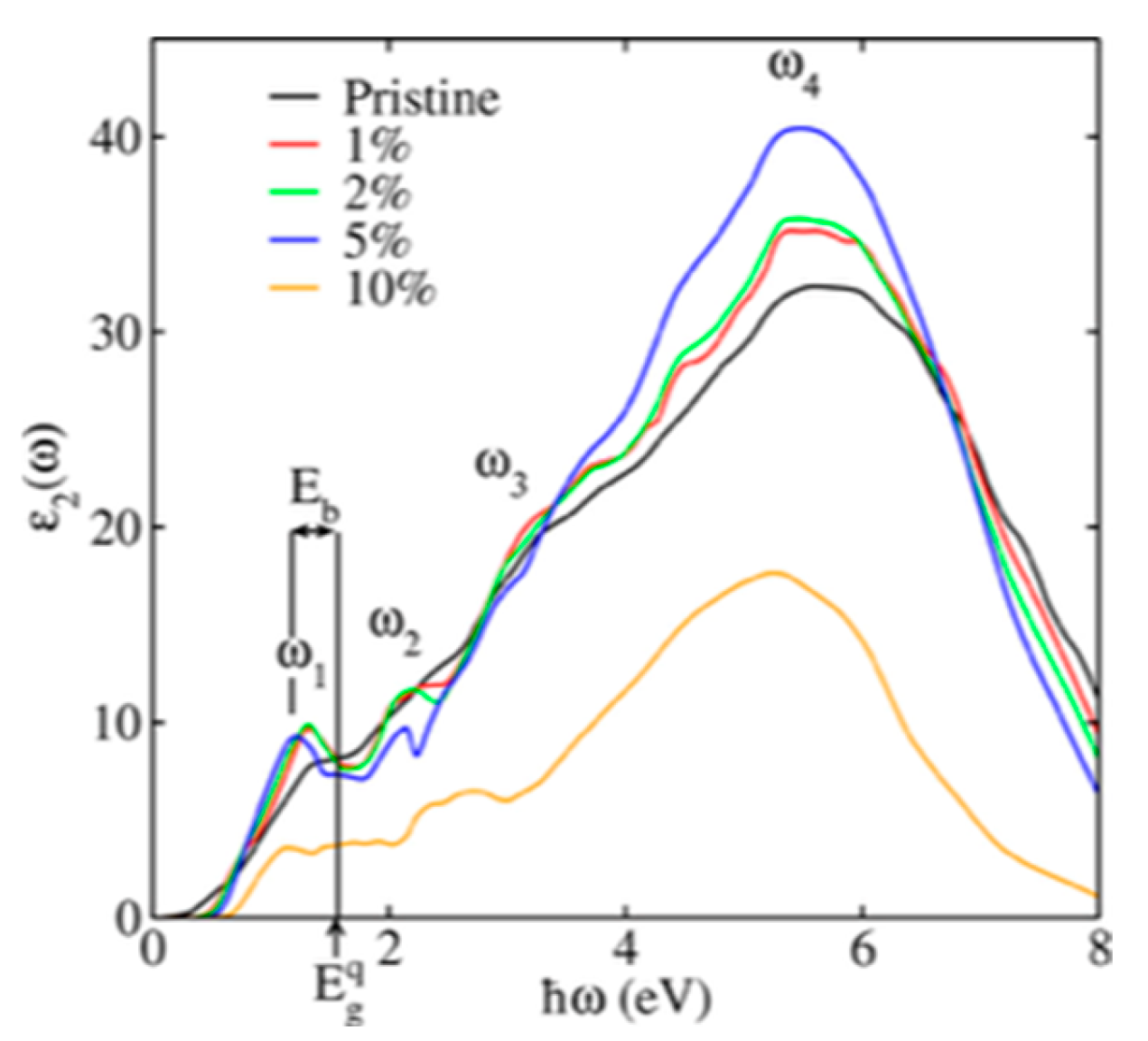
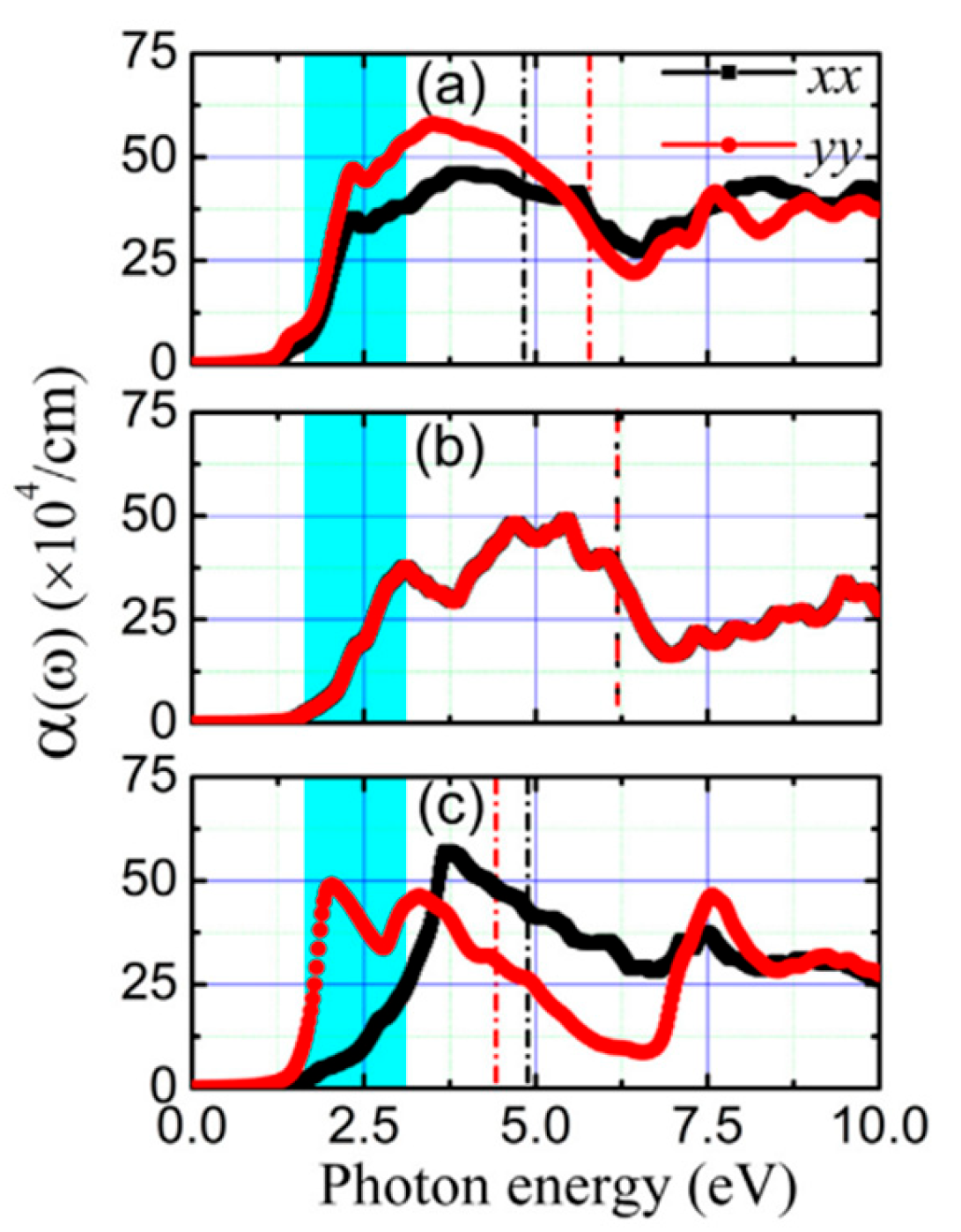
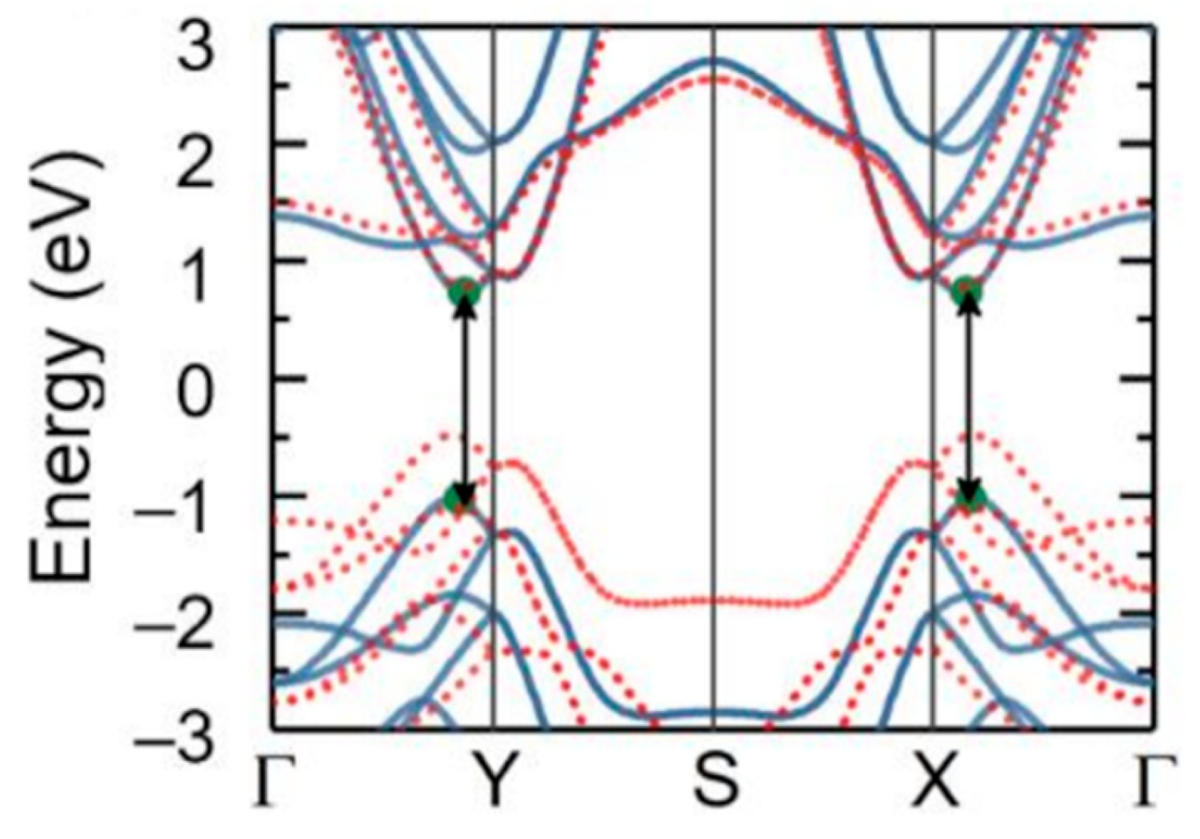
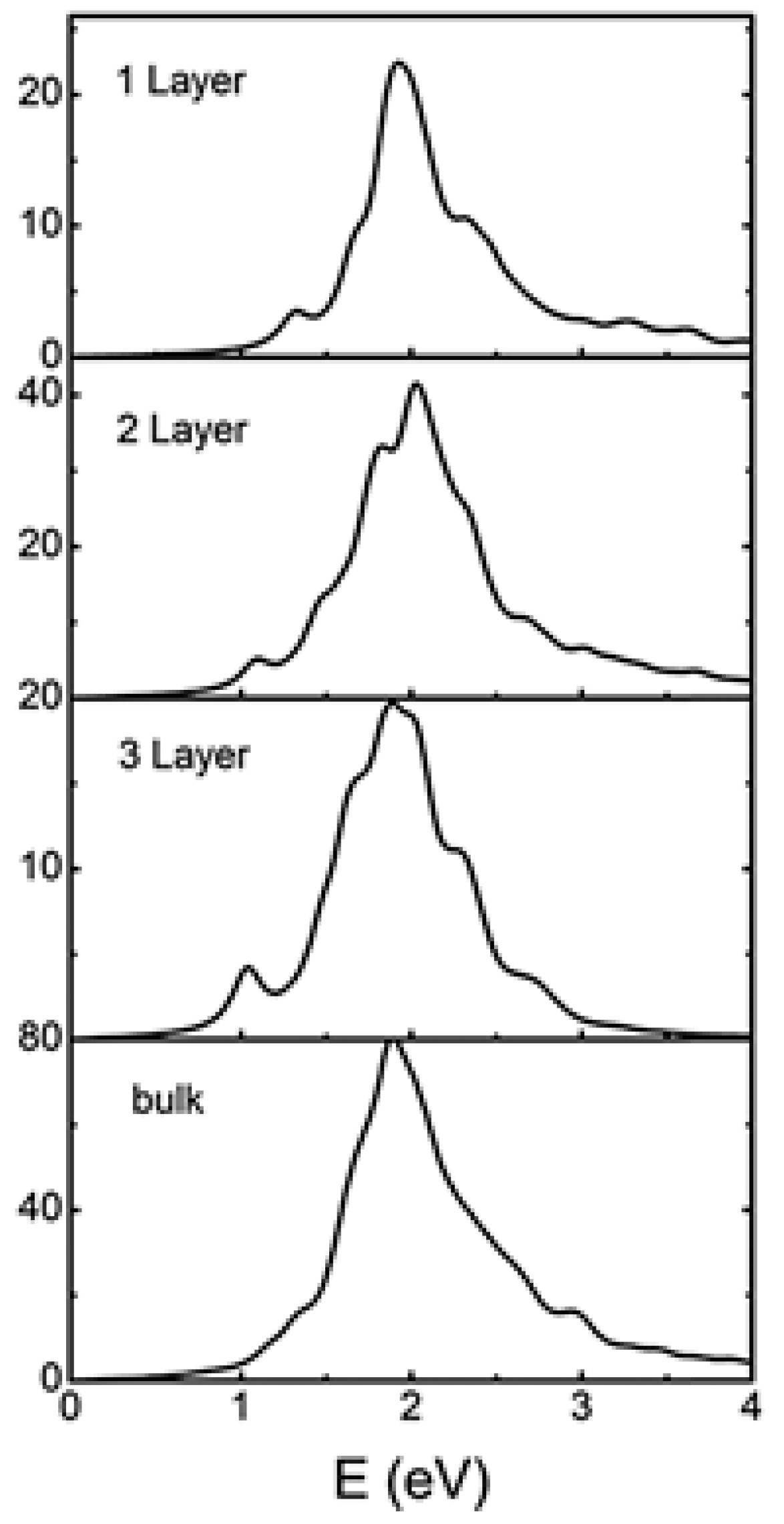
5.1. SnSe Optical Properties
5.2. SnS Optical Properties
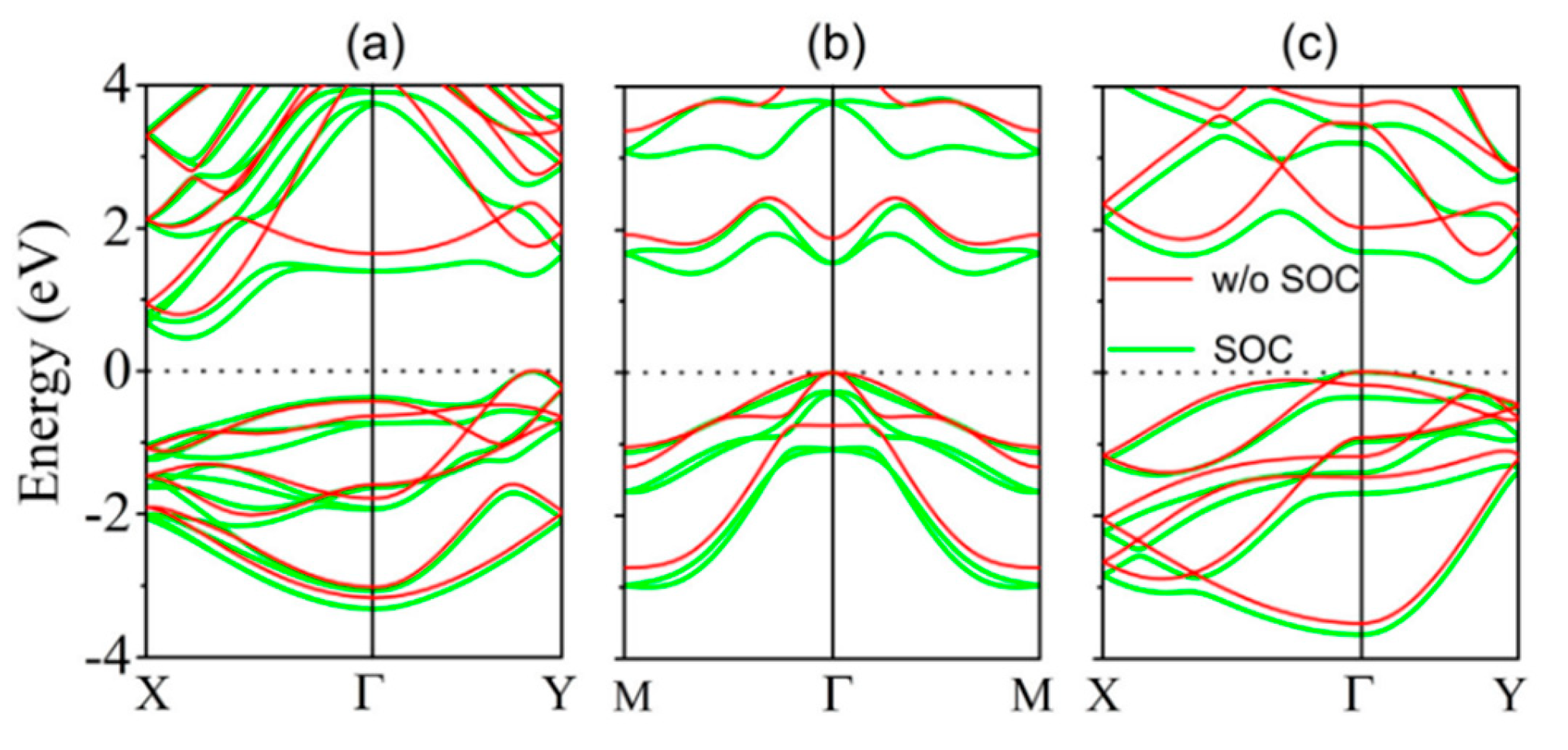
5.3. SnTe Optical Properties
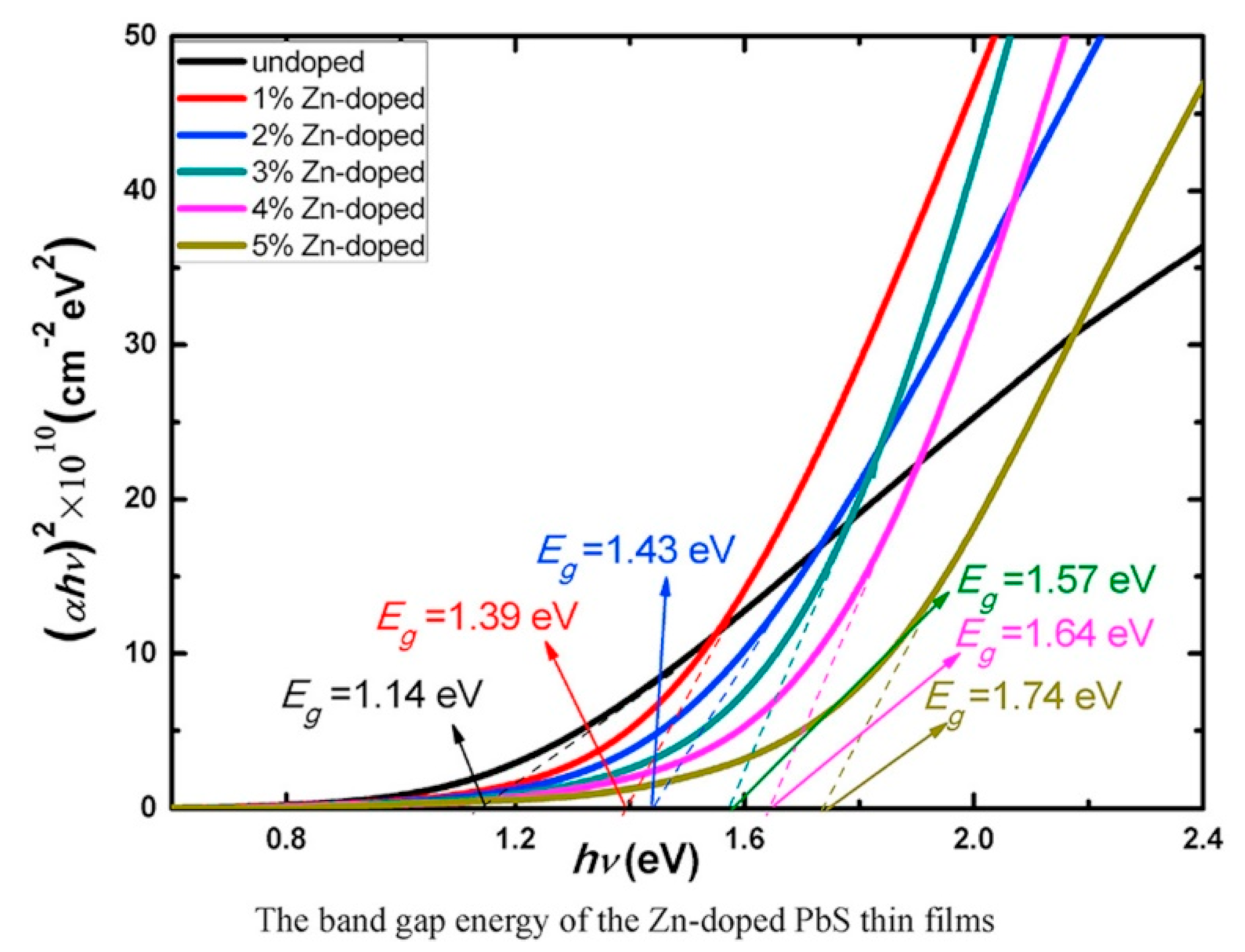
5.4. PbS Optical Properties
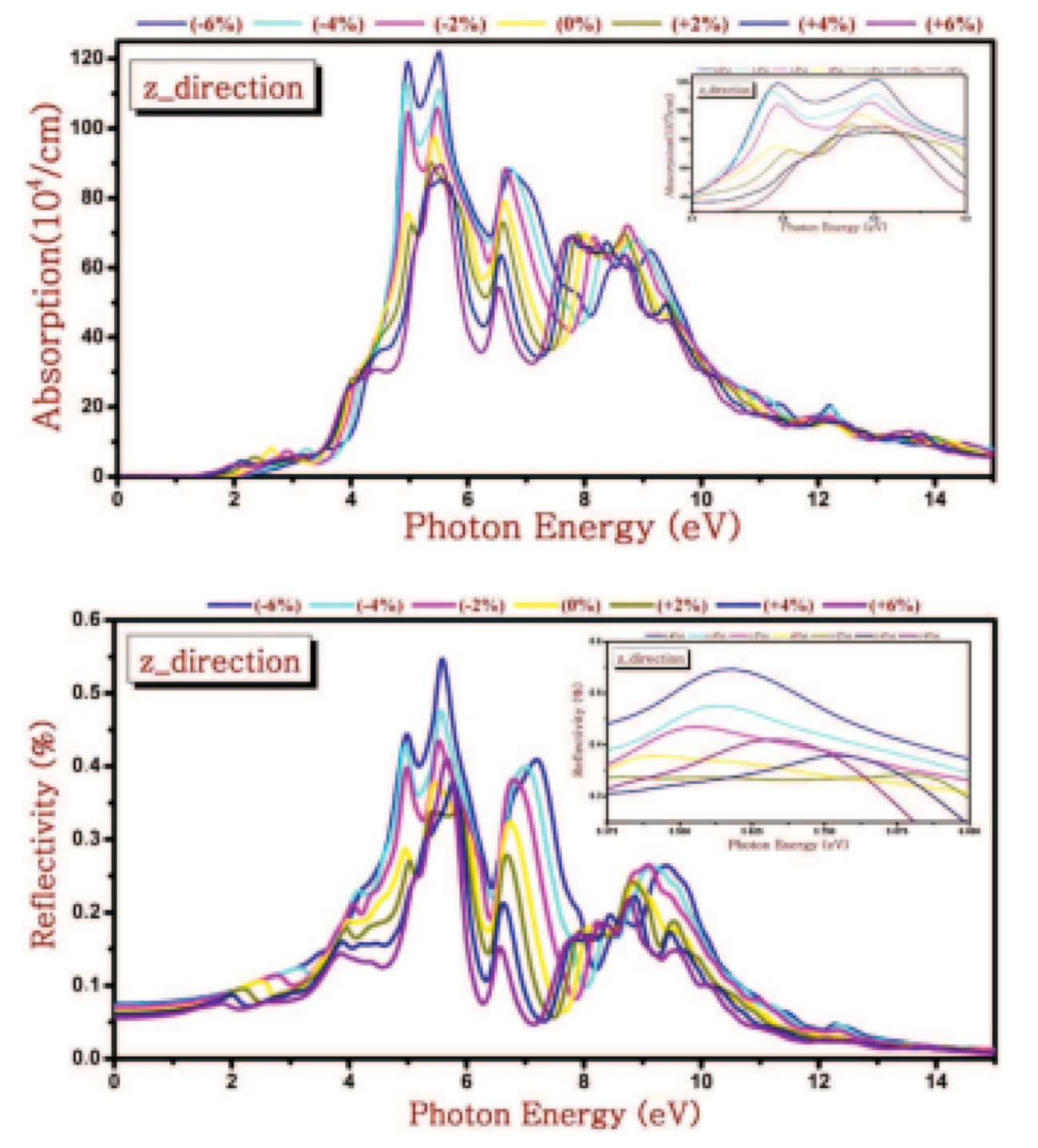
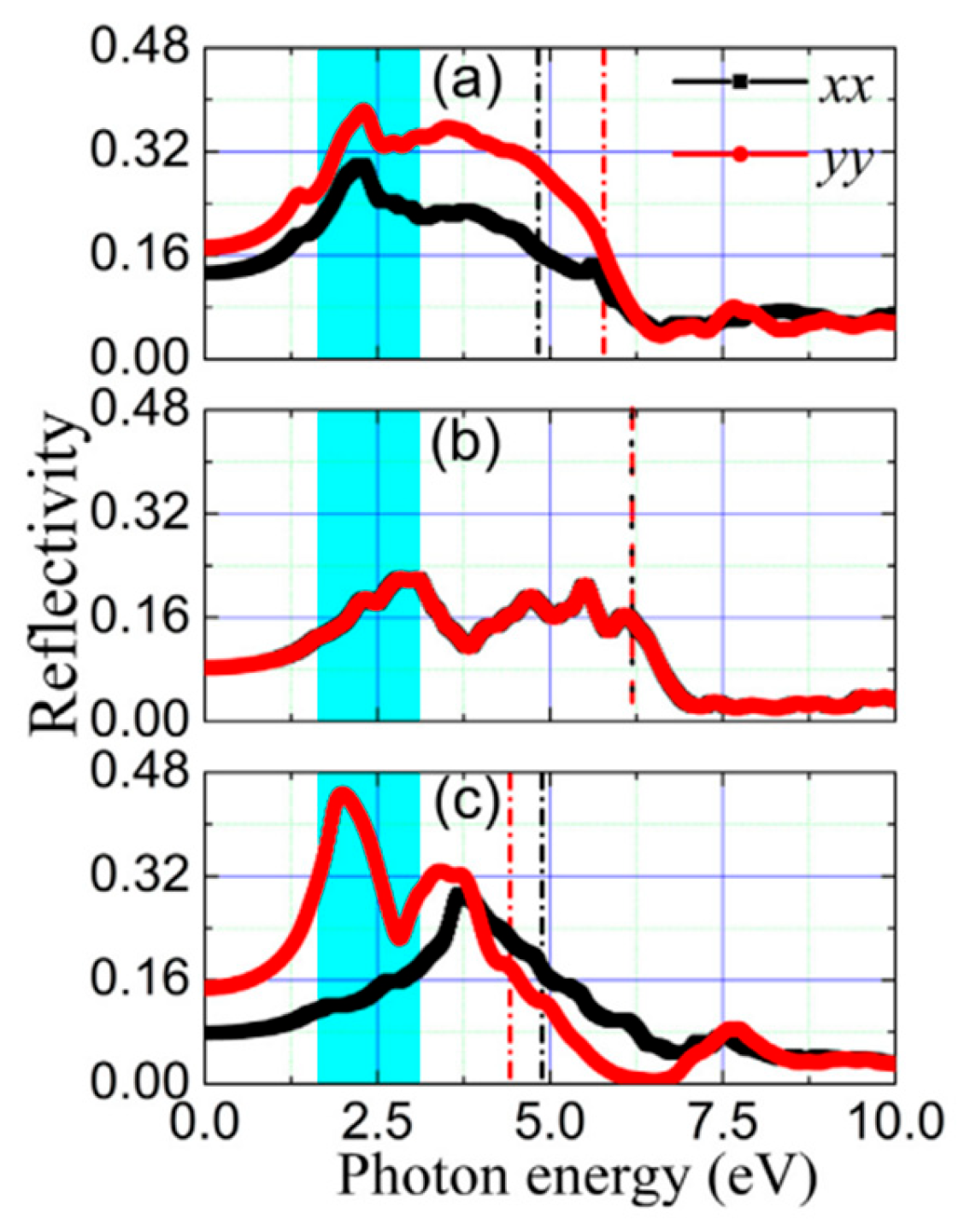
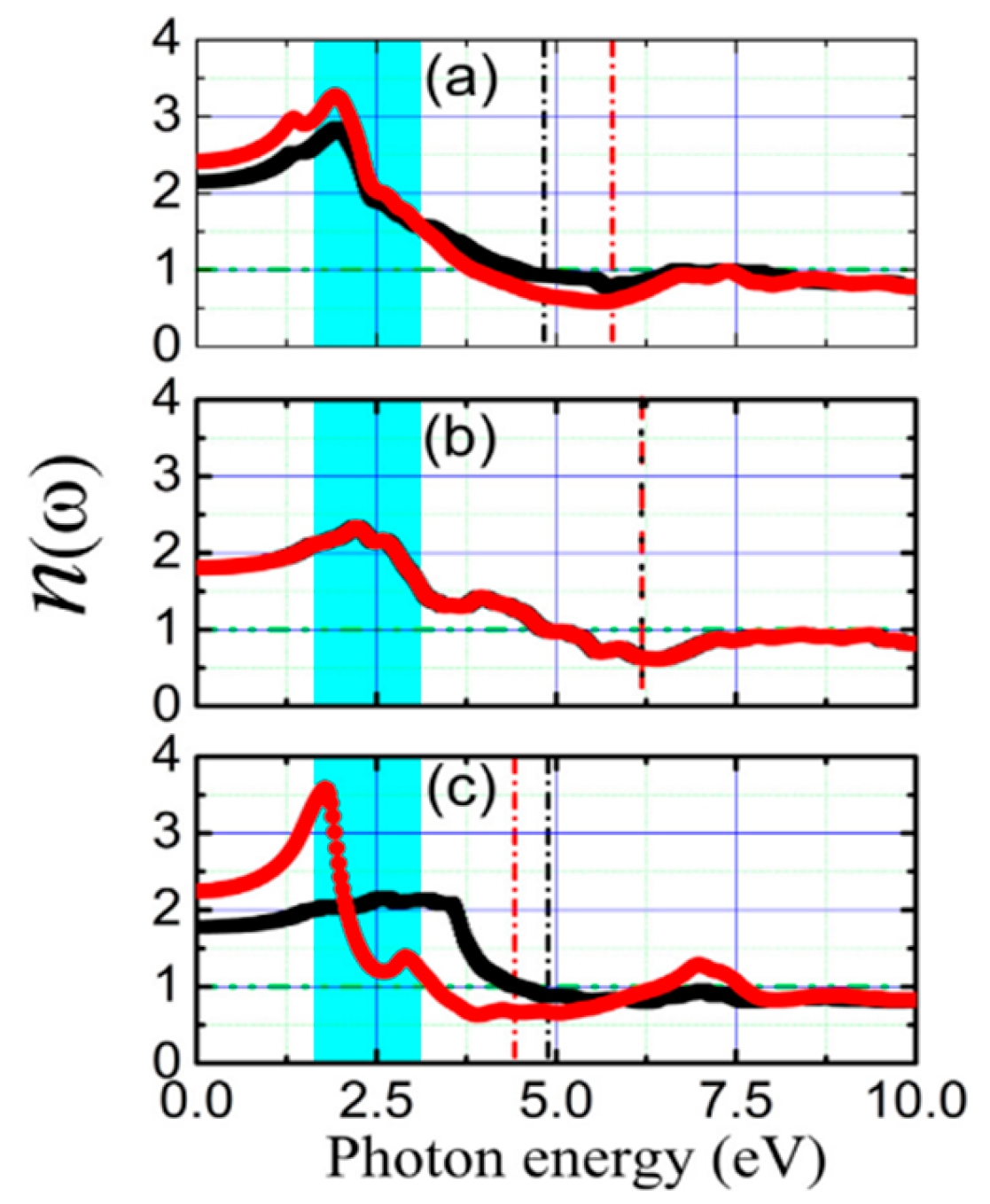
5.5. PbSe Optical Properties
5.6. PbTe Optical Properties
6. Applications
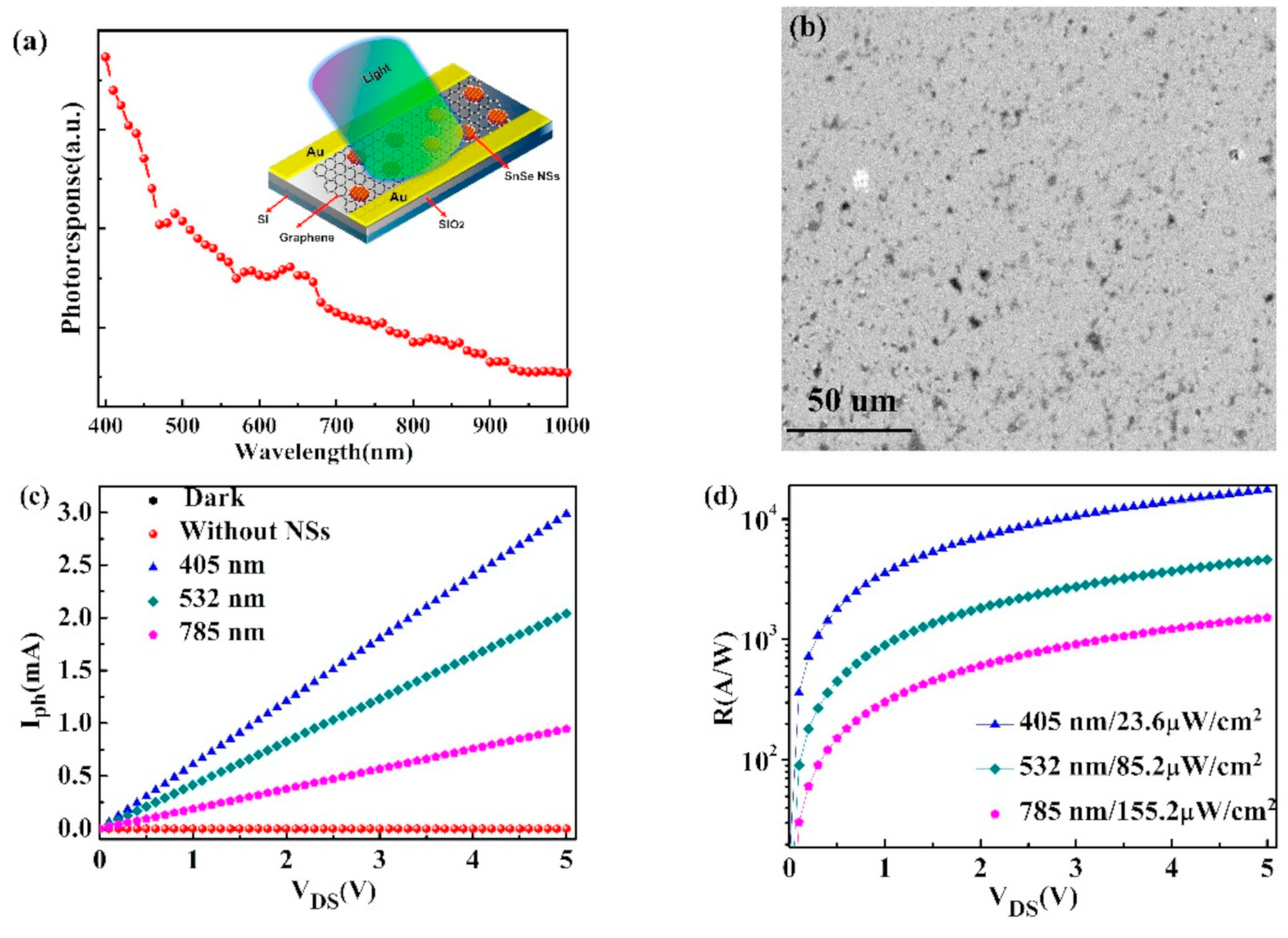
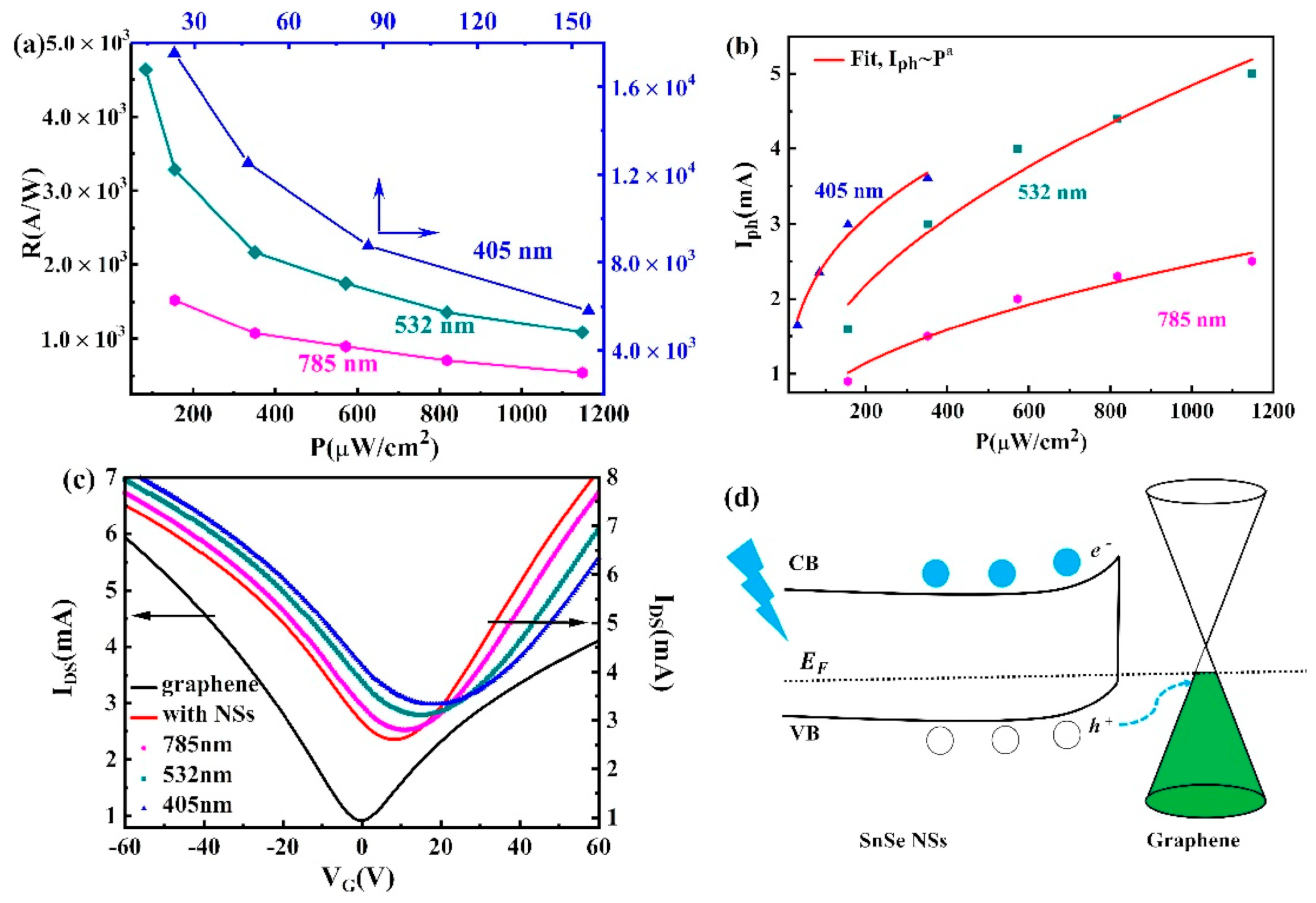
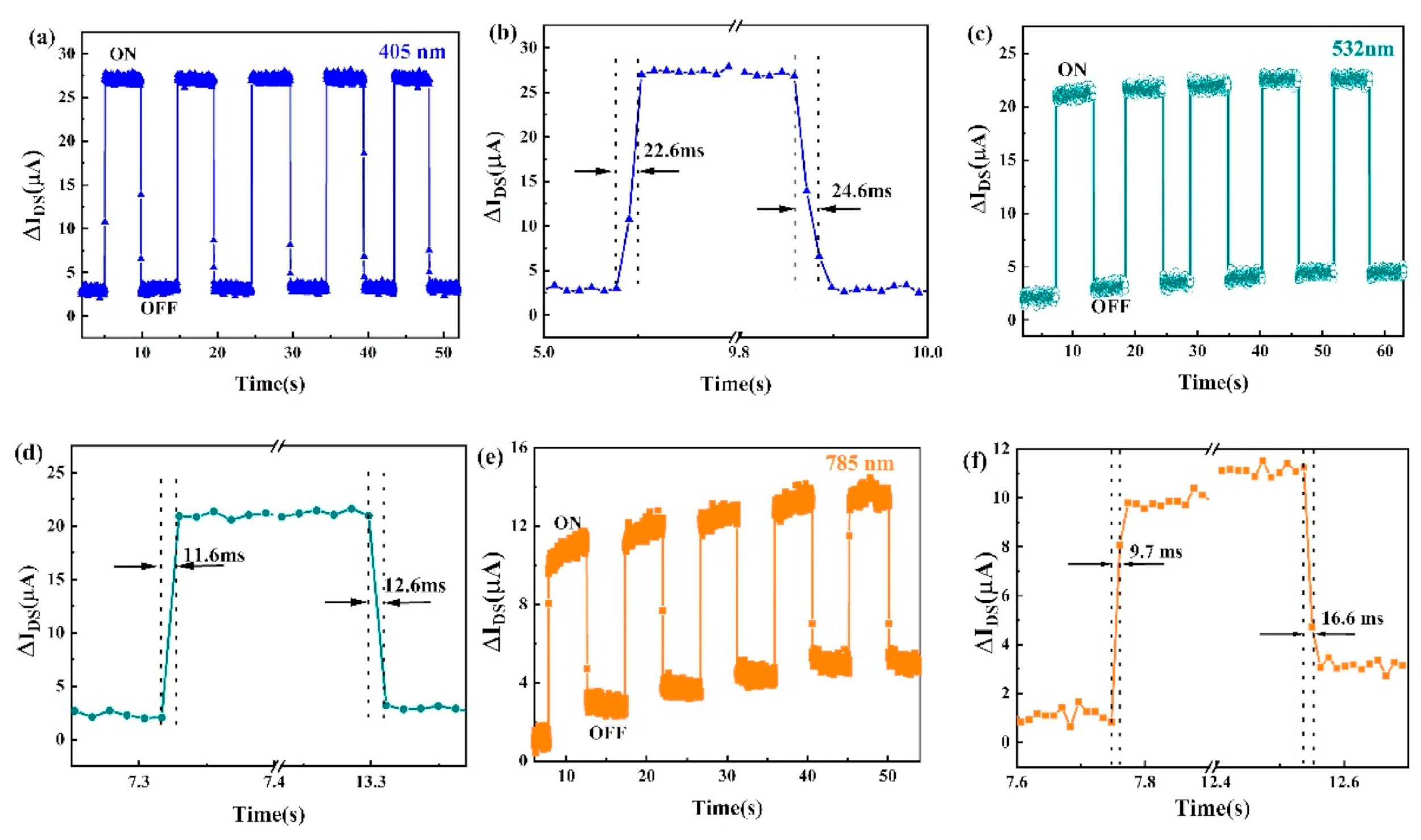
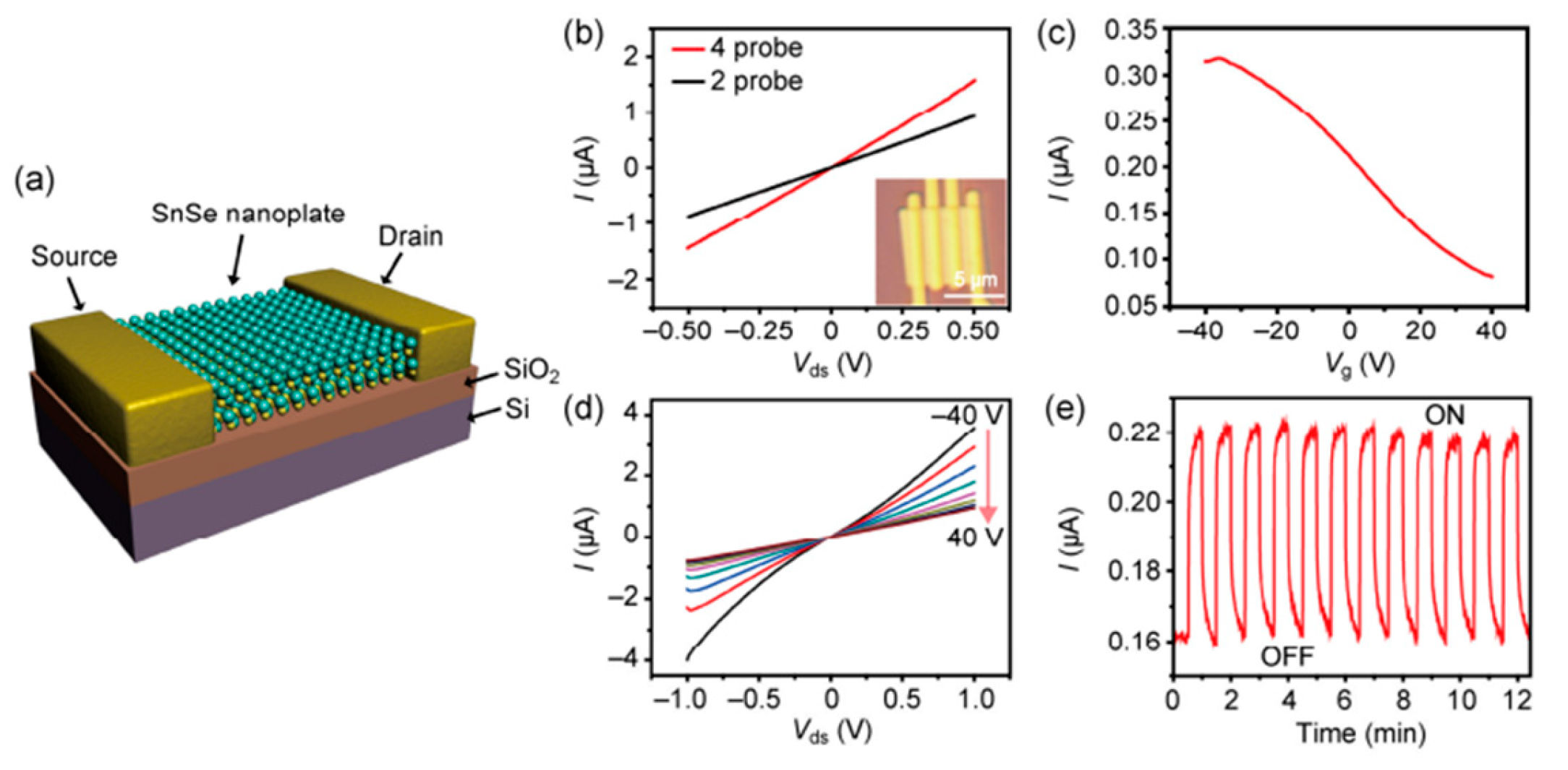
6.1. SnSe Applications
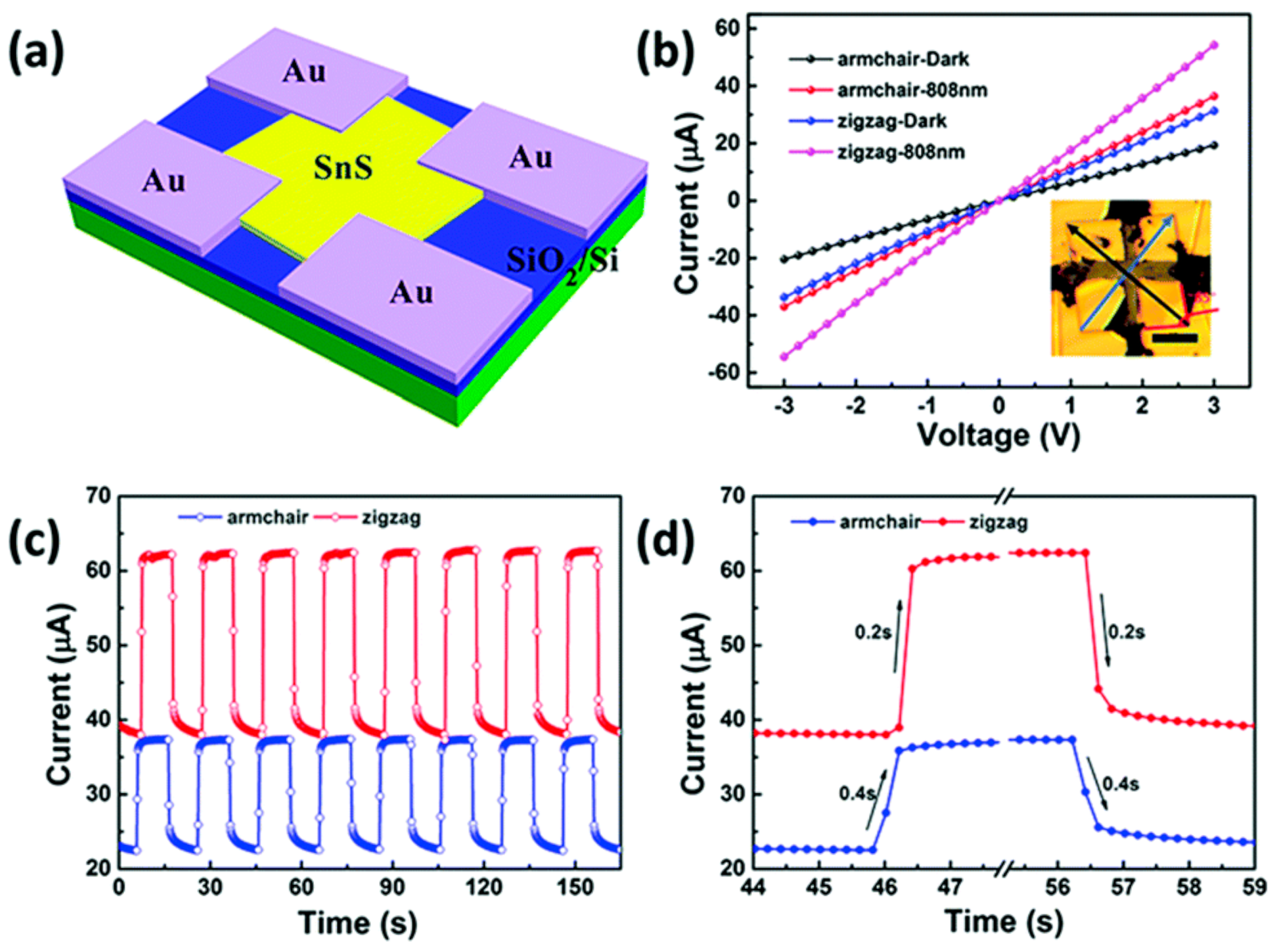
6.2. SnS Applications
6.3. SnTe Applications
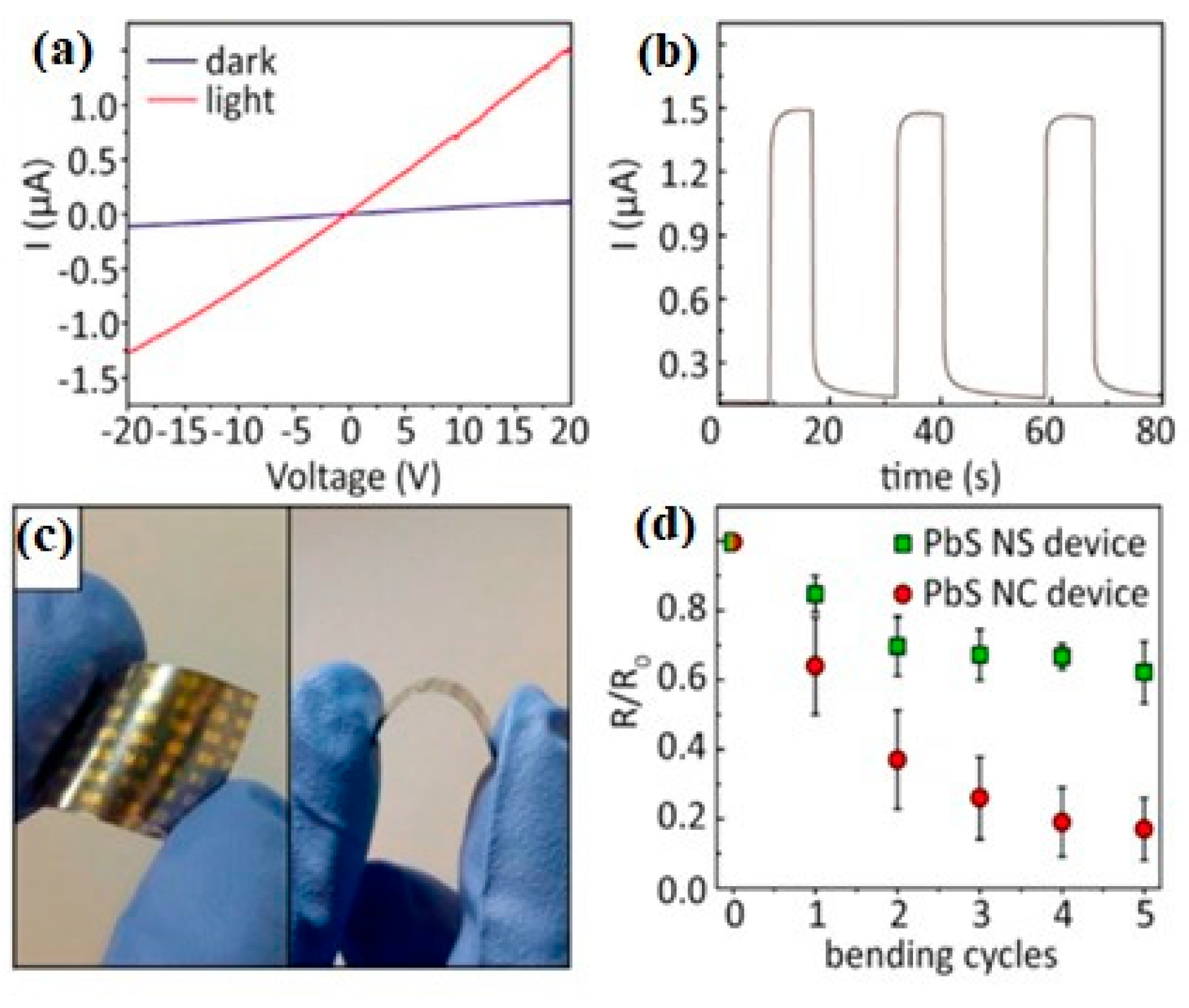
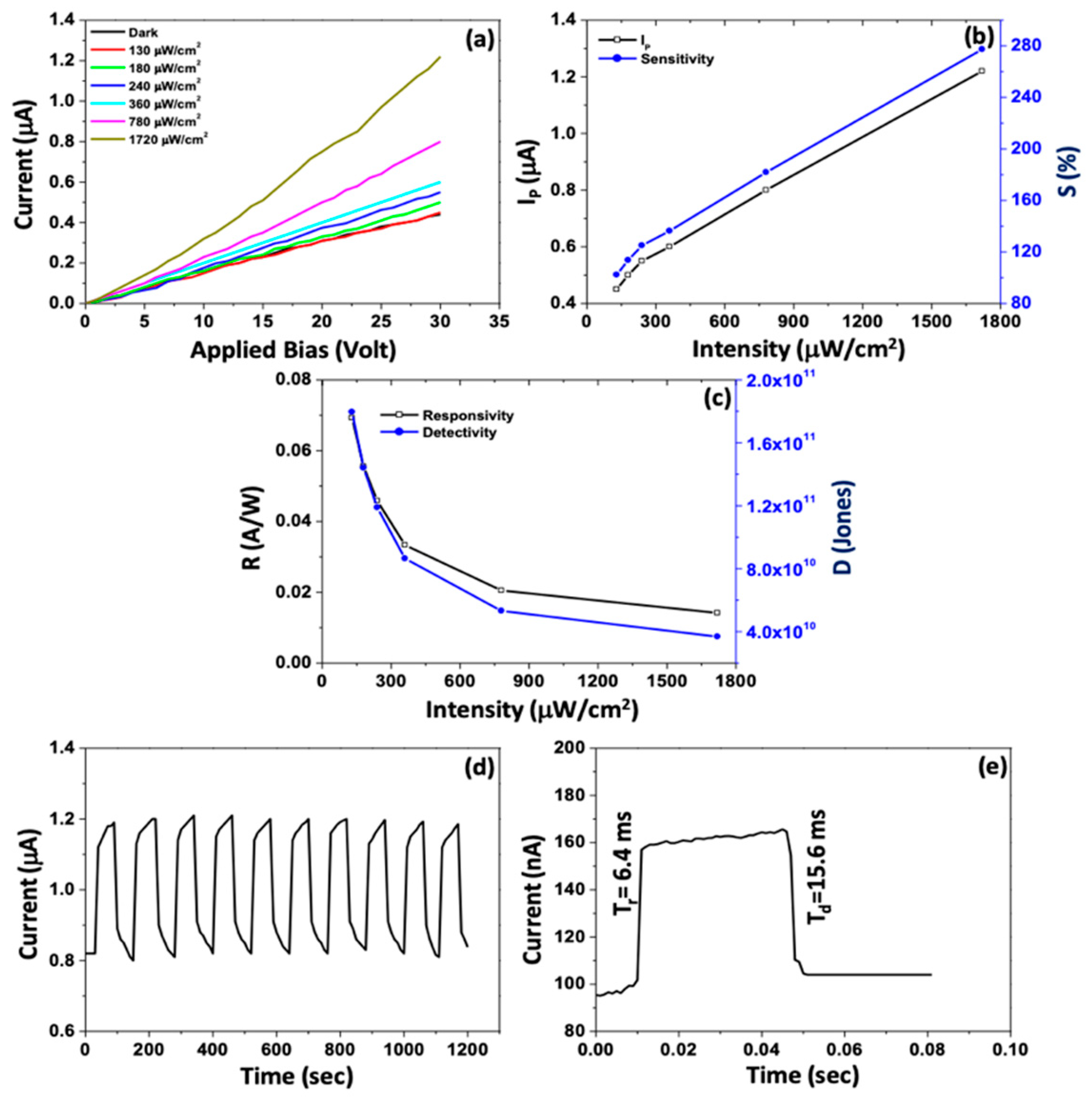
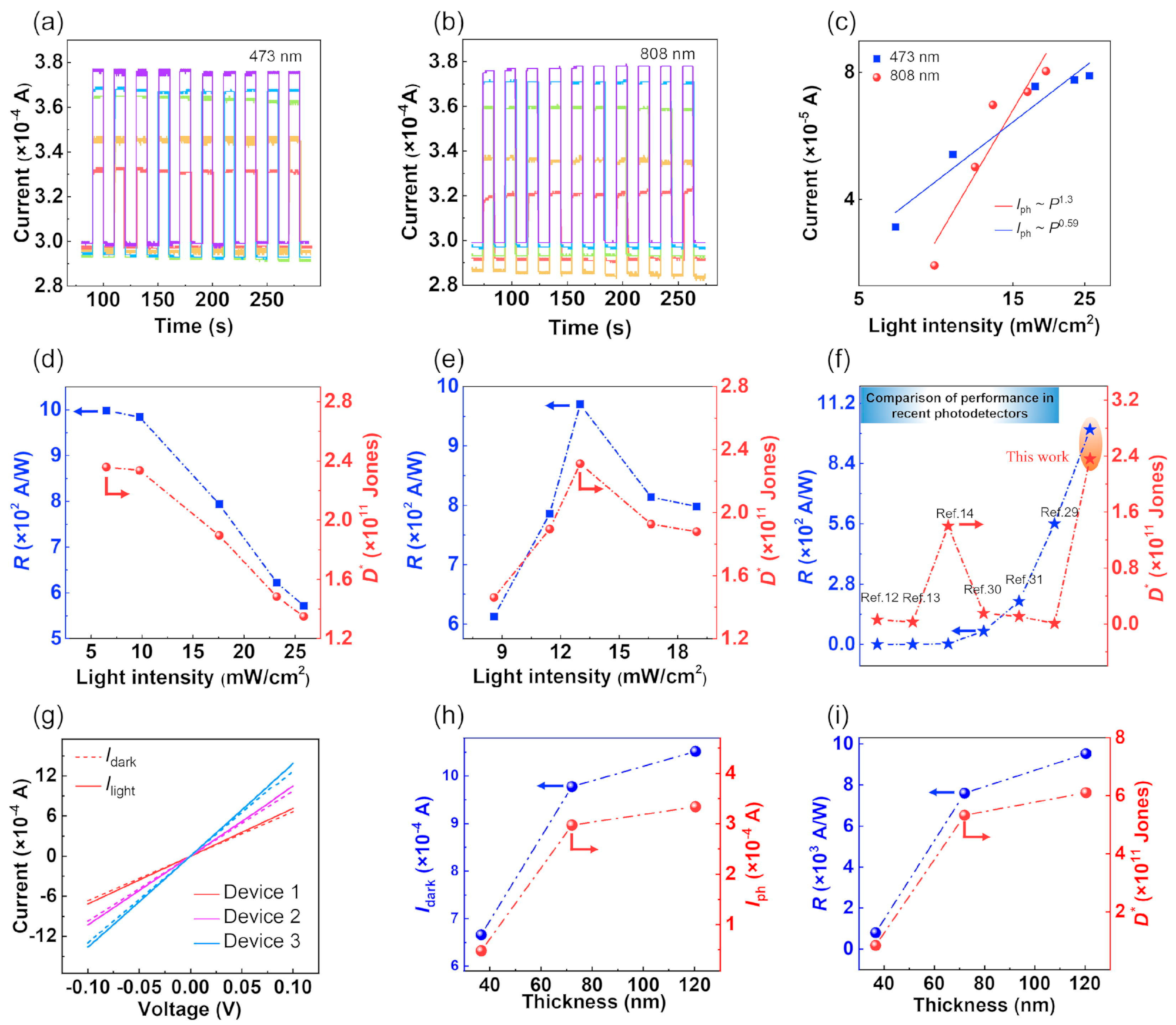
6.4. PbS Applications
6.5. PbSe Applications
6.6. PbTe Applications
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Titirici, M.-M.; Antonietti, M. Chemistry and materials options of sustainable carbon materials made by hydrothermal carbonization. Chem. Soc. Rev. 2009, 39, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.; Shi, Y.; Wang, X. The Sub-Nanometer Scale as a New Focus in Nanoscience. Adv. Mater. 2018, 30, e1802031. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Wu, X.; Zhu, Y.; Xia, C.; Han, Z.; Yu, H.; Chen, C.; Feng, T.; Mao, J.; Chen, Y.; et al. Boosting room-temperature thermoelectric performance of Mg3Sb1.5Bi0.5 material through breaking the contradiction between carrier concentration and carrier mobility. Acta Mater. 2024, 265, 119636. [Google Scholar] [CrossRef]
- Jiang, F.; Feng, T.; Zhu, Y.; Han, Z.; Shu, R.; Chen, C.; Zhang, Y.; Xia, C.; Wu, X.; Yu, H.; et al. Extraordinary thermoelectric performance, thermal stability and mechanical properties of n-type Mg3Sb1.5Bi0.5 through multi-dopants at interstitial site. Mater. Today Phys. 2022, 27, 100835. [Google Scholar] [CrossRef]
- Shuai, J.; Tan, X.; Guo, Q.; Xu, J.; Gellé, A.; Gautier, R.; Halet, J.-F.; Failamani, F.; Jiang, J.; Mori, T. Enhanced thermoelectric performance through crystal field engineering in transition metal–doped GeTe. Mater. Today Phys. 2019, 9, 100094. [Google Scholar] [CrossRef]
- Mao, J.; Liu, Z.; Zhou, J.; Zhu, H.; Zhang, Q.; Chen, G.; Ren, Z. Advances in thermoelectrics. Adv. Phys. 2018, 67, 69–147. [Google Scholar] [CrossRef]
- Wang, M.; Xiao, Y.; Li, Y.; Han, L.; Sun, Z.; He, L.; Liu, R.; Hu, K. Recent Progress on Graphene Flexible Photodetectors. Materials 2022, 15, 4820. [Google Scholar] [CrossRef]
- Das, T.; Ahn, J.-H. Development of electronic devices based on two-dimensional materials. FlatChem 2017, 3, 43–63. [Google Scholar] [CrossRef]
- Banik, A.; Roychowdhury, S.; Biswas, K. The journey of tin chalcogenides towards high-performance thermoelectrics and topological materials. Chem. Commun. 2018, 54, 6573–6590. [Google Scholar] [CrossRef]
- Solayman; Sarker, A.; Muntasir, M.; Sharme, R.K.; Islam, R. Pressure-induced investigation of structural, electronic, optical, and mechanical properties of BaCeO3. Opt. Mater. 2024, 148, 114699. [Google Scholar] [CrossRef]
- Thapa, D. Tunable Electronic and Magnetic Properties in 2D-WSe2 Monolayer via Vanadium (V) Doping and Chalcogenide (Se) Vacancies: A First-Principle Investigations; Mississippi State University: Starkville, MS, USA, 2021; Available online: https://search.proquest.com/openview/71a47e7ba937aa70fdbf029126887646/1?pq-origsite=gscholar&cbl=18750&diss=y (accessed on 3 September 2024).
- Majid, A.; Jabeen, A. (Eds.) Characteristics, Strategies and Applications of Layered Materials: An Introduction. In Layeredness in Materials: Characteristics, Strategies and Applications; Springer Nature: Singapore, 2023; pp. 1–16. [Google Scholar] [CrossRef]
- Xia, C.; Li, J. Recent advances in optoelectronic properties and applications of two-dimensional metal chalcogenides. J. Semicond. 2016, 37, 051001. [Google Scholar] [CrossRef]
- Yu, M.; Hilse, M.; Zhang, Q.; Liu, Y.; Wang, Z.; Law, S. Review of Nanolayered Post-transition Metal Monochalcogenides: Synthesis, Properties, and Applications. ACS Appl. Nano Mater. 2024. [Google Scholar] [CrossRef]
- Gomes, L.C.; Carvalho, A. Electronic and optical properties of low-dimensional group-IV monochalcogenides. J. Appl. Phys. 2020, 128, 121101. [Google Scholar] [CrossRef]
- Li, T.; Luo, S.; Wang, X.; Zhang, L. Alternative Lone-Pair ns2-Cation-Based Semiconductors beyond Lead Halide Perovskites for Optoelectronic Applications. Adv. Mater. 2021, 33, 2008574. [Google Scholar] [CrossRef]
- Jadaun, P. First-Principles Study of Electronic and Topological Properties of Graphene and Graphene-like Materials. 2013. Available online: http://hdl.handle.net/2152/21246 (accessed on 3 September 2024).
- Sharme, R.K.; Islam, R.; Sarker, A.; Solayman; Momin, A.; Islam, R. First-principles study on electronic, mechanical, and optical properties of pressure-induced vanadium-based perovskite KVO3. Phys. B Condens. Matter 2024, 681, 415785. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, N.; Li, D.; Zhu, J. Thermal Properties of Two Dimensional Layered Materials. Adv. Funct. Mater. 2017, 27, 1604134. [Google Scholar] [CrossRef]
- Butler, S.Z.; Hollen, S.M.; Cao, L.; Cui, Y.; Gupta, J.A.; Gutiérrez, H.R.; Heinz, T.F.; Hong, S.S.; Huang, J.; Ismach, A.F.; et al. Progress, Challenges, and Opportunities in Two-Dimensional Materials Beyond Graphene. ACS Nano 2013, 7, 2898–2926. [Google Scholar] [CrossRef]
- Terada, T.; Uematsu, Y.; Ishibe, T.; Naruse, N.; Sato, K.; Nguyen, T.Q.; Kobayashi, E.; Nakano, H.; Nakamura, Y. Giant Enhancement of Seebeck Coefficient by Deformation of Silicene Buckled Structure in Calcium-Intercalated Layered Silicene Film. Adv. Mater. Interfaces 2021, 9, 2101752. [Google Scholar] [CrossRef]
- Wei, Y.; Nukala, P.; Salverda, M.; Matzen, S.; Zhao, H.J.; Momand, J.; Everhardt, A.S.; Agnus, G.; Blake, G.R.; Lecoeur, P.; et al. A rhombohedral ferroelectric phase in epitaxially strained Hf0.5Zr0.5O2 thin films. Nat. Mater. 2018, 17, 1095–1100. [Google Scholar] [CrossRef]
- Yang, D.; Su, X.; Yan, Y.; Hu, T.; Xie, H.; He, J.; Uher, C.; Kanatzidis, M.G.; Tang, X. Manipulating the Combustion Wave during Self-Propagating Synthesis for High Thermoelectric Performance of Layered Oxychalcogenide Bi1–xPbxCuSeO. Chem. Mater. 2016, 28, 4628–4640. [Google Scholar] [CrossRef]
- Corletto, A.; Ellis, A.V.; Shepelin, N.A.; Fronzi, M.; Winkler, D.A.; Shapter, J.G.; Sherrell, P.C. Energy Interplay in Materials: Unlocking Next-Generation Synchronous Multisource Energy Conversion with Layered 2D Crystals. Adv. Mater. 2022, 34, e2203849. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Hennig, R.G. Computational prediction of two-dimensional group-IV mono-chalcogenides. Appl. Phys. Lett. 2014, 105, 042103. [Google Scholar] [CrossRef]
- Chang, K.; Parkin, S.S.P. Experimental formation of monolayer group-IV monochalcogenides. J. Appl. Phys. 2020, 127, 220902. [Google Scholar] [CrossRef]
- Yang, S.; Wu, M.; Wang, B.; Zhao, L.-D.; Huang, L.; Jiang, C.; Wei, S.-H. Enhanced Electrical and Optoelectronic Characteristics of Few-Layer Type-II SnSe/MoS2 van der Waals Heterojunctions. ACS Appl. Mater. Interfaces 2017, 9, 42149–42155. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-H.; Cho, K.; Park, N.-W.; Park, S.; Koh, J.-H.; Lee, S.-K. Multi-Layer SnSe Nanoflake Field-Effect Transistors with Low-Resistance Au Ohmic Contacts. Nanoscale Res. Lett. 2017, 12, 373. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Y.; Wu, M.; Zhao, L.-D.; Lin, Z.; Cheng, H.-C.; Wang, Y.; Jiang, C.; Wei, S.-H.; Huang, L.; et al. Highly-anisotropic optical and electrical properties in layered SnSe. Nano Res. 2017, 11, 554–564. [Google Scholar] [CrossRef]
- Guo, J.; Wang, L.; Yu, Y.; Wang, P.; Huang, Y.; Duan, X. SnSe/MoS2 van der Waals Heterostructure Junction Field-Effect Transistors with Nearly Ideal Subthreshold Slope. Adv. Mater. 2019, 31, e1902962. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Y.; Yang, S.; Jiang, C. SnSe field-effect transistors with improved electrical properties. Nano Res. 2021, 15, 1532–1537. [Google Scholar] [CrossRef]
- Liu, M.; Shi, Y.; Wu, M.; Tian, Y.; Wei, H.; Sun, Q.; Shafi, M.; Man, B. UV surface-enhanced Raman scattering properties of SnSe2 nanoflakes. J. Raman Spectrosc. 2020, 51, 750–755. [Google Scholar] [CrossRef]
- Huang, Y.; Li, L.; Lin, Y.-H.; Nan, C.-W. Liquid Exfoliation Few-Layer SnSe Nanosheets with Tunable Band Gap. J. Phys. Chem. C 2017, 121, 17530–17537. [Google Scholar] [CrossRef]
- Ye, Y.; Xian, Y.; Cai, J.; Lu, K.; Liu, Z.; Shi, T.; Du, J.; Leng, Y.; Wei, R.; Wang, W.; et al. Linear and Nonlinear Optical Properties of Few-Layer Exfoliated SnSe Nanosheets. Adv. Opt. Mater. 2019, 7, 1800579. [Google Scholar] [CrossRef]
- Ren, X.; Qi, X.; Shen, Y.; Xu, G.; Li, J.; Li, Z.; Huang, Z.; Zhong, J. Synthesis of SnSe nanosheets by hydrothermal intercalation and exfoliation route and their photoresponse properties. Mater. Sci. Eng. B 2016, 214, 46–50. [Google Scholar] [CrossRef]
- Qiao, H.; Huang, Z.; Ren, X.; Yao, H.; Luo, S.; Tang, P.; Qi, X.; Zhong, J. Photoresponse improvement in liquid-exfoliated SnSe nanosheets by reduced graphene oxide hybridization. J. Mater. Sci. 2018, 53, 4371–4377. [Google Scholar] [CrossRef]
- Ju, H.; Kim, J. Chemically Exfoliated SnSe Nanosheets and Their SnSe/Poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate) Composite Films for Polymer Based Thermoelectric Applications. ACS Nano 2016, 10, 5730–5739. [Google Scholar] [CrossRef]
- Song, H.-Y.; Lü, J.-T. Density functional theory study of inter-layer coupling in bulk tin selenide. Chem. Phys. Lett. 2018, 695, 200–204. [Google Scholar] [CrossRef]
- Van Duong, T.; Chung, N.X.; Phan, H.N.; Nguyen, H.T.; Nguyen, D.D.; Le, L.T.; Van Pham, N. A facile way to optimize thermoelectric properties of SnSe thin films via sonication-assisted liquid-phase exfoliation. J. Mater. Sci. Mater. Electron. 2022, 33, 15385–15392. [Google Scholar] [CrossRef]
- Mathews, N. Electrodeposited tin selenide thin films for photovoltaic applications. Sol. Energy 2011, 86, 1010–1016. [Google Scholar] [CrossRef]
- Shi, G.; Kioupakis, E. Anisotropic Spin Transport and Strong Visible-Light Absorbance in Few-Layer SnSe and GeSe. Nano Lett. 2015, 15, 6926–6931. [Google Scholar] [CrossRef]
- Sarkar, A.S.; Mushtaq, A.; Kushavah, D.; Pal, S.K. Liquid exfoliation of electronic grade ultrathin tin(II) sulfide (SnS) with intriguing optical response. NPJ 2D Mater. Appl. 2020, 4, 1–9. [Google Scholar] [CrossRef]
- Gurnani, C.; Hawken, S.L.; Hector, A.L.; Huang, R.; Jura, M.; Levason, W.; Perkins, J.; Reid, G.; Stenning, G.B.G. Tin(IV) chalcogenoether complexes as single source precursors for the chemical vapour deposition of SnE2 and SnE (E = S, Se) thin films. Dalton Trans. 2018, 47, 2628–2637. [Google Scholar] [CrossRef]
- Butt, F.K.; Mirza, M.; Cao, C.; Idrees, F.; Tahir, M.; Safdar, M.; Ali, Z.; Tanveer, M.; Aslam, I. Synthesis of mid-infrared SnSe nanowires and their optoelectronic properties. CrystEngComm 2014, 16, 3470–3473. [Google Scholar] [CrossRef]
- Khan, M.D.; Aamir, M.; Sohail, M.; Sher, M.; Baig, N.; Akhtar, J.; Malik, M.A.; Revaprasadu, N. Bis(selenobenzoato)dibutyltin(iv) as a single source precursor for the synthesis of SnSe nanosheets and their photo-electrochemical study for water splitting. Dalton Trans. 2018, 47, 5465–5473. [Google Scholar] [CrossRef]
- Kevin, P.; Malik, S.N.; Malik, M.A.; O’Brien, P. The aerosol assisted chemical vapour deposition of SnSe and Cu2SnSe3 thin films from molecular precursors. Chem. Commun. 2014, 50, 14328–14330. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Salt-assisted chemical vapor deposition of two-dimensional transition metal dichalcogenides. iScience 2021, 24, 103229. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wong, C.P.Y.; Zou, J.; Li, S.; Wang, Q.; Chen, J.; Qi, D.; Wang, H.; Eda, G.; Chua, D.H.C.; et al. Two-step fabrication of single-layer rectangular SnSe flakes. 2D Mater. 2017, 4, 021026. [Google Scholar] [CrossRef]
- Yu, Y.; Xiong, T.; Guo, Z.; Hou, S.; Yang, J.; Liu, Y.-Y.; Gu, H.; Wei, Z. Wide-spectrum polarization-sensitive and fast-response photodetector based on 2D group IV-VI semiconductor tin selenide. Fundam. Res. 2022, 2, 985–992. [Google Scholar] [CrossRef]
- Muntasir, M.; Sharme, R.K.; Uddin, M.B.; Haque, M.S.; Khan, F. Modeling Microstructural and Mechanical Properties of Solidified Al-Sn-Cu System. Malays. J. Compos. Sci. Manuf. 2023, 12, 84–101. [Google Scholar] [CrossRef]
- Hao, L.; Du, Y.; Wang, Z.; Wu, Y.; Xu, H.; Dong, S.; Liu, H.; Liu, Y.; Xue, Q.; Han, Z.; et al. Wafer-size growth of 2D layered SnSe films for UV-Visible-NIR photodetector arrays with high responsitivity. Nanoscale 2020, 12, 7358–7365. [Google Scholar] [CrossRef]
- Chiu, M.; Ji, X.; Zhang, T.; Mao, N.; Luo, Y.; Shi, C.; Zheng, X.; Liu, H.; Han, Y.; Wilson, W.L.; et al. Growth of Large-Sized 2D Ultrathin SnSe Crystals with In-Plane Ferroelectricity. Adv. Electron. Mater. 2023, 9, 2201031. [Google Scholar] [CrossRef]
- Jiang, J.; Wong, C.P.Y.; Zhang, W.; Wee, A.T.S. Atmospheric Pressure Fabrication of Large-Sized Single-Layer Rectangular SnSe Flakes. J. Vis. Exp. JoVE 2018, 133, 57023. [Google Scholar]
- Sarkar, D.; Sharme, R.K.; Khan, F. A study on the tensile properties from the composition of an annealed structural steel sample. In Proceedings of the International Conference on Mechanical Engineering and Renewable Energy, Chattogram, Bangladesh, 12–14 December 2021. [Google Scholar]
- Zhou, Q.; Wang, M.; Li, Y.; Liu, Y.; Chen, Y.; Wu, Q.; Wang, S. Fabrication of highly textured 2D SnSe layers with tunable electronic properties for hydrogen evolution. Molecules 2021, 26, 3319. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Küster, F.; Miller, B.J.; Ji, J.-R.; Zhang, J.-L.; Sessi, P.; Barraza-Lopez, S.; Parkin, S.S.P. Microscopic Manipulation of Ferroelectric Domains in SnSe Monolayers at Room Temperature. Nano Lett. 2020, 20, 6590–6597. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.; Brune, V.; Lüggert, S.; Hegemann, C.; van Gerven, D.; Wilhelm, M.; Ji, S.; Choi, H.; Mathur, S. Direct Synthesis of Two-Dimensional SnSe and SnSe2 through Molecular Scale Preorganization. Inorg. Chem. 2023, 62, 6274–6287. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, H.; Huang, R.; Chen, W.; Zhang, H. Recent Advances in SnSe Nanostructures beyond Thermoelectricity. Adv. Funct. Mater. 2022, 32, 2200516. [Google Scholar] [CrossRef]
- Li, X.; Song, Z.; Zhao, H.; Zhang, W.; Sun, Z.; Liang, H.; Zhu, H.; Pei, J.; Li, L.; Ruan, S. SnSe nanosheets: From facile synthesis to applications in broadband photodetections. Nanomaterials 2020, 11, 49. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.; Zhang, K.; Chen, S.; Mei, F.; Shen, G. Anisotropic photoresponse of layered 2D SnS-based near infrared photodetectors. J. Mater. Chem. C 2017, 5, 11288–11293. [Google Scholar] [CrossRef]
- Brent, J.R.; Lewis, D.J.; Lorenz, T.; Lewis, E.A.; Savjani, N.; Haigh, S.J.; Seifert, G.; Derby, B.; O’brien, P. Tin(II) Sulfide (SnS) Nanosheets by Liquid-Phase Exfoliation of Herzenbergite: IV–VI Main Group Two-Dimensional Atomic Crystals. J. Am. Chem. Soc. 2015, 137, 12689–12696. [Google Scholar] [CrossRef]
- Khan, H.; Mahmood, N.; Zavabeti, A.; Elbourne, A.; Rahman, A.; Zhang, B.Y.; Krishnamurthi, V.; Atkin, P.; Ghasemian, M.B.; Yang, J.; et al. Liquid metal-based synthesis of high performance monolayer SnS piezoelectric nanogenerators. Nat. Commun. 2020, 11, 3449. [Google Scholar] [CrossRef]
- Khan, M.D.; Akhtar, J.; Malik, M.A.; Akhtar, M.; Revaprasadu, N. Phase-pure fabrication and shape evolution studies of SnS nanosheets. New J. Chem. 2015, 39, 9569–9574. [Google Scholar] [CrossRef]
- Jamali-Sheini, F.; Cheraghizade, M.; Yousefi, R. SnS nanosheet films deposited via thermal evaporation: The effects of buffer layers on photovoltaic performance. Sol. Energy Mater. Sol. Cells 2016, 154, 49–56. [Google Scholar] [CrossRef]
- Modi, K.H.; Pataniya, P.M.; Patel, V.; Sumesh, C. Microwave assisted synthesis of SnS nanosheets for fabrication of large area SnS/Si heterojunction. Sol. Energy 2021, 221, 412–417. [Google Scholar] [CrossRef]
- Ju, H.; Park, D.; Kim, J. Fabrication of porous SnS nanosheets and their combination with conductive polymer for hybrid thermoelectric application. Chem. Eng. J. 2018, 356, 950–954. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, G.; Tian, H.; Fan, C.; Wang, M.; Li, E. Facile synthesis of tin monosulfide nanosheets via physical vapour deposition and their near-infrared photoresponse. AIP Adv. 2019, 9, 095205. [Google Scholar] [CrossRef]
- Kang, J.-G.; Park, J.-G.; Kim, D.-W. Superior rate capabilities of SnS nanosheet electrodes for Li ion batteries. Electrochem. Commun. 2010, 12, 307–310. [Google Scholar] [CrossRef]
- Shan, W.; Fu, Z.; Ma, M.; Liu, Z.; Xue, Z.; Xu, J.; Zhang, F.; Li, Y. Facile chemical bath synthesis of SnS nanosheets and their ethanol sensing properties. Sensors 2019, 19, 2581. [Google Scholar] [CrossRef]
- Sutter, E.; Wang, J.; Sutter, P. Surface Passivation by Excess Sulfur for Controlled Synthesis of Large, Thin SnS Flakes. Chem. Mater. 2020, 32, 8034–8042. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Li, B.; Tian, H.; Fan, C.; Zhang, Y.; Hua, Z.; Wang, M.; Zheng, H.; Li, E. High-performance photodetectors based on two-dimensional tin (II) sulfide (SnS) nanoflakes. J. Mater. Chem. C 2018, 6, 10036–10041. [Google Scholar] [CrossRef]
- Sarkar, A.S.; Stratakis, E. Dispersion behaviour of two dimensional monochalcogenides. J. Colloid Interface Sci. 2021, 594, 334–341. [Google Scholar] [CrossRef]
- Li, S.; Zheng, J.; Zuo, S.; Wu, Z.; Yan, P.; Pan, F. 2D hybrid anode based on SnS nanosheet bonded with graphene to enhance electrochemical performance for lithium-ion batteries. RSC Adv. 2015, 5, 46941–46946. [Google Scholar] [CrossRef]
- Xia, J.; Li, X.-Z.; Huang, X.; Mao, N.; Zhu, D.-D.; Wang, L.; Xu, H.; Meng, X.-M. Physical vapor deposition synthesis of two-dimensional orthorhombic SnS flakes with strong angle/temperature-dependent Raman responses. Nanoscale 2015, 8, 2063–2070. [Google Scholar] [CrossRef]
- Krishnamurthi, V.; Khan, H.; Ahmed, T.; Zavabeti, A.; Tawfik, S.A.; Jain, S.K.; Spencer, M.J.S.; Balendhran, S.; Crozier, K.B.; Li, Z.; et al. Liquid-Metal Synthesized Ultrathin SnS Layers for High-Performance Broadband Photodetectors. Adv. Mater. 2020, 32, e2004247. [Google Scholar] [CrossRef] [PubMed]
- Higashitarumizu, N.; Kawamoto, H.; Nakamura, M.; Shimamura, K.; Ohashi, N.; Ueno, K.; Nagashio, K. Self-passivated ultra-thin SnS layers via mechanical exfoliation and post-oxidation. Nanoscale 2018, 10, 22474–22483. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Guo, C.; Zhao, M.; Li, R.; Xue, J. Two-Dimensional SnS: A Phosphorene Analogue with Strong In-Plane Electronic Anisotropy. ACS Nano 2017, 11, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Kim, J.; Kim, Y.K. Growth of Large-Area SnS Films with Oriented 2D SnS Layers for Energy-Efficient Broadband Optoelectronics. Adv. Funct. Mater. 2018, 28, 1804737. [Google Scholar] [CrossRef]
- Li, F.; Moayed, M.M.R.; Klein, E.; Lesyuk, R.; Klinke, C. In-Plane Anisotropic Faceting of Ultralarge and Thin Single-Crystalline Colloidal SnS Nanosheets. J. Phys. Chem. Lett. 2019, 10, 993–999. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, J.; Shen, S.; Xu, H.; Wang, Q. Ultralarge single crystal SnS rectangular nanosheets. Chem. Commun. 2011, 47, 5226–5228. [Google Scholar] [CrossRef]
- Li, F.; Fu, J.; Torche, A.; Kull, S.; Kornowski, A.; Lesyuk, R.; Bester, G.; Klinke, C. Single-Crystalline Colloidal Quasi-2D Tin Telluride. Adv. Mater. Interfaces 2020, 7, 2000410. [Google Scholar] [CrossRef]
- He, F.; Klein, E.; Bartling, S.; Saeidpour, S.; Corzilius, B.; Lesyuk, R.; Klinke, C. Template-Mediated Formation of Colloidal Two-Dimensional Tin Telluride Nanosheets and the Role of the Ligands. J. Phys. Chem. C 2022, 126, 20498–20504. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Wang, H.; Yuan, G.; Suvorova, A.; Gain, S.; Ren, Y.; Lei, W. Ultrathin High-Quality SnTe Nanoplates for Fabricating Flexible Near-Infrared Photodetectors. ACS Appl. Mater. Interfaces 2020, 12, 31810–31822. [Google Scholar] [CrossRef]
- Song, L.; Tang, L.; Hao, Q.; Yang, C.; Teng, K.S.; Wang, H.; Yue, B.; Li, J.; Wei, H. Large-area SnTe nanofilm: Preparation and its broadband photodetector with ultra-low dark current. Opt. Express 2022, 30, 14828–14838. [Google Scholar] [CrossRef]
- Chang, K.; Liu, J.; Lin, H.; Wang, N.; Zhao, K.; Zhang, A.; Jin, F.; Zhong, Y.; Hu, X.; Duan, W.; et al. Discovery of robust in-plane ferroelectricity in atomic-thick SnTe. Science 2016, 353, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yin, Q.; Huang, H.; Yu, Q.; Liu, B.; Yang, J.; Dong, Z.; Shen, Z.; Zhu, B.; Liao, L.; et al. Van der Waals epitaxy of ultrathin crystalline PbTe nanosheets with high near-infrared photoelectric response. Nano Res. 2020, 14, 1955–1960. [Google Scholar] [CrossRef]
- Zhu, T.J.; Chen, X.; Meng, X.Y.; Zhao, X.B.; He, J. Anisotropic Growth of Cubic PbTe Nanoparticles to Nanosheets: Controlled Synthesis and Growth Mechanisms. Cryst. Growth Des. 2010, 10, 3727–3731. [Google Scholar] [CrossRef]
- Jiang, J.; Cheng, R.; Yin, L.; Wen, Y.; Wang, H.; Zhai, B.; Liu, C.; Shan, C.; He, J. Van der Waals epitaxial growth of two-dimensional PbSe and its high-performance heterostructure devices. Sci. Bull. 2022, 67, 1659–1668. [Google Scholar] [CrossRef]
- Koh, W.-K.; Dandu, N.K.; Fidler, A.F.; Klimov, V.I.; Pietryga, J.M.; Kilina, S.V. Thickness-Controlled Quasi-Two-Dimensional Colloidal PbSe Nanoplatelets. J. Am. Chem. Soc. 2017, 139, 2152–2155. [Google Scholar] [CrossRef]
- Klepzig, L.F.; Biesterfeld, L.; Romain, M.; Niebur, A.; Schlosser, A.; Hübner, J.; Lauth, J. Colloidal 2D PbSe nanoplatelets with efficient emission reaching the telecom O-, E-and S-band. Nanoscale Adv. 2022, 4, 590–599. [Google Scholar] [CrossRef]
- Bhandari, G.B.; Subedi, K.; He, Y.; Jiang, Z.; Leopold, M.; Reilly, N.; Lu, H.P.; Zayak, A.T.; Sun, L. Thickness-Controlled Synthesis of Colloidal PbS Nanosheets and Their Thickness-Dependent Energy Gaps. Chem. Mater. 2014, 26, 5433–5436. [Google Scholar] [CrossRef]
- Gao, L.; Chen, H.; Wang, R.; Wei, S.; Kuklin, A.V.; Mei, S.; Zhang, F.; Zhang, Y.; Jiang, X.; Luo, Z.; et al. Ultra-Small 2D PbS Nanoplatelets: Liquid-Phase Exfoliation and Emerging Applications for Photo-Electrochemical Photodetectors. Small 2021, 17, e2005913. [Google Scholar] [CrossRef]
- Moayed, M.M.R.; Bielewicz, T.; Noei, H.; Stierle, A.; Klinke, C. High-Performance n- and p-Type Field-Effect Transistors Based on Hybridly Surface-Passivated Colloidal PbS Nanosheets. Adv. Funct. Mater. 2018, 28, 1706815. [Google Scholar] [CrossRef]
- Aerts, M.; Bielewicz, T.; Klinke, C.; Grozema, F.C.; Houtepen, A.J.; Schins, J.M.; Siebbeles, L.D.A. Highly efficient carrier multiplication in PbS nanosheets. Nat. Commun. 2014, 5, 3789. [Google Scholar] [CrossRef]
- Zhu, Z.; Qu, Y.; Wang, Z.; Zhou, F.; Zhao, C.; Lin, Y.; Li, L.; Yao, Y.; Wu, Y. 2D PbS Nanosheets with Zigzag Edges for Efficient CO2 Photoconversion. Chem. A Eur. J. 2020, 26, 13601–13605. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Wang, Q.; Yin, L.; Liu, Q.; Wang, F.; Wang, F.; Wang, Z.; Liu, K.; Xu, K.; Huang, Y.; et al. Epitaxial 2D PbS nanoplates arrays with highly efficient infrared response. Adv. Mater. 2016, 28, 8051–8057. [Google Scholar] [CrossRef] [PubMed]
- Schliehe, C.; Juarez, B.H.; Pelletier, M.; Jander, S.; Greshnykh, D.; Nagel, M.; Meyer, A.; Foerster, S.; Kornowski, A.; Klinke, C.; et al. Ultrathin PbS Sheets by Two-Dimensional Oriented Attachment. Science 2010, 329, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Savitzky, B.H.; Yang, J.; Newman, J.T.; Perez, K.A.; Hyun, B.-R.; Kourkoutis, L.F.; Hanrath, T.; Wise, F.W. Colloidal Synthesis of PbS and PbS/CdS Nanosheets Using Acetate-Free Precursors. Chem. Mater. 2015, 28, 127–134. [Google Scholar] [CrossRef]
- Wu, W.-Y.; Chakrabortty, S.; Guchhait, A.; Wong, G.Y.Z.; Dalapati, G.K.; Lin, M.; Chan, Y. Solution-Processed 2D PbS Nanoplates with Residual Cu2S Exhibiting Low Resistivity and High Infrared Responsivity. Chem. Mater. 2016, 28, 9132–9138. [Google Scholar] [CrossRef]
- Acharya, S.; Das, B.; Thupakula, U.; Ariga, K.; Sarma, D.D.; Israelachvili, J.; Golan, Y. A Bottom-Up Approach toward Fabrication of Ultrathin PbS Sheets. Nano Lett. 2013, 13, 409–415. [Google Scholar] [CrossRef]
- Vázquez, F.M.; Yu, Q.; Klepzig, L.F.; Siebbeles, L.D.A.; Crisp, R.W.; Lauth, J. Probing Excitons in Ultrathin PbS Nanoplatelets with Enhanced Near-Infrared Emission. J. Phys. Chem. Lett. 2021, 12, 680–685. [Google Scholar] [CrossRef]
- Khan, A.H.; Brescia, R.; Polovitsyn, A.; Angeloni, I.; Martín-García, B.; Moreels, I. Near-Infrared Emitting Colloidal PbS Nanoplatelets: Lateral Size Control and Optical Spectroscopy. Chem. Mater. 2017, 29, 2883–2889. [Google Scholar] [CrossRef]
- Jiang, Z.; Bhandari, G.B.; Premathilaka, S.M.; Khan, S.; Dimick, D.M.; Stombaugh, C.; Mandell, A.; He, Y.; Lu, H.P.; Sun, L. Growth of colloidal PbS nanosheets and the enhancement of their photoluminescence. Phys. Chem. Chem. Phys. 2015, 17, 23303–23307. [Google Scholar] [CrossRef]
- Premathilaka, S.M.; Tang, Y.; Jiang, Z.; Weeraddana, T.M.; Antu, A.D.; Bischoff, S.; Sun, L. Controlling the Lateral Size and Excitonic Properties of Colloidal PbS Nanosheets. ChemNanoMat 2019, 6, 816–820. [Google Scholar] [CrossRef]
- Sonntag, L.; Shamraienko, V.; Fan, X.; Khoshkhoo, M.S.; Kneppe, D.; Koitzsch, A.; Gemming, T.; Hiekel, K.; Leo, K.; Lesnyak, V.; et al. Colloidal PbS nanoplatelets synthesized via cation exchange for electronic applications. Nanoscale 2019, 11, 19370–19379. [Google Scholar] [CrossRef] [PubMed]
- Bielewicz, T.; Klein, E.; Klinke, C. New ways to synthesize lead sulfide nanosheets—Substituted alkanes direct the growth of 2D nanostructures. Nanotechnology 2016, 27, 355602. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.-F.; Jin, F.; Yang, W.-J.; Sun, D.-P.; Wang, X. Liquid–liquid interfacial synthesis of single-crystalline PbS nanoplates and nanocube-based microspheres. Mater. Lett. 2012, 69, 10–12. [Google Scholar] [CrossRef]
- Shkir, M.; AlFaify, S.; Ganesh, V.; Yahia, I. Facile one pot synthesis of PbS nanosheets and their characterization. Solid State Sci. 2017, 70, 81–85. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Martín-García, B.; Buha, J.; Almeida, G.; Toso, S.; Marras, S.; Bonaccorso, F.; Petralanda, U.; Infante, I.; Manna, L. Ultrathin Orthorhombic PbS Nanosheets. Chem. Mater. 2019, 31, 8145–8153. [Google Scholar] [CrossRef]
- Gu, Y.-Y.; Wang, Y.-F.; Xia, J.; Meng, X.-M. Chemical vapor deposition of two-dimensional PbS nanoplates for photodetection. Chin. Phys. Lett. 2020, 37, 048101. [Google Scholar] [CrossRef]
- Pallikara, I.; Skelton, J.M. Phase stability of the tin monochalcogenides SnS and SnSe: A quasi-harmonic lattice-dynamics study. Phys. Chem. Chem. Phys. 2021, 23, 19219–19236. [Google Scholar] [CrossRef]
- Shi, X.; Tao, X.; Zou, J.; Chen, Z. High-Performance Thermoelectric SnSe: Aqueous Synthesis, Innovations, and Challenges. Adv. Sci. 2020, 7, 1902923. [Google Scholar] [CrossRef]
- Qin, B.; Wang, D.; He, W.; Zhang, Y.; Wu, H.; Pennycook, S.J.; Zhao, L.-D. Realizing High Thermoelectric Performance in p-Type SnSe through Crystal Structure Modification. J. Am. Chem. Soc. 2018, 141, 1141–1149. [Google Scholar] [CrossRef]
- Chattopadhyay, T.; Pannetier, J.; Von Schnering, H. Neutron diffraction study of the structural phase transition in SnS and SnSe. J. Phys. Chem. Solids 1986, 47, 879–885. [Google Scholar] [CrossRef]
- Serrano-Sánchez, F.; Nemes, N.M.; Dura, O.J.; Fernandez-Diaz, M.T.; Martínez, J.L.; Alonso, J.A. Structural phase transition in polycrystalline SnSe: A neutron diffraction study in correlation with thermoelectric properties. J. Appl. Crystallogr. 2016, 49, 2138–2144. [Google Scholar] [CrossRef]
- Erdemir, A. Crystal Chemistry and Solid Lubricating Properties of the Monochalcogenides Gallium Selenide and Tin Selenide. Tribol. Trans. 1994, 37, 471–478. [Google Scholar] [CrossRef]
- Chen, Z.-G.; Shi, X.; Zhao, L.-D.; Zou, J. High-performance SnSe thermoelectric materials: Progress and future challenge. Prog. Mater. Sci. 2018, 97, 283–346. [Google Scholar] [CrossRef]
- Shi, X.-L.; Chen, W.-Y.; Tao, X.; Zou, J.; Chen, Z.-G. Rational structural design and manipulation advance SnSe thermoelectrics. Mater. Horiz. 2020, 7, 3065–3096. [Google Scholar] [CrossRef]
- Kim, S.-U.; Duong, A.-T.; Cho, S.; Rhim, S.; Kim, J. A microscopic study investigating the structure of SnSe surfaces. Surf. Sci. 2016, 651, 5–9. [Google Scholar] [CrossRef]
- Flitcroft, J.M.; Pallikara, I.; Skelton, J.M. Thermoelectric Properties of Pnma and Rocksalt SnS and SnSe. Solids 2022, 3, 155–176. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, X.; Ding, Y.; Li, M. Exploration work function and optical properties of monolayer SnSe allotropes. Superlattices Microstruct. 2018, 114, 251–258. [Google Scholar] [CrossRef]
- Hu, Z.-Y.; Li, K.-Y.; Lu, Y.; Huang, Y.; Shao, X.-H. High thermoelectric performances of monolayer SnSe allotropes. Nanoscale 2017, 9, 16093–16100. [Google Scholar] [CrossRef]
- Greyson, E.C.; Barton, J.E.; Odom, T.W. Tetrahedral Zinc Blende Tin Sulfide Nano- and Microcrystals. Small 2006, 2, 368–371. [Google Scholar] [CrossRef]
- Abutbul, R.E.; Segev, E.; Zeiri, L.; Ezersky, V.; Makov, G.; Golan, Y. Synthesis and properties of nanocrystalline π-SnS–a new cubic phase of tin sulphide. RSC Adv. 2016, 6, 5848–5855. [Google Scholar] [CrossRef]
- von Schnering, H.G.; Wiedemeier, H. The high temperature structure of ß-SnS and ß-SnSe and the B16-to-B33 type λ-transition path. Z. Fur Krist. Mater. 1981, 156, 143–150. [Google Scholar] [CrossRef]
- Mariano, A.N.; Chopra, K.L. Polymorphism in some IV-VI compounds induced by high pressure and thin-film epitaxial growth. Appl. Phys. Lett. 1967, 10, 282–284. [Google Scholar] [CrossRef]
- Moshwan, R.; Yang, L.; Zou, J.; Chen, Z. Eco-Friendly SnTe Thermoelectric Materials: Progress and Future Challenges. Adv. Funct. Mater. 2017, 27, 1703278. [Google Scholar] [CrossRef]
- Siddique, S.; Gowda, C.C.; Demiss, S.; Tromer, R.; Paul, S.; Sadasivuni, K.K.; Olu, E.F.; Chandra, A.; Kochat, V.; Galvão, D.S.; et al. Emerging two-dimensional tellurides. Mater. Today Proc. 2021, 51, 402–426. [Google Scholar] [CrossRef]
- Chang, K.; Kaloni, T.P.; Lin, H.; Bedoya-Pinto, A.; Pandeya, A.K.; Kostanovskiy, I.; Zhao, K.; Zhong, Y.; Hu, X.; Xue, Q.; et al. Enhanced Spontaneous Polarization in Ultrathin SnTe Films with Layered Antipolar Structure. Adv. Mater. 2018, 31, e1804428. [Google Scholar] [CrossRef]
- Madelung, O. Landolt-Bornstein Group III: Condensed Matter; Springer: Berlin/Heidelberg, Germany, 1983. [Google Scholar]
- Lin, J.-C.; Sharma, R.C.; Chang, Y.A. The Pb- S (Lead-Sulfur) system. Bull. Alloy Phase Diagr. 1986, 7, 374–381. [Google Scholar] [CrossRef]
- Macias-Pinilla, D.F.; Echeverría-Arrondo, C.; Reyes, A.F.G.; Agouram, S.; Muñoz-Sanjosé, V.; Planelles, J.; Mora-Seró, I.; Climente, J.I. Morphology and Band Structure of Orthorhombic PbS Nanoplatelets: An Indirect Band Gap Material. Chem. Mater. 2021, 33, 420–429. [Google Scholar] [CrossRef]
- Wan, W.; Yao, Y.; Sun, L.; Liu, C.; Zhang, F. Topological, valleytronic, and optical properties of monolayer PbS. arXiv 2016, arXiv:1608.04536. [Google Scholar] [CrossRef]
- Streltsov, S.V.; Manakov, A.Y.; Vokhmyanin, A.P.; Ovsyannikov, S.V.; Shchennikov, V.V. Crystal lattice and band structure of the intermediate high-pressure phase of PbSe. J. Phys. Condens. Matter 2009, 21, 385501. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; Pang, J.; Chen, H.; Wei, G.; Wei, S.; Yan, J.; Jin, S. Study on SO2 and Cl2 sensor application of 2D PbSe based on first principles calculations. RSC Adv. 2022, 12, 8530–8535. [Google Scholar] [CrossRef]
- Tang, S.; Bai, S.; Wu, M.; Luo, D.; Wang, D.; Yang, S.; Zhao, L.-D. Honeycomb-like puckered PbSe with wide bandgap as promising thermoelectric material: A first-principles prediction. Mater. Today Energy 2021, 23, 100914. [Google Scholar] [CrossRef]
- Qadri, S.B.; Singh, A.; Yousuf, M. Structural stability of PbS films as a function of temperature. Thin Solid Films 2003, 431–432, 506–510. [Google Scholar] [CrossRef]
- Pereira, P.B.; Sergueev, I.; Gorsse, S.; Dadda, J.; Müller, E.; Hermann, R.P. Lattice dynamics and structure of GeTe, SnTe and PbTe. Phys. Status Solidi (b) 2012, 250, 1300–1307. [Google Scholar] [CrossRef]
- Xue, F.; Wang, J.; Liu, X.; Sun, X. Study of electronic structure, elastic properties and thermodynamic properties of three PbTe phases: First-principles calculations. Optik 2021, 232, 166533. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, G.; Li, Y. Hydrostatic Pressure Tuning of Thermal Conductivity for PbTe and PbSe Considering Pressure-Induced Phase Transitions. ACS Omega 2021, 6, 3980–3990. [Google Scholar] [CrossRef]
- Banai, R.; Horn, M.; Brownson, J. A review of tin (II) monosulfide and its potential as a photovoltaic absorber. Sol. Energy Mater. Sol. Cells 2016, 150, 112–129. [Google Scholar] [CrossRef]
- Ninan, G.G.; Kartha, C.S.; Vijayakumar, K. Spray pyrolysed SnS thin films in n and p type: Optimization of deposition process and characterization of samples. J. Anal. Appl. Pyrolysis 2016, 120, 121–125. [Google Scholar] [CrossRef]
- Burton, L.A.; Walsh, A. Phase Stability of the Earth-Abundant Tin Sulfides SnS, SnS2, and Sn2S3. J. Phys. Chem. C 2012, 116, 24262–24267. [Google Scholar] [CrossRef]
- Skelton, J.M.; Burton, L.A.; Jackson, A.J.; Oba, F.; Parker, S.C.; Walsh, A. Lattice dynamics of the tin sulphides SnS2, SnS and Sn2S3: Vibrational spectra and thermal transport. Phys. Chem. Chem. Phys. 2017, 19, 12452–12465. [Google Scholar] [CrossRef]
- Shin, D.; Sato, S.A.; Hübener, H.; De Giovannini, U.; Park, N.; Rubio, A. Dynamical amplification of electric polarization through nonlinear phononics in 2D SnTe. NPJ Comput. Mater. 2020, 6, 182. [Google Scholar] [CrossRef]
- Pal, S.; Arora, R.; Roychowdhury, S.; Harnagea, L.; Saurabh, K.; Shenoy, S.; Muthu, D.V.S.; Biswas, K.; Waghmare, U.V.; Sood, A.K. Pressure-induced phase transitions in the topological crystalline insulator SnTe and its comparison with semiconducting SnSe: Raman and first-principles studies. Phys. Rev. B 2020, 101, 155202. [Google Scholar] [CrossRef]
- Sadovnikov, S.I.; Gusev, A.I. Structure and properties of PbS films. J. Alloys Compd. 2013, 573, 65–75. [Google Scholar] [CrossRef]
- Ravich, I.I. Semiconducting Lead Chalcogenides; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 5, Available online: https://books.google.com/books?hl=en&lr=&id=XmfmBwAAQBAJ&oi=fnd&pg=PA1&dq=Ravich+Y+I,+Efimova+B+A+and+Smirnov+I+A+1970+Semiconducting+Lead+Chalcogenides+(New+York:+Plenum)+&ots=0g2kk2yiSJ&sig=uqTrFN1XJBI2H836ank2xN4aIB8 (accessed on 5 September 2024).
- Begum, A.; Hussain, A.; Rahman, A. Effect of deposition temperature on the structural and optical properties of chemically prepared nanocrystalline lead selenide thin films. Beilstein J. Nanotechnol. 2012, 3, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Ovsyannikov, S.V.; Shchennikov, V.V.; Manakov, A.Y.; Likhacheva, A.Y.; Ponosov, Y.S.; Mogilenskikh, V.E.; Vokhmyanin, A.P.; Ancharov, A.I.; Skipetrov, E.P. Unusual B1–B2 transition in PbSe under high pressure: Evidence for two intermediate phases; transport, structural, and optical properties. Phys. Status Solidi (b) 2009, 246, 615–621. [Google Scholar] [CrossRef]
- Rousse, G.; Klotz, S.; Saitta, A.M.; Rodriguez-Carvajal, J.; McMahon, M.I.; Couzinet, B.; Mezouar, M. Structure of the intermediate phase of PbTe at high pressure. Phys. Rev. B 2005, 71, 224116. [Google Scholar] [CrossRef]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Chen, G.; Tang, M.Y.; Yang, R.G.; Lee, H.; Wang, D.Z.; Ren, Z.F.; Fleurial, J.; Gogna, P. New Directions for Low-Dimensional Thermoelectric Materials. Adv. Mater. 2007, 19, 1043–1053. [Google Scholar] [CrossRef]
- Slack, G.A.; Ross, R.G. Thermal conductivity under pressure and through phase transitions in solid alkali halides. II. Theory. J. Phys. C Solid State Phys. 1985, 18, 3957–3980. [Google Scholar] [CrossRef]
- Fu, L.; Yin, M.; Wu, D.; Li, W.; Feng, D.; Huang, L.; He, J. Large enhancement of thermoelectric properties in n-type PbTe via dual-site point defects. Energy Environ. Sci. 2017, 10, 2030–2040. [Google Scholar] [CrossRef]
- Ishibe, T.; Okuhata, R.; Kaneko, T.; Yoshiya, M.; Nakashima, S.; Ishida, A.; Nakamura, Y. Heat transport through propagon-phonon interaction in epitaxial amorphous-crystalline multilayers. Commun. Phys. 2021, 4, 153. [Google Scholar] [CrossRef]
- Momand, J.; Wang, R.; Boschker, J.E.; Verheijen, M.A.; Calarco, R.; Kooi, B.J. Interface formation of two- and three-dimensionally bonded materials in the case of GeTe–Sb2Te3 superlattices. Nanoscale 2015, 7, 19136–19143. [Google Scholar] [CrossRef]
- Yang, S.H.; Zhu, T.J.; Sun, T.; He, J.; Zhang, S.N.; Zhao, X.B. Nanostructures in high-performance (GeTe)x(AgSbTe2)100−x thermoelectric materials. Nanotechnology 2008, 19, 245707. [Google Scholar] [CrossRef] [PubMed]
- González-Barrios, M.; Tabuyo-Martínez, M.; Ávila-Brande, D.; Prado-Gonjal, J. Perspective on Crystal Structures, Synthetic Methods, and New Directions in Thermoelectric Materials. Small Struct. 2024, 2400136. [Google Scholar] [CrossRef]
- Gao, W.; Zheng, Z.; Wen, P.; Huo, N.; Li, J. Novel two-dimensional monoelemental and ternary materials: Growth, physics and application. Nanophotonics 2020, 9, 2147–2168. [Google Scholar] [CrossRef]
- Feng, J.-J.; Zhu, W.; Deng, Y. An overview of thermoelectric films: Fabrication techniques, classification, and regulation methods. Chin. Phys. B 2018, 27, 047210. [Google Scholar] [CrossRef]
- Jiang, Y.; Wei, T.-R.; Shi, X. Beyond phase-change materials: Pseudo-binary (GeTe)n(Sb2Te3)m alloys as promising thermoelectric materials. Mater. Today Phys. 2023, 36, 101167. [Google Scholar] [CrossRef]
- Barraza-Lopez, S.; Kaloni, T.P.; Poudel, S.P.; Kumar, P. Tuning the ferroelectric-to-paraelectric transition temperature and dipole orientation of group-IV monochalcogenide monolayers. Phys. Rev. B 2018, 97, 024110. [Google Scholar] [CrossRef]
- Gupta, R.; Dongre, B.; Carrete, J.; Bera, C. Thermoelectric properties of the SnS monolayer: Fully ab initio and accelerated calculations. J. Appl. Phys. 2021, 130, 054301. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Q.; Yang, X.; Song, S.; Wang, J.; Wang, S.-F. Quasi-commercial production of SnS-based nanosheets with enhanced thermoelectric performance via a wet chemical synthesis. Chem. Eng. J. 2022, 430, 133049. [Google Scholar] [CrossRef]
- Li, Y.; Wu, M.; Song, D.K.; Ding, T.; Liu, F.; Li, J.; Zhang, H.; Xie, H. Transport and Thermoelectric Properties of SnX (X = S or Se) Bilayers and Heterostructures. ACS Appl. Energy Mater. 2020, 3, 6946–6955. [Google Scholar] [CrossRef]
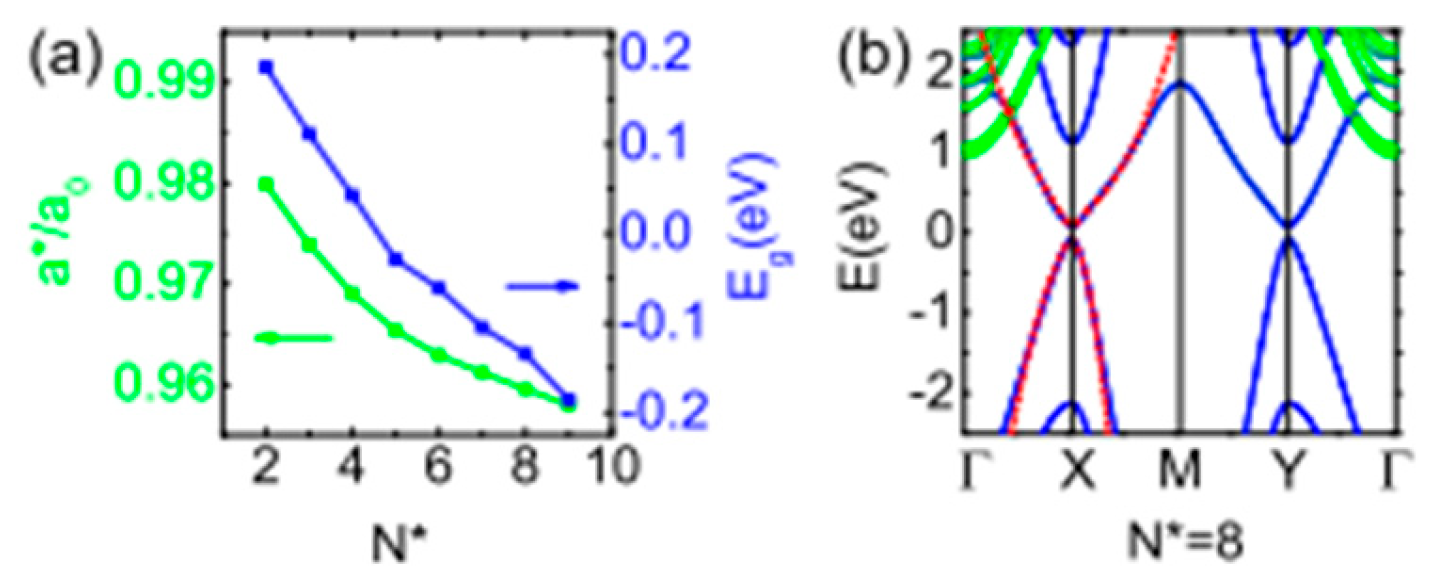
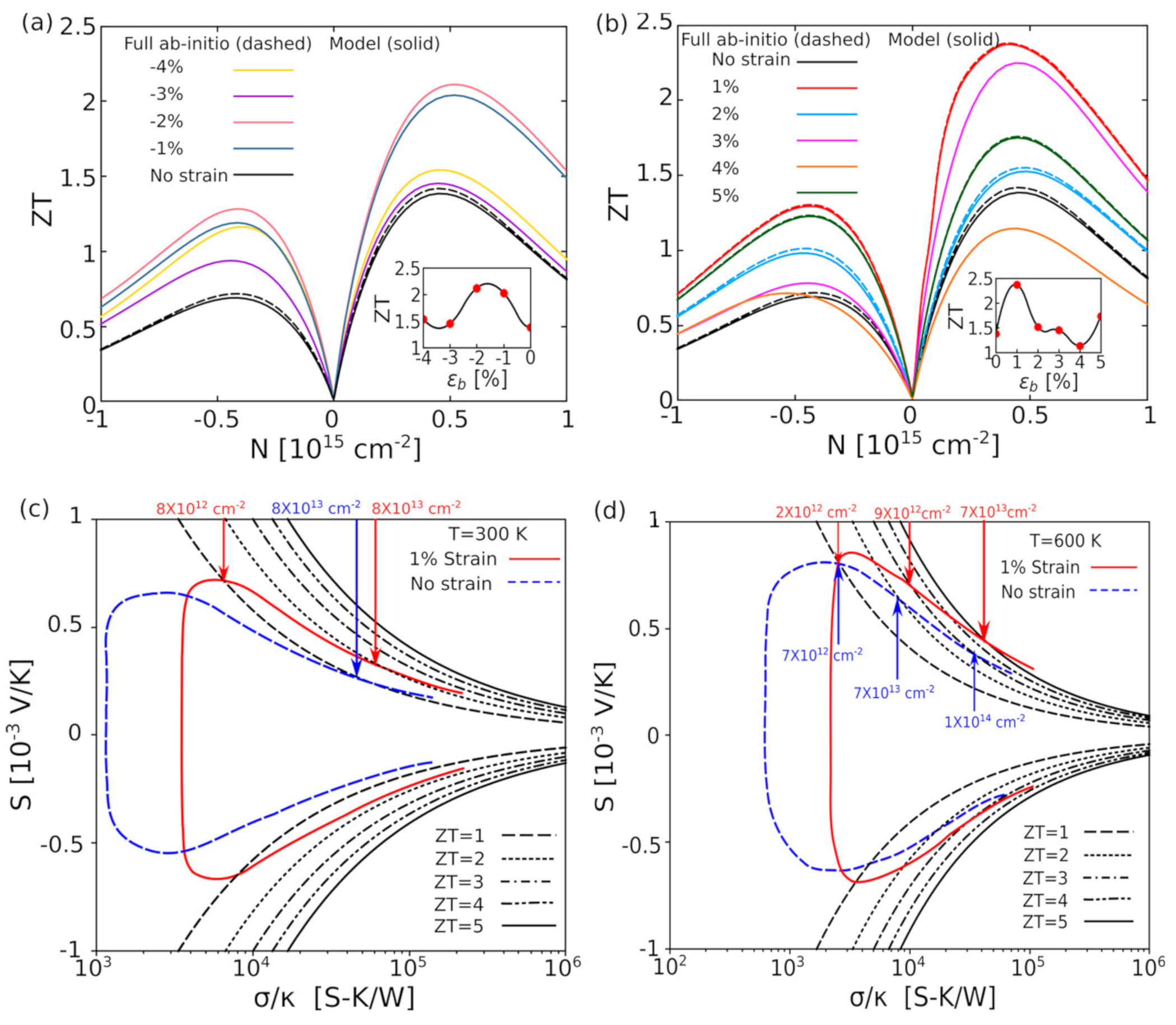
- Gupta, R.; Kakkar, S.; Dongre, B.; Carrete, J.; Bera, C. Enhancement in the Thermoelectric Performance of SnS Monolayer by Strain Engineering. ACS Appl. Energy Mater. 2023, 6, 3944–3952. [Google Scholar] [CrossRef]
- Shafique, A.; Shin, Y.-H. Thermoelectric and phonon transport properties of two-dimensional IV–VI compounds. Sci. Rep. 2017, 7, 506. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.; Hamad, B. Thermoelectric and lattice dynamics properties of layered MX (M= Sn, Pb; X= S, Te) compounds. Appl. Surf. Sci. 2021, 538, 147911. [Google Scholar] [CrossRef]
- Fang, W.; Wei, H.; Xiao, X.; Chen, Y.; Li, M.; He, Y. Monolayer SnX (X = O, S, Se): Two-Dimensional Materials with Low Lattice Thermal Conductivities and High Thermoelectric Figures of Merit. ACS Appl. Energy Mater. 2022, 5, 7802–7812. [Google Scholar] [CrossRef]
- Sandonas, L.M.; Teich, D.; Gutierrez, R.; Lorenz, T.; Pecchia, A.; Seifert, G.; Cuniberti, G. Anisotropic Thermoelectric Response in Two-Dimensional Puckered Structures. J. Phys. Chem. C 2016, 120, 18841–18849. [Google Scholar] [CrossRef]
- Dong, B.; Wang, Z.; Hung, N.T.; Oganov, A.R.; Yang, T.; Saito, R.; Zhang, Z. New two-dimensional phase of tin chalcogenides: Candidates for high-performance thermoelectric materials. Phys. Rev. Mater. 2019, 3, 013405. [Google Scholar] [CrossRef]
- Li, Y.; Wu, M.N.; Ding, T.; Ma, K.; Liu, F.S.; Ao, W.Q.; Li, J.Q. Promising thermoelectric properties and anisotropic electrical and thermal transport of monolayer SnTe. Appl. Phys. Lett. 2019, 114, 083901. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, S.; Sun, Y.; Liu, X.; Wang, H.; Wang, H.; Chen, Y.; Ni, Y. XTe (X= Ge, Sn, Pb) monolayers: Promising thermoelectric materials with ultralow lattice thermal conductivity and high-power factor. ES Energy Environ. 2020, 10, 59–65. [Google Scholar] [CrossRef]
- Tang, S.; Li, X.; Bai, S.; Wan, D.; Zhang, J.; Wu, M.; Luo, D. First-principles study of wrinkled SnTe monolayer as p-type thermoelectric material. Vacuum 2023, 217, 112533. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, D.; Chen, Y.; Wang, H.; Ou, K.; Xia, Y.; Wang, H.; Ni, Y. The thermoelectric properties of XTe (X= Ge, Sn and Pb) monolayers from first-principles calculations. Phys. Scr. 2022, 97, 125709. [Google Scholar] [CrossRef]
- Lubis, P.; Amalia, N.; Wella, S.A.; Sholihun, S. Thermoelectric properties of monolayer and bilayer buckled XTe (X= Ge, Sn, and Pb). Adv. Nat. Sci. Nanosci. Nanotechnol. 2022, 13, 025008. [Google Scholar] [CrossRef]
- Pandit, A.; Haleoot, R.; Hamad, B. Thermal conductivity and enhanced thermoelectric performance of SnTe bilayer. J. Mater. Sci. 2021, 56, 10424–10437. [Google Scholar] [CrossRef]
- Ju, H.; Kim, J. Anion-exchanged porous SnTe nanosheets for ultra-low thermal conductivity and high-performance thermoelectrics. Chem. Eng. J. 2020, 402, 126274. [Google Scholar] [CrossRef]
- Panwar, A.K.; Tanwar, P.; Singh, S.; Srivastava, A.K. Investigation of vacuum evaporated SnTe thin films for their structural, electrical and thermoelectric properties. Indian J. Pure Appl. Phys. (IJPAP) 2020, 58, 740–749. [Google Scholar]
- Asfandiyar Wei, T.R.; Li, Z.; Sun, F.H.; Pan, Y.; Wu, C.F.; Farooq, M.U.; Tang, H.; Li, F.; Li, B.; Li, J.F. Thermoelectric SnS and SnS-SnSe solid solutions prepared by mechanical alloying and spark plasma sintering: Anisotropic thermoelectric properties. Sci. Rep. 2017, 7, 43262. [Google Scholar] [CrossRef]
- Zhao, L.-D.; Lo, S.-H.; Zhang, Y.; Sun, H.; Tan, G.; Uher, C.; Wolverton, C.; Dravid, V.P.; Kanatzidis, M.G. Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals. Nature 2014, 508, 373–377. [Google Scholar] [CrossRef]
- Chandra, S.; Biswas, K. Realization of High Thermoelectric Figure of Merit in Solution Synthesized 2D SnSe Nanoplates via Ge Alloying. J. Am. Chem. Soc. 2019, 141, 6141–6145. [Google Scholar] [CrossRef]
- Li, S.; Hou, Y.; Li, D.; Zou, B.; Zhang, Q.; Cao, Y.; Tang, G. Realization of high thermoelectric performance in solution-synthesized porous Zn and Ga codoped SnSe nanosheets. J. Mater. Chem. A 2022, 10, 12429–12437. [Google Scholar] [CrossRef]
- Wang, F.Q.; Zhang, S.; Yu, J.; Wang, Q. Thermoelectric properties of single-layered SnSe sheet. Nanoscale 2015, 7, 15962–15970. [Google Scholar] [CrossRef]
- Ding, G.; Hu, Y.; Li, D.; Wang, X. A comparative study of thermoelectric properties between bulk and monolayer SnSe. Results Phys. 2019, 15, 102631. [Google Scholar] [CrossRef]
- Guo, S.-D.; Wang, Y.-H. Thermoelectric properties of orthorhombic group IV–VI monolayers from the first-principles calculations. J. Appl. Phys. 2017, 121, 034302. [Google Scholar] [CrossRef]
- Zhu, X.-L.; Hou, C.-H.; Zhang, P.; Liu, P.-F.; Xie, G.; Wang, B.-T. High Thermoelectric Performance of New Two-Dimensional IV–VI Compounds: A First-Principles Study. J. Phys. Chem. C 2019, 124, 1812–1819. [Google Scholar] [CrossRef]
- Ding, D.; Wang, D.; Zhao, M.; Lv, J.; Jiang, H.; Lu, C.; Tang, Z. Interface Engineering in Solution-Processed Nanocrystal Thin Films for Improved Thermoelectric Performance. Adv. Mater. 2016, 29. [Google Scholar] [CrossRef] [PubMed]
- Haq, B.U.; AlFaify, S.; Alshahrani, T.; Ahmed, R.; Mahmood, Q.; Tahir, S.; Alhashim, H.H.; Laref, A. Exploring the potential of lead-chalcogenide monolayers for room-temperature thermoelectric applications. Ceram. Int. 2020, 47, 3380–3388. [Google Scholar] [CrossRef]
- Yin, Z.P.; Sheng, C.Y.; Hu, R.; Han, S.; Fan, D.; Cao, G.H.; Liu, H. Strong interlayer coupling in two-dimensional PbSe with high thermoelectric performance. J. Phys. Condens. Matter 2020, 33, 325701. [Google Scholar] [CrossRef]
- Gayner, C.; Kar, K.K.; Kim, W. Recent progress and futuristic development of PbSe thermoelectric materials and devices. Mater. Today Energy 2018, 9, 359–376. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, L.-D. Charge and phonon transport in PbTe-based thermoelectric materials. npj Quantum Mater. 2018, 3, 55. [Google Scholar] [CrossRef]
- Pei, Y.; Shi, X.; LaLonde, A.; Wang, H.; Chen, L.; Snyder, G.J. Convergence of electronic bands for high performance bulk thermoelectrics. Nature 2011, 473, 66–69. [Google Scholar] [CrossRef]
- Girard, S.N.; He, J.; Zhou, X.; Shoemaker, D.; Jaworski, C.M.; Uher, C.; Dravid, V.P.; Heremans, J.P.; Kanatzidis, M.G. High Performance Na-doped PbTe–PbS Thermoelectric Materials: Electronic Density of States Modification and Shape-Controlled Nanostructures. J. Am. Chem. Soc. 2011, 133, 16588–16597. [Google Scholar] [CrossRef]
- Heremans, J.P.; Wiendlocha, B.; Chamoire, A.M. Resonant levels in bulk thermoelectric semiconductors. Energy Environ. Sci. 2011, 5, 5510–5530. [Google Scholar] [CrossRef]
- Biswas, K.; He, J.; Zhang, Q.; Wang, G.; Uher, C.; Dravid, V.P.; Kanatzidis, M.G. Strained endotaxial nanostructures with high thermoelectric figure of merit. Nat. Chem. 2011, 3, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wu, H.; Cui, J.; Wang, D.; Fu, L.; Zhang, Y.; Chen, Y.; He, J.; Pennycook, S.J.; Zhao, L.-D. Realizing high performance n-type PbTe by synergistically optimizing effective mass and carrier mobility and suppressing bipolar thermal conductivity. Energy Environ. Sci. 2018, 11, 2486–2495. [Google Scholar] [CrossRef]
- Volkov, B.A.; Ryabova, L.I.; Khokhlov, D.R. Mixed-valence impurities in lead telluride-based solid solutions. Physics-Uspekhi 2002, 45, 819. [Google Scholar] [CrossRef]
- Su, X.; Hao, S.; Bailey, T.P.; Wang, S.; Hadar, I.; Tan, G.; Song, T.; Zhang, Q.; Uher, C.; Wolverton, C.; et al. Weak Electron Phonon Coupling and Deep Level Impurity for High Thermoelectric Performance Pb1−xGaxTe. Adv. Energy Mater. 2018, 8, 1800659. [Google Scholar] [CrossRef]
- Hao, X.; Chen, X.; Zhou, X.; Zhang, L.; Tao, J.; Wang, C.; Wu, T.; Dai, W. Performance Optimization for PbTe-Based Thermoelectric Materials. Front. Energy Res. 2021, 9, 754532. [Google Scholar] [CrossRef]
- Bafekry, A.; Stampfl, C.; Peeters, F.M. The Electronic, Optical, and Thermoelectric Properties of Monolayer PbTe and the Tunability of the Electronic Structure by External Fields and Defects. Phys. Status Solidi (b) 2020, 257, 2000182. [Google Scholar] [CrossRef]
- Tang, S.; Wu, M.; Bai, S.; Luo, D.; Zhang, J.; Yang, S. Honeycomb-like puckered PbTe monolayer: A promising n-type thermoelectric material with ultralow lattice thermal conductivity. J. Alloys Compd. 2022, 907, 164439. [Google Scholar] [CrossRef]
- Al Bouzieh, N.; Sattar, M.A.; Benkraouda, M.; Amrane, N. A Comparative Study of Electronic, Optical, and Thermoelectric Properties of Zn-Doped Bulk and Monolayer SnSe Using Ab Initio Calculations. Nanomaterials 2023, 13, 2084. [Google Scholar] [CrossRef]
- Khoa, D.Q.; Nguyen, C.V.; Phuc, H.V.; Ilyasov, V.V.; Vu, T.V.; Cuong, N.Q.; Hoi, B.D.; Lu, D.V.; Feddi, E.; El-Yadri, M.; et al. Effect of strains on electronic and optical properties of monolayer SnS: Ab-initio study. Phys. B Condens. Matter 2018, 545, 255–261. [Google Scholar] [CrossRef]
- Do, T.-N.; Idrees, M.; Amin, B.; Hieu, N.N.; Phuc, H.V.; Hoa, L.T.; Nguyen, C.V. First principles study of structural, optoelectronic and photocatalytic properties of SnS, SnSe monolayers and their van der Waals heterostructure. Chem. Phys. 2020, 539, 110939. [Google Scholar] [CrossRef]
- Fatahi, N.; Hoat, D.M.; Laref, A.; Amirian, S.; Reshak, A.H.; Naseri, M. 2D Hexagonal SnTe monolayer: A quasi direct band gap semiconductor with strain sensitive electronic and optical properties. Eur. Phys. J. B 2020, 93, 32. [Google Scholar] [CrossRef]
- Xu, L.; Yang, M.; Wang, S.J.; Feng, Y.P. Electronic and optical properties of the monolayer group-IV monochalcogenides MX (M = Ge, Sn; X = S, Se, Te). Phys. Rev. B 2017, 95, 235434. [Google Scholar] [CrossRef]
- Liu, M.; Zhan, Q.; Li, W.; Li, R.; He, Q.; Wang, Y. Effect of Zn doping concentration on optical band gap of PbS thin films. J. Alloys Compd. 2019, 792, 1000–1007. [Google Scholar] [CrossRef]
- Ekuma, C.E. Effects of Vacancy Defects on the Electronic and Optical Properties of Monolayer PbSe. J. Phys. Chem. Lett. 2018, 9, 3680–3685. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wu, L.; Urban, A.; Cao, H.; Lu, P. Anisotropic to Isotropic Transition in Monolayer Group-IV Tellurides. Materials 2021, 14, 4495. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-B.; Guo, P.; Zhang, Y.-N.; Peng, R.-F.; Zhang, H.; Liu, L.-M. High carrier mobility of few-layer PbX (X= S, Se, Te). J. Mater. Chem. C 2015, 3, 6284–6290. [Google Scholar] [CrossRef]
- Stotter, J.; Show, Y.; Wang, S.; Swain, G. Comparison of the Electrical, Optical, and Electrochemical Properties of Diamond and Indium Tin Oxide Thin-Film Electrodes. Chem. Mater. 2005, 17, 4880–4888. [Google Scholar] [CrossRef]
- Ellmer, K. Past achievements and future challenges in the development of optically transparent electrodes. Nat. Photon 2012, 6, 809–817. [Google Scholar] [CrossRef]
- Azani, M.R.; Hassanpour, A.; Torres, T. Benefits, Problems, and Solutions of Silver Nanowire Transparent Conductive Electrodes in Indium Tin Oxide (ITO)-Free Flexible Solar Cells. Adv. Energy Mater. 2020, 10, 2002536. [Google Scholar] [CrossRef]
- Guerrero, A.; Marchesi, L.F.; Boix, P.P.; Ruiz-Raga, S.; Ripolles-Sanchis, T.; Garcia-Belmonte, G.; Bisquert, J. How the Charge-Neutrality Level of Interface States Controls Energy Level Alignment in Cathode Contacts of Organic Bulk-Heterojunction Solar Cells. ACS Nano 2012, 6, 3453–3460. [Google Scholar] [CrossRef]
- Ishii, H.; Hayashi, N.; Ito, E.; Washizu, Y.; Sugi, K.; Kimura, Y.; Niwano, M.; Ouchi, Y.; Seki, K. Kelvin probe study of band bending at organic semiconductor/metal interfaces: Examination of Fermi level alignment. Phys. Status Solidi (a) 2004, 201, 1075–1094. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, L.; Sun, M.; Wang, M.; Chen, W.; Lin, S.; Xie, D.; Zhu, H. Large scale self-assembly of SnSe nanosheets prepared by the hot-injection method for photodetector and capacitor applications. Mater. Today Energy 2019, 12, 418–425. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, H.; Zhou, Y.; Liao, L.; Jiang, Y.; Yang, X.; Chen, G.; Lin, M.; Wang, Y.; Peng, H.; et al. Controlled synthesis of single-crystal SnSe nanoplates. Nano Res. 2015, 8, 288–295. [Google Scholar] [CrossRef]
- Xu, H.; Hao, L.; Liu, H.; Dong, S.; Wu, Y.; Liu, Y.; Cao, B.; Wang, Z.; Ling, C.; Li, S.; et al. Flexible SnSe Photodetectors with Ultrabroad Spectral Response up to 10.6 μm Enabled by Photobolometric Effect. ACS Appl. Mater. Interfaces 2020, 12, 35250–35258. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Ding, Y.; Hu, X.; Zhou, W.; Yu, X.; Zhang, S. Recent progress in 2D group IV–IV monochalcogenides: Synthesis, properties and applications. Nanotechnology 2019, 30, 252001. [Google Scholar] [CrossRef] [PubMed]
- Taher, T.; Chakraborty, R.; Ahmed, S.; Subrina, S. First-principles study on strain engineered SnS monolayer. In Proceedings of the 2018 10th International Conference on Electrical and Computer Engineering (ICECE), Dhaka, Bangladesh, 20–22 December 2018; pp. 1–4. Available online: https://ieeexplore.ieee.org/abstract/document/8636748/ (accessed on 6 September 2024).
- Dong, A.; Tian, T.; Zhao, H.; Li, S.; Deng, S.; Song, X.; Yan, Y.; Xia, C.; Li, J. Low-temperature deposition of 2D SnS nanoflakes on PET substrates for flexible photodetectors with broadband response. Semicond. Sci. Technol. 2020, 35, 115016. [Google Scholar] [CrossRef]
- Wen, H.; Xiong, L.; Tan, C.; Zhu, K.; Tang, Y.; Wang, J.; Zhong, X. Localized electric-field-enhanced low-light detection by a 2D SnS visible-light photodetector. Chin. Phys. B 2021, 30, 057803. [Google Scholar] [CrossRef]
- Luo, J.; Song, X.; Lu, Y.; Hu, Y.; Lv, X.; Li, L.; Li, X.; Deng, J.; Yan, Y.; Jiang, Y.; et al. Phase-controlled synthesis of SnS2 and SnS flakes and photodetection properties. J. Phys. Condens. Matter 2022, 34, 285701. [Google Scholar] [CrossRef]
- Dong, W.; Lu, C.; Luo, M.; Liu, Y.; Han, T.; Ge, Y.; Xue, X.; Zhou, Y.; Xu, X. Enhanced UV–Vis photodetector performance by optimizing interfacial charge transportation in the heterostructure by SnS and SnSe2. J. Colloid Interface Sci. 2022, 621, 374–384. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, T.; Seliverstov, A.; Zhang, H.; Wang, Y.; Wang, F.; Peng, X.; Lu, Q.; Qin, C.; Pan, X.; et al. Layer-Dependent Optoelectronic Properties of 2D van der Waals SnS Grown by Pulsed Laser Deposition. Adv. Electron. Mater. 2019, 6, 1901020. [Google Scholar] [CrossRef]
- Li, X.; Ruan, S.; Zhu, H. Easy fabrication of performant and broadband response SnS/Si photodetector. Mater. Sci. Semicond. Process. 2022, 151, 106991. [Google Scholar] [CrossRef]
- Kumar, M.; Patel, M.; Kim, J.; Lim, D. Enhanced broadband photoresponse of a self-powered photodetector based on vertically grown SnS layers via the pyro-phototronic effect. Nanoscale 2017, 9, 19201–19208. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Xie, Z.; Fan, T.; Li, J.; Wang, Y.; Wu, L.; Ma, D.; Li, Z.; Ge, Y.; Huang, Z.N.; et al. Black-phosphorus-analogue tin monosulfide: An emerging optoelectronic two-dimensional material for high-performance photodetection with improved stability under ambient/harsh conditions. J. Mater. Chem. C 2018, 6, 9582–9593. [Google Scholar] [CrossRef]
- Vinoth, E.; Archana, J.; Harish, S.; Navaneethan, M. Hydrothermally Derived Layered 2D SnS Nanosheets for Near Infra-Red (NIR) Photodetectors. IEEE Photon- Technol. Lett. 2021, 33, 1499–1502. [Google Scholar] [CrossRef]
- Mahdi, M.S.; Ibrahim, K.; Ahmed, N.M.; Hmood, A.; Mustafa, F.I.; Azzez, S.A.; Bououdina, M. High performance and low-cost UV–Visible–NIR photodetector based on tin sulphide nanostructures. J. Alloys Compd. 2018, 735, 2256–2262. [Google Scholar] [CrossRef]
- He, B.; Jing, Y.; Zhu, X.; Fan, C.; Sun, C.; Zhang, Y.; Wang, M.; Zheng, H.; Li, E. Visible to Near-Infrared Light Detectors Based on Au/SnS/Ag Schottky Structure With Ultrahigh Responsivity and Fast Response. IEEE Trans. Electron Devices 2023, 70, 4251–4256. [Google Scholar] [CrossRef]
- Balakarthikeyan, R.; Santhanam, A.; Khan, A.; El-Toni, A.M.; Ansari, A.A.; Imran, A.; Shkir, M.; AlFaify, S. Performance analysis of SnS thin films fabricated using thermal evaporation technique for photodetector applications. Optik 2021, 244, 167460. [Google Scholar] [CrossRef]
- Wang, J.; Lian, G.; Xu, Z.; Fu, C.; Lin, Z.; Li, L.; Wang, Q.; Cui, D.; Wong, C.-P. Growth of Large-Size SnS Thin Crystals Driven by Oriented Attachment and Applications to Gas Sensors and Photodetectors. ACS Appl. Mater. Interfaces 2016, 8, 9545–9551. [Google Scholar] [CrossRef]
- Patel, M.; Kim, H.-S.; Kim, J. Wafer-scale production of vertical SnS multilayers for high-performing photoelectric devices. Nanoscale 2017, 9, 15804–15812. [Google Scholar] [CrossRef]
- Jiang, T.; Zang, Y.; Sun, H.; Zheng, X.; Liu, Y.; Gong, Y.; Fang, L.; Cheng, X.; He, K. Broadband High-Responsivity Photodetectors Based on Large-Scale Topological Crystalline Insulator SnTe Ultrathin Film Grown by Molecular Beam Epitaxy. Adv. Opt. Mater. 2016, 5, 1600727. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Dong, S.; Xu, H.; Wu, Y.; Hao, L.; Cao, B.; Li, M.; Wang, Z.; Han, Z.; et al. Photothermoelectric SnTe Photodetector with Broad Spectral Response and High On/Off Ratio. ACS Appl. Mater. Interfaces 2020, 12, 49830–49839. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, W.; Pan, Z.; Yu, Q.; Yin, Q.; Guo, L.; Zhao, Y.; Sun, T.; Bao, Q.; Zhang, K. Ultra-Broadband Flexible Photodetector Based on Topological Crystalline Insulator SnTe with High Responsivity. Small 2018, 14, e1802598. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xia, J.; Li, X.; Ru, F.; Chen, X.; Hua, Z.; Shao, R.; Wang, X.; Zhang, W.; Lee, C.-S.; et al. Vapor phase epitaxy of PbS single-crystal films on water-soluble substrates and application to photodetectors. Nano Res. 2022, 15, 5402–5409. [Google Scholar] [CrossRef]
- Thabit, M.Y.H.; Kaawash, N.M.S.; Begum, S.; Halge, D.I.; Narwade, V.N.; Alegaonkar, P.S.; Bogle, K.A. Simple and Productive Method to Develop Highly Sensitive and Fast Infrared Photodetector Using Spray Deposited Nanocrystalline PbS Thin Film. J. Phys. Conf. Ser. 2023, 2426, 012004. [Google Scholar] [CrossRef]
- Khanzode, P.M.; Halge, D.I.; Narwade, V.N.; Dadge, J.W.; Bogle, K.A. Highly photoresponsive visible light photodetector using nano PbS thin film on paper. Optik 2021, 226, 165933. [Google Scholar] [CrossRef]
- Ren, Y.; Dai, T.; Luo, W.; Liu, X. Fabrication of lead selenide thin film photodiode for near-infrared detection via O2-plasma treatment. J. Alloys Compd. 2018, 753, 6–10. [Google Scholar] [CrossRef]
- Peng, S.; Li, H.; Zhang, C.; Han, J.; Zhang, X.; Zhou, H.; Liu, X.; Wang, J. Promoted Mid-Infrared Photodetection of PbSe Film by Iodine Sensitization Based on Chemical Bath Deposition. Nanomaterials 2022, 12, 1391. [Google Scholar] [CrossRef]
- He, B.; Ren, Y.-X.; Dai, T.-J.; Hou, S.; Liu, X.-Z. Characterization and performance of graphene–PbSe thin film heterojunction. Rare Met. 2019, 40, 219–224. [Google Scholar] [CrossRef]
- Che, Y.; Cao, X.; Yao, J. A PbSe nanocrystal vertical phototransistor with graphene electrode. Opt. Mater. 2019, 89, 138–141. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, Z.; Wang, P.; Wei, G.; He, A.; Guo, W.; Wang, M. Schottky–ohmic converted contact, fast-response, infrared PbTe photodetector with stable photoresponse in air. RSC Adv. 2016, 6, 107878–107885. [Google Scholar] [CrossRef]
- Han, Z.; Singh, V.; Kita, D.; Monmeyran, C.; Becla, P.; Su, P.; Li, J.; Huang, X.; Kimerling, L.C.; Hu, J.; et al. On-chip chalcogenide glass waveguide-integrated mid-infrared PbTe detectors. Appl. Phys. Lett. 2016, 109, 071111. [Google Scholar] [CrossRef]










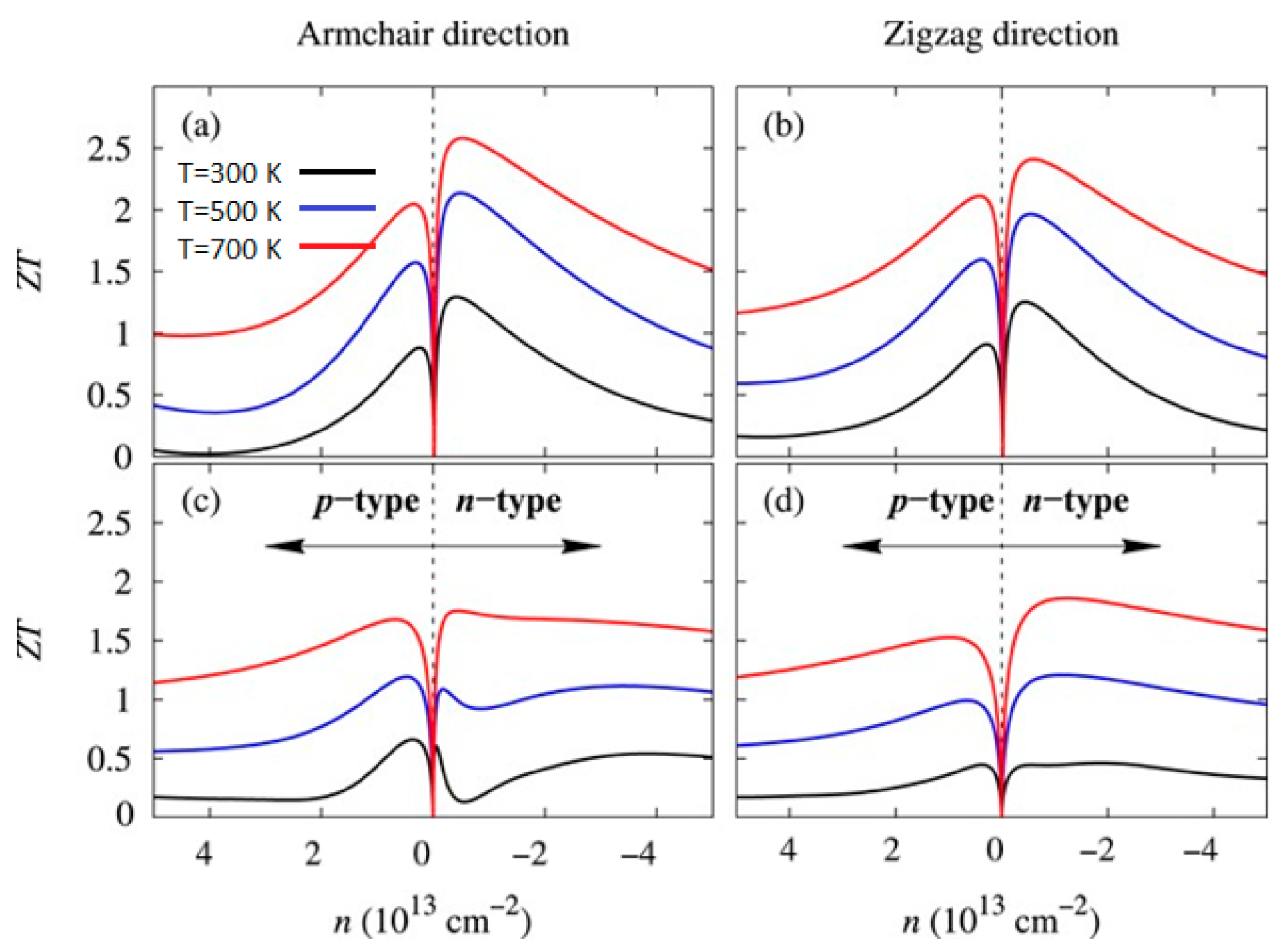

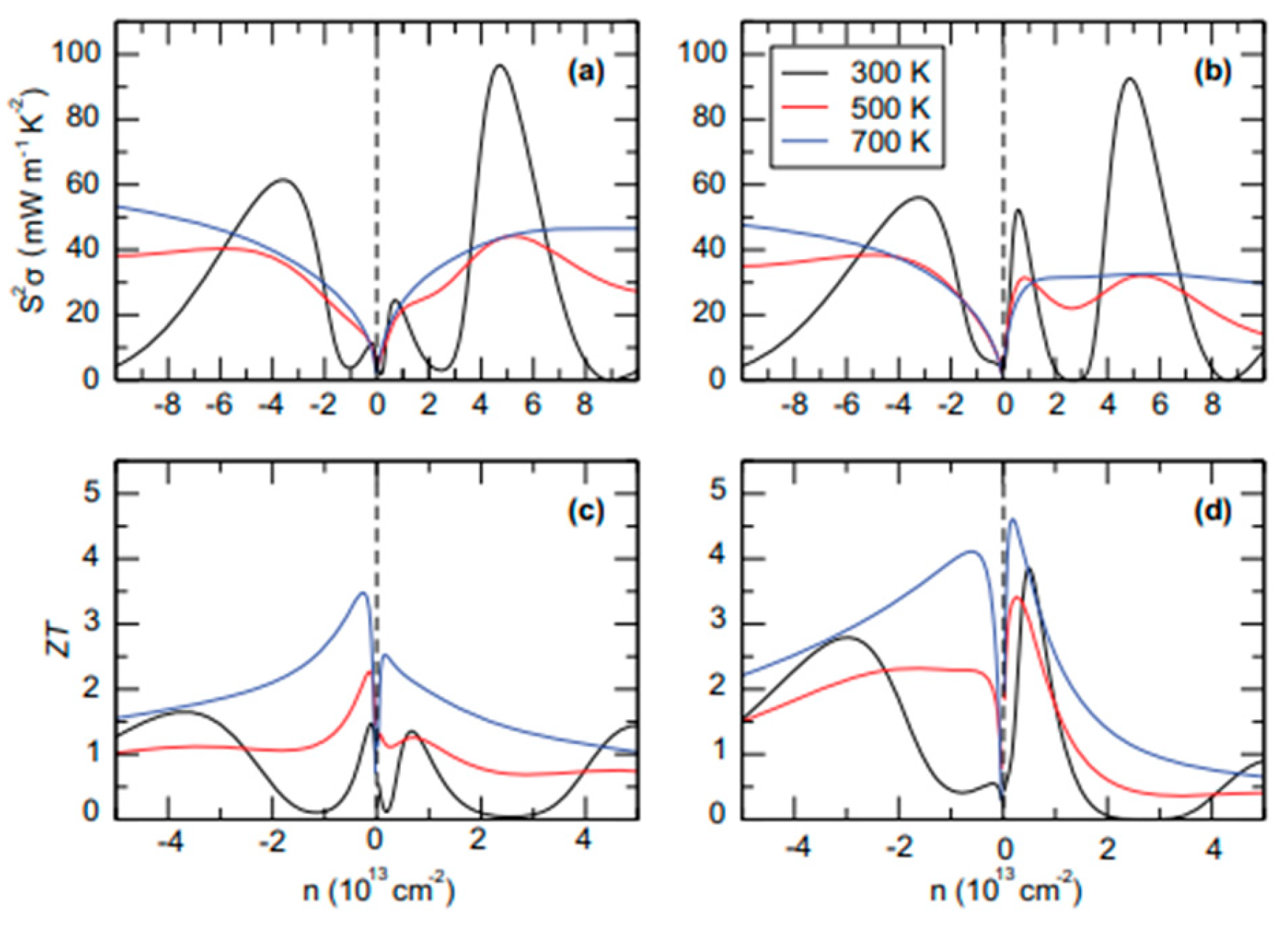
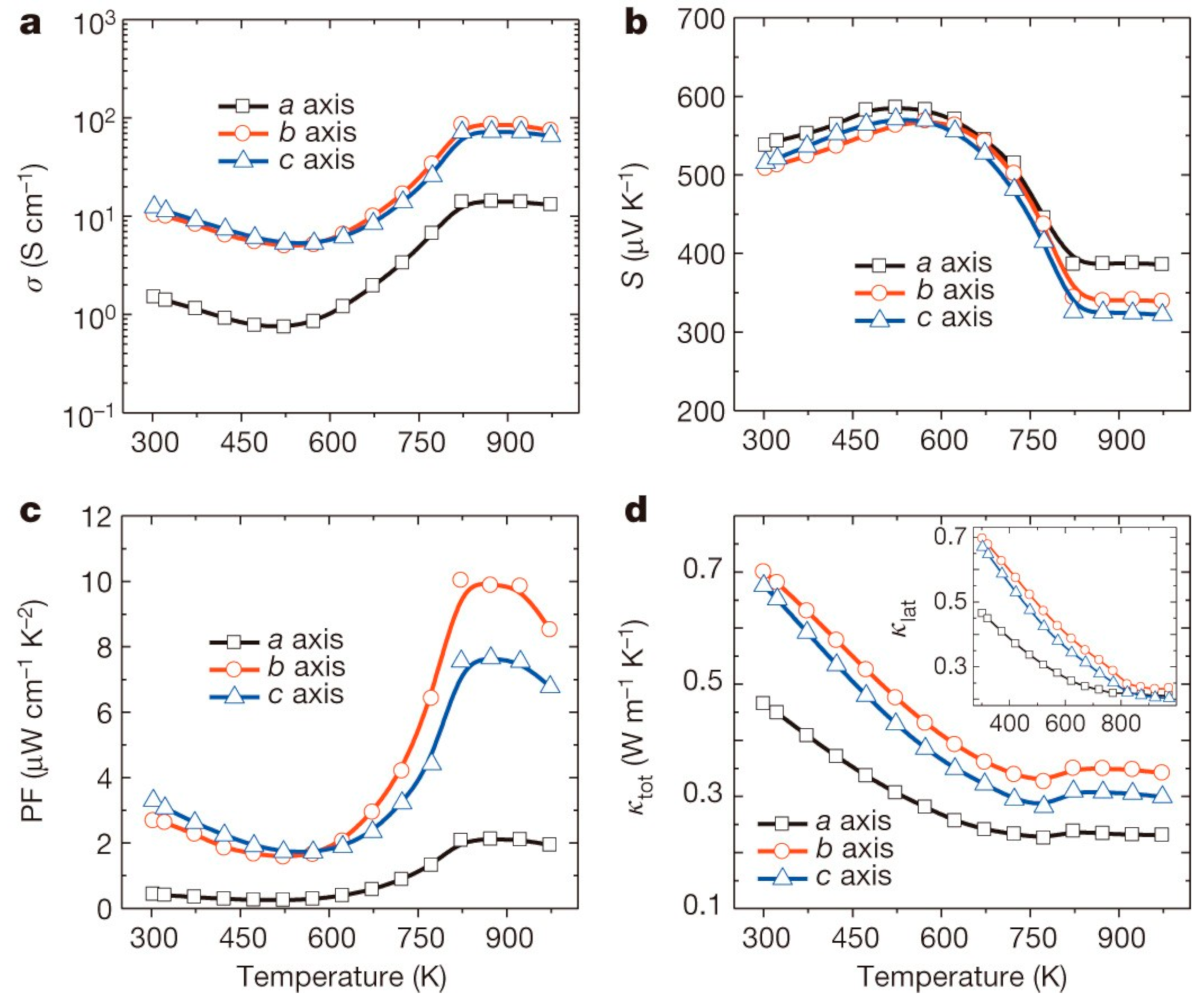
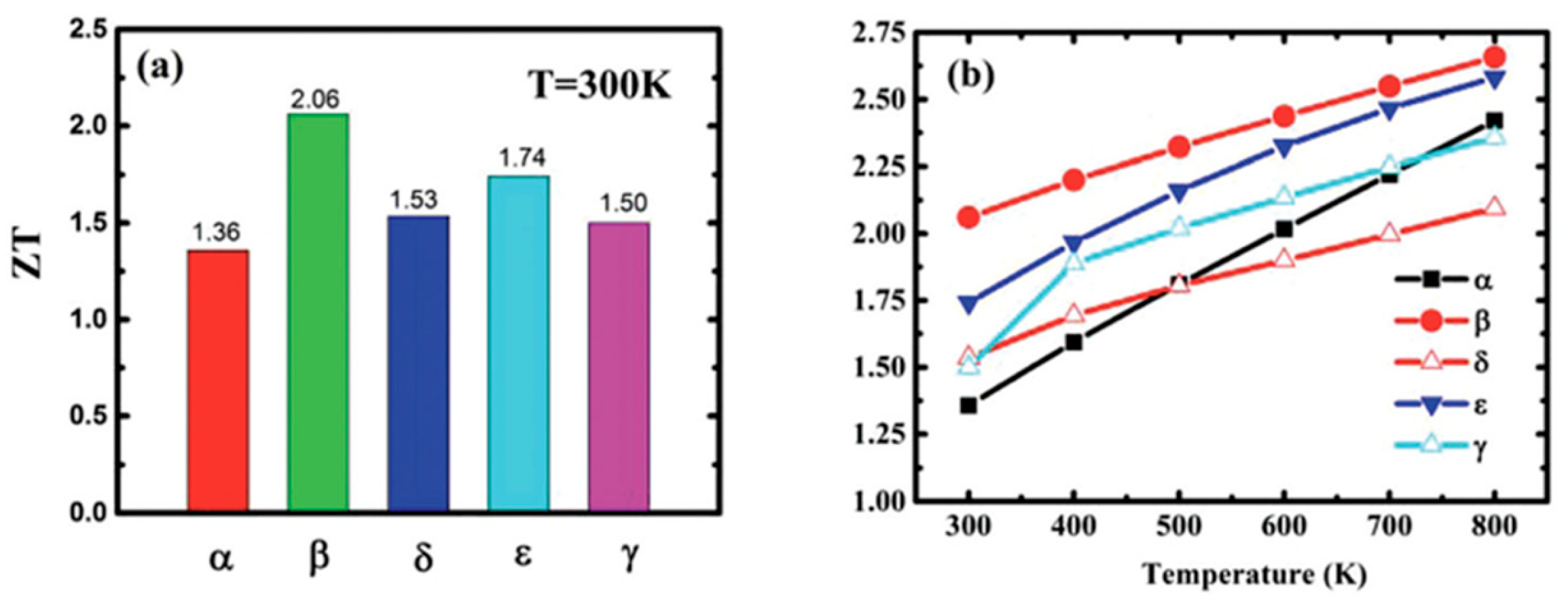


















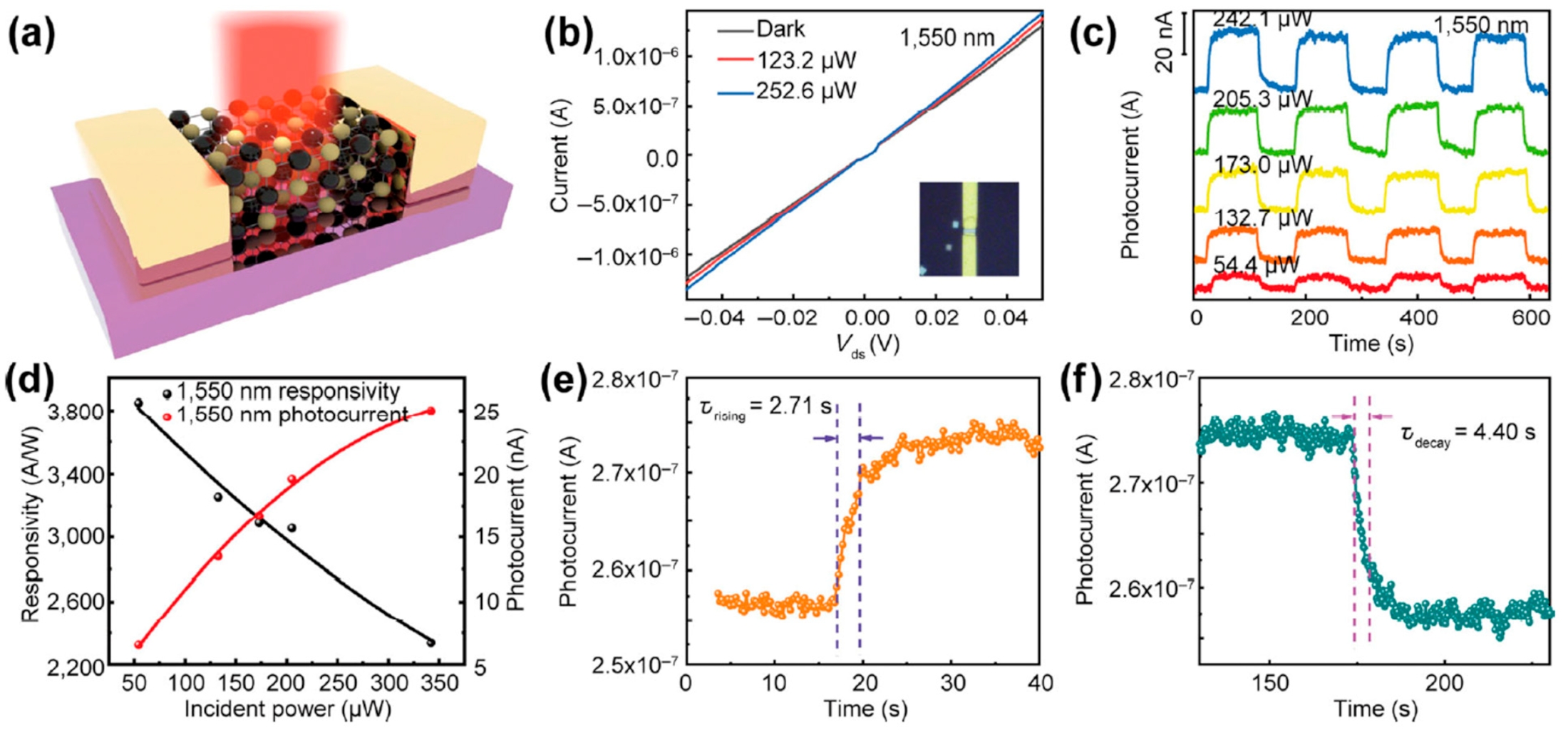
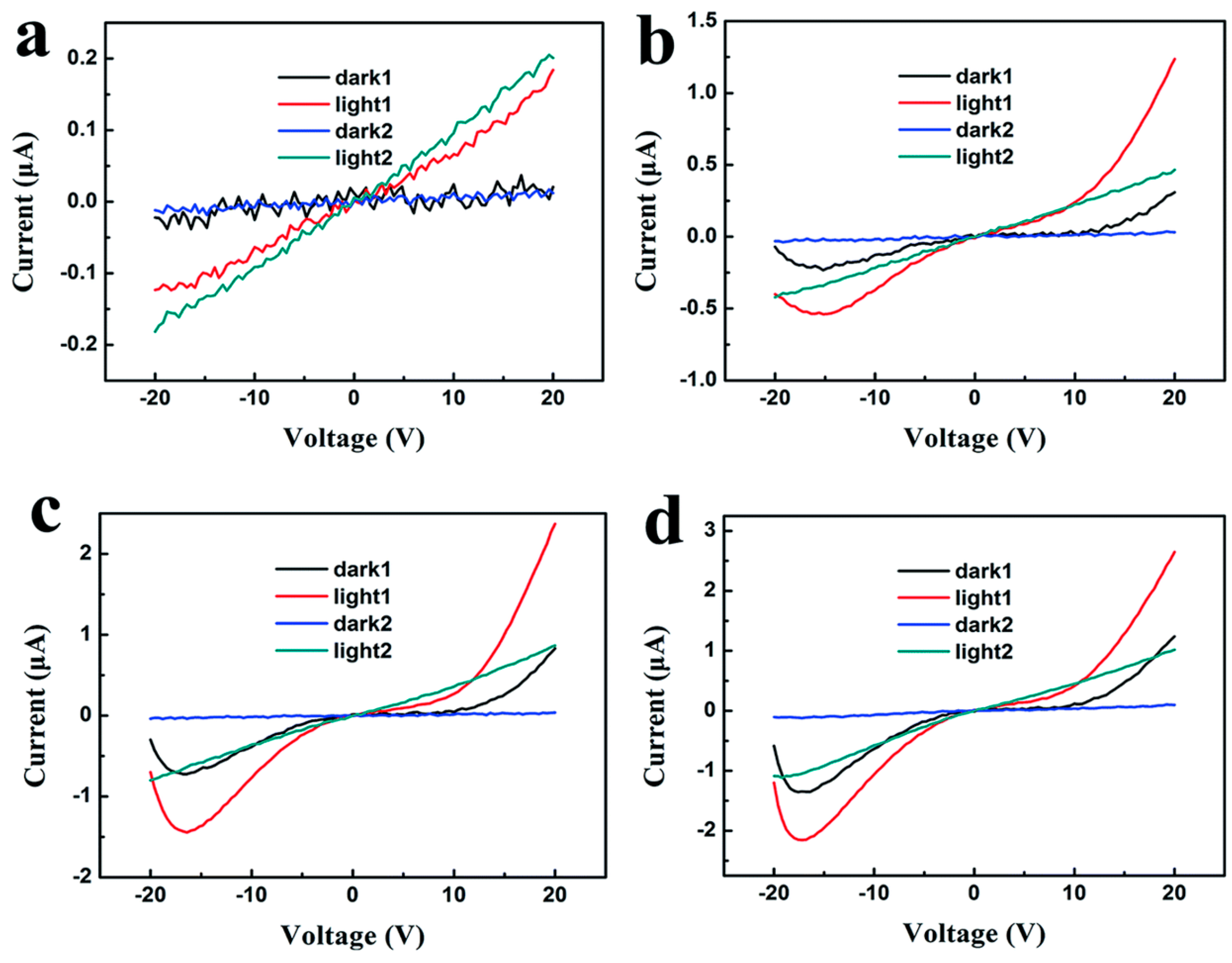
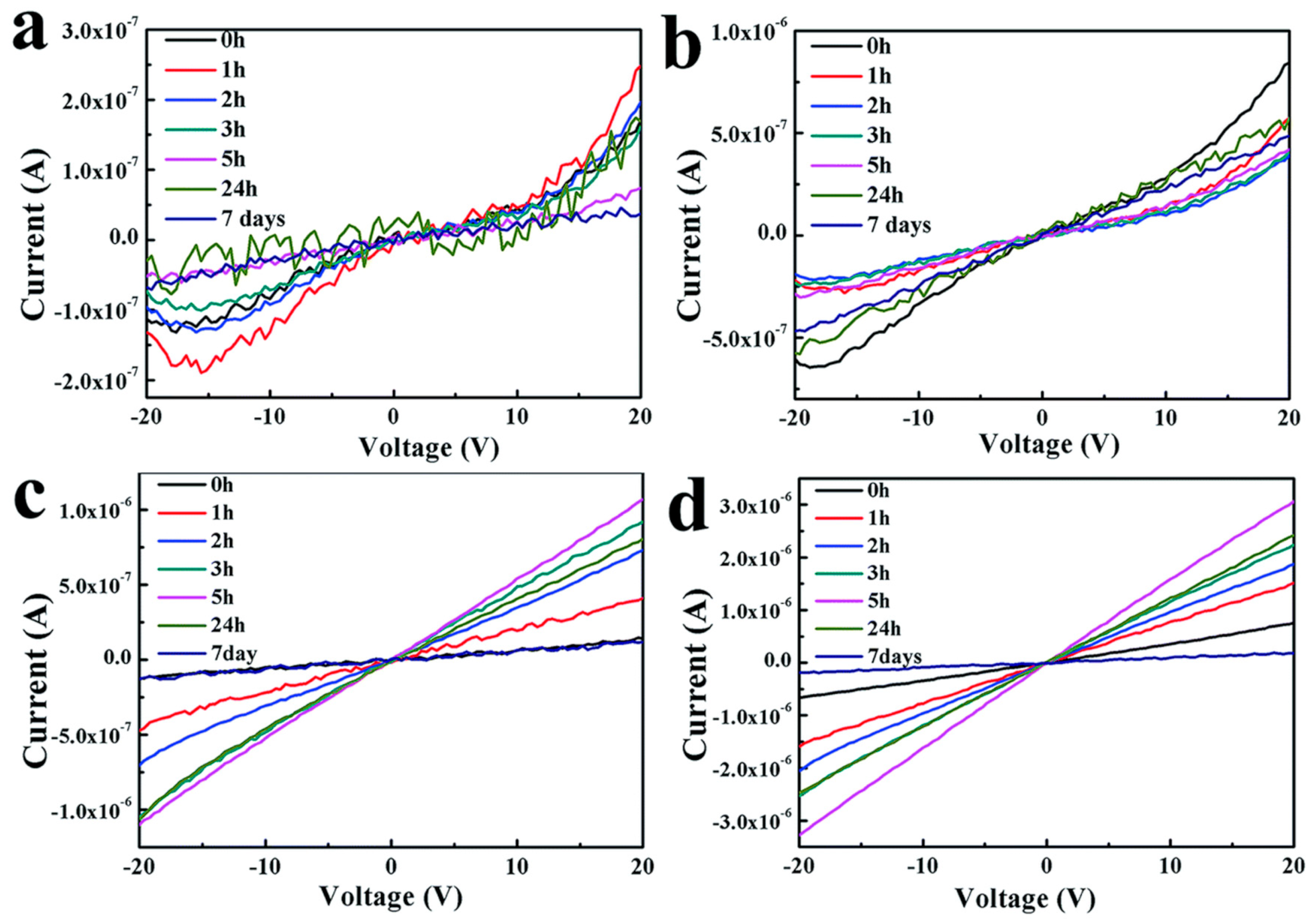
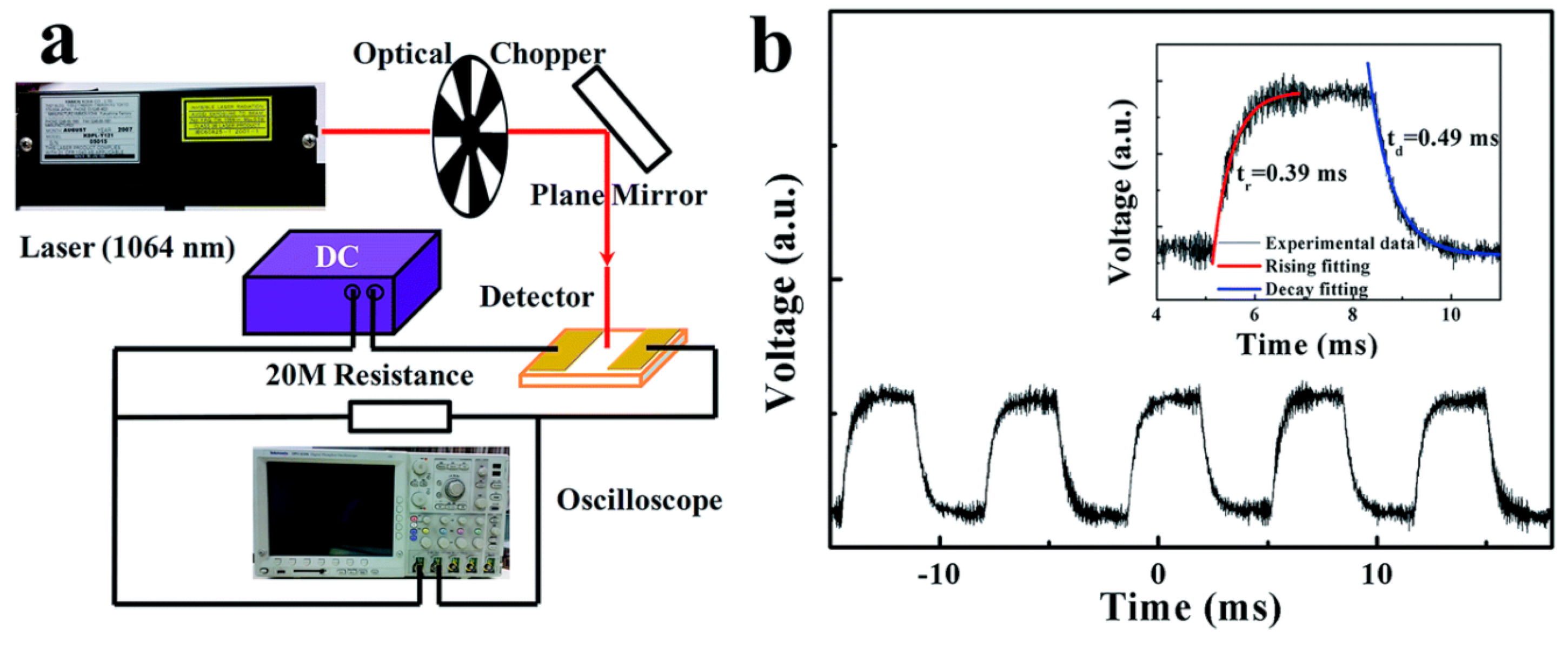
| Material | Morphology | Process Used | Thickness (nm) | Reference |
|---|---|---|---|---|
| SnSe | Nanoflakes | ME | >10 | Song et al. [38] |
| SnSe | Nanoflakes | Micromechanical Cleavage | 90 | Cho et al. [28] |
| SnSe | Nanoflakes | Micromechanical Cleavage | ~7 and 28 | Yang et al. [27] |
| SnSe | Nanoflakes | ME | 50 | Guo et al. [30] |
| SnSe | Nanoflakes | ME | 71 | Yang et al. [29] |
| SnSe | Nanoflakes | ME | 20.2–31.5 | Liu et al. [31] |
| SnSe | Nanoflakes | ME | 1.82 | Liu et al. [32] |
| SnSe | 2D films | LPE | N/A | Li et al. [58] |
| Monolayer | LPE | N/A | ||
| SnSe | NSs | LPE | 3000 rpm: 8.9 8000 rpm: 5.9 12,000 rpm: 4.3 | Huang et al. [33] |
| SnSe | NSs | LPE | 3000 rpm: 9.5 8000 rpm: 6 12,000 rpm: 2.5 | Ye et al. [34] |
| SnSe | NSs | LPE | 8 | Li et al. [59] |
| SnSe | NSs | Li Intercalation Assisted LPE | 3.4 | Ju and Kim [37] |
| SnSe | NSs | Li Intercalation Assisted LPE | 6 | Ren et al. [35] |
| SnSe | NSs | Li Intercalation Assisted LPE | 0.68 | Qiao et al. [36] |
| SnSe | NSs | Molecule intercalation method and LPE (sonication-assisted) | 1.82 | Doung et al. [39] |
| SnSe | Nanoflakes | Electrochemical and ME | 2.7 | Lee et al. [40] |
| SnSe | Nanoflakes | Vapor transport deposition and nitrogen etching | 0.68 | Jiang et al. [48] |
| SnSe | NSs | Chemical Vapor Transport and ME | 6 | Yu et al. [49] |
| SnSe | Nanoflakes | Sputtering | 5 | Hao et al. [51] |
| SnSe | Nanoflakes | PVD | ~2.0 | Chiu et al. [53] |
| SnSe | Nanoflakes | Vapor Transport Deposition and Nitrogen Etching | 0.68 | Jiang et al. [54] |
| SnSe | Nanoflakes | MBE | 0.68 | Zhou et al. [57] |
| SnSe | Monolayer | Van der Waals MBE | 0.60 | Chang et al. [56] |
| SnSe | Nanoflakes | Microwave-assisted solvothermal synthesis | N/A | Hartl et al. [58] |
| SnS | NSs | PVD | N/A | Zhang et al. [60] |
| SnS | NSs | LPE | 4.1 | Brent et al. [61] |
| SnS | Monolayer | Van der Waals Adhesion Exfoliation Method | 0.7 | Khan et al. [62] |
| SnS | NSs | N/A | N/A | Khan et al. [63] |
| SnS | NSs | Thermal Evaporation | Free of buffer layer-: 80 With Ag buffer layer-: 37 With Au buffer layer-: 51 | Jamali-Sheini et al. [64] |
| SnS | NSs | Microwave-assisted Assisted Solvothermal Synthesis | N/A | Modi et al. [65] |
| SnS | Porous NSs | Chemical Exfoliation Process | N/A | Ju et al. [66] |
| SnS | NSs | PVD | 12 | Yuan et al. [67] |
| SnS | NSs | Laser ablation vapor transport process | N/A | Kang et al. [68] |
| SnS | NSs | Chemical Bath Deposition (CBD) | 100 | Shan et al. [69] |
| SnS | Nanoflakes | Thermal evaporation | 5–160 | Sutter et al. [70] |
| SnS | Nanoflakes | CVD | 25–95 | Liu et al. [71] |
| SnS | NSs | LPE | ~4.5 and ~10 | Sarkar and Stratakis [72] |
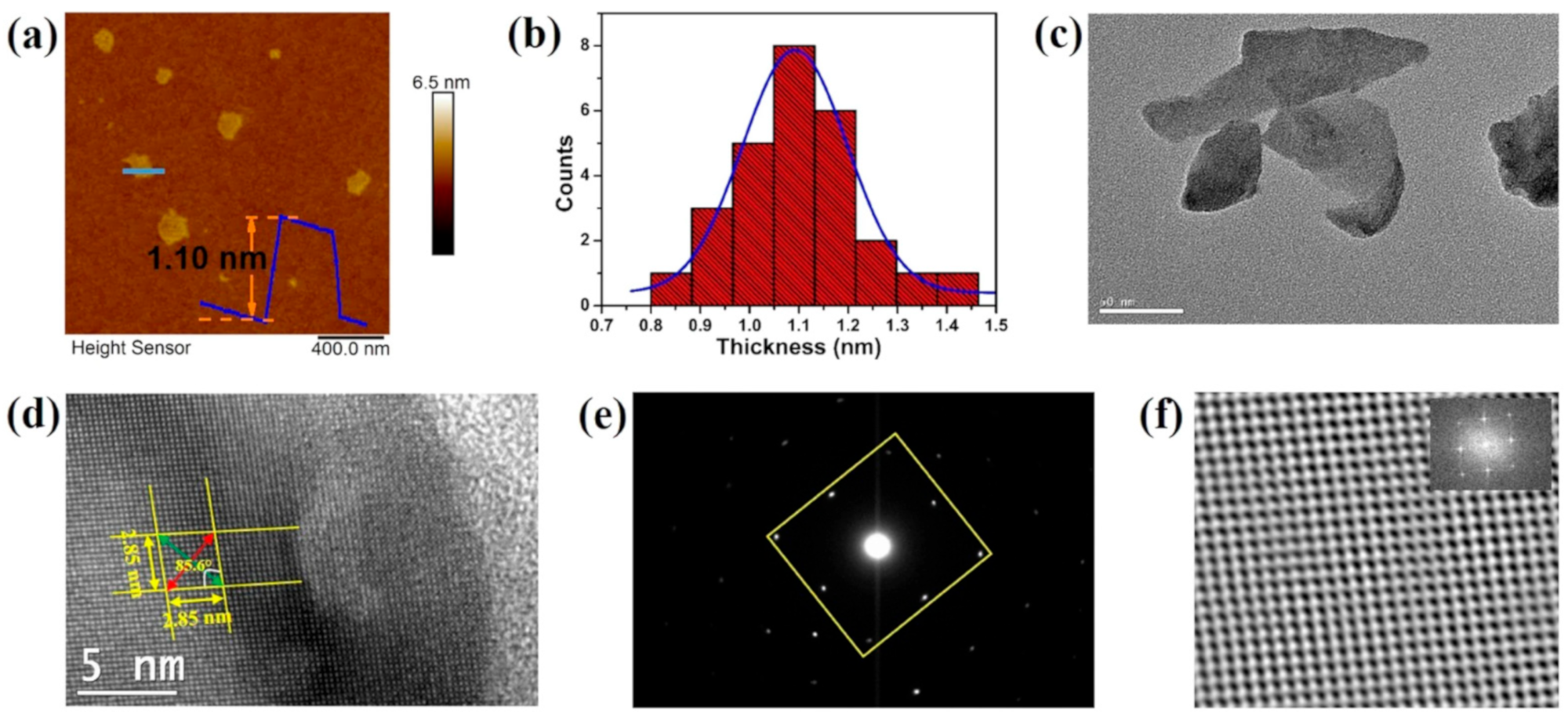
| SnS | NSs | LPE | 1.10 | Sarker et al. [42] |
| SnS | NSs | Solvothermal Process | 10 | Li et al. [73] |
| SnS | Nanoflakes | PVD | 5.5 | Xia et al. [74] |
| SnS | NSs | ME | ~0.8 and ~1.8 | Krishnamurti et al. [75] |
| SnS | Nanoflakes | ME | 13.8, 10.5 and 4.3 | Higashitarumizu et al. [76] |
| SnS | Nanoplates | Physical vapor transport | 6 | Tian et al. [77] |
| SnS | NSs | Magnetron Sputtering | 11.2 | Patel et al. [78] |
| SnS | NSs | Colloidal synthesis | 7 | Li et al. [79] |
| SnS | NSs | N/A | 20 | Zhang et al. [80] |
| SnTe | 2D layers | DFT calculations | N/A | Chang et al. [26] |
| SnTe | Nanocrystals | Colloidal synthesis (hot injection) | 30 | Li et al. [81] |
| SnTe | 2D layers | N/A | N/A | He et al. [82] |
| SnTe | 2D Nanoplates | CVD | 3.6 | Liu et al. [83] |
| SnTe | NSs | N/A | N/A | Song et al. [84] |
| Nanofilms | Magnetron Sputtering | N/A | ||
| SnTe | NSs | Low-temperature STM and MBE | N/A | Chang et al. [85] |
| PbTe | NSs | PVD | 7.1 | Zhao et al. [86] |
| PbTe | NSs | Hydrothermal synthesis | N/A | Zhu et al. [87] |
| PbSe | NSs | Atmospheric pressure chemical vapor deposition (APCVD) | ~19.6, ~47.6, and ~101 | Jiang et al. [88] |
| PbSe | Nanoplates | N/A | 2–4 | Koh et al. [89] |
| PbSe | Nanoplates | Colloidal synthesis | 0.8 ± 0.1 | Klepig et al. [90] |
| PbS | NSs | Colloidal Synthesis | 1.2–4.6 | Bhandari et al. [91] |
| PbS | Nanoplatelets | LPE | 3.7 and 11.2 | Gao et al. [92] |
| PbS | NSs | Colloidal synthesis | N/A | Moayed et al. [93] |
| PbS | NSs | Oriented attachment of nanocrystals | 4, 5.9, and 7 | Aerts et al. [94] |
| PbS | NSs | Solvothermal method | 25 | Zhu et al. [95] |
| PbS | Nanoplates | CVD | 5–35 | Wen et al. [96] |
| PbS | NSs | 2D Oriented attachment of nanocrystals | 2.2 | Schleicher et al. [97] |
| PbS | NSs | Colloidal synthesis (Precursor-based) | 2.0 and 3.6 | Zhang et al. [98] |
| PbS | Nanoplates | WCS | 3.5 | Wu et al. [99] |
| PbS | NSs | Bottom-up approach | 1.8 | Acharya et al. [100] |
| PbS | Nanoplatelets | Colloidal synthesis | 1–2 | Manteiga Vázquez et al. [101] |
| PbS | Nanoplates | Colloidal synthesis (single-molecule precursor approach) | 1.8 | Khan et al. [102] |
| PbS | NSs | Colloidal synthesis | 2.2 | áPeter Lu [103] |
| PbS | NSs | Colloidal synthesis | 2.4 | Premathilaka et al. [104] |
| PbS | Nanoplates | Hot-Injection | 5 | Sontangg et al. [105] |
| PbS | NSs | Colloidal synthesis | 10 ± 0.2 | Bielewicz et al. [106] |
| PbS | Nanoplates | Chemical synthesis | 50 | Han et al. [107] |
| PbS | NSs | One pot synthesis | <5 | Shkir et al. [108] |
| PbS | NSs | Colloidal synthesis | 1.2 | Akkerman et al. [109] |
| PbS | NSs | CVD | 50 | Gu et al. [110] |
| Material Phase | Type of Lattice | Process Used for Synthesis/Calculation Method | Pressure (GPa) | Type of Film | Lattice Parameters a, b, c or a, b or a (Å) | Reference |
|---|---|---|---|---|---|---|
| α-SnSe | Orthogonal, Pnma | N/A | N/A | N/A | 11.5524, 4.1777, 4.4261 | [114] |
| β-SnSe | Orthogonal, Cnma | N/A | N/A | N/A | 4.3062, 11.7243, 4.3178 | [117] |
| α-SnSe | Orthogonal, Pnma | Arc Melting | N/A | Multilayer | 11.5524, 4.1777 4.4261 | [118] |
| β-SnSe | Orthogonal, Cmcm | Arc Melting with isothermal heating | N/A | Multilayer | 4.3062, 11.7243, 4.3178 | [118] |
| π-SnSe | Cubic, P213 | N/A | N/A | Multilayer | 11.9702 | [119] |
| Rock salt SnSe | N/A | N/A | N/A | Monolayer | N/A | [122] |
| π-SnSe | Honeycomb | DFT, as implemented in the VASP | N/A | Monolayer | N/A | [121] |
| α-SnSe | Orthorhombic | N/A | N/A | Monolayer | 3.95, 4.82 | [121] |
| β-SnSe | 2D hexagonal | N/A | N/A | Monolayer | 3.78 | [121] |
| SnSe | N/A | N/A | N/A | Nanosheet | N/A | [49] |
| Rock salt SnSe | Cubic, Fmm | DFT, as implemented in the VASP | N/A | Multilayer | 5.71 | [123] |
| α-SnSe | Orthorhombic, P21mn | ab initio DFT (PBE and HSE) | N/A | Monolayer | a = 3.95, b = 4.82 | [124] |
| β-SnSe | Hexagonal, P3m1 | ab initio DFT (PBE and HSE) | N/A | Monolayer | 3.78, 3.78 | [124] |
| γ-SnSe | Honeycomb, P21mn | ab initio DFT (PBE and HSE) | N/A | Monolayer | 3.78, 6.11 | [124] |
| δ-SnSe | Honeycomb, P21ca | ab initio DFT (PBE and HSE) | N/A | Monolayer | 6.14, 6.23 | [124] |
| ε-SnSe | Honeycomb, P21ca | ab initio DFT (PBE and HSE) | N/A | Monolayer | 7.1, 6.6 | [124] |
| α-SnSe | N/A | N/A | N/A | Multilayer | 11.32, 4.05, 4.24 | [125] |
| β-SnSe | N/A | N/A | N/A | Multilayer | 4.148, 11.480, 4.177 | [125] |
| π-SnS | N/A | N/A | N/A | N/A | 11.595 | [124] |
| α-SnS | Orthogonal, Pnma | N/A | N/A | Multilayer | 11.32, 4.05, 4.24 | [126] |
| β-SnS | Orthogonal, Cmcm | N/A | N/A | Multilayer | 4.148, 11.480 4.177 | [126] |
| α-SnTe | rhombohedral or orthorhombic | N/A | N/A | N/A | a = 6.235 Å and α = 89.895 Å | [127] |
| β-SnTe | rhombohedral or orthorhombic, Fmm | N/A | Room temperature and pressure | N/A | a = 6.318 Å and α = 90 Å | [85] |
| γ-SnTe | rhombohedral or orthorhombic | N/A | N/A | N/A | N/A | [127] |
| Rock salt SnS | Cubic, Fmm | Epitaxial growth at high-pressure | N/A | Multilayer | 5.80 | [26] |
| π-SnS | Cubic, P213 | Chemical Synthesis | N/A | Multilayer | 11.595 | [125] |
| α-SnTe | rhombohedral or orthorhombic, R3m | Arc Melting | N/A | Multilayer | a = 6.235 and α = 89.895 | [128] |
| β-SnTe | Cubic, Fmm | Arc Melting with isothermal heating | >18 kbar | Multilayer | a = 6.318 and α = 90 | [128] |
| γ-SnTe | Orthorhombic, Pnma | DFT, as implemented in the VASP | 18 kbar | Multilayer | 11.95, 4.37, 4.48 | [128] |
| A and β-SnTe | N/A | N/A | N/A | Ultrathin | N/A | [129] |
| γ-SnTe, Rock salt | N/A | Variable temperature scanning tunneling microscopy (VT-STM) | N/A | Nanoplate | N/A | [129] |
| PbS-Rock salt | Cubic, Fmm | N/A | N/A | N/A | 5.936 | [130] |
| B1-PbS to B33-PbS | Orthorhombic | N/A | 2.5 GPa | N/A | N/A | [131] |
| PbS | -Pnma -Orthorhombic | N/A | 3.0 GPa | -Nanocrystal -Nanofilms | 10.96, 4.02, 4.28 | [132] |
| Bulk PbS | N/A | First-principles calculations using the projector augmented plane waves method | N/A | Monolayer | 6.00 | [133] |
| B1-PbS | Cubic, Fmm | CGA | Normal pressure | Multilayer | a = 6.12 | [132,134,135] |
| PbS-Orthorhombic | Pnma | CGA | ~9.5 GPa | Multilayer | 11.18, 4.17, 4.05 [Pnma] 4.06, 10.85, 4.16 [Cmcm] | [136] |
| B2-PbS | cubic, Pmm | CGA | >16 GPa | Multilayer | a = 3.56 | [137] |
| B1-PbSe | Cubic, Fmm | Arc Melting | N/A | Multilayer | a = 5.936 | [132] |
| B33-PbSe | Orthorhombic, Pnma | Chemical synthesis after one hour | N/A | Multilayer | 10.96, 4.02, 4.28 | [137] |
| PbSe | Orthorhombic, Pnma | N/A | ~9.5 GPa | N/A | 11.18, 4.17, 4.05 | [134] |
| CsCl PbSe | Orthorhombic, Cmcm | N/A | ~9.5 GPa | N/A | 3.56 | [134] |
| Honeycomb Puckered PbSe | P3m1 | DFT calculation | Normal pressure | Monolayer | a = b = 4.091 | [136] |
| B1-PbTe | Cubic, Fmm | Chemical Synthesis | Normal pressure | Multilayer | a = 6.461 | [138] |
| PbTe | Pnma Pmm CsCl | N/A | N/A | N/A | N/A | [139] |
| Orthorhombic PbTe | Pnma | GGA | ~9.5 GPa | Multilayer | 8.11, 4.5, 6.26 | [140] |
| B2-PbTe | cubic, Pmm | GGA | >24.7 GPa | Multilayer | a = 3.57 | [140] |
| Material | Method | Type of Layer | Value | Temperature | PF (Power Factor) | Reference: |
|---|---|---|---|---|---|---|
| SnS | DFT and Boltzmann | Monolayer | 1.36–5 | 300–600 K | N/A | Gupta et al. [164] |
| SnS | WCS | NSs | 0.8 | 873 K | 0.48 | Li et al. [165] |
| SnS | Synthesized porous and coated with PANI | NSs | 0.078 | 450 K | N/A | Ju et al. [66] |
| SnS | First-Principle Calculations and Boltzmann Transport | Monolayer | N/A | N/A | ~1.1 W-m−1-K−2 | Gupta et al. [167] |
| SnS SnSe | DFT and Boltzmann transport | 2D monochalcogenides | 1.75, 1.88 2.63, 2.46 | 700 K | N/A | Shafique et al. [168] |
| SnS | Density functional theory and semi-classical Boltzmann transport | Monolayer | 1.04 | 700 K | N/A | Pandit et al. [169] |
| SnS | First principal calculation | Monolayer | 0.13–0.89 | 300–700 K | N/A | Fang et al. [170] |
| SnS | Green’s function-based transport | Monolayer | ZigZag-0.95 Armchair-1.6 | Room temperature | N/A | Sandonas et al. [179] |
| SnS | USPEX and VASP | Monolayer | ~1.19–3.18 | 300–900 K | N/A | Dong et al. [172] |
| SnTe | Density functional theory and semi-classical Boltzmann transport | Monolayer | n-type: 2.9 p-type: 2.2 | N/A | 37 mW/(m K2) | Li et al. [173] |
| SnTe | Phonon dispersion calculation and elasticity criteria | Monolayer | ~0.96–3.81 | 300–900 K | N/A | Dong et al. [172] |
| SnTe | First-principles calculation and Boltzmann transport | Monolayer | 1.58 | 900 K | N/A | Zhang et al. [174] |
| SnTe | First-principles calculation and Boltzmann transport | Monolayer | 1.44 | 900 K | N/A | Tang et al. [175] |
| SnTe | First-principles calculation and Boltzmann transport | Monolayer | 2.61 | 500 K | N/A | Liu et al. [176] |
| SnTe | DFT and Boltzmann transport | Monolayer and bilayer | >1.6 | 900 K | N/A | Lubis et al. [177] |
| SnTe | First-principle calculations | Bilayer | 3.48 | 700 K | N/A | Pandit et al. [178] |
| SnTe | DFT and semi-classical Boltzmann transport | Monolayer | 1.46 | 700 K | N/A | Pandit et al. [169] |
| SnTe | Synthesized porous | NSs | 1.1 | 923 K | N/A | Ju and Kim [179] |
| SnTe | Vacuum deposition method | Thin films | ~1.0 | 300 K | N/A | Panwar et al. [180] |
| SnSe | N/A | Single crystal | 2.6 | 973 K | N/A | Zhao et al. [182] |
| SnSe | Hydrothermal synthesis and SPS | Nanoplates | ~2.1 ~1.75 | 873 K | N/A | Chandra et al. [183] |
| SnSe | Synthesized porous by solution synthesis | NSs | 0.8 to ~1.86 | 873 K | N/A | Li et al. [184] |
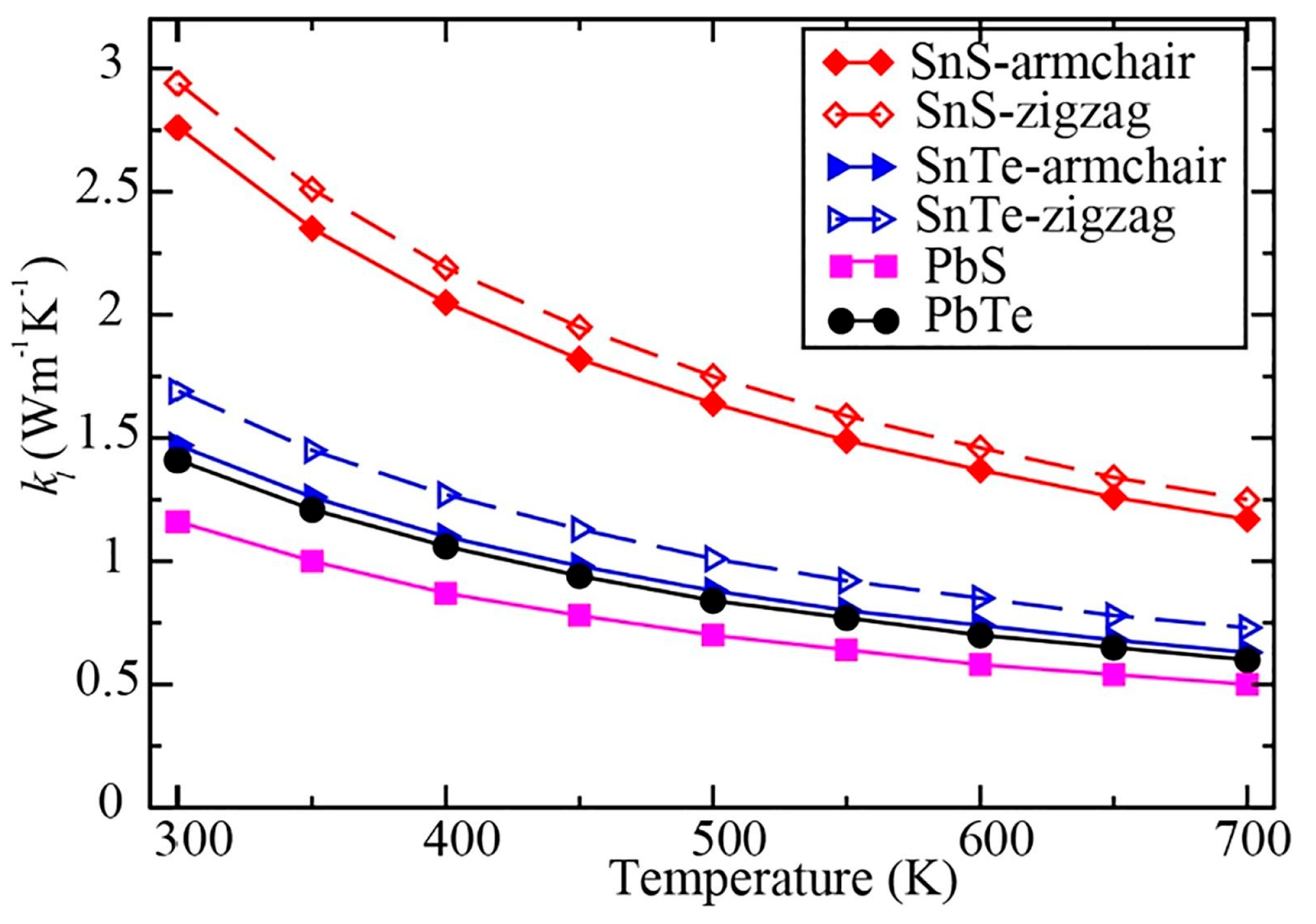
| SnSe | First principle calculations | Single sheet Single sheet | 3.27 N/A | 700 K room temperature | 2.57 W/mK (zigzag) and 2.02 W/mK (armchair) | Wang et al. [185] |
| SnSe | USPEX combined with VASP | Monolayer | ~0.93 to 2.51 | 300 K to 900 K | N/A | Dong et al. [172] |
| SnSe | DFT combined with Boltzmann transport theory | Monolayer | 2.63 and 2.46 | 700 K | N/A | Shafique et al. [168] |
| SnSe | N/A | Monolayer | N/A | -Room temperature | 0.91–0.97 W-m−1-K−2 | Fang et al. [170] |
| N/A | Monolayer | ~0.25–1.41 | 300–700 K | N/A | ||
| SnSe | First-principles electronic structure calculations and Boltzmann transport theory | Monolayer | p-type: 1.2 n-type: 2.5 | N/A | N/A | Ding et al. [186] |
| SnSe | Ge-doped synthesized by simple hydrothermal route followed by SPS | 2D nanoplates | ~2.1 | 873 K | N/A | Chandra et al. [183] |
| Ge doping (3 mol%) | Nanoplates | ~1.75 | 873 K | N/A | ||
| N/A | Nanoplates | N/A | 873 K | ~0.18 W/mK | ||
| SnSe | First-principles calculations, semi-classical Boltzmann, with SOC | Monolayer | N/A | N/A | N/A | Guo and Wang [187] |
| Monolayer | N/A | N/A | N/A | |||
| β−SnSe | DFT | Monolayer | 2.06 | 300 K | N/A | Hu et al. [122] |
| 2D hexagonal | 2.32 | 700 K | N/A | |||
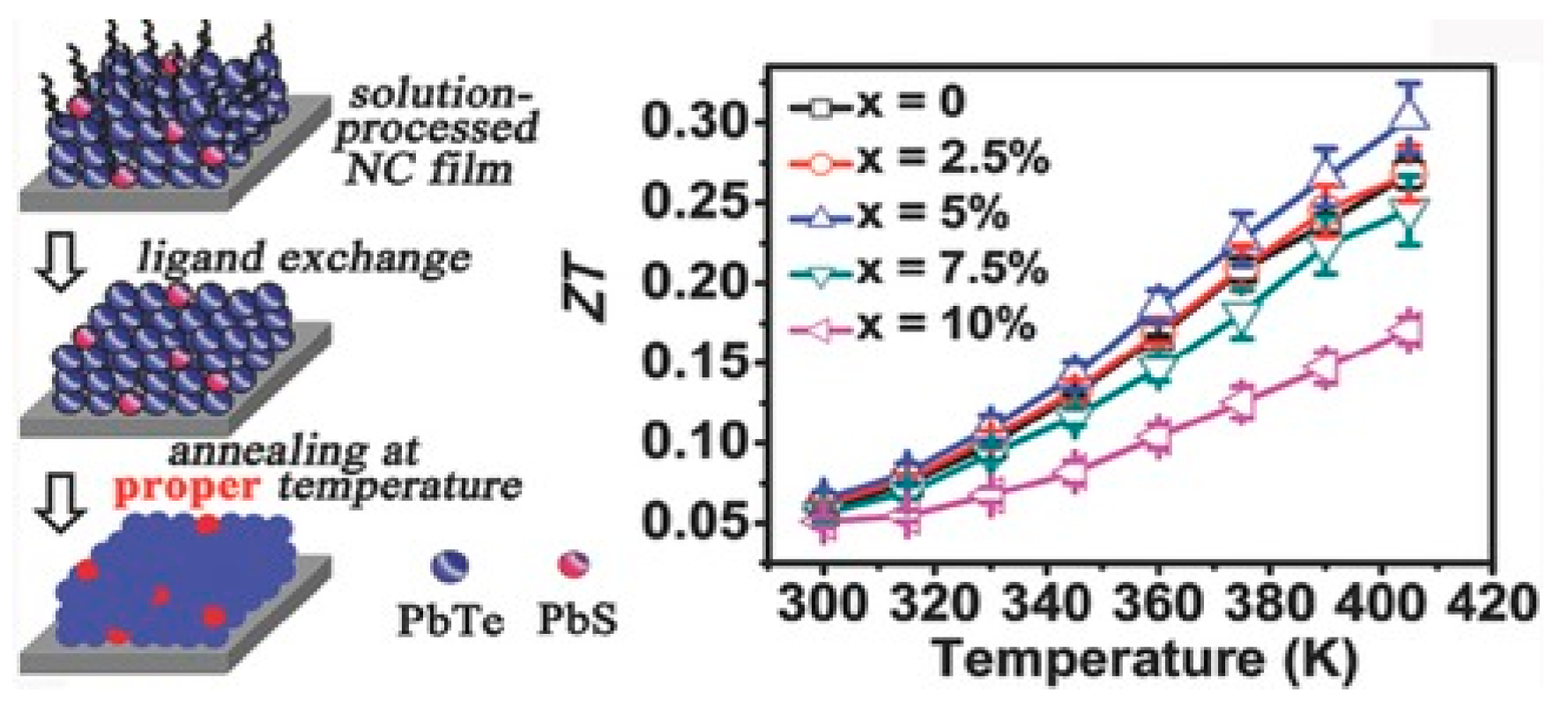
| PbS | Layer-by-layer method via ligand exchange | Nanocrystal films | 0.30 | 405 K | N/A | Ding et al. [189] |
| Layer-by-layer method via ligand exchange | Nanocrystal | 0.30 | 405 K | N/A | ||
| PbS | Ab initio approach | Monolayer | ~1.0 | N/A | 6.1 × 1010 W/mK2 | Haq et al. [190] |
| PbS | DFT combined with semi-classical Boltzmann transport theory | 2D monolayers | n-type: 1.51 | 700 K | N/A | Pandit et al. [169] |
| PbSe | p-type doped | Monolayer | p-type: ~1.3 | 900 K | N/A | Tang et al. [136] |
| PbSe | DFT and p + n doped | N/A | 0.3 and 0.8 | Room temperature | N/A | Yin et al. [191] |
| DFT and p + n doped | N/A | 2.2 | 900 K | N/A | ||
| PbSe | Doped p and n dopants | N/A | ≤0.1 to 0.3 | Room temperature | N/A | Gayner et al. [192] |
| Doped p and n dopants | N/A | ≥1 | 650–873 K | N/A | ||
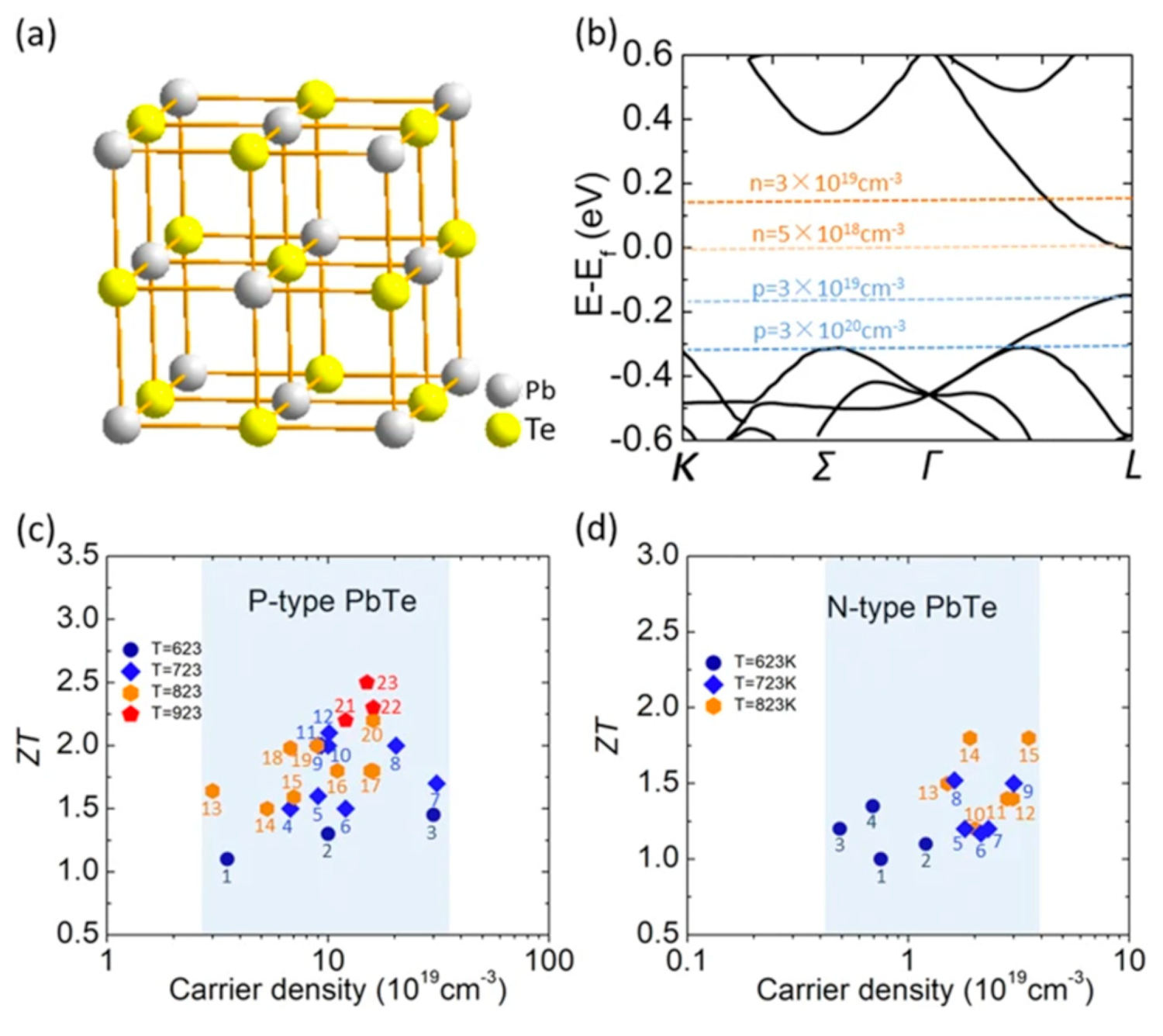
| PbTe | N/A | N/A | ~2.5 | 923 K | ~1.5 × 1020 cm−3 | [193] |
| PbTe | First-principles calculations and semi-classical Boltmazz Transport | Monolayer | ~1.55 | 900 K | N/A | [203] |
| PbTe | First-principles calculations and semi-classical Boltzmann transport theory | Monolayer | >1.58 | 900 K | N/A | Zhang et al. [174] |
| SnTe | First-principles calculations and semi-classical Boltzmann transport theory | Monolayer | N/A | N/A | N/A | Liu et al. [176] |
| First-principles calculations and semi-classical Boltzmann transport theory | Monolayer | 5.91 | 500 K | N/A | ||
| PbTe | DFT combined with semi-classical Boltzmann transport theory and SOC | 2D monolayer | 1.94 | 700 K | N/A | Pandit et al. [169] |
| Material | Type of Lattice | Process Used for Synthesis/Calculation Method | Type of Film | -Value and Temp. | Optical Bandgap | Reference |
|---|---|---|---|---|---|---|
| SnS | Monolayer | DFT, Boltzmann transport equation | Thin film | ~5 and ~1.36 at 600 K and 300 K | N/A | [165] |
| SnS | Nanosheet | WCS | Thin film | N/A | N/A | [66] |
| SnS | NSs | Coated with conductive PANI | Thin film | N/A | N/A | [166] |
| SnTe | NSs | Synthesized porous | Thin film | 1.1 at 923 K | ||
| SnS | Monolayer | Piezoelectric nanogenerators and PFM | Thin film | N/A | N/A | [64] |
| SnS SnSe | 2D heterojunction | First-principle calculations and DFT | Thin film | N/A | 1.3 eV | [167] |
| SnS | Monolayer | First-principle calculations and Boltzmann transport equation | Thin film | N/A | N/A | [168] |
| SnS | 2D monochalcogenides | DFT and Boltzmann transport theory | Thin film | 1.75, 1.88 at 700 K | N/A | [169] |
| SnSe | Monolayer | DFT and Boltzmann transport theory | Thin film | 2.63, 2.46 at 700 K | N/A | |
| SnS | 2D monolayers | DFT and semi-classical Boltzmann transport theory | Thin film | 1.04 at 700 K | N/A | [170] |
| SnS | Monolayer | First-principle calculations | Thin film | ~0.13–0.89 at 300–700 K | N/A | [179] |
| SnSe | Monolayer | N/A | N/A | ~0.25–1.41 at 300–700 K | N/A | |
| SnS | Monolayer | Green’s function-based transport techniques | Thin film | Zigzag~0.95 Armchair~1.6 | N/A | [172] |
| SnS | Monolayer | USPEX combined with VASP | Thin film | ~1.19 to 3.18 at 300K to 900 K | N/A | [173] |
| SnTe | Monolayer | Phonon dispersion calculation | Thin film | ~0.96 to 3.81 at 300K to 900 K | N/A | |
| SnSe | Monolayer | USPEX combined with VASP | Thin film | ~0.93 to 2.51 at 300K to 900 K | N/A | |
| SnTe | monolayer | DFT, Boltzmann transport theory | Thin film | 2.9 (n-type, armchair), 2.2 (p-type, armchair) | 1.05 eV | [174] |
| SnTe | Monolayer | First-principles calculation, Boltzmann transport theory | Thin film | More than 1.58 at 900 K | N/A | [175] |
| PbTe | Monolayer | First-principles calculation, the Boltzmann transport theory | N/A | ~1.58 at 900 K | N/A | |
| SnTe | 2D wrinkled monolayer | First-principles calculations, Boltzmann transport theory | Thin film | 1.44 at 900 K | N/A | [176] |
| SnTe | Monolayer | First-principles calculations, Boltzmann transport equation | Thin film | ~2.61 at 500 K | indirect band gap semiconductors | [177] |
| PbTe | Monolayer | First-principles calculations, Boltzmann transport equation | N/A | ~5.91 at 500 K | ||
| SnTe | Monolayer Bilayer | DFT, Linearised Boltzmann transport theory | Single-layer thickness | >1.6 at 900 K | N/A | [178] |
| SnTe | Bilayer | First-principle calculations | Thin film | 3.48 at 700 K | N/A | [169] |
| SnTe | Monolayer | DFT, semi-classical Boltzmann transport theory | Thin film | 1.46 at 700 K | N/A | [180] |
| PbS | 2D monolayers | DFT, semi-classical Boltzmann transport theory | N/A | n-type: 1.51 at 700 K | N/A | |
| PbTe | 2D monolayers | DFT, Semi-classical Boltzmann Transport Theory | N/A | -p-type: 1.94 at 700 K | N/A | |
| SnTe | N/A | Vacuum Deposition Method | Thin film | ~1.0 at 300 K | N/A | [181] |
| SnSe | Monolayer | N/A | N/A | N/A | N/A | [182] |
| SnSe | Single crystal | N/A | N/A | 2.6 at 973 K | N/A | [183] |
| SnSe | Nanoplates | Hydrothermal Synthesis Anisotropic Measurement Parallel to the SPS | N/A | N/A | N/A | [184] |
| SnSe | N/A | N/A | Thin film | 0.055 at 501 K | N/A | [185] |
| SnSe | Single sheet | First-Principle Calculations | N/A | 3.27 at 700 K | N/A | [186] |
| SnSe | Monolayer | First-principles calculations, Boltzmann transport theory | N/A | 1.2 (p-type), 2.5 (n-type) | N/A | [183] |
| SnSe | 2D nanoplates | Hydrothermal synthesis, SPS | N/A | ~2.1 at 873 K ~1.75 at 873 K | N/A | [187] |
| SnSe | Monolayer | First-principles calculations, semi-classical Boltzmann transport theory, SOC | N/A | N/A | N/A | [188] |
| SnS | Monolayer | |||||
| SnSe | Monolayer | Ab initio DFT calculations | N/A | β−SnSe: 2.06 at 300 K | N/A | [121] |
| SnSe | 2D hexagonal | First-principle study | N/A | 2.32 at 700 K | N/A | [189] |
| Pbs PbTe | N/A | Layer-by-layer method via ligand exchange | Nanocrystal films | 0.30 at 405 K | N/A | [190] |
| PbSe | Monolayer | N/A | N/A | 1.3 (p-type, zigzag) at 900 K | N/A | [114] |
| PbSe | 2D | First-principles calculations | N/A | 3.95 at 500 K | N/A | [191] |
| PbSe | N/A | DFT | N/A | 0.8 (p-type, room temperature), 0.3 (n-type, room temperature), 2.2 (p-type, 900 K) | N/A | [192] |
| PbSe | N/A | N/A | N/A | ≤0.1 to 0.3 (room temperature), ≥1 (650–873 K) | N/A | [193] |
| PbTe | 2D | Doped with n and p-type impurities | N/A | n and p-type: ~2.5 at 923 K | N/A | [194] |
| PbTe | N/A | Band convergence | N/A | N/A | N/A | [195] |
| PbTe | N/A | Band convergence | N/A | N/A | N/A | [196] |
| PbTe | N/A | Introducing an excess density of state near Fermi level and increasing energy-dependence (μE) | N/A | N/A | N/A | [197] |
| PbTe | Nanostructures | Introducing foreign atom doping | N/A | N/A | N/A | [202] |
| PbTe | Monolayer Bilayer Trilayer Tetralayer | First-principles calculations, DFT | N/A | N/A | Bandgap increases with a decrease in layer thickness 0.4 eV 0.25 eV 0.2 eV | [203] |
| PbTe | Honeycomb-like puckered monolayer | First-principles calculations, the semi-classical Boltzmann transport theory | N/A | ~1.55 at 900 K | Wide bandgap of 2.251 eV | [203] |
| SnSe | Nanolayers | Typical bath sonication exfoliation method | 8.9,5.9, and 4.3 nm corresponding to 15, 10, 7 layers | N/A | Indirect bandgap: 0.91, 1.13, 1.27, and 1.35 eV Calculated bandgap: 0.93 to 1.79 eV | [15] |
| SnSe | Monolayers | PBE method and HSE06 calculations | N/A | N/A | A: 1.04 eV Β: 2.22 eV Γ: 1.52 eV δ: 1.55 eV | [121] |
| SnSe | Monolayer | GW with BSE method | N/A | N/A | 1.41 eV | [204] |
| SnSe | Monolayer | DFT calculation | N/A | N/A | 0.986, 1.067, 1.471, 1.67 eV | [205] |
| SnS | Monolayer | First-principles calculations | N/A | N/A | 1.63 eV | [208] |
| SnS | Monolayer | First-principles calculations, DFT | N/A | N/A | 1.38 eV | [209] |
| SnS | Monolayer | First-principles calculations | 200 nm | N/A | 2.89 eV | [86] |
| SnS | NSs | UV–vis–NIR Fractioned SnS sol B | N/A | N/A | 1.03 eV 1.65 eV | [62] |
| SnTe | Hexagonal monolayer | Ab initio approach | N/A | N/A | PBE theory: ~2.00 eV HSE06: ~2.60 eV | [210] |
| SnX (X = S, Se, Te) | Monolayer | N/A | N/A | N/A | Indirect Bandgap | [190] |
| PbS | NSs | CBD | N/A | N/A | 1.14, 1.39, 1.43, 1.57, 1.64, 1.74 eV | [211] |
| PbS (001) | Monolayer | HSE + SOC | Hex-layer | N/A | N/A | [212] |
| Few layers | N/A | 0.24 to 1.92 | ||||
| Kf layer | N/A | N/A | ||||
| PbSe | Monolayer | DFT calculations, first-principle-based typical medium approximation | N/A | N/A | 0.21 eV | [218] |
| PbSe | Monolayers | First-principles calculations within DFT full-potential linearized-augmented-plus-local-orbit method within DFT WIEN2k computational package | N/A | N/A | Indirect Bandgaps: 0.45, 1.39, and 1.26 eV | [219] |
| PbTe | Monolayer | DFT Calculations adopting the GCA of the PBE functional | N/A | N/A | 1.742 eV Mono, bi, tri, and bulk: 1.23–3.20 eV | [220] |
| PbSe PbTe | N/A | SOC | N/A | N/A | 2 eV | [221] |
| SnSe | NSs | Typical bath sonication method | N/A | N/A | 0.91, 1.13, 1.27, and 1.35 eV | [33] |
| SnSe | Monolayer | BSE method | N/A | N/A | 1.41 eV | [15] |
| SnSe | Monolayer | GCA, SCAN, HSE06, and other DFT methods | N/A | N/A | 0.986, 1.067, 1.471, 1.67 eV | [204] |
| SnS | Monolayer | First-principles calculations | N/A | N/A | 1.63 eV | [205] |
| SnS | Monolayer | N/A | N/A | N/A | 1.3 eV | [166] |
| SnS | Monolayer | N/A | N/A | N/A | [205] | |
| SnS | Monolayer | First-principle calculations based on DFT | N/A | N/A | 1.38 eV | [222] |
| SnS | Monolayer | First-principle calculations | N/A | N/A | 2.89 eV | [86] |
| SnS | NSs | UV–vis–NIR absorbance spectroscopy | Thin film | N/A | 1.03 eV | [61] |
| SnS | Monolayer | First-principle calculations | 200 nm | N/A | 3.75 eV | [206] |
| SnTe | Hexagonal Monolayer | PBE theory and HSE06 | N/A | N/A | PBE theory: ~2.00 eV HSE06: ~2.60 eV | [207] |
| SnX (X = S, Se, Te) | Monolayer | GW-BSE method | N/A | N/A | N/A | [208] |
| PbS | NSs | CBD | N/A | N/A | 1.14, 1.39, 1.43, 1.57, 1.64, 1.74 eV | [209] |
| PbS | Monolayer to hex-layer | HSE + SOC method | N/A | N/A | 0.24 to 1.92 eV | [133] |
| PbSe | Monolayer | DFT calculations with First-principles-based typical medium approximation | N/A | N/A | 0.21 eV | [210] |
| PbSe | Monolayer | First-principles calculations within DFT | N/A | N/A | α-0.45 β-1.39 γ-1.26 | [190] |
| PbTe | Monolayer | First-Principle Calculations | N/A | N/A | 0.4 eV: bilayer 0.25 eV: trilayer 0.2 eV: tetralayer | [202] |
| PbTe | Monolayer | GCA implemented in the VASP | N/A | N/A | 1.742 eV | [211] |
| PbTe | Mono, bi, tri, and bulk | First-principle calculations | N/A | N/A | 1.23–3.20 eV | [212] |
| Material | Maximum Responsivity | Maximum Detectivity | Excitation Wavelength and Power | Rise and Fall Time | Spectral Range | Reference |
|---|---|---|---|---|---|---|
| SnSe | 405, 532, and 785 nm wavelengths as 1.75 × 104 A/W, 4.63 × 103 A/W, and 1.52 × 103 A/W | N/A | N/A | N/A | N/A | Li et al. [51] |
| SnSe NSs synthesized magnetron sputtering method | 277.3 A/W | 7.6 × 1011 Jones | UV-visible-NIR range | N/A | UV to NIR | Hao et al. [52] |
| SnSe NSs synthesized by hot injection method | 30 mA/W (400 nm)/11 mA/W (1050 nm) | N/A | 400 nm (0.46 mW/cm2)/1050 nm (0.4 mW/cm2) | N/A | Visible to NIR | Zhong et al. [218] |
| SnSe NSs synthesized by electron beam lithography | ~330 A/W | N/A | White light, bias voltage of 0.1 V | N/A | N/A | Zhao et al. [219] |
| SnSe NSs | 9.27 A/W | 4.08 × 1010 Jones | 808 nm | N/A | 360 to 1550 nm | Li et al. [166] |
| SnSe thin film photodetectors by depositing SnSe thin films on PET templates | 1745.5 A/W (404 nm)/0.16 A/W (10.6 µm) | 4.2 × 1011 Jones (404 nm)/3.9 × 107 Jones (10.6 µm) | 404 nm laser/10.6 µm laser | N/A | UV to IR | Xu et al. [220] |
| SnS nanoflakes | N/A | N/A | N/A | Fast response and recovery time | N/A | Hu et al. [221] |
| SnS nanosheet-based photodetector (monolayer) SnS nanosheet-based photodetector (Au-decorated) | 365 A/W 635 A/W | 5.70 × 104% 9.92 × 104% | 808 nm, 40 mW/cm2 808 nm, 40 mW/cm2 | Response: 0.35 s, Recovery: 0.35 s N/A | NIR NIR | Zhang et al. [60] |
| SnS nanoflakes synthesized by CVD | 156.0 A/W | 4.77 × 104% | N/A | N/A | N/A | Liu et al. [71] |
| SnS nanoflakes fabricated by poly-ethylene terephthalate substrates | 1280 A/W (355 nm)/69 A/W (1550 nm) | 3.02 × 1011 Jones | 355 nm/1550 nm | N/A | 355 nm to 1550 nm | Dong et al. [223] |
| SnS NSs synthesized by PVD | 161 A/W | 4.45 × 104% | 450 nm blue light | N/A | N/A | Wen et al. [224] |
| SnS synthesized by metallic liquid tin | 927 A/W (660 nm, single unit cell)/3.51 × 103 A/W (660 nm, multiple unit cells) | 1.09 × 109 Jones (660 nm, single unit cell)/6.83 × 1010 Jones (660 nm, multiple unit cells) | 280–850 nm | 0.12 ms (single unit cell)/0.16 ms (multiple unit cells) | Deep UV to NIR | Krishnamurthi et al. [75] |
| SnS and SnS2 nanoflakes synthesized by phase-controlled synthesis | 3390 mA/W | 1.1 × 1010 Jones | N/A | 3.10 ms (response)/1.59 ms (recovery) | N/A | Luo et al. [225] |
| SnS NSs synthesized by solvothermal process | 86.2 mA/W | 1010 Jones | Low intensity (0.02 mW/cm2) | 150 ms | N/A | Modi et al. [65] |
| SnS films | N/A | N/A | 365 nm, 80 µWcm−2 | τg: 60 ms, τd: 10 ms | Broadband | Wang et al. [226] |
| SnS films | Up to 1.17 A/W (980 nm)/17.31 mA/W (1550 nm) | N/A | 980 nm/1550 nm | 11.0 ms | Visible to NIR | Li et al. [227] |
| Sns layers grown vertically on Si substrates | 12 mA/W | ~3.2 × 1014 Jones | 760 nm, 7 mWcm−2 | Rise: ~12 µs, Decay: ~55 µs | UV to NIR | Kumar et al. [228] |
| SnS NSs synthesized by the LPE method | 59.8 μA/W | 9.43 × 107 Jones | N/A | 0.1–0.3 s | UV to Visible | Huang et al. [229] |
| SnS films synthesized by the PVD method | 14.78 µA/W | N/A | N/A | N/A | UV to visible | Dong et al. [230] |
| SnS | 0.19 A/W | 9.213 × 1011 Jones | 1030 nm | Response: 2 s, Recovery: 4 s | UV to NIR | Vinoth et al. [231] |
| SnS NSs synthesized by the PVD method | 1604 A/W | 3.42 × 1011 Jones | 850 nm | Response: 7.6 ms, Recovery: 29.9 ms | NIR | Yuan et al. [67] |
| SnS nanoflakes synthesized by CBD | 0.01 mA/W | N/A | 530 nm | Rise: 0.36 s, Decay: 0.38 s | UV to NIR | Mahdi et al. [232] |
| SnS | 1652.87 A/W | 8.05 × 1012 Jones | 850 nm | Response: 6.5 ms | Visible to NIR | He et al. [233] |
| SnS thin films fabricated by a thermal evaporation method | 6.4 × 102 A/W | 6.05 × 109 Jones | N/A | Response: 1.5 s, Recovery: 2.5 s | N/A | Balakarthikeyan et al. [234] |
| SnS thin crystals synthesized by solvothermal method | 2040 A/W | ~3 × 109 Jones | ~4.75 × 105% | ~90 ms | N/A | Wang et al. [235] |
| SnS layers on n-Si substrates fabricated photodetectors | 0.25 A/W (850 nm, −1 V bias)/1.19 A/W (10 µW/cm2) | 1.3 × 1011 Jones (850 nm, −1 V bias)/7.1 × 1011 Jones (10 µW/cm2) | 850 nm/850 nm | Rise: 41 µs/222 µs | NIR | Patel et al. [236] |
| SnTe nanoplates are synthesized by the van der Waals growth process and then deposited on mica substrates | 698 mA/W (3.6 nm thick)/1.468 A/W (35 nm thick) | 3.89 × 108 Jones | 980 nm | N/A | NIR | Liu et al. [83] |
| SnTe nanofilms synthesized by magnetron sputtering technique on a quartz substrate | 1.71 A/W | 3.46 × 1011 cmHz1/2W−1 | 940 nm, 0.2 mWcm−2 | N/A | UV to NIR | Song et al. [84] |
| SnTe NSs synthesized by MBE on SrTiO3 substrate | 3.75 A/W (2003 nm) | N/A | 405 nm, 632 nm, 808 nm, 1550 nm, 2003 nm | N/A | Visible to mid-infrared | Jiang et al. [237] |
| SnTe thin films synthesized by a magnetron sputtering method deposited on polyethylene terephthalate templates used Pd as electrodes | 3.9 mA/W | 1.3 × 1010 Jones | 404 nm | Response: 78 ms, Recovery: 84 ms | UV to MIR | Liu et al. [238] |
| SnTe nanoflakes deposited by depositing 100 nm to 120 nm thick SnTe nanoflakes on Si/SiO2 substrates with Cr/Au as electrodes | 71.11 A/W (254 nm)/49.03 A/W (635 nm)/10.91 A/W (1550 nm)/4.17 A/W (4650 nm) | N/A | 254 nm, 635 nm, 1550 nm, 4650 nm | N/A | Deep UV to MIR | Yang et al. [239] |
| PbS photodetectors by fabrication on PET substrate under dark and illuminated conditions | 0.013 A/W (100 μm gap)/0.1 A/W (10 μm gap) | 1.9 × 109 Jones (100 μm gap)/1.3 × 109 Jones (10 μm gap) | 400–750 nm, 100 mWcm−2 | N/A | Visible | Akkerman et al. [109] |
| PbS NSs by CVD method | 7.5 A/W | 1.44 × 1012 Jones | 450 nm, 40 mW/cm2 | ~0.25 s | Visible | Wang et al. [240] |
| PbS nanoplates by CVD method | 37 A/W to 119 A/W | N/A | N/A | N/A | N/A | Gu et al. [110] |
| PbS nanoplates with Cu2S residues by Cu2S residues on Si/SiO2 substrate and used Au as electrode | ~1739 A/W | 2.55 × 1011 Jones | 808 nm, 0.5 mW | N/A | NIR | Wu et al. [99] |
| PbS thin layer by synthesization on a glass substrate | 70 mA/W | 1.8 × 1011 Jones | N/A | Response: 6.4 ms, Recovery: 15.6 ms | NIR | Thabit et al. [241] |
| PbS films on paper by spray pyrolysis method and graphite electrolyte used on them | 0.0356 A/W | N/A | N/A | Response: 14.7 ms, Recovery: 6.3 ms | NIR | Khandoz et al. [242] |
| PbS NSs by LPE on ITO substrates | 27.81 mA/W | 3.96 × 1010 Jones | N/A | N/A | UV-Vis-NIR | Gao et al. [92] |
| PbSe NSs | ~998.15 A/W (473 nm)/~970.05 A/W (808 nm) | ~2.36 × 1011 Jones (473 nm)/~2.31 × 1011 Jones (808 nm) | 473 nm, 6.50–25.85 mW/cm2/808 nm, variable | N/A | NIR | Jiang et al. [88] |
| PbSe thin films by PVD | 0.35 A/W | 1.2 × 1011 Jones | N/A | N/A | NIR | Ren et al. [243] |
| PbSe films by using the CBD | 30.27 A/W | N/A | 808 nm, 233 µW/cm2 | N/A | NIR | Peng et al. [244] |
| PbSe thin films by depositing graphene and PbSe thin films on SiO2/Si substrates | 420 A/W | 5.9 × 1011 Jones | N/A | N/A | NIR | He et al. [245] |
| PbSe thin films by depositing spin-coated PbSe thin films with graphene on SiO2/Si substrate | 1.1 × 104 A/W | 1.3 × 1010 Jones | NIR, 36 mWcm−2 | Response: 7 ms, Recovery: 10 ms | NIR | Che et al. [246] |
| PbTe 2D thin films by PVD method | 3847.1 A/W | N/A | 1550 nm, 54.4 μW | 2.71 s | 4.40 s | Zhao et al. [86] |
| PbTe quantum dot thin film by a layer-by-layer spin-coating method | 0.13–1.9 mA/W (depending on thickness) | N/A | N/A | 0.39 ms | 0.49 ms | Lin et al. [247] |
| PbTe thin films | ~1.0 A/W | 2 × 1012 Jones | 2.1–2.5 μm | N/A | N/A | Han et al. [248] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, S.; Sharme, R.K.; Terrones, M.; Rana, M.M. Recent Progress on Layered Sn and Pb-Based Mono Chalcogenides: Synthesis, Structure, Optical, and Thermoelectric Properties and Related Applications. Nanomaterials 2024, 14, 1530. https://doi.org/10.3390/nano14181530
Rahman S, Sharme RK, Terrones M, Rana MM. Recent Progress on Layered Sn and Pb-Based Mono Chalcogenides: Synthesis, Structure, Optical, and Thermoelectric Properties and Related Applications. Nanomaterials. 2024; 14(18):1530. https://doi.org/10.3390/nano14181530
Chicago/Turabian StyleRahman, Safwan, Razia Khan Sharme, Mauricio Terrones, and Mukti M. Rana. 2024. "Recent Progress on Layered Sn and Pb-Based Mono Chalcogenides: Synthesis, Structure, Optical, and Thermoelectric Properties and Related Applications" Nanomaterials 14, no. 18: 1530. https://doi.org/10.3390/nano14181530
APA StyleRahman, S., Sharme, R. K., Terrones, M., & Rana, M. M. (2024). Recent Progress on Layered Sn and Pb-Based Mono Chalcogenides: Synthesis, Structure, Optical, and Thermoelectric Properties and Related Applications. Nanomaterials, 14(18), 1530. https://doi.org/10.3390/nano14181530








