Lignin Nanoparticles: Transforming Environmental Remediation
Abstract
:1. Introduction
2. Lignin Nanoparticles: Synthesis and Tailoring for Environmental Applications
2.1. Precipitation Techniques
2.1.1. Controlled Solvent Evaporation
2.1.2. Anti-Solvent Precipitation
2.1.3. Electrostatic Precipitation
2.2. Emulsion Techniques for Lignin Nanoparticle Synthesis and Targeted Pollutant Removal
2.2.1. Oil-in-Water (O/W) Emulsions
2.2.2. Water-in-Oil (W/O) Emulsions
2.2.3. Reverse Emulsion Polymerization
2.2.4. Solvent Evaporation from Emulsions
2.3. Enzymatic Modification Techniques
2.3.1. Laccase-Mediated Oxidation
2.3.2. Peroxidase-Catalyzed Modification
2.3.3. Ligninase Treatment
2.3.4. Cellulase-Catalyzed Modification
3. Unique Properties of Lignin Nanoparticles for Environmental Remediation
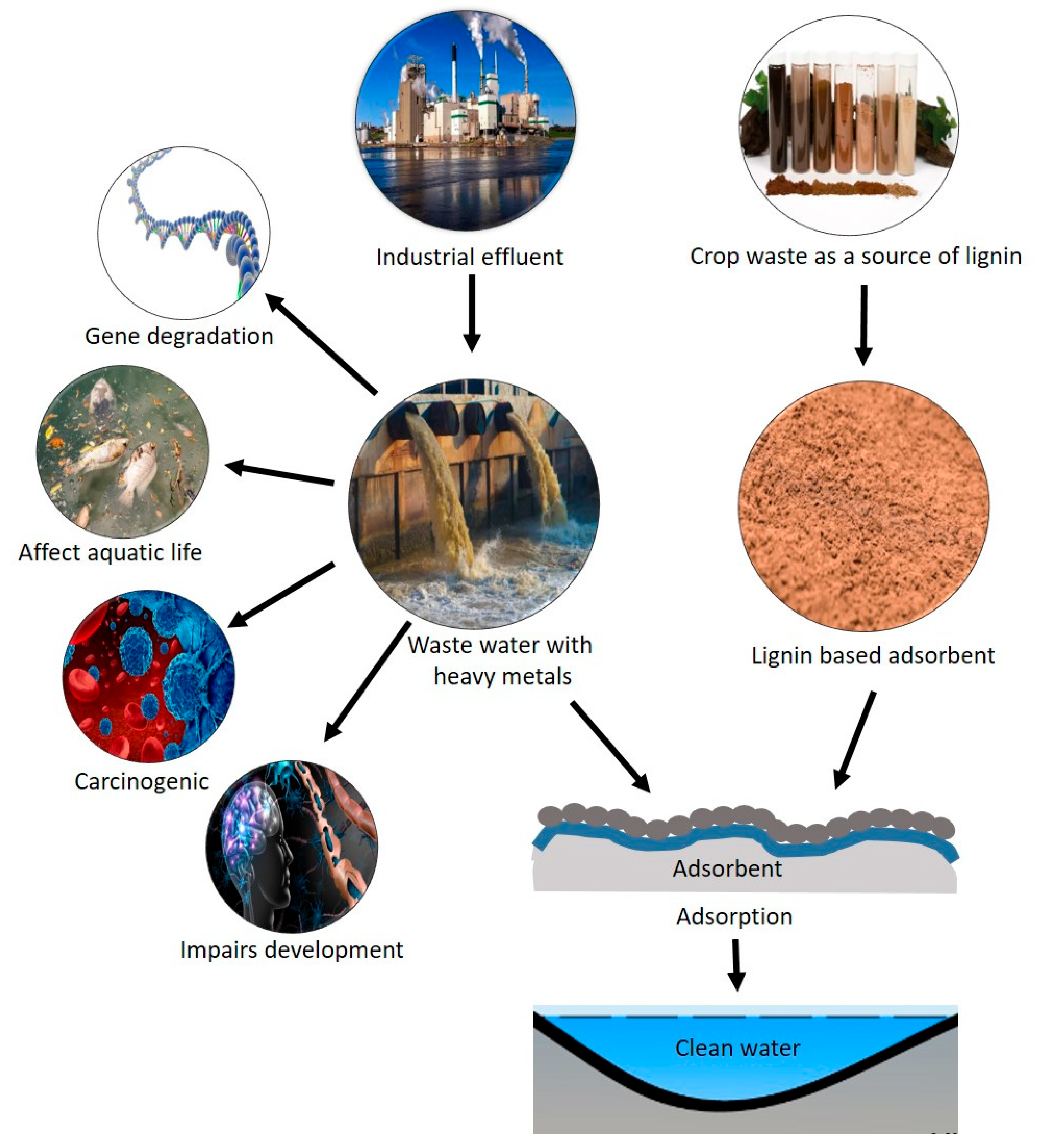
4. Application of Lignin Nanoparticles in Water Purification
- Electrostatic Interaction: Lignin nanoparticles can interact with charged organic contaminants through electrostatic attraction. Negatively charged functional groups on lignin nanoparticles can attract positively charged organic molecules (Wang et al., 2020).
- π-π Stacking: Aromatic organic contaminants often undergo π-π stacking interactions with lignin nanoparticles, facilitated by the presence of phenolic rings in lignin structures (Shen et al., 2017).Hydrogen Bonding: The hydroxyl and carbonyl groups on lignin nanoparticle surfaces can form hydrogen bonds with organic compounds, promoting their adsorption [124].
- Pore Entrapment: The porous structure of lignin nanoparticles allows for the physical trapping and immobilization of organic molecules within their pores [124].
5. Utilizing Lignin Nanoparticles for Soil Remediation
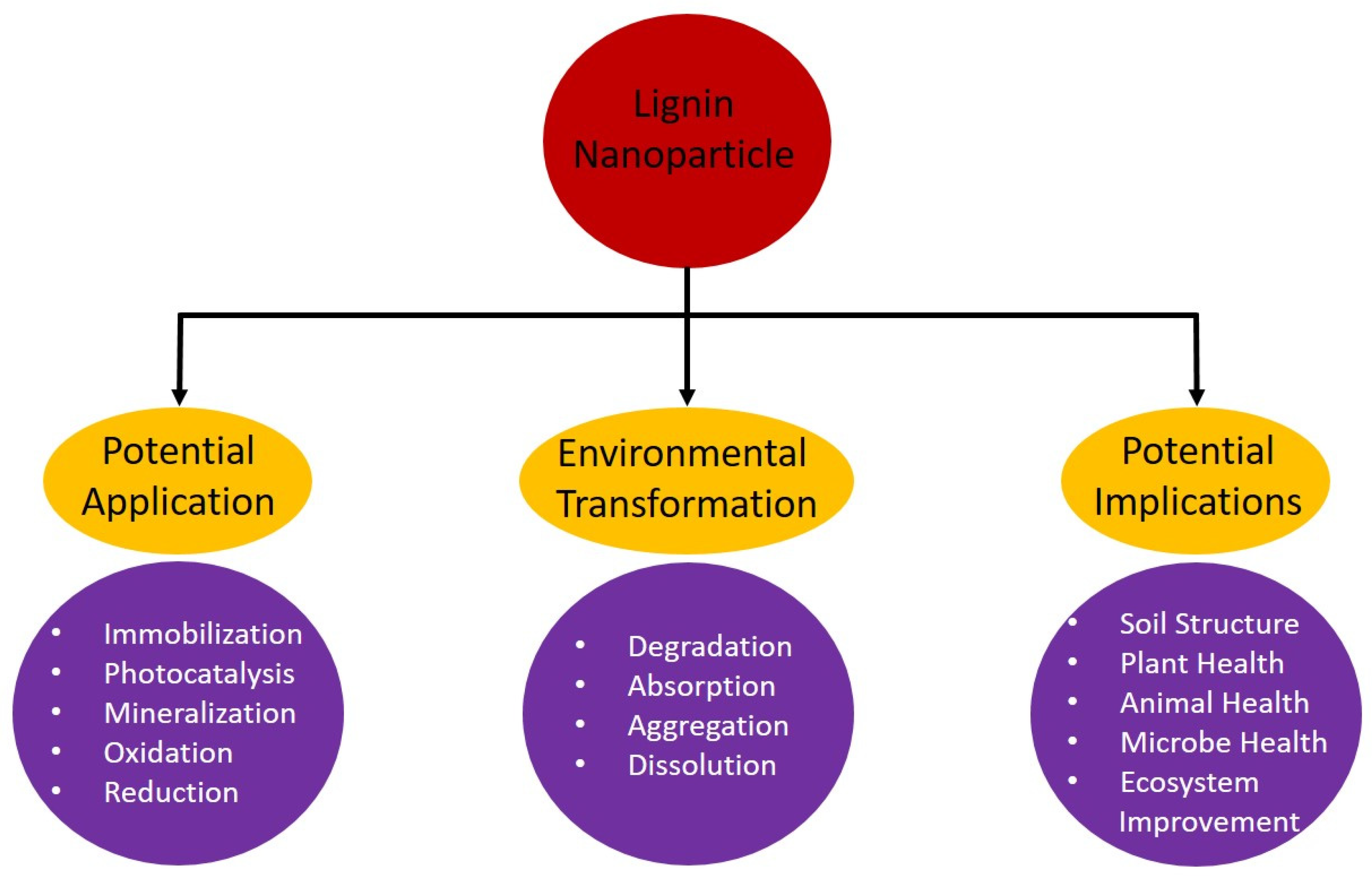
6. Challenges in Deploying Lignin Nanoparticles for Environmental Remediation
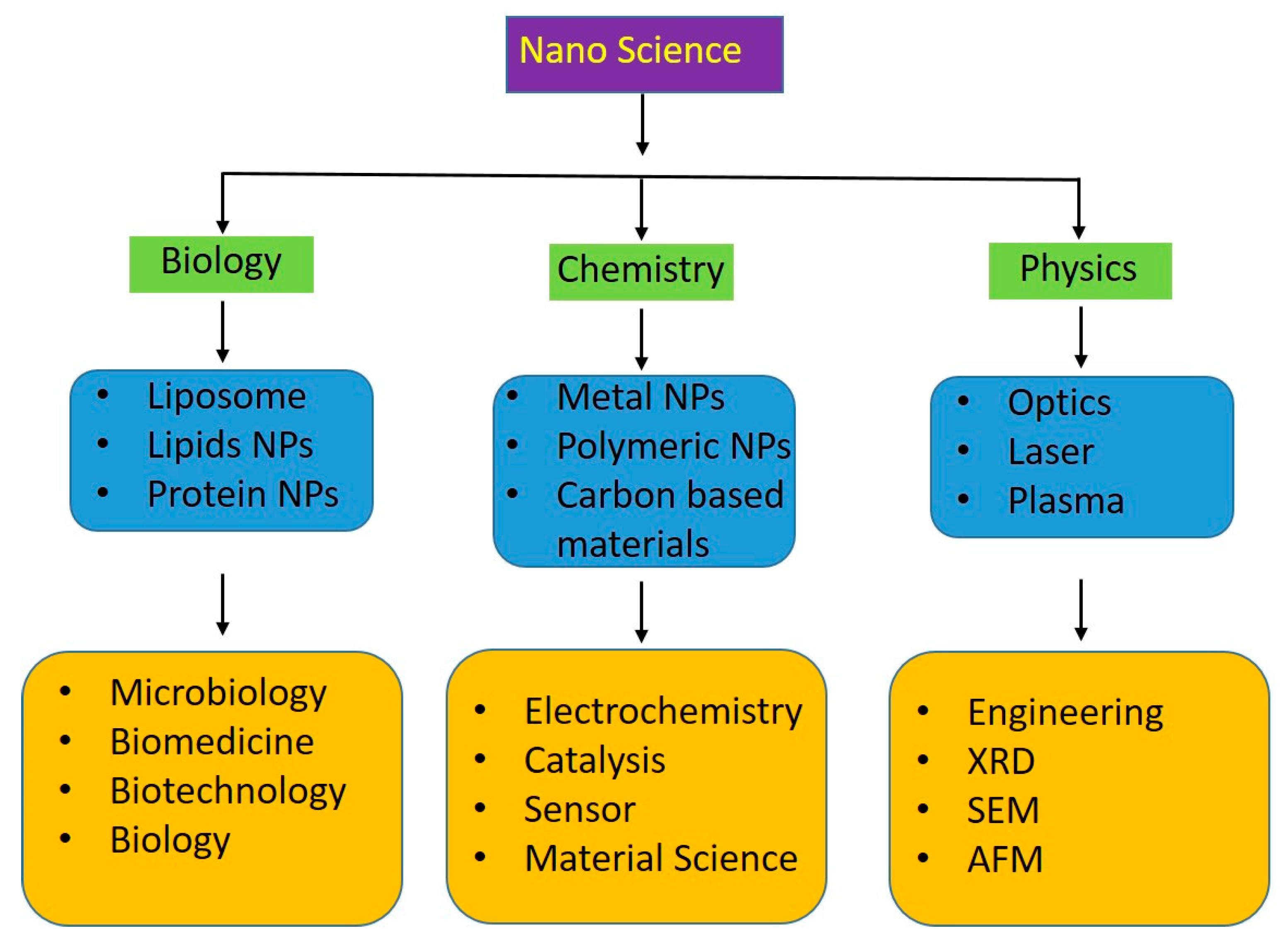
7. Multidisciplinary Approach to Harnessing Lignin Nanoparticles
8. Future Prospects and Implications of Lignin Nanoparticles in Sustainable Remediation
8.1. Potential Transformative Impact on Environmental Cleanup
8.2. Advancing Circular and Sustainable Paradigms
8.3. Coexistence between Humanity and Nature through Nanoparticles Solutions
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Potts, R.; Dommain, R.; Moerman, J.W.; Behrensmeyer, A.K.; Deino, A.L.; Riedl, S.; Beverly, E.J.; Brown, E.T.; Deocampo, D.; Kinyanjui, R. Increased ecological resource variability during a critical transition in hominin evolution. Sci. Adv. 2020, 6, eabc8975. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, R.V.; Aragón-Correa, J.A.; Marano, V.; Tashman, P.A. The corporate governance of environmental sustainability: A review and proposal for more integrated research. J. Manag. 2021, 47, 1468–1497. [Google Scholar]
- Lubchenco, J. Entering the century of the environment: A new social contract for science. Science 1998, 279, 491–497. [Google Scholar] [CrossRef]
- Leonard, A. The Story of Stuff: How Our Obsession with Stuff Is Trashing the Planet, Our Communities, and Our Health-and a Vision for Change; Simon and Schuster: New York, NY, USA, 2010. [Google Scholar]
- Becker, P. Sustainability Science: Managing Risk and Resilience for Sustainable Development; Newnes: Oxford, UK, 2014. [Google Scholar]
- Lucertini, G.; Musco, F. Circular urban metabolism framework. One Earth 2020, 2, 138–142. [Google Scholar] [CrossRef]
- Meadows, D.; Randers, J. The Limits to Growth: The 30-Year Update; Routledge: Oxfordshire, UK, 2012. [Google Scholar]
- Singh, S.B. Emerging sustainable nanomaterials and their applications in catalysis and corrosion control. Curr. Nanosci. 2021, 17, 540–553. [Google Scholar] [CrossRef]
- Payne, C.M.; Knott, B.C.; Mayes, H.B.; Hansson, H.; Himmel, M.E.; Sandgren, M.; Stahlberg, J.; Beckham, G.T. Fungal cellulases. Chem. Rev. 2015, 115, 1308–1448. [Google Scholar]
- Council, N.R. A New Biology for the 21st Century; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Sharma, V.K.; Filip, J.; Zboril, R.; Varma, R.S. Natural inorganic nanoparticles–formation, fate, and toxicity in the environment. Chem. Soc. Rev. 2015, 44, 8410–8423. [Google Scholar] [CrossRef]
- Yuan, J.-C.; Huang, R.; Jiang, L.-Y.; Liu, G.-D.; Liu, P.-D.; Xu, W.-R. Facile production of cellulose nanofibers from raw elephant grass by an aluminum chloride-enhanced acidic deep eutectic solvent. Int. J. Biol. Macromol. 2023, 246, 125687. [Google Scholar] [CrossRef]
- Dale, V.H.; Kline, K.L.; Wright, L.L.; Perlack, R.D.; Downing, M.; Graham, R.L. Interactions among bioenergy feedstock choices, landscape dynamics, and land use. Ecol. Appl. 2011, 21, 1039–1054. [Google Scholar] [CrossRef]
- Vasseghian, Y.; Arunkumar, P.; Joo, S.-W.; Gnanasekaran, L.; Kamyab, H.; Rajendran, S.; Balakrishnan, D.; Chelliapan, S.; Klemeš, J.J. Metal-organic framework-enabled pesticides are an emerging tool for sustainable cleaner production and environmental hazard reduction. J. Clean. Prod. 2022, 373, 133966. [Google Scholar] [CrossRef]
- Hermann, E.; Hochuli, P.A.; Méhay, S.; Bucher, H.; Brühwiler, T.; Ware, D.; Hautmann, M.; Roohi, G.; Yaseen, A. Organic matter and palaeoenvironmental signals during the Early Triassic biotic recovery: The Salt Range and Surghar Range records. Sediment. Geol. 2011, 234, 19–41. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Leadbeater, D.R. Bioprospecting Halotolerant Lignocellulolytic Enzymes from Salt Marsh Ecosystems; University of York: York, UK, 2018. [Google Scholar]
- Lizundia, E.; Sipponen, M.H.; Greca, L.G.; Balakshin, M.; Tardy, B.L.; Rojas, O.J.; Puglia, D. Multifunctional lignin-based nanocomposites and nanohybrids. Green Chem. 2021, 23, 6698–6760. [Google Scholar] [CrossRef]
- Mudhoo, A.; Ramasamy, D.L.; Bhatnagar, A.; Usman, M.; Sillanpää, M. An analysis of the versatility and effectiveness of composts for sequestering heavy metal ions, dyes and xenobiotics from soils and aqueous milieus. Ecotoxicol. Environ. Saf. 2020, 197, 110587. [Google Scholar] [CrossRef] [PubMed]
- Abraham, B.; Syamnath, V.; Arun, K.; Zahra, P.F.; Anjusha, P.; Kothakotta, A.; Chen, Y.-H.; Ponnusamy, V.K.; Nisha, P. Lignin-based nanomaterials for food and pharmaceutical applications: Recent trends and future outlook. Sci. Total Environ. 2023, 881, 163316. [Google Scholar] [CrossRef]
- Roy, A.; Sharma, A.; Yadav, S.; Jule, L.T.; Krishnaraj, R. Nanomaterials for remediation of environmental pollutants. Bioinorg. Chem. Appl. 2021, 2021, 1764647. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Qi, P.; Wang, Y.; Liu, Y.; Sui, K. Electrostatic assembly construction of polysaccharide functionalized hybrid membrane for enhanced antimony removal. J. Hazard. Mater. 2021, 410, 124633. [Google Scholar] [CrossRef]
- Fu, L.; Wang, S.; Lin, G.; Zhang, L.; Liu, Q.; Fang, J.; Wei, C.; Liu, G. Post-functionalization of UiO-66-NH2 by 2, 5-Dimercapto-1, 3, 4-thiadiazole for the high efficient removal of Hg (II) in water. J. Hazard. Mater. 2019, 368, 42–51. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kadam, A.; Shinde, S.; Saratale, R.G.; Patra, J.; Ghodake, G. Recent developments in nanotechnology transforming the agricultural sector: A transition replete with opportunities. J. Sci. Food Agric. 2018, 98, 849–864. [Google Scholar] [CrossRef]
- Matveeva, V.G.; Bronstein, L.M. From renewable biomass to nanomaterials: Does biomass origin matter? Prog. Mater. Sci. 2022, 130, 100999. [Google Scholar] [CrossRef]
- Barhoum, A.; Jeevanandam, J.; Rastogi, A.; Samyn, P.; Boluk, Y.; Dufresne, A.; Danquah, M.K.; Bechelany, M. Plant celluloses, hemicelluloses, lignins, and volatile oils for the synthesis of nanoparticles and nanostructured materials. Nanoscale 2020, 12, 22845–22890. [Google Scholar] [CrossRef] [PubMed]
- Sethupathy, S.; Morales, G.M.; Gao, L.; Wang, H.; Yang, B.; Jiang, J.; Sun, J.; Zhu, D. Lignin valorization: Status, challenges and opportunities. Bioresour. Technol. 2022, 347, 126696. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Ashraf, S.S.; Barceló, D.; Iqbal, H.M. Biocatalytic degradation/redefining “removal” fate of pharmaceutically active compounds and antibiotics in the aquatic environment. Sci. Total Environ. 2019, 691, 1190–1211. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, M.R.; Lowry, G.V.; Alvarez, P.; Dionysiou, D.; Biswas, P. Assessing the risks of manufactured nanomaterials. Environ. Sci. Technol. 2006, 40, 4336–4345. [Google Scholar] [CrossRef] [PubMed]
- Gatto, A. A pluralistic approach to economic and business sustainability: A critical meta-synthesis of foundations, metrics, and evidence of human and local development. Corp. Soc. Responsib. Environ. Manag. 2020, 27, 1525–1539. [Google Scholar] [CrossRef]
- Du, B.; Li, W.; Bai, Y.; Pan, Z.; Wang, Q.; Wang, X.; Ding, H.; Lv, G.; Zhou, J. Fabrication of uniform lignin nanoparticles with tunable size for potential wound healing application. Int. J. Biol. Macromol. 2022, 214, 170–180. [Google Scholar] [CrossRef]
- Nagar, R.; Mathur, P.; Chaturvedi, P.; Sharma, C.; Bhatnagar, P. Secondary Metabolites in Green Synthesis of Nanoparticles. In Microbial Approaches for Sustainable Green Technologies; CRC Press: Boca Raton, FL, USA, 2024; pp. 287–303. [Google Scholar]
- Rao, B.G.; Mukherjee, D.; Reddy, B.M. Novel approaches for preparation of nanoparticles. In Nanostructures for Novel Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–36. [Google Scholar]
- Azimvand, J.; Didehban, K.; Mirshokrai, S.A. Preparation and characterization of lignin polymeric nanoparticles using the green solvent ethylene glycol: Acid precipitation technology. BioResources 2018, 13, 2887–2897. [Google Scholar] [CrossRef]
- Sameni, J.; Krigstin, S.; Sain, M. Solubility of lignin and acetylated lignin in organic solvents. BioResources 2017, 12, 1548–1565. [Google Scholar] [CrossRef]
- Kumar, R.; Gupta, A.; Chawla, M.; Aadil, K.R.; Dutt, S.; Kumar, V.B.; Chaudhary, A. Advances in nanotechnology based strategies for synthesis of nanoparticles of lignin. In Lignin: Biosynthesis and Transformation for Industrial Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 203–229. [Google Scholar]
- Shenashen, M.A.; El-Safty, S.A.; Elshehy, E.A. Synthesis, morphological control, and properties of silver nanoparticles in potential applications. Part. Part. Syst. Charact. 2014, 31, 293–316. [Google Scholar] [CrossRef]
- Chacon, W.D.C.; Verruck, S.; Monteiro, A.R.; Valencia, G.A. The mechanism, biopolymers and active compounds for the production of nanoparticles by anti-solvent precipitation: A review. Food Res. Int. 2023, 168, 112728. [Google Scholar] [CrossRef]
- Rieland, J. Towards Sustainable Textiles: Microplastics, Coffee, and Closing the Loop. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, 2023. [Google Scholar]
- Joye, I.J.; McClements, D.J. Production of nanoparticles by anti-solvent precipitation for use in food systems. Trends Food Sci. Technol. 2013, 34, 109–123. [Google Scholar] [CrossRef]
- Chauhan, P.S. Lignin nanoparticles: Eco-friendly and versatile tool for new era. Bioresour. Technol. Rep. 2020, 9, 100374. [Google Scholar] [CrossRef]
- Zhang, Z.; Terrasson, V.; Guénin, E. Lignin nanoparticles and their nanocomposites. Nanomaterials 2021, 11, 1336. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Liu, L. Advances in Preparation and Applications of Lignin Nanoparticles. In Lignin Chemistry: Characterization, Isolation, and Valorization; Wiley: Hoboken, NJ, USA, 2024; pp. 369–400. [Google Scholar]
- Sekeri, S.H.B. Preparation of a Stabilized Nano-Lignin Emulsifier from Oil Palm Empty Fruit Bunch. Master’s Thesis, Universiti Sains Malaysia, Gelugor, Pulau Pinang, Malaysia, 2022. [Google Scholar]
- Beckers, S. Controlled Drug Delivery by Lignin Nanocarriers for Sustainable Plant Protection; Johannes Gutenberg-Universität Mainz: Mainz, Germany, 2020. [Google Scholar]
- Mikkonen, K.S. Strategies for structuring diverse emulsion systems by using wood lignocellulose-derived stabilizers. Green Chem. 2020, 22, 1019–1037. [Google Scholar] [CrossRef]
- Nypelö, T.E.; Carrillo, C.A.; Rojas, O.J. Lignin supracolloids synthesized from (W/O) microemulsions: Use in the interfacial stabilization of Pickering systems and organic carriers for silver metal. Soft Matter 2015, 11, 2046–2054. [Google Scholar] [CrossRef]
- Mudassir, M.A.; Aslam, H.Z.; Ansari, T.M.; Zhang, H.; Hussain, I. Fundamentals and Design-Led Synthesis of Emulsion-Templated Porous Materials for Environmental Applications. Adv. Sci. 2021, 8, 2102540. [Google Scholar] [CrossRef]
- Tang, Q.; Qian, Y.; Yang, D.; Qiu, X.; Qin, Y.; Zhou, M. Lignin-based nanoparticles: A review on their preparations and applications. Polymers 2020, 12, 2471. [Google Scholar] [CrossRef] [PubMed]
- Yusefi, M.; Chan, H.; Teow, S.; Kia, P.; Lee-Kiun Soon, M.; Sidik, N.; Shameli, K. 5-Fluorouracil Encapsulated Chitosan-Cellulose Fiber Bionanocomposites: Synthesis, Characterization and In Vitro Analysis towards Colorectal Cancer Cells. Nanomaterials 2021, 11, 1691. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Meng, X.; Pu, Y.; Ragauskas, A.J. Recent advances in the application of functionalized lignin in value-added polymeric materials. Polymers 2020, 12, 2277. [Google Scholar] [CrossRef]
- O’Donnell, P.B.; McGinity, J.W. Preparation of microspheres by the solvent evaporation technique. Adv. Drug Deliv. Rev. 1997, 28, 25–42. [Google Scholar] [CrossRef]
- Reddy, Y.N.; Gogde, K.; Paul, S.; Bhaumik, J. Lignin to Platform Chemicals and Biomaterials: Chemical and Biological Perspectives. In Biomass for Bioenergy and Biomaterials; CRC Press: Boca Raton, FL, USA, 2021; pp. 31–64. [Google Scholar]
- Malhotra, M.; Suman, S.K. Laccase-mediated delignification and detoxification of lignocellulosic biomass: Removing obstacles in energy generation. Environ. Sci. Pollut. Res. 2021, 28, 58929–58944. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Bilal, M.; Iqbal, H.M.; Raj, A. Lignin peroxidase in focus for catalytic elimination of contaminants—A critical review on recent progress and perspectives. Int. J. Biol. Macromol. 2021, 177, 58–82. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.; Kelkar, A.; Baraniya, D.; Molaei, A.; Moulick, A.; Meena, R.S.; Formanek, P. Enzymatic degradation of lignin in soil: A review. Sustainability 2017, 9, 1163. [Google Scholar] [CrossRef]
- Sellami, K.; Couvert, A.; Nasrallah, N.; Maachi, R.; Abouseoud, M.; Amrane, A. Peroxidase enzymes as green catalysts for bioremediation and biotechnological applications: A review. Sci. Total Environ. 2022, 806, 150500. [Google Scholar] [CrossRef]
- Koradiya, M. Advances in Enzymatic Hydrolysis and Saccharification Technology. In Biofuels; CRC Press: Boca Raton, FL, USA, 2024; pp. 83–114. [Google Scholar]
- Rath, S.; Pradhan, D.; Du, H.; Mohapatra, S.; Thatoi, H. Transforming lignin into value-added products: Perspectives on lignin chemistry, lignin-based biocomposites, and pathways for augmenting ligninolytic enzyme production. Adv. Compos. Hybrid Mater. 2024, 7, 27. [Google Scholar] [CrossRef]
- Hu, Y.; Priya, A.; Chen, C.; Liang, C.; Wang, W.; Wang, Q.; Lin, C.S.K.; Qi, W. Recent advances in substrate-enzyme interactions facilitating efficient biodegradation of lignocellulosic biomass: A review. Int. Biodeterior. Biodegrad. 2023, 180, 105594. [Google Scholar] [CrossRef]
- Di Iorio, E.; Colombo, C.; Cheng, Z.; Capitani, G.; Mele, D.; Ventruti, G.; Angelico, R. Characterization of magnetite nanoparticles synthetized from Fe (II)/nitrate solutions for arsenic removal from water. J. Environ. Chem. Eng. 2019, 7, 102986. [Google Scholar] [CrossRef]
- Ariyanta, H.A.; Sari, F.P.; Sohail, A.; Restu, W.K.; Septiyanti, M.; Aryana, N.; Fatriasari, W.; Kumar, A. Current roles of lignin for the agroindustry: Applications, challenges, and opportunities. Int. J. Biol. Macromol. 2023, 240, 124523. [Google Scholar] [CrossRef]
- Wang, M.; Mohanty, S.K.; Mahendra, S. Nanomaterial-supported enzymes for water purification and monitoring in point-of-use water supply systems. Acc. Chem. Res. 2019, 52, 876–885. [Google Scholar] [CrossRef]
- Gao, W.; Fatehi, P. Lignin for polymer and nanoparticle production: Current status and challenges. Can. J. Chem. Eng. 2019, 97, 2827–2842. [Google Scholar] [CrossRef]
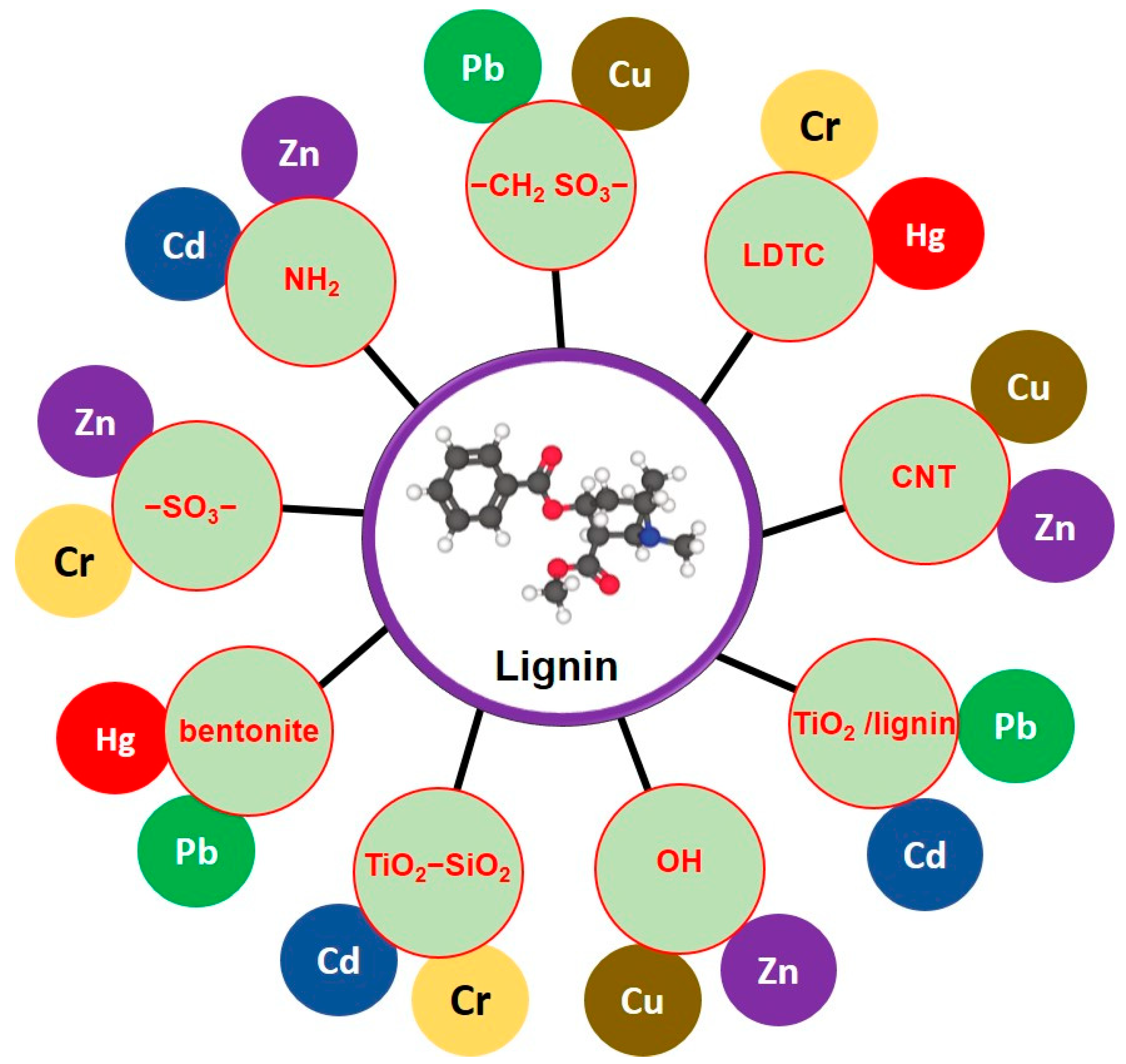
- Ge, Y.; Li, Z. Application of lignin and its derivatives in adsorption of heavy metal ions in water: A review. ACS Sustain. Chem. Eng. 2018, 6, 7181–7192. [Google Scholar] [CrossRef]
- Agustiany, E.A.; Rasyidur Ridho, M.; Rahmi DN, M.; Madyaratri, E.W.; Falah, F.; Lubis, M.A.R.; Solihat, N.N.; Syamani, F.A.; Karungamye, P.; Sohail, A. Recent developments in lignin modification and its application in lignin-based green composites: A review. Polym. Compos. 2022, 43, 4848–4865. [Google Scholar] [CrossRef]
- Dahiya, S.; Katakojwala, R.; Ramakrishna, S.; Mohan, S.V. Biobased products and life cycle assessment in the context of circular economy and sustainability. Mater. Circ. Econ. 2020, 2, 1–28. [Google Scholar] [CrossRef]
- Pham, C.D.; Dang, M.D.; Ly, T.B.; Tran, K.D.; Vo, N.T.; Do, N.H.; Mai, P.T.; Le, P.K. A review of the extraction methods and advanced applications of lignin-silica hybrids derived from natural sources. Int. J. Biol. Macromol. 2023, 230, 123175. [Google Scholar] [CrossRef]
- Duarah, P.; Haldar, D.; Purkait, M.K. Technological advancement in the synthesis and applications of lignin-based nanoparticles derived from agro-industrial waste residues: A review. Int. J. Biol. Macromol. 2020, 163, 1828–1843. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for environmental remediation: Materials and applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, A.; Jellouli Ennigrou, D.; Proietto, F.; Hamzaoui, A.H.; Jaouadi, M. Electrochemical Degradation of Phenol in Aqueous Solutions Using Activated Carbon-ZnO Composite. Environ. Eng. Sci. 2023, 40, 349–361. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Li, D.; Tang, H.; Yu, D.; Wang, L.; Jiang, L. Effect of anionic polysaccharides on conformational changes and antioxidant properties of protein-polyphenol binary covalently-linked complexes. Process Biochem. 2020, 89, 89–97. [Google Scholar] [CrossRef]
- De Melo, L.F.M.; Martins, V.G.d.Q.A.; da Silva, A.P.; de Oliveira Rocha, H.A.; Scortecci, K.C. Biological and pharmacological aspects of tannins and potential biotechnological applications. Food Chem. 2023, 414, 135645. [Google Scholar] [CrossRef]
- Nobahar, A.; Carlier, J.D.; Miguel, M.G.; Costa, M.C. A review of plant metabolites with metal interaction capacity: A green approach for industrial applications. BioMetals 2021, 34, 761–793. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.M.; Shaikh, S.M.; Jalab, R.; Gulied, M.H.; Nasser, M.S.; Benamor, A.; Adham, S. Adsorption of organic pollutants by natural and modified clays: A comprehensive review. Sep. Purif. Technol. 2019, 228, 115719. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, J.; Wang, J.; Qi, X.; Rosenholm, J.M.; Cai, K. Polydopamine coatings in confined nanopore space: Toward improved retention and release of hydrophilic cargo. J. Phys. Chem. C 2015, 119, 24512–24521. [Google Scholar] [CrossRef]
- Rzepecka-Stojko, A.; Stojko, J.; Kurek-Górecka, A.; Górecki, M.; Kabała-Dzik, A.; Kubina, R.; Moździerz, A.; Buszman, E. Polyphenols from bee pollen: Structure, absorption, metabolism and biological activity. Molecules 2015, 20, 21732–21749. [Google Scholar] [CrossRef]
- Wu, L.; Du, C.; He, J.; Yang, Z.; Li, H. Effective adsorption of diclofenac sodium from neutral aqueous solution by low-cost lignite activated cokes. J. Hazard. Mater. 2020, 384, 121284. [Google Scholar] [CrossRef]
- Ma, C.-Y.; Xu, L.-H.; Sun, Q.; Shen, X.-J.; Wen, J.-L.; Yuan, T.-Q. Tailored one-pot lignocellulose fractionation to maximize biorefinery toward controllable producing lignin nanoparticles and facilitating enzymatic hydrolysis. Chem. Eng. J. 2022, 450, 138315. [Google Scholar] [CrossRef]
- Zhang, W.; Qiu, X.; Wang, C.; Zhong, L.; Fu, F.; Zhu, J.; Zhang, Z.; Qin, Y.; Yang, D.; Xu, C.C. Lignin derived carbon materials: Current status and future trends. Carbon Res. 2022, 1, 14. [Google Scholar] [CrossRef]
- Grabber, J.H. How do lignin composition, structure, and cross-linking affect degradability? A review of cell wall model studies. Crop Sci. 2005, 45, 820–831. [Google Scholar] [CrossRef]
- Khalil, H.A.; Bhat, A.; Yusra, A.I. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Ma, Q.; Cui, L.; Zhou, S.; Li, Y.; Shi, W.; Ai, S. Iron nanoparticles in situ encapsulated in lignin-derived hydrochar as an effective catalyst for phenol removal. Environ. Sci. Pollut. Res. 2018, 25, 20833–20840. [Google Scholar] [CrossRef]
- Custodis, V.B.; Karakoulia, S.A.; Triantafyllidis, K.S.; van Bokhoven, J.A. Catalytic fast pyrolysis of lignin over high-surface-area mesoporous aluminosilicates: Effect of porosity and acidity. ChemSusChem 2016, 9, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- HPS, A.K.; Saurabh, C.K.; Adnan, A.; Fazita, M.N.; Syakir, M.; Davoudpour, Y.; Rafatullah, M.; Abdullah, C.; Haafiz, M.; Dungani, R. A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications. Carbohydr. Polym. 2016, 150, 216–226. [Google Scholar]
- Liu, Z.-H.; Hao, N.; Shinde, S.; Pu, Y.; Kang, X.; Ragauskas, A.J.; Yuan, J.S. Defining lignin nanoparticle properties through tailored lignin reactivity by sequential organosolv fragmentation approach (SOFA). Green Chem. 2019, 21, 245–260. [Google Scholar] [CrossRef]
- Shangguan, J.; Hensley, A.J.; Gradiski, M.V.; Pfriem, N.; McEwen, J.-S.; Morris, R.H.; Chin, Y.-H.C. The role of protons and hydrides in the catalytic hydrogenolysis of guaiacol at the ruthenium nanoparticle–water interface. ACS Catal. 2020, 10, 12310–12332. [Google Scholar] [CrossRef]
- Ersöz, A.; Denizli, A.; Şener, İ.; Atılır, A.; Diltemiz, S.; Say, R. Removal of phenolic compounds with nitrophenol-imprinted polymer based on π–π and hydrogen-bonding interactions. Sep. Purif. Technol. 2004, 38, 173–179. [Google Scholar] [CrossRef]
- Mohanapriya, V.; Sakthivel, R.; Pham, N.D.K.; Cheng, C.K.; Le, H.S.; Dong, T.M.H. Nanotechnology-A ray of hope for heavy metals removal. Chemosphere 2023, 311, 136989. [Google Scholar] [CrossRef]
- Dutta, S.; Bhaumik, A.; Wu, K.C.-W. Hierarchically porous carbon derived from polymers and biomass: Effect of interconnected pores on energy applications. Energy Environ. Sci. 2014, 7, 3574–3592. [Google Scholar] [CrossRef]
- Pandey, A.; Kalamdhad, A.; Sharma, Y.C. Recent advances of nanocellulose as biobased adsorbent for heavy metal ions removal: A sustainable approach integrating with waste management. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100791. [Google Scholar] [CrossRef]
- Sohni, S.; Hassan, T.; Khan, S.B.; Akhtar, K.; Bakhsh, E.M.; Hashim, R.; Nidaullah, H.; Khan, M.; Khan, S.A. Lignin nanoparticles-reduced graphene oxide based hydrogel: A novel strategy for environmental applications. Int. J. Biol. Macromol. 2023, 225, 1426–1436. [Google Scholar] [CrossRef]
- Selvasembian, R.; Singh, P. Biosorption for Wastewater Contaminants; Wiley Online Library: Hoboken, NJ, USA, 2022. [Google Scholar]
- Du, B.; Chai, L.; Wang, Y.; Wang, X.; Chen, X.; Zhou, J.; Sun, R.-C. Fabrication of demethylated lignin-based micro-particle for efficient adsorption of malachite green from aqueous solution. J. Mol. Liq. 2023, 382, 121935. [Google Scholar] [CrossRef]
- Sohni, S.; Hashim, R.; Nidaullah, H.; Lamaming, J.; Sulaiman, O. Chitosan/nano-lignin based composite as a new sorbent for enhanced removal of dye pollution from aqueous solutions. Int. J. Biol. Macromol. 2019, 132, 1304–1317. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Ghatak, H.R.; Ahuja, S. A review on photocatalytic remediation of environmental pollutants and H2 production through water splitting: A sustainable approach. Environ. Technol. Innov. 2020, 19, 100893. [Google Scholar] [CrossRef]
- Sinha, V.; Chakma, S. Advances in the preparation of hydrogel for wastewater treatment: A concise review. J. Environ. Chem. Eng. 2019, 7, 103295. [Google Scholar] [CrossRef]
- Li, Q.; Zhan, Z.; Jin, S.; Tan, B. Wettable magnetic hypercrosslinked microporous nanoparticle as an efficient adsorbent for water treatment. Chem. Eng. J. 2017, 326, 109–116. [Google Scholar] [CrossRef]
- Meng, Y.; Liu, T.; Yu, S.; Cheng, Y.; Lu, J.; Wang, H. A lignin-based carbon aerogel enhanced by graphene oxide and application in oil/water separation. Fuel 2020, 278, 118376. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Meng, Y.; Aihemaiti, A.; Xu, Y.; Xiang, H.; Gao, Y.; Chen, X. Preparation, environmental application and prospect of biochar-supported metal nanoparticles: A review. J. Hazard. Mater. 2020, 388, 122026. [Google Scholar] [CrossRef] [PubMed]
- Muthukumaran, P.; Babu, P.S.; Shyamalagowri, S.; Aravind, J.; Kamaraj, M.; Govarthanan, M. Polymeric biomolecules based nanomaterials: Production strategies and pollutant mitigation as an emerging tool for environmental application. Chemosphere 2022, 307, 136008. [Google Scholar] [CrossRef] [PubMed]
- Dey, N.; Vickram, S.; Thanigaivel, S.; Subbaiya, R.; Kim, W.; Karmegam, N.; Govarthanan, M. Nanomaterials for transforming barrier properties of lignocellulosic biomass towards potential applications–A review. Fuel 2022, 316, 123444. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs)(arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef]
- Senila, M. Metal and metalloid monitoring in water by passive sampling–A review. Rev. Anal. Chem. 2023, 42, 20230065. [Google Scholar] [CrossRef]
- Senila, M. Recent Advances in the Determination of Major and Trace Elements in Plants Using Inductively Coupled Plasma Optical Emission Spectrometry. Molecules 2024, 29, 3169. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Sharma, R.; Agrawal, P.R.; Kumar, R.; Ittishree; Gupta, G. Biosorption for Eliminating Inorganic Contaminants (IOCs) from Wastewater. In Biosorption for Wastewater Contaminants; Wiley Online Library: Hoboken, NJ, USA, 2022; pp. 42–62. [Google Scholar]
- Yang, X.; Wan, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Wang, H.; Ok, Y.S.; Jiang, Y.; Gao, B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. Chem. Eng. J. 2019, 366, 608–621. [Google Scholar] [CrossRef]
- Kayan, G.Ö.; Kayan, A. Composite of natural polymers and their adsorbent properties on the dyes and heavy metal ions. J. Polym. Environ. 2021, 29, 3477–3496. [Google Scholar] [CrossRef]
- Yoosaf, K.; Ipe, B.I.; Suresh, C.H.; Thomas, K.G. In situ synthesis of metal nanoparticles and selective naked-eye detection of lead ions from aqueous media. J. Phys. Chem. C 2007, 111, 12839–12847. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, P.; Zhou, H.-C.; Sharma, V.K. Water-stable metal-organic frameworks for aqueous removal of heavy metals and radionuclides: A review. Chemosphere 2018, 209, 783–800. [Google Scholar] [CrossRef]
- Amonette, J.E.; Joseph, S. Characteristics of biochar: Microchemical properties. In Biochar for Environmental Management; Routledge: Oxfordshire, UK, 2012; pp. 65–84. [Google Scholar]
- Luo, M.; Lin, H.; Li, B.; Dong, Y.; He, Y.; Wang, L. A novel modification of lignin on corncob-based biochar to enhance removal of cadmium from water. Bioresour. Technol. 2018, 259, 312–318. [Google Scholar] [CrossRef]
- Santander, P.; Butter, B.; Oyarce, E.; Yáñez, M.; Xiao, L.-P.; Sánchez, J. Lignin-based adsorbent materials for metal ion removal from wastewater: A review. Ind. Crops Prod. 2021, 167, 113510. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Duttagupta, S.; Mukherjee, A. Emerging organic contaminants in global community drinking water sources and supply: A review of occurrence, processes and remediation. J. Environ. Chem. Eng. 2022, 10, 107560. [Google Scholar] [CrossRef]
- Arun, J.; Nirmala, N.; Priyadharsini, P.; Dawn, S.; Santhosh, A.; Gopinath, K.; Govarthanan, M. A mini-review on bioderived carbon and its nanocomposites for removal of organic pollutants from wastewater. Mater. Lett. 2022, 310, 131476. [Google Scholar] [CrossRef]
- Zhang, B.; Fang, C.; Ning, J.; Dai, R.; Liu, Y.; Wu, Q.; Zhang, F.; Zhang, W.; Dou, S.; Liu, X. Unusual aliovalent Cd doped γ-Bi2MoO6 nanomaterial for efficient photocatalytic degradation of sulfamethoxazole and rhodamine B under visible light irradiation. Carbon Neutralization 2023, 2, 646–660. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, G.; Shi, H.; Wu, Q.; Xue, S.; Shao, T.; Zhang, F.; Liu, X. Density functional theory study of electronic structure and optical properties of ln3+-doped γ-bi2moo6 (ln= gd, ho, yb). Crystals 2023, 13, 1158. [Google Scholar] [CrossRef]
- Kumar, R.; Sudhaik, A.; Raizada, P.; Hosseini-Bandegharaei, A.; Thakur, V.K.; Saini, A.; Saini, V.; Singh, P. An overview on bismuth molybdate based photocatalytic systems: Controlled morphology and enhancement strategies for photocatalytic water purification. J. Environ. Chem. Eng. 2020, 8, 104291. [Google Scholar] [CrossRef]
- Li, K.; Liu, C.; Li, J.; Wang, G.; Wang, K. Architecting inorganic/organic S-scheme heterojunction of Bi4Ti3O12 coupling with g-C3N4 for photocatalytic H2O2 production from pure water. Acta Phys.-Chim. Sin. 2024, 40, 2403009. [Google Scholar] [CrossRef]
- Shao, X.; Li, K.; Li, J.; Cheng, Q.; Wang, G.; Wang, K. Investigating S-scheme charge transfer pathways in NiS@Ta2O5 hybrid nanofibers for photocatalytic CO2 conversion. Chin. J. Catal. 2023, 51, 193–203. [Google Scholar] [CrossRef]
- Pasini, S.M.; Valerio, A.; Yin, G.; Wang, J.; de Souza, S.M.G.U.; Hotza, D.; de Souza, A.A.U. An overview on nanostructured TiO2–containing fibers for photocatalytic degradation of organic pollutants in wastewater treatment. J. Water Process Eng. 2021, 40, 101827. [Google Scholar] [CrossRef]
- Tan, X.-F.; Zhu, S.-S.; Wang, R.-P.; Chen, Y.-D.; Show, P.-L.; Zhang, F.-F.; Ho, S.-H. Role of biochar surface characteristics in the adsorption of aromatic compounds: Pore structure and functional groups. Chin. Chem. Lett. 2021, 32, 2939–2946. [Google Scholar] [CrossRef]
- Pandey, S.; Mandari, K.K.; Kim, J.; Kang, M.; Fosso-Kankeu, E. Recent advancement in visible-light-responsive photocatalysts in heterogeneous photocatalytic water treatment technology. In Photocatalysts in Advanced Oxidation Processes for Wastewater Treatment; Wiley Online Library: Hoboken, NJ, USA, 2020; pp. 167–196. [Google Scholar]
- Schneider, W.D.H.; Dillon, A.J.P.; Camassola, M. Lignin nanoparticles enter the scene: A promising versatile green tool for multiple applications. Biotechnol. Adv. 2021, 47, 107685. [Google Scholar] [CrossRef]
- Saravanan, A.; Swaminaathan, P.; Kumar, P.S.; Yaashikaa, P.; Kamalesh, R.; Rangasamy, G. A comprehensive review on immobilized microbes-biochar and their environmental remediation: Mechanism, challenges and future perspectives. Environ. Res. 2023, 236, 116723. [Google Scholar] [CrossRef]
- Uddin, M.M.; Zakeel, M.C.M.; Zavahir, J.S.; Marikar, F.M.; Jahan, I. Heavy metal accumulation in rice and aquatic plants used as human food: A general review. Toxics 2021, 9, 360. [Google Scholar] [CrossRef]
- Di Giulio, M.; Holderegger, R.; Tobias, S. Effects of habitat and landscape fragmentation on humans and biodiversity in densely populated landscapes. J. Environ. Manag. 2009, 90, 2959–2968. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Anjana; Rana, H.; Goswami, S. A comprehensive review on the heavy metal removal for water remediation by the application of lignocellulosic biomass-derived nanocellulose. J. Polym. Environ. 2022, 30, 1–18. [Google Scholar] [CrossRef]
- Wu, Y.; Guan, C.-Y.; Griswold, N.; Hou, L.-y.; Fang, X.; Hu, A.; Hu, Z.-q.; Yu, C.-P. Zero-valent iron-based technologies for removal of heavy metal (loid) s and organic pollutants from the aquatic environment: Recent advances and perspectives. J. Clean. Prod. 2020, 277, 123478. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Greener synthesis of lignin nanoparticles and their applications. Green Chem. 2020, 22, 612–636. [Google Scholar] [CrossRef]
- Wang, L.; Rinklebe, J.; Tack, F.M.; Hou, D. A review of green remediation strategies for heavy metal contaminated soil. Soil Use Manag. 2021, 37, 936–963. [Google Scholar] [CrossRef]
- Bolan, N.; Hoang, S.A.; Beiyuan, J.; Gupta, S.; Hou, D.; Karakoti, A.; Joseph, S.; Jung, S.; Kim, K.-H.; Kirkham, M. Multifunctional applications of biochar beyond carbon storage. Int. Mater. Rev. 2022, 67, 150–200. [Google Scholar] [CrossRef]
- Kacprzak, M.; Kupich, I.; Jasinska, A.; Fijalkowski, K. Bio-based waste’substrates for degraded soil improvement—Advantages and challenges in European context. Energies 2022, 15, 385. [Google Scholar] [CrossRef]
- Zhu, J.; Agarwal, U.P.; Ciesielski, P.N.; Himmel, M.E.; Gao, R.; Deng, Y.; Morits, M.; Österberg, M. Towards sustainable production and utilization of plant-biomass-based nanomaterials: A review and analysis of recent developments. Biotechnol. Biofuels 2021, 14, 114. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.; Vladimirova, D.; Holgado, M.; van Fossen, K.; Yang, M.; Silva, E.A.; Barlow, C.Y. Business model innovation for sustainability: Towards a unified perspective for creation of sustainable business models. Bus. Strategy Environ. 2017, 26, 597–608. [Google Scholar] [CrossRef]
- Ganie, A.S.; Bano, S.; Khan, N.; Sultana, S.; Rehman, Z.; Rahman, M.M.; Sabir, S.; Coulon, F.; Khan, M.Z. Nanoremediation technologies for sustainable remediation of contaminated environments: Recent advances and challenges. Chemosphere 2021, 275, 130065. [Google Scholar] [CrossRef]
- Qian, Y.; Qin, C.; Chen, M.; Lin, S. Nanotechnology in soil remediation− applications vs. implications. Ecotoxicol. Environ. Saf. 2020, 201, 110815. [Google Scholar] [CrossRef] [PubMed]
- Demirer, G.S.; Silva, T.N.; Jackson, C.T.; Thomas, J.B.; Ehrhardt, D.W.; Rhee, S.Y.; Mortimer, J.C.; Landry, M.P. Nanotechnology to advance CRISPR–Cas genetic engineering of plants. Nat. Nanotechnol. 2021, 16, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Yadav, K.; Ahmad, R.; Abd-Elsalam, K.A. Emerging Frontiers in Nanotechnology for Precision Agriculture: Advancements, Hurdles and Prospects. Agrochemicals 2023, 2, 220–256. [Google Scholar] [CrossRef]
- Xu, D.-M.; Fu, R.-B.; Wang, J.-X.; Shi, Y.-X.; Guo, X.-P. Chemical stabilization remediation for heavy metals in contaminated soils on the latest decade: Available stabilizing materials and associated evaluation methods-A critical review. J. Clean. Prod. 2021, 321, 128730. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, W.; Ciesielski, P.N.; Fang, Z.; Zhu, J.; Henriksson, G.; Himmel, M.E.; Hu, L. Wood-derived materials for green electronics, biological devices, and energy applications. Chem. Rev. 2016, 116, 9305–9374. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Smith, S.M.; Wantala, K.; Kajitvichyanukul, P. Photocatalytic remediation of persistent organic pollutants (POPs): A review. Arab. J. Chem. 2020, 13, 8309–8337. [Google Scholar] [CrossRef]
- Aven, T.; Zio, E. Foundational issues in risk assessment and risk management. Risk Anal. 2014, 34, 1164–1172. [Google Scholar] [CrossRef]
- Santo Pereira, A.d.E.; de Oliveira, J.L.; Savassa, S.M.; Rogério, C.B.; de Medeiros, G.A.; Fraceto, L.F. Lignin nanoparticles: New insights for a sustainable agriculture. J. Clean. Prod. 2022, 345, 131145. [Google Scholar] [CrossRef]
- Zhang, Z.; Cissoko, N.; Wo, J.; Xu, X. Factors influencing the dechlorination of 2,4-dichlorophenol by Ni–Fe nanoparticles in the presence of humic acid. J. Hazard. Mater. 2009, 165, 78–86. [Google Scholar] [CrossRef]
- HogenEsch, H.; O’Hagan, D.T.; Fox, C.B. Optimizing the utilization of aluminum adjuvants in vaccines: You might just get what you want. npj Vaccines 2018, 3, 51. [Google Scholar] [CrossRef]
- Haynes, H.; Asmatulu, R. Nanotechnology safety in the aerospace industry. In Nanotechnology Safety; Elsevier: Amsterdam, The Netherlands, 2013; pp. 85–97. [Google Scholar]
- Saleem, H.; Zaidi, S.J. Nanoparticles in reverse osmosis membranes for desalination: A state of the art review. Desalination 2020, 475, 114171. [Google Scholar] [CrossRef]
- Jaldurgam, F.F.; Ahmad, Z.; Touati, F. Synthesis and performance of large-scale cost-effective environment-friendly nanostructured thermoelectric materials. Nanomaterials 2021, 11, 1091. [Google Scholar] [CrossRef] [PubMed]
- Candan, Z.; Tozluoglu, A.; Gonultas, O.; Yildirim, M.; Fidan, H.; Alma, M.H.; Salan, T. Nanocellulose: Sustainable biomaterial for developing novel adhesives and composites. In Industrial Applications of Nanocellulose and Its Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2022; pp. 49–137. [Google Scholar]
- Khaddage, F.; Müller, W.; Flintoff, K. Advancing mobile learning in formal and informal settings via mobile app technology: Where to from here, and how? J. Educ. Technol. Soc. 2016, 19, 16–26. [Google Scholar]
- Stavis, S.M.; Fagan, J.A.; Stopa, M.; Liddle, J.A. Nanoparticle manufacturing–heterogeneity through processes to products. ACS Appl. Nano Mater. 2018, 1, 4358–4385. [Google Scholar] [CrossRef]
- Cortese, A.D. The critical role of higher education in creating a sustainable future. Plan. High. Educ. 2003, 31, 15–22. [Google Scholar]
- Kinzig, A.P. Bridging disciplinary divides to address environmental and intellectual challenges. Ecosystems 2001, 4, 709–715. [Google Scholar] [CrossRef]
- Livingston, A.; Trout, B.L.; Horvath, I.T.; Johnson, M.D.; Vaccaro, L.; Coronas, J.; Babbitt, C.W.; Zhang, X.; Pradeep, T.; Drioli, E. Challenges and directions for green chemical engineering—Role of nanoscale materials. In Sustainable Nanoscale Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–18. [Google Scholar]
- Acciardo, E.; Tabasso, S.; Cravotto, G.; Bensaid, S. Process intensification strategies for lignin valorization. Chem. Eng. Process.Process Intensif. 2022, 171, 108732. [Google Scholar] [CrossRef]
- Braithwaite, J.; Churruca, K.; Long, J.C.; Ellis, L.A.; Herkes, J. When complexity science meets implementation science: A theoretical and empirical analysis of systems change. BMC Med. 2018, 16, 63. [Google Scholar] [CrossRef]
- Viswanathan, M.; Sridharan, S. Product Development for the BoP: Insights on Concept and Prototype Development from University-Based Student Projects in I ndia. J. Prod. Innov. Manag. 2012, 29, 52–69. [Google Scholar] [CrossRef]
- Gorelick, S.M.; Zheng, C. Global change and the groundwater management challenge. Water Resour. Res. 2015, 51, 3031–3051. [Google Scholar] [CrossRef]
- Dragone, G.; Kerssemakers, A.A.; Driessen, J.L.; Yamakawa, C.K.; Brumano, L.P.; Mussatto, S.I. Innovation and strategic orientations for the development of advanced biorefineries. Bioresour. Technol. 2020, 302, 122847. [Google Scholar] [CrossRef]
- Simenstad, C.; Tanner, C.; Crandell, C.; White, J.; Cordell, J. Challenges of habitat restoration in a heavily urbanized estuary: Evaluating the investment. J. Coast. Res. 2005, 6–23. [Google Scholar]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Sharma, P.; Bano, A.; Singh, S.P.; Sharma, S.; Xia, C.; Nadda, A.K.; Lam, S.S.; Tong, Y.W. Engineered microbes as effective tools for the remediation of polyaromatic aromatic hydrocarbons and heavy metals. Chemosphere 2022, 306, 135538. [Google Scholar] [CrossRef] [PubMed]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Kylili, A.; Koutinas, M.; Georgali, P.-Z.; Fokaides, P.A. Lignin valorisation: Life Cycle Assessment (LCA) considerations for enabling Circular Bioeconomy. Int. J. Sustain. Energy 2023, 42, 1008–1027. [Google Scholar] [CrossRef]
- Kumar, B.; Verma, P. Biomass-based biorefineries: An important architype towards a circular economy. Fuel 2021, 288, 119622. [Google Scholar] [CrossRef]
- Morganti, P.; Danti, S.; Coltelli, M.B. Chitin and lignin to produce biocompatible tissues. Res. Clin. Dermatol. 2018, 1, 5–11. [Google Scholar] [CrossRef]
- Wertz, J.-L.; Mengal, P.; Perez, S. Biomass in the Bioeconomy: Focus on the EU and US; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Fabbri, F.; Bischof, S.; Mayr, S.; Gritsch, S.; Jimenez Bartolome, M.; Schwaiger, N.; Guebitz, G.M.; Weiss, R. The Biomodified Lignin Platform: A Review. Polymers 2023, 15, 1694. [Google Scholar] [CrossRef]
- Manjunatha, S.; Biradar, D.; Aladakatti, Y.R. Nanotechnology and its applications in agriculture: A review. J. Farm. Sci. 2016, 29, 1–13. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, P.; Ali, S.; Jan, R.; Kim, K.-M. Lignin Nanoparticles: Transforming Environmental Remediation. Nanomaterials 2024, 14, 1541. https://doi.org/10.3390/nano14181541
Khan P, Ali S, Jan R, Kim K-M. Lignin Nanoparticles: Transforming Environmental Remediation. Nanomaterials. 2024; 14(18):1541. https://doi.org/10.3390/nano14181541
Chicago/Turabian StyleKhan, Pirzada, Sajid Ali, Rahmatullah Jan, and Kyung-Min Kim. 2024. "Lignin Nanoparticles: Transforming Environmental Remediation" Nanomaterials 14, no. 18: 1541. https://doi.org/10.3390/nano14181541







