Eco-Friendly Facile Conversion of Waste Eggshells into CaO Nanoparticles for Environmental Applications
Abstract
1. Introduction
2. Experimental Details
2.1. Antimicrobial Activity
2.2. Photocatalytic Study
3. Results and Analysis
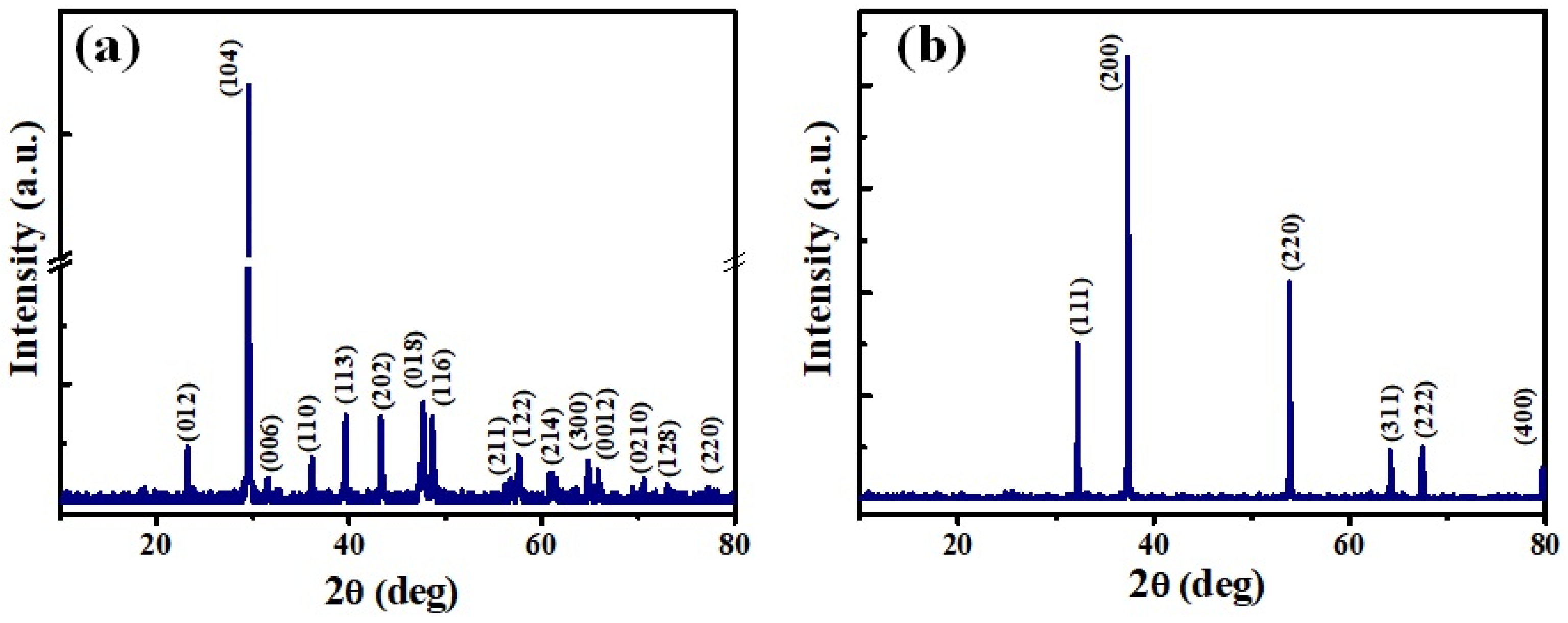
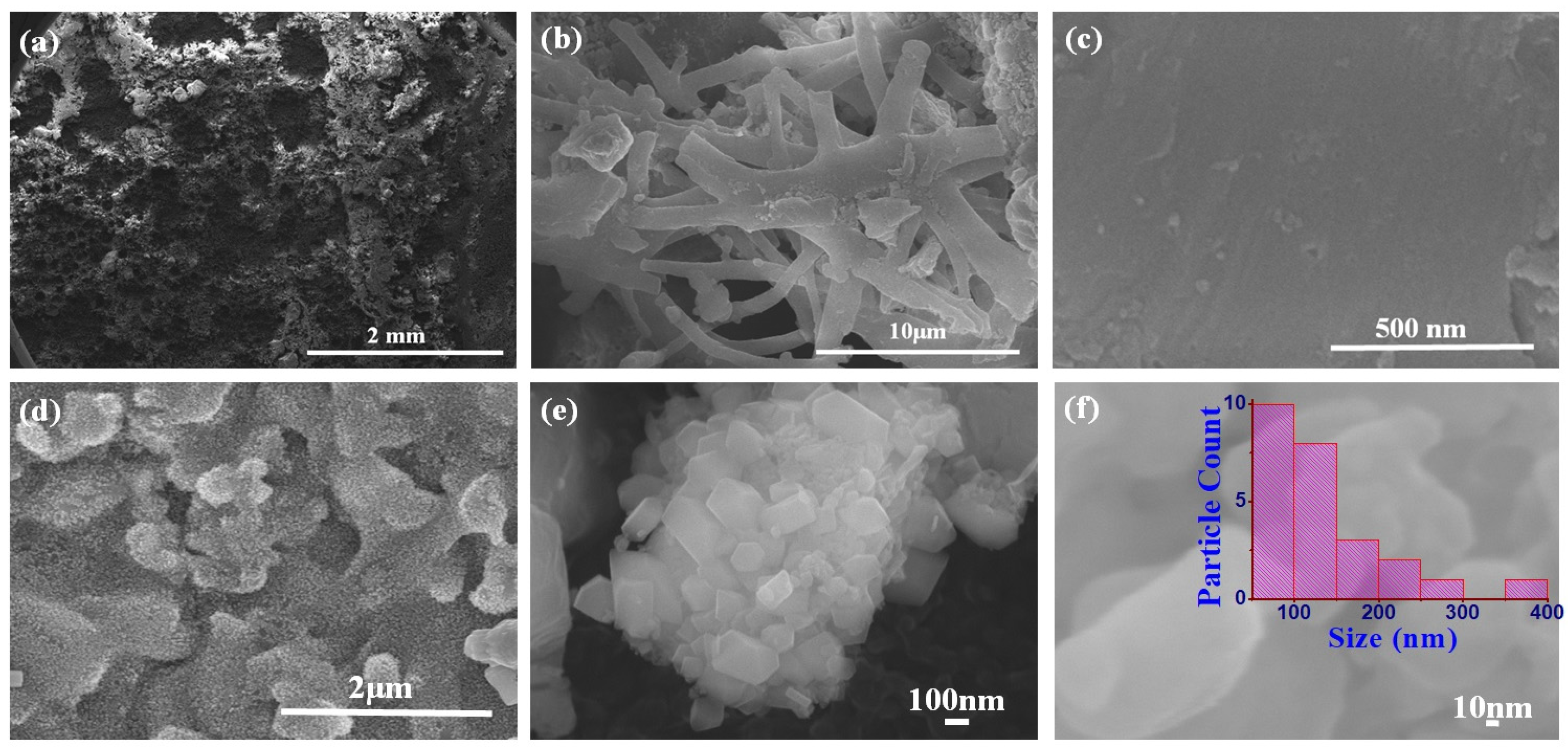
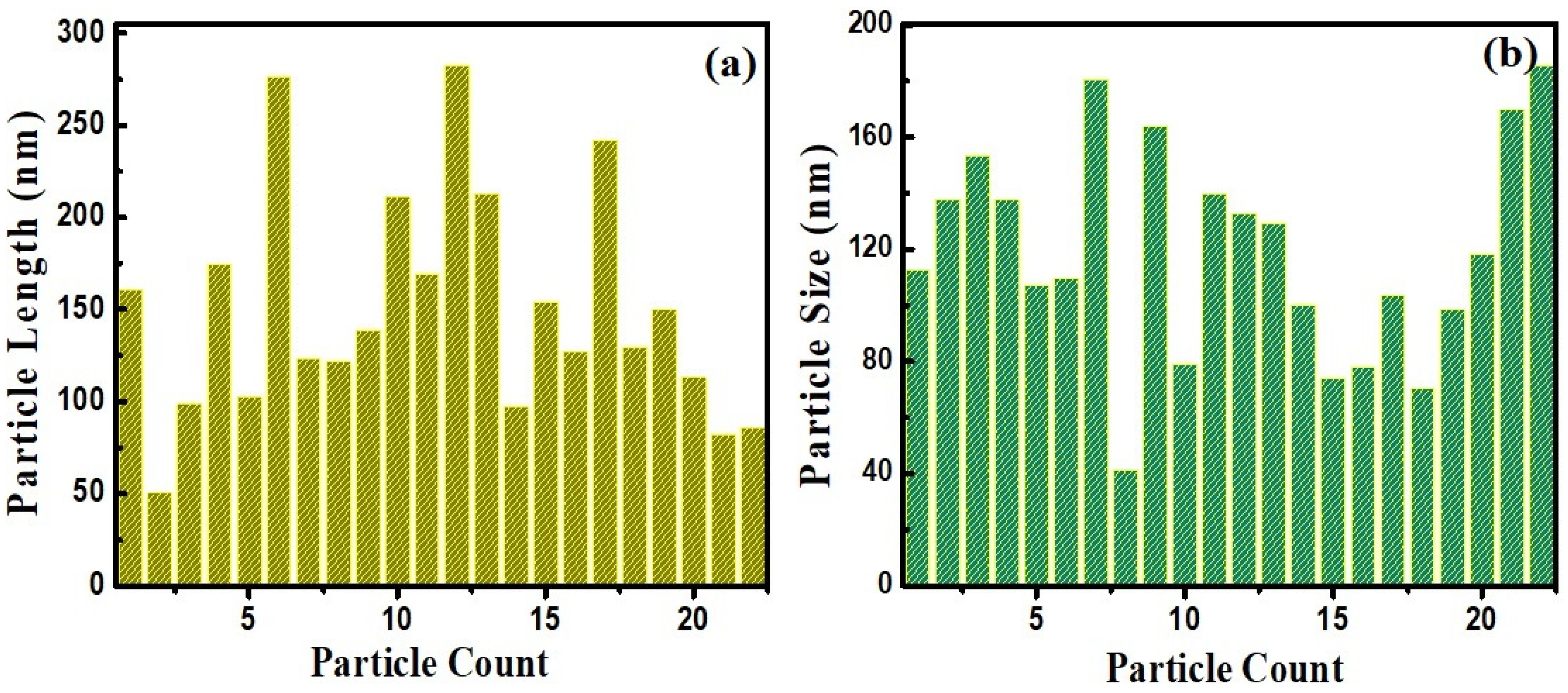
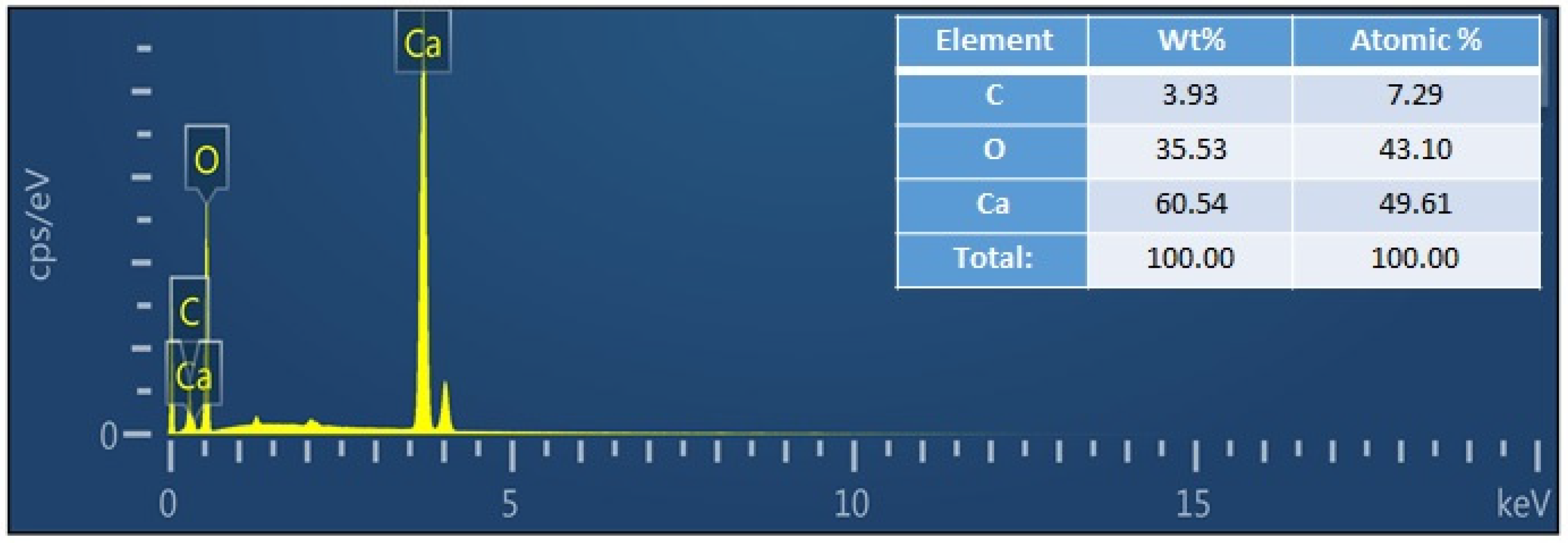
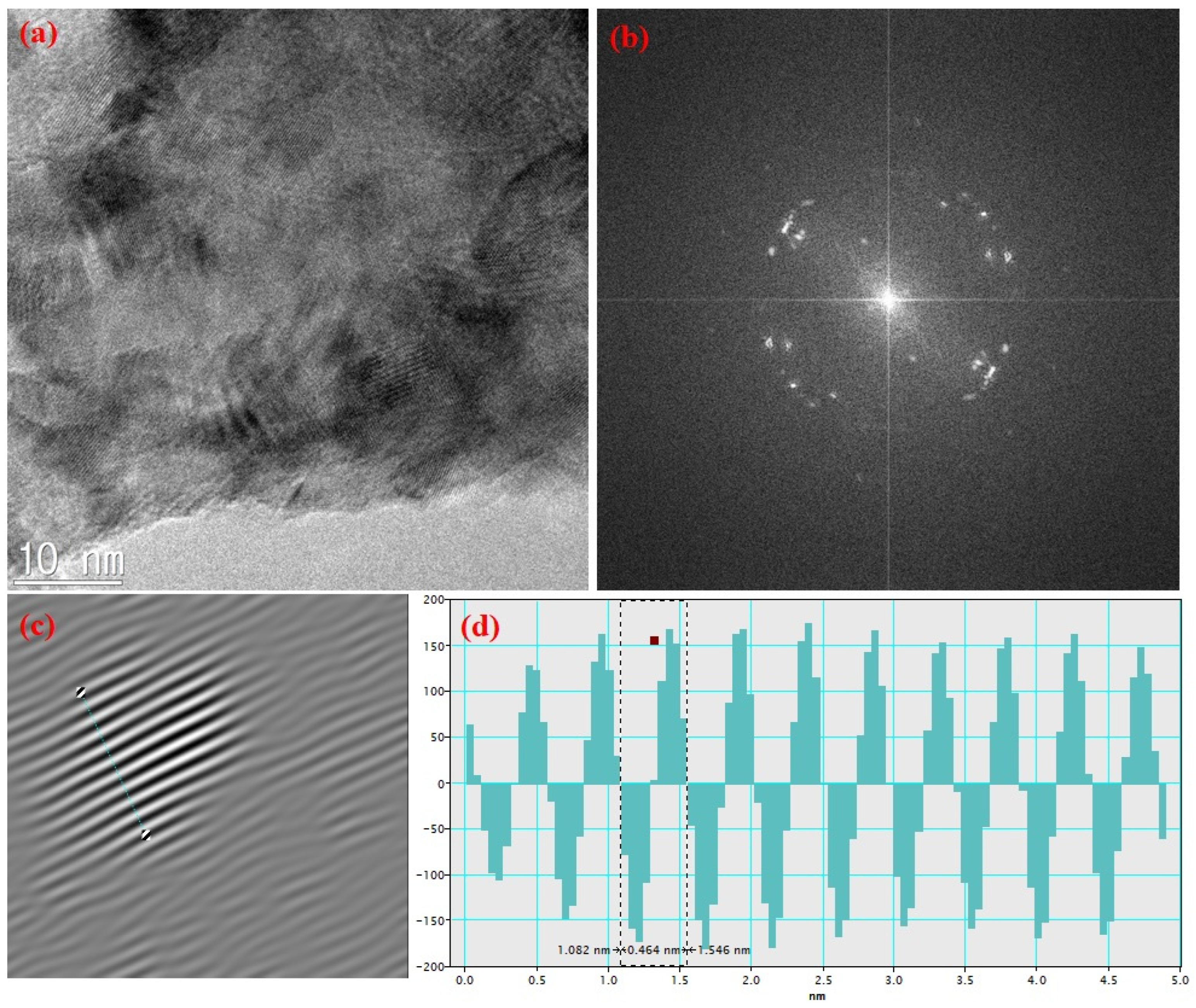
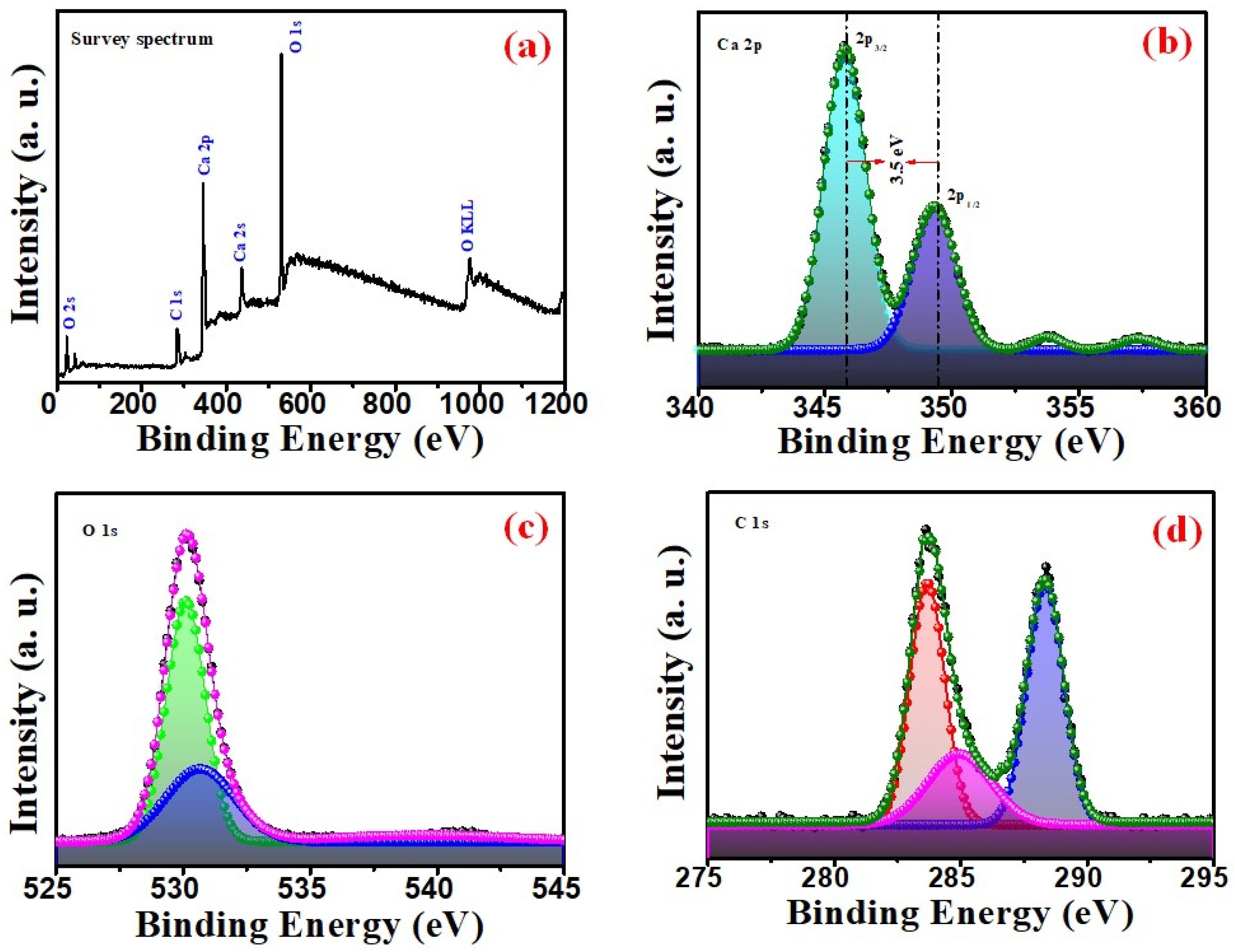
3.1. Structural and Chemical Analysis
3.2. Optical Characterization
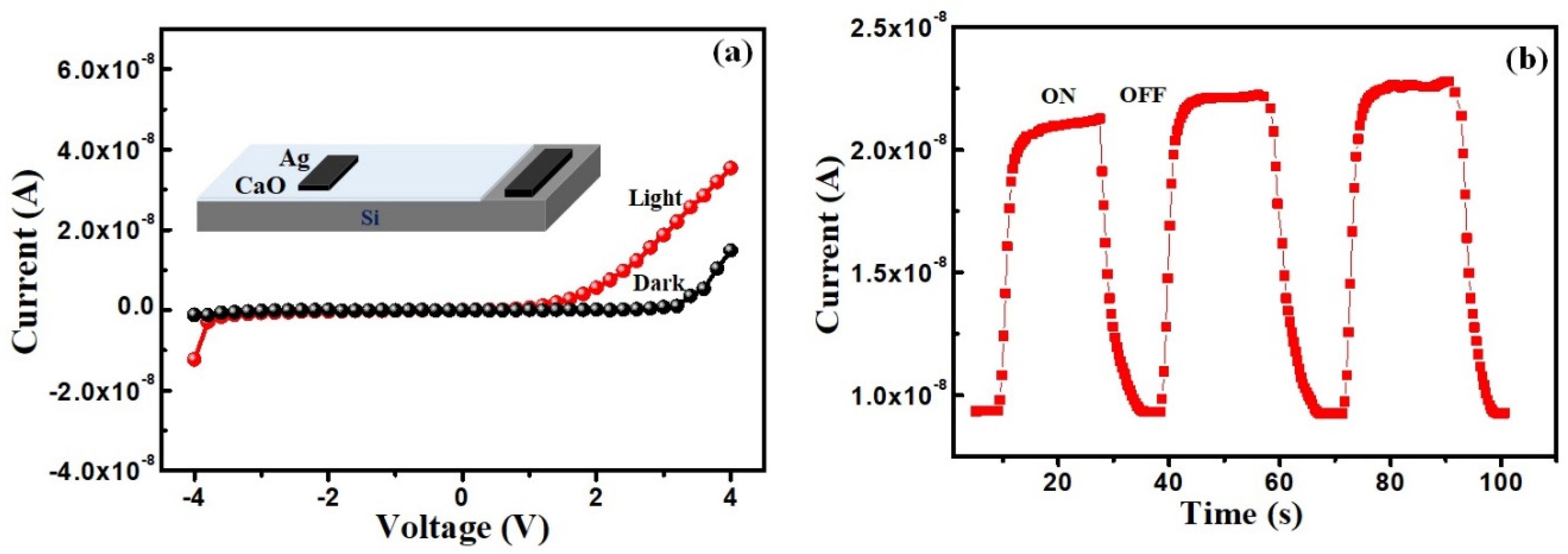
3.3. Optical Sensing Property
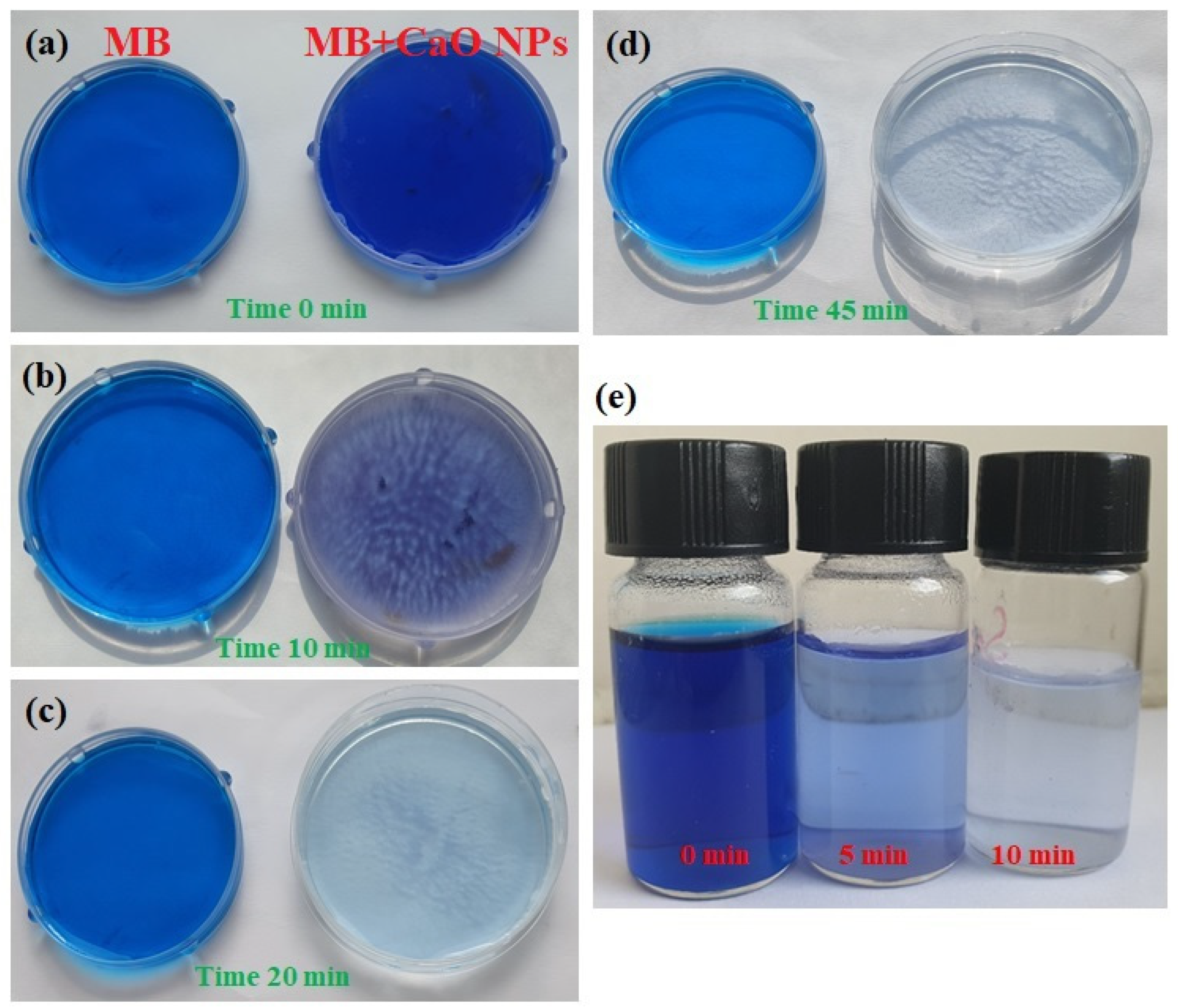
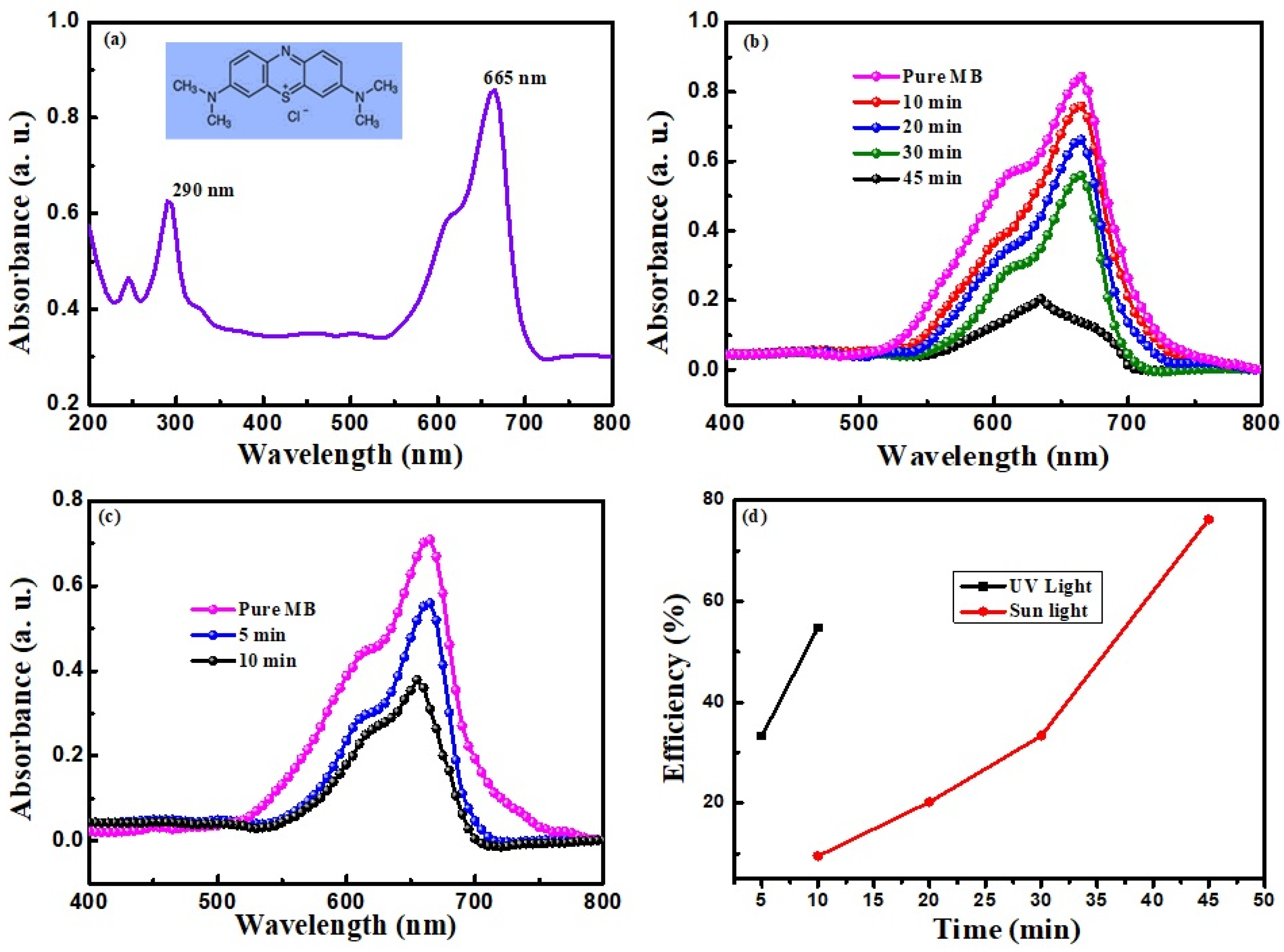
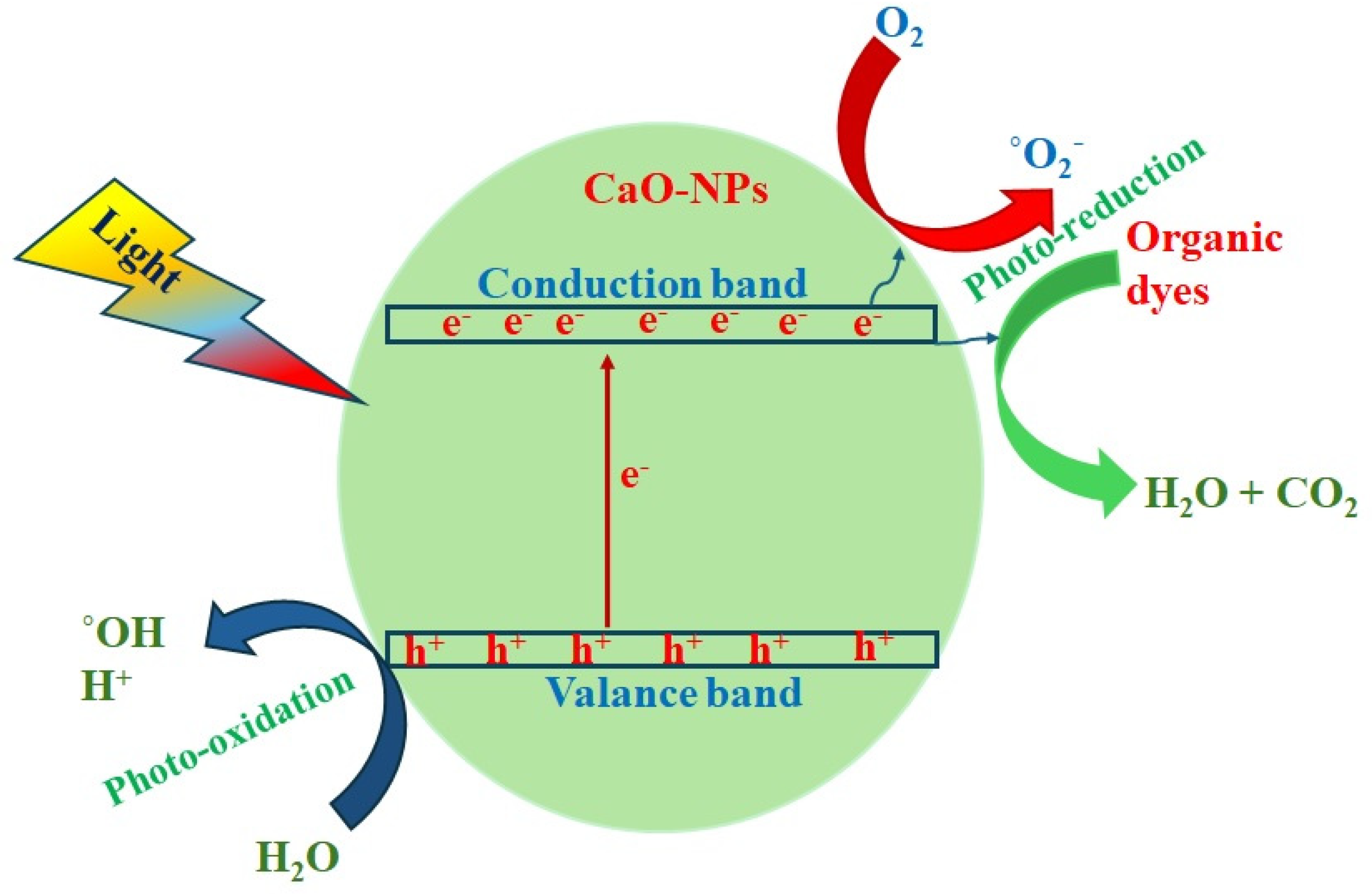
3.4. Photocatalytic Study
3.5. Electrocatalytic Study
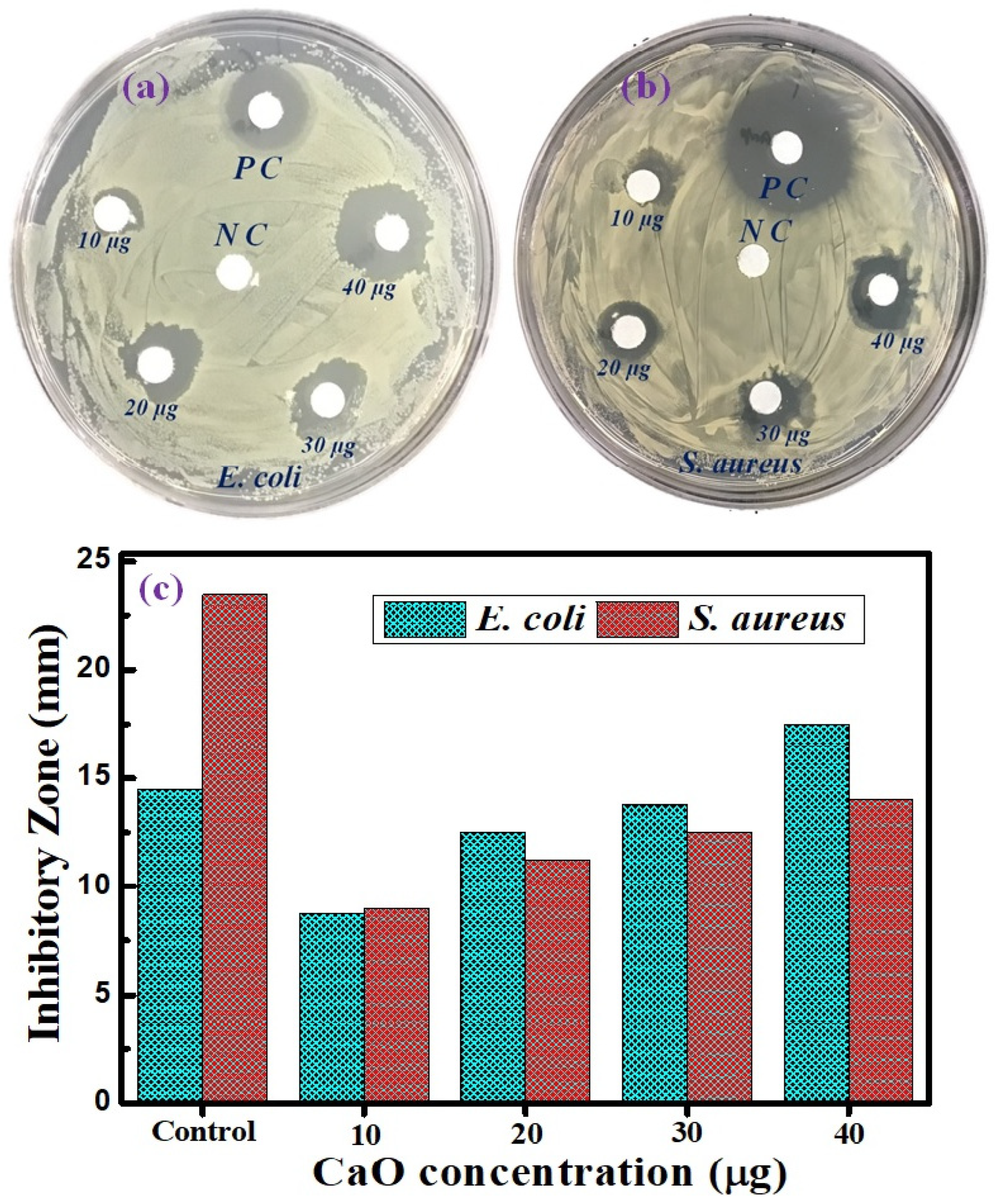
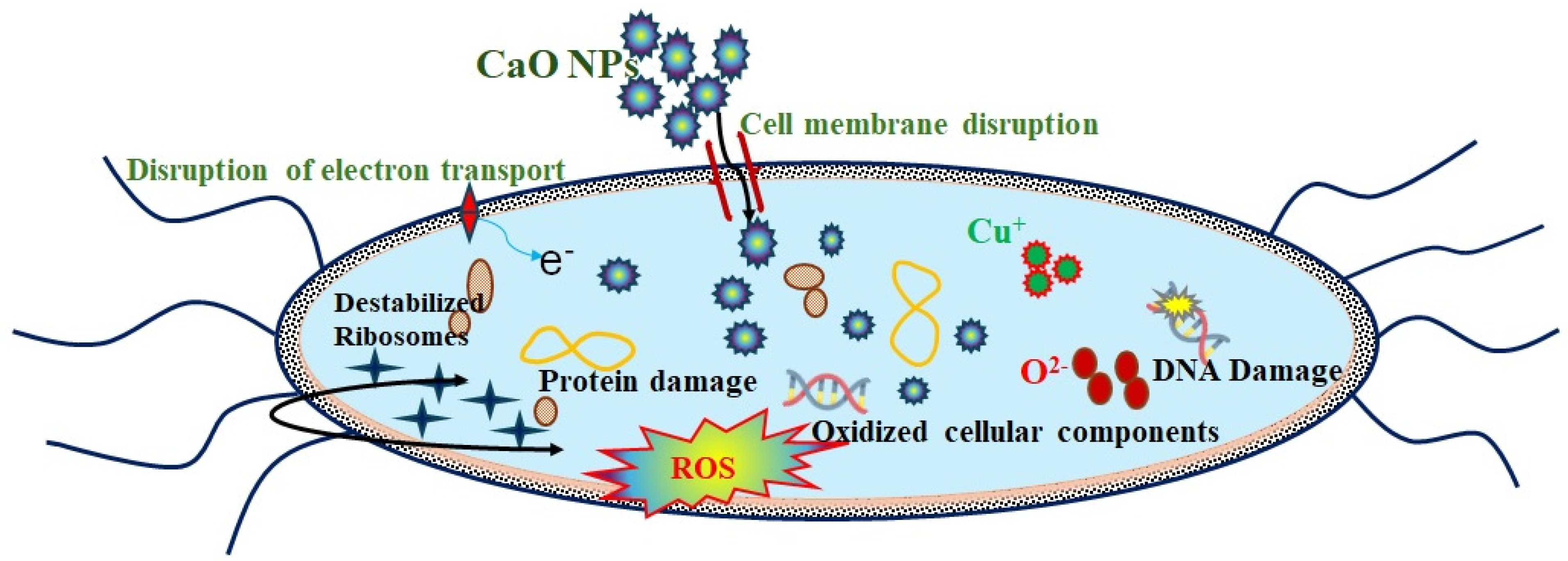
3.6. Antibacterial Activity of CaO NPs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Helwani, Z.; Ramli, M.; Saputra, E.; Bahruddin, B.; Yolanda, D.; Fatra, W.; Idroes, G.M.; Muslem, M.; Mahlia, T.M.I.; Idroes, R. Impregnation of CaO from eggshell waste with magnetite as a solid catalyst (Fe3O4/CaO) for transesterification of palm oil off-grade. Catalysts 2020, 10, 164. [Google Scholar] [CrossRef]
- Francis, A.; Rahman, M.A. The environmental sustainability of calcined calcium phosphates production from the milling of eggshell wastes and phosphoric acid. J. Clean. Prod. 2016, 137, 1432–1438. [Google Scholar] [CrossRef]
- Adaikalam, K.; Teli, A.M.; Marimuthu, K.P.; Ramesh, S.; Lee, H.; Kim, H.S.; Kim, H.-S. Energy Storage Application of CaO/Graphite Nanocomposite Powder Obtained from Waste Eggshells and Used Lithium-Ion Batteries as a Sustainable Development Approach. Nanomaterials 2024, 14, 1129. [Google Scholar] [CrossRef]
- Chou, M.-Y.; Lee, T.-A.; Lin, Y.-S.; Hsu, S.-Y.; Wang, M.-F.; Li, P.-H.; Huang, P.-H.; Lu, W.-C.; Ho, J.-H. On the removal efficiency of copper ions in wastewater using calcined waste eggshells as natural adsorbents. Sci. Rep. 2023, 13, 437. [Google Scholar] [CrossRef]
- Wong, Y.C.; Ang, R.X. Study of calcined eggshell as potential catalyst for biodiesel formation using used cooking oil. Open Chem. 2018, 16, 1166–1175. [Google Scholar] [CrossRef]
- Anantharaman, A.; Ramalakshmi, S.; George, M. Green synthesis of calcium oxide nanoparticles and its applications. Int. J. Eng. Res. Appl. 2016, 6, 27–31. [Google Scholar]
- Tang, Z.-X.; Yu, Z.; Zhang, Z.-L.; Zhang, X.-Y.; Pan, Q.-Q.; Shi, L.-E. Sonication-assisted preparation of CaO nanoparticles for antibacterial agents. Quím. Nova 2013, 36, 933–936. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Sutha, M.; Sowndarya, K.; Chandran, M.; Yuvaraj, D.; Praveen Kumar, R. Calcium Oxide Nanoparticles as An Effective Filtration Aid for Purification of Vehicle Gas Exhaust. In Advances in Internal Combustion Engine Research; Srivastava, D.K., Agarwal, A.K., Datta, A., Maurya, R.K., Eds.; Springer: Singapore, 2018; pp. 181–192. [Google Scholar]
- Zhou, Y.; Zhou, Z.; Liu, L.; She, X.; Xu, R.; Sun, J.; Xu, M. Enhanced thermochemical energy storage stability of CaO-based composite pellets incorporated with a Zr-based stabilizer. Energy Fuels 2021, 35, 18778–18788. [Google Scholar] [CrossRef]
- Kathalingam, A.; Park, H.-C.; Kim, S.-D.; Kim, H.-S.; Velumani, S.; Mahalingam, T. Synthesis of ZnO nanorods using different precursor solutions and their two terminal device characterization. J. Mater. Sci. Mater. Electron. 2015, 26, 5724–5734. [Google Scholar] [CrossRef]
- Kumari, M.; Sarkar, B.; Mukherjee, K. Nanoscale calcium oxide and its biomedical applications: A comprehensive review. Biocatal. Agric. Biotechnol. 2023, 47, 102506. [Google Scholar] [CrossRef]
- Khine, E.E.; Koncz-Horvath, D.; Kristaly, F.; Ferenczi, T.; Karacs, G.; Baumli, P.; Kaptay, G. Synthesis and characterization of calcium oxide nanoparticles for CO2 capture. J. Nanoparticle Res. 2022, 24, 139. [Google Scholar] [CrossRef]
- Habte, L.; Shiferaw, N.; Mulatu, D.; Thenepalli, T.; Chilakala, R.; Ahn, J.W. Synthesis of nano-calcium oxide from waste eggshell by sol-gel method. Sustainability 2019, 11, 3196. [Google Scholar] [CrossRef]
- Fatima, F.; Siddiqui, S.; Khan, W.A. Nanoparticles as Novel Emerging Therapeutic Antibacterial Agents in the Antibiotics Resistant Era. Biol. Trace Elem. Res. 2021, 199, 2552–2564. [Google Scholar] [CrossRef]
- Mondal, S.K.; Chakraborty, S.; Manna, S.; Mandal, S.M. Antimicrobial nanoparticles: Current landscape and future challenges. RSC Pharm. 2024, 1, 388–402. [Google Scholar] [CrossRef]
- Ikram, M.; Khalid, A.; Shahzadi, A.; Haider, A.; Naz, S.; Naz, M.; Shahzadi, I.; Ul-Hamid, A.; Haider, J.; Nabgan, W.; et al. Enhanced Photocatalytic Degradation with Sustainable CaO Nanorods Doped with Ce and Cellulose Nanocrystals: In Silico Molecular Docking Studies. ACS Omega 2022, 7, 27503–27515. [Google Scholar] [CrossRef]
- Asep Bayu Dani, N.; Brigitta Stacia, M.; Risti, R. Calcium Oxide Nanoparticle Production and its Application as Photocatalyst. J. Adv. Res. Appl. Sci. Eng. Technol. 2023, 30, 168–181. [Google Scholar] [CrossRef]
- Hou, J.; Xiong, X.; Jiao, C.; Huang, X.; Fu, D.; Zhao, H.; Li, Y. Cleaner production of disperse florescent dyes in supercritical CO2 and their applications in dyeing polyester fabric. Dye. Pigment. 2022, 202, 110250. [Google Scholar] [CrossRef]
- Do, K.L.; Su, M.; Zhao, F. From historical dye to bio-colourant: Processing, identification in historical textiles and potential applications of anthraquinone-based morindone. Dye. Pigment. 2022, 205, 110482. [Google Scholar] [CrossRef]
- Devarahosahalli Veeranna, K.; Theeta Lakshamaiah, M.; Thimmasandra Narayan, R. Photocatalytic Degradation of Indigo Carmine Dye Using Calcium Oxide. Int. J. Photochem. 2014, 2014, 530570. [Google Scholar] [CrossRef]
- Zhu, C.-Y.; Shen, M.-T.; Qi, M.-J.; Zhao, Y.-Y.; Xu, Z.; Li, P.; Ru, J.; Gao, W.; Zhang, X.-M. Constructed CdS/Mn-MOF heterostructure for promoting photocatalytic degradation of Rhodamine B. Dye. Pigment. 2023, 219, 111607. [Google Scholar] [CrossRef]
- Li, S.; Wang, P.; Yang, K.; Liu, Y.; Cheng, D.; He, L. Methylene blue-based near-infrared activatable probes for bioimaging. Dye. Pigment. 2023, 211, 111083. [Google Scholar] [CrossRef]
- Vanthana Sree, G.; Nagaraaj, P.; Kalanidhi, K.; Aswathy, C.A.; Rajasekaran, P. Calcium oxide a sustainable photocatalyst derived from eggshell for efficient photo-degradation of organic pollutants. J. Clean. Prod. 2020, 270, 122294. [Google Scholar] [CrossRef]
- Jadhav, V.; Bhagare, A.; Wahab, S.; Lokhande, D.; Vaidya, C.; Dhayagude, A.; Khalid, M.; Aher, J.; Mezni, A.; Dutta, M. Green Synthesized Calcium Oxide Nanoparticles (CaO NPs) Using Leaves Aqueous Extract of Moringa oleifera and Evaluation of Their Antibacterial Activities. J. Nanomater. 2022, 2022, 9047507. [Google Scholar] [CrossRef]
- Alobaidi, Y.M.; Ali, M.M.; Mohammed, A.M. Synthesis of Calcium Oxide Nanoparticles from Waste Eggshell by Thermal Decomposition and their Applications. Jordan J. Biol. Sci. 2022, 15, 269–274. [Google Scholar]
- Münchow, E.A.; Pankajakshan, D.; Albuquerque, M.T.; Kamocki, K.; Piva, E.; Gregory, R.L.; Bottino, M.C. Synthesis and characterization of CaO-loaded electrospun matrices for bone tissue engineering. Clin. Oral Investig. 2016, 20, 1921–1933. [Google Scholar] [CrossRef]
- Abraham, S.; Sarathy, V. Biomedical Applications of Calcium Oxide Nanoparticles-A Spectroscopic Study. Int. J. Pharm. Sci. Rev. Res. 2018, 49, 121. [Google Scholar]
- Lanzón, M.; Madrid-Mendoza, J.A.; Navarro-Moreno, D.; García-Vera, V.E. Use of eggshell waste: A green and effective method for the synthesis of pure calcium hydroxide suspensions. Constr. Build. Mater. 2023, 377, 131106. [Google Scholar] [CrossRef]
- Sáez-Guinoa, J.; Senante, I.; Llera-Sastresa, E.; Romeo, L.M. Techno-economic assessment of solar photovoltaic electrification and calcium looping technology as decarbonisation pathways of alumina industry. Results Eng. 2024, 23, 102456. [Google Scholar] [CrossRef]
- Lisbona, P.; Bailera, M.; Hills, T.; Sceats, M.; Díez, L.I.; Romeo, L.M. Energy consumption minimization for a solar lime calciner operating in a concentrated solar power plant for thermal energy storage. Renew. Energy 2020, 156, 1019–1027. [Google Scholar] [CrossRef]
- Moumin, G.; Ryssel, M.; Zhao, L.; Markewitz, P.; Sattler, C.; Robinius, M.; Stolten, D. CO2 emission reduction in the cement industry by using a solar calciner. Renew. Energy 2020, 145, 1578–1596. [Google Scholar] [CrossRef]
- Davis, D.; Müller, F.; Saw, W.L.; Steinfeld, A.; Nathan, G.J. Solar-driven alumina calcination for CO2 mitigation and improved product quality. Green Chem. 2017, 19, 2992–3005. [Google Scholar] [CrossRef]
- Panagiotou, E.; Kafa, N.; Koutsokeras, L.; Kouis, P.; Nikolaou, P.; Constantinides, G.; Vyrides, I. Turning calcined waste egg shells and wastewater to Brushite: Phosphorus adsorption from aqua media and anaerobic sludge leach water. J. Clean. Prod. 2018, 178, 419–428. [Google Scholar] [CrossRef]
- Urnukhsaikhan, E.; Bold, B.-E.; Gunbileg, A.; Sukhbaatar, N.; Mishig-Ochir, T. Antibacterial activity and characteristics of silver nanoparticles biosynthesized from Carduus crispus. Sci. Rep. 2021, 11, 21047. [Google Scholar] [CrossRef] [PubMed]
- Supriyanto, N.S.W.; Sukarni, S.; Puspitasari, P.; Permanasari, A.A. Synthesis and characterization of CaO/CaCO3 from quail eggshell waste by solid state reaction process. In Proceedings of the AIP Conference Proceedings, Bodrum, Turkey, 4–8 September 2019. [Google Scholar]
- Witoon, T. Characterization of calcium oxide derived from waste eggshell and its application as CO2 sorbent. Ceram. Int. 2011, 37, 3291–3298. [Google Scholar] [CrossRef]
- Fayyazi, E.; Ghobadian, B.; van de Bovenkamp, H.H.; Najafi, G.; Hosseinzadehsamani, B.; Heeres, H.J.; Yue, J. Optimization of biodiesel production over chicken eggshell-derived CaO catalyst in a continuous centrifugal contactor separator. Ind. Eng. Chem. Res. 2018, 57, 12742–12755. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Gauri, S.S.; Bhattacharya, M.; Bhattacharya, J. Antimicrobial activity of CaO nanoparticles. J. Biomed. Nanotechnol. 2013, 9, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Mirghiasi, Z.; Bakhtiari, F.; Darezereshki, E.; Esmaeilzadeh, E. Preparation and characterization of CaO nanoparticles from Ca(OH)2 by direct thermal decomposition method. J. Ind. Eng. Chem. 2014, 20, 113–117. [Google Scholar] [CrossRef]
- Li, B.; Wu, C.; Xu, J.; Hu, D.; Zhang, T.; Fang, X.; Tong, J.J. One-pot redox synthesis of graphene from waste graphite of spent lithium ion batteries with peracetic acid assistance. Mater. Chem. Phys. 2020, 241, 122397. [Google Scholar] [CrossRef]
- Minakshi, M.; Higley, S.; Baur, C.; Mitchell, D.R.; Jones, R.T.; Fichtner, M.J. Calcined chicken eggshell electrode for battery and supercapacitor applications. RSC Adv. 2019, 9, 26981–26995. [Google Scholar] [CrossRef]
- Medeiros, S.; Albuquerque, E.L.; Maia, F., Jr.; Caetano, E.; Farias, G.; Freire, V.; Cavada, B.; Pessati, M.; Pessati, T. Structural and optical properties of CaO. Microelectron. J. 2005, 36, 1058–1061. [Google Scholar] [CrossRef]
- Jaiswal, K.K.; Dutta, S.; Pohrmen, C.B.; Verma, R.; Kumar, A.; Ramaswamy, A.P. Bio-waste chicken eggshell-derived calcium oxide for photocatalytic application in methylene blue dye degradation under natural sunlight irradiation. Inorg. Nano-Met. Chem. 2021, 51, 995–1004. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, V.; Pradhan, J.K.; Sharma, S.K.; Singh, P.; Sharma, J.K. Structural, Optical and Antibacterial Response of CaO Nanoparticles Synthesized via Direct Precipitation Technique. Nano Biomed. Eng. 2021, 13, 172–178. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Hussain, T.; Karuppasamy, K.; Santhoshkumar, P.; Kim, K.-Y.; Manikandan, R.; Jung, J.; Kim, H.-S. Diverse chalcogen bonded molybdenum dichalcogenide alloy for the efficient photo- and electro-catalytic activity to eradicate the methylene blue and Congo red dyes. J. Clean. Prod. 2023, 426, 139127. [Google Scholar] [CrossRef]
- Zhou, Y.; Kong, Y.; Kundu, S.; Cirillo, J.D.; Liang, H. Antibacterial activities of gold and silver nanoparticles against Escherichia coli and bacillus Calmette-Guérin. J. Nanobiotechnology 2012, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, J.A.P.; Salapare, H.S.; Villamayor, M.M.S.; Siringan, M.A.T.; Ramos, H.J. Antibacterial efficiency of magnetron sputtered TiO2 on poly(methyl methacrylate). Surf. Interfaces 2017, 8, 28–35. [Google Scholar] [CrossRef]
- Çatıker, E.; Stakleff, K.S.; Carr, K.B.; Sancaktar, E. Laser-perforated polymer films for possible use in tissue engineering. Surf. Innov. 2016, 4, 23–32. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adaikalam, K.; Hussain, S.; Anbu, P.; Rajaram, A.; Sivanesan, I.; Kim, H.-S. Eco-Friendly Facile Conversion of Waste Eggshells into CaO Nanoparticles for Environmental Applications. Nanomaterials 2024, 14, 1620. https://doi.org/10.3390/nano14201620
Adaikalam K, Hussain S, Anbu P, Rajaram A, Sivanesan I, Kim H-S. Eco-Friendly Facile Conversion of Waste Eggshells into CaO Nanoparticles for Environmental Applications. Nanomaterials. 2024; 14(20):1620. https://doi.org/10.3390/nano14201620
Chicago/Turabian StyleAdaikalam, Kathalingam, Sajjad Hussain, Periasamy Anbu, Arulmozhi Rajaram, Iyyakkannu Sivanesan, and Hyun-Seok Kim. 2024. "Eco-Friendly Facile Conversion of Waste Eggshells into CaO Nanoparticles for Environmental Applications" Nanomaterials 14, no. 20: 1620. https://doi.org/10.3390/nano14201620
APA StyleAdaikalam, K., Hussain, S., Anbu, P., Rajaram, A., Sivanesan, I., & Kim, H.-S. (2024). Eco-Friendly Facile Conversion of Waste Eggshells into CaO Nanoparticles for Environmental Applications. Nanomaterials, 14(20), 1620. https://doi.org/10.3390/nano14201620








