The Influence of Thickness and Spectral Properties of Green Color-Emitting Polymer Thin Films on Their Implementation in Wearable PLED Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ink Formulation
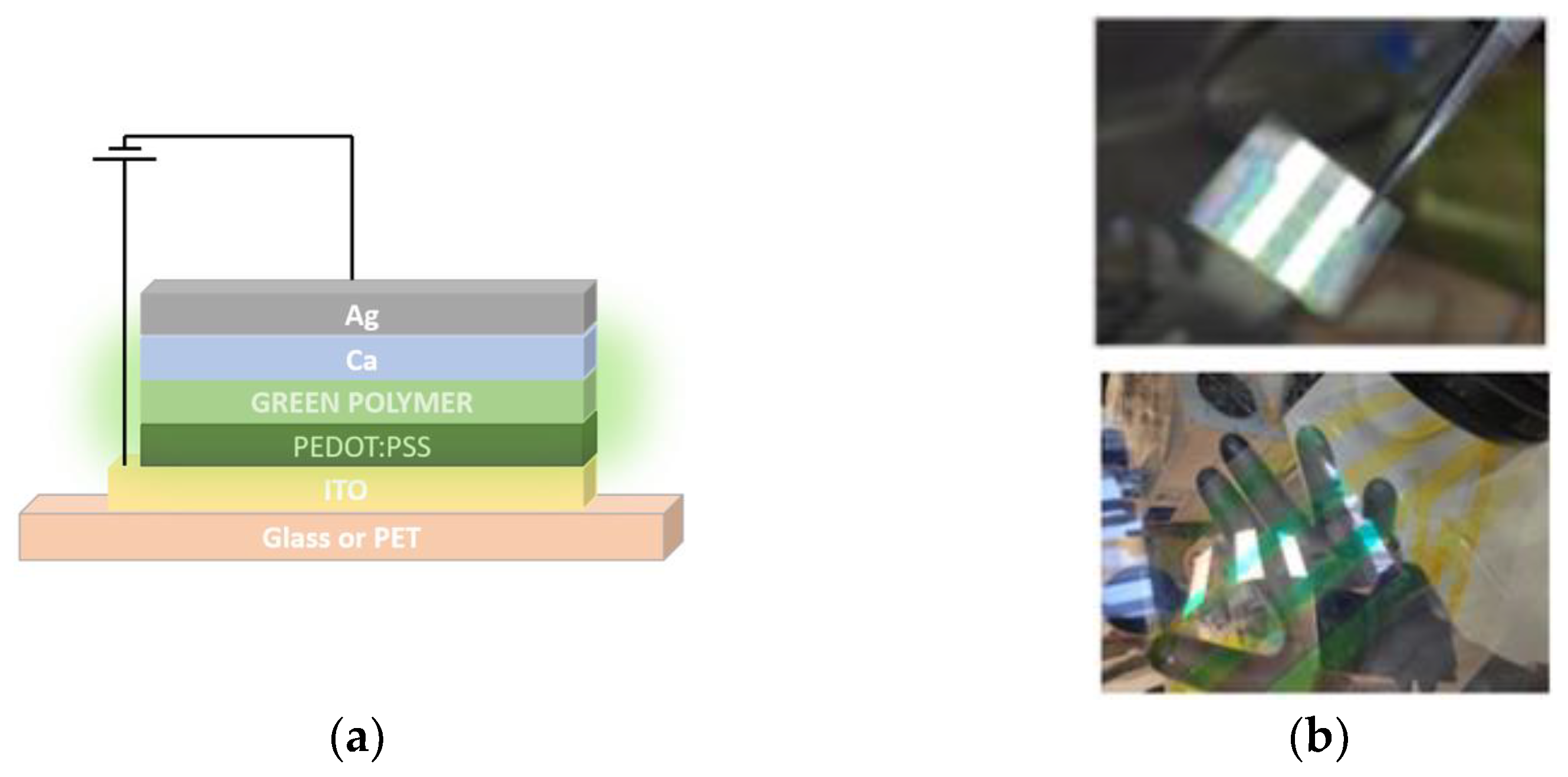
2.2. PLED Fabrication
2.3. Thin Film and Device Characterization
3. Results
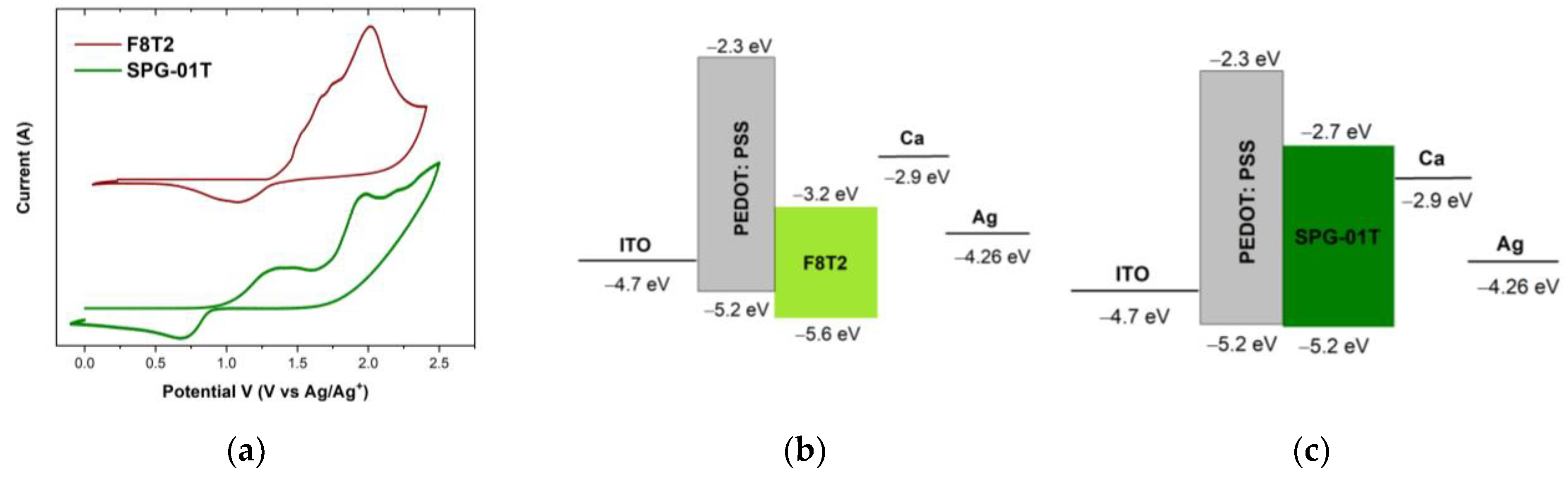
3.1. Photophysical and Electrochemical Characterization of Thin Films
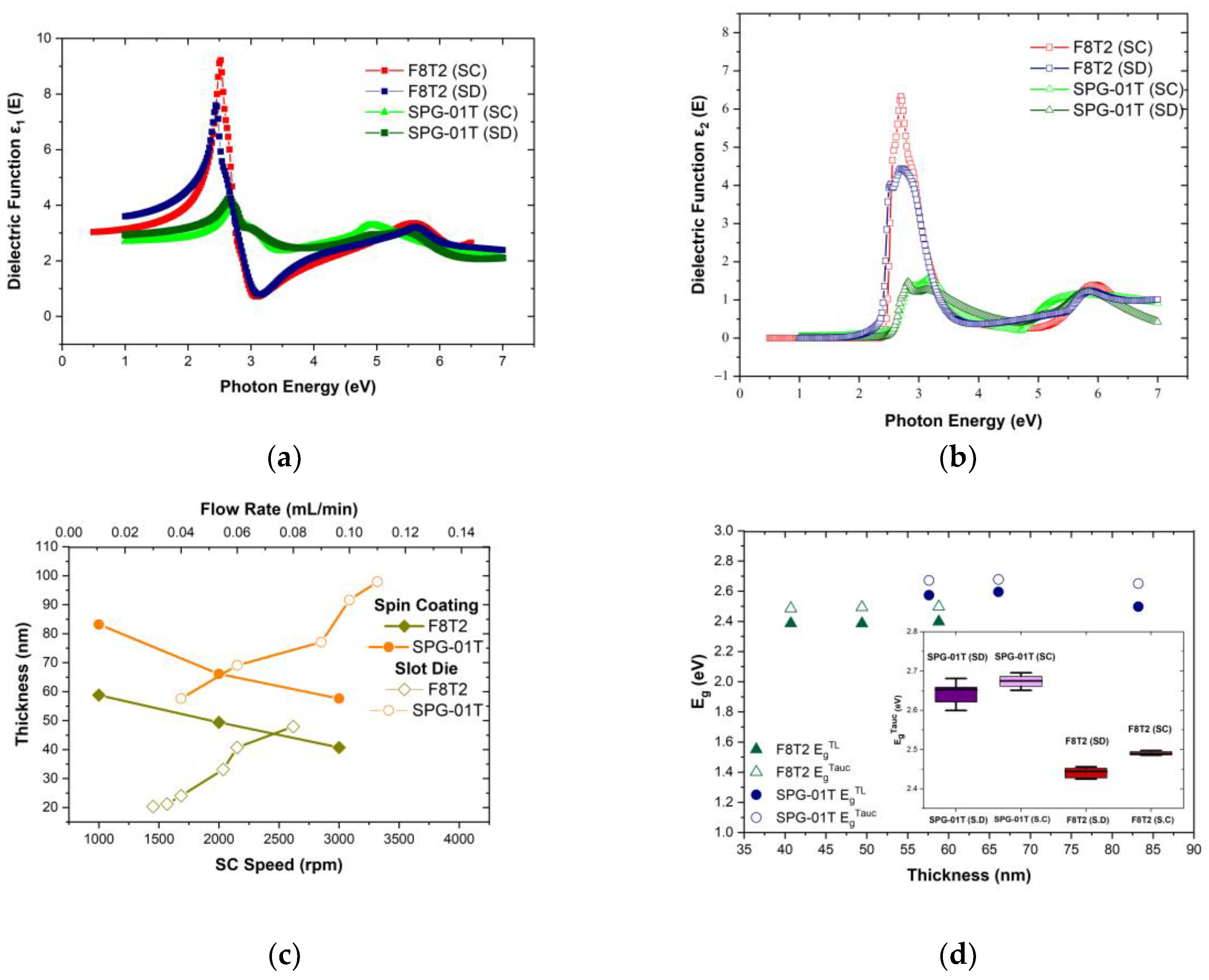
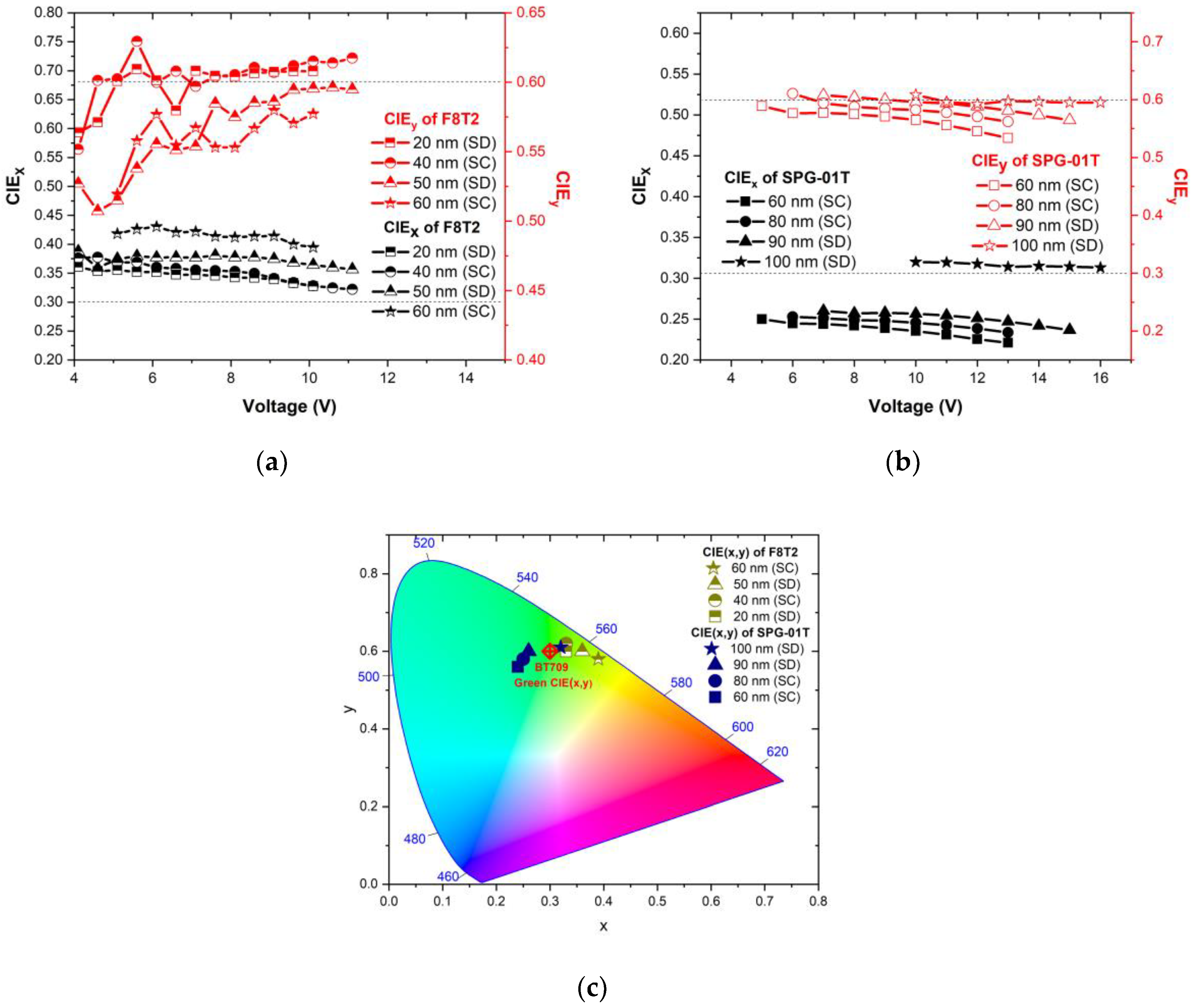
3.2. Dependence of Optical Characteristics and Morphological Features of F8T2 and SPG-01T on Thickness
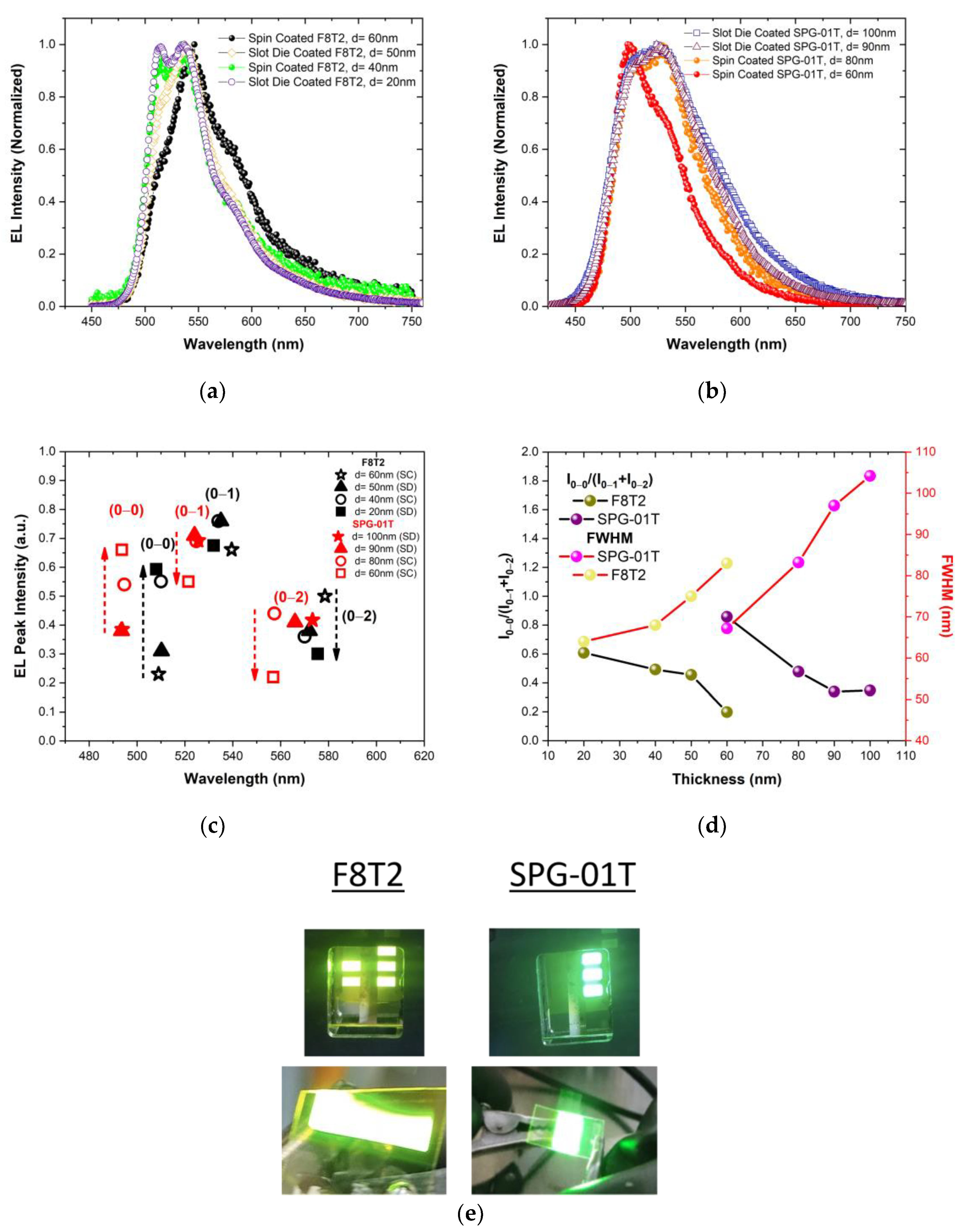
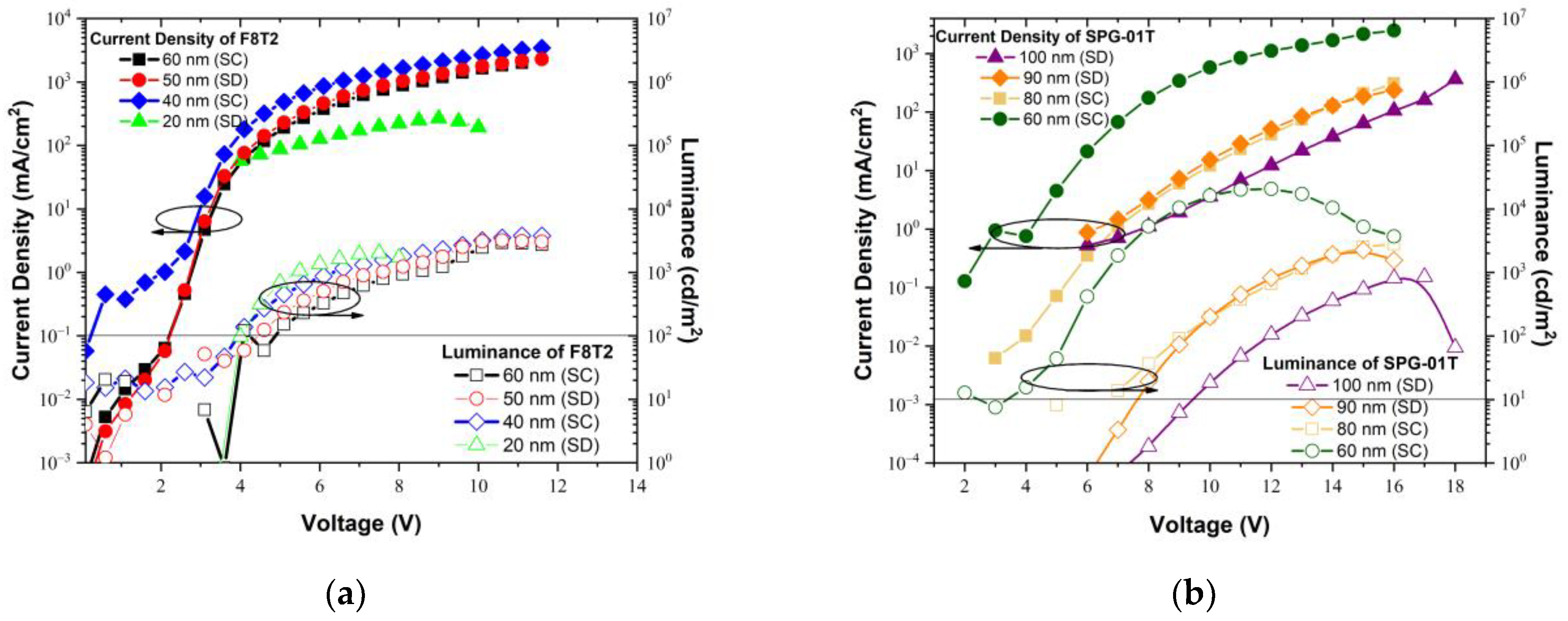
3.3. Dependence of Electroluminescence on Emissive Material’s Thickness
3.4. Electrical Performance of the Fabricated PLEDs
3.5. Detection of Rhodamine 6G
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, J.; Lee, H.; Jeong, E.G.; Choi, K.C.; Yoo, S. Organic Light-Emitting Diodes: Pushing Toward the Limits and Beyond. Adv. Mater. 2020, 32, 1907539. [Google Scholar] [CrossRef] [PubMed]
- Dhanabalan, S.S.; Sriram, S.; Walia, S.; Avaninathan, S.R.; Carrasco, M.F.; Bhaskaran, M. Wearable Label-Free Optical Biodetectors: Progress and Perspectives. Adv. Photonics Res. 2021, 2, 2000076. [Google Scholar] [CrossRef]
- Ku, M.; Hwang, J.C.; Oh, B.; Park, J.-U. Smart Sensing Systems Using Wearable Optoelectronics. Adv. Intell. Syst. 2020, 2, 1900144. [Google Scholar] [CrossRef]
- Shinar, J.; Shinar, R. Organic light-emitting devices (OLEDs) and OLED-based chemical and biological sensors: An overview. J. Phys. D Appl. Phys. 2008, 41, 133001. [Google Scholar] [CrossRef]
- Ramuz, M.; Leuenberger, D.; Bürgi, L. Optical biosensors based on integrated polymer light source and polymer photodiode. J. Polym. Sci. B Polym. Phys. 2011, 49, 80–87. [Google Scholar] [CrossRef]
- Bansal, A.K.; Hou, S.; Kulyk, O.; Bowman, E.M.; Samuel, I.D.W. Wearable Organic Optoelectronic Sensors for Medicine. Adv. Mater. 2015, 27, 7638–7644. [Google Scholar] [CrossRef] [PubMed]
- Krujatz, F.; Or, H.; Fehse, K.; Jahnel, M.; Werner, A.; Bley, T. Exploiting the Potential of OLED-Based Photo-Organic Sensors for Biotechnological Applications. Chem. Sci. 2016, 7, 1000134. [Google Scholar] [CrossRef]
- Keum, C.; Murawski, C.; Archer, E.; Kwon, S.; Mischok, A.; Gather, M.C. A substrateless, flexible, and water-resistant organic light-emitting diode. Nat. Commun. 2020, 11, 6250. [Google Scholar] [CrossRef]
- Han, D.; Khan, Y.; Ting, S.; King, N.; Yaacobi-Gross, N.; Humphries, M.J.; Newsome, C.J.; Arias, A.C. Flexible Blade-Coated Multicolor Polymer Light-Emitting Diodes for Optoelectronic Sensors. Adv. Mater. 2017, 29, 1606206. [Google Scholar] [CrossRef]
- Yokota, T.; Zalar, P.; Kaltenbrunner, M.; Jinno, H.; Matsuhisa, N.; Kitanosako, H.; Tachibana, Y.; Yukita, W.; Koizumi, M.; Someya, Τ. Ultraflexible organic photonic skin. Sci. Adv. 2016, 2, 1501856. [Google Scholar] [CrossRef]
- Elsamnah, F.; Bilgaiyan, A.; Affiq, M.; Shim, C.H.; Ishidai, H.; Hattori, R. Comparative Design Study for Power Reduction in Organic Optoelectronic Pulse Meter Sensor. Biosensors 2019, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Dimitriev, O.P. Dynamics of Excitons in Conjugated Molecules and Organic Semiconductor Systems. Chem. Rev. 2022, 122, 8487–8593. [Google Scholar] [CrossRef] [PubMed]
- De Jong, M.; Seijo, L.; Meijerink, A.; Rabouw, F.T. Resolving the ambiguity in the relation between Stokes shift and Huang–Rhys parameter. Phys. Chem. Chem. Phys. 2015, 17, 16959–16969. [Google Scholar] [CrossRef]
- Jahnel, M.; Beyer, B.; Thomschke, M.; Fehse, K.; Krujatz, F.; Leo, K. Narrow Bandwidth Top-Emitting OLEDs Designed for Rhodamine 6G Excitation in Biological Sensing Applications. Electronics 2015, 4, 982–994. [Google Scholar] [CrossRef]
- Fukagawa, H.; Oono, T.; Iwasaki, Y.; Hatakeyama, T.; Shimizu, T. High-efficiency ultrapure green organic light-emitting diodes. Mater. Chem. Front. 2018, 2, 704–709. [Google Scholar] [CrossRef]
- Gioti, M. Optical, photophysical, and electrooptical studies on slot-die polyfluorene-based flexible OLED devices. Opt. Mater. Express 2021, 11, 1442–1456. [Google Scholar] [CrossRef]
- De Azevedo, D.; Freitas, J.N.; Domingues, R.A.; Faleiros, M.M.; De Almeida Santos, T.E.; Atvars, T.D.Z. Tuning the emission color of a single-layer polymer light-emitting diode with a solution-processed external layer. Synth. Met. 2016, 222, 205–210. [Google Scholar] [CrossRef]
- Rajasekar, M.; Ranjitha, V.; Rajasekar, K. Recent advances in Fluorescent-based cation sensors for biomedical applications. Results Chem. 2023, 5, 100850. [Google Scholar] [CrossRef]
- Zeng, S.; Xiaosheng, L.; Kafuti, Y.S.; Kim, H.; Wang, J.; Peng, X.; Li, H.; Yoon, J. Fluorescent dyes based on rhodamine derivatives for bioimaging and therapeutics: Recent progress, challenges, and prospects. Chem. Soc. Rev. 2023, 52, 5607–5651. [Google Scholar] [CrossRef]
- Lochner, C.M.; Khan, Y.; Pierre, A.; Arias, A.C. All-organic optoelectronic sensor for pulse oximetry. Nat. Commun. 2014, 5, 5745. [Google Scholar] [CrossRef]
- Choudhury, B.; Shinar, R.; Shinar, J. Glucose biosensors based on organic light-emitting devices structurally integrated with a luminescent sensing element. J. Appl. Phys. 2004, 96, 2949–2954. [Google Scholar] [CrossRef]
- Mbarek, M.; Almoneef, M.M.; Βen Saleh, Y.; Alimi, K. Organic optoelectronic copolymer involving PVK and F8T2: Synthesis and characterization. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 252, 119509. [Google Scholar] [CrossRef]
- Wyatt-Moon, G.; Georgiadou, D.G.; Zoladek-Lemanczyk, A.; Castro, F.A.; Anthopoulos, T.D. Flexible nanogap polymer light-emitting diodes fabricated via adhesion lithography (a-Lith). J. Phys. Mater. 2018, 1, 01LT01. [Google Scholar] [CrossRef]
- Chuang, C.N.; Chang, C.Y.; Chang, C.L.; Wang, Y.X.; Lin, Y.S.; Leung, M.K. Poly(9,9-dialkylfluorene-co-triphenylamine)s—A family of photo-curable hole-transport polymers for polymer light-emitting diodes applications. Eur. Polym. J. 2014, 56, 33–44. [Google Scholar] [CrossRef]
- Da Costa, R.G.M.; Farias, F.R.L.; Maqueira, L.; Neto, C.S.; Carneiro, L.S.A.; Almeida, J.M.S.; Buarque, C.D.; Aucelio, R.Q.; Limberger, J. Synthesis, Photophysical and Electrochemical Properties of Novel D-π-D and D-π-A Triphenylamino-Chalcones and β-Arylchalcones. J. Braz. Chem. Soc. 2019, 30, 81–89. [Google Scholar] [CrossRef]
- Liu, N.; Mei, S.; Sun, D.; Shi, W.; Feng, J.; Zhou, Y.; Mei, F.; Xu, J.; Jiang, Y.; Cao, X. Effects of Charge Transport Materials on Blue Fluorescent Organic Light-Emitting Diodes with a Host-Dopant System. Micromachines 2019, 10, 344. [Google Scholar] [CrossRef]
- Yadav, R.A.K.; Dubey, D.K.; Chen, S.Z.; Liang, T.W.; Jou, J.H. Role of Molecular Orbital Energy Levels in OLED Performance. Sci. Rep. 2020, 10, 9915. [Google Scholar] [CrossRef]
- Howlader, C.; Hasan, M.; Zakhidov, A.; Chen, M.Y. Determining the refractive index and the dielectric constant of PPDT2FBT thin film using spectroscopic ellipsometry. Opt. Mater. 2020, 110, 110445. [Google Scholar] [CrossRef]
- Losurdo, M.; Giangregorio, M.M.; Capezzuto, P.; Bruno, G.; Babudri, F.; Colangiuli, D.; Farinola, G.M.; Naso, F. Spectroscopic ellipsometry for characterization of organic semiconductor polymeric thin films. Synth. Met. 2003, 138, 49–53. [Google Scholar] [CrossRef]
- Tselekidou, D.; Papadopoulos, K.; Zachariadis, A.; Kyriazopoulos, V.; Kassavetis, S.; Laskarakis, A.; Gioti, M. Solution-processable red phosphorescent OLEDs based on Ir(dmpq)2(acac) doped in small molecules as emitting layer. Mater. Sci. Semicond. Process. 2023, 163, 107546. [Google Scholar] [CrossRef]
- Jellison, G.E.; Merkulov, V.I.; Puretzky, A.A.; Geohegan, D.B.; Eres, G.; Lowndes, D.H.; Caughman, J.B. Characterization of thin-film amorphous semiconductors using spectroscopic ellipsometry. Thin Solid Film. 2000, 377–378, 68–73. [Google Scholar] [CrossRef]
- Gioti, Μ. Spectroscopic Ellipsometry Studies on Solution-Processed OLED Devices: Optical Properties and Interfacial Layers. Materials 2022, 15, 9077. [Google Scholar] [CrossRef]
- Shuchi, N.; Mower, J.; Stinson, V.; McLamb, M.; Boreman, G.; Walter, M.; Hofmann, T. Complex dielectric function of thiazolothiazole thin films determined by spectroscopic ellipsometry. Opt. Mater. Express 2023, 13, 1589–1595. [Google Scholar] [CrossRef]
- Witkowska, E.; Glowacki, I.; Ke, T.H.; Malinowski, P.; Heremans, P. Efficient OLEDs Based on Slot-Die-Coated Multicomponent Emissive Layer. Polymers 2022, 14, 3363. [Google Scholar] [CrossRef]
- Eder, T.; Stangl, T.; Gmelch, M.; Remmerssen, K.; Laux, D.; Höger, S.; Lupton, J.M.; Vogelsang, J. Switching between H- and J-type electronic coupling in single conjugated polymer aggregates. Nat. Commun. 2017, 8, 1641. [Google Scholar] [CrossRef]
- Ghasemi, B.; Ševčík, J.; Nádaždy, V.; Végsö, K.; Šiffalovič, P.; Urbánek, P.; Kuřitka, I. Thickness Dependence of Electronic Structure and Optical Properties of F8BT Thin Films. Polymers 2022, 14, 641. [Google Scholar] [CrossRef]
- Yuan, K.; Wang, X.; Mellerup, S.K.; Kozin, I.; Wang, S. Spiro-BODIPYs with a Diaryl Chelate: Impact on Aggregation and Luminescence. J. Org. Chem. 2017, 82, 13481–13487. [Google Scholar] [CrossRef]
- Ha, J.M.; Hur, S.H.; Pathak, A.; Jeong, J.E.; Woo, H.Y. Recent Advances in Organic Luminescent Materials with Narrowband Emission. NPG Asia Mater. 2021, 13, 53. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, L.; Wu, J.; Yu, J. Spectral Variation of Solution-Processed Blue Bis[(4,6-Difluorophenyl)-Pyridinato-N,C2′] (Picolinate) Iridium(III) Phosphor. Dyes Pigments 2014, 102, 234–240. [Google Scholar] [CrossRef]
- Ma, T.; Wang, Z.; Song, C.; Song, N.; Ren, J.; Liu, B.; Zhang, H.; Zhang, H.; Dan, L. J-Aggregate Behavior of Poly[(9,9- dioctyluorenyl-2,7-diyl)- alt-co-(bithiophene)] (F8T2) in Crystal and Liquid Crystal Phases. J. Phys. Chem. C 2019, 123, 24321–24327. [Google Scholar] [CrossRef]
- O’Neill, M.; Kelly, S.M. Ordered materials for organic electronics and photonics. Adv. Mater. 2011, 23, 566–584. [Google Scholar] [CrossRef]
- Qu, Y.; Zheng, Q.; Fan, J.; Liao, L.S.; Jiang, Z.Q. Spiro compounds for organic light-emitting diodes. Acc. Mater. Res. 2021, 2, 1261–1271. [Google Scholar] [CrossRef]
- Höfle, S.; Lutz, T.; Egel, A.; Nickel, F.; Kettlitz, S.W.; Gomard, G.; Lemmer, U.; Colsmann, A. Influence of the Emission Layer Thickness on the Optoelectronic Properties of Solution Processed Organic Light-Emitting Diodes. ACS Photonics 2014, 1, 968–973. [Google Scholar] [CrossRef]
- Tyagi, P.; Srivastava, R.; Giri, L.I.; Tuli, S.; Lee, C. Degradation of Organic Light Emitting Diode: Heat Related Issues and Solutions. Synth. Met. 2016, 216, 40–50. [Google Scholar] [CrossRef]
- Fadavieslam, M.R. The effect of thickness of light emitting layer on physical properties of OLED devices. Optik 2019, 182, 452–457. [Google Scholar] [CrossRef]
- Ameen, M.A.; Saeed, M.A.; Mahmood, A.S. Dip-coating sol–gel preparation and characterization of Rhodamine 6G-doped nanostructured TiO2 thin films on structural and spectroscopic properties. J. Sol Gel Sci. Technol. 2022, 101, 655–671. [Google Scholar] [CrossRef]
- Chapman, M.; Euler, W.B. Rhodamine 6G Structural Changes in Water/Ethanol Mixed Solvent. J. Fluoresc. 2018, 28, 1431–1437. [Google Scholar] [CrossRef]
- Lian, C.; Yoshida, K.; Nogues, C.; Samuel, I.D.W. Organic Light-Emitting Diode Based Fluorescence Sensing System for DNA Detection. Adv. Mater. Technol. 2022, 7, 2100806. [Google Scholar] [CrossRef]
- Lian, C.; Young, D.; Randall, R.E.; Samuel, I.D.W. Organic Light-Emitting Diode Based Fluorescence-Linked Immunosorbent Assay for SARS-CoV-2 Antibody Detection. Biosensors 2022, 12, 1125. [Google Scholar] [CrossRef]

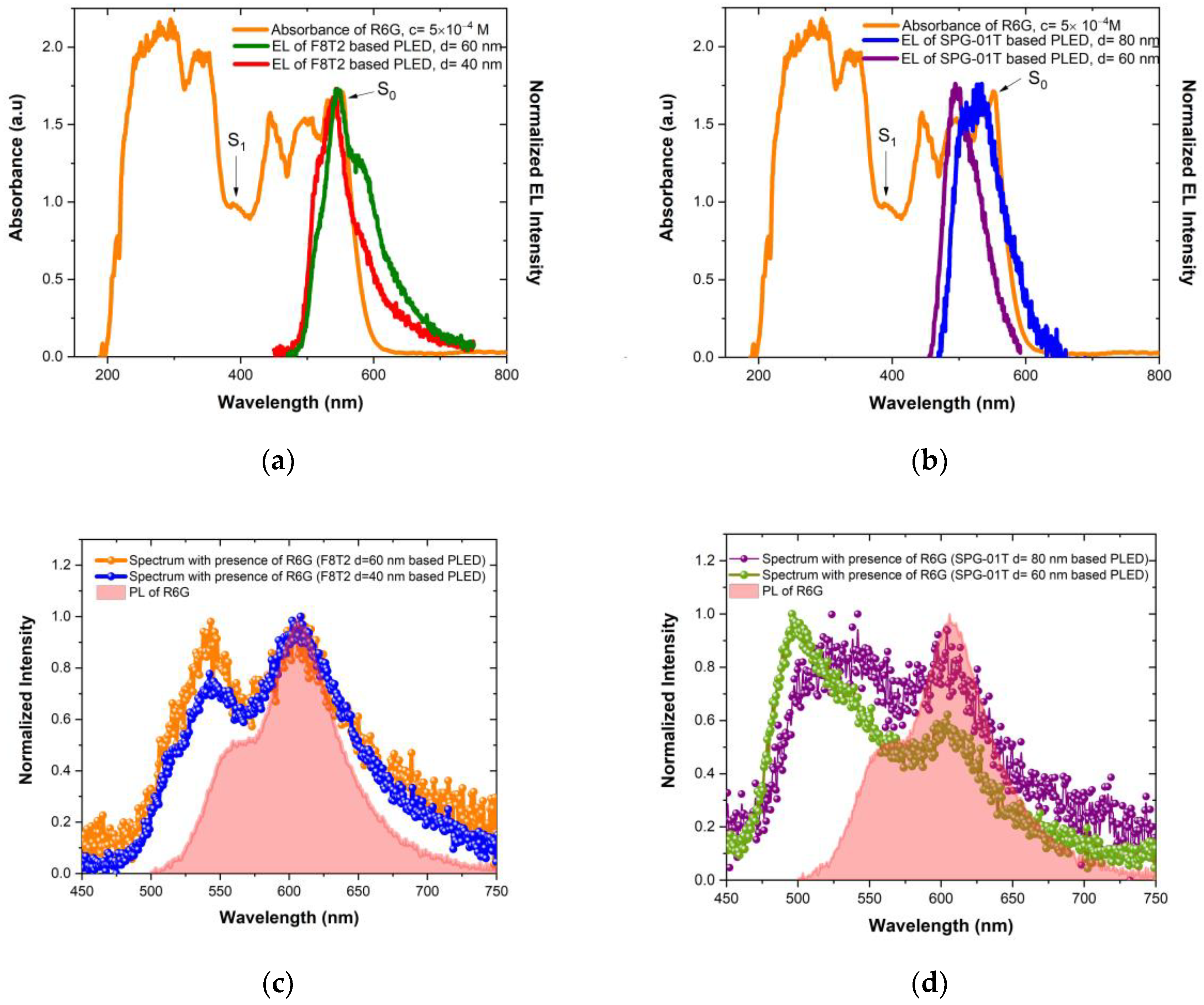
| Polymer | Photophysical Properties | Electrochemical Properties | |||||
|---|---|---|---|---|---|---|---|
| λAbs (nm) | λonset (nm) | EgOpt (eV) | λPL (nm) | Eonset (V) | EHOMO (eV) | ELUMO (eV) | |
| F8T2 | 453 (0–1), 484 (0–0) | 519 | 2.39 | 510 (0–0), 542 (0–1), 581 (0–2) | 1.23 | −5.6 | −3.2 |
| SPG-01T | 343 (0–3), 367 (0–2), 397 (0–1), 442 (0–0) | 490 | 2.53 | 509 (0–0), 537 (0–1) | 0.91 | −5.2 | −2.7 |
| Polymer | Fabrication Method | |||||||
|---|---|---|---|---|---|---|---|---|
| Spin Coating | Slot-Die Coating | |||||||
| Speed (rpm) | Thickness (nm) | EgTL (eV) | EgTauc (eV) | Flow Rate (mL/min) | Thickness (nm) | EgTL (eV) | EgTauc (eV) | |
| F8T2 | 1000 | 58.8 | 2.40 | 2.4972 | 0.03 | 20.5 | 2.27 | 2.45 |
| 2000 | 49.4 | 2.39 | 2.4933 | 0.035 | 21.4 | 2.27 | 2.46 | |
| 3000 | 40.7 | 2.39 | 2.4856 | 0.04 | 24.2 | 2.25 | 2.45 | |
| 0.05 | 33.3 | 2.29 | 2.44 | |||||
| 0.06 | 40.9 | 2.31 | 2.43 | |||||
| 0.08 | 48.1 | 2.31 | 2.42 | |||||
| SPG-01T | 1000 | 83.2 | 2.50 | 2.6508 | 0.04 | 57.8 | 1.81 | 2.78 |
| 2000 | 66.1 | 2.60 | 2.6778 | 0.06 | 69.2 | 1.90 | 2.80 | |
| 3000 | 57.6 | 2.57 | 2.6713 | 0.08 | 78.4 | 1.92 | 2.70 | |
| 0.10 | 91.8 | 1.58 | 2.74 | |||||
| 0.11 | 98.1 | 1.36 | 2.70 | |||||
| Polymer | Thickness (Fabrication Method) (nm) | Electrical Characteristics | |||
|---|---|---|---|---|---|
| FWHM (nm) | 1 Von (V) | Max. Luminance (cd/m2) | 2 Vop (V) | ||
| F8T2 | 60 (SC) | 83 | 4.9 | 2907 | 4.7 |
| 50 (SD) | 75 | 4.6 | 3183 | 4.4 | |
| 40 (SC) | 68 | 4.1 | 1963 | 3.8 | |
| 20 (SD) | 64 | 3.9 | 2023 | 5.3 | |
| SPG-01T | 100 (SD) | 104.2 | 9.2 | 854 | 16 |
| 90 (SD) | 97 | 7.5 | 2432 | 13.6 | |
| 80 (SC) | 83.2 | 7 | 2808 | 13.5 | |
| 60 (SC) | 67.2 | 5 | 20,690 | 7.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulos, K.; Tselekidou, D.; Zachariadis, A.; Laskarakis, A.; Logothetidis, S.; Gioti, M. The Influence of Thickness and Spectral Properties of Green Color-Emitting Polymer Thin Films on Their Implementation in Wearable PLED Applications. Nanomaterials 2024, 14, 1608. https://doi.org/10.3390/nano14191608
Papadopoulos K, Tselekidou D, Zachariadis A, Laskarakis A, Logothetidis S, Gioti M. The Influence of Thickness and Spectral Properties of Green Color-Emitting Polymer Thin Films on Their Implementation in Wearable PLED Applications. Nanomaterials. 2024; 14(19):1608. https://doi.org/10.3390/nano14191608
Chicago/Turabian StylePapadopoulos, Kyparisis, Despoina Tselekidou, Alexandros Zachariadis, Argiris Laskarakis, Stergios Logothetidis, and Maria Gioti. 2024. "The Influence of Thickness and Spectral Properties of Green Color-Emitting Polymer Thin Films on Their Implementation in Wearable PLED Applications" Nanomaterials 14, no. 19: 1608. https://doi.org/10.3390/nano14191608





