A Hybrid Perovskite-Based Electromagnetic Wave Absorber with Enhanced Conduction Loss and Interfacial Polarization through Carbon Sphere Embedding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of MAPbI3 Microcrystals and Carbon Sphere
2.2. Synthesis of MAPbI3/CS Composites
2.3. Structural Characterization
2.4. Material Microwave Absorption Characterization
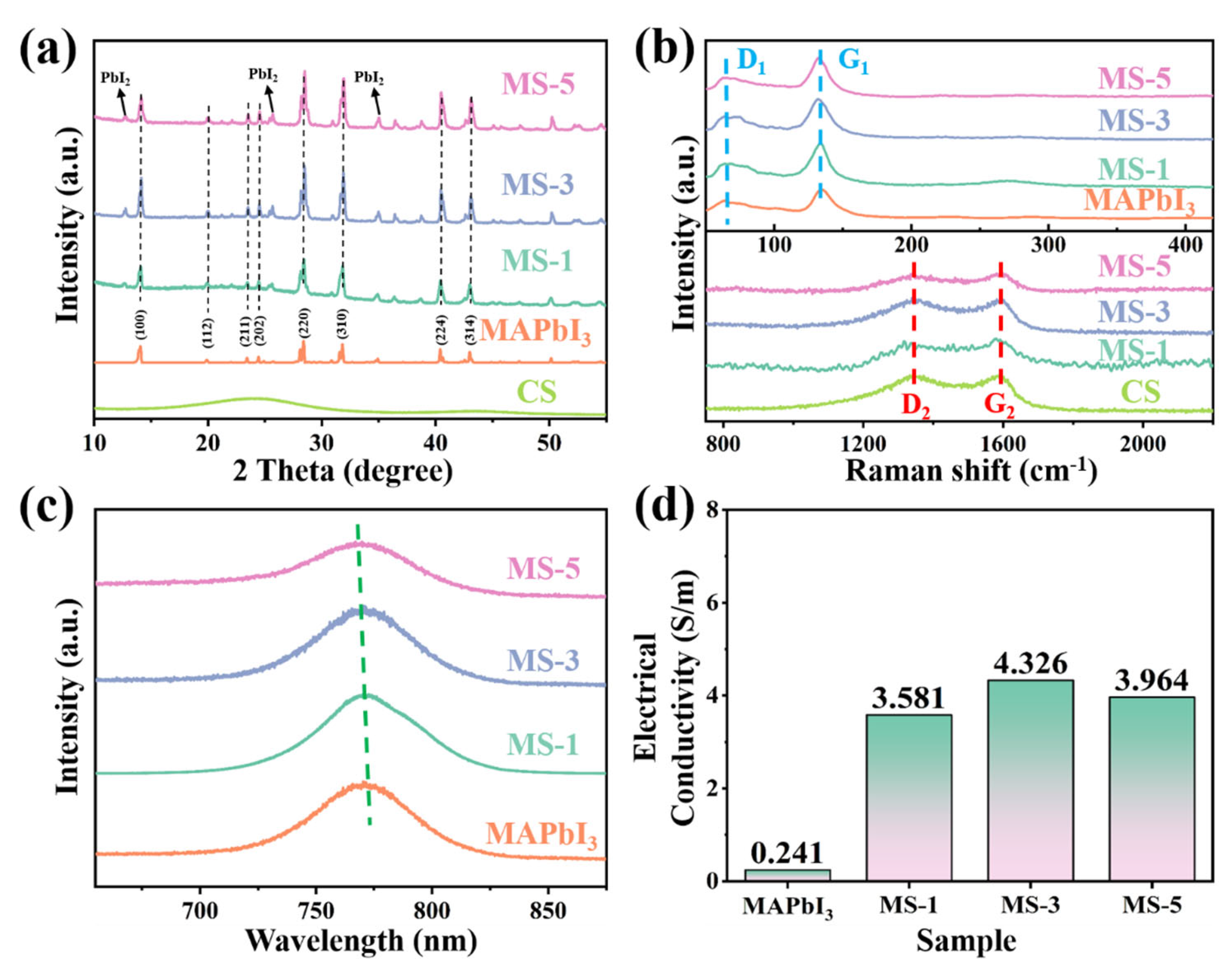
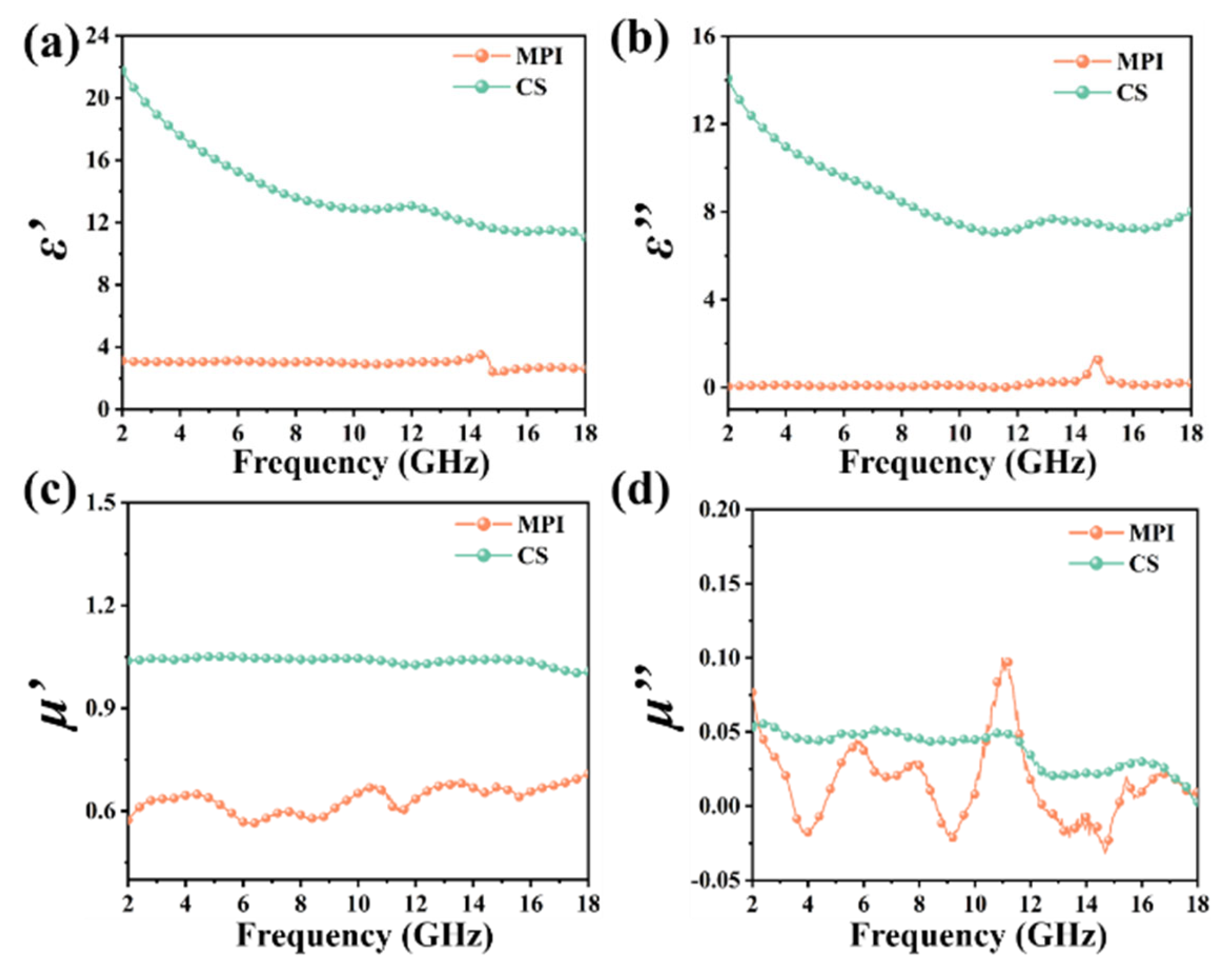
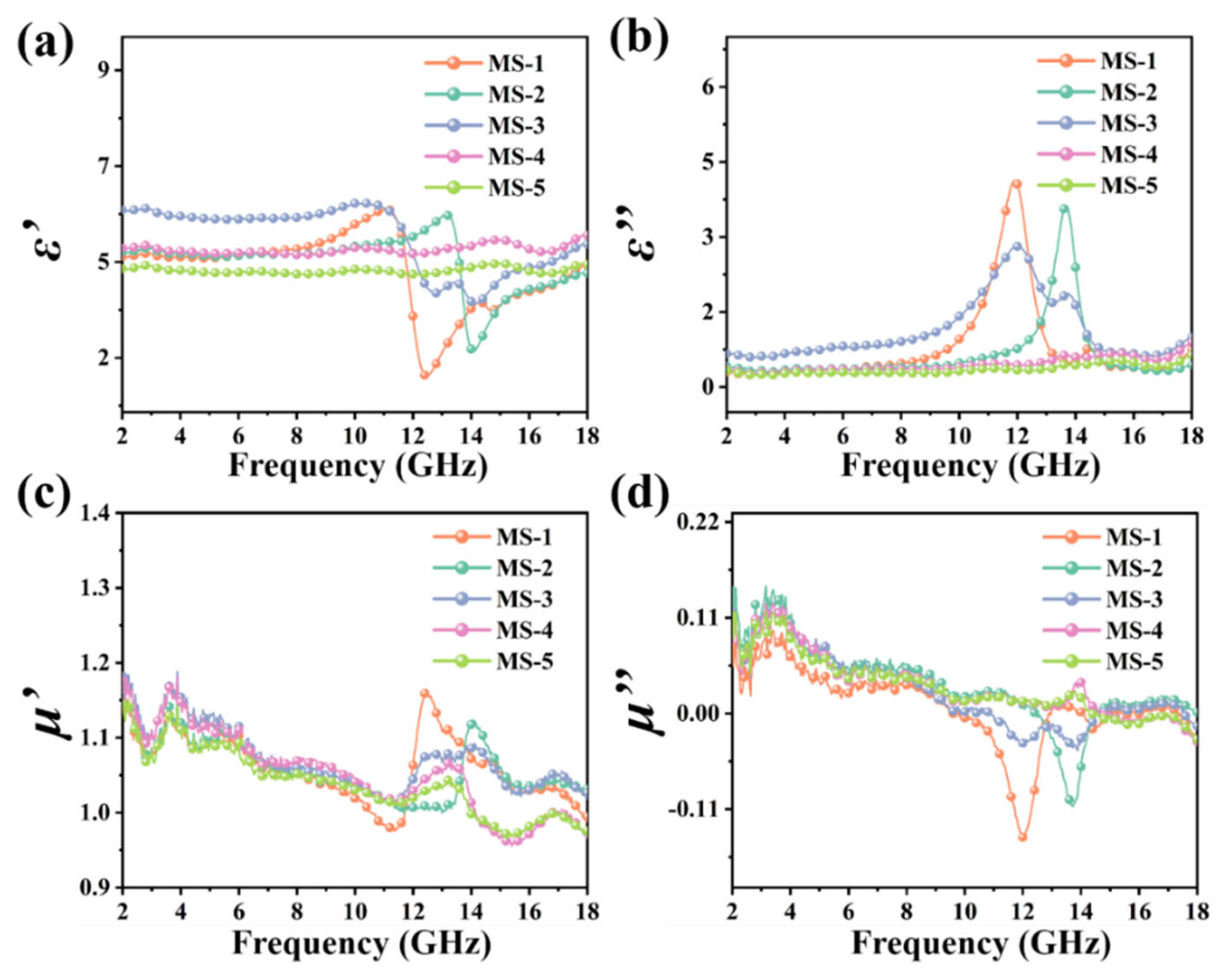
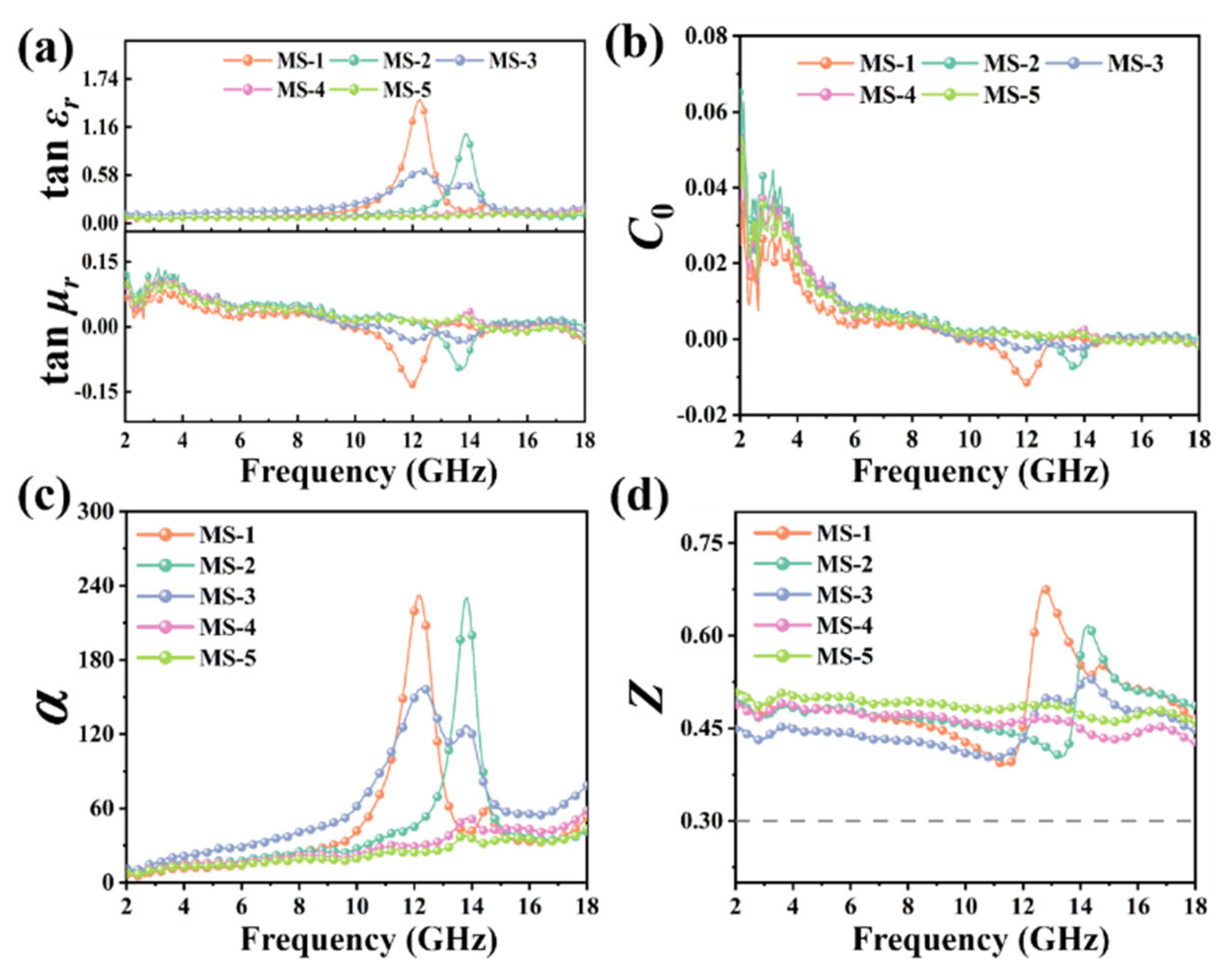
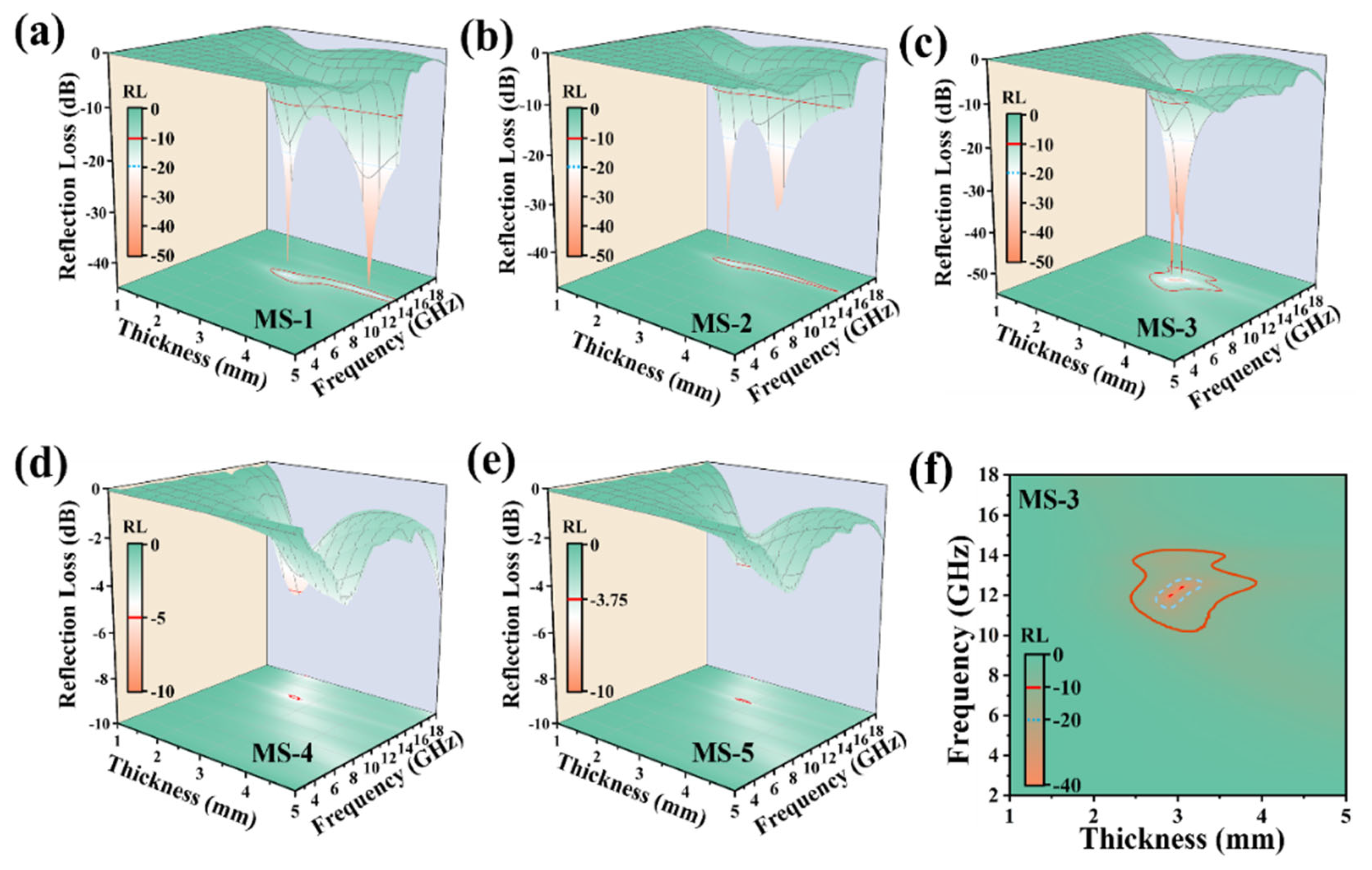
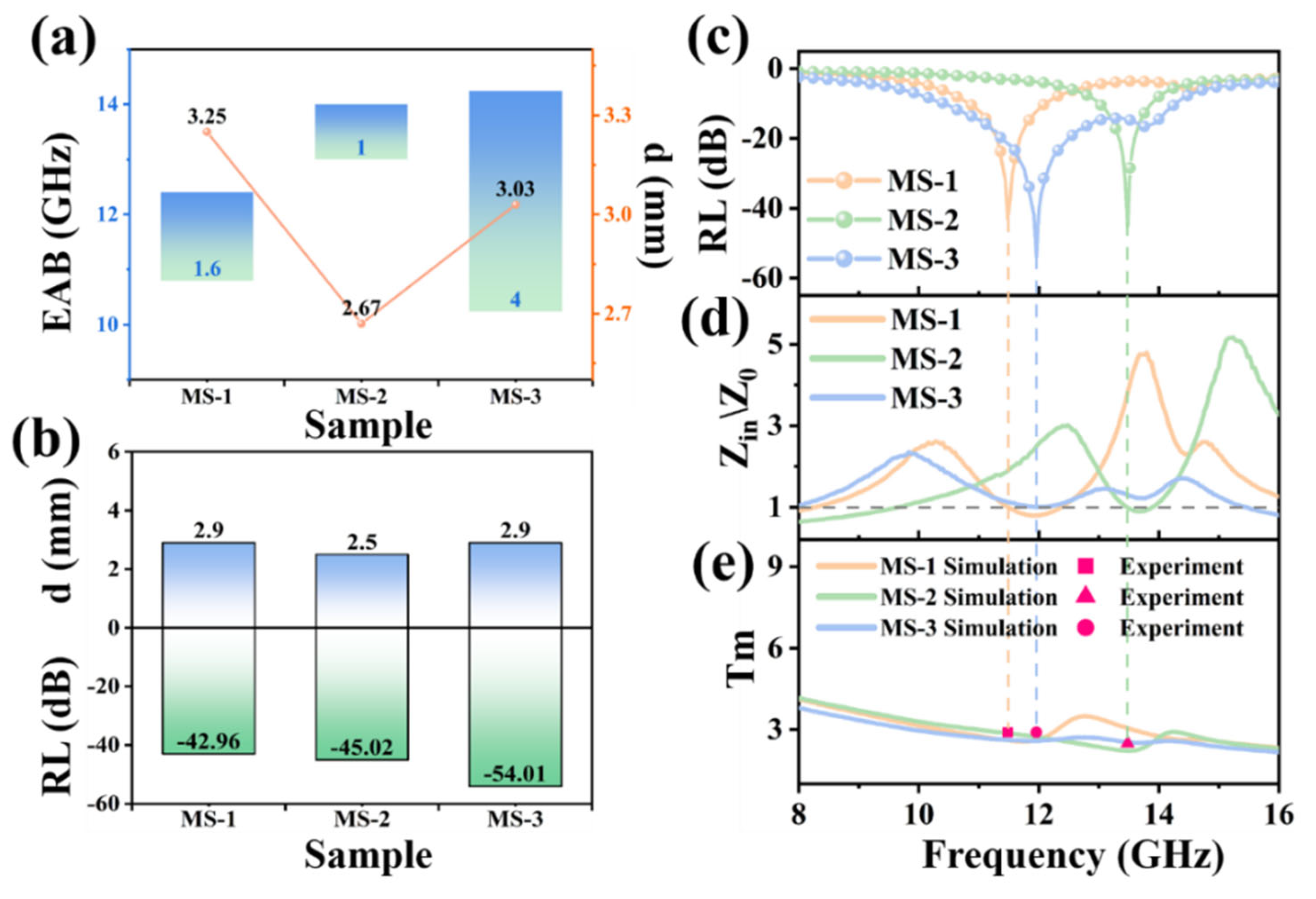
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zeng, X.; Zhao, C.; Jiang, X.; Yu, R.; Che, R. Functional Tailoring of Multi-dimensional Pure MXene Nanostructures for Significantly Accelerated electromagnetic Wave Absorption. Small 2023, 19, 2303393. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yao, L.; Zheng, Q.; Cao, M.-S. Graphene-wrapped multiloculated nickel ferrite: A highly efficient electromagnetic attenuation material for microwave absorbing and green shielding. Nano Res. 2022, 15, 6751–6760. [Google Scholar] [CrossRef]
- Cao, M.; Han, C.; Wang, X.; Zhang, M.; Zhang, Y.; Shu, J.; Yang, H.; Fang, X.; Yuan, J. Graphene nanohybrids: Excellent electromagnetic properties for the absorbing and shielding of electromagnetic waves. J. Mater. Chem. C 2018, 6, 4586–4602. [Google Scholar] [CrossRef]
- Wang, F.; Gu, W.; Chen, J.; Wu, Y.; Zhou, M.; Tang, S.; Cao, X.; Zhang, P.; Ji, G. The point defect and electronic structure of K doped LaCo0.9Fe0.1O3 perovskite with enhanced microwave absorbing ability. Nano Res. 2021, 15, 3720–3728. [Google Scholar] [CrossRef]
- Miao, P.; Yang, J.; Liu, Y.; Xie, H.; Chen, K.-J.; Kong, J. Emerging Perovskite Electromagnetic Wave Absorbers from Bi-Metal-Organic Frameworks. Cryst. Growth Des. 2020, 20, 4818–4826. [Google Scholar] [CrossRef]
- Miao, P.; Qu, N.; Chen, W.; Wang, T.; Zhao, W.; Kong, J. A two-dimensional semiconductive Cu-S metal-organic framework for broadband microwave absorption. Chem. Eng. J. 2023, 454, 140445. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, Y.; Zhang, J.; Ma, Y.; Xu, P.; Zhang, P.; Yang, Z.; Zhou, W. Two-dimensional (PEA)2PbBr4 perovskite modified with conductive network for high-performance electromagnetic wave absorber. Mater. Lett. 2022, 326, 132926. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, Z.; Yao, Y.; Wang, D.; Yang, Z.; Zhang, P.; Zhao, Q.; Zhou, W. Constructing Conductive Network in Hybrid Perovskite for a Highly Efficient Microwave Absorption System. Adv. Funct. Mater. 2022, 32, 2206053. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, Z.; Yao, Y.; Shi, X.; Zhang, P.; Yang, Z.; Zhao, Q.; Zhou, W. Inorganic Halide Perovskite Electromagnetic Wave Absorption System with Ultra-Wide Absorption Bandwidth and High Thermal-Stability. Adv. Electron. Mater. 2022, 9, 2201179. [Google Scholar] [CrossRef]
- Qin, F.; Chen, J.; Liu, J.; Liu, L.; Tang, C.; Tang, B.; Li, G.; Zeng, L.; Li, H.; Yi, Z. Design of high efficiency perovskite solar cells based on inorganic and organic undoped double hole layer. Sol. Energy 2023, 262, 111796. [Google Scholar] [CrossRef]
- Guo, H.; Yang, J.; Pu, B.; Chen, H.; Li, Y.; Wang, Z.; Niu, X. Excellent microwave absorption of lead halide perovskites with high stability. J. Mater. Chem. C 2018, 6, 4201–4207. [Google Scholar] [CrossRef]
- Zhao, B.; Li, Y.; Wang, Z.; Ding, J.; Zhang, R.; Che, R. Galvanic Replacement Reaction Involving Core-Shell Magnetic Chains and Orientation-Tunable Microwave Absorption Properties. Small 2020, 16, 2003502. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Pei, K.; Xing, L.; Yu, X.; You, W.; Che, R. Enhanced Microwave Absorption Performance from Magnetic Coupling of Magnetic Nanoparticles Suspended within Hierarchically Tubular Composite. Adv. Funct. Mater. 2019, 29, 1901448. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Z.; Ge, C.; Qi, X.; Bao, Q.; Liu, C. Constructing MXene-based mixed-dimensional structure with multiple interfaces to optimize dielectric-magnetic synergistic effect for effective electromagnetic wave absorption. J. Colloid Interf. Sci. 2024, 667, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, L.; Wu, H.; Zang, D. Boosted electromagnetic wave absorption performance from vacancies, defects and interfaces engineering in Co(OH)F/Zn0.76Co0.24S/Co3S4 composite. Chem. Eng. J. 2021, 411, 128601. [Google Scholar] [CrossRef]
- Zhao, B.; Yan, Z.; Liu, L.; Zhang, Y.; Guan, L.; Guo, X.; Li, R.; Che, R.; Zhang, R. A Liquid-Metal-Assisted Competitive Galvanic Reaction Strategy Toward Indium/Oxide Core-Shell Nanoparticles with Enhanced Microwave Absorption. Adv. Funct. Mater. 2024, 34, 2314008. [Google Scholar] [CrossRef]
- Pan, Y.; Lan, D.; Jia, Z.; Feng, A.; Wu, G.; Yin, P. Multi-mode tunable electromagnetic wave absorber based on hollow nano-cage structure and self-anticorrosion performance. Adv. Compos. Hybrid Mater. 2024, 7, 40. [Google Scholar] [CrossRef]
- Zhong, X.; He, M.; Zhang, C.; Guo, Y.; Hu, J.; Gu, J. Heterostructured BN@Co-C@C Endowing Polyester Composites Excellent Thermal Conductivity and Microwave Absorption at C Band. Adv. Funct. Mater. 2024, 34, 231355. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, G.; Zhang, R.; Wu, Z.; Xu, C.; Man, H.; Che, R. Charge modulation of CNTs-based conductive network for oxygen reduction reaction and microwave absorption. Carbon 2021, 178, 310–319. [Google Scholar] [CrossRef]
- Hu, Q.; Yang, R.; Mo, Z.; Lu, D.; Yang, L.; He, Z.; Zhu, H.; Tang, Z.; Gui, X. Nitrogen-doped and Fe-filled CNTs/NiCo2O4 porous sponge with tunable microwave absorption performance. Carbon 2019, 153, 737–744. [Google Scholar] [CrossRef]
- Gao, Z.; Zhao, Z.; Lan, D.; Kou, K.; Zhang, J.; Wu, H. Accessory ligand strategies for hexacyanometallate networks deriving perovskite polycrystalline electromagnetic absorbents. J. Mater. Sci. Technol. 2021, 82, 69–79. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, S.; Chu, W.; Li, H.; Chen, Y.; Chen, B.; Chen, B.; Liu, H. Tailoring conductive network nanostructures of ZIF-derived cobalt-decorated N-doped graphene/carbon nanotubes for microwave absorption applications. J. Colloid Interf. Sci. 2021, 591, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.A.; Mhlanga, S.D.; Coville, N.J. Carbon spheres. Mater. Sci. Eng. R Rep. 2010, 70, 1–28. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, C.; Qu, X.; Liu, J.; Lu, Y.; Yang, Z. Porous carbon and carbon composite hollow spheres. Colloid Polym. Sci. 2008, 286, 1093–1096. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, Z.; Li, Y.; Dai, S.; Ji, G.; Zhao, H.; Cao, J.; Du, Y. Rationally regulating complex dielectric parameters of mesoporous carbon hollow spheres to carry out efficient microwave absorption. Carbon 2018, 127, 643–652. [Google Scholar] [CrossRef]
- Wang, Y.; Han, X.; Xu, P.; Liu, D.; Cui, L.; Zhao, H.; Du, Y. Synthesis of pomegranate-like Mo2C@C nanospheres for highly efficient microwave absorption. Chem. Eng. J. 2019, 372, 312–320. [Google Scholar] [CrossRef]
- Weir, W.B. Automatic Measurement of Complex Dielectric Constant and Permeability at Microwave Frequencies. Proc. IEEE 1974, 62, 33–36. [Google Scholar] [CrossRef]
- Nicolson, A.M.; Ross, G.F. Measurement of the Intrinsic Properties of materials by Time-Domain Techniques. IEEE Trans. Instrum. Meas. 1970, 19, 377–382. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Cui, D.; Ren, X.; Sun, J.; Liu, X.; Zhang, J.; Wei, Q.; Fan, H.; Yu, F.; et al. Two-Inch-Sized Perovskite CH3NH3PbX3 (X = Cl, Br, I) Crystals: Growth and Characterization. Adv. Mater. 2015, 27, 5176–5183. [Google Scholar] [CrossRef]
- Zhang, C.; Mu, C.; Xiang, J.; Wang, B.; Wen, F.; Song, J.; Wang, C.; Liu, Z. Microwave absorption characteristics of CH3NH3PbI3 perovskite/carbon nanotube composites. J. Mater. Sci. 2017, 52, 13023–13032. [Google Scholar] [CrossRef]
- Chang, S.; Chen, C.; Chen, L.; Tien, C.; Cheng, H.; Huang, W.; Lin, H.; Chen, S.; Wu, G. Unraveling the multifunctional capabilities of PCBM thin films in inverted-type CH3NH3PbI3 based photovoltaics. Sol. Energy Mater. Sol. Cells 2017, 169, 40–46. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Y.; Wang, Z.; Wu, R.; Cheng, H.; Chui, H. Characterization of a CH3NH3PbI3 perovskite microwire by Raman spectroscopy. J. Raman Spectrosc. 2022, 53, 288–296. [Google Scholar] [CrossRef]
- Pérez-Osorio, M.A.; Milot, R.L.; Filip, M.R.; Patel, J.B.; Herz, L.M.; Johnston, M.B.; Giustino, F. Vibrational Properties of the Organic-Inorganic Halide Perovskite CH3NH3PbI3 from Theory and Experiment: Factor Group Analysis, First-Principles Calculations, and Low-Temperature Infrared Spectra. J. Phys. Chem. C 2015, 119, 25703–25718. [Google Scholar] [CrossRef]
- Ray, S.C.; Tetana, Z.N.; Erasmus, R.; Mathur, A.; Coville, N.J. Carbon spheres for energy applications: Raman and X-ray photoemission spectroscopy studies. Int. J. Energy Res. 2014, 38, 444–451. [Google Scholar] [CrossRef]
- Jia, X.; Hu, Z.; Zhu, Y.; Weng, T.; Wang, J.; Zhang, J.; Zhu, Y. Facile synthesis of organic-inorganic hybrid perovskite CH3NH3PbI3 microcrystals. J. Alloys Compd. 2017, 725, 270–274. [Google Scholar] [CrossRef]
- Wang, P.; Wei, J.; Huang, B.; Qin, X.; Yao, S.; Zhang, Q.; Wang, Z.; Xu, G.; Jing, X. Synthesis and characterization of carbon spheres prepared by chemical vapour deposition. Mater. Lett. 2007, 61, 4854–4856. [Google Scholar] [CrossRef]
- Quan, B.; Liang, X.; Xu, G.; Cheng, Y.; Zhang, Y.; Liu, W.; Ji, G.; Du, Y. A permittivity regulating strategy to achieve high performance electromagnetic wave absorbers with compatibility of impedance matching and energy conservation. New J. Chem. 2017, 41, 1259–1266. [Google Scholar] [CrossRef]
- Wang, N.; You, K.; Mohanavel, V.; Mehrez, S.; Alamri, S.; Nag, K.; Mahariq, I. Comprehensive study of electromagnetic wave absaorption properties of GdMnO3-MoSe2 hybrid composites. Ceram. Int. 2023, 49, 9191–9202. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, D.; Wang, R.; Wang, M.; Cao, M.; Ye, W.; Long, X.; Yuan, G. Drude-Lorentz resonance absorption in quasi-2D NiFe hydrotalcite/less layer graphite composites. Appl. Phys. A Mater. Sci. Process. 2023, 129, 5. [Google Scholar] [CrossRef]
- Zhao, B.; Shao, G.; Fan, B.; Zhao, W.; Zhang, R. Investigation of the electromagnetic absorption properties of Ni@TiO2 and Ni@SiO2 composite microspheres with core-shell structure. Phys. Chem. Chem. Phys. 2015, 17, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Shao, G.; Fan, B.; Zhao, W.; Xie, Y.; Zhang, R. Facile preparation and enhanced microwave absorption properties of core-shell composite spheres composited of Ni cores and TiO2 shells. Phys. Chem. Chem. Phys. 2015, 17, 8802–8810. [Google Scholar] [CrossRef]
- Xie, A.; Sun, M.; Zhang, K.; Jiang, W.; Wu, F.; He, M. In situ growth of MoS2 nanosheets on reduced graphene oxide (RGO) surfaces: Interfacial enhancement of absorbing performance against electromagnetic pollution. Phys. Chem. Chem. Phys. 2016, 18, 24931–24936. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yuan, X.; Li, X.; Li, L.; Song, Q.; Lv, X.; Gu, G.; Sui, M. Hollow cube-like CuS derived from Cu2O crystals for the highly efficient elimination of electromagnetic pollution. New J. Chem. 2018, 42, 6735–6741. [Google Scholar] [CrossRef]
- Moitra, D.; Dhole, S.; Ghosh, B.K.; Chandel, M.; Jani, R.K.; Patra, M.K.; Vadera, S.R.; Ghosh, N.N. Synthesis and microwave absorption properties of BiFeO3 nanowire-RGO nanocomposite and first-principles calculations for insight of electromagnetic properties and electronic structures. J. Phys. Chem. C 2017, 121, 21290. [Google Scholar] [CrossRef]
- Qin, M.; Zhang, L.; Wu, H. Dielectric Loss Mechanism in Electromagnetic Wave Absorbing Materials. Adv. Sci. 2022, 9, 2105553. [Google Scholar] [CrossRef]
- Song, L.; Duan, Y.; Liu, J.; Pang, H. Transformation between nanosheets and nanowires structure in MnO2 upon providing Co2+ ions and applications for microwave absorption. Nano Res. 2020, 13, 95–104. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lian, X.; Yao, Y.; Xiong, Z.; Duan, Y.; Wang, J.; Fu, S.; Dai, Y.; Zhou, W.; Zhang, Z. A Hybrid Perovskite-Based Electromagnetic Wave Absorber with Enhanced Conduction Loss and Interfacial Polarization through Carbon Sphere Embedding. Nanomaterials 2024, 14, 1566. https://doi.org/10.3390/nano14191566
Lian X, Yao Y, Xiong Z, Duan Y, Wang J, Fu S, Dai Y, Zhou W, Zhang Z. A Hybrid Perovskite-Based Electromagnetic Wave Absorber with Enhanced Conduction Loss and Interfacial Polarization through Carbon Sphere Embedding. Nanomaterials. 2024; 14(19):1566. https://doi.org/10.3390/nano14191566
Chicago/Turabian StyleLian, Xuehua, Yao Yao, Ziming Xiong, Yantao Duan, Jianbao Wang, Shangchen Fu, Yinsuo Dai, Wenke Zhou, and Zhi Zhang. 2024. "A Hybrid Perovskite-Based Electromagnetic Wave Absorber with Enhanced Conduction Loss and Interfacial Polarization through Carbon Sphere Embedding" Nanomaterials 14, no. 19: 1566. https://doi.org/10.3390/nano14191566





