Non-Woven Fibrous Polylactic Acid/Hydroxyapatite Nanocomposites Obtained via Solution Blow Spinning: Morphology, Thermal and Mechanical Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization Techniques
3. Results and Discussion
3.1. Structural and Morphological Characterization
3.2. Thermal Characterization
3.3. Mechanical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amini, S.; Salehi, H.; Setayeshmehr, M.; Ghorbani, M. Masoud Ghorbani Natural and Synthetic Polymeric Scaffolds Used in Peripheral Nerve Tissue Engineering: Advantages and Disadvantages. Polym. Adv. Technol. 2021, 32, 2267–2289. [Google Scholar] [CrossRef]
- Shahverdi, M.; Seifi, S.; Akbari, A.; Mohammadi, K.; Shamloo, A.; Movahhedy, M.R. Melt Electrowriting of PLA, PCL, and Composite PLA/PCL Scaffolds for Tissue Engineering Application. Sci. Rep. 2022, 12, 19935. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, J.; Lee, M.K.; Park, C.; Jung, H.D.; Kim, H.E.; Jang, T.S. Fabrication of Strong, Bioactive Vascular Grafts with PCL/Collagen and PCL/Silica Bilayers for Small-Diameter Vascular Applications. Mater. Des. 2019, 181, 108079. [Google Scholar] [CrossRef]
- Raja, I.S.; Preeth, D.R.; Vedhanayagam, M.; Hyon, S.H.; Lim, D.; Kim, B.; Rajalakshmi, S.; Han, D.W. Polyphenols-Loaded Electrospun Nanofibers in Bone Tissue Engineering and Regeneration. Biomater. Res. 2021, 25, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yan, D.; Zhao, L.; Lin, J. Composite Fibrous Membrane Comprising PLA and PCL Fibers for Biomedical Application. Compos. Commun. 2022, 34, 101268. [Google Scholar] [CrossRef]
- Zhang, S.; Rind, N.A.; Tang, N.; Liu, H.; Yin, X.; Yu, J.; Ding, B. Electrospun Nanofibers for Air Filtration. In Electrospinning: Nanofabrication and Applications; William Andrew: Norwich, NY, USA, 2019; pp. 365–389. [Google Scholar] [CrossRef]
- Gomes, N.O.; Paschoalin, R.T.; Bilatto, S.; Sorigotti, A.R.; Farinas, C.S.; Mattoso, L.H.C.; Machado, S.A.S.; Oliveira, O.N.; Raymundo-Pereira, P.A. Flexible, Bifunctional Sensing Platform Made with Biodegradable Mats for Detecting Glucose in Urine. ACS Sustain. Chem. Eng. 2023, 11, 2209–2218. [Google Scholar] [CrossRef]
- Immich, A.P.S.; Arias, M.L.; Carreras, N.; Boemo, R.L.; Tornero, J.A. Drug Delivery Systems Using Sandwich Configurations of Electrospun Poly(Lactic Acid) Nanofiber Membranes and Ibuprofen. Mater. Sci. Eng. C 2013, 33, 4002–4008. [Google Scholar] [CrossRef]
- Gama-Castañeda, N.O.; Franco-Colín, M.; Aguilar-Méndez, M.Á.; San Martin-Martinez, E.; Cano-Europa, E.; Casañas-Pimentel, R.G. Polymeric Nanofiber Dressings with Incorporated Rifampicin for Transdermal Administration. Int. J. Polym. Mater. Polym. Biomater. 2022, 72, 1032–1041. [Google Scholar] [CrossRef]
- Hardingham, T.; Tew, S.; Murdoch, A. Tissue Engineering: Chondrocytes and Cartilage. Arthritis Res. 2002, 4 (Suppl. S3), S63–S68. [Google Scholar] [CrossRef]
- Langer, R.; Lacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Lu, T.; Li, Y.; Chen, T. Techniques for Fabrication and Construction of Three-Dimensional Scaffolds for Tissue Engineering. Int. J. Nanomed. 2013, 8, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N. Biodegradable Polymer-Based Scaffolds for Tissue Engineering; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-34802-0. [Google Scholar]
- Tang, G.; Liu, Z.; Liu, Y.; Yu, J.; Wang, X.; Tan, Z.; Ye, X. Recent Trends in the Development of Bone Regenerative Biomaterials. Front. Cell Dev. Biol. 2021, 9, 665813. [Google Scholar] [CrossRef]
- Aslankoohi, N.; Mondal, D.; Rizkalla, A.S.; Mequanint, K. Bone Repair and Regenerative Biomaterials: Towards Recapitulating the Microenvironment. Polymers 2019, 11, 1437. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Cui, S.; Luo, D.; Liu, Y. Novel Inorganic Nanomaterial-Based Therapy for Bone Tissue Regeneration. Nanomaterials 2021, 11, 789. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D Bioactive Composite Scaffolds for Bone Tissue Engineering. Bioact. Mater. 2017, 3, 278–314. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Kundu, B.; Naskar, D.; Kim, H.W.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Silk Scaffolds in Bone Tissue Engineering: An Overview. Acta Biomater. 2017, 63, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic Acid (PLA) Controlled Delivery Carriers for Biomedical Applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Luciano, B. Polyhydroxyalkanoates Based Systems: The Future of Drug Delivery and Tissue Engineering Devices. In Bio-Based Nanomaterials: Synthesis Protocols, Mechanisms and Applications Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2022; pp. 133–169. [Google Scholar] [CrossRef]
- Lizundia, E.; Mateos, P.; Vilas, J.L. Tuneable Hydrolytic Degradation of Poly(L-Lactide) Scaffolds Triggered by ZnO Nanoparticles. Mater. Sci. Eng. C 2017, 75, 714–720. [Google Scholar] [CrossRef]
- García-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of Bone Grafting Materials for Bone Repair and Regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef]
- Szewczyk, A.; Skwira, A.; Konopacka, A.; Sądej, R.; Prokopowicz, M. Mesoporous Silica-Bioglass Composite Pellets as Bone Drug Delivery System with Mineralization Potential. Int. J. Mol. Sci. 2021, 22, 4708. [Google Scholar] [CrossRef]
- Deng, Y.; Kuiper, J. (Eds) Functional 3D Tissue Engineering Scaffolds. Materials, Technologies and Applications; Deng, Y., Kuiper, J., Eds.; Woodhead Publishing: Sawston, UK, 2017; ISBN 9780081009802. [Google Scholar]
- Kim, S.-M.; Kang, I.-G.; Cheon, K.-H.; Jang, T.-S.; Kim, H.-E.; Jung, H.-D.; Kang, M.-H. Enhanced Bioactivity of Micropatterned Hydroxyapatite Embedded Poly(L-Lactic) Acid for a Load-Bearing Implant. Polymers 2020, 12, 2390. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kim, J.; Han, G.; Kim, D.E.; Cheon, K.H.; Lee, H.; Kim, H.E.; Kim, Y.J.; Jang, T.S.; Jung, H.D. 3D-Printed Biodegradable Composite Scaffolds with Significantly Enhanced Mechanical Properties via the Combination of Binder Jetting and Capillary Rise Infiltration Process. Addit. Manuf. 2021, 41, 101988. [Google Scholar] [CrossRef]
- Wang, M. Biomaterials and Tissue Engineering. Biological and Medical Physics, Biomedical Engineering.; Shi, D., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2004. [Google Scholar]
- Prasad, A.; Sankar, M.R.; Katiyar, V. State of Art on Solvent Casting Particulate Leaching Method for Orthopedic ScaffoldsFabrication. Mater. Today Proc. 2017, 4, 898–907. [Google Scholar] [CrossRef]
- Medeiros, E.S.; Glenn, G.M.; Klamczynski, A.P.; Orts, W.J.; Mattoso, L.H. Solution Blow Spinning: A New Method to Produce Micro- and Nanofibers from Polymer Solutions. J. Appl. Sci. 2009, 113, 2322–2330. [Google Scholar] [CrossRef]
- da Silva Parize, D.D.; Foschini, M.M.; de Oliveira, J.E.; Klamczynski, A.P.; Glenn, G.M.; Marconcini, J.M.; Mattoso, L.H.C. Solution Blow Spinning: Parameters Optimization and Effects on the Properties of Nanofibers from Poly(Lactic Acid)/Dimethyl Carbonate Solutions. J. Mater. Sci. 2016, 51, 4627–4638. [Google Scholar] [CrossRef]
- Gonzalez-Benito, J.; Teno, J.; Torres, D.; Díaz, M. Solution Blow Spinning and Obtaining Submicrometric Fibers of Different Polymers. Int. J. Nanoparticles Nanotechnol. 2017, 3, 007. [Google Scholar]
- González-Benito, J.; Teno, J.; González-Gaitano, G.; Xu, S.; Chiang, M.Y. PVDF/TiO2 Nanocomposites Prepared by Solution Blow Spinning: Surface Properties and Their Relation with S. Mutans Adhesion. Polym. Test. 2017, 58, 21–30. [Google Scholar] [CrossRef]
- Zhang, T.; Tian, H.; Yin, X.; Li, Z.; Zhang, X.; Yang, J.; Zhu, L. Solution Blow Spinning of Polylactic Acid to Prepare Fibrous Oil Adsorbents Through Morphology Optimization with Response Surface Methodology. J. Polym. Environ. 2020, 28, 812–825. [Google Scholar] [CrossRef]
- González-Benito, J.; Torres, D.; Ballesteros, C.; Ruiz, V.M.; Teno, J. PVDF Based Nanocomposites Produced by Solution Blow Spinning, Structure and Morphology Induced by the Presence of MWCNT and Their Consequences on Some Properties. Colloid Polym. Sci. 2019, 297, 1105–1118. [Google Scholar] [CrossRef]
- Teno, J.; González-Gaitano, G.; González-Benito, J. Poly (Ethylene-Co-Vinyl Acetate) Films Prepared by Solution Blow Spinning: Surface Characterization and Its Relation with E. coli Adhesion. Polym. Test. 2017, 60, 140–148. [Google Scholar] [CrossRef]
- González-Benito, J.; Lorente, M.A.; Olmos, D.; Kramar, A. Solution Blow Spinning to Prepare Preferred Oriented Poly(Ethylene Oxide) Submicrometric Fibers. Fibers 2023, 11, 79. [Google Scholar] [CrossRef]
- Moradienayat, M.; Olmos, D.; González-Benito, J. Airbrushed Polysulfone (PSF)/Hydroxyapatite (HA) Nanocomposites: Effect of the Presence of Nanoparticles on Mechanical Behavior. Polymers 2022, 14, 753. [Google Scholar] [CrossRef] [PubMed]
- Kuska, R.; Milovanovic, S.; Frerich, S.; Ivanovic, J. Thermal Analysis of Polylactic Acid under High CO2 Pressure Applied in Supercritical Impregnation and Foaming Process Design. J. Supercrit. Fluids 2019, 144, 71–80. [Google Scholar] [CrossRef]
- Nofar, M.; Ameli, A.; Park, C.B. Development of Polylactide Bead Foams with Double Crystal Melting Peaks. Polymer 2015, 69, 83–94. [Google Scholar] [CrossRef]
- Kim, H.S.; Hong, S.I.; Kim, S.J. On the Rule of Mixtures for Predicting the Mechanical Properties of Composites with Homogeneously Distributed Soft and Hard Particles. J. Mater. Process. Technol. 2001, 112, 109–113. [Google Scholar] [CrossRef]
- Mayer, G. New Classes of Tough Composite Materials-Lessons from Natural Rigid Biological Systems. Mater. Sci. Eng. C 2006, 26, 1261–1268. [Google Scholar] [CrossRef]
- Segura González, E.A.; Olmos, D.; Lorente, M.Á.; Vélaz, I.; González-Benito, J. Preparation and Characterization of Polymer Composite Materials Based on PLA/TiO2 for Antibacterial Packaging. Polymers 2018, 10, 1365. [Google Scholar] [CrossRef]
- Ruz-Cruz, M.A.; Herrera-Franco, P.J.; Flores-Johnson, E.A.; Moreno-Chulim, M.V.; Galera-Manzano, L.M.; Valadez-González, A. Thermal and Mechanical Properties of PLA-Based Multiscale Cellulosic Biocomposites. J. Mater. Res. Technol. 2022, 18, 485–495. [Google Scholar] [CrossRef]
- Sahadat Hossain, M.; Ahmed, S. FTIR Spectrum Analysis to Predict the Crystalline and Amorphous Phases of Hydroxyapatite: A Comparison of Vibrational Motion to Reflection. RSC Adv. 2023, 13, 14625–14630. [Google Scholar] [CrossRef]
- Capitelli, F.; Dida, B.; Della Ventura, G.; Baldassarre, F.; Capelli, D.; Senesi, G.; Mele, A.; Siliqi, D. Functional Nano-Hydroxyapatite for Applications in Conservation of Stony Monuments of Cultural Heritage. Multidiscip. Digit. Publ. Inst. Proc. 2021, 62, 11. [Google Scholar]
- El Khouri, A.; Zegzouti, A.; Elaatmani, M.; Capitelli, F. Bismuth-Substituted Hydroxyapatite Ceramics Synthesis: Morphological, Structural, Vibrational and Dielectric Properties. Inorg. Chem. Commun. 2019, 110, 107568. [Google Scholar] [CrossRef]
- Vadas, D.; Nagy, Z.K.; Csontos, I.; Marosi, G.; Bocz, K. Effects of Thermal Annealing and Solvent-Induced Crystallization on the Structure and Properties of Poly(Lactic Acid) Microfibres Produced by High-Speed Electrospinning. J. Therm. Anal. Calorim. 2020, 142, 581–594. [Google Scholar] [CrossRef]
- Leonés, A.; Peponi, L.; Lieblich, M.; Benavente, R.; Fiori, S. In Vitro Degradation of Plasticized PLA Electrospun Fiber Mats: Morphological, Thermal and Crystalline Evolution. Polymers 2020, 12, 2975. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Green, T.B.; Joo, Y.L. The Thermal Effects on Electrospinning of Polylactic Acid Melts. Polymer 2006, 47, 7497–7505. [Google Scholar] [CrossRef]
- Liu, C.; Wong, H.M.; Yeung, K.W.K.; Tjong, S.C. Novel Electrospun Polylactic Acid Nanocomposite Fiber Mats with Hybrid Graphene Oxide and Nanohydroxyapatite Reinforcements Having Enhanced Biocompatibility. Polymers 2016, 8, 287. [Google Scholar] [CrossRef]
- Moradienayat, M.; González-Benito, J.; Olmos, D. Airbrushed PSF/ZnO Composite Coatings as a Novel Approach for the Consolidation of Historical Bones. Nanomaterials 2023, 13, 625. [Google Scholar] [CrossRef]
- Nguyen, N.M.; Kakarla, A.B.; Nukala, S.G.; Kong, C.; Baji, A.; Kong, I. Evaluation of Physicochemical Properties of a Hydroxyapatite Polymer Nanocomposite for Use in Fused Filament Fabrication. Polymers 2023, 15, 3980. [Google Scholar] [CrossRef]
- Xu, W.; Shen, R.; Yan, Y.; Gao, J. Preparation and Characterization of Electrospun Alginate/PLA Nanofibers as Tissue Engineering Material by Emulsion Eletrospinning. J. Mech. Behav. Biomed. Mater. 2017, 65, 428–438. [Google Scholar] [CrossRef]
- Wright, Z.M.; Arnold, A.M.; Holt, B.D.; Eckhart, K.E.; Sydlik, S.A. Functional Graphenic Materials, Graphene Oxide, and Graphene as Scaffolds for Bone Regeneration. Regen. Eng. Transl. Med. 2019, 5, 190–209. [Google Scholar] [CrossRef]
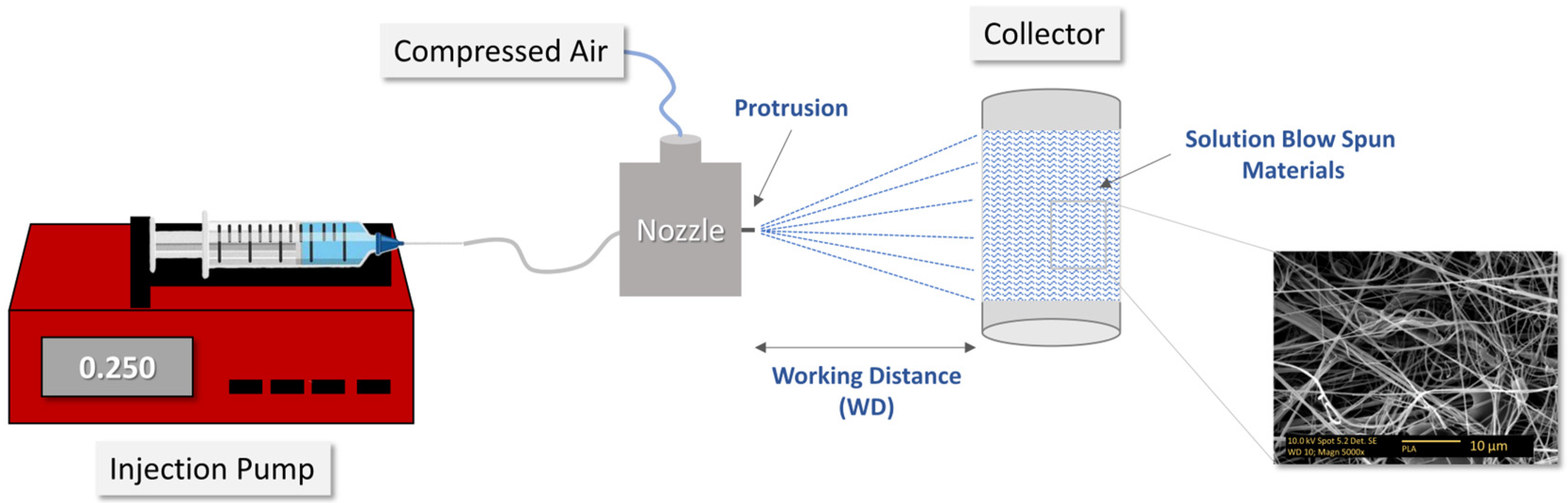
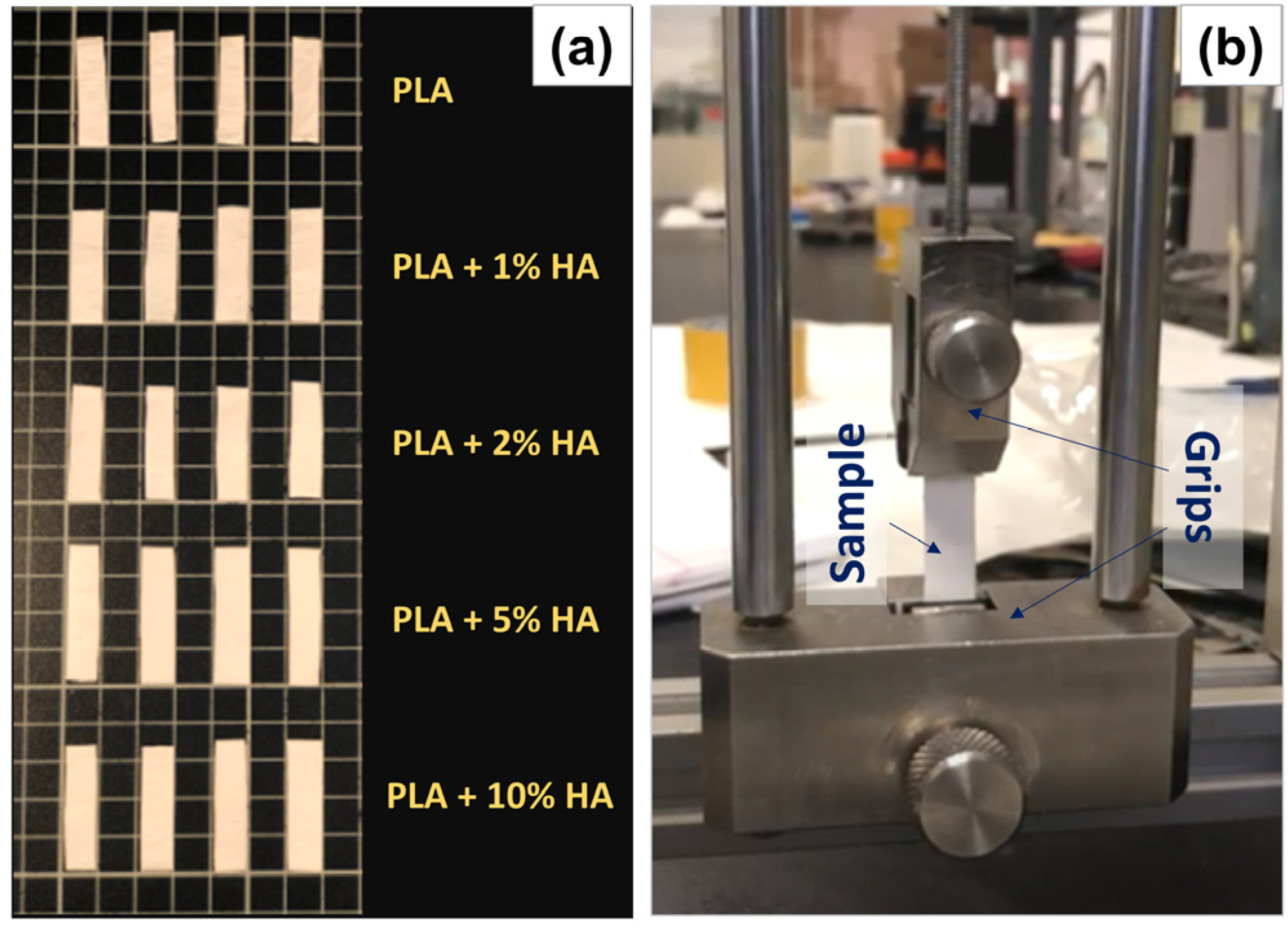
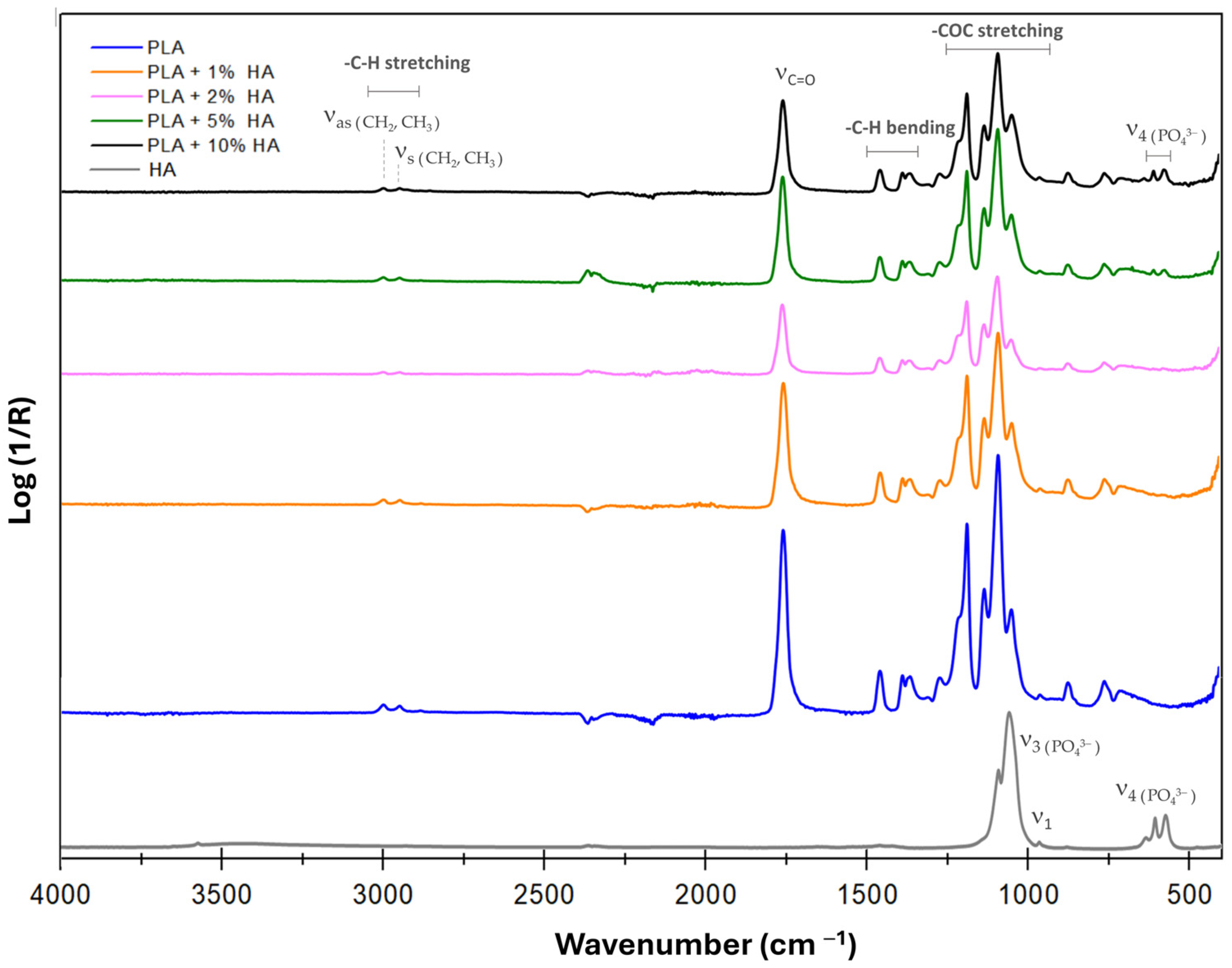
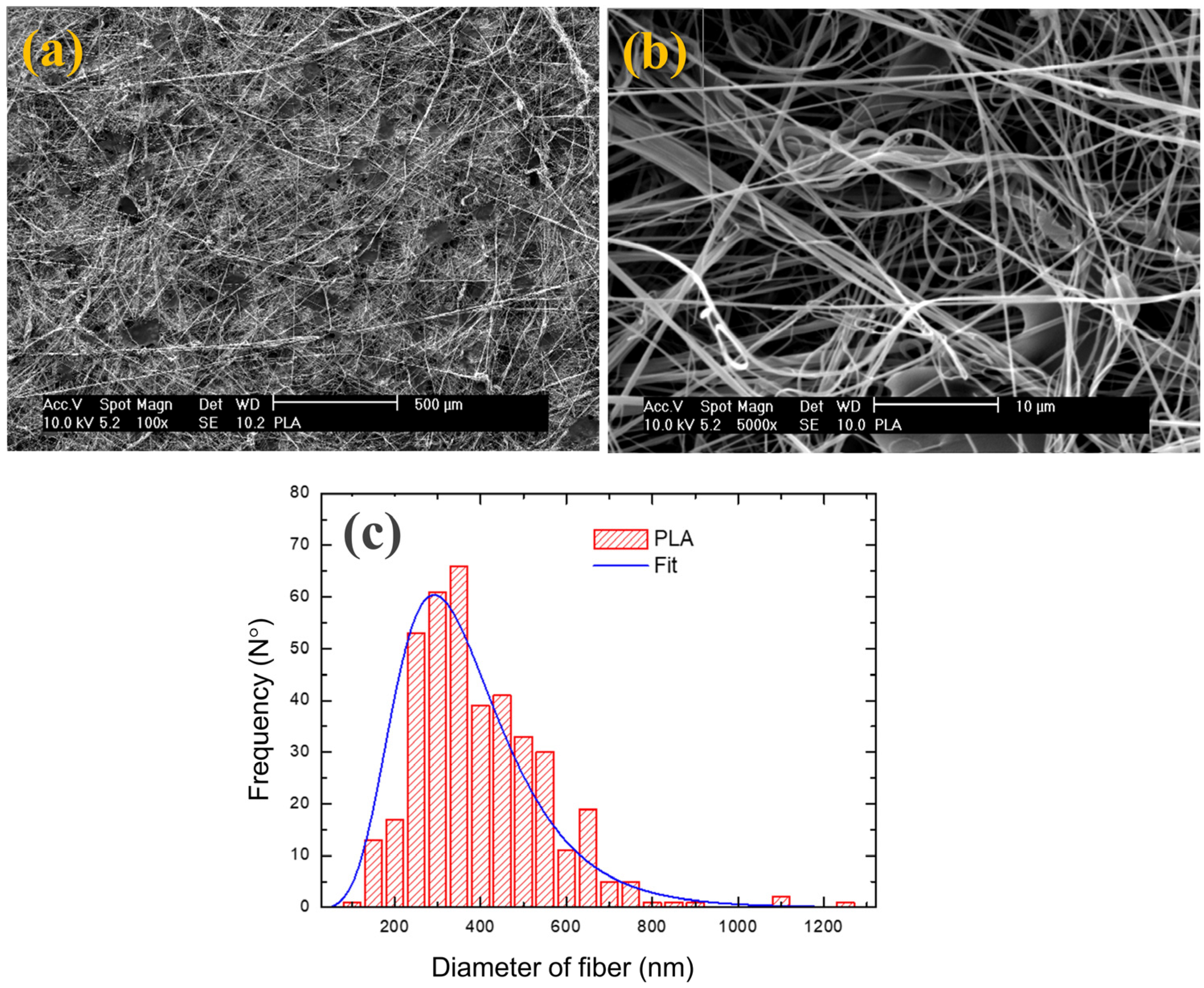
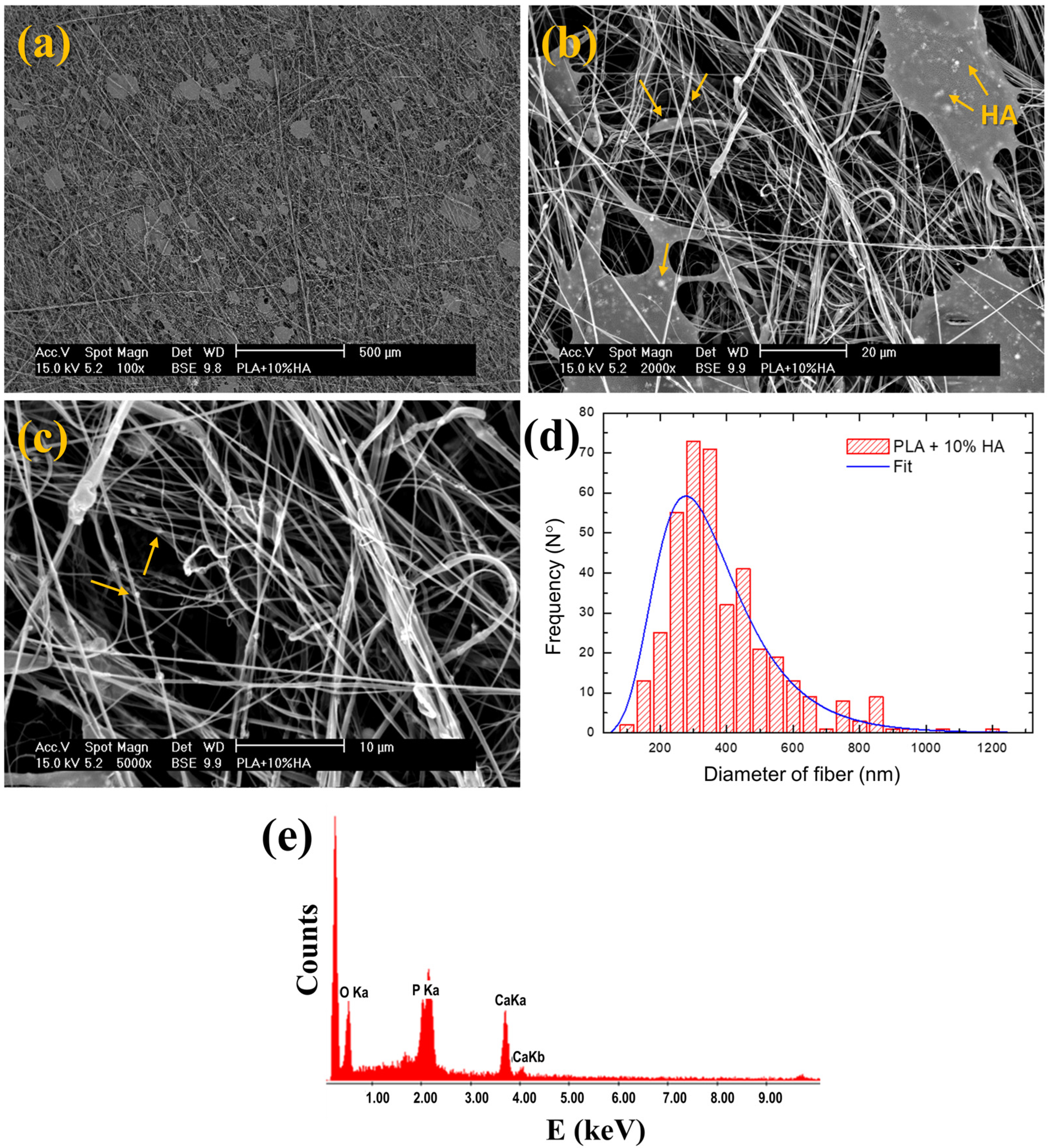


| Pressure (bars) | Working Distance (cm) | Injection Rate (mL/min) | Collector Rotation Speed (rpm) | Volume Injected (mL) |
|---|---|---|---|---|
| 2 | 11 | 0.25 | 200 | 25 |
| Sample | Diameter of Fiber (nm) | Estimated Porosity (%) |
|---|---|---|
| PLA | 370 ± 140 | 84 ± 1 |
| PLA + 1% HA | 430 ± 170 | 80 ± 4 |
| PLA + 2% HA | 380 ± 150 | 80 ± 1 |
| PLA + 5% HA | 400 ± 150 | 80 ± 2 |
| PLA + 10% HA | 360 ± 160 | 80 ± 1 |
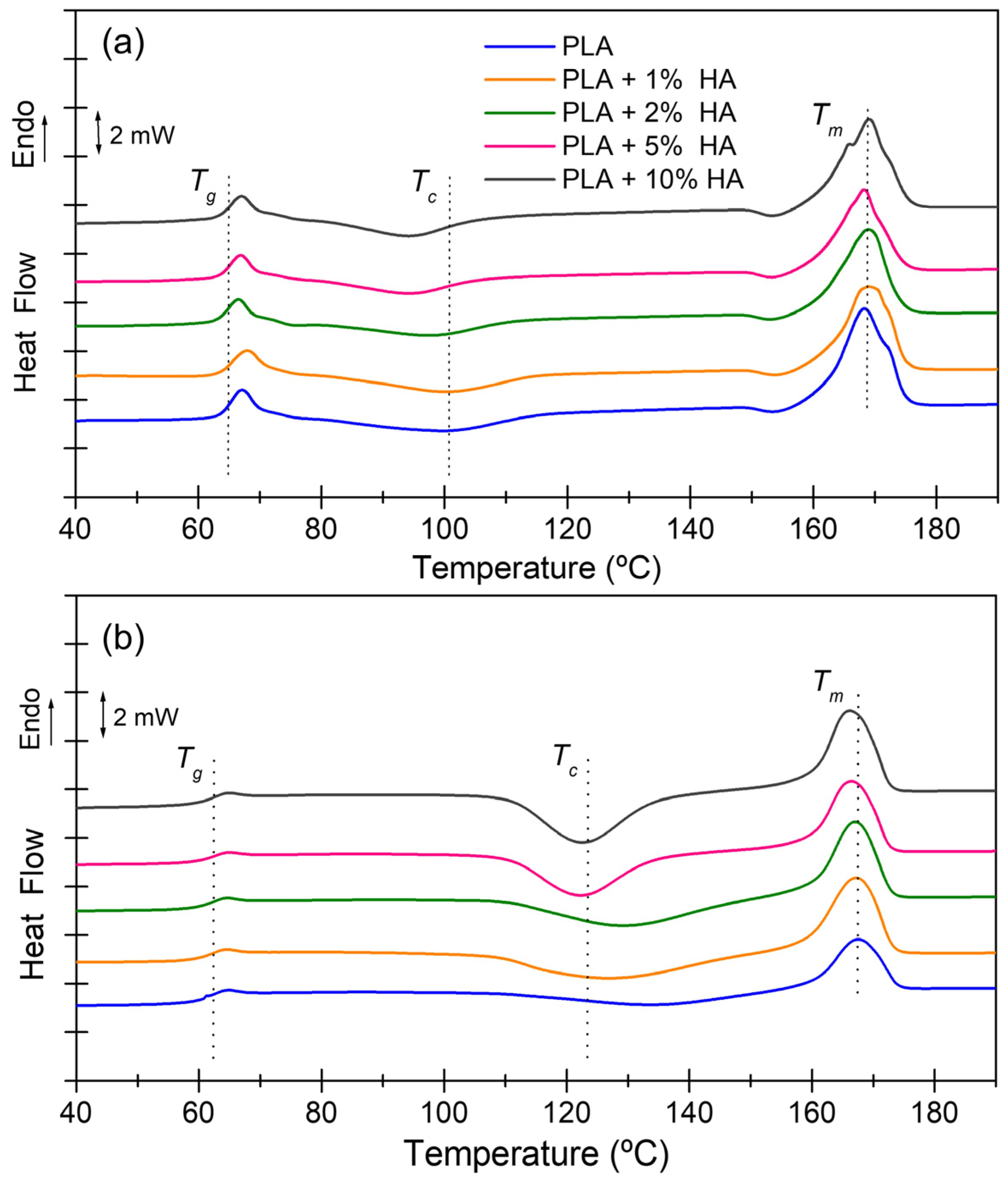
| Sample | NPs (%) | Tg (°C) | Tc (°C) | Tm (°C) | ΔHcc (J·g−1) | ΔHm (J·g−1) | χc before 1 (%) | χc after 2 (%) |
|---|---|---|---|---|---|---|---|---|
| PLA | 0 | 65.4 | 100.5 | 168.3 | 17.0 | 41.1 | 25.8 | 44.0 |
| PLA + 1% HA | 1 | 65.6 | 100.5 | 169.1 | 16.4 | 38.9 | 24.0 | 41.5 |
| PLA + 2% HA | 2 | 67.7 | 97.9 | 168.9 | 12.5 | 41.7 | 31.3 | 44.6 |
| PLA + 5% HA | 5 | 65.2 | 94.5 | 168.2 | 14.6 | 39.0 | 26.1 | 41.7 |
| PLA + 10% HA | 10 | 65.2 | 94.4 | 169.1 | 16.5 | 43.3 | 28.6 | 46.3 |
| Sample | NPs (%) | Tg (°C) | Tc (°C) | Tm (°C) | ΔHcc (J·g−1) | ΔHm (J·g−1) | χc after 2 (%) |
| PLA | 0 | 60.7 | 134.2 | 167.5 | 20.2 | 20.1 | 21.5 |
| PLA + 1% HA | 1 | 62.2 | 127.3 | 167.2 | 40.8 | 30.8 | 32.9 |
| PLA + 2% HA | 2 | 62.4 | 129.5 | 167.1 | 42.8 | 31.3 | 33.4 |
| PLA + 5% HA | 5 | 62.5 | 122.3 | 166.5 | 45.9 | 29.0 | 30.9 |
| PLA + 10% HA | 10 | 62.6 | 122.7 | 166.3 | 43.6 | 35.9 | 38.3 |
| Sample | NPs (%) | Elastic Modulus (MPa) | Tensile Strength (MPa) | Maximum Strain (1/1) | Area under Curve (MPa) |
|---|---|---|---|---|---|
| PLA | 0 | 19.0 ± 9.0 | 1.07 ± 0.43 | 0.15 ± 0.03 | 0.093 ± 0.05 |
| PLA + 1% HA | 1 | 23.0 ± 10 | 1.28 ± 0.31 | 0.25 ± 0.08 | 0.153 ± 0.05 |
| PLA + 2% HA | 2 | 22.5 ± 3.0 | 1.01 ± 0.35 | 0.14 ± 0.04 | 0.082 ± 0.05 |
| PLA + 5% HA | 5 | 30.4 ± 0.4 | 1.20 ± 0.37 | 0.13 ± 0.04 | 0.105 ± 0.06 |
| PLA + 10% HA | 10 | 38.0 ± 5.5 | 1.07 ± 0.44 | 0.12 ± 0.08 | 0.099 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Benito, J.; Zuñiga-Prado, S.; Najera, J.; Olmos, D. Non-Woven Fibrous Polylactic Acid/Hydroxyapatite Nanocomposites Obtained via Solution Blow Spinning: Morphology, Thermal and Mechanical Behavior. Nanomaterials 2024, 14, 196. https://doi.org/10.3390/nano14020196
González-Benito J, Zuñiga-Prado S, Najera J, Olmos D. Non-Woven Fibrous Polylactic Acid/Hydroxyapatite Nanocomposites Obtained via Solution Blow Spinning: Morphology, Thermal and Mechanical Behavior. Nanomaterials. 2024; 14(2):196. https://doi.org/10.3390/nano14020196
Chicago/Turabian StyleGonzález-Benito, Javier, Stephania Zuñiga-Prado, Julian Najera, and Dania Olmos. 2024. "Non-Woven Fibrous Polylactic Acid/Hydroxyapatite Nanocomposites Obtained via Solution Blow Spinning: Morphology, Thermal and Mechanical Behavior" Nanomaterials 14, no. 2: 196. https://doi.org/10.3390/nano14020196







