Tuneable Red and Blue Emission of Bi3+-Co-Doped SrF2:Eu3+ Nanophosphors for LEDs in Agricultural Applications
Abstract
1. Introduction
2. Experimental
2.1. Synthesis of SrF2: x mol% Eu3+ (x = 1, 5, 10, 15, 20) and SrF2: 10 mol% Eu3+, y mol% Bi3+ (y = 5, 10, 15, 20, 30, 40, 50) Nanoparticles
2.2. Characterization
3. Results and Discussion
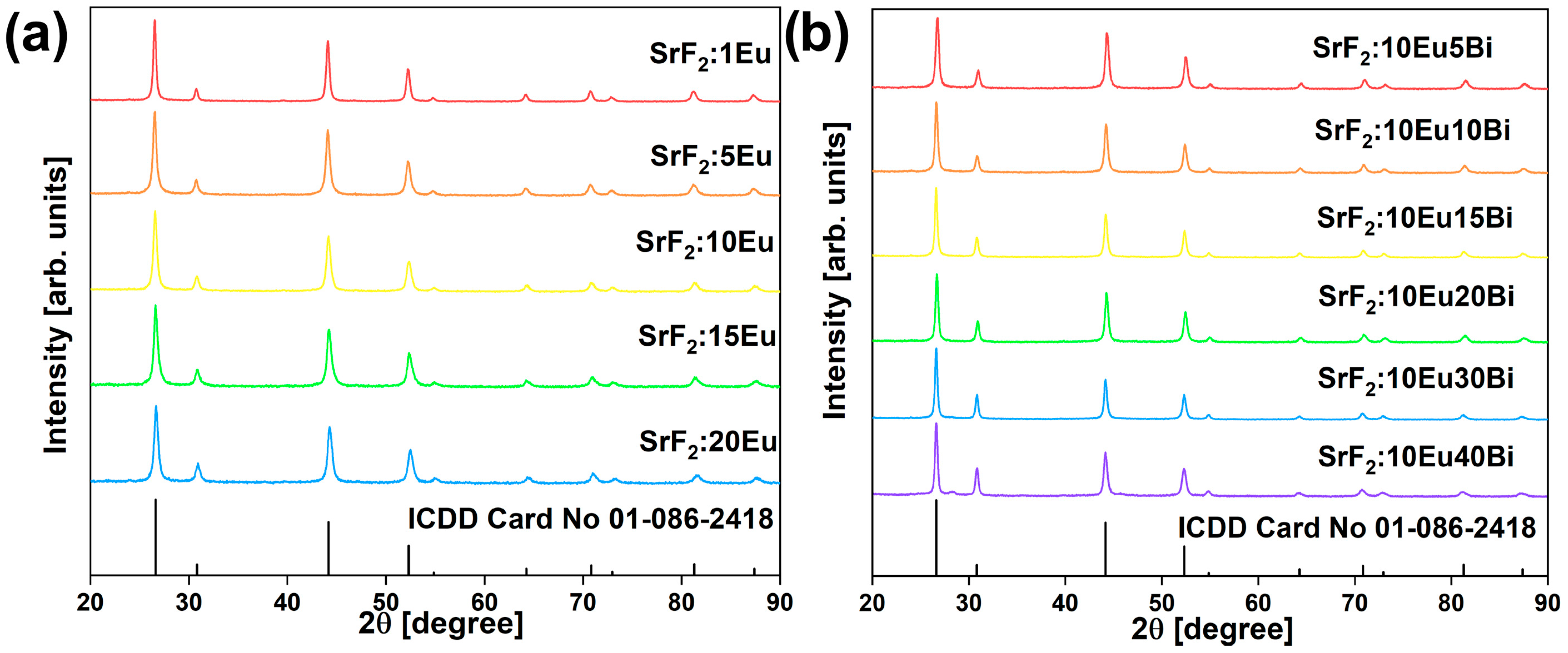
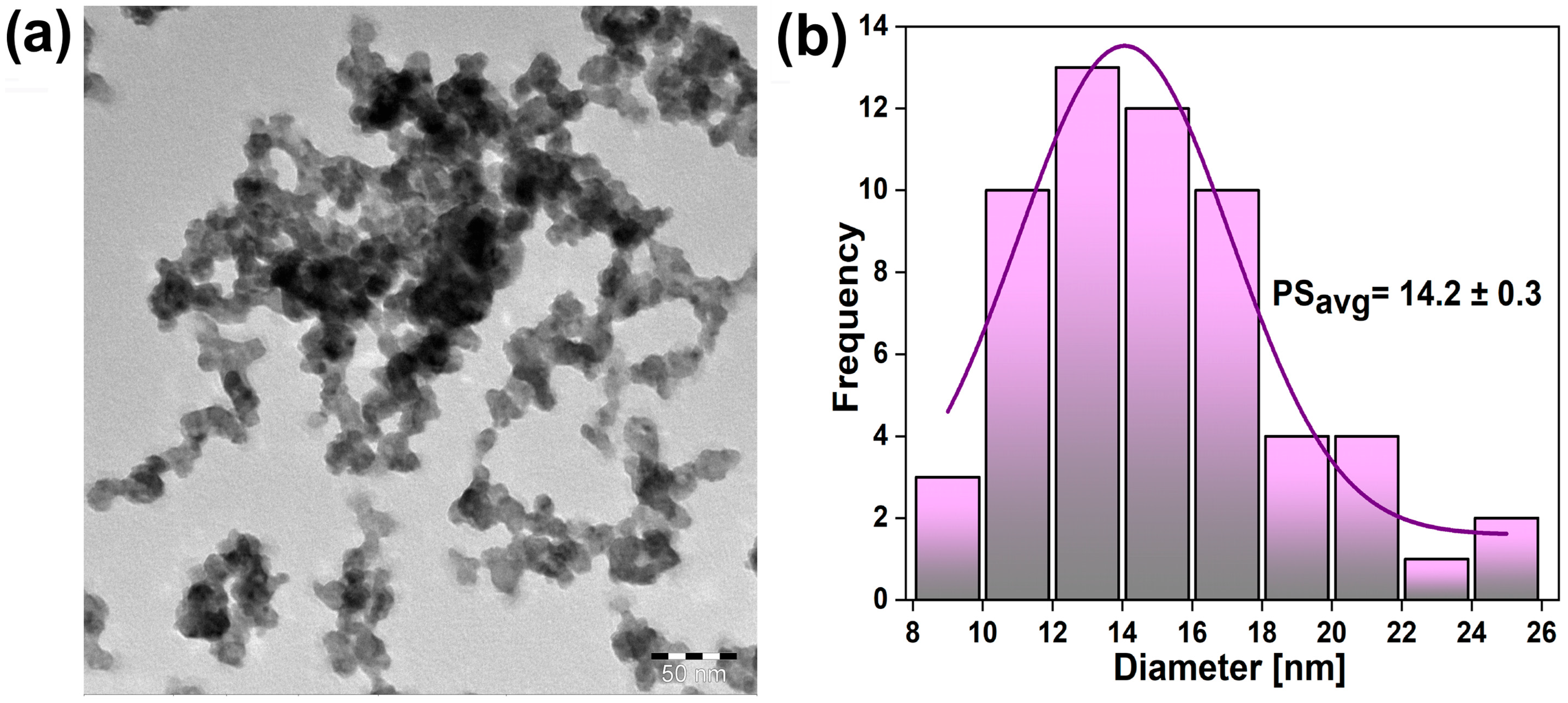
3.1. Structure and Morphology
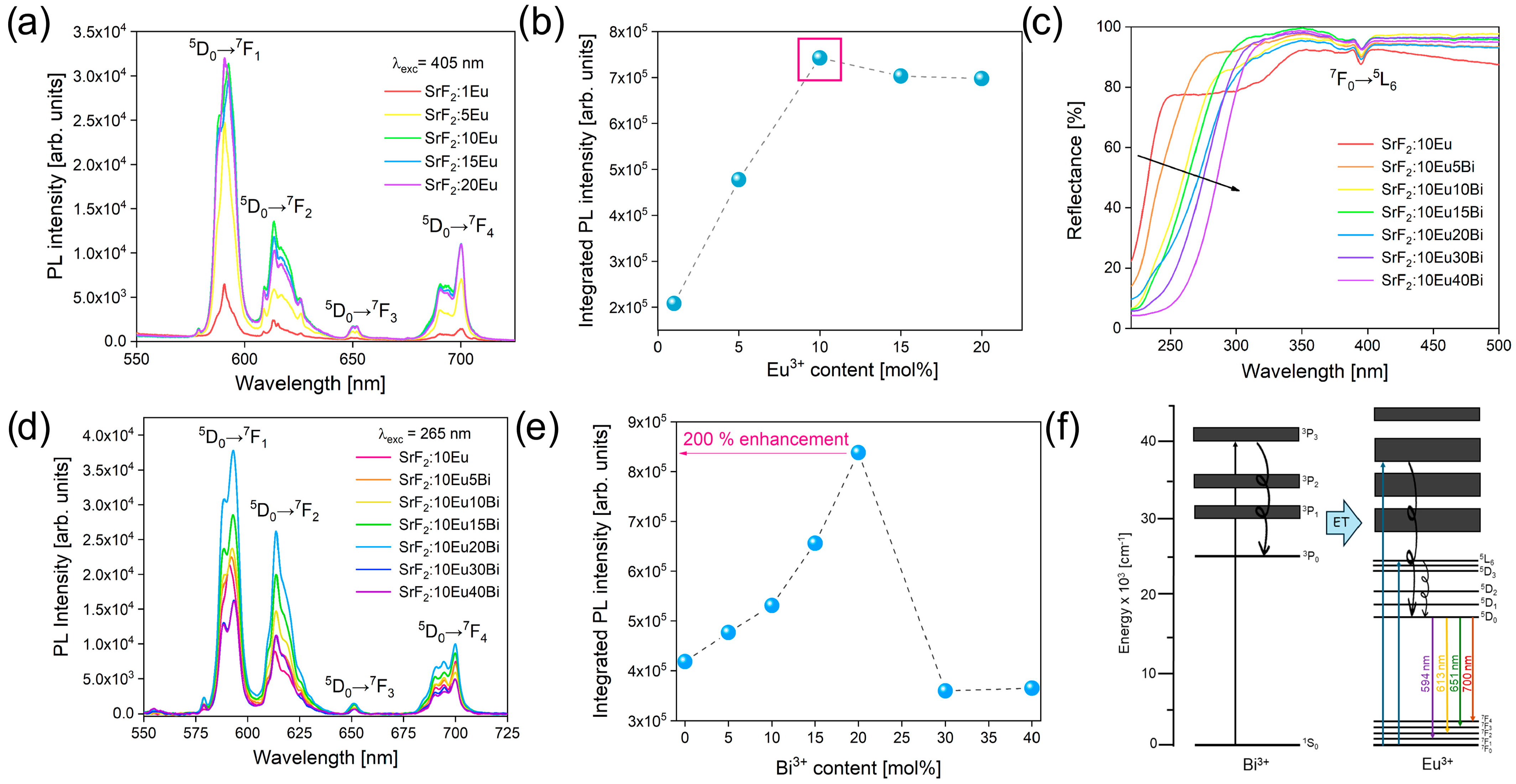
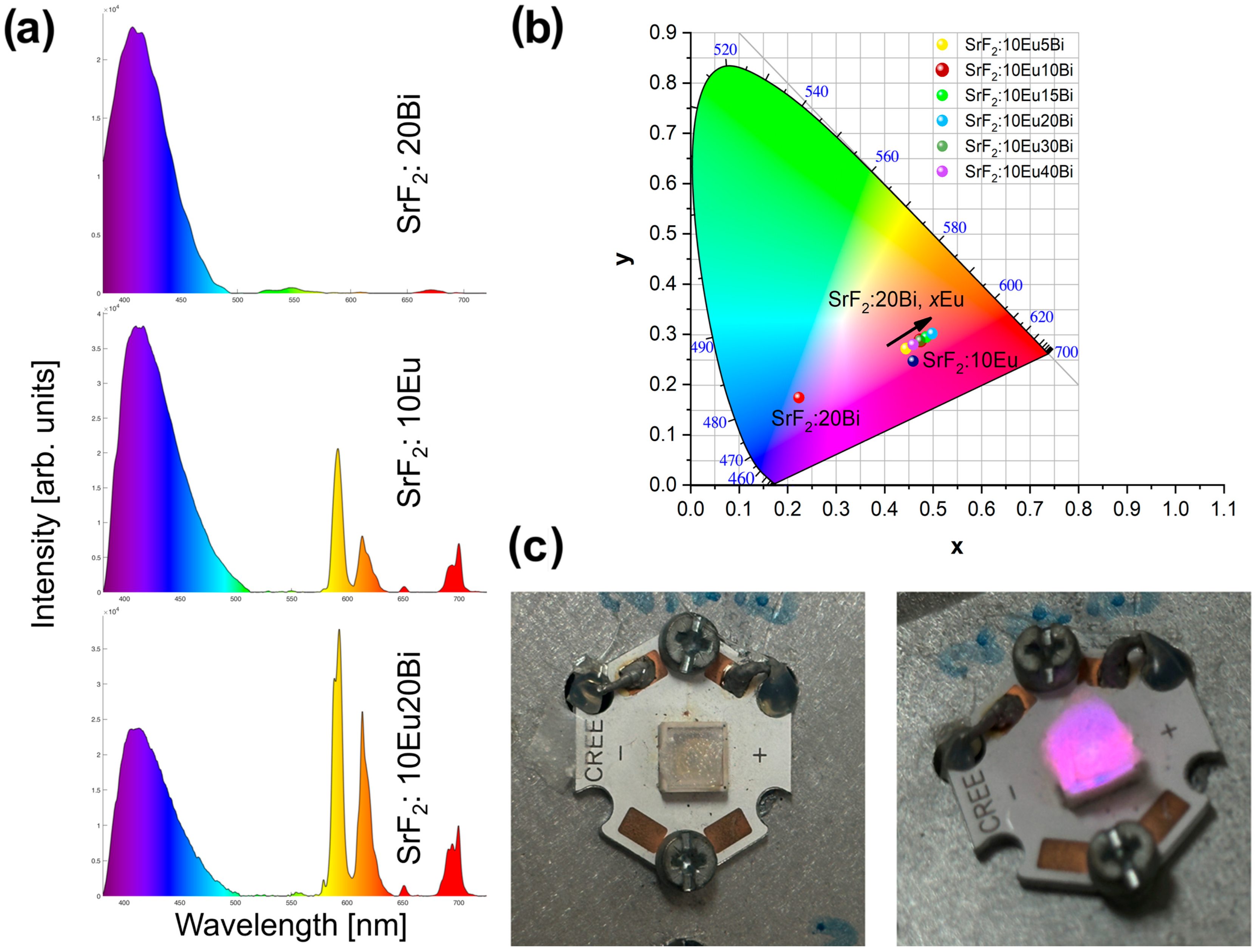
3.2. Photoluminescence Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McCree, K.J. The Action Spectrum, Absorptance and Quantum Yield of Photosynthesis in Crop Plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Gao, Q.; Liao, Q.; Li, Q.; Yang, Q.; Wang, F.; Li, J. Effects of LED Red and Blue Light Component on Growth and Photosynthetic Characteristics of Coriander in Plant Factory. Horticulturae 2022, 8, 1165. [Google Scholar] [CrossRef]
- Sena, S.; Kumari, S.; Kumar, V.; Husen, A. Light Emitting Diode (LED) Lights for the Improvement of Plant Performance and Production: A comprehensive review. Curr. Res. Biotechnol. 2024, 7, 100184. [Google Scholar] [CrossRef]
- Fang, S.; Lang, T.; Cai, M.; Han, T. Light Keys Open Locks of Plant Photoresponses: A Review of Phosphors for Plant Cultivation LEDs. J. Alloys Compd. 2022, 902, 163825. [Google Scholar] [CrossRef]
- Zhen, S.; Bugbee, B. Far-red photons have equivalent efficiency to traditional photosynthetic photons: Implications for redefining photosynthetically active radiation. Plant Cell Environ. 2020, 43, 259–1272. [Google Scholar] [CrossRef]
- Chen, X.L.; Li, Y.L.; Wang, L.C.; Guo, W.Z. Red and Blue Wavelengths Affect the Morphology, Energy Use Efficiency and Nutritional Content of Lettuce (Lactuca sativa L.). Sci. Rep. 2021, 11, 8374. [Google Scholar] [CrossRef]
- Wollaeger, H.M.; Runkle, E.S. Growth of Impatiens, Petunia, Salvia, and Tomato Seedlings Under Blue, Green, and Red Light-Emitting Diodes. Hortic. Sci. 2014, 49, 734–740. [Google Scholar]
- Hogewoning, S.; Trouwborst, G.; Maljaars, H.; Poorter, H.; Van Ieperen, W.; Harbinson, J. Blue Light Dose-Responses of Leaf Photosynthesis, Morphology, and Chemical Composition of Cucumis sativus Grown Under Different Combinations of Red and Blue Light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Trivellini, A.; Toscano, S.; Romano, D.; Ferrante, A. The Role of Blue and Red Light in the Orchestration of Secondary Metabolites, Nutrient Transport and Plant Quality. Plants 2023, 12, 2026. [Google Scholar] [CrossRef]
- Islam, M.A.; Kuwar, G.; Clarke, J.L.; Blystad, D.R.; Gislerød, H.R.; Olsen, J.E.; Torre, S. Artificial Light from Light Emitting Diodes (LEDs) with a High Portion of Blue Light Results in Shorter Poinsettias Compared to High Pressure Sodium (HPS) Lamps. Sci. Hortic. 2012, 147, 136–143. [Google Scholar] [CrossRef]
- Owen, W.G.; Lopez, R.G. Comparison of Sole-Source and Supplemental Lighting on Callus Formation and Initial Rhizogenesis of Gaura and Salvia Cuttings. Hortic. Sci. 2019, 54, 684–691. [Google Scholar] [CrossRef]
- Yang, J.; Li, C.; Kong, D.; Guo, F.; Wei, H. Light-Mediated Signaling and Metabolic Changes Coordinate Stomatal Opening and Closure. Front. Plant Sci. 2020, 11, 601478. [Google Scholar] [CrossRef]
- Serna-Gallén, P.; Beltrán-Mir, H.; Cordoncillo, E. Practical Guidance for Easily interpreting the Emission and Physicochemical Parameters of Eu3+ in Solid-State Hosts. Ceram. Int. 2023, 49, 41078–41089. [Google Scholar] [CrossRef]
- Dang, P.; Liang, S.; Li, G.; Wei, Y.; Cheng, Z.; Lian, H.; Shang, M.; Hod, S.J.; Lin, J. Controllable Optical Tuning and Improvement in Li+,Eu3+-codoped BaSc2O4:Bi3+ Based on Energy Transfer and Charge Compensation. J. Mater. Chem. C 2018, 6, 6449–6459. [Google Scholar] [CrossRef]
- Liu, M.; Yang, C.; Liu, W.; Zhou, X.; Liu, S.; You, Q.; Jiang, X. Synthesis of Bi3+ and Eu3+ Co-doped Na4CaSi3O9 Blue-Red Light Tunable Emission Phosphors for Inducing Plant Growth. Ceram. Int. 2024, 50, 9058–9069. [Google Scholar] [CrossRef]
- Wang, H.; Chen, X.Y.; Teng, L.M.; Xu, D.K.; Chen, W.P.; Wei, R.F.; Hu, F.F.; Sun, X.Y.; Guo, H. Adjustable Emission and Energy Transfer Process in BaGd2O4:Bi3+,Eu3+ Phosphors. J. Lumin. 2019, 206, 185–191. [Google Scholar] [CrossRef]
- Guo, Y.; Park, S.H.; Choi, B.C.; Jeong, J.H.; Kim, J.H. Dual-Mode Manipulating Multicenter Photoluminescence in a Single-Phased Ba9Lu2Si6O24:Bi3+, Eu3+ Phosphor to Realize White Light/Tunable Emissions. Sci. Rep. 2017, 7, 15884. [Google Scholar] [CrossRef]
- Kong, H.; Jia, G.; Li, H.; Meng, Z.; Zhang, N.; Zhang, C. Deep Blue, Cyan, Orange-Red, and White Multicolor Emissions Generated by Bi3+/Eu3+ Activated KBaYSi2O7 Luminescent Materials for White Light-Emitting Diodes. Ceram. Int. 2023, 49, 15320–15332. [Google Scholar] [CrossRef]
- Sun, L.; Devakumar, B.; Liang, J.; Wang, S.; Sun, Q.; Huang, X. Novel High-Efficiency Violet-Red Dual-Emitting Lu2GeO5: Bi3+, Eu3+ Phosphors for Indoor Plant Growth Lighting. J. Lumin. 2019, 214, 116544. [Google Scholar] [CrossRef]
- Du, C.; Gao, D.; Hou, X.; Zhang, X.; Pang, Q.; Yun, S. Deep trap engineering in Gd3GaO6:Bi3+ persistent phosphors through co-doping lanthanide ions. J. Mater. Chem. C 2024, 12, 9284–9292. [Google Scholar] [CrossRef]
- Krishnaiah, K.V.; Venkatalakshmamma, P.; Kumar, K.U.; Haritha, P.; Lavin, V.; Martin, I.R.; Ravi, N.; Reddy, H.S.K.; Venkatramu, V.; Nallabala, N.K.R.; et al. Structure, Morphology, Photonconversion and Energy Tansfer Characteristics of Er3+/Yb3+:BaYF5 Nanocrystals Synthesized by Hydrothermal Method for Photovoltaics. Ceramamics Int. 2023, 49, 26879–26889. [Google Scholar] [CrossRef]
- Cortelletti, P.; Pedroni, M.; Boschi, F.; Pin, S.; Ghigna, P.; Canton, P.; Vetrone, F.; Speghini, A. Luminescence of Eu3+ Activated CaF2 and SrF2 Nanoparticles: Effect of the Particle Size and Codoping with Alkaline Ions. Cryst. Growth Des. 2018, 18, 686–694. [Google Scholar] [CrossRef]
- Đačanin Far, L.J.; Zeković, I.; Periša, J.; Ristić, Z.; Alodhayb, A.; Dramićanin, M.D.; Antić, Ž. Luminescent Eu3+ Doped SrF2 Nanoparticles for Fluorescent Detection of Fertilizers. Opt. Mater. 2023, 142, 114061. [Google Scholar] [CrossRef]
- Lorbeer, C.; Behrends, F.; Cybinska, J.; Eckert, H.; Mudring, A.-V. Charge Compensation in RE3+ (RE = Eu, Gd) and M+ (M = Li, Na, K) Do-doped Alkaline Earth Nanofluorides Obtained by Microwave Reaction with Reactive Ionic Liquids Leading to Improved Optical Properties. J. Mater. Chem. C 2014, 2, 9439–9450. [Google Scholar] [CrossRef]
- Mancebo, D.G.; Becerro, A.I.; Corral, A.; Moros, M.; Balcerzyk, M.; de la Fuente, J.M.; Ocaña, M. Enhancing Luminescence and X-ray Absorption Capacity of Eu3+:LaF3 Nanoparticles by Bi3+ Codoping. ACS Omega 2019, 4, 765–774. [Google Scholar] [CrossRef]
- Luo, R.; Li, Q.; Wang, J.; Ning, Z.; Zhao, Y.; Liu, M.; Lai, X.; Zhong, C.; Bi, J.; Gao, D. Bi3+ Ion Doping: An Effective Strategy to Significantly Enhance Luminescence Performances of NaGdF4: Eu3+ Red-emitting Nanocrystals. J. Alloys Compd. 2020, 828, 154375. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, X.; Zhang, J. The effects of structural characterization on the luminescence of Eu3+-doped fluoride nano/microcrystals. CrystEngComm 2014, 16, 11115–11121. [Google Scholar] [CrossRef]
- Forsyth, J.B.; Wilson, C.C.; Sabine, T.M. Time-of-Flight Neutron Diffraction Study of Anharmonic Thermal Vibrations in SrF2, at the Spallation Neutron Source ISIS. Acta Crystallogr. Sect. A Found. Adv. 1989, 45, 244. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, Y.; Zhang, G.-B.; Wu, C.; Yang, G.-T.; Wang, C.; Li, G.-H. Concentration and Temperature Dependences of YBO3:Bi3+ Luminescence Under Vacuum Ultraviolet Excitation. Chin. Phys. Lett. 2008, 25, 1884–1887. [Google Scholar] [CrossRef]
- Sontakke, A.D.; Tarafder, A.; Biswas, K.; Annapurna, K. Sensitized Red Luminescence from Bi3+ Do-Doped Eu3+: ZnO–B2O3 Glasses. Phys. B Condens. Matter 2009, 404, 3525–3529. [Google Scholar] [CrossRef]
- Weber, M.J.; Monchamp, R.R. Luminescence of Bi4Ge3O12: Spectral and Decay Properties. J. Appl. Phys. 1973, 44, 5495–5499. [Google Scholar] [CrossRef]
- Hull, R.; Parisi, J.; Osgood, R.M.; Warlimont, H.; Liu, G.; Jacquier, B. (Eds.) Spectroscopic Properties of Rare Earths in Optical Materials; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar] [CrossRef]
- Bourcet, J.C.; Moine, B.; Boulon, G.; Reisfeld, R.; Kalisky, Y. Energy Transfer between Bi3+ and Eu3+ in Germanite Glasses using Time Resolved Spectroscopy. Chem. Phys. Lett. 1979, 61, 23–24. [Google Scholar] [CrossRef]
- Cao, R.; Liu, N.; Zhong, Q.; Tu, Y.; Xu, Y.; Zhang, H.; Luo, W.; Chen, T. Luminescence Properties and Tunable Emission of Ca3MgSi2O8:Eu3+, Bi3+ Phosphor with Bi3+ → Eu3+ Energy Transfer. J. Mater. Sci. Mater. Electron. 2021, 32, 26620–26630. [Google Scholar] [CrossRef]
- Giraldo, O.; Fei, M.; Wei, R.; Liming, T.; Zheng, Z.; Guo, H. Energy Transfer and White Luminescence in Bi3+/Eu3+ Co-Doped Oxide Glasses. J. Lumin. 2020, 219, 116918. [Google Scholar] [CrossRef]
| Sample | Abbreviated Name | Sr(NO3)2 (g) | Eu(NO3)3·6H2O (g) | NaF (g) | EG (ml) |
|---|---|---|---|---|---|
| Sr0.99Eu0.01F2 | SrF2:1Eu | 0.2095 | 0.00446 | 0.0840 | 15 |
| Sr0.95Eu0.05F2 | SrF2:5Eu | 0.2010 | 0.0223 | ||
| Sr0.9Eu0.1F2 | SrF2:10Eu | 0.1905 | 0.0446 | ||
| Sr0.85Eu0.15F2 | SrF2:15Eu | 0.1799 | 0.0669 | ||
| Sr0.8Eu0.2F2 | SrF2:20Eu | 0.1693 | 0.0892 |
| Sample | Abbreviated Name | Sr(NO3)2 (g) | Eu(NO3)3·6H2O (g) | Bi(NO3)3·5H2O (g) | NaF (g) | EG (ml) |
|---|---|---|---|---|---|---|
| Sr0.4Eu0.1Bi0.5F2 | SrF2:10Eu5Bi | 0.1799 | 0.0446 | 0.0243 | 0.0840 | 15 |
| Sr0.8Eu0.1Bi0.1F2 | SrF2:10Eu10Bi | 0.1693 | 0.0485 | |||
| Sr0.75Eu0.1Bi0.15F2 | SrF2:10Eu15Bi | 0.1587 | 0.0725 | |||
| Sr0.7Eu0.1Bi0.2F2 | SrF2:10Eu20Bi | 0.1481 | 0.0970 | |||
| Sr0.6Eu0.1Bi0.3F2 | SrF2:10Eu30Bi | 0.1269 | 0.1455 | |||
| Sr0.5Eu0.1Bi0.4F2 | SrF2:10Eu40Bi | 0.1058 | 0.1940 | |||
| Sr0.4Eu0.1Bi0.5F2 | SrF2:10Eu50Bi | 0.0846 | 0.2425 |
| Sample | % Blue | % Red | CIE (x, y) Coordinates |
|---|---|---|---|
| SrF2:20Bi | 100.0 | 0.0 | (0.223, 0.174) |
| SrF2:10Eu | 85.0 | 15.0 | (0.399, 0.247) |
| SrF2:10Eu5Bi | 76.5 | 23.5 | (0.444, 0.271) |
| SrF2:10Eu10Bi | 71.6 | 28.4 | (0.474, 0.288) |
| SrF2:10Eu15Bi | 61.9 | 38.1 | (0.486, 0.294) |
| SrF2:10Eu20Bi | 59.2 | 40.8 | (0.498, 0.301) |
| SrF2:10Eu30Bi | 66.7 | 33.3 | (0.473, 0.287) |
| SrF2:10Eu40Bi | 73.1 | 26.9 | (0.459, 0.279) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Periša, J.; Kuzman, S.; Ćirić, A.; Ristić, Z.; Antić, Ž.; Dramićanin, M.D.; Milićević, B. Tuneable Red and Blue Emission of Bi3+-Co-Doped SrF2:Eu3+ Nanophosphors for LEDs in Agricultural Applications. Nanomaterials 2024, 14, 1617. https://doi.org/10.3390/nano14201617
Periša J, Kuzman S, Ćirić A, Ristić Z, Antić Ž, Dramićanin MD, Milićević B. Tuneable Red and Blue Emission of Bi3+-Co-Doped SrF2:Eu3+ Nanophosphors for LEDs in Agricultural Applications. Nanomaterials. 2024; 14(20):1617. https://doi.org/10.3390/nano14201617
Chicago/Turabian StylePeriša, Jovana, Sanja Kuzman, Aleksandar Ćirić, Zoran Ristić, Željka Antić, Miroslav D. Dramićanin, and Bojana Milićević. 2024. "Tuneable Red and Blue Emission of Bi3+-Co-Doped SrF2:Eu3+ Nanophosphors for LEDs in Agricultural Applications" Nanomaterials 14, no. 20: 1617. https://doi.org/10.3390/nano14201617
APA StylePeriša, J., Kuzman, S., Ćirić, A., Ristić, Z., Antić, Ž., Dramićanin, M. D., & Milićević, B. (2024). Tuneable Red and Blue Emission of Bi3+-Co-Doped SrF2:Eu3+ Nanophosphors for LEDs in Agricultural Applications. Nanomaterials, 14(20), 1617. https://doi.org/10.3390/nano14201617









