Features of High-Precision Photothermal Analysis of Liquid Systems by Dual-Beam Thermal Lens Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Test Samples
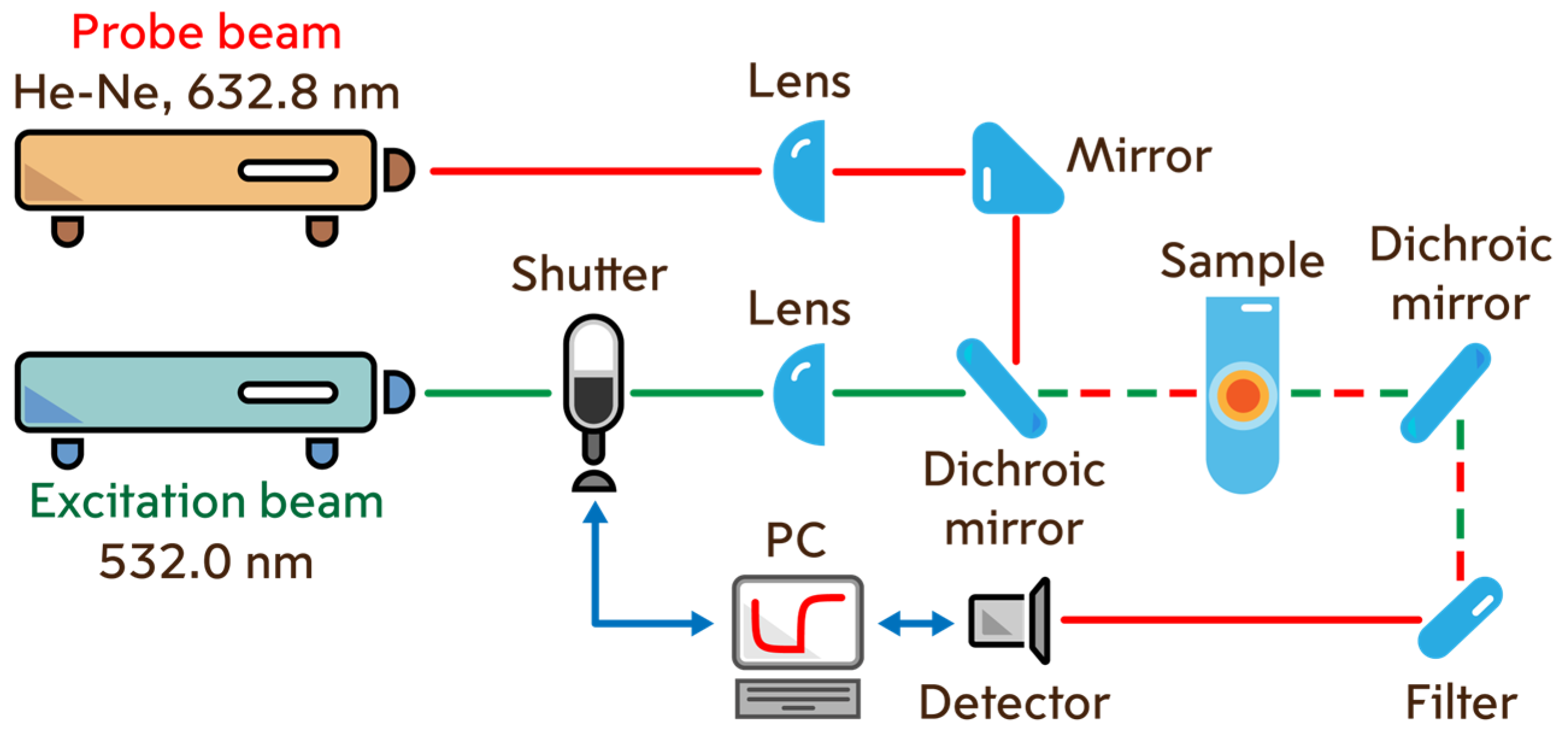
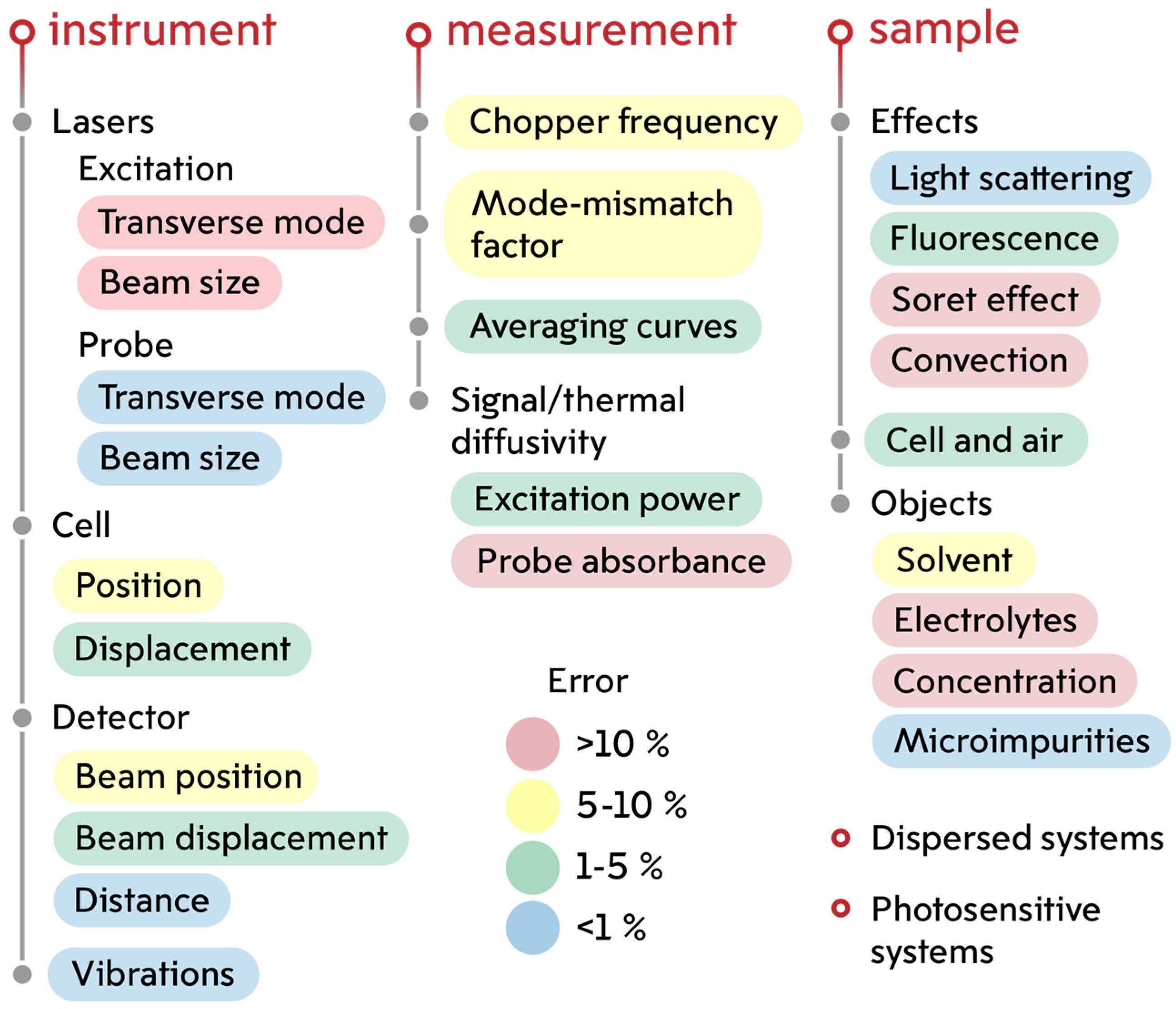
2.2. Thermal Lens Measurements
2.3. Other Measurements
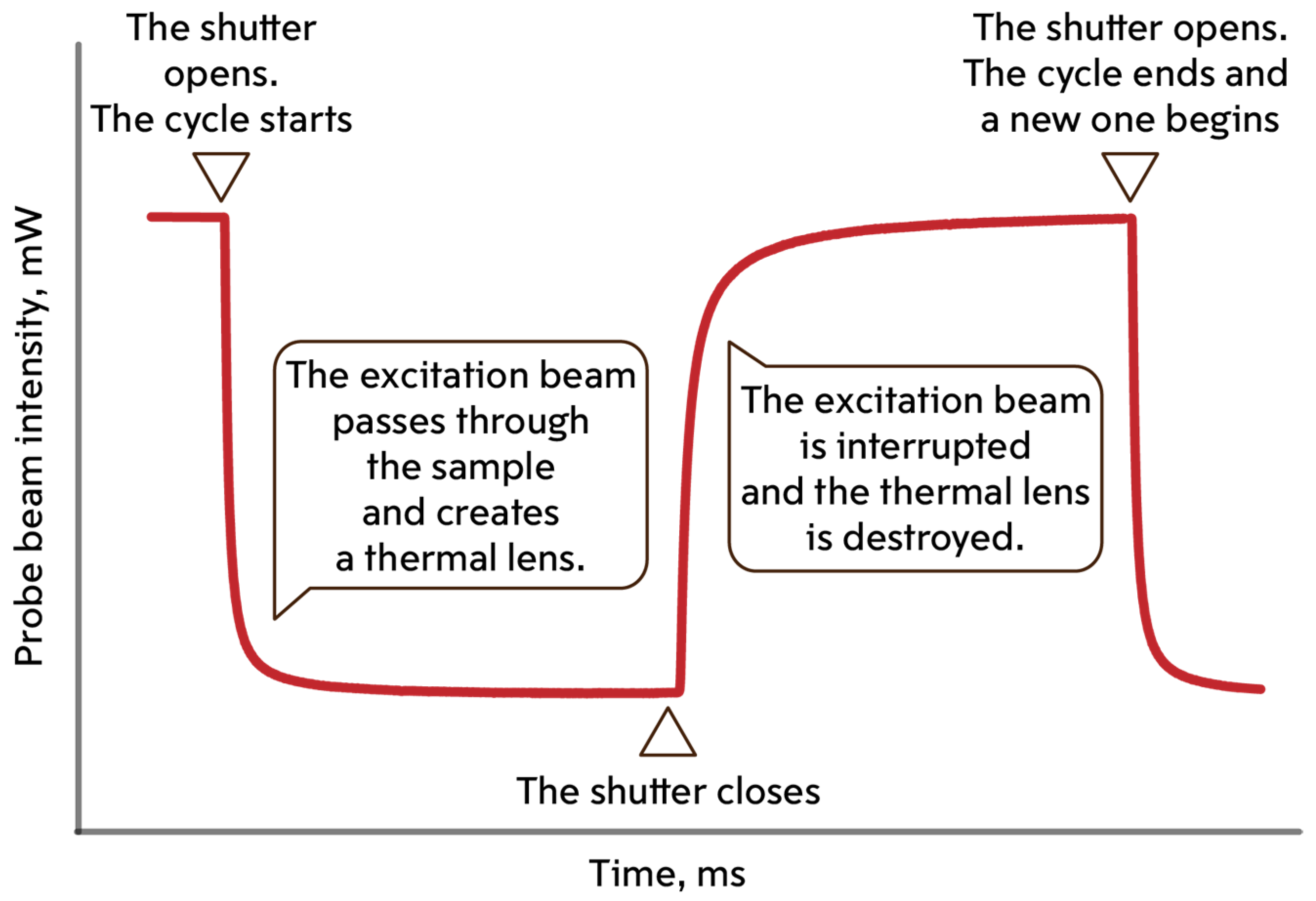
2.4. Finding the Thermal Lens Signal and Thermal Diffusivity
- The sample thickness is low compared to the confocal distances of the beams to ensure that the beam sizes are constant throughout the sample.
- To prevent edge effects, the surface of the sample is large compared to the area of the excitation beam in the sample, .
- The laser power absorbed by the sample is small and there is no convection flow.
- The change in refractive index with temperature dn/dT is constant with increasing sample temperature.
3. Results and Discussion
3.1. Photothermal Effect of Quartz Cell and Air
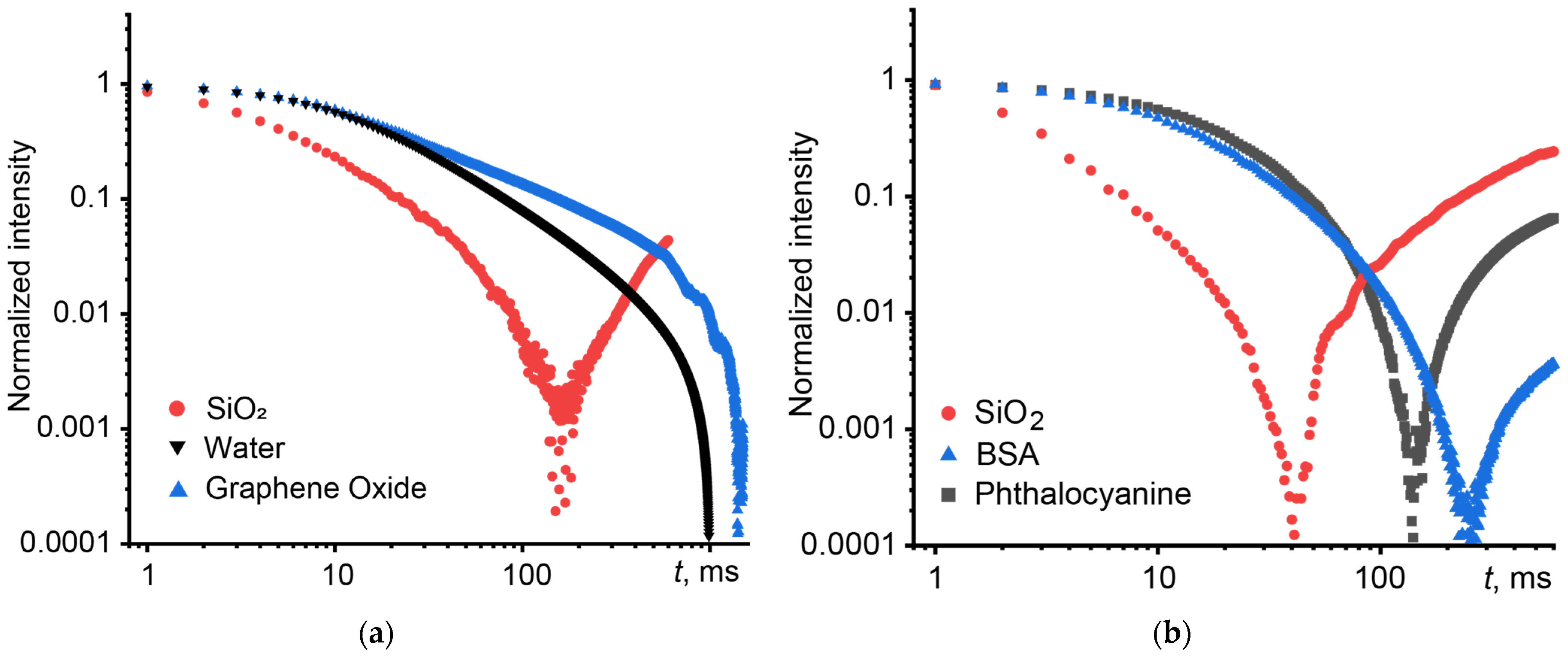
3.2. Light Scattering
3.3. Fluorescence
3.4. Convection and Mass Transfer
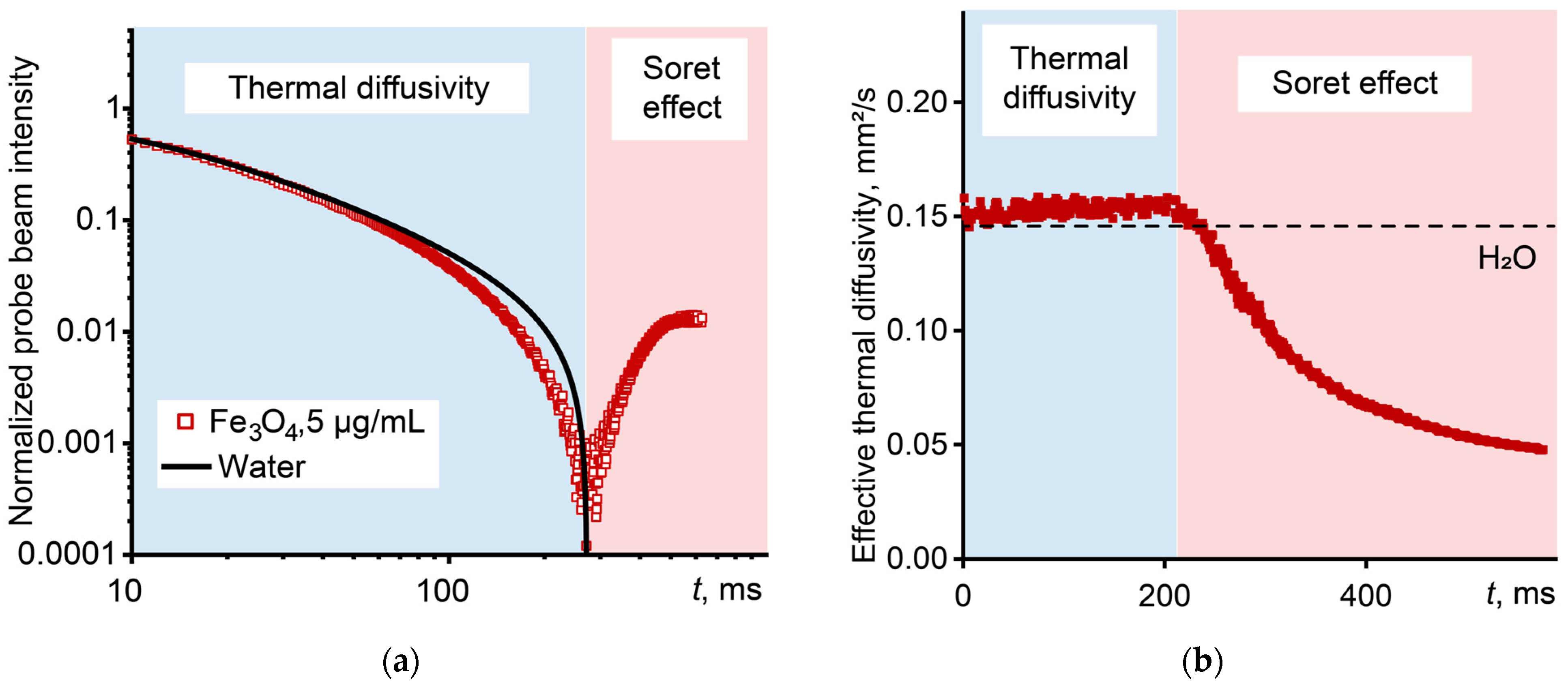
3.5. Ludwig–Soret Effect
3.6. Effect of Solvent
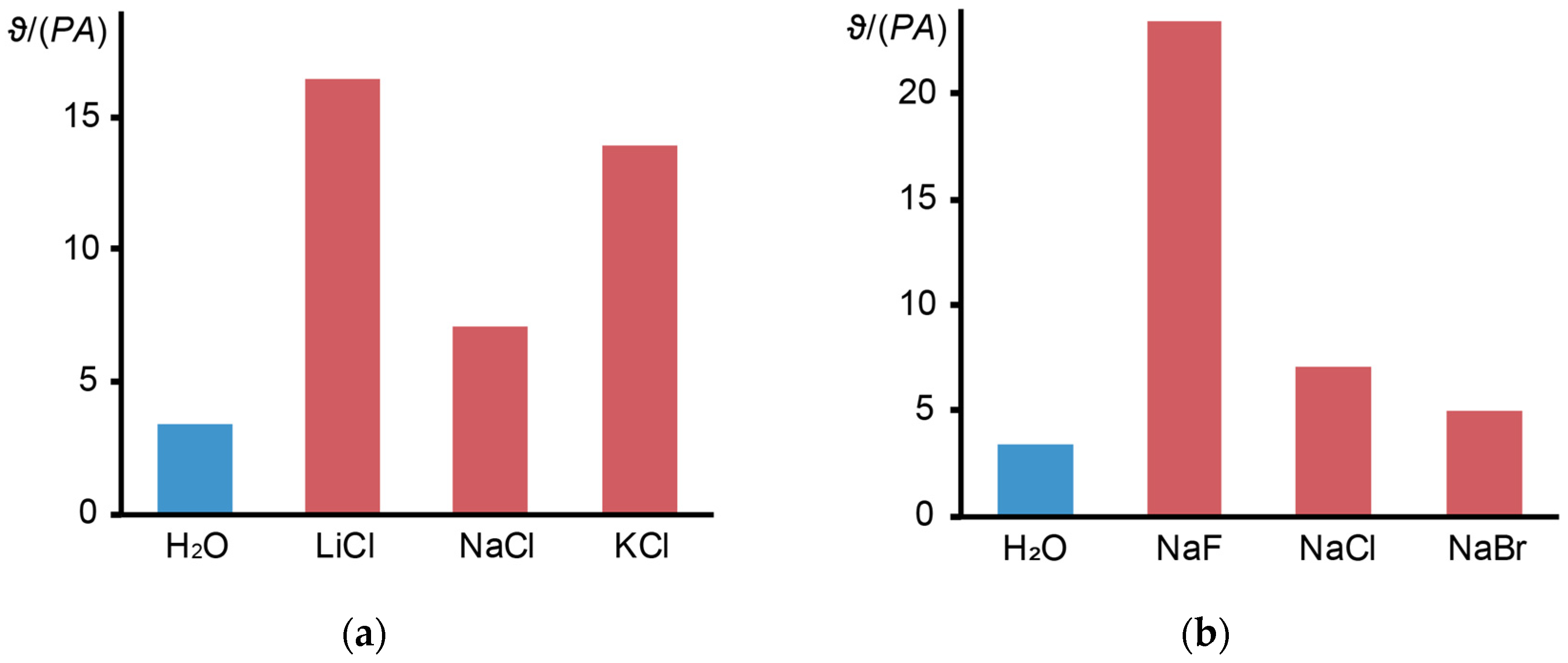
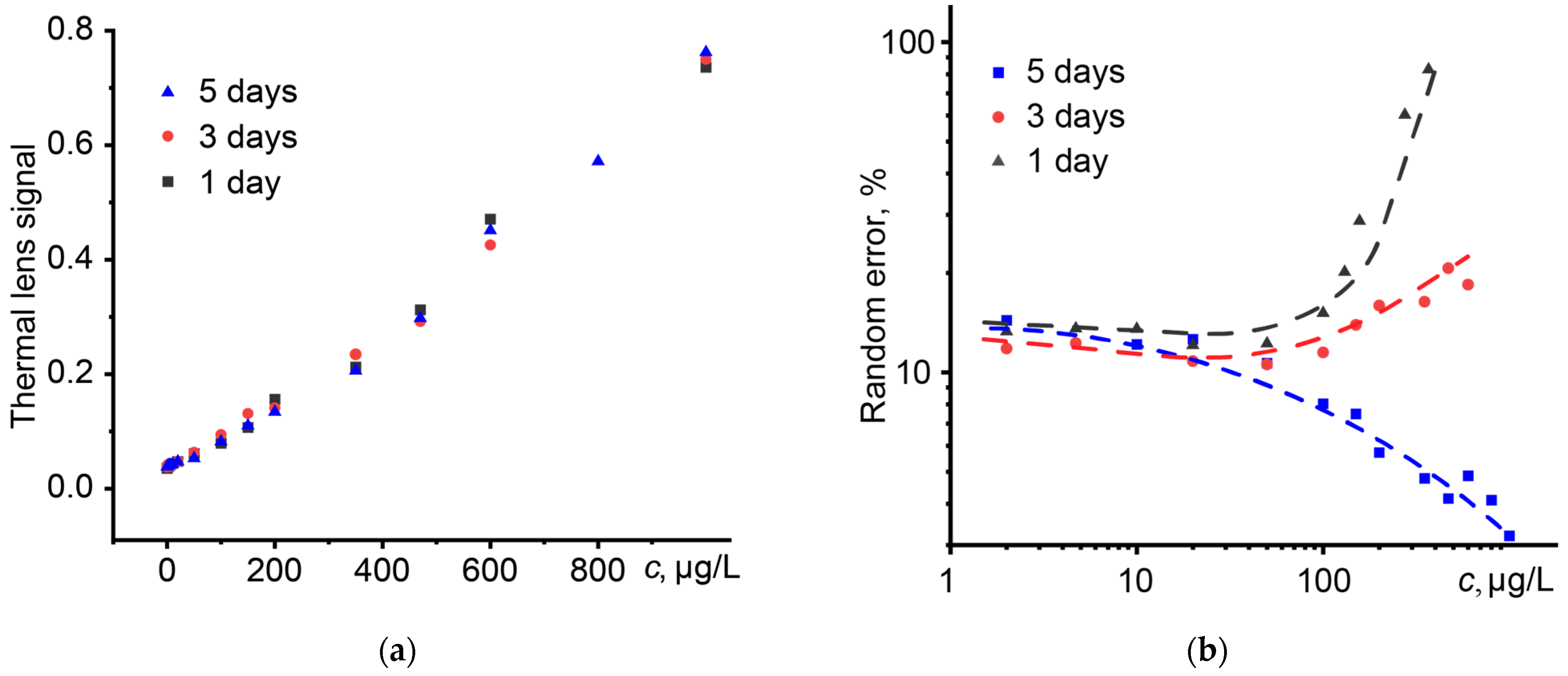
3.7. Electrolytes
3.8. Photometric Analysis of Multiphase Systems
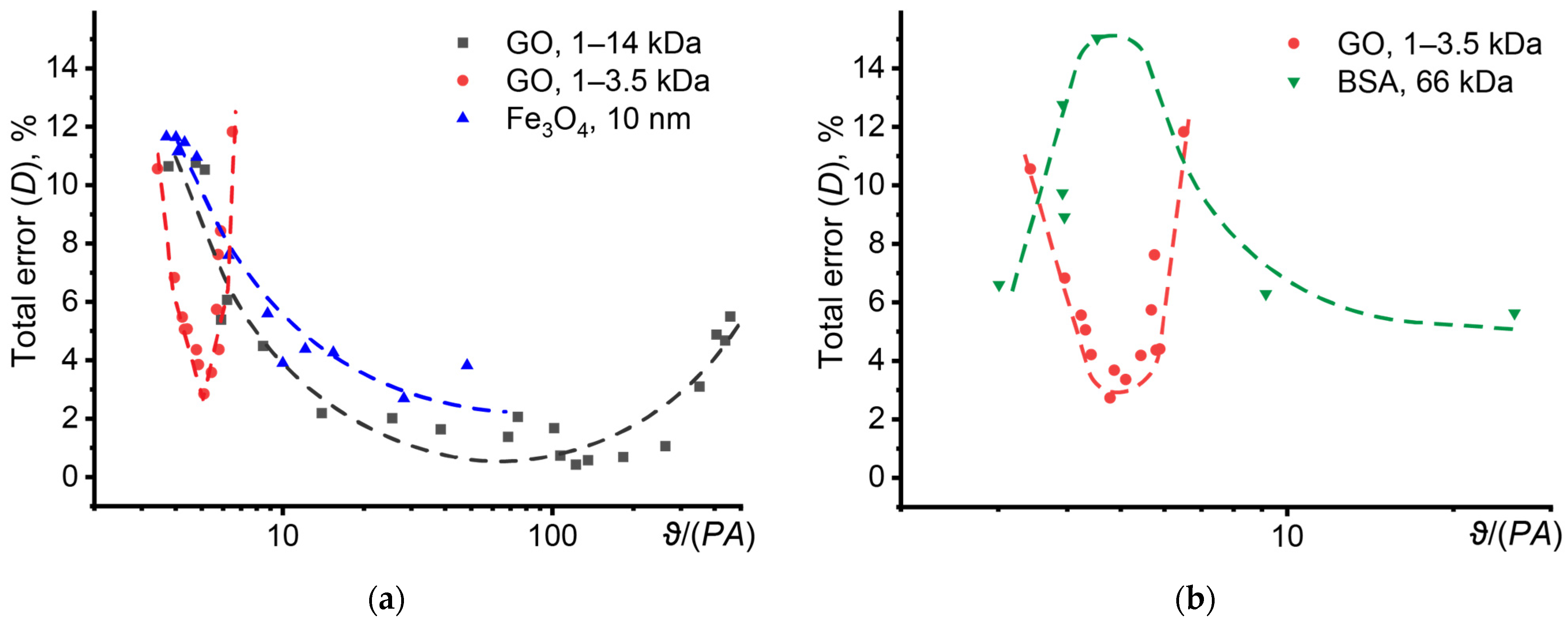
3.8.1. Collection and Processing of Photothermal Data in Disperse Systems
3.8.2. Optical Properties
3.8.3. Thermal Diffusivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bialkowski, S.E.; Astrath, N.G.C.; Proskurnin, M.A. Photothermal Spectroscopy Methods; Wiley: Hoboken, NJ, USA, 2019; p. 512. [Google Scholar]
- Jansen, K.L.; Harris, J.M. Thermal lens measurements of optical computation of the laser beam spot size. Anal. Chem. 1985, 57, 1698–1703. [Google Scholar] [CrossRef]
- Jansen, K.L.; Harris, J.M. Double-beam thermal lens spectrometry. Anal. Chem. 1985, 57, 2434–2436. [Google Scholar] [CrossRef]
- Dovichi, N.J.; Harris, J.M. Time-resolved thermal lens calorimetry. Anal. Chem. 1981, 53, 106–109. [Google Scholar] [CrossRef]
- Hannachi, R. Photothermal lens spectrometry: Experimental optimization and direct quantification of permanganate in water. Sens. Actuat. B 2021, 333, 129542. [Google Scholar] [CrossRef]
- Ramis-Ramos, G. Analytical characteristics, applications and perspectives in thermal lens spectrometry. Anal. Chim. Acta 1993, 283, 623–634. [Google Scholar] [CrossRef]
- Soto, C.; Saavedra, R.; Toral, M.I.; Nacaratte, F.; Poza, C. Preliminary studies for ciclopirox olamine determination by thermal lens spectrophotometry. Microchem. J. 2016, 129, 36–40. [Google Scholar] [CrossRef]
- Cruz, R.A.; Filadelpho, M.C.; Castro, M.P.; Andrade, A.A.; Souza, C.M.; Catunda, T. Very low optical absorptions and analyte concentrations in water measured by Optimized Thermal Lens Spectrometry. Talanta 2011, 85, 850–858. [Google Scholar] [CrossRef]
- Kazemi, E.; Haji Shabani, A.M.; Dadfarnia, S.; Abbasi, A.; Rashidian Vaziri, M.R.; Behjat, A. Development of a novel mixed hemimicelles dispersive micro solid phase extraction using 1-hexadecyl-3-methylimidazolium bromide coated magnetic graphene for the separation and preconcentration of fluoxetine in different matrices before its determination by fiber optic linear array spectrophotometry and mode-mismatched thermal lens spectroscopy. Anal. Chim. Acta 2016, 905, 85–92. [Google Scholar] [CrossRef]
- Proskurnin, M.A.; Kononets, M.Y. Modern analytical thermooptical spectroscopy. Russ. Chem. Rev. 2004, 73, 1143–1172. [Google Scholar] [CrossRef]
- Martelanc, M.; Ziberna, L.; Passamonti, S.; Franko, M. Application of high-performance liquid chromatography combined with ultra-sensitive thermal lens spectrometric detection for simultaneous biliverdin and bilirubin assessment at trace levels in human serum. Talanta 2016, 154, 92–98. [Google Scholar] [CrossRef]
- Franko, M. Thermal Lens Spectrometric Detection in Flow Injection Analysis and Separation Techniques. Appl. Spectrosc. Rev. 2008, 43, 358–388. [Google Scholar] [CrossRef]
- Šikovec, M.; Novič, M.; Franko, M. Application of thermal lens spectrometric detection to the determination of heavy metals by ion chromatography. J. Chromatogr. A 1996, 739, 111–117. [Google Scholar] [CrossRef]
- Hibara, A.; Fukuyama, M.; Chung, M.; Priest, C.; Proskurnin, M.A. Interfacial Phenomena and Fluid Control in Micro/Nanofluidics. Anal. Sci. 2016, 32, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Kurian, A.; Bindhu, C.V.; Nampoori, V.P.N. Kinetic Studies of Chemical Reaction using Laser Induced Thermal Lens Technique. J. Opt. 2015, 37, 43–50. [Google Scholar] [CrossRef]
- Proskurnin, M.A.; Chernysh, V.V.; Pakhomova, S.V.; Kononets, M.Y.; Sheshenev, A.A. Investigation of the reaction of copper(I) with 2,9-dimethyl-1,10-phenanthroline at trace level by thermal lensing. Talanta 2002, 57, 831–839. [Google Scholar] [CrossRef]
- Pakhomova, S.V.; Proskurnin, M.A.; Chernysh, V.V.; Kononets, M.Y.; Ivanova, E.K. Determination of stability constants of copper(I) chelates with 1,10-phenanthroline by thermal lensing. J. Anal. Chem. 2001, 56, 910–917. [Google Scholar] [CrossRef]
- Nedosekin, D.A.; Proskurnin, M.A.; Kononets, M.Y. Model for continuous-wave laser-induced thermal lens spectrometry of optically transparent surface-absorbing solids. Appl. Opt. 2005, 44, 6296–6306. [Google Scholar] [CrossRef]
- Li, S.-H.; He, H.-B.; Shan, Y.-G.; Li, D.-W.; Zhao, Y.-A.; Fan, Z.-X. Enhanced surface thermal lensing for absorption evaluation and defect identification of optical films. Appl. Opt. 2010, 49, 2417–2421. [Google Scholar] [CrossRef]
- Bourgoin, J.-P.; Allogho, G.-G.; Haché, A. Thermal conduction in thin films measured by optical surface thermal lensing. J. Appl. Phys. 2010, 108, 073520. [Google Scholar] [CrossRef]
- Seidman, K.; Payne, A. The Determination of the Heat Capacities of Liquids with Time Resolved Thermal Lens Calorimetry: A More Accurate Procedure. J. Chem. Educ. 1998, 75, 897–900. [Google Scholar] [CrossRef]
- Mohebbifar, M.R. Experimental comparison of methods based on falling and rising signal regions for thermal diffusivity measurement by pulsed dual-beam thermal lens spectroscopy. Measurement 2020, 156, 107611. [Google Scholar] [CrossRef]
- Boudebs, G.; Zinoune, J.B.; Cassagne, C.; Chis, M. Thermal lens Z-scan measurements: Theoretical and experimental uncertainties for low and high fluorescence quantum yields. Appl. Opt. 2023, 62, 7669–7677. [Google Scholar] [CrossRef] [PubMed]
- Astrath, N.G.; Astrath, F.B.; Shen, J.; Zhou, J.; Michaelian, K.H.; Fairbridge, C.; Malacarne, L.C.; Pedreira, P.R.; Medina, A.N.; Baesso, M.L. Thermal-lens study of photochemical reaction kinetics. Opt. Lett. 2009, 34, 3460–3462. [Google Scholar] [CrossRef] [PubMed]
- Brennetot, R.; Georges, J. Investigation of non-linear absorption effects in pulsed-laser thermal lens spectrometry of dye solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1999, 55, 381–395. [Google Scholar] [CrossRef]
- Liu, M.; Ding, C.; Wang, J. Modeling of thermal conductivity of nanofluids considering aggregation and interfacial thermal resistance. RSC Adv. 2016, 6, 3571–3577. [Google Scholar] [CrossRef]
- Herculano, L.S.; Malacarne, L.C.; Zanuto, V.S.; Lukasievicz, G.V.; Capeloto, O.A.; Astrath, N.G. Investigation of the photobleaching process of eosin Y in aqueous solution by thermal lens spectroscopy. J. Phys. Chem. B 2013, 117, 1932–1937. [Google Scholar] [CrossRef]
- Sindhu Swapna, M.N.; Raj, V.; Cabrera, H.; Sankararaman, S.I. Thermal Lensing of Multi-walled Carbon Nanotube Solutions as Heat Transfer Nanofluids. ACS Appl. Nano Mater. 2021, 4, 3416–3425. [Google Scholar] [CrossRef]
- Swapna, M.S.; Raj, V.; Sankararaman, S. Allotropic transformation instigated thermal diffusivity of soot nanofluid: Thermal lens study. Phys. Fluids 2019, 31, 117106. [Google Scholar] [CrossRef]
- Usoltseva, L.O.; Volkov, D.S.; Avramenko, N.V.; Korobov, M.V.; Proskurnin, M.A. Nanodiamond aqueous dispersions as potential nanofluids: The determination of properties by thermal lensing and other techniques. Nanosyst. Phys. Chem. Math. 2018, 9, 17–20. [Google Scholar] [CrossRef]
- Lopes, C.S.; Lenart, V.M.; Turchiello, R.F.; Gómez, S.L. Determination of the Thermal Diffusivity of Plasmonic Nanofluids Containing PVP-Coated Ag Nanoparticles Using Mode-Mismatched Dual-Beam Thermal Lens Technique. Adv. Condens. Matter Phys. 2018, 2018, 3052793. [Google Scholar] [CrossRef]
- Georges, J. Advantages and limitations of thermal lens spectrometry over conventional spectrophotometry for absorbance measurements. Talanta 1999, 48, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Thorne, J.B.; Bobbitt, D.R. Comparison of Beer’s Law and Thermal Lens Techniques for Absorption Measurements under Conditions of High Scattering Backgrounds. Appl. Spectrosc. 1993, 47, 360–365. [Google Scholar] [CrossRef]
- Proskurnin, M.A.; Volkov, D.S.; Gor’kova, T.A.; Bendrysheva, S.N.; Smirnova, A.P.; Nedosekin, D.A. Advances in thermal lens spectrometry. J. Anal. Chem. 2015, 70, 249–276. [Google Scholar] [CrossRef]
- Proskurnin, M.A.; Khabibullin, V.R.; Usoltseva, L.O.; Vyrko, E.A.; Mikheev, I.V.; Volkov, D.S. Photothermal and optoacoustic spectroscopy: State of the art and prospects. Phys. Uspekhi 2022, 65, 270–312. [Google Scholar] [CrossRef]
- Bauer, S.; De Reggi, A.S. Pulsed electrothermal technique for measuring the thermal diffusivity of dielectric films on conducting substrates. J. Appl. Phys. 1996, 80, 6124–6128. [Google Scholar] [CrossRef]
- Prado, J.I.; Calviño, U.; Lugo, L. Experimental Methodology to Determine Thermal Conductivity of Nanofluids by Using a Commercial Transient Hot-Wire Device. Appl. Sci. 2022, 12, 329. [Google Scholar] [CrossRef]
- Hu, R.; Ma, A.; Wang, Y. Transient hot wire measures thermophysical properties of organic foam thermal insulation materials. Exp. Therm Fluid Sci. 2018, 98, 674–682. [Google Scholar] [CrossRef]
- Jaber, W.; Chapuis, P.-O. Non-idealities in the 3ω method for thermal characterization in the low- and high-frequency regimes. AIP Adv. 2018, 8, 045111. [Google Scholar] [CrossRef]
- Usoltseva, L.O.; Volkov, D.S.; Karpushkin, E.A.; Korobov, M.V.; Proskurnin, M.A. Thermal Conductivity of Detonation Nanodiamond Hydrogels and Hydrosols by Direct Heat Flux Measurements. Gels 2021, 7, 248. [Google Scholar] [CrossRef]
- Souza, R.R.; Faustino, V.; Gonçalves, I.M.; Moita, A.S.; Bañobre-López, M.; Lima, R. A Review of the Advances and Challenges in Measuring the Thermal Conductivity of Nanofluids. Nanomaterials 2022, 12, 2526. [Google Scholar] [CrossRef]
- Usoltseva, L.O.; Korobov, M.V.; Proskurnin, M.A. Photothermal spectroscopy: A promising tool for nanofluids. J. Appl. Phys. 2020, 128, 190901. [Google Scholar] [CrossRef]
- Qiu, L.; Ma, Y.; Wang, S.; Ouyang, Y.; Feng, Y. High-accuracy thermophysical property measurement technique based on Labview-3ω method. AIP Adv. 2023, 13, 045014. [Google Scholar] [CrossRef]
- Khabibullin, V.R.; Usoltseva, L.O.; Mikheev, I.V.; Proskurnin, M.A. Thermal Diffusivity of Aqueous Dispersions of Silicon Oxide Nanoparticles by Dual-Beam Thermal Lens Spectrometry. Nanomaterials 2023, 13, 1006. [Google Scholar] [CrossRef]
- Nunes dos Santos, W.; Mummery, P.; Wallwork, A. Thermal diffusivity of polymers by the laser flash technique. Polym. Test. 2005, 24, 628–634. [Google Scholar] [CrossRef]
- Venerus, D.C.; Schieber, J.D.; Iddir, H.; Guzman, J.D.; Broerman, A.W. Measurement of thermal diffusivity in polymer melts using forced Rayleigh light scattering. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 1069–1078. [Google Scholar] [CrossRef]
- Bennett, C.A., Jr.; Patty, R.R. Thermal wave interferometry: A potential application of the photoacoustic effect. Appl. Opt. 1982, 21, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.B. Technique for the measurement of thermal diffusivity based on the laser intensity modulation method (LIMM). Ferroelectrics 1989, 93, 87–93. [Google Scholar] [CrossRef]
- Habiba, U.; Hebert, R.J. Powder Bed Thermal Diffusivity Using Laser Flash Three Layer Analysis. Materials 2023, 16, 6494. [Google Scholar] [CrossRef]
- Philipp, A.; Eichinger, J.F.; Aydin, R.C.; Georgiadis, A.; Cyron, C.J.; Retsch, M. The accuracy of laser flash analysis explored by finite element method and numerical fitting. Heat Mass Transf. 2019, 56, 811–823. [Google Scholar] [CrossRef]
- Bento, A.C.; Almond, D.P. The accuracy of thermal wave interferometry for the evaluation of thermophysical properties of plasma-sprayed coatings. Meas. Sci. Technol. 1995, 6, 1022–1027. [Google Scholar] [CrossRef]
- Khabibullin, V.R.; Usoltseva, L.O.; Galkina, P.A.; Galimova, V.R.; Volkov, D.S.; Mikheev, I.V.; Proskurnin, M.A. Measurement Precision and Thermal and Absorption Properties of Nanostructures in Aqueous Solutions by Transient and Steady-State Thermal-Lens Spectrometry. Physchem 2023, 3, 156–197. [Google Scholar] [CrossRef]
- Khabibullin, V.R.; Ratova, D.V.; Stolbov, D.N.; Mikheev, I.V.; Proskurnin, M.A. The Thermophysical and Physicochemical Properties of the Aqueous Dispersion of Graphene Oxide Dual-Beam Thermal Lens Spectrometry. Nanomaterials 2023, 13, 2126. [Google Scholar] [CrossRef] [PubMed]
- Khabibullin, V.R.; Franko, M.; Proskurnin, M.A. Accuracy of Measurements of Thermophysical Parameters by Dual-Beam Thermal-Lens Spectrometry. Nanomaterials 2023, 13, 430. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, I.; Sekiya, R.; Haino, T. A protocol for size separation of nanographenes. RSC Adv. 2019, 9, 33843–33846. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, N.; Tomsik, E.; Laishevkina, S.; Iakobson, O.; Pankova, G. Cross-linked polyelectrolyte microspheres: Preparation and new insights into electro-surface properties. Soft Matter 2021, 17, 2290–2301. [Google Scholar] [CrossRef]
- Shakirova, J.R.; Shevchenko, N.N.; Baigildin, V.A.; Chelushkin, P.S.; Khlebnikov, A.F.; Tomashenko, O.A.; Solomatina, A.I.; Starova, G.L.; Tunik, S.P. Eu-Based Phosphorescence Lifetime Polymer Nanothermometer: A Nanoemulsion Polymerization Approach to Eliminate Quenching of Eu Emission in Aqueous Media. ACS Appl. Polym. Mater. 2019, 2, 537–547. [Google Scholar] [CrossRef]
- Kazimirova, K.O.; Shtykov, S.N. Sorption and concentration anionic azo dyes on nanomagnetite modified with cationic polyelectrolytes. Sorbtsionnye I Khromatograficheskie Protsessy 2024, 23, 980–992. [Google Scholar] [CrossRef]
- Egunova, O.R.; Reshetnikova, I.S.; Kazimirova, K.O.; Shtykov, S.N. Magnetic Solid-Phase Extraction and Fluorimetric Determination of Some Fluoroquinolones. J. Anal. Chem. 2020, 75, 24–33. [Google Scholar] [CrossRef]
- Dubinina, T.V.; Tomilova, L.G.; Zefirov, N.S. Synthesis of phthalocyanines with an extended system of π-electron conjugation. Russ. Chem. Rev. 2013, 82, 865–895. [Google Scholar] [CrossRef]
- Dubinina, T.V.; Paramonova, K.V.; Trashin, S.A.; Borisova, N.E.; Tomilova, L.G.; Zefirov, N.S. Novel near-IR absorbing phenyl-substituted phthalo- and naphthalocyanine complexes of lanthanide(III): Synthesis and spectral and electrochemical properties. Dalton Trans. 2014, 43, 2799–2809. [Google Scholar] [CrossRef]
- Carter, C.A.; Harris, J.M. Comparison of models describing the thermal lens effect. Appl. Opt. 1984, 23, 476. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.P.; Leite, R.C.C.; Moore, R.S.; Porto, S.P.S.; Whinnery, J.R. Long-Transient Effects in Lasers with Inserted Liquid Samples. J. Appl. Phys. 1965, 36, 3–8. [Google Scholar] [CrossRef]
- Hu, C.; Whinnery, J.R. New thermooptical measurement method and a comparison with other methods. Appl. Opt. 1973, 12, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Lowe, R.D.; Snook, R.D. A model for cw laser induced mode-mismatched dual-beam thermal lens spectrometry. Chem. Phys. 1992, 165, 385–396. [Google Scholar] [CrossRef]
- Sheldon, S.J.; Knight, L.V.; Thorne, J.M. Laser-induced thermal lens effect: A new theoretical model. Appl. Opt. 1982, 21, 1663–1669. [Google Scholar] [CrossRef]
- Vargas-Vargas, A.D.; Mejorada-Sánchez, J.L.; Castellanos-Durán, F.R.; Vargas, E.; Isidro-Ojeda, M.A.; Cedeño, E.; Rojas-Trigos, J.B.; Calderón, A.; Marín, E. Dual beam transient thermal lens spectroscopy with high repetition pulsed IR-Laser Excitation: Photothermal and fluorescence quantum yields determination. Infrared Phys. Technol. 2023, 129, 104561. [Google Scholar] [CrossRef]
- Proskurnin, M.A.; Usoltseva, L.O.; Volkov, D.S.; Nedosekin, D.A.; Korobov, M.V.; Zharov, V.P. Photothermal and Heat-Transfer Properties of Aqueous Detonation Nanodiamonds by Photothermal Microscopy and Transient Spectroscopy. J. Phys. Chem. C 2021, 125, 7808–7823. [Google Scholar] [CrossRef]
- Mikheev, I.V.; Usoltseva, L.O.; Ivshukov, D.A.; Volkov, D.S.; Korobov, M.V.; Proskurnin, M.A. Approach to the Assessment of Size-Dependent Thermal Properties of Disperse Solutions: Time-Resolved Photothermal Lensing of Aqueous Pristine Fullerenes C60and C70. J. Phys. Chem. C 2016, 120, 28270–28287. [Google Scholar] [CrossRef]
- Dovichi, N.J.; Bialkowski, S.E. Thermo-Optical Spectrophotometries in Analytical Chemistry. C R C Crit. Rev. Anal. Chem. 1987, 17, 357–423. [Google Scholar] [CrossRef]
- Franko, M.; Tran, C.D. Analytical thermal lens instrumentation. Rev. Sci. Instrum. 1996, 67, 1–18. [Google Scholar] [CrossRef]
- Soyeh, I.; Hannachi, R.; Sammouda, H. Investigation of the quartz cuvette surface contamination used for permanganate quantification in tap water by photothermal lens spectrometry. Appl. Phys. B 2023, 130, 15. [Google Scholar] [CrossRef]
- Proskurnin, M.A.; Bendrysheva, S.N.; Ragozina, N.; Heissler, S.; Faubel, W.; Pyell, U. Optimization of instrumental parameters of a near-field thermal-lens detector for capillary electrophoresis. Appl. Spectrosc. 2005, 59, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Zidan, M.D.; Allahham, A.; Ghanem, A.; Mousa, N.; Abdallah, B.; Al Salman, A. Thermal lens investigation of the CdSe quantum dots using dual beam z-scan technique. Results Opt. 2024, 16, 100692. [Google Scholar] [CrossRef]
- Arnaud, N.; Georges, J. Thermal lens spectrometry in aqueous solutions of Brij 35: Investigation of micelle effects on the time-resolved and steady-state signals. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2001, 57A, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Escalona, R. Study of a convective field induced by thermal lensing using interferometry. Opt. Commun. 2008, 281, 388–394. [Google Scholar] [CrossRef]
- Vyrko, E.; Volkov, D.S.; Proskurnin, M.A. Numerical Simulation of Photothermal Lens Spectrometry Models Relevant for Analytical Chemistry. Int. J. Thermophys. 2018, 39, 117. [Google Scholar] [CrossRef]
- Carter, C.A.; Harris, J.M. Thermal lens absorption measurements on small volume samples. Anal. Chem. 1984, 56, 922–925. [Google Scholar] [CrossRef]
- Dada, O.O.; Jorgensen, M.R.; Bialkowski, S.E. Continuous laser-excited photothermal spectrometry of CdSxSe1-x doped glasses. Appl. Spectrosc. 2007, 61, 1373–1378. [Google Scholar] [CrossRef]
- Joshi, P.R.; Dada, O.O.; Bialkowski, S.E. Pulsed laser excited photothermal lens spectrometry of cadmium sulfoselenide doped silica glasses. J. Phys. Conf. Ser. 2010, 214, 012117. [Google Scholar] [CrossRef]
- Power, J.F.; Langford, C.H. Optical absorbance of dissolved organic matter in natural water studies using the thermal lens effect. Anal. Chem. 1988, 60, 842–846. [Google Scholar] [CrossRef]
- Yasa, Z.A.; Jackson, W.B.; Amer, N.M. Photothermal spectroscopy of scattering media. Appl. Opt. 1982, 21, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Šikovec, M.; Cruz, F.G.; Franko, M.; Katz, S.A. Determination of Hexavalent Chromium in Extracts of CCA-Treated Building Timbers By Thermal Lens Spectrometry: A Comparison to Spectrophotometry and Atomic Absorption Spectrometry. Spectrosc. Lett. 1996, 29, 465–475. [Google Scholar] [CrossRef]
- Marcano, A.; Basaldua, I.; Villette, A.; Edziah, R.; Liu, J.; Ziane, O.; Melikechi, N. Photothermal lens spectrometry measurements in highly turbid media. Appl. Spectrosc. 2013, 67, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Georges, J.; Mermet, J.M. Measurement of thermal energy recovery using thermal lens spectrometry in fluorescence quenching experiments. Spectrochim. Acta Part A Mol. Spectrosc. 1994, 50, 953–959. [Google Scholar] [CrossRef]
- Fischer, M.; Georges, J. Fluorescence quantum yield of rhodamine 6G in ethanol as a function of concentration using thermal lens spectrometry. Chem. Phys. Lett. 1996, 260, 115–118. [Google Scholar] [CrossRef]
- Fischer, M.; Georges, J. Use of thermal lens spectrometry for the investigation of dimerization equilibria of rhodamine 6G in water and aqueous micellar solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1997, 53, 1419–1430. [Google Scholar] [CrossRef]
- Dy, E.; Gu, C.; Shen, J.; Qu, W.; Xie, Z.; Wang, X.; Baesso, M.L.; Astrath, N.G.C. Sensitivity enhancement of thermal lens spectrometry. J. Appl. Phys. 2022, 131, 063102. [Google Scholar] [CrossRef]
- Malacarne, L.C.; Savi, E.L.; Baesso, M.L.; Lenzi, E.K.; Astrath, N.G.C. Role of Photophysics Processes in Thermal Lens Spectroscopy of Fluids: A Theoretical Study. J. Phys. Chem. A 2014, 118, 5983–5988. [Google Scholar] [CrossRef]
- Georges, J.; Arnaud, N.; Parise, L. Limitations arising from optical saturation in fluorescence and thermal lens spectrometries using pulsed laser excitation: Application to the determination of the fluorescence quantum yield of rhodamine 6G. Appl. Spectrosc. 1996, 50, 1505–1511. [Google Scholar] [CrossRef]
- Shemeena Basheer, N.; Rajesh Kumar, B.; Kurian, A.; George, S.D. Thermal lens probing of distant dependent fluorescence quenching of Rhodamine 6G by silver nanoparticles. J. Lumin. 2013, 137, 225–229. [Google Scholar] [CrossRef]
- Estupiñán-López, C.; Dominguez, C.T.; Filho, P.E.C.; Santos, B.S.; Fontes, A.; de Araujo, R.E. A pH dependence study of CdTe quantum dots fluorescence quantum yields using eclipsing thermal lens spectroscopy. J. Lumin. 2016, 174, 17–21. [Google Scholar] [CrossRef]
- Fischer, M.; Georges, J. Limitations arising in the study of the fluorescence quenching of rhodamine 6G by iodides using cw-laser thermal lens spectrometry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1998, 54, 101–110. [Google Scholar] [CrossRef]
- Singhal, S.; Goswami, D. Unraveling the molecular dependence of femtosecond laser-induced thermal lens spectroscopy in fluids. Analyst 2020, 145, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Amador-Hernandez, J.; Fernandez-Romero, J.M.; Ramis-Ramos, G.; De Castro, M.D.L. Pulse Thermal Lens Spectrometry of β-Carotene in Flow Systems at Atmospheric- and High-Pressure Conditions. Appl. Spectrosc. 1998, 52, 1465–1471. [Google Scholar] [CrossRef]
- Buffett, C.E.; Morris, M.D. Convective Effects in Thermal Lens Spectroscopy. Appl. Spectrosc. 1983, 37, 455–458. [Google Scholar] [CrossRef]
- Dovichi, N.J.; Harris, J.M. Thermal lens calorimetry for flowing samples. Anal. Chem. 1981, 53, 689–692. [Google Scholar] [CrossRef]
- Skogerboe, K.J.; Yeung, E.S. Single laser thermal lens detector for microbore liquid chromatography based on high-frequency modulation. Anal. Chem. 1986, 58, 1014–1018. [Google Scholar] [CrossRef]
- Nickolaisen, S.L.; Bialkowski, S.E. Pulsed laser thermal lens spectrophotometry for flowing liquid detection. Anal Chem 1986, 58, 215–220. [Google Scholar] [CrossRef]
- Alfheim, J.A.; Langford, C.H. Determination of formaldehyde with the thermal lens effect. Anal. Chem. 1985, 57, 861–864. [Google Scholar] [CrossRef]
- Mohebbifar, M.R. Study of the effect of temperature on thermophysical properties of ethyl myristate by dual-beam thermal lens technique. Optik 2021, 247, 168000. [Google Scholar] [CrossRef]
- Martín-Biosca, Y.; Medina-Hernández, M.J.; García-Alvarez-Coque, M.C.; Ramis-Ramos, G. Effect of the nature of the solvent on the limit of detection in thermal lens spectrometry. Anal. Chim. Acta 1994, 296, 285–294. [Google Scholar] [CrossRef]
- Arnaud, N.; Georges, J. Cw-laser thermal lens spectrometry in binary mixtures of water and organic solvents: Composition dependence of the steady-state and time-resolved signals. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2004, 60, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Georges, J.; Paris, T. Influence of the Soret effect on the analytical signal in cw-laser thermal lens spectrometry of micellar solutions. Anal. Chim. Acta 1999, 386, 287–296. [Google Scholar] [CrossRef]
- Arnaud, N.; Georges, J. On the analytical use of the Soret-enhanced thermal lens signal in aqueous solutions. Anal. Chim. Acta 2001, 445, 239–244. [Google Scholar] [CrossRef]
- Arnaud, N.; Georges, J. Investigation of the thermal lens effect in water-ethanol mixtures: Composition dependence of the refractive index gradient, the enhancement factor and the Soret effect. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2001, 57, 1295–1301. [Google Scholar] [CrossRef]
- Cabrera, H.; Sira, E.; Rahn, K.; García-Sucre, M. A thermal lens model including the Soret effect. Appl. Phys. Lett. 2009, 94, 051103. [Google Scholar] [CrossRef]
- Proskurnin, M.A.; Chernysh, V.V.; Filichkina, V.A. Some Metrological Aspects of the Optimization of Thermal-Lens Procedures. J. Anal. Chem. 2004, 59, 818–827. [Google Scholar] [CrossRef]
- Franko, M.; Tran, C.D. Thermal lens effect in electrolyte and surfactant media. J. Phys. Chem. 2002, 95, 6688–6696. [Google Scholar] [CrossRef]
- Hornig, D. On the Spectrum and Structure of Water and Ionic Solutions. J. Chem. Phys. 1964, 40, 3119–3120. [Google Scholar] [CrossRef]
- Pope, R.M.; Fry, E.S. Absorption spectrum (380–700 nm) of pure water. 2. Integrating cavity measurements. Appl. Opt. 1997, 36, 8710–8723. [Google Scholar] [CrossRef]
- Sogandares, F.M.; Fry, E.S. Absorption spectrum (340–640 nm) of pure water. I. Photothermal measurements. Appl. Opt. 1997, 36, 8699–8709. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.K.N.; Tam, A.C. Optical absorption coefficients of water. Nature 1979, 280, 302–304. [Google Scholar] [CrossRef]
- Phillips, C.M.; Crouch, S.R.; Leroi, G.E. Matrix effects in thermal lensing spectrometry: Determination of phosphate in saline solutions. Anal. Chem. 1986, 58, 1710–1714. [Google Scholar] [CrossRef]
- Zhirkov, A.A.; Nikiforov, A.A.; Tsar’kov, D.S.; Volkov, D.S.; Proskurnin, M.A.; Zuev, B.K. Effect of electrolytes on the sensitivity of the thermal lens determination. J. Anal. Chem. 2012, 67, 290–296. [Google Scholar] [CrossRef]
- Colcombe, S.M.; Lowe, R.D.; Snook, R.D. Thermal lens investigation of the temperature dependence of the refractive index of aqueous electrolyte solutions. Anal. Chim. Acta 1997, 356, 277–288. [Google Scholar] [CrossRef]
- Georges, J. Matrix effects in thermal lens spectrometry: Influence of salts, surfactants, polymers and solvent mixtures. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2008, 69, 1063–1072. [Google Scholar] [CrossRef]
- Simon, J.; Anugop, B.; Nampoori, V.P.N.; Kailasnath, M. Effect of pulsed laser irradiation on the thermal diffusivity of bimetallic Au/Ag nanoparticles. Opt. Laser Technol. 2021, 139, 106954. [Google Scholar] [CrossRef]
- Jiménez-Pérez, J.L.; López-Gamboa, G.; Sánchez-Ramírez, J.F.; Correa-Pacheco, Z.N.; Netzahual-Lopantzi, A.; Cruz-Orea, A. Thermal Diffusivity Dependence with Highly Concentrated Graphene Oxide/Water Nanofluids by Mode-Mismatched Dual-Beam Thermal Lens Technique. Int. J. Thermophys. 2021, 42, 107. [Google Scholar] [CrossRef]
- Nideep, T.K.; Ramya, M.; Nampoori, V.P.N.; Kailasnath, M. The size dependent thermal diffusivity of water soluble CdTe quantum dots using dual beam thermal lens spectroscopy. Phys. E Low-Dimens. Syst. Nanostructures 2020, 116, 113724. [Google Scholar] [CrossRef]
- Swapna, M.N.S.; Korte, D.; Sankararaman, S.I. Solid-Volume-Fraction Retained Tailoring of Thermal Diffusivity of Multiwalled Carbon Nanotube Nanofluid: A Photothermal Investigation. Phys. Status Solidi 2023, 220, 2200797. [Google Scholar] [CrossRef]
- Netzahual-Lopantzi, Á.; Sánchez-Ramírez, J.F.; Jiménez-Pérez, J.L.; Cornejo-Monroy, D.; López-Gamboa, G.; Correa-Pacheco, Z.N. Study of the thermal diffusivity of nanofluids containing SiO2 decorated with Au nanoparticles by thermal lens spectroscopy. Appl. Phys. A 2019, 125, 588. [Google Scholar] [CrossRef]
- Prakash, A.; Pathrose, B.P.; Nampoori, V.P.N.; Radhakrishnan, P.; Mujeeb, A. Thermal diffusivity of neutral red dye using dual beam thermal lens technique: A comparison on the effects using nano pulsed laser ablated silver and gold nanoparticles. Phys. E Low-Dimens. Syst. Nanostructures 2019, 107, 203–208. [Google Scholar] [CrossRef]
- Luna-Sánchez, J.L.; Jiménez-Pérez, J.L.; Carbajal-Valdez, R.; Lopez-Gamboa, G.; Pérez-González, M.; Correa-Pacheco, Z.N. Green synthesis of silver nanoparticles using Jalapeño Chili extract and thermal lens study of acrylic resin nanocomposites. Thermochim. Acta 2019, 678, 178314. [Google Scholar] [CrossRef]
- Lopez-Munoz, G.A.; Pescador-Rojas, J.A.; Ortega-Lopez, J.; Salazar, J.S.; Balderas-Lopez, J.A. Thermal diffusivity measurement of spherical gold nanofluids of different sizes/concentrations. Nanoscale Res. Lett. 2012, 7, 423. [Google Scholar] [CrossRef]
- Mathew, R.M.; Zachariah, E.S.; Jose, J.; Thomas, T.; John, J.; Titus, T.; Unni, N.G.; Mathew, S.; Mujeeb, A.; Thomas, V. Synthesis, characterization and evaluation of tunable thermal diffusivity of phosphorus-doped carbon nanodot. Appl. Phys. A 2020, 126, 828. [Google Scholar] [CrossRef]
- Ramya, M.; Nideep, T.K.; Nampoori, V.P.N.; Kailasnath, M. Particle size and concentration effect on thermal diffusivity of water-based ZnO nanofluid using the dual-beam thermal lens technique. Appl. Phys. B 2019, 125, 181. [Google Scholar] [CrossRef]
- Jiménez-Pérez, J.L.; Gutiérrez Fuentes, R.; Sánchez-Sosa, R.; Zapata Torres, M.G.; Correa-Pacheco, Z.N.; Sánchez Ramírez, J.F. Thermal diffusivity study of nanoparticles and nanorods of titanium dioxide (TiO2) and titanium dioxide coated with cadmium sulfide (TiO2CdS). Mater. Sci. Semicond. Process. 2015, 37, 62–67. [Google Scholar] [CrossRef]
- González-Araoz, M.P.; Sánchez-Ramírez, J.F.; Jiménez-Pérez, J.L.; Chigo-Anota, E.; Herrera-Pérez, J.L.; Mendoza-Álvarez, J.G.J.N.S. Negative thermal diffusivity enhancement in semiconductor nanofluids. Nat. Sci. 2012, 4, 1022–1028. [Google Scholar] [CrossRef]
- Augustine, A.K.; Mathew, S.; Girijavallabhan, C.P.; Radhakrishnan, P.; Nampoori, V.P.N.; Kailasnath, M. Size dependent variation of thermal diffusivity of CdSe nanoparticles based nanofluid using laser induced mode-matched thermal lens technique. J. Opt. 2014, 44, 85–91. [Google Scholar] [CrossRef]
- Moreira, T.A.; Moreira, D.C.; Ribatski, G. Nanofluids for heat transfer applications: A review. J. Braz. Soc. Mech. Sci. Eng. 2018, 40, 303. [Google Scholar] [CrossRef]
- Tao, Q.; Zhong, F.; Deng, Y.; Wang, Y.; Su, C. A Review of Nanofluids as Coolants for Thermal Management Systems in Fuel Cell Vehicles. Nanomaterials 2023, 13, 2861. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, I.; Valiente, R.; Ortiz, F.; Renedo, C.J.; Ortiz, A. Effect of TiO2 and ZnO Nanoparticles on the Performance of Dielectric Nanofluids Based on Vegetable Esters During Their Aging. Nanomaterials 2020, 10, 692. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Aquino, R.; Jiménez-Pérez, J.L.; Altamirano-Juárez, D.C.; López-Gamboa, G.; Correa-Pacheco, Z.N.; Carbajal-Valdéz, R. Green Synthesis of Silver Nanoparticles Contained in Centrifuged Citrus Oil and Their Thermal Diffusivity Study by Using Thermal Lens Technique. Int. J. Thermophys. 2018, 40, 3. [Google Scholar] [CrossRef]
- Pedreira, P.R.B.; Hirsch, L.R.; Pereira, J.R.D.; Medina, A.N.; Bento, A.C.; Baesso, M.L.; Rollemberg, M.C.E.; Franko, M. Observation of laser induced photochemical reaction of Cr(VI) species in water during thermal lens measurements. Chem. Phys. Lett. 2004, 396, 221–225. [Google Scholar] [CrossRef]
- Pedreira, P.R.B.; Hirsch, L.R.; Pereira, J.R.D.; Medina, A.N.; Bento, A.C.; Baesso, M.L.; Rollemberg, M.C.; Franko, M.; Shen, J. Real-time quantitative investigation of photochemical reaction using thermal lens measurements: Theory and experiment. J. Appl. Phys. 2006, 100, 044906. [Google Scholar] [CrossRef]
- Savi, E.L.; Malacarne, L.C.; Baesso, M.L.; Pintro, P.T.M.; Croge, C.; Shen, J.; Astrath, N.G.C. Investigation into photostability of soybean oils by thermal lens spectroscopy. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2015, 145, 125–129. [Google Scholar] [CrossRef]
- Rodriguez, L.G.; Iza, P.; Paz, J.L. Study of dependence between thermal diffusivity and sample concentration measured by means of frequency-resolved thermal lens experiment. J. Nonlinear Opt. Phys. Mater. 2016, 25, 1650022. [Google Scholar] [CrossRef]
- Mathew, S.; Francis, F.; Joseph, S.A.; Kala, M.S. Enhanced thermal diffusivity of water based ZnO nanoflower/rGO nanofluid using the dual-beam thermal lens technique. Nano-Struct. Nano-Objects 2021, 28, 100784. [Google Scholar] [CrossRef]
- Francis, F.; Anila, E.I.; Joseph, S.A. Dependence of thermal diffusivity on nanoparticle shape deduced through thermal lens technique taking ZnO nanoparticles and nanorods as inclusions in homogeneous dye solution. Optik 2020, 219, 165210. [Google Scholar] [CrossRef]
- Lenart, V.M.; Astrath, N.G.C.; Turchiello, R.F.; Goya, G.F.; Gómez, S.L. Thermal diffusivity of ferrofluids as a function of particle size determined using the mode-mismatched dual-beam thermal lens technique. J. Appl. Phys. 2018, 123, 085107. [Google Scholar] [CrossRef]
- Hari, M.; Joseph, S.A.; Mathew, S.; Nithyaja, B.; Nampoori, V.P.N.; Radhakrishnan, P. Thermal diffusivity of nanofluids composed of rod-shaped silver nanoparticles. Int. J. Therm. Sci. 2013, 64, 188–194. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Tran, H.-V.; Xu, S.; Lee, T.R. Fe3O4 Nanoparticles: Structures, Synthesis, Magnetic Properties, Surface Functionalization, and Emerging Applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef] [PubMed]
- Khabibullin, V.R.; Chetyrkina, M.R.; Obydennyy, S.I.; Maksimov, S.V.; Stepanov, G.V.; Shtykov, S.N. Study on Doxorubicin Loading on Differently Functionalized Iron Oxide Nanoparticles: Implications for Controlled Drug-Delivery Application. Int. J. Mol. Sci. 2023, 24, 4480. [Google Scholar] [CrossRef] [PubMed]
- Constantino, R.; Lenzi, G.G.; Franco, M.G.; Lenzi, E.K.; Bento, A.C.; Astrath, N.G.C.; Malacarne, L.C.; Baesso, M.L. Thermal Lens Temperature Scanning technique for evaluation of oxidative stability and time of transesterification during biodiesel synthesis. Fuel 2017, 202, 78–84. [Google Scholar] [CrossRef]
- Deus, W.B.; Ventura, M.; Silva, J.R.; Andrade, L.H.C.; Catunda, T.; Lima, S.M. Monitoring of the ester production by near-near infrared thermal lens spectroscopy. Fuel 2019, 253, 1090–1096. [Google Scholar] [CrossRef]
- Gvozdev, D.A.; Maksimov, E.G.; Paschenko, V.Z. Photobleaching of Phthalocyanine Molecules within a Complex with Colloidal Quantum Dots. Mosc. Univ. Biol. Sci. Bull. 2020, 75, 7–12. [Google Scholar] [CrossRef]




| Sample | Excitation Power, mW | |
|---|---|---|
| 200.0 | 300.0 | |
| air | 0.00006 | 0.00022 |
| quartz cell (10.00 mm) | 0.00030 | 0.00124 |
| Solvent | Thermal Diffusivity, mm2/s | Thermal Lens Signal | |
|---|---|---|---|
| Experiment | Theory [70] | ||
| Water (Milli-Q) | 0.147 ± 0.005 | 0.145 | 0.036 ± 0.003 |
| Water (distilled) | 0.149 ± 0.009 | 0.038 ± 0.003 | |
| Water (central water supply) | 0.15 ± 0.01 | 0.046 ± 0.004 | |
| Ethanol | 0.088 ± 0.002 | 0.089 | 0.38 ± 0.02 |
| Chloroform | 0.085 ± 0.003 | 0.083 | 0.16 ± 0.06 |
| Toluene | 0.090 ± 0.008 | 0.091 | 1.1 ± 0.3 |
| Acetonitrile | 0.115 ± 0.008 | 0.111 | 1.120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khabibullin, V.R.; Mikheev, I.V.; Proskurnin, M.A. Features of High-Precision Photothermal Analysis of Liquid Systems by Dual-Beam Thermal Lens Spectrometry. Nanomaterials 2024, 14, 1586. https://doi.org/10.3390/nano14191586
Khabibullin VR, Mikheev IV, Proskurnin MA. Features of High-Precision Photothermal Analysis of Liquid Systems by Dual-Beam Thermal Lens Spectrometry. Nanomaterials. 2024; 14(19):1586. https://doi.org/10.3390/nano14191586
Chicago/Turabian StyleKhabibullin, Vladislav R., Ivan V. Mikheev, and Mikhail A. Proskurnin. 2024. "Features of High-Precision Photothermal Analysis of Liquid Systems by Dual-Beam Thermal Lens Spectrometry" Nanomaterials 14, no. 19: 1586. https://doi.org/10.3390/nano14191586






