Novel Bis(4-aminophenoxy) Benzene-Based Aramid Copolymers with Enhanced Solution Processability
Abstract
1. Introduction
2. Experimental Procedure
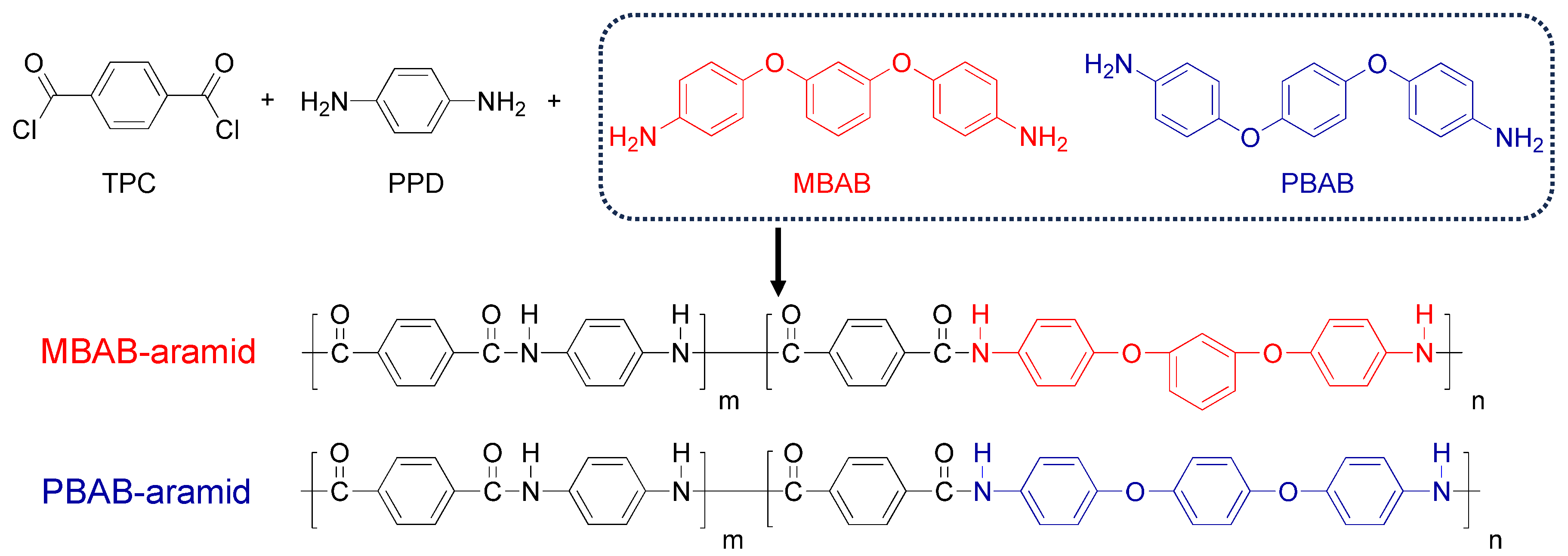
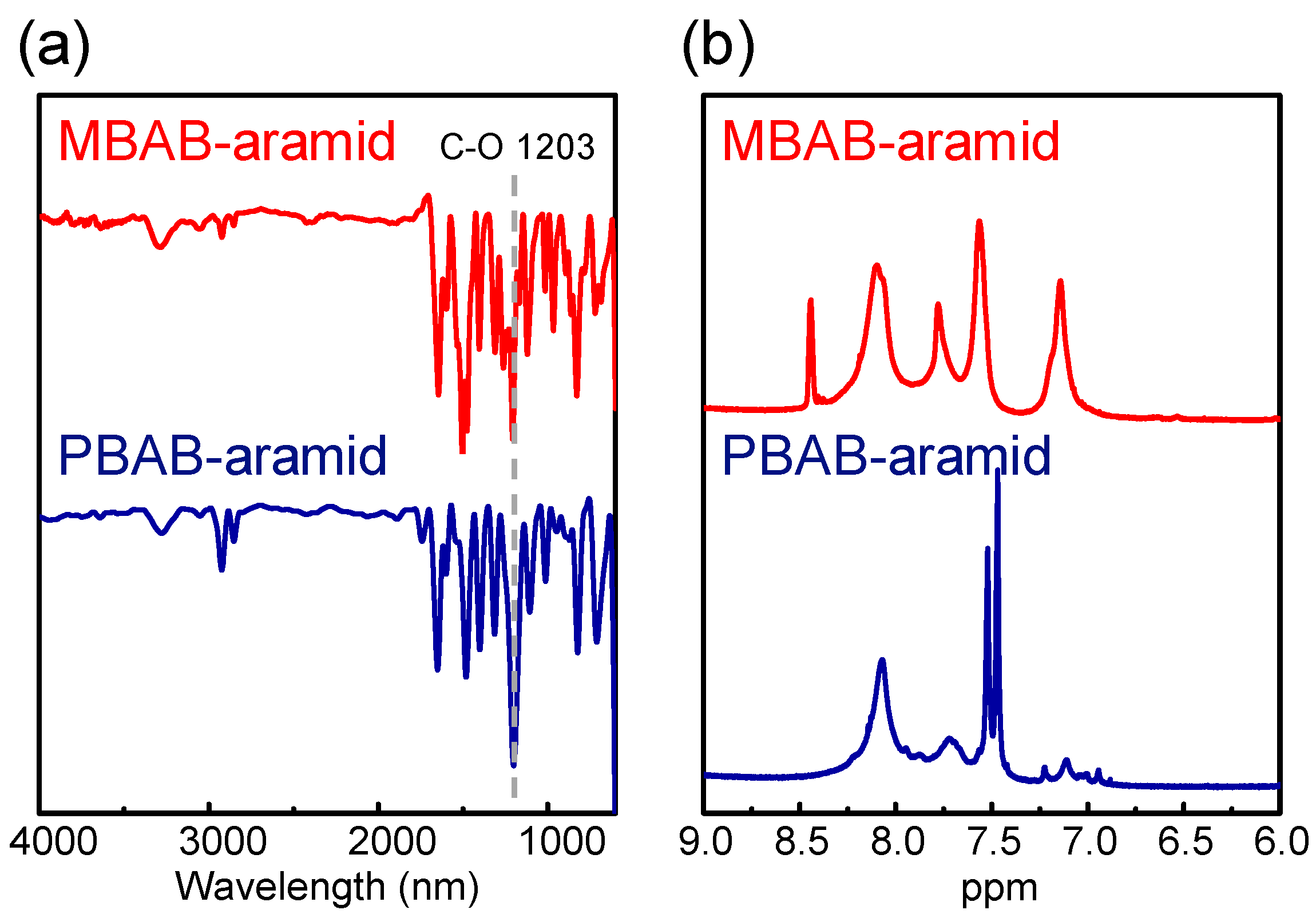
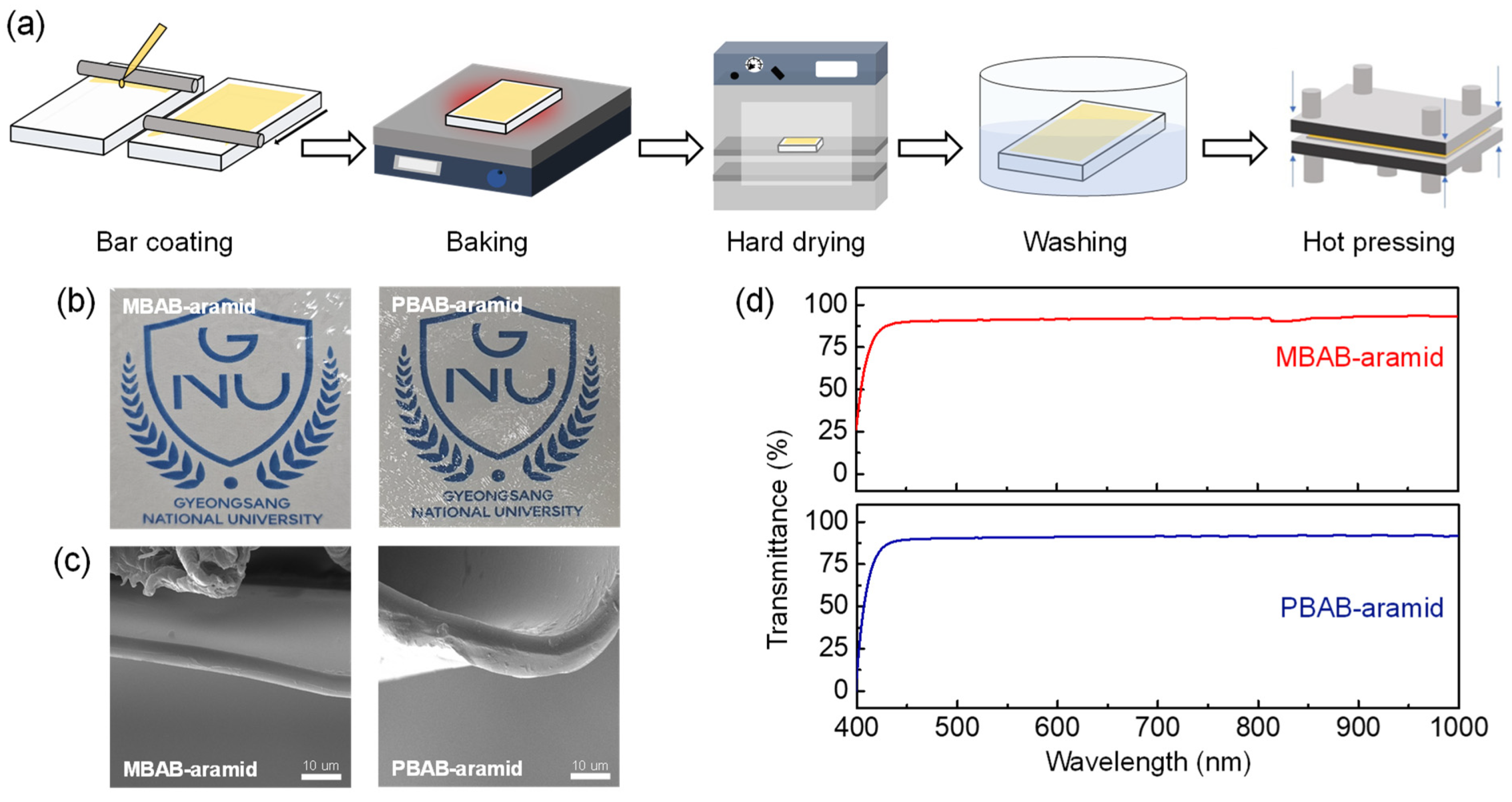
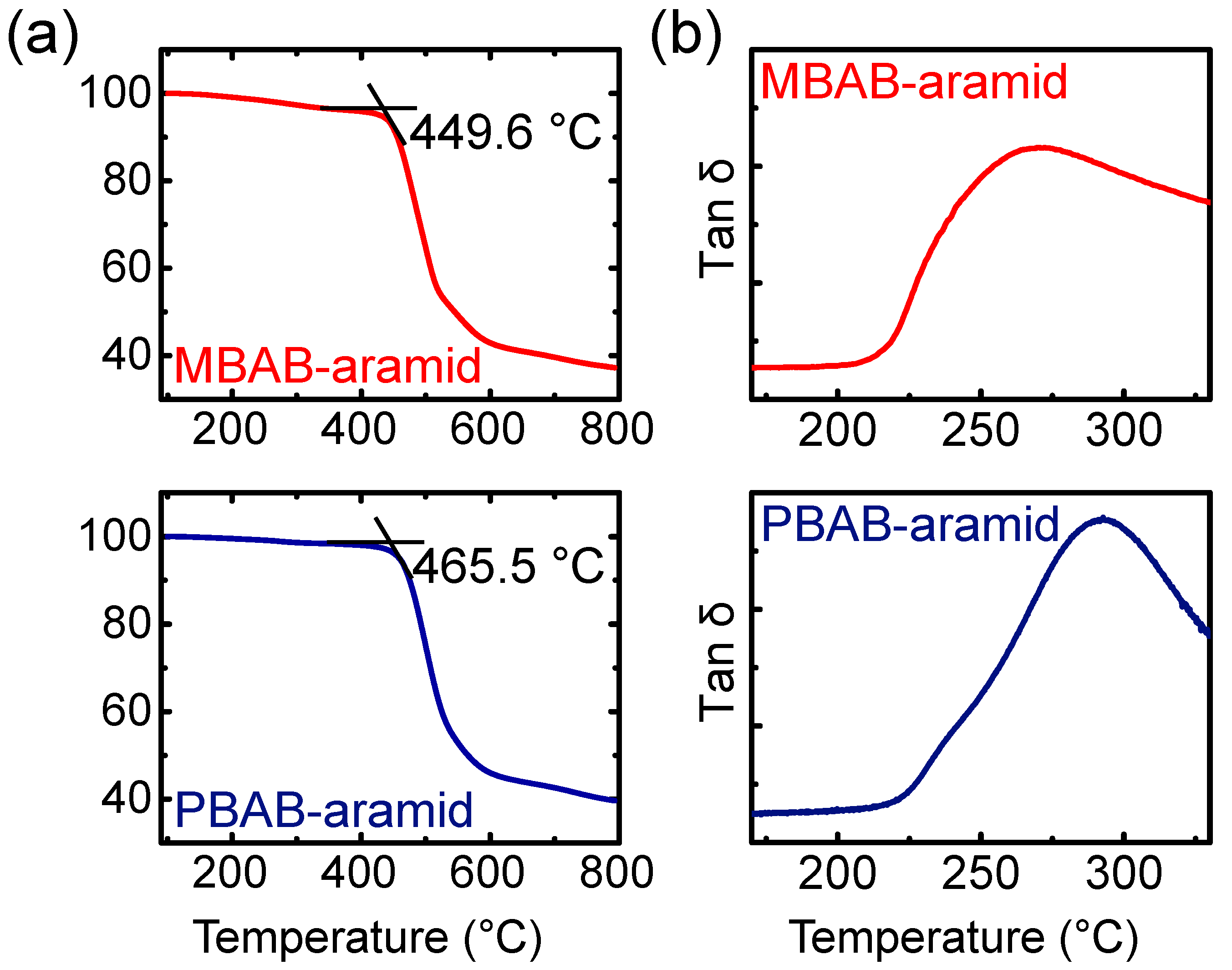
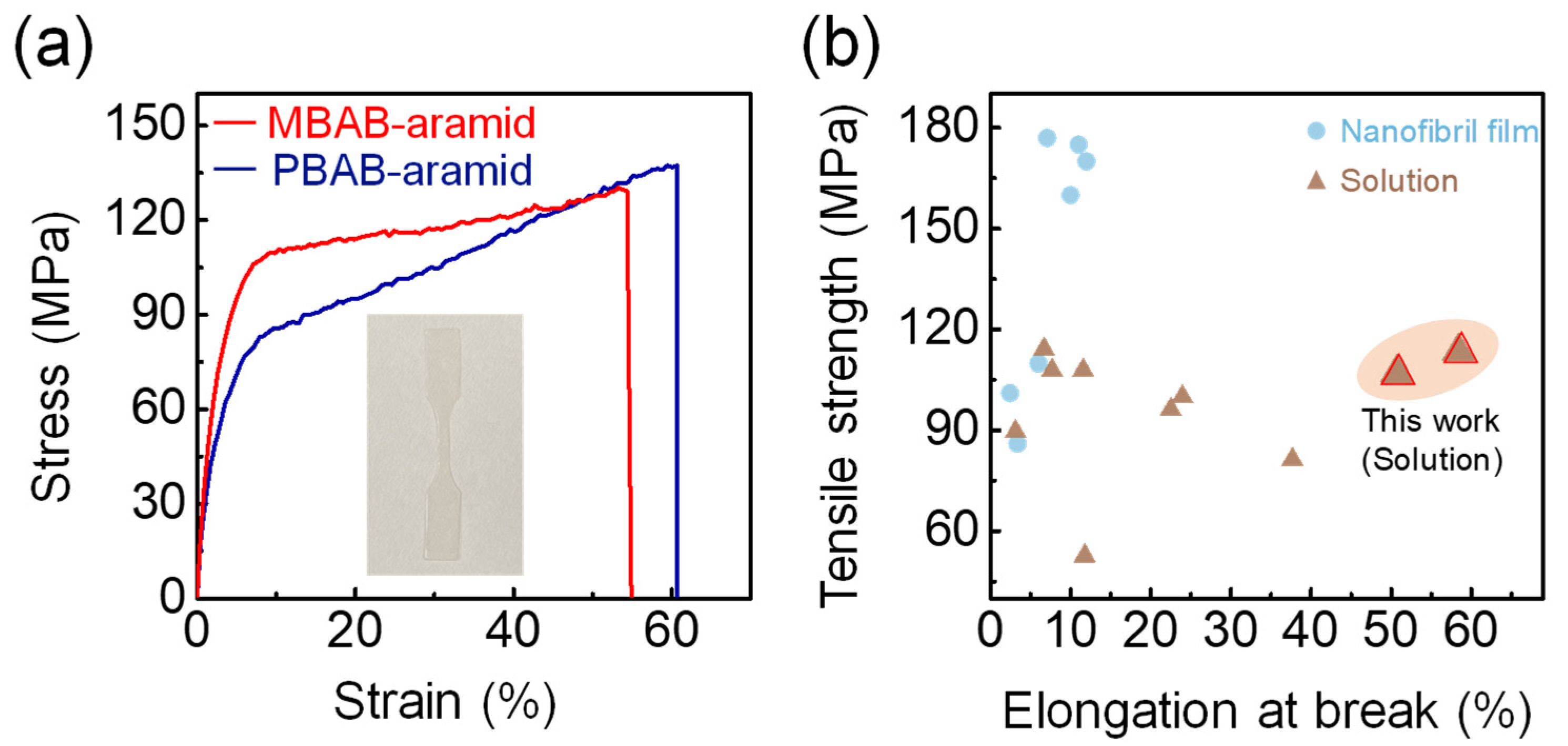
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhai, L.; Huang, Z.; Luo, Y.; Yang, H.; Xing, T.; He, A.; Yu, Z.; Liu, J.; Zhang, X.; Xu, W.; et al. Decorating aramid fibers with chemically-bonded amorphous TiO2 for improving UV resistance in the simulated extreme environment. Chem. Eng. J. 2022, 440, 135724. [Google Scholar] [CrossRef]
- Huang, S.; Lin, P.; Yao, S.; Wu, M.; Zhu, Z.; Wang, L.; Wang, H. High-performance para-aramid paper strengthened by ultrafine fiber pulp of polyphenylene sulfide. Compos. Sci. Technol. 2021, 216, 109073. [Google Scholar] [CrossRef]
- Zhang, B.; Jia, L.; Tian, M.; Ning, N.; Zhang, L.; Wang, W. Surface and interface modification of aramid fiber and its reinforcement for polymer composites: A review. Eur. Polym. J. 2021, 147, 110352. [Google Scholar] [CrossRef]
- Faruk, O.; Yang, Y.; Zhang, J.; Yu, J.; Lv, J.; Lv, W.; Du, Y.; Wu, J.; Qi, D. A Comprehensive Review of Ultrahigh Molecular Weight Polyethylene Fibers for Applications Based on Their Different Preparation Techniques. Adv. Polym. Technol. 2023, 2023, 6656692. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Boronat, T.; Balart, R.; Torres-Giner, S. Evaluation of the engineering performance of different bio-based aliphatic homopolyamide tubes prepared by profile extrusion. Polym. Test. 2017, 61, 421–429. [Google Scholar] [CrossRef]
- Lee, J.A.; Ahn, J.H.; Kim, I.; Li, S.; Lee, S.Y. Synthesis, Characterization, and Application of Fully Biobased and Biodegradable Nylon-4,4 and -5,4. ACS Sustain. Chem. Eng. 2020, 8, 5604–5614. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, C.; Yang, S.; He, R.; Guo, Y.; Guo, Z.-X.; Guo, B.; Qiu, T.; Tuo, X. High performance all-para-aramid paper prepared by impregnating heterocyclic aramid into poly(p-phenylene terephthalamide) microfiber/nanofiber-based paper. Compos. Sci. Technol. 2023, 242, 110203. [Google Scholar] [CrossRef]
- Ramani, R.; Kotresh, T.M.; Indu Shekar, R.; Sanal, F.; Singh, U.K.; Renjith, R.; Amarendra, G. Positronium probes free volume to identify para- and meta-aramid fibers and correlation with mechanical strength. Polym. J. 2018, 135, 39–49. [Google Scholar] [CrossRef]
- Sikkema, D.J. Chapter 4: Rigid-chain polymers: Aromatic polyamides, heterocyclic rigid rod polymers, and polyesters. Adv. Ind. Eng. Polym. Res. 2022, 5, 80–89. [Google Scholar] [CrossRef]
- Cosimbescu, L.; Malhotra, D.; Pallaka, M.R.; Swita, M.S. Kevlar-like Aramid Polymers from Mixed PET Waste. ACS Omega 2022, 7, 32026–32037. [Google Scholar] [CrossRef]
- Zhang, Z.; Hong, W.; Wang, X.; Xu, C.; Jiang, Y.; Du, J.; Miao, D.; Xiao, G. Preparation and structure–property relationship of flexible aramid films with enhanced strength by introducing asymmetric and symmetric aromatic ether bond structures. Polym. Chem. 2023, 14, 3906–3915. [Google Scholar] [CrossRef]
- Lee, D.; Jung, A.; Son, J.G.; Yeom, B. Phase-inversion Induced Co-assembly of Poly(ether imide)/Aramid Nanofibrillar Composite Separators for High-speed Lithium-metal Batteries. Energy Storage Mater. 2023, 61, 102902. [Google Scholar] [CrossRef]
- Murthy, N.S. Hydrogen bonding, mobility, and structural transitions in aliphatic polyamides. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 1763–1782. [Google Scholar] [CrossRef]
- Termonia, Y.; Meakin, P.; Smith, P. Theoretical study of the influence of the molecular weight on the maximum tensile strength of polymer fibers. Macromolecules 1985, 18, 2246–2252. [Google Scholar] [CrossRef]
- García, J.M.; García, F.C.; Serna, F.; de la Peña, J.L. High-performance aromatic polyamides. Prog. Polym. Sci. 2010, 35, 623–686. [Google Scholar] [CrossRef]
- Gómez-Valdemoro, A.; Martínez-Máñez, R.; Sancenón, F.; García, F.C.; García, J.M. Functional Aromatic Polyethers: Polymers with Tunable Chromogenic and Fluorogenic Properties. Macromolecules 2010, 43, 7111–7121. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Ahmad, Z.; Sarwar, M.I. Soluble aromatic polyamide bearing ether linkages: Synthesis and characterization. Colloid Polym. Sci. 2007, 285, 1749–1754. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Lin, K.-H. Soluble aromatic polyamides bearing asymmetrical diaryl ether groups. Polym. J. 2004, 45, 7877–7885. [Google Scholar] [CrossRef]
- Mallakpour, S.; Taghavi, M. Direct polyamidation in green media: Studies on thermal degradation of novel organosoluble and optically active flame retardant polyamides. React. Funct. Polym. 2009, 69, 206–215. [Google Scholar] [CrossRef]
- Dickhudt, R.J.; Devineni, G.; Kostal, J.; Boyes, S.G. Synthesis of well-defined aromatic poly(amide-ether)s via chain-growth condensation polymerization. J. Polym. Sci. 2024, 62, 1446–1459. [Google Scholar] [CrossRef]
- Hu, K.; Zhao, D.; Wu, G.; Ma, J. Aromatic poly (ether ester) s derived from a naturally occurring building block nipagin and linear aliphatic α, ω-diols. RSC Adv. 2017, 7, 32989–33000. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Chang, Y.-H. New soluble aromatic polyamides containing ether linkages and laterally attached p-terphenyls. Eur. Polym. J. 2004, 40, 1749–1757. [Google Scholar] [CrossRef]
- Yan, X.; Bai, L.; Feng, B.; Zheng, J. Mechanically strong, thermally stable, and reprocessable poly(dimethylsiloxane) elastomers enabled by dynamic silyl ether linkages. Eur. Polym. J. 2022, 173, 111267. [Google Scholar] [CrossRef]
- Kim, E.-K.; Jung, J.; Cho, K.; Yun, G.J.; Lee, J.-C. Synthesis of polybenzimidazoles having improved processability by introducing two and three ether groups in a repeating unit. Eur. Polym. J. 2022, 162, 110900. [Google Scholar] [CrossRef]
- Espeso, J.F.; De La Campa, J.G.; Lozano, A.E.; De Abajo, J. Synthesis and characterization of new soluble aromatic polyamides based on 4-(1-adamantyl)-1, 3-bis(4-aminophenoxy)benzene. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 1014–1023. [Google Scholar] [CrossRef]
- Yang, C.-P.; Chen, Y.-P.; Woo, E.M. Fluorinated polyamides and poly(amide imide)s based on 1,4-bis(4-amino-2-trifluromethylphenoxy)benzene, aromatic dicarboxylic acids, and various monotrimellitimides and bistrimellitimides: Syntheses and properties. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3116–3129. [Google Scholar] [CrossRef]
- Yang, C.-P.; Cherng, J.-J. Synthesis and properties of aromatic polyamides derived from 1,2-bis(4-aminophenoxy)benzene and aromatic dicarboxylic acids. J. Polym. Sci. Part A Polym. Chem. 1995, 33, 2209–2220. [Google Scholar] [CrossRef]
- Xu, L.-Y.; Wang, W.; Yang, X.; Wang, S.; Shao, Y.; Chen, M.; Sun, R.; Min, J. Real-time monitoring polymerization degree of organic photovoltaic materials toward no batch-to-batch variations in device performance. Nat. Commun. 2024, 15, 1248. [Google Scholar] [CrossRef]
- Yokozawa, T.; Yokoyama, A. Chain-Growth Condensation Polymerization for the Synthesis of Well-Defined Condensation Polymers and π-Conjugated Polymers. Chem. Rev. 2009, 109, 5595–5619. [Google Scholar] [CrossRef]
- Mukherjee, M.; Kumar, S.; Bose, S.; Das, C.; Kharitonov, A. Study on the mechanical, rheological, and morphological properties of short Kevlar™ fiber/s-PS composites. Polym.-Plast. Technol. Eng. 2008, 47, 623–629. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Soria, R.B.; Volodine, A.; Van der Bruggen, B. Aramid nanofiber and modified ZIF-8 constructed porous nanocomposite membrane for organic solvent nanofiltration. J. Membr. Sci. 2020, 603, 118002. [Google Scholar] [CrossRef]
- Salim, M.S.; Ariawan, D.; Ahmad Rasyid, M.F.; Ahmad Thirmizir, M.Z.; Mat Taib, R.; Mohd. Ishak, Z.A. Effect of fibre surface treatment on interfacial and mechanical properties of non-woven kenaf fibre reinforced acrylic based polyester composites. Polym. Compos. 2019, 40, E214–E226. [Google Scholar] [CrossRef]
- Kwon, D.-J.; Kwon, I.-J.; Milam-Guerrero, J.; Yang, S.B.; Yeum, J.H.; Choi, H.H. Aramid nanofiber-reinforced multilayer electromagnetic-interference (EMI) shielding composites with high interfacial durability. Mater. Des. 2022, 215, 110452. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Q.; Fu, L.; Shen, G.; Zhang, W.; Yang, R.; Sun, K.; Lv, F.; Fan, S. Superhydrophobic meta-aramid papers prepared by the surface-embedded spray coating strategy. Appl. Surf. Sci. 2024, 648, 159044. [Google Scholar] [CrossRef]
- Murayama, S.; Kuroda, S.-i.; Osawa, Z. Hydrophobic and hydrophilic interpenetrating polymer networks composed of polystyrene and poly(2-hydroxyethyl methacrylate): 1. PS-PHEMA sequential IPNs synthesized in the presence of a common solvent. Polym. J. 1993, 34, 2845–2852. [Google Scholar] [CrossRef]
- Yang, C.; Wu, H.; Dai, Y.; Tang, S.; Luo, L.; Liu, X. Self-enhancement in aramid fiber by filling free hydrogen bonding interaction sites in macromolecular chains with its oligomer. Polym. J. 2019, 180, 121687. [Google Scholar] [CrossRef]
- Trigo-López, M.; Vallejos, S.; Reglero Ruiz, J.A.; García-Gómez, A.; Seara-Martínez, M.; García, F.C.; García, J.M. High-performance nanoporous aramid films reinforced with functionalized carbon nanocharges using ionic liquids. Polym. J. 2020, 202, 122629. [Google Scholar] [CrossRef]
- de Ruijter, C.; Mendes, E.; Boerstoel, H.; Picken, S.J. Orientational order and mechanical properties of poly(amide-block-aramid) alternating block copolymer films and fibers. Polym. J. 2006, 47, 8517–8526. [Google Scholar] [CrossRef]
- E, S.; Ma, Q.; Huang, J.; Jin, Z.; Lu, Z. Enhancing mechanical strength and toughness of aramid nanofibers by synergetic interactions of covalent and hydrogen bonding. Compos. Part A 2020, 137, 106031. [Google Scholar] [CrossRef]
- E, S.; Ma, Q.; Ning, D.; Huang, J.; Jin, Z.; Lu, Z. Bio-inspired covalent crosslink of aramid nanofibers film for improved mechanical performances. Compos. Sci. Technol. 2021, 201, 108514. [Google Scholar] [CrossRef]
- Kim, H.; Noh, J.-H.; Kim, Y.-R.; Kim, H.; Kwak, G. Preparation and Properties of Mechanically Robust, Colorless, and Transparent Aramid Films. Polymers 2024, 16, 575. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Geng, B.; Ma, Q.; Ning, D.; Zhao, R.; Kong, F.; E, S. Polymer induced strengthening and toughening of aramid nanofiber film: The importance of densification and hydrogen bonding. Appl. Surf. Sci. 2023, 607, 155045. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, M.; Yang, B.; Liu, G.; Song, S. Fabrication and characterization of differentiated aramid nanofibers and transparent films. Appl. Nanosci. 2019, 9, 631–645. [Google Scholar] [CrossRef]
- Yang, B.; Wang, L.; Zhang, M.; Luo, J.; Ding, X. Timesaving, High-Efficiency Approaches To Fabricate Aramid Nanofibers. ACS Nano 2019, 13, 7886–7897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hong, W.; Wang, X.; Wang, J.; Xu, C.; Xiao, G.; Shao, D.; Du, J. Meta-aramid film with both flexibility and strength: Combined effect of aromatic ether bond and micro-branched structure. Polym. J. 2024, 299, 126971. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, X.; Zhang, W.; Zhang, M.; Zhang, X.; Zhao, N.; Liu, R.; Xu, J.; Shen, Z.; Fan, X. Facile preparation and characterization of soluble aramid. J. Appl. Polym. Sci. 2018, 135, 4634159. [Google Scholar] [CrossRef]

| Sample | Mn (g/mol) | Mw (g/mol) | Viscosity (Pa·s) | ||
|---|---|---|---|---|---|
| 30 °C | 50 °C | 80 °C | |||
| MBAB-aramid | 190,000 | 495,000 | 193.9 | 91.0 | 35.2 |
| PBAB-aramid | 149,000 | 432,000 | 194.3 | 98.7 | 42.7 |
| Properties | MBAB-Aramid | PBAB-Aramid | |
|---|---|---|---|
| Surface energy (mJ/m2) | Polar | 39.0 | 34.1 |
| Dispersive | 9.3 | 14.3 | |
| Total | 48.2 | 48.4 | |
| Td5 (°C) | 451.3 | 459.3 | |
| Tonset (°C) | 449.6 | 495.5 | |
| Tg (°C) | 270.1 | 292.7 | |
| Tensile strength (MPa) | 107.1 ± 21.2 (130.8) | 113.5 ± 23.1 (138.2) | |
| Elongation at break (%) | 50.7 ± 4.2 (54.9) | 58.4 ± 14.8 (61.3) | |
| Modulus (GPa) | 3.99 ± 0.60 (4.39) | 2.95 ± 0.98 (3.07) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, W.; Jadhav, A.M.; Ryu, Y.; Kim, S.; Im, J.; Jeong, Y.; Vanessa; Kim, Y.; Sung, Y.; Kim, Y.; et al. Novel Bis(4-aminophenoxy) Benzene-Based Aramid Copolymers with Enhanced Solution Processability. Nanomaterials 2024, 14, 1632. https://doi.org/10.3390/nano14201632
Song W, Jadhav AM, Ryu Y, Kim S, Im J, Jeong Y, Vanessa, Kim Y, Sung Y, Kim Y, et al. Novel Bis(4-aminophenoxy) Benzene-Based Aramid Copolymers with Enhanced Solution Processability. Nanomaterials. 2024; 14(20):1632. https://doi.org/10.3390/nano14201632
Chicago/Turabian StyleSong, Wonseong, Amol M. Jadhav, Yeonhae Ryu, Soojin Kim, Jaemin Im, Yujeong Jeong, Vanessa, Youngjin Kim, Yerin Sung, Yuri Kim, and et al. 2024. "Novel Bis(4-aminophenoxy) Benzene-Based Aramid Copolymers with Enhanced Solution Processability" Nanomaterials 14, no. 20: 1632. https://doi.org/10.3390/nano14201632
APA StyleSong, W., Jadhav, A. M., Ryu, Y., Kim, S., Im, J., Jeong, Y., Vanessa, Kim, Y., Sung, Y., Kim, Y., & Choi, H. H. (2024). Novel Bis(4-aminophenoxy) Benzene-Based Aramid Copolymers with Enhanced Solution Processability. Nanomaterials, 14(20), 1632. https://doi.org/10.3390/nano14201632







