Facile Preparation of Three-Dimensional Cubic MnSe2/CNTs and Their Application in Aqueous Copper Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
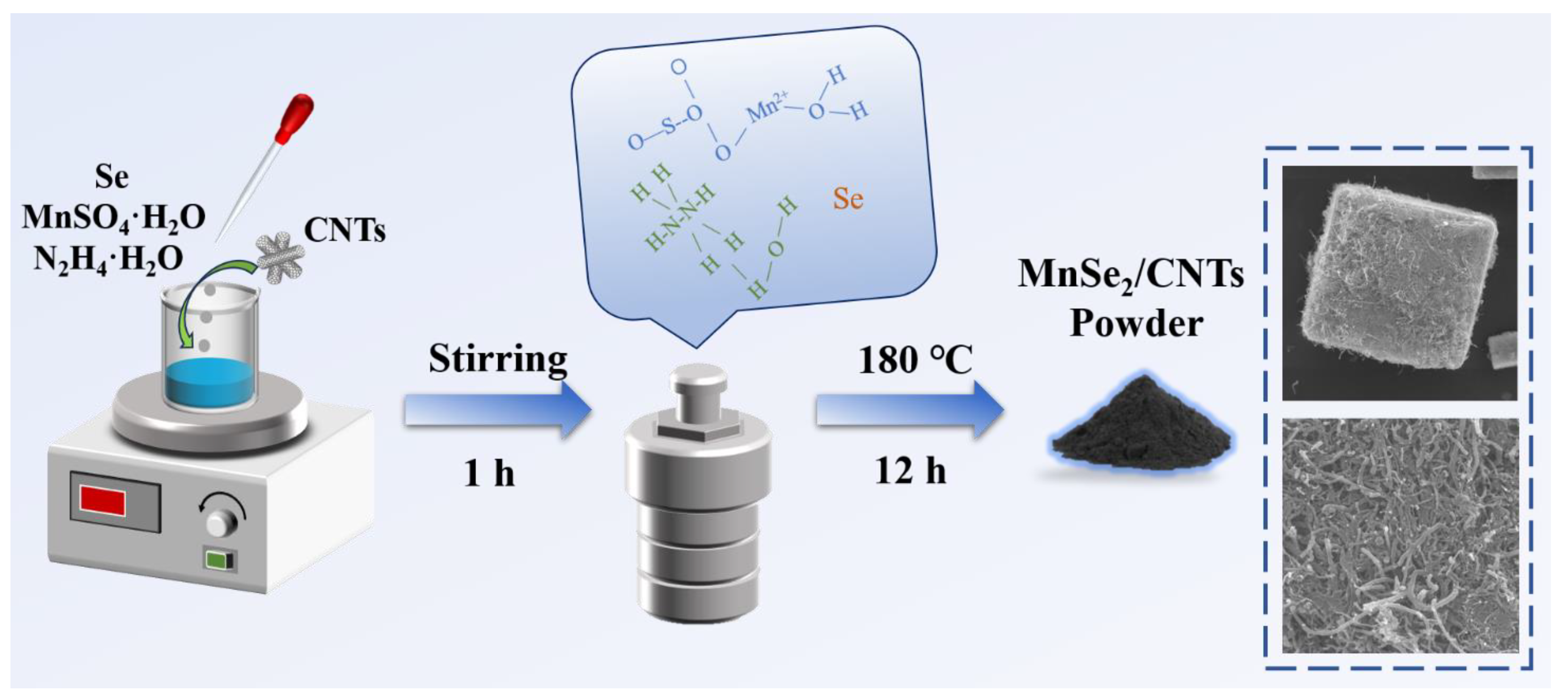
2.2. Synthesis of MnSe2/CNTs Composites
2.3. Materials Characterizations
2.4. Electrochemical Measurements
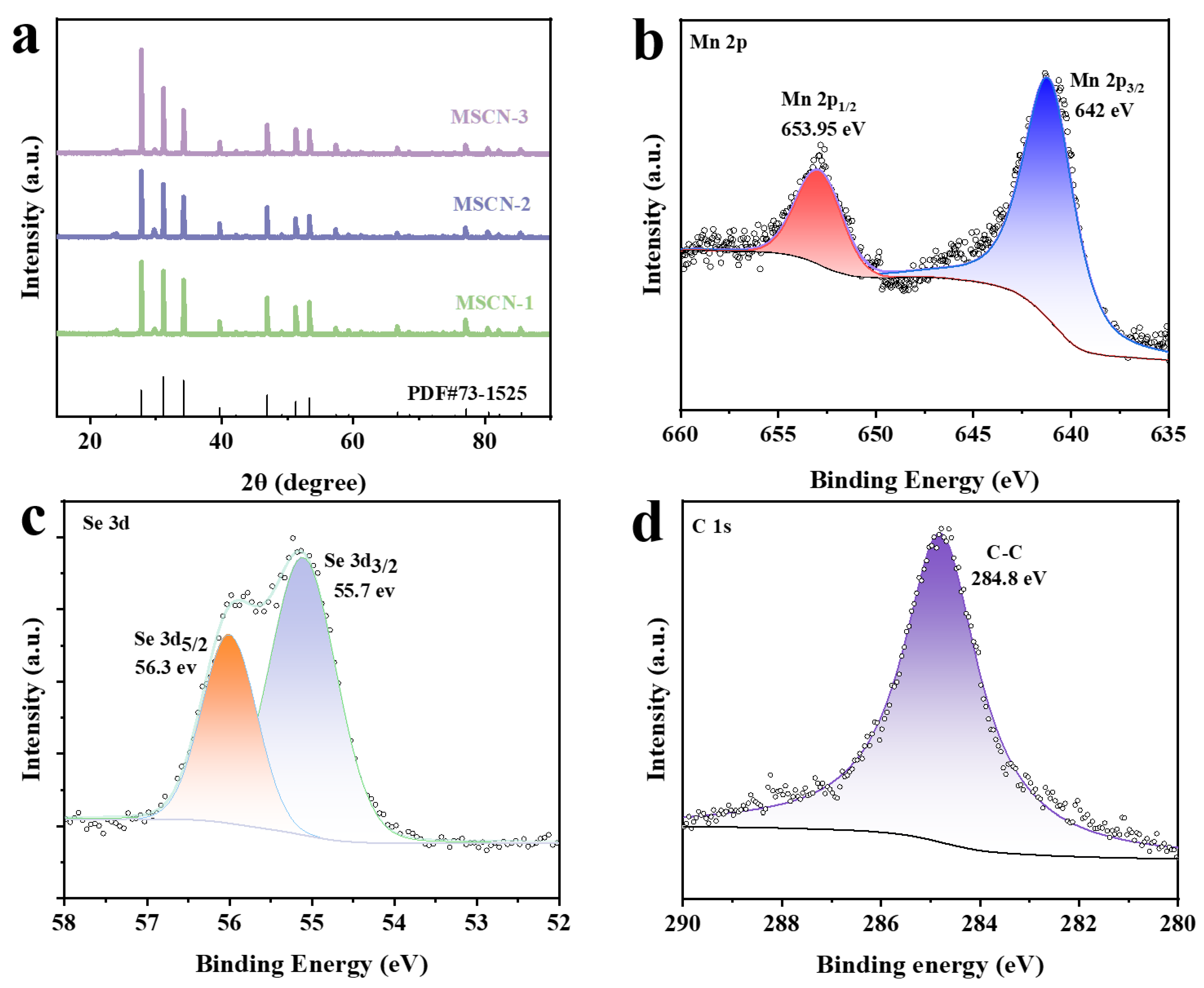
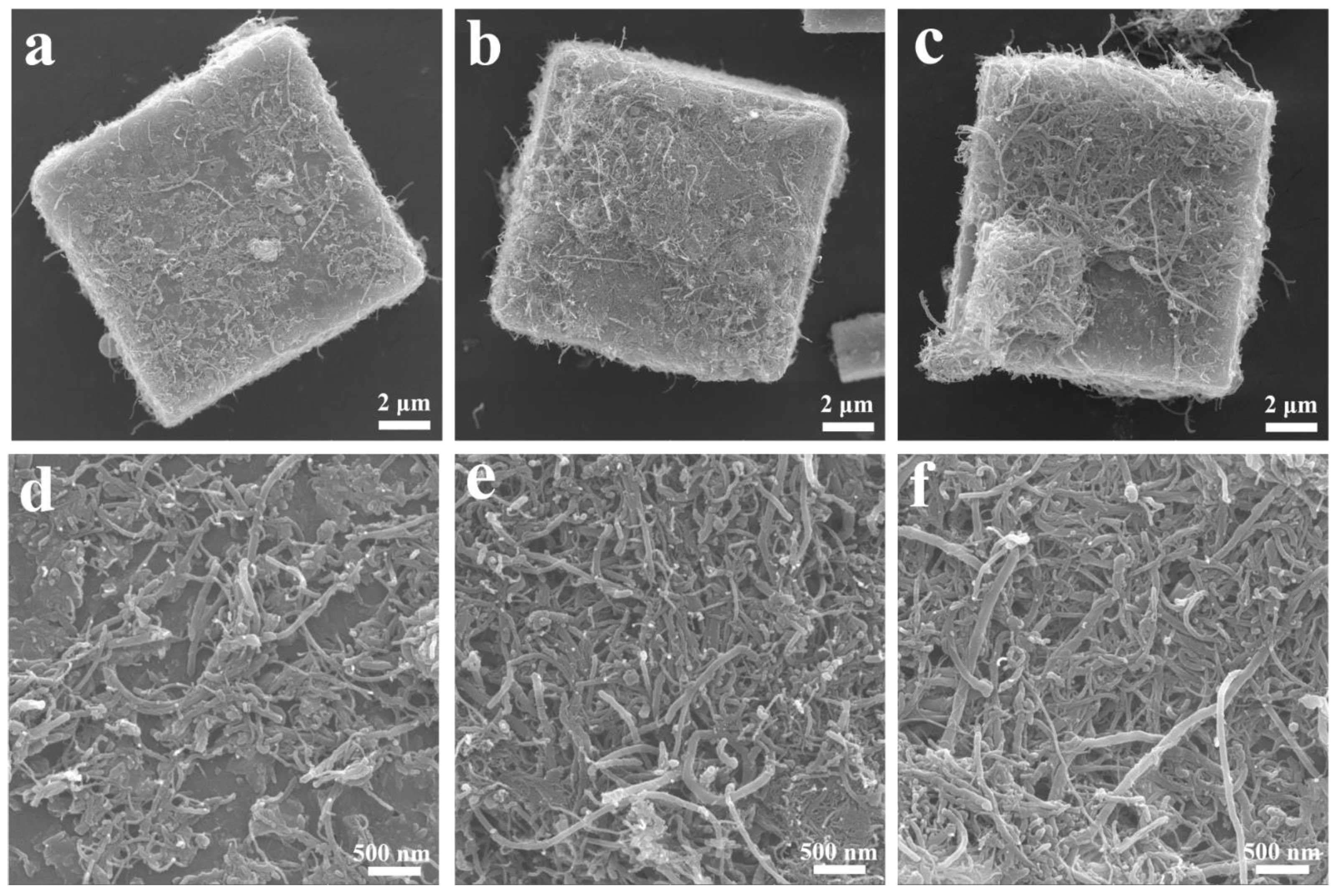
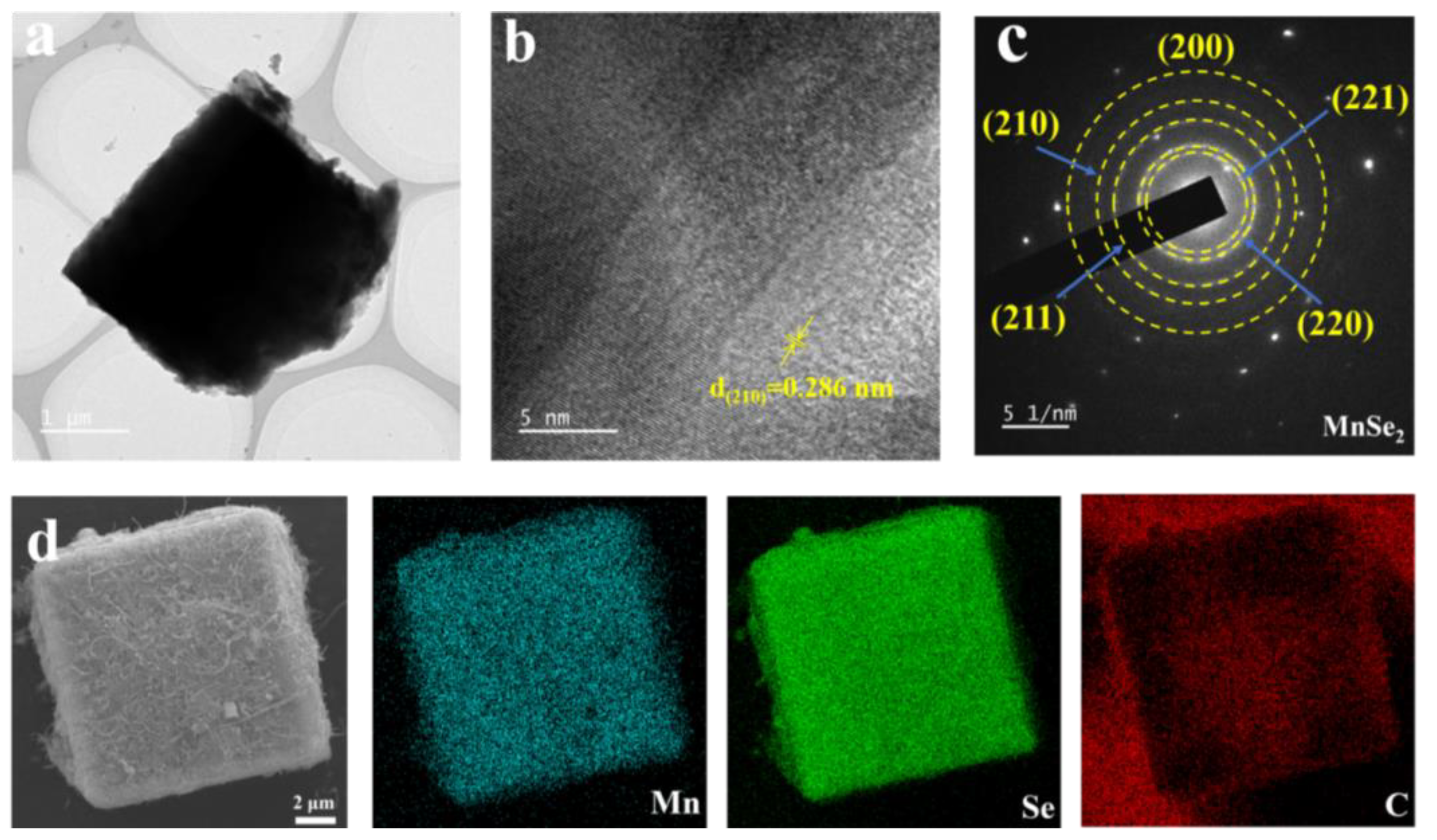
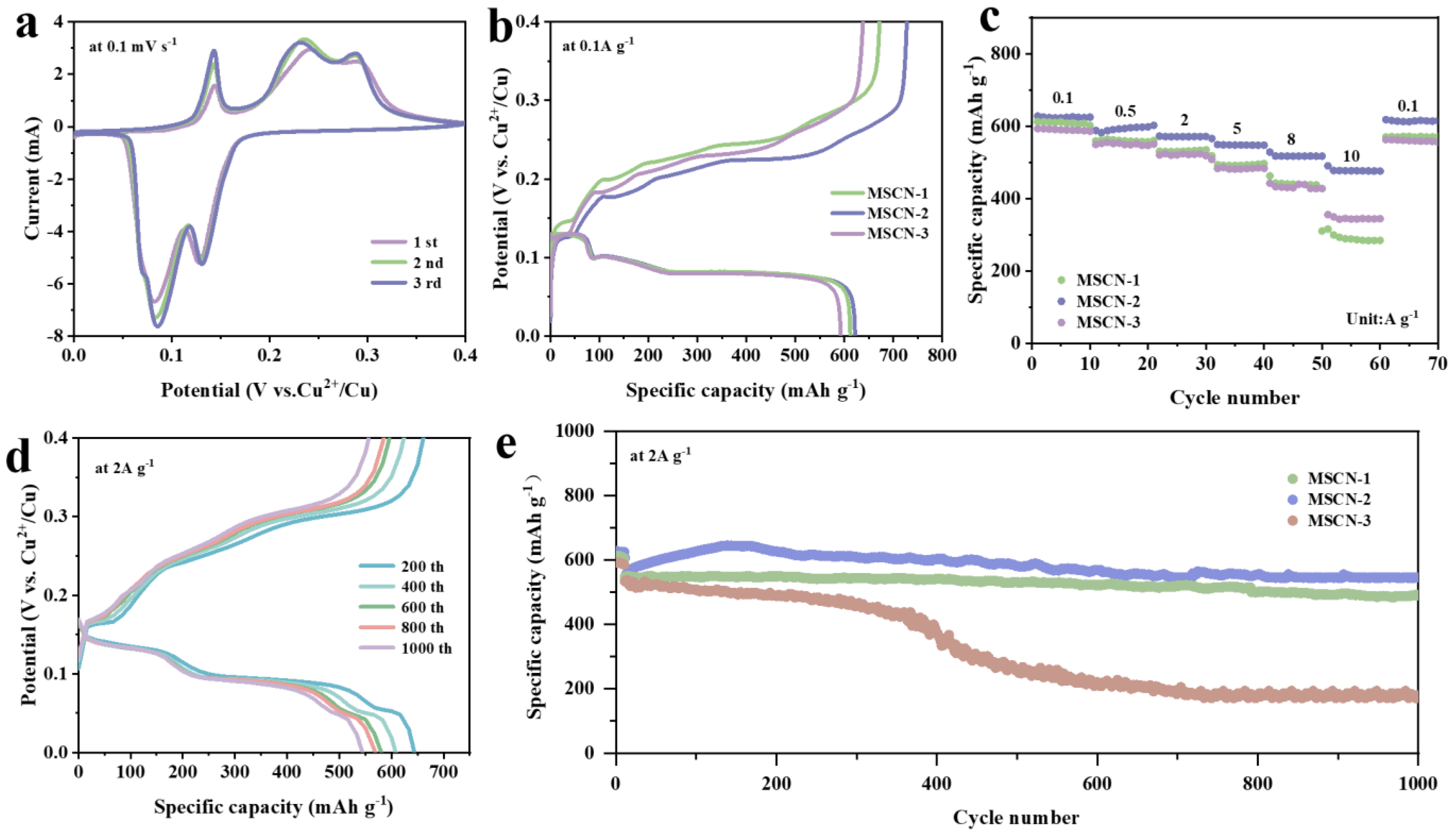
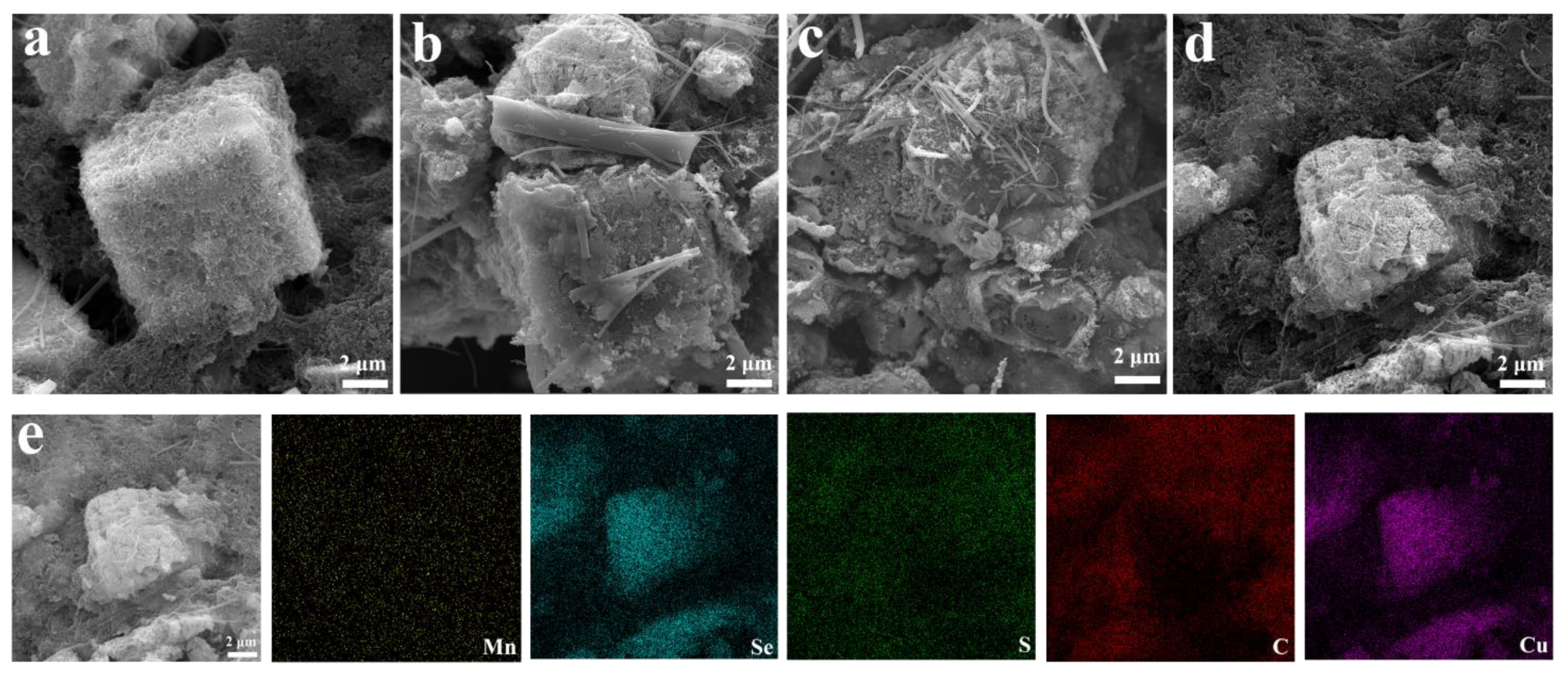
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fan, X.; Xiang, K.; Zhou, W.; Deng, W.; Zhu, H.; Chen, L.; Chen, H. A novel improvement strategy and a comprehensive mechanism insight for α-MnO2 energy storage in rechargeable aqueous zinc-ion batteries. Carbon Energy 2024, 6, e536. [Google Scholar] [CrossRef]
- Guo, Z.; Han, X.; Zhang, C.; He, S.; Liu, K.; Hu, J.; Yang, W.; Jian, S.; Jiang, S.; Duan, G. Activation of biomass-derived porous carbon for supercapacitors: A review. Chin. Chem. Lett. 2024, 35, 109007. [Google Scholar] [CrossRef]
- Shang, Z.; An, X.; Nie, S.; Li, N.; Cao, H.; Cheng, Z.; Liu, H.; Ni, Y.; Liu, L. Design of B/N Co-doped micro/meso porous carbon electrodes from CNF/BNNS/ZIF-8 nanocomposites for advanced supercapacitors. J. Bioresour. Bioprod. 2023, 8, 292–305. [Google Scholar] [CrossRef]
- Guo, F.; He, W.; Wang, R.; Wei, B.; Liang, S.; Qiulin, J.; Hu, G.; Li, W. Preparation of nano-structured wood aerogel-based composite electrode and its electrochemical performance. J. For. Eng. 2023, 8, 122–130. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Liu, Y.; Duan, G.; Liang, Z.; Han, J.; Huang, Y.; Han, X.; Zhang, C.; He, S.; et al. Design of cyclic carbonate-based electrolytes for HC anodes towards improved low-temperature performance in lithium-ion batteries system. Fuel 2025, 379, 133048. [Google Scholar] [CrossRef]
- Wen, X.; Luo, J.; Xiang, K.; Zhou, W.; Zhang, C.; Chen, H. High-performance monoclinic WO3 nanospheres with the novel NH4+ diffusion behaviors for aqueous ammonium-ion batteries. Chem. Eng. J. 2023, 458, 141381. [Google Scholar] [CrossRef]
- Huang, Y.-z.; Xing, L.; Pei, S.; Zhou, W.; Hu, Y.-j.; Deng, W.-N.; Chen, L.; Zhu, H.; Chen, H. Co9S8/CNTs microspheres as superior-performance cathodes in aqueous ammonium-ion batteries. Trans. Nonferrous Met. Soc. China 2023, 33, 3452–3464. [Google Scholar] [CrossRef]
- Guo, T.; Xiang, K.; Wen, X.; Zhou, W.; Chen, H. Facile construction on flower-like CuS microspheres and their applications for the high-performance aqueous ammonium-ion batteries. Mater. Res. Bull. 2024, 170, 112595. [Google Scholar] [CrossRef]
- Liu, Y.; Xiang, K.; Zhou, W.; Deng, W.; Zhu, H.; Chen, H. Investigations on Tunnel-Structure MnO2 for Utilization as a High-Voltage and Long-Life Cathode Material in Aqueous Ammonium-Ion and Hybrid-Ion Batteries. Small 2024, 20, 2308741. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, H.; Zhang, W.; Lu, Y. Bioanode boosts efficacy of chlorobenzenes-powered microbial fuel cell: Performance, kinetics, and mechanism. Bioresour. Technol. 2024, 405, 130936. [Google Scholar] [CrossRef]
- Li, L.; Nam, J.S.; Kim, M.S.; Wang, Y.; Jiang, S.; Hou, H.; Kim, I.D. Sulfur–Carbon Electrode with PEO-LiFSI-PVDF Composite Coating for High-Rate and Long-Life Lithium–Sulfur Batteries. Adv. Energy Mater. 2023, 13, 2302139. [Google Scholar] [CrossRef]
- Tian, Z.; Yang, C.; Zhang, C.; Han, X.; Han, J.; Liu, K.; He, S.; Duan, G.; Jian, S.; Hu, J.; et al. In-situ activation of resorcinol-furfural resin derived hierarchical porous carbon for supercapacitors and zinc-ion hybrid capacitors. J. Energy Storage 2024, 85, 111130. [Google Scholar] [CrossRef]
- Andrade, A.M.; Liu, Z.; Grewal, S.; Nelson, A.J.; Nasef, Z.; Diaz, G.; Lee, M.H. MOF-derived Co/Cu-embedded N-doped carbon for trifunctional ORR/OER/HER catalysis in alkaline media. Dalton Trans. 2021, 50, 5473–5482. [Google Scholar] [CrossRef]
- Jiang, L.; Wu, Z.; Wang, Y.; Tian, W.; Yi, Z.; Cai, C.; Jiang, Y.; Hu, L. Ultrafast zinc-ion diffusion ability observed in 6.0-nanometer spinel nanodots. ACS Nano 2019, 13, 10376–10385. [Google Scholar] [CrossRef]
- Wang, L.; Shi, J.L.; Su, H.; Li, G.; Zubair, M.; Guo, Y.G.; Yu, H. Composite-structure material design for high-energy lithium storage. Small 2018, 14, 1800887. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, Z.; Lan, D.; Liu, Y.; Ma, L.; Cui, J. N-doped carbon/V2O3 microfibers as high-rate and ultralong-life cathode for rechargeable aqueous zinc-ion batteries. J. Alloys Compd. 2021, 861, 158560. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Lv, T.-T.; Wang, H.; Guo, X.-T.; Liu, C.-S.; Pang, H. Nsutite-type VO2 microcrystals as highly durable cathode materials for aqueous zinc-Ion batteries. Chem. Eng. J. 2021, 417, 128408. [Google Scholar] [CrossRef]
- Liang, Y.; Dong, H.; Aurbach, D.; Yao, Y. Current status and future directions of multivalent metal-ion batteries. Nat. Energy 2020, 5, 646–656. [Google Scholar] [CrossRef]
- Wang, D.; Lv, H.; Hussain, T.; Yang, Q.; Liang, G.; Zhao, Y.; Ma, L.; Li, Q.; Li, H.; Dong, B. A manganese hexacyanoferrate framework with enlarged ion tunnels and two-species redox reaction for aqueous Al-ion batteries. Nano Energy 2021, 84, 105945. [Google Scholar] [CrossRef]
- Cai, H.; Bi, S.; Wang, R.; Liu, L.; Niu, Z. A lattice-matching strategy for highly reversible copper-metal anodes in aqueous batteries. Angew. Chem. Int. Ed. 2022, 61, e202205472. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, C.; Yan, H.; Liu, Y.; Yue, C.; Zhang, L.; Shui, M.; Hu, F.; Shu, J. Laser-induced graphene assisting self-conversion reaction for sulfur-free aqueous Cu-S battery. Adv. Funct. Mater. 2021, 31, 2103893. [Google Scholar] [CrossRef]
- Wu, X.; Markir, A.; Ma, L.; Xu, Y.; Jiang, H.; Leonard, D.P.; Shin, W.; Wu, T.; Lu, J.; Ji, X. A four-electron sulfur electrode hosting a Cu2+/Cu+ redox charge carrier. Angew. Chem. Int. Ed. 2019, 58, 12640–12645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Yu, M.; Ni, J.; Li, L. Understanding the role of topotactic anion exchange in the robust Cu ion storage of CuS1–x Se x. ACS Energy Lett. 2022, 7, 1835–1841. [Google Scholar] [CrossRef]
- Javed, M.S.; Najim, T.; Hussain, I.; Batool, S.; Idrees, M.; Mehmood, A.; Imran, M.; Assiri, M.A.; Ahmad, A.; Shah, S.S.A. 2D V2O5 nanoflakes as a binder-free electrode material for high-performance pseudocapacitor. Ceram. Int. 2021, 47, 25152–25157. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Zhong, Y.; Tade, M.; Zhou, W.; Shao, Z. Molecular design of mesoporous NiCo2O4 and NiCo2S4 with sub-micrometer-polyhedron architectures for efficient pseudocapacitive energy storage. Adv. Funct. Mater. 2017, 27, 1701229. [Google Scholar] [CrossRef]
- Javed, M.S.; Najam, T.; Hussain, I.; Shah, S.S.A.; Ibraheem, S.; Mahmood, A.; Imran, M.; Assiri, M.A.; Siyal, S.H. Novel 2D vanadium oxysulfide nano-spindles decorated carbon textile composite as an advanced electrode for high-performance pseudocapacitors. Mater. Lett. 2021, 303, 130478. [Google Scholar] [CrossRef]
- Hussain, I.; Mohamed, S.G.; Ali, A.; Abbas, N.; Ammar, S.M.; Al Zoubi, W. Uniform growth of Zn-Mn-Co ternary oxide nanoneedles for high-performance energy-storage applications. J. Electroanal. Chem. 2019, 837, 39–47. [Google Scholar] [CrossRef]
- Dan, H.; Tao, K.; Hai, Y.; Liu, L.; Gong, Y. (Co, Mn)-Doped NiSe2-diethylenetriamine (dien) nanosheets and (Co, Mn, Sn)-doped NiSe2 nanowires for high performance supercapacitors: Compositional/morphological evolution and (Co, Mn)-induced electron transfer. Nanoscale 2019, 11, 16810–16827. [Google Scholar] [CrossRef]
- Ni, D.; Chen, Y.; Yang, X.; Liu, C.; Cai, K. Microwave-assisted synthesis method for rapid synthesis of tin selenide electrode material for supercapacitors. J. Alloys Compd. 2018, 737, 623–629. [Google Scholar] [CrossRef]
- Ali, L.; Shittu, T.; Kuttiyathil, M.S.; Alam, A.; Iqbal, M.Z.; Khaleel, A.; Sivaramakrishnan, K.; Altarawneh, M. Catalytic upgrading of bio-oil from halophyte seeds into transportation fuels. J. Bioresour. Bioprod. 2023, 8, 444–460. [Google Scholar] [CrossRef]
- Sree Raj, K.; Shajahan, A.S.; Chakraborty, B.; Rout, C.S. Two-dimensional layered metallic VSe2/SWCNTs/rGO based ternary hybrid materials for high performance energy storage applications. Chem.—A Eur. J. 2020, 26, 6662–6669. [Google Scholar] [CrossRef]
- Li, M.; Bai, L.; Jiang, S.; Sillanpää, M.; Huang, Y.; Liu, Y. Electrocatalytic transformation of oxygen to hydroxyl radicals via three-electron pathway using nitrogen-doped carbon nanotube-encapsulated nickel nanocatalysts for effective organic decontamination. J. Hazard. Mater. 2023, 452, 131352. [Google Scholar] [CrossRef]
- Rout, C.S.; Mondal, S.; Samal, R.; Gangan, A.S.; Nayak, S.K.; Chakraborty, B. An experimental and computational study of enhanced charge storage capacity of chemical vapor deposited Ni3S2-reduced graphene oxide hybrids. Appl. Surf. Sci. 2019, 497, 143789. [Google Scholar] [CrossRef]
- Jiang, S.; Xie, M.; Jin, L.; Ren, Y.; Zheng, W.; Ji, S.; Liu, Y. New insights into electrocatalytic singlet oxygen generation for effective and selective water decontamination. Chin. Chem. Lett. 2024. [Google Scholar] [CrossRef]
- Samal, R.; Mondal, S.; Gangan, A.S.; Chakraborty, B.; Rout, C.S. Comparative electrochemical energy storage performance of cobalt sulfide and cobalt oxide nanosheets: Experimental and theoretical insights from density functional theory simulations. Phys. Chem. Chem. Phys. 2020, 22, 7903–7911. [Google Scholar] [CrossRef]
- Shi, X.; Wang, H.; Ji, S.; Linkov, V.; Liu, F.; Wang, R. CoNiSe2 nanorods directly grown on Ni foam as advanced cathodes for asymmetric supercapacitors. Chem. Eng. J. 2019, 364, 320–327. [Google Scholar] [CrossRef]
- Pathak, M.; Tamang, D.; Kandasamy, M.; Chakraborty, B.; Rout, C.S. A comparative experimental and theoretical investigation on energy storage performance of CoSe2, NiSe2 and MnSe2 nanostructures. Appl. Mater. Today 2020, 19, 100568. [Google Scholar] [CrossRef]
- Dong, H.; Li, X.; Niu, L.; Liu, Z. Preparation and electrochemical performance of nanocellulose/carbon nanotube carbon aerogel. J. For. Eng. 2023, 8, 121–129. [Google Scholar] [CrossRef]
- Javed, M.S.; Shah, S.S.A.; Hussain, S.; Tan, S.; Mai, W. Mesoporous manganese-selenide microflowers with enhanced electrochemical performance as a flexible symmetric 1.8 V supercapacitor. Chem. Eng. J. 2020, 382, 122814. [Google Scholar] [CrossRef]
- Momma, T.; Liu, X.; Osaka, T.; Ushio, Y.; Sawada, Y. Electrochemical modification of active carbon fiber electrode and its application to double-layer capacitor. J. Power Sources 1996, 60, 249–253. [Google Scholar] [CrossRef]
- Samal, R.; Bhat, M.; Kapse, S.; Thapa, R.; Late, D.J.; Rout, C.S. Enhanced energy storage performance and theoretical studies of 3D cuboidal manganese diselenides embedded with multiwalled carbon nanotubes. J. Colloid Interface Sci. 2021, 598, 500–510. [Google Scholar] [CrossRef]
- Ma, W.; Ding, Y.; Li, Y.; Gao, S.; Jiang, Z.; Cui, J.; Huang, C.; Fu, G. Durable, self-healing superhydrophobic nanofibrous membrane with self-cleaning ability for highly-efficient oily wastewater purification. J. Membr. Sci. 2021, 634, 119402. [Google Scholar] [CrossRef]
- Cao, J.; He, W.; Zhou, P.; Wei, B.; Wang, R.; Liang, S.; Ji, Q. Preparation of wood aerogel membrane and adsorption performance on Cu2+ in waste water. J. For. Eng. 2023, 8, 113–120. [Google Scholar] [CrossRef]
- Lu, T.; Liang, H.; Cao, W.; Deng, Y.; Qu, Q.; Ma, W.; Xiong, R.; Huang, C. Blow-spun nanofibrous composite Self-cleaning membrane for enhanced purification of oily wastewater. J. Colloid Interface Sci. 2022, 608, 2860–2869. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Lu, T.; Zhang, X.; Zeng, Z.; Tao, R.; Qu, Q.; Zhang, Y.; Zhu, M.; Xiong, R.; Huang, C. Multi-hierarchical nanofiber membrane with typical curved-ribbon structure fabricated by green electrospinning for efficient, breathable and sustainable air filtration. J. Membr. Sci. 2022, 660, 120857. [Google Scholar] [CrossRef]
- Xie, J.; Liu, G.; Sun, J.; Zheng, R.; Zhao, W.; Chu, T.; Lin, H.; Xu, Y.; Gao, S.; Sui, Z. α-MnO2/CNTs with cross-linked reticular structure: Towards ultralong life zinc-ion batteries. Diam. Relat. Mater. 2022, 125, 109024. [Google Scholar] [CrossRef]
- Xu, C.; Peng, S.; Tan, C.; Ang, H.; Tan, H.; Zhang, H.; Yan, Q. Ultrathin S-doped MoSe2 nanosheets for efficient hydrogen evolution. J. Mater. Chem. A 2014, 2, 5597–5601. [Google Scholar] [CrossRef]
- Xie, J.; Liu, G.; Jiang, X.; Sui, Z.; Gao, S. One-step co-precipitation of MnSe2/CNTs as a high-performance cathode material for zinc-ion batteries. Ceram. Int. 2023, 49, 10165–10171. [Google Scholar] [CrossRef]
- Zhu, H.; Qin, G.; Zhou, W.; Li, Y.; Zhou, X. Constructing flake-like ternary rare earth Pr3Si2C2 ceramic on SiC whiskers to enhance electromagnetic wave absorption properties. Ceram. Int. 2024, 50, 134–142. [Google Scholar] [CrossRef]
- Qu, Q.; Zhang, X.; Yang, A.; Wang, J.; Cheng, W.; Zhou, A.; Deng, Y.; Xiong, R.; Huang, C. Spatial confinement of multi-enzyme for cascade catalysis in cell-inspired all-aqueous multicompartmental microcapsules. J. Colloid Interface Sci. 2022, 626, 768–774. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Y.; Zhao, J.; Liu, J.; Lei, J.; Wang, L.; Huang, C. A novel green lignosulfonic acid/Nafion composite membrane with reduced cost and enhanced thermal stability. Chem. Commun. 2021, 57, 9288–9291. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zeng, G.; Jin, H.; Jiang, S.; Huang, M.; Zhang, C.; Chen, H. Bio-Template Synthesis of V2O3@ Carbonized Dictyophora Composites for Advanced Aqueous Zinc-Ion Batteries. Molecules 2023, 28, 2147. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Liu, X.; Li, Z.; Fan, H.; Zhang, Y.; Yang, H.Y. Cobalt-nickel bimetallic sulfide (NiS2/CoS2) based dual-carbon framework for super sodium ion storage. J. Colloid Interface Sci. 2023, 633, 480–488. [Google Scholar] [CrossRef]
- Li, X.; Liang, H.; Qin, B.; Wang, M.; Zhang, Y.; Fan, H. Rational design of heterostructured bimetallic sulfides (CoS2/NC@VS4) with VS4 nanodots decorated on CoS2 dodecahedron for high-performance sodium and potassium ion batteries. J. Colloid Interface Sci. 2022, 625, 41–49. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Tai, L.; Zhou, W.; Chen, H.; Liu, J.; Jiang, S. Facile Preparation of Three-Dimensional Cubic MnSe2/CNTs and Their Application in Aqueous Copper Ion Batteries. Nanomaterials 2024, 14, 1621. https://doi.org/10.3390/nano14201621
Wang J, Tai L, Zhou W, Chen H, Liu J, Jiang S. Facile Preparation of Three-Dimensional Cubic MnSe2/CNTs and Their Application in Aqueous Copper Ion Batteries. Nanomaterials. 2024; 14(20):1621. https://doi.org/10.3390/nano14201621
Chicago/Turabian StyleWang, Junjun, Linlin Tai, Wei Zhou, Han Chen, Jingxiong Liu, and Shaohua Jiang. 2024. "Facile Preparation of Three-Dimensional Cubic MnSe2/CNTs and Their Application in Aqueous Copper Ion Batteries" Nanomaterials 14, no. 20: 1621. https://doi.org/10.3390/nano14201621







