Coordination-Polymer-Derived Cu-CoO/C Nanocomposite Used in Fenton-like Reaction to Achieve Efficient Degradation of Organic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Aqueous Solution of Metal Methacrylate Salts
2.3. Preparation of Cu-CoO/C Nanoribbon
2.4. Catalytic Performance
2.5. Characterization
3. Results and Discussion
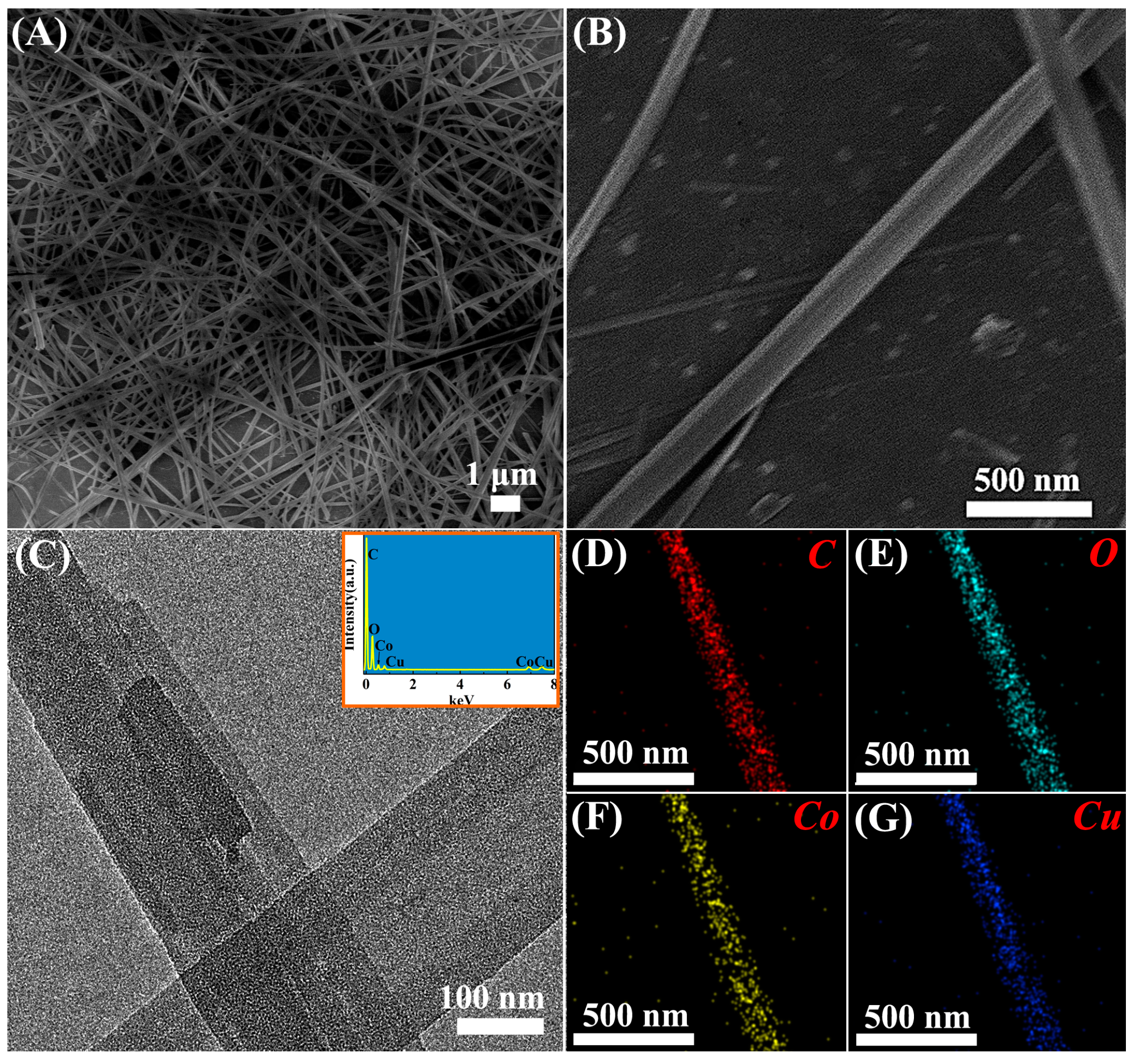
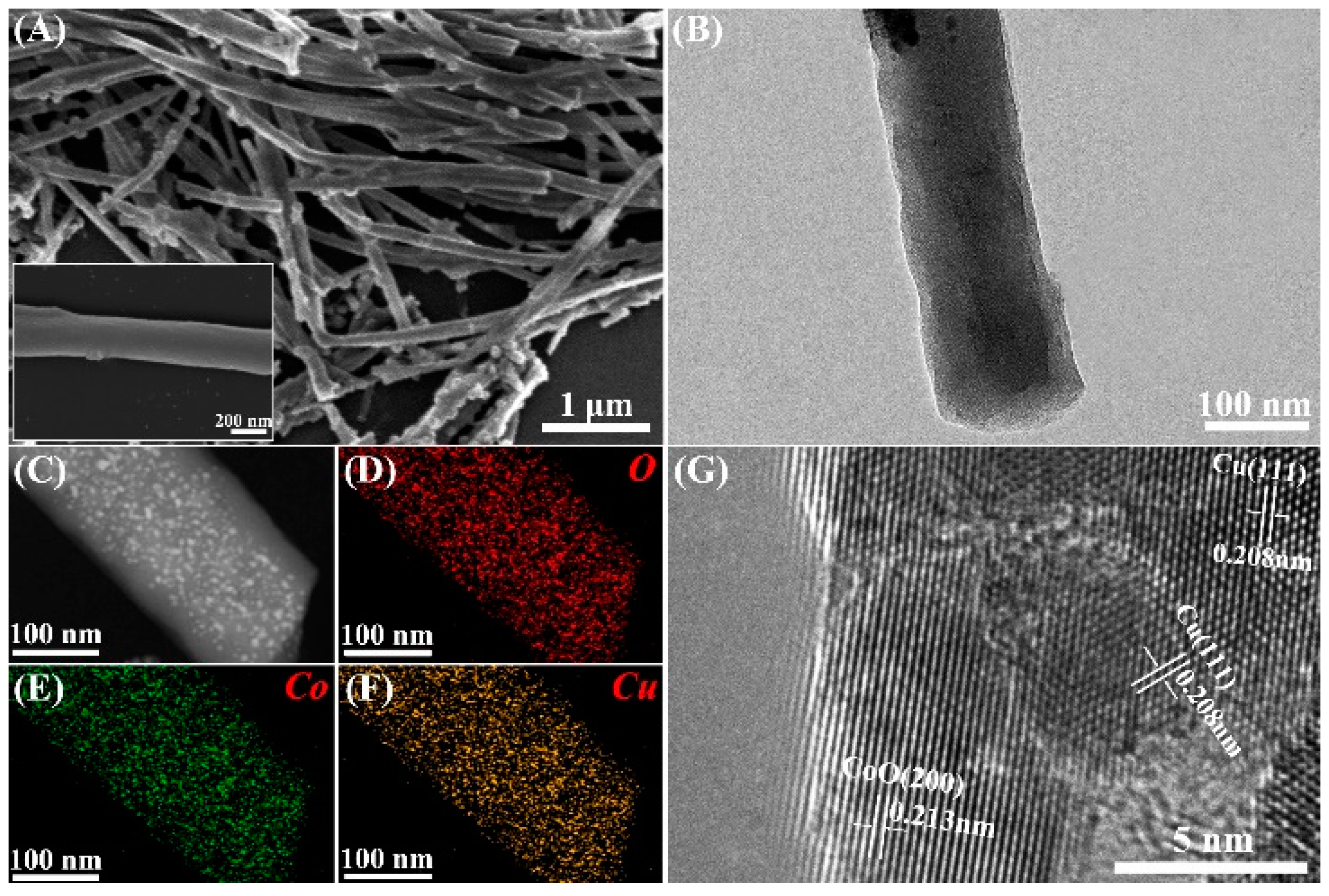
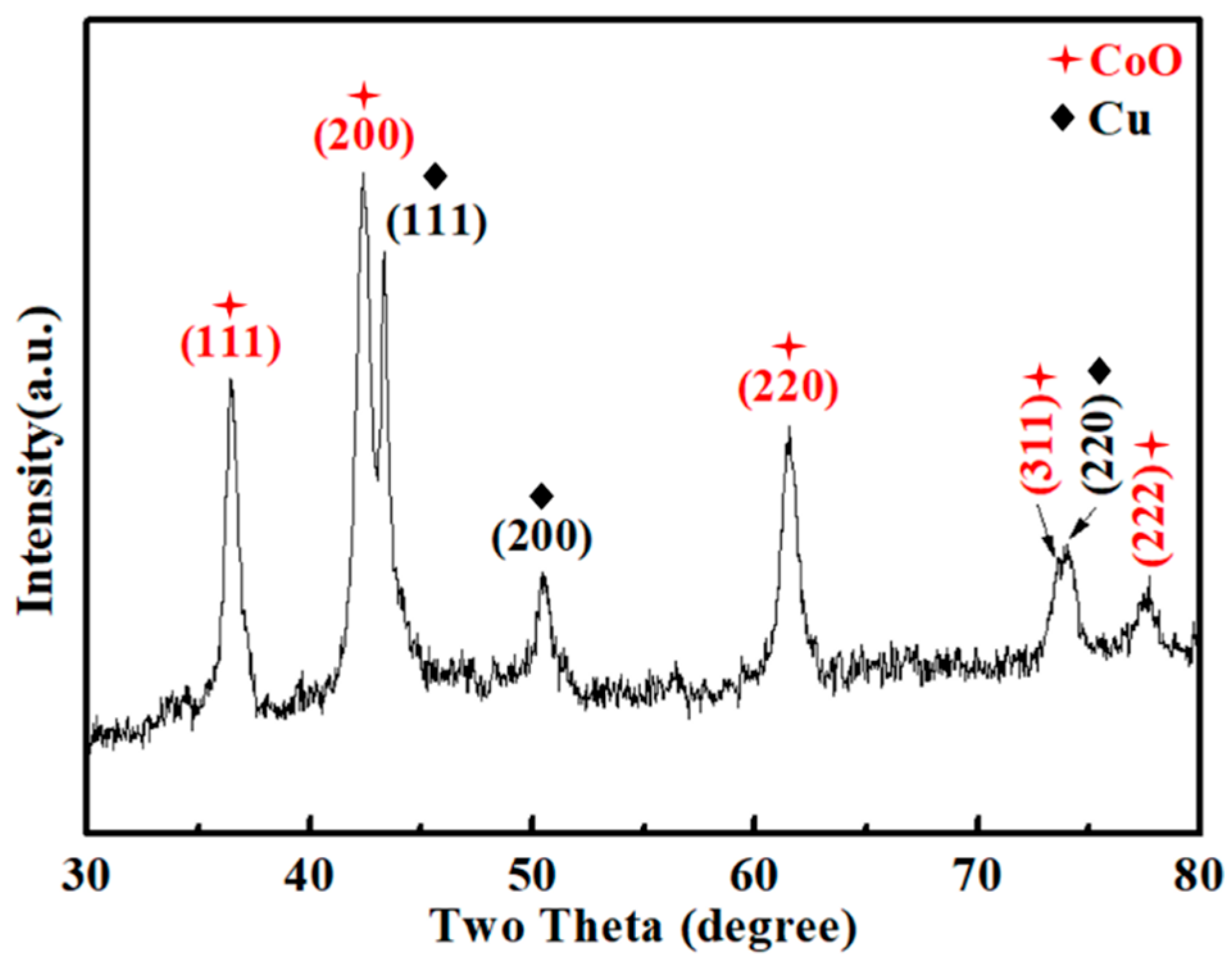
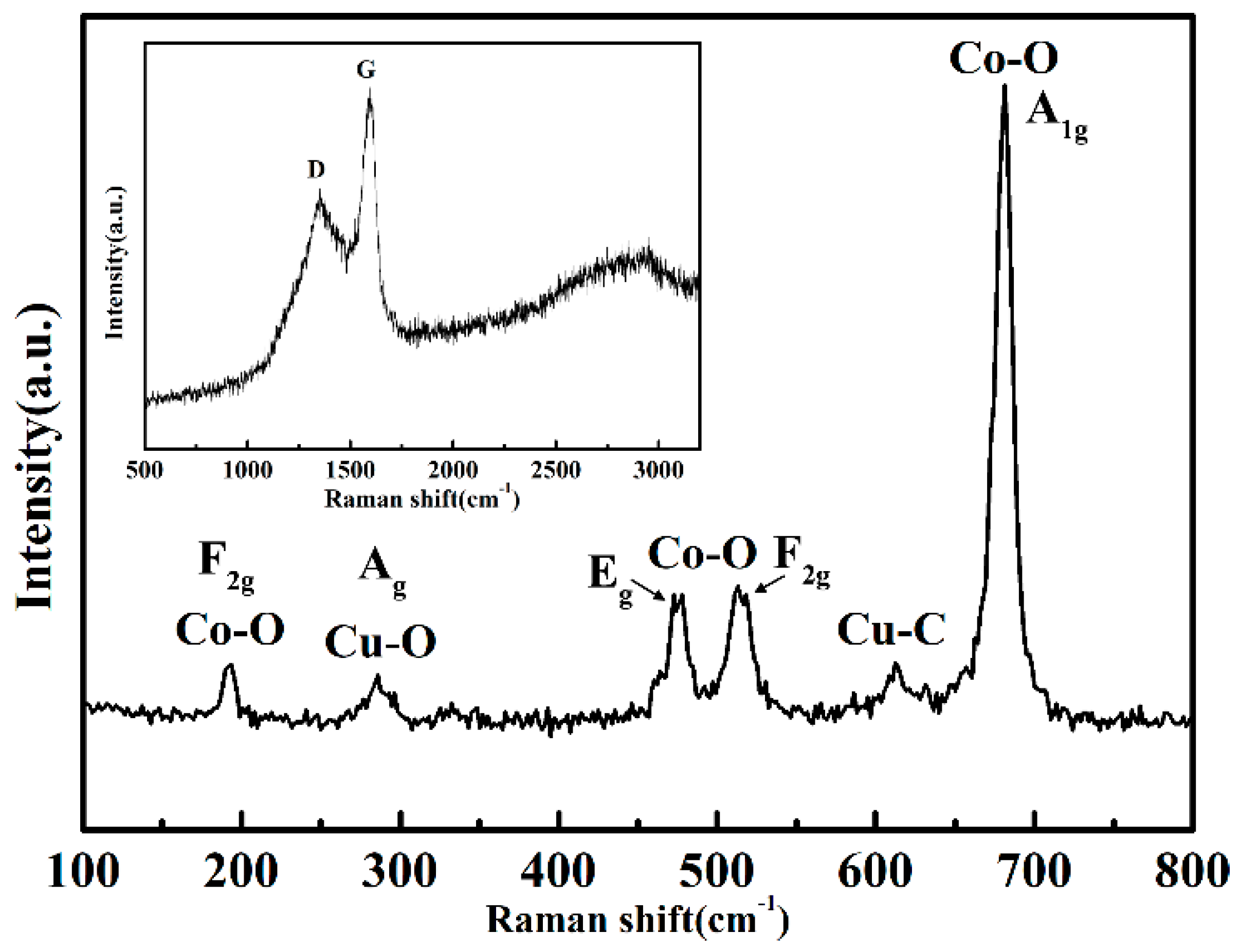
3.1. Characterization
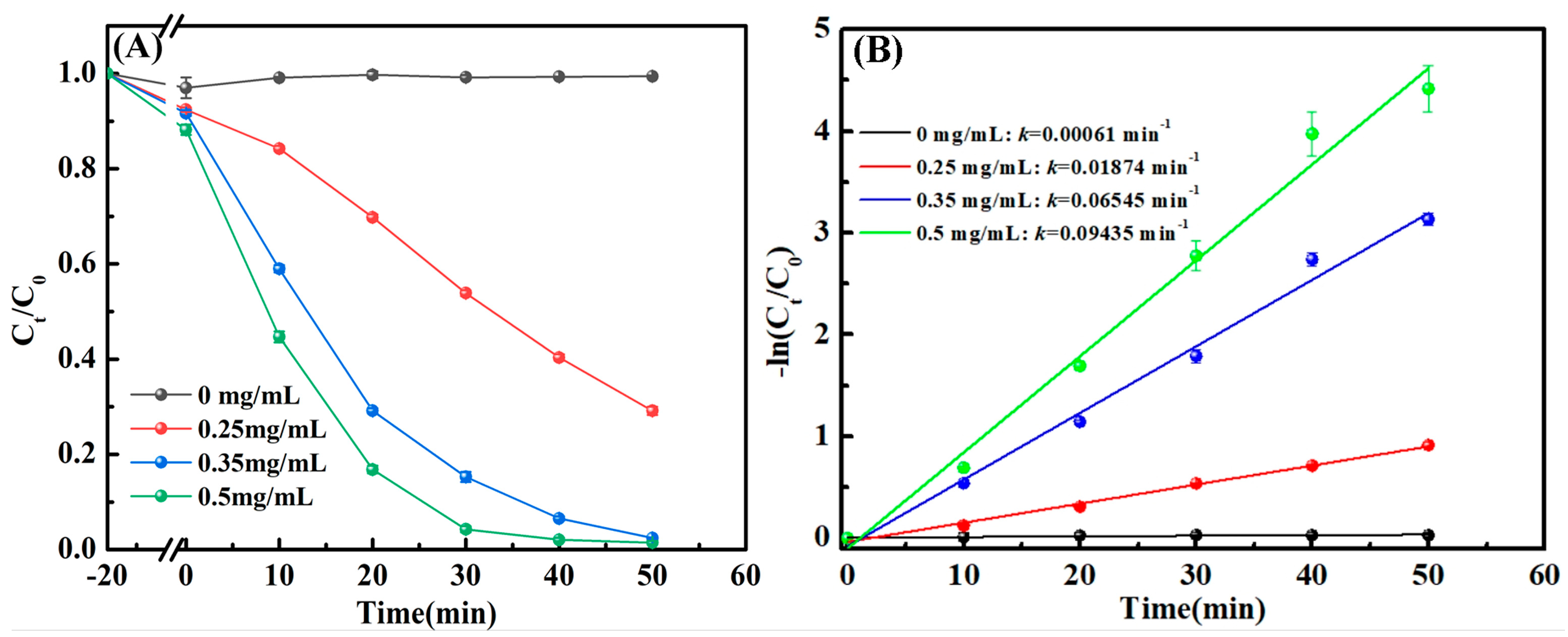
3.2. Effect of Catalyst Dosage
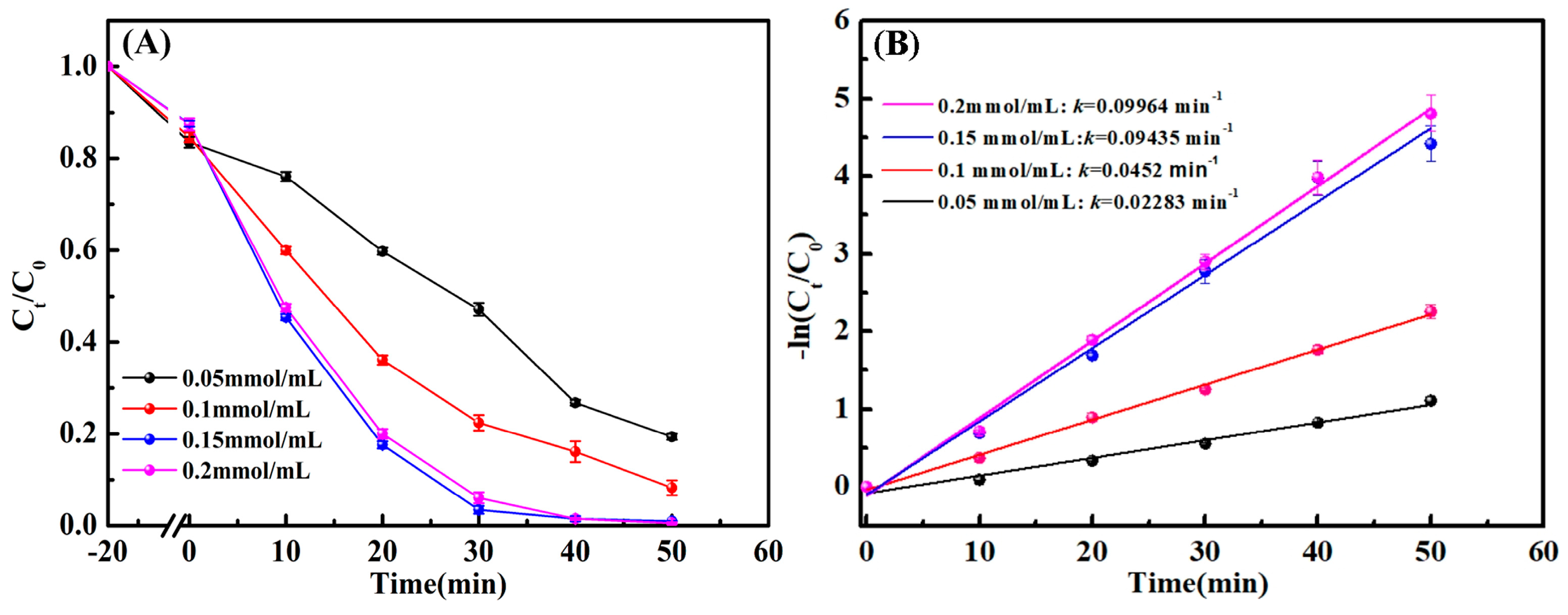
3.3. Effect of H2O2 Dosage
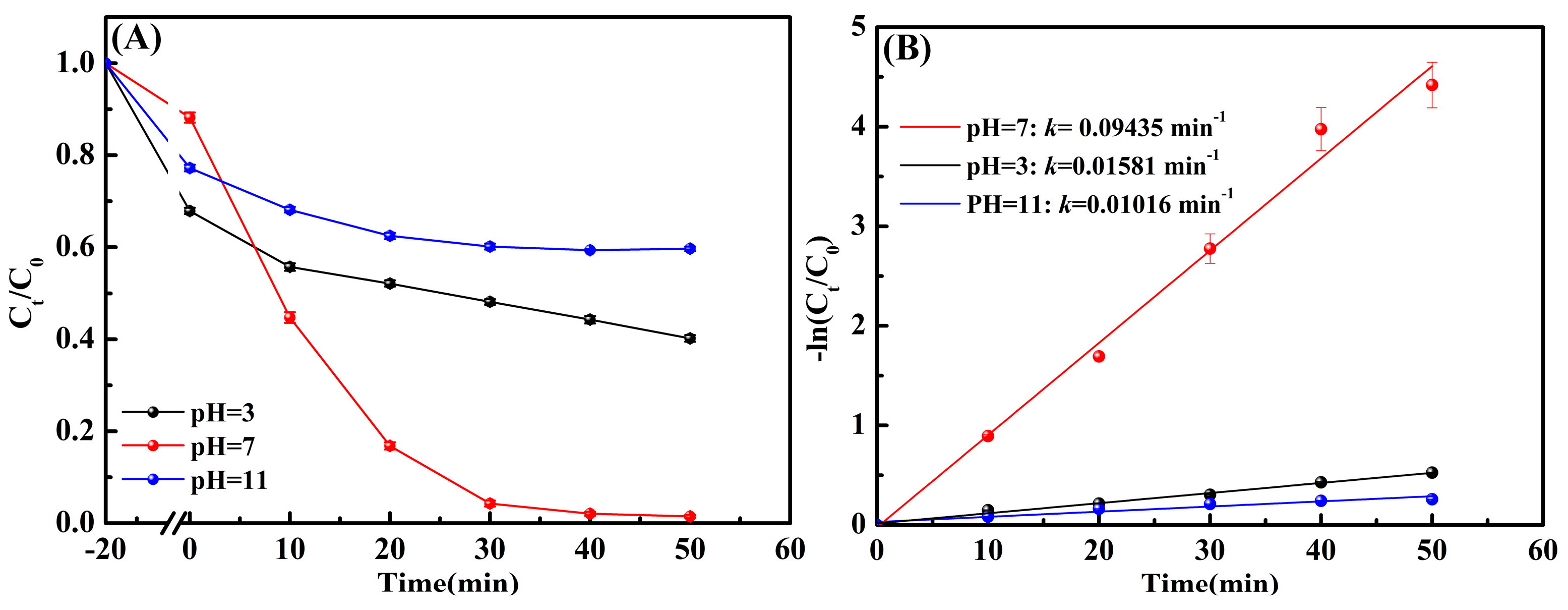
3.4. Effect of pH Value
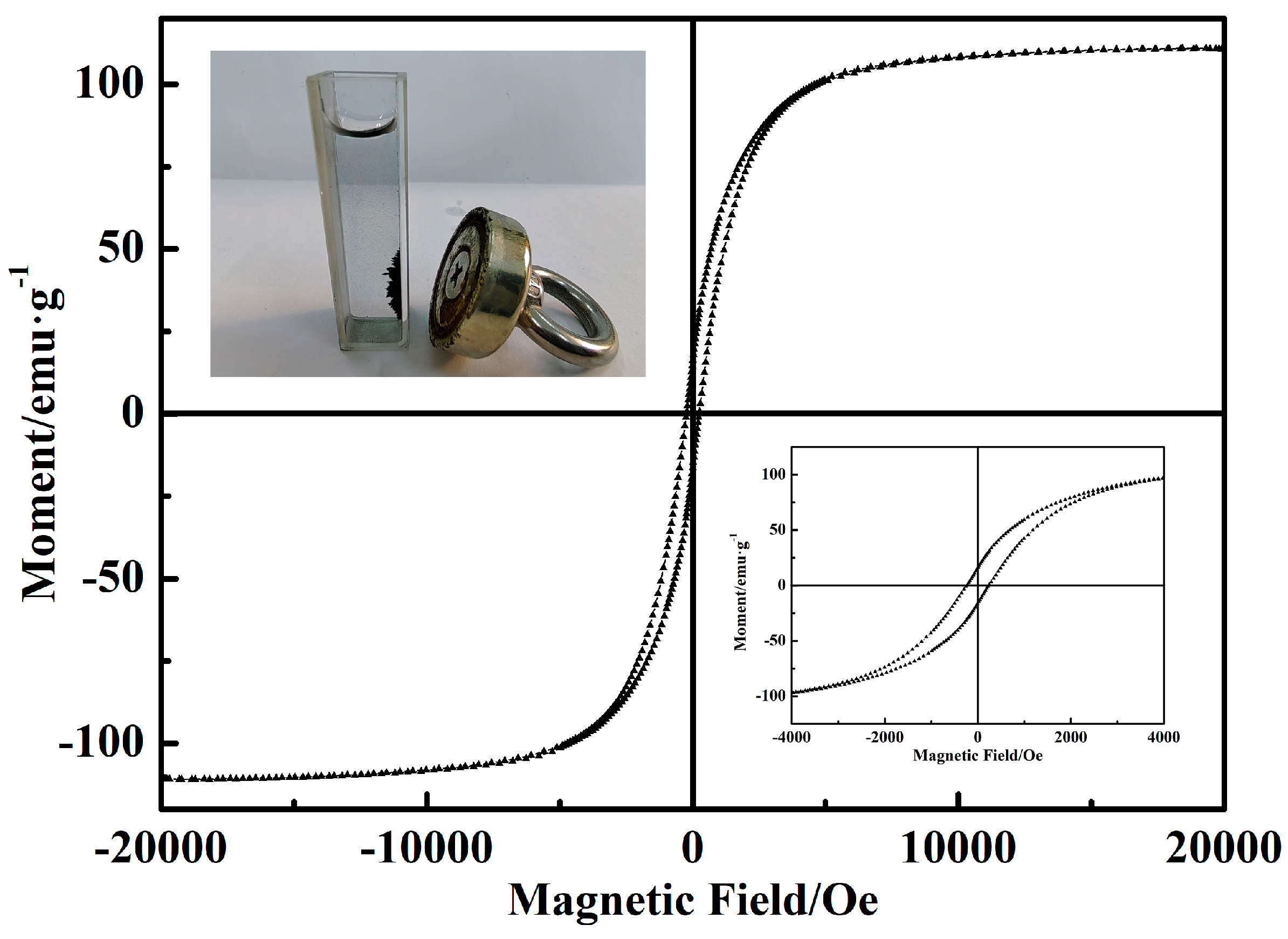
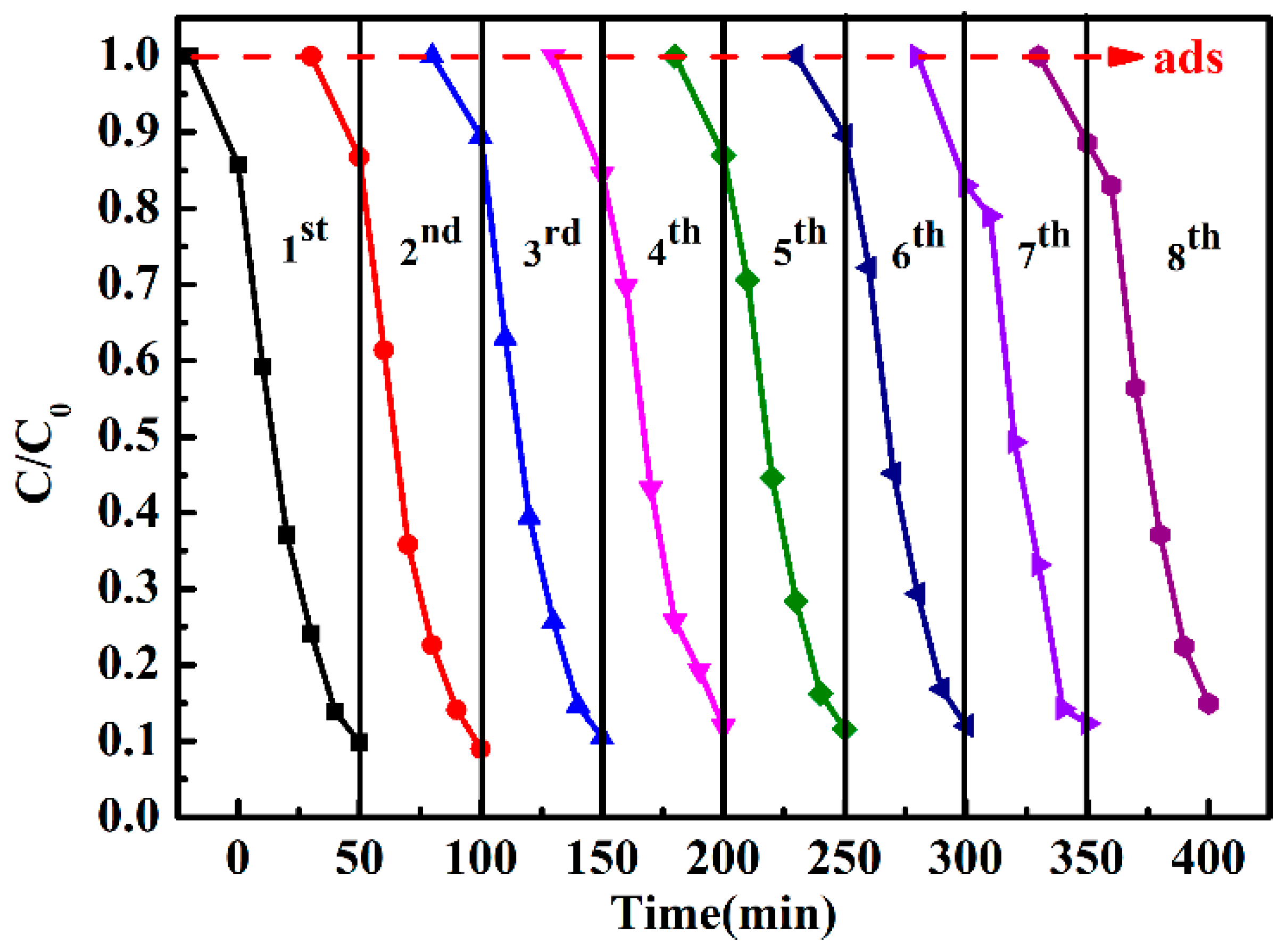
3.5. Reusability and Stability of Catalyst
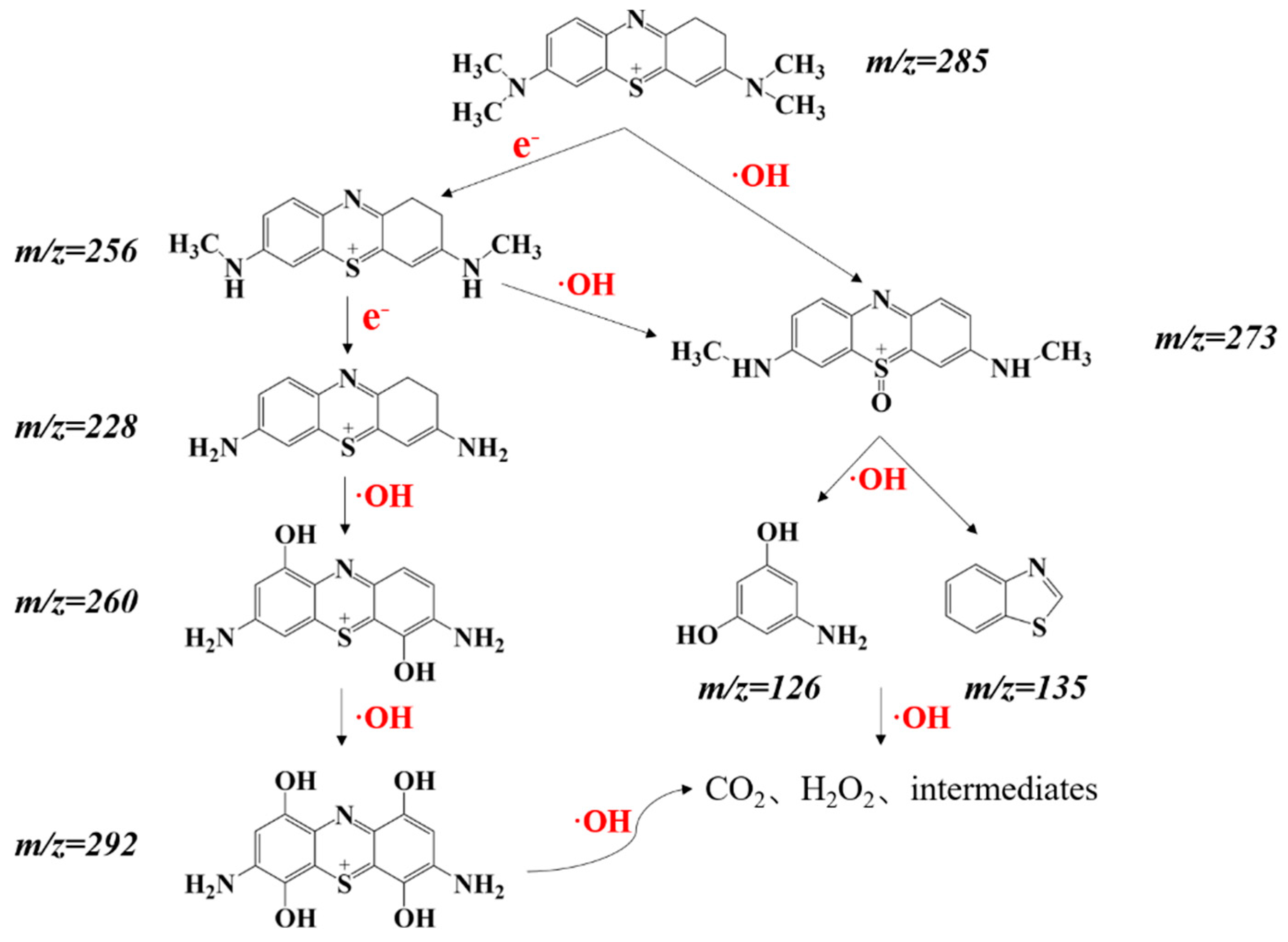
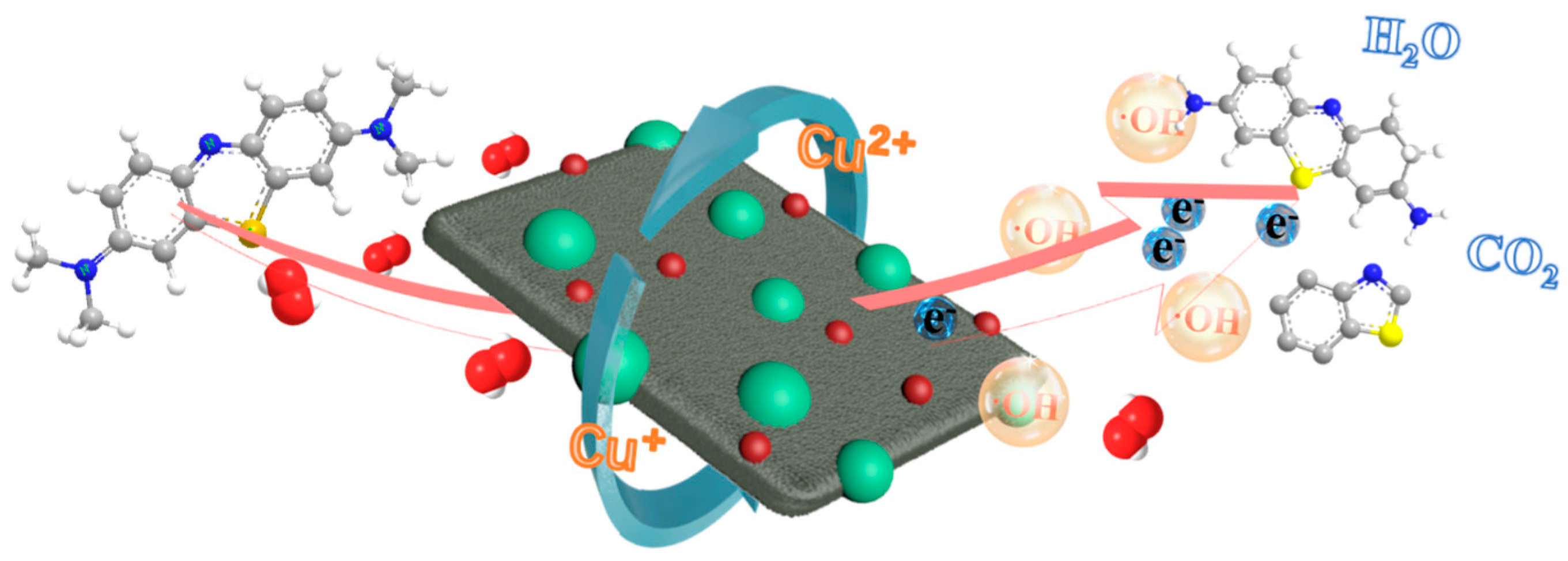
3.6. Possible Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hussain, S.; Aneggi, E.; Trovarelli, A.; Goi, D. Removal of organics from landfill leachate by heterogeneous Fenton-like oxidation over copper-based catalyst. Catalysts 2022, 12, 338. [Google Scholar] [CrossRef]
- Pliego, G.; Zazo, J.A.; Garcia, M.M.; Munoz, M.; Casas, J.A.; Rodriguez, J.J. Trends in the intensification of the Fenton process for wastewater treatment: An overview. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2611–2692. [Google Scholar] [CrossRef]
- Macarena, M.; Zahara, M.P.; Jose, A.C.; Juan, J.R. Preparation of magnetite-based catalysts and their application in heterogeneous Fenton oxidation-a review. Appl. Catal. B Environ. 2015, 176, 249–265. [Google Scholar]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, M.; Fang, J.; Fu, J.; Chen, X. Visible-light photo-Fenton oxidation of phenol with rGO-α-FeOOH supported on Al-doped mesoporous silica (MCM-41) at neutral pH: Performance and optimization of the catalyst. Chemosphere 2017, 182, 468–476. [Google Scholar] [CrossRef]
- Tang, J.T.; Wang, J.L. Iron-copper bimetallic metal-organic frameworks for efficient Fenton like degradation of sulfamethoxazole under mild conditions. Chemosphere 2020, 241, 125002. [Google Scholar] [CrossRef]
- Li, J.Y.; Ninh, P.A.; Dai, R.B.; Wang, Z.W.; David, W.T. Recent advances in Cu-Fenton systems for the treatment of industrial wastewaters: Role of Cu complexes and Cu composites. J. Hazard. Mater. 2020, 392, 122261. [Google Scholar] [CrossRef]
- Shima, R.P.; Abdul, A.A.R.; Wan, M.A.W.D. Review on the application of modified iron oxides as heterogeneous catalysts in Fenton reactions. J. Clean. Prod. 2014, 64, 24–35. [Google Scholar]
- Qin, Y.; Li, G.; Gao, Y.; Zhang, L.; Ok, Y.S.; An, T. Persistent free radicals in carbon-based materials on transformation of refractory organic contaminants (ROCs) in water: A critical review. Water Res. 2018, 137, 130–143. [Google Scholar] [CrossRef]
- Yang, Z.C.; Shan, C.; Pignatello, J.J.; Pan, B.C. Mn(II) acceleration of the picolinic acid-assisted Fenton reaction: New insight into the role of manganese in homogeneous Fenton AOPs. Environ. Sci. Technol. 2022, 56, 6621–6630. [Google Scholar] [CrossRef]
- Hou, X.; Huang, X.; Ai, Z.; Zhao, J.; Zhang, L. Ascorbic acid/Fe@Fe2O3: A highly efficient combined Fenton reagent to remove organic contaminants. J. Hazard. Mater. 2016, 310, 170–178. [Google Scholar] [CrossRef]
- Salazar, R.; Brillas, E.; Sirés, I. Finding the best Fe2+/Cu2+ combination for the solar photoelectro-Fenton treatment of simulated wastewater containing the industrial textile dye Disperse Blue 3. Appl. Catal. B Environ. 2012, 115, 107–116. [Google Scholar] [CrossRef]
- Southworth, B.A.; Voelker, B.M. Hydroxyl radical production via the photo-Fenton reaction in the presence of fulvic acid. Environ. Sci. Technol. 2003, 37, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tian, P.F.; Ding, D.D.; Yang, Z.X.; Wang, W.Z.; Xin, H.; Xu, J.; Han, Y.F. Revealing the active species of Cu-based catalysts for heterogeneous Fenton reaction. Appl. Catal. B Environ. 2019, 258, 117985. [Google Scholar] [CrossRef]
- Shi, H.; He, Y.; Li, Y.B.; Luo, P.Y. 2D MOF derived cobalt and nitrogen-doped ultrathin oxygen-rich carbon nanosheets for efficient Fenton-like catalysis: Tuning effect of oxygen functional groups in close vicinity to Co-N sites. J. Hazard. Mater. 2023, 443, 130345. [Google Scholar] [CrossRef]
- Huang, P.P.; Chang, Q.; Jiang, G.D.; Xiao, K.R.; Wang, X. MIL-101(FeII3, Mn) with dual-reaction center as Fenton-like catalyst for highly efficient peroxide activation and phenol degradation. Sep. Purif. Technol. 2023, 306, 122582. [Google Scholar] [CrossRef]
- Bhaumik, M.; Maity, A.; Brink, H.G. Metallic nickel nanoparticles supported polyaniline nanotubes as heterogeneous Fenton-like catalyst for the degradation of brilliant green dye in aqueous solution. J. Colloid Interface Sci. 2022, 611, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Mao, N.; Jiao, Y.; Duan, X.L. g-C3N4/ZnO heterojunction as Fenton-like catalyst for degradation of organic pollution. Mater. Res. Bull. 2022, 151, 111818. [Google Scholar] [CrossRef]
- Sharmoukh, W.; Abdelhamid, H.N. Fenton-like cerium metal-organic frameworks (Ce-MOFs) for catalytic oxidation of olefins, alcohol, and dyes degradation. J. Clust. Sci. 2023, 34, 2509–2519. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Bussche, A.V.D.; Kabadi, P.K.; Kane, A.B.; Kane, R.H. Biological and Environmental Transformations of Copper-Based Nanomaterials. ACS Nano 2013, 7, 8715–8727. [Google Scholar] [CrossRef]
- Wang, L.H.; Jiang, J.; Ma, J.; Pang, S.Y.; Zhang, T. A review on advanced oxidation processes homogeneously initiated by copper(II). Chem. Eng. J. 2022, 427, 131721. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Ma, L.L.; Li, J.L.; Yu, Y. In situ Fenton reagent generated from TiO2/Cu2O composite film: A new way to utilize TiO2 under visible light irradiation. Environ. Sci. Technol. 2007, 41, 6264–6269. [Google Scholar] [CrossRef] [PubMed]
- Ai, Z.H.; Xiao, H.Y.; Mei, T.; Liu, J.; Zhang, L.Z.; Deng, K.J.; Qiu, J.R. Electro-fenton degradation of rhodamine B based on a composite cathode of Cu2O nanocubes and carbon nanotubes. J. Phys. Chem. C 2008, 112, 11929–11935. [Google Scholar] [CrossRef]
- Hussain, S.; Aneggi, E.; Goi, D. Catalytic activity of metals in heterogeneous Fenton-like oxidation of wastewater contaminants: A review. Environ. Chem. Lett. 2021, 19, 2405–2424. [Google Scholar] [CrossRef]
- Xu, S.Q.; Zhu, H.X.; Cao, W.R.; Wen, Z.B.; Wang, J.N.; Francois-Xavier, C.P.; Wintgens, T. Cu-Al2O3-g-C3N4 and Cu-Al2O3-C-dots with dual-reaction centres for simultaneous enhancement of Fenton-like catalytic activity and selective H2O2 conversion to hydroxyl radicals. Appl. Catal. B Environ. 2018, 234, 223–233. [Google Scholar] [CrossRef]
- Jin, H.; Tian, X.K.; Nie, Y.L.; Zhou, Z.X.; Yang, C.; Li, Y.; Lu, L.Q. Oxygen vacancy promoted heterogeneous Fenton-like degradation of ofloxacin at pH 3.2–9.0 by Cu substituted magnetic Fe3O4@FeOOH nanocomposite. Environ. Sci. Technol. 2017, 51, 12699–12706. [Google Scholar] [CrossRef]
- Duan, J.B.; Pang, S.Y.; Wang, Z.; Zhou, Y.; Gao, Y.; Li, J.; Guo, Q.; Jiang, J. Hydroxylamine driven advanced oxidation processes for water treatment: A review. Chemosphere 2020, 262, 128390. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Du, X.Y.; Zhu, X.Y.; Huang, E.H.; Liu, L.; An, Y.L. Regulate the behaviors of photo-generated carriers in CuFe-LDH/TiO2 based on positive bias voltage to enhance the capability of photo-Fenton on nitrobenzene degradation. Appl. Surf. Sci. 2022, 602, 154312. [Google Scholar] [CrossRef]
- Lyu, L.; Zhang, L.L.; Hu, C. Enhanced Fenton-like degradation of pharmaceuticals over framework copper species in copper-doped mesoporous silica microspheres. Chem. Eng. J. 2015, 274, 298–306. [Google Scholar] [CrossRef]
- Wang, C.Q.; Cao, Y.J.; Wang, H. Copper-based catalyst from waste printed circuit boards for effective Fenton-like discoloration of Rhodamine B at neutral pH. Chemosphere 2019, 230, 278–285. [Google Scholar] [CrossRef]
- Zhu, J.N.; Zhu, X.Q.; Cheng, F.F.; Li, P.; Wang, F.; Xiao, Y.W.; Xiong, W.W. Preparing copper doped carbon nitride from melamine templated crystalline copper chloride for Fenton-like catalysis. Appl. Catal. B Environ. 2019, 256, 117830. [Google Scholar] [CrossRef]
- Xu, J.W.; Zheng, X.L.; Feng, Z.P.; Lu, Z.Y. Organic wastewater treatment by a single-atom catalyst and electrolytically produced H2O2. Nat. Sustain. 2020, 4, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wu, X.L.; Shi, C.Y.; Lin, H.J.; Chen, J.R.; Shi, Y.P.; Wang, S.B.; Duan, X.G. Molecular engineering toward pyrrolic N-rich M-N-4 (M = Cr, Mn, Fe, Co, Cu) single-atom sites for enhanced heterogeneous Fenton-like reaction. Adv. Funct. Mater. 2021, 31, 2007877. [Google Scholar] [CrossRef]
- Cui, F.; Shao, Q.; Cui, T.Y.; Xu, L.X.; Yao, T.J.; Zhang, X. Janus building block-enabled fabrication of dualmetal equipped coordination polymers: An ideal precursor for noble metal/metal oxide nanocomposites with excellent catalytic performance. J. Mater. Chem. A 2015, 3, 20073. [Google Scholar] [CrossRef]
- Taft, R.W.; Kamlet, M.J. The solvatochromic comparison method. I. The. beta.-scale of solvent hydrogen-bond acceptor (HBA) basicities. J. Am. Chem. Soc. 1976, 19, 2886–2894. [Google Scholar] [CrossRef]
- Li, H.F.; Liu, S.L.; Xie, Z.; Bai, C.Y. Copper-carbon clusters CunCm (n, m = 1–6): Segregation, bonding and Raman spectra. Mater. Today Commun. 2021, 26, 102035. [Google Scholar] [CrossRef]
- Chen, L.J.; Li, G.S.; Li, L.P. CuO Nanocrystals in Thermal Decomposition of Ammonium Perchlorate-Stabilization, Structural Characterization and Catalytic Activities. J. Therm. Anal. Calorim. 2008, 91, 581–587. [Google Scholar] [CrossRef]
- Ma, Q.H.; Liu, M.F.; Cui, F.; Zhang, J.J.; Cui, T.Y. Fabrication of 3D graphene microstructures with uniform metal oxide nanoparticles via molecular self-assembly strategy and their supercapacitor performance. Carbon 2023, 204, 336–345. [Google Scholar] [CrossRef]
- Kim, H.; Jae, W.; Song, J.; Kim, J. Skein-shaped ZnO/N-doped carbon microstructures as a high-performance anode material for lithium-ion batteries. J. Alloys Compd. 2019, 772, 507–515. [Google Scholar] [CrossRef]
- Liu, S.Y.; Teng, L.; Zhao, Y.M.; Liu, Z.; Zhang, J.W.; Muhammad, I.; Afrasiab, U.R.; Shi, K.N. Facile route to synthesize porous hierarchical Co3O4/CuO nanosheets with high porosity and excellent NOx sensing properties at room temperature. Appl. Surf. Sci. 2018, 450, 91–101. [Google Scholar] [CrossRef]
- Cao, L.Y.; Kang, Q.; Li, J.Y.; Huang, J.F.; Cheng, Y.Y. Assembly control of CoO/reduced graphene oxide composites for their enhanced lithium storage behavior. Appl. Surf. Sci. 2018, 455, 96–105. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, N.P.; Gong, Y.S.; Wang, R.T.; Zhang, X.D. Cu-Fe embedded cross-linked 3D hydrogel for enhanced reductive removal of Cr(VI): Characterization, performance, and mechanisms. Chemosphere 2021, 380, 130663. [Google Scholar] [CrossRef] [PubMed]
- Herney-Ramirez, J.; Vicente, M.A.; Madeira, L.M. Heterogeneous photo-Fenton oxidation with pillared clay-based catalysts for wastewater treatment: A review. Appl. Catal. B Environ. 2010, 98, 10–26. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, C.; Wei, H.; Wang, Q.; Li, K. In situ synthesis and immobilization of a Cu (II)epyridyl complex on silica microspheres as a novel Fenton like catalyst for RhB degradation at near-neutral pH. RSC Adv. 2017, 7, 22825–22835. [Google Scholar] [CrossRef]
- Anfruns, A.; García-Suarez, E.J.; Montes-Moran, M.A.; Gonzalez-Olmos, R.; Martin, M.J. New insights into the influence of activated carbon surface oxygen groups on H2O2 decomposition and oxidation of pre-adsorbed volatile organic compounds. Carbon 2014, 77, 89–98. [Google Scholar] [CrossRef]
- Wu, L.H.; Xie, Q.H.; Lv, Y.B.; Zhang, Z.Y.; Wu, Z.Y.; Liang, X.J.; Lu, M.Z.; Nie, Y. Degradation of methylene blue by dielectric barrier discharge plasma coupled with activated carbon supported on polyurethane foam. RSC Adv. 2019, 9, 25967–25975. [Google Scholar] [CrossRef]
- Lopez-Ramon, M.V.; Alvarez, M.A.; Moreno-Castilla, C.; Fontecha-Camara, M.A.; Yebra-Rodríguez, A.; Bailon-García, E. Effect of calcination temperature of a copper ferrite synthesized by a sol-gel method on its structural characteristics and performance as Fenton catalyst to remove gallic acid from water. J. Colloid Interface Sci. 2018, 511, 193–202. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Liu, R.; Zhao, Y.; Shen, X.; Sun, C.; Yang, Z.; Wang, J.; Du, Y.; Geng, S.; Chen, F. Coordination-Polymer-Derived Cu-CoO/C Nanocomposite Used in Fenton-like Reaction to Achieve Efficient Degradation of Organic Compounds. Nanomaterials 2024, 14, 132. https://doi.org/10.3390/nano14020132
Xu L, Liu R, Zhao Y, Shen X, Sun C, Yang Z, Wang J, Du Y, Geng S, Chen F. Coordination-Polymer-Derived Cu-CoO/C Nanocomposite Used in Fenton-like Reaction to Achieve Efficient Degradation of Organic Compounds. Nanomaterials. 2024; 14(2):132. https://doi.org/10.3390/nano14020132
Chicago/Turabian StyleXu, Linxu, Rupeng Liu, Yubo Zhao, Xue Shen, Cuizhen Sun, Zhigang Yang, Jin Wang, Yufeng Du, Shuying Geng, and Feiyong Chen. 2024. "Coordination-Polymer-Derived Cu-CoO/C Nanocomposite Used in Fenton-like Reaction to Achieve Efficient Degradation of Organic Compounds" Nanomaterials 14, no. 2: 132. https://doi.org/10.3390/nano14020132





