Sustainable and Reusable Modified Membrane Based on Green Gold Nanoparticles for Efficient Methylene Blue Water Decontamination by a Photocatalytic Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Grape Marc Extract Preparation
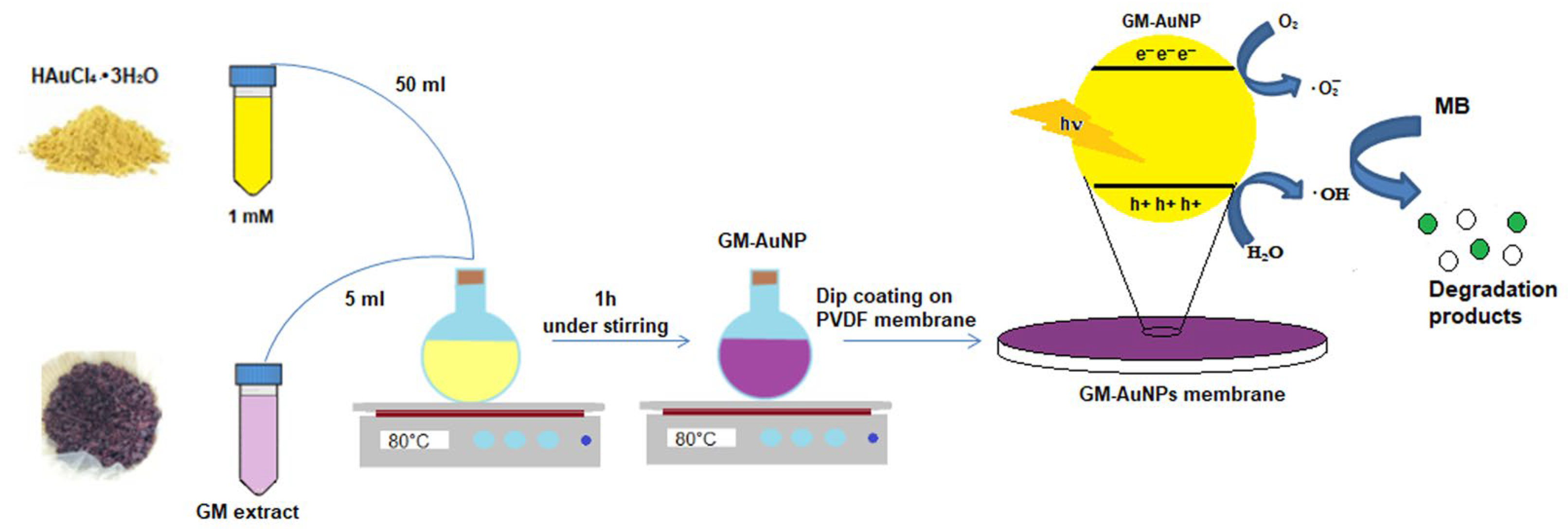
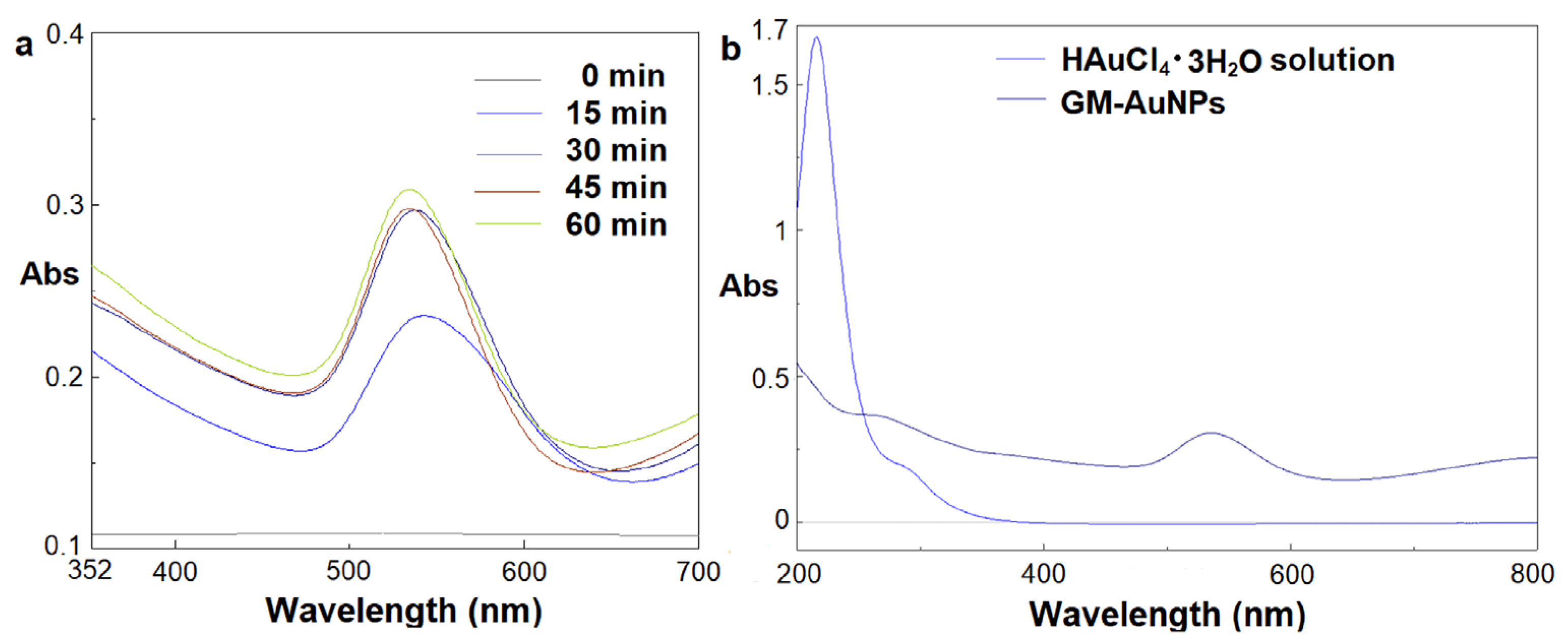
2.3. Synthesis of Green Gold Nanoparticles
2.4. Modified PVDF Membrane Preparation
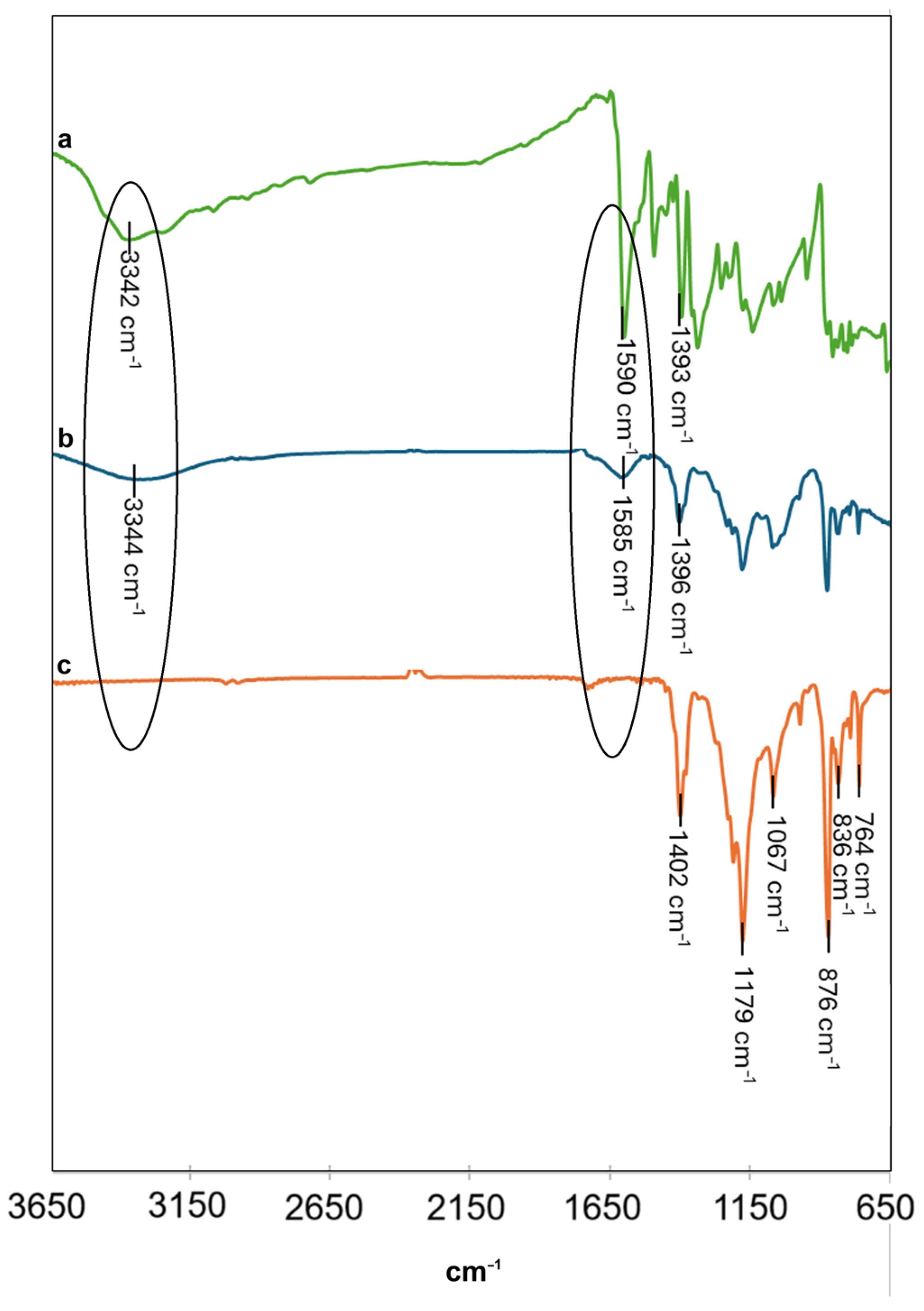
2.5. Characterization Studies
2.6. Photocatalytic Activity and Kinetic Studies of Modified Membrane
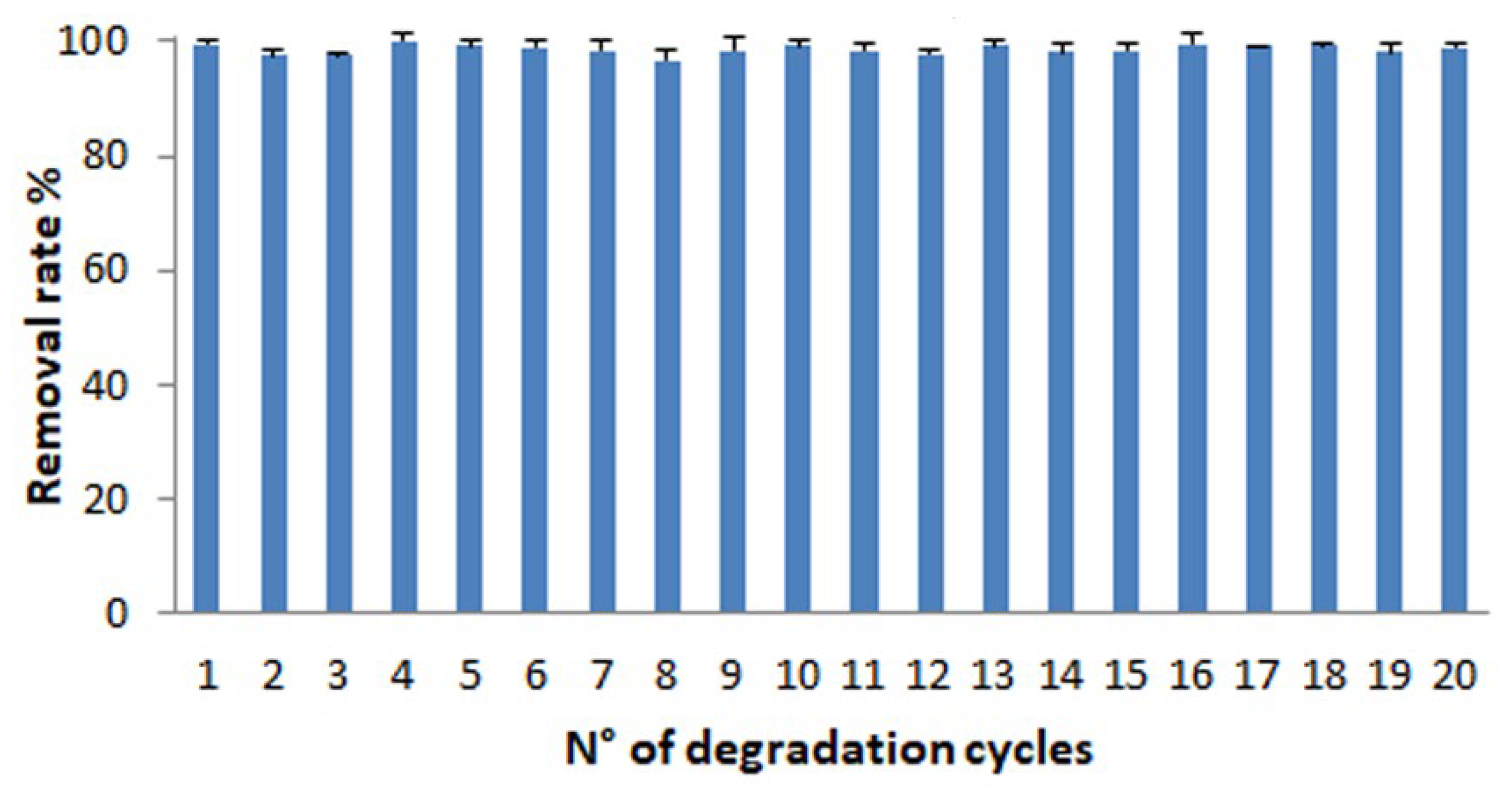
2.7. Reusability of Modified Membrane in MB Degradation
3. Results and Discussion
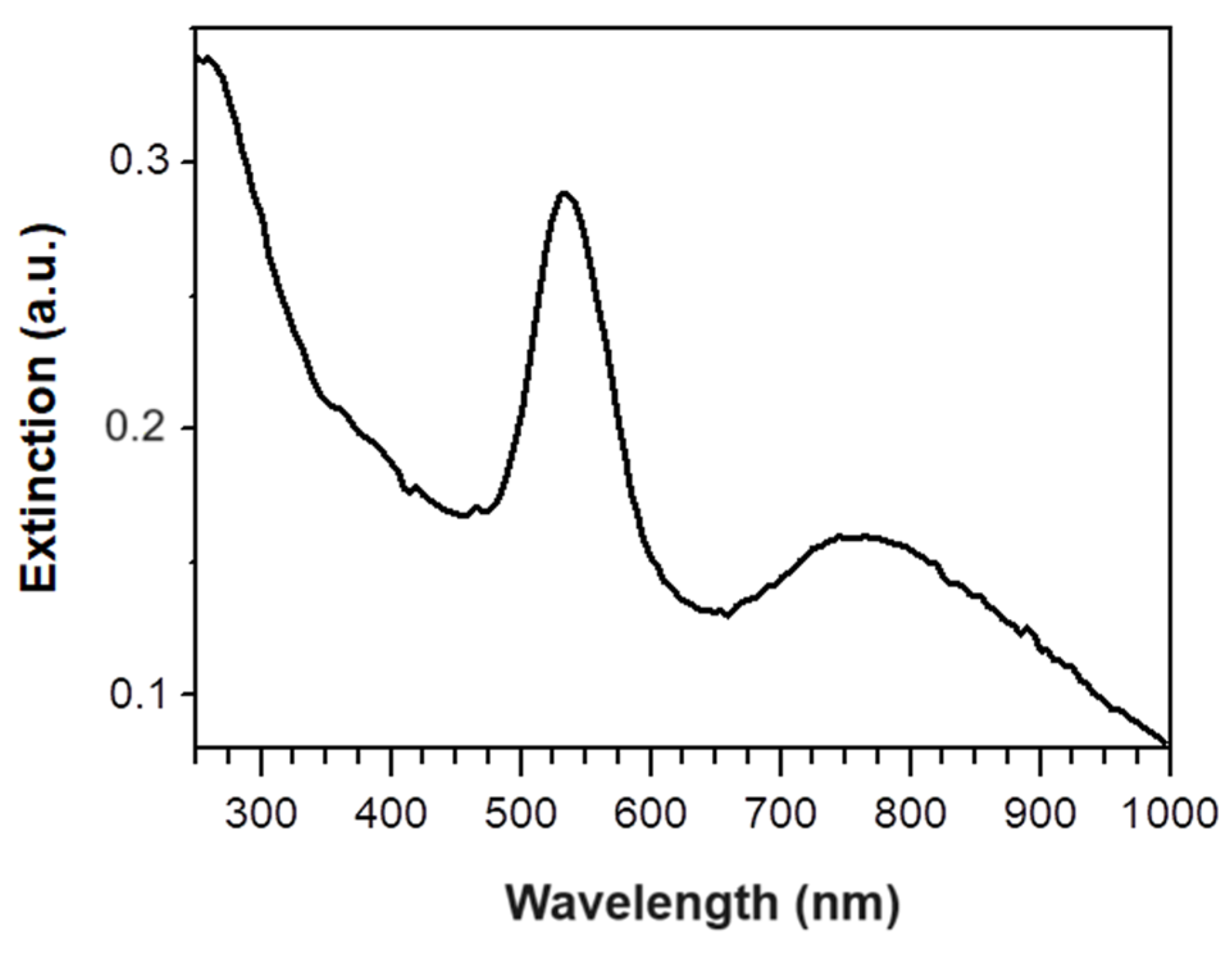
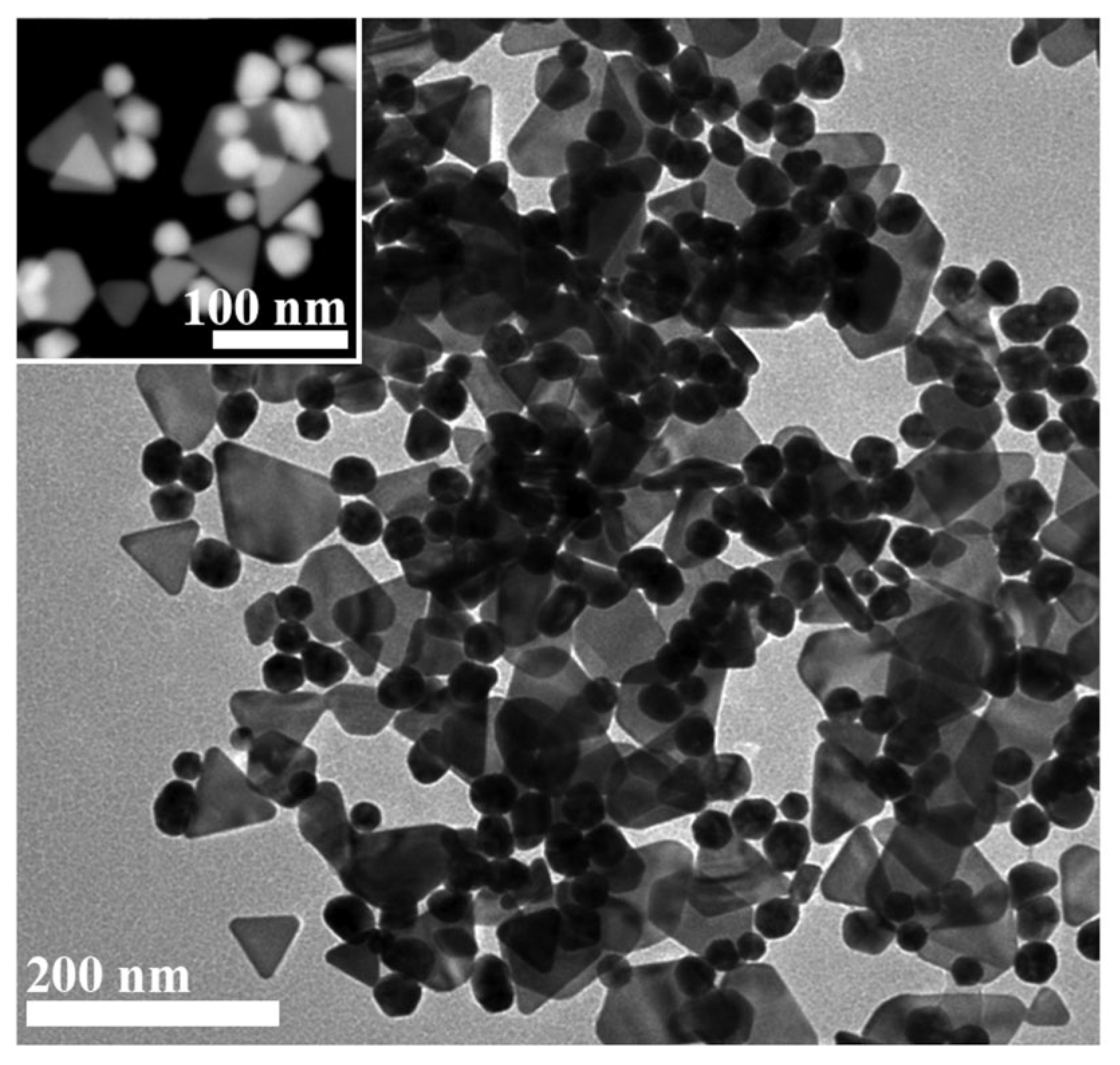
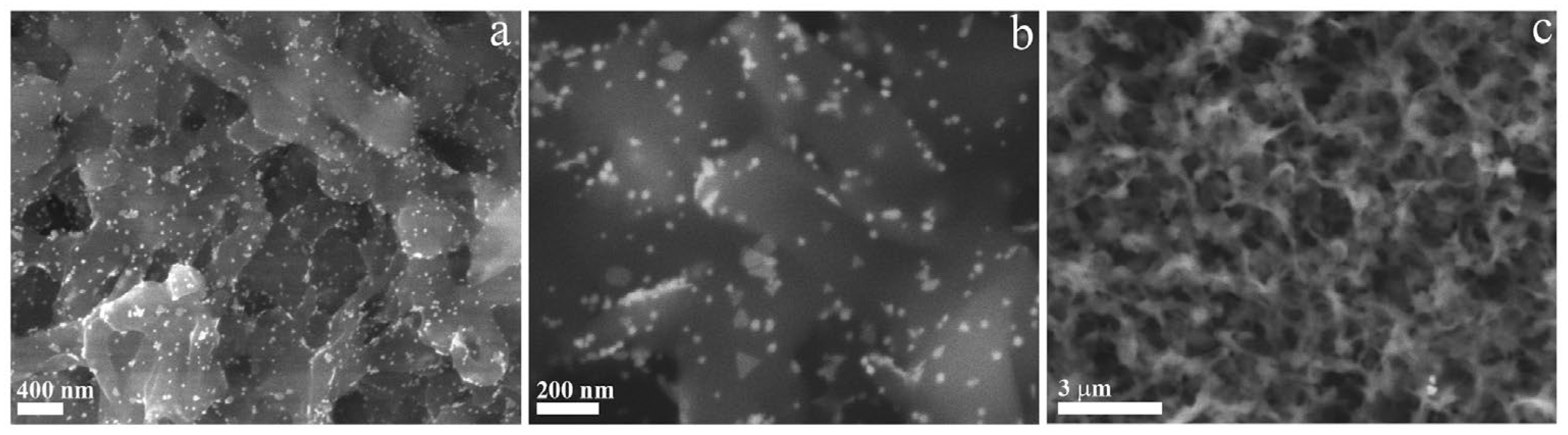
3.1. Synthesis and Characterization of Green Gold Nanoparticles
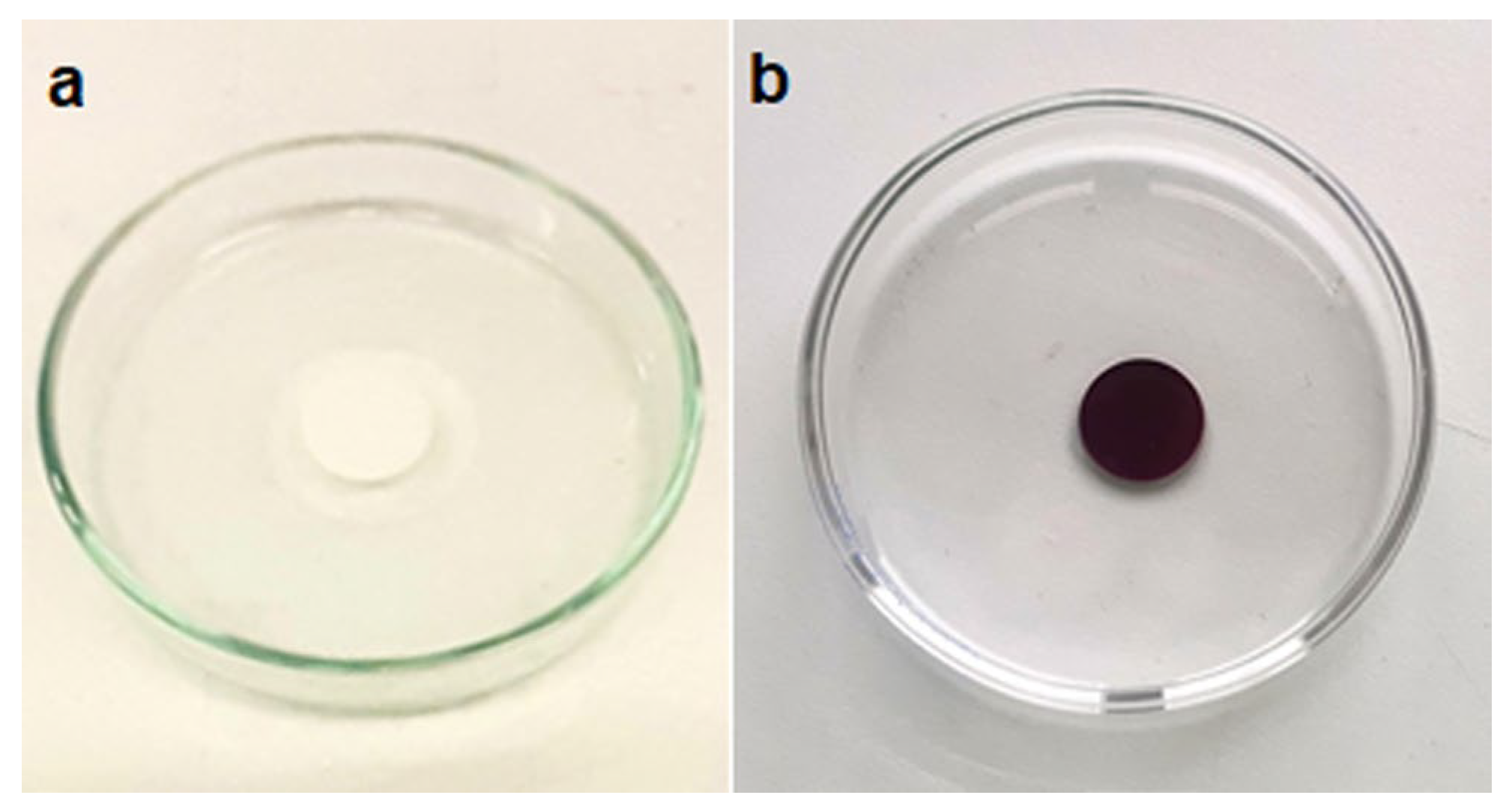
3.2. Dip Coating of PVDF Membrane in GM-AuNPs Dispersion
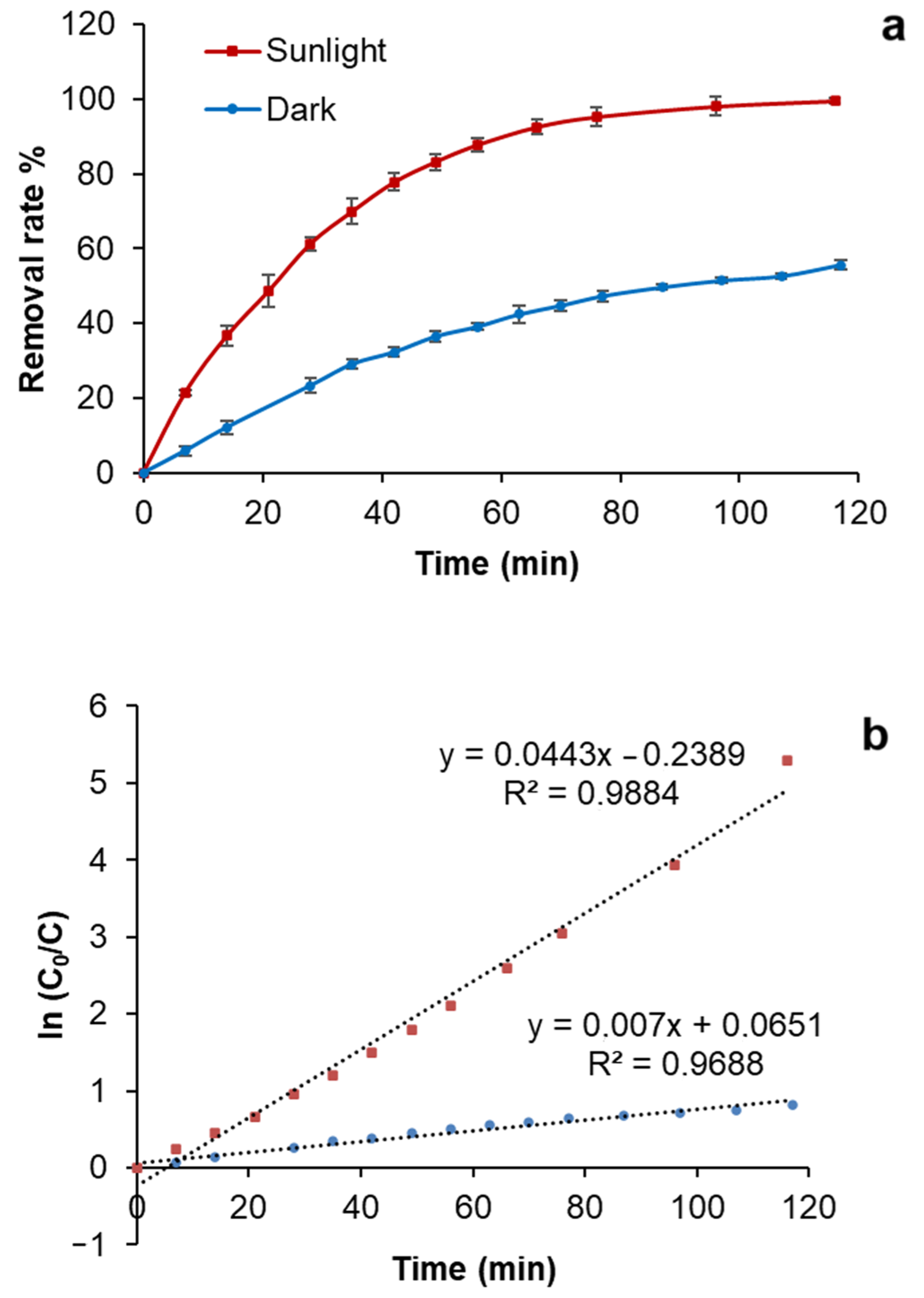
3.3. Photocatalytic Studies
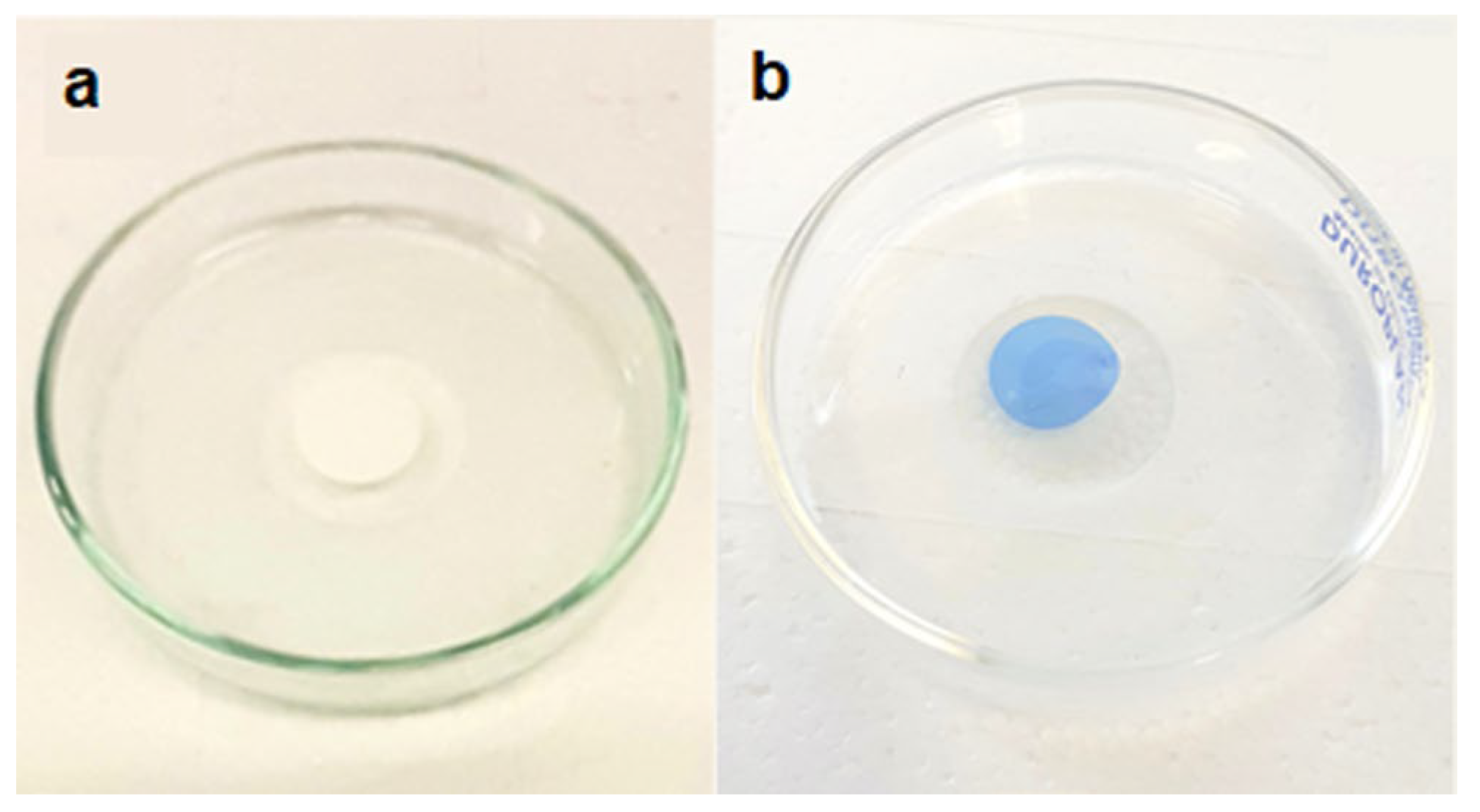
3.4. Modified Membrane Behavior with Other Organic Pollutants
3.5. Reusability and Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alprol, A.E.; Mansour, A.T.; Abdelwahab, A.M.; Ashour, M. Advances in Green Synthesis of Metal Oxide Nanoparticles by Marine Algae for Wastewater Treatment by Adsorption and Photocatalysis Techniques. Catalysts 2023, 13, 888. [Google Scholar] [CrossRef]
- Policarpo Tonelli, F.M.; Santos Silva, C.; Silva Delgado, V.M.; Policarpo Tonelli, F.C. Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators. Green Process. Synth. 2023, 12, 20230008. [Google Scholar] [CrossRef]
- Din, M.I.; Khalid, R.; Najee, J.; Hussain, Z. Fundamentals and photocatalysis of methylene blue dye using various nanocatalytic assemblies—A critical review. J. Clean. Prod. 2021, 298, 126567. [Google Scholar] [CrossRef]
- Omran, B.A.; Baek, K.H. Valorization of agro-industrial biowaste to green nanomaterials for wastewater treatment: Approaching green chemistry and circular economy principles. J. Environ. Manag. 2022, 311, 114806. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Li, Q.; Pi, Z.; Zhang, J.; Sun, L.-W.; Xu, J.; Cao, Y.; Cheng, J.; Bian, Y. Metal–Organic-Framework-Derived Ball-Flower-like Porous Co3O4/Fe2O3 Heterostructure with Enhanced Visible-Light-Driven Photocatalytic Activity. Nanomaterials 2022, 12, 904. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Luo, J.; Dai, Z.; Feng, M.; Gu, M.; Xie, Y. Graphic carbon nitride/ferroferric oxide/reduced graphene oxide nanocomposite as highly active visible light photocatalyst. Nano Res. 2023, 1, 371–376. [Google Scholar] [CrossRef]
- Del Sole, R.; Fogel, A.A.; Somin, V.A.; Vasapollo, G.; Mergola, L. Evaluation of Effective Composite Biosorbents Based on Wood Sawdust and Natural Clay for Heavy Metals Removal from Water. Materials 2023, 16, 5322. [Google Scholar] [CrossRef]
- Mamyachenkov, S.V.; Adryshev, A.K.; Seraya, N.V.; Khairullina, A.A.; Daumova, G.K. Nanostructured complex sorbent for cleaning heavy metal ions from industrial effluent. Metallurgist 2017, 61, 615–623. [Google Scholar] [CrossRef]
- Li, R.; Wang, B.; Niu, A.; Cheng, N.; Chen, M.; Zhang, X.; Yu, Z.; Wang, S. Application of biochar immobilized microorganisms for pollutants removal from wastewater: A review. Sci. Total Environ. 2022, 837, 155563. [Google Scholar] [CrossRef]
- Del Sole, R.; Maggio, A.; Mergola, L. Green grape marc biosorbents preparation for mercury removal in aqueous media. Chem. Ind. Chem. Eng. Q. 2023, 29, 1–10. [Google Scholar] [CrossRef]
- Arias Arias, F.E.; Beneduci, A.; Chidichimo, F.; Furia, E.; Straface, S. Study of the adsorption of mercury (II) on lignocellulosic materials under static and dynamic conditions. Chemosphere 2017, 180, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Mora Alvarez, N.M.; Pastrana, J.M.; Lagos, Y.; Lozada, J.J. Evaluation of mercury (Hg2+) adsorption capacity using exhausted coffee waste. Sustain. Chem. Pharm. 2018, 10, 60–70. [Google Scholar] [CrossRef]
- Husien, S.; El-taweel, R.M.; Salim, A.I.; Fahim, I.S.; Said, L.A.; Radwan, A.G. Review of activated carbon adsorbent material for textile dyes removal: Preparation, and modelling. Curr. Res. Green Sustain. Chem. 2022, 5, 100325. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Ali, N.; Khan, I.; Zhang, B.; Sadiq, M. Heterogeneous photodegradation of industrial dyes: An insight to different mechanisms and rate affecting parameters. J. Environ. Chem. Eng. 2020, 8, 104364. [Google Scholar] [CrossRef]
- Khan, I.; Shah, T.; Tariq, M.R.; Ahmad, M.; Zhang, B. Understanding the toxicity of trinitrophenol and promising decontamination strategies for its neutralization: Challenges and future perspectives. J. Environ. Chem. Eng. 2024, 12, 112720. [Google Scholar] [CrossRef]
- Xu, D.; Wu, S.; Yan, A.; Chen, Z.; Xu, J.; Gu, C.; Qi, Y.; Wu, S. Efficient recycling of sewage water in a polyester integrated industry: A case study. Desalin. Water Treat. 2024, 319, 100508. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, P.; Ye, L.; Xu, D. Progress of anaerobic membrane bioreactor in municipal wastewater treatment. Sci. Adv. Mater. 2023, 15, 1277–1298. [Google Scholar] [CrossRef]
- Seoudi, R.; Al-Marhab, F.A. Synthesis, characterization and photocatalytic application of different sizes of gold nanoparticles on 4-Nitrophenol. World J. Nano Sci. Eng. 2016, 6, 120. [Google Scholar] [CrossRef]
- Silva-Holguín, P.N.; Garibay-Alvarado, J.A.; Reyes-López, S.Y. Silver nanoparticles: Multifunctional tool in environmental water remediation. Materials 2024, 17, 1939. [Google Scholar] [CrossRef]
- Palani, G.; Trilaksana, H.; Merlyn Sujatha, R.; Kannan, K.; Rajendran, S.; Korniejenko, K.; Nykiel, M.; Uthayakumar, M. Silver Nanoparticles for waste water management. Molecules 2023, 28, 3520. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E. Gold nanoparticle-based plasmonic biosensors. Biosensors 2023, 13, 411. [Google Scholar] [CrossRef] [PubMed]
- Kumari, H.; Suman, S.; Ranga, R.; Chahal, S.; Devi, S.; Sharma, S.; Kumar, S.; Kumar, P.; Kumar, S.; Kumar, A.; et al. A Review on photocatalysis used for wastewater treatment: Dye degradation. Water Air Soil Pollut. 2023, 234, 349. [Google Scholar] [CrossRef] [PubMed]
- Huggias, S.; Serradell, M.A.; Azcárate, J.C.; Casella, M.L.; Peruzzo, P.J.; Bolla, P.A. Catalytic performance in nitroarene reduction of nanocatalyst based on noble metal nanoparticles supported on polymer/s-layer protein hybrids. J. Phys. Chem. B 2024, 128, 4809. [Google Scholar] [CrossRef] [PubMed]
- Mergola, L.; Carbone, L.; Stomeo, T.; Del Sole, R. Green synthesis of iridium nanoparticles from winery waste and their catalytic effectiveness in water decontamination. Materials 2023, 16, 2060. [Google Scholar] [CrossRef]
- Lizundia, E.; Luzi, F.; Puglia, D. Organic waste valorisation towards circular and sustainable biocomposites. Green Chem. 2022, 24, 5429. [Google Scholar] [CrossRef]
- Baiocco, D.; Lavecchia, R.; Natali, S.; Zuorro, A. Production of Metal Nanoparticles by Agro-Industrial Wastes: A Green Opportunity for Nanotechnology. Chem. Eng. Trans. 2016, 47, 67. [Google Scholar] [CrossRef]
- Cui, M.; Huang, X.; Zhang, X.; Xie, Q.; Yang, D. Ultra-small iridium nanoparticles as active catalysts for the selective and efficient reduction of nitroarenes. New J. Chem. 2020, 44, 18274. [Google Scholar] [CrossRef]
- Bordiwala, R.V. Green synthesis and Applications of Metal Nanoparticles—A Review Article. Results Chem. 2023, 5, 100832. [Google Scholar] [CrossRef]
- Song, W.C.; Kim, B.; Park, S.Y.; Park, G.; Oh, J.W. Biosynthesis of silver and gold nanoparticles using Sargassum horneri extract as catalyst for industrial dye degradation. Arab. J. Chem. 2022, 15, 104056. [Google Scholar] [CrossRef]
- Kulkarni, R.; Harip, S.; Kumar, A.R.; Deobagkar, D.; Zinjarde, S. Peptide stabilized gold and silver nanoparticles derived from the mangrove isolate Pseudoalteromonas lipolytica mediate dye decolorization. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 180. [Google Scholar] [CrossRef]
- Rajput, G.; Pandya, N. Catalytic Degradation of Methylene Blue Using Gold Nanoparticles Capped by Polyoxyethylene Cholesteryl Ether. Adv. Sci. Eng. Med. 2020, 12, 1236. [Google Scholar] [CrossRef]
- Baruah, D.; Goswami, M.; Yadav, R.N.S.; Yadav, A.; Das, A.M. Biogenic synthesis of gold nanoparticles and their application in photocatalytic degradation of toxic dyes. J. Photochem. Photobiol. B Biol. 2018, 186, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manag. 2018, 72, 99. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, Z.; Liu, C.; Dai, X.; Zhou, W.; Jiang, K.; Zhang, T.; Yin, J.; Gao, J.; Yin, H.; et al. Continuous in situ portable SERS analysis of pollutants in water and air by a highly sensitive gold nanoparticle-decorated PVDF substrate. Anal. Bioanal. Chem. 2021, 413, 5469. [Google Scholar] [CrossRef]
- Tran, H.D.; Nguyen, D.Q.; Do, P.T.; Tran, U.N.P. Kinetics of photocatalytic degradation of organic compounds: A mini-review and new approach. RSC Adv. 2023, 13, 16915. [Google Scholar] [CrossRef]
- Iqbal, A.; Ibrahim, N.H.; Rahman, N.R.A.; Saharudin, K.A.; Adam, F.; Sreekantan, S.; Yusop, R.M.; Jaafar, N.F.; Wilson, L.D. ZnO Surface Doping to Enhance the Photocatalytic Activity of Lithium Titanate/TiO2 for Methylene Blue Photodegradation under Visible Light Irradiation. Surfaces 2020, 3, 301–318. [Google Scholar] [CrossRef]
- Basak, S.; Ali, S.; Das, D.; Sikdar, S.; Roy, M.N. Synthesis, Characterization and Visible Light Induced Photo-Degradation of Acid Orange II Dye in Aqueous Medium using a Novel Synthesized Al2MoZnO7 Nanocomposite. J. Adv. Chem. Sci. 2020, 6, 676. [Google Scholar] [CrossRef]
- Singh, R.K.; Behera, S.S.; Singh, K.R.; Mishra, S.; Panigrahi, B.; Sahoo, T.R.; Parhia, P.K.; Mandala, D. Biosynthesized gold nanoparticles as photocatalysts for selective degradation of cationic dye and their antimicrobial activity. J. Photochem. Photobiol. A Chem. 2020, 400, 112704. [Google Scholar] [CrossRef]
- Fiévet, F.; Ammar-Merah, S.; Brayner, R.; Chau, F.; Giraud, M.; Mammeri, F.; Peron, J.; Piquemal, J.-Y.; Sicard, L.; Viau, G. The polyol process: A unique method for easy access to metal nanoparticles with tailored sizes, shapes and compositions. Chem. Soc. Rev. 2018, 47, 5187–5233. [Google Scholar] [CrossRef]
- Rosas-García, V.M.; Rodríguez-Nava, O.; Cuenca-Álvarez, R.; Garrido-Hernandez, A.; García-Hernández, M.; Morales-Ramírez, A.J. Photocatalytic Degradation of Methylene Blue (MB) and Methyl Orange (MO) by the Highly Oxidative Properties of SnO2Sb2O3 Particles. Mater. Trans. 2022, 63, 1188. [Google Scholar] [CrossRef]
- Lyu, P.; Espinoza, R.; Nguyen, S.C. Photocatalysis of Metallic Nanoparticles: Interband vs. Intraband Induced Mechanisms. J. Phys. Chem. C 2023, 127, 15685–15698. [Google Scholar] [CrossRef] [PubMed]
- Baumberg, J.J. Hot electron science in plasmonics and catalysis: What we argue about. Faraday Discuss. 2019, 214, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Han, S.F.; Zhang, Q.W.; Zhang, N.; Zhao, D.D. Photocatalytic oxidation degradation mechanism study of methylene blue dye waste water with GR/iTO2. MATEC Web Conf. 2018, 238, 03006. [Google Scholar] [CrossRef]
- Zuo, W.; Chen, G.; Chen, F.; Li, S.; Wang, B. Green synthesis and characterization of gold nanoparticles embedded into magnetic carbon nanocages and their highly efficient degradation of methylene blue. RSC Adv. 2016, 6, 28774. [Google Scholar] [CrossRef]




| MB Concentration (mg L−1) | Degradation % ± SD (%) |
|---|---|
| 5.6 | 99.5 ± 0.7 |
| 15 | 86.8 ± 1.5 |
| 30 | 73.8 ± 2.1 |
| 40 | 63.7 ± 2.3 |
| Polluttants | Pseudo-First-Order | Pseudo-Second-Order | ||
|---|---|---|---|---|
| k1 (min−1) | R2 | K2 (L mg−1 min−1) | R2 | |
| MB | 0.0443 | 0.9884 | 0.8340 | 0.5179 |
| RO | 0.0043 | 0.7359 | 0.0822 | 0.9974 |
| 4NP | 0.0010 | 0.8184 | 0.1640 | 0.9991 |
| MO | 0.0003 | 0.3387 | 11.891 | 0.9994 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mergola, L.; Carbone, L.; Bloise, E.; Lazzoi, M.R.; Del Sole, R. Sustainable and Reusable Modified Membrane Based on Green Gold Nanoparticles for Efficient Methylene Blue Water Decontamination by a Photocatalytic Process. Nanomaterials 2024, 14, 1611. https://doi.org/10.3390/nano14191611
Mergola L, Carbone L, Bloise E, Lazzoi MR, Del Sole R. Sustainable and Reusable Modified Membrane Based on Green Gold Nanoparticles for Efficient Methylene Blue Water Decontamination by a Photocatalytic Process. Nanomaterials. 2024; 14(19):1611. https://doi.org/10.3390/nano14191611
Chicago/Turabian StyleMergola, Lucia, Luigi Carbone, Ermelinda Bloise, Maria Rosaria Lazzoi, and Roberta Del Sole. 2024. "Sustainable and Reusable Modified Membrane Based on Green Gold Nanoparticles for Efficient Methylene Blue Water Decontamination by a Photocatalytic Process" Nanomaterials 14, no. 19: 1611. https://doi.org/10.3390/nano14191611






