Exploring the Potential of Bimetallic PtPd/C Cathode Catalysts to Enhance the Performance of PEM Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Study of the Catalyst’s Composition and Structure
2.3. Electrochemical Methods of This Study
2.4. Testing of the Catalysts in Membrane Electrode Assemblies
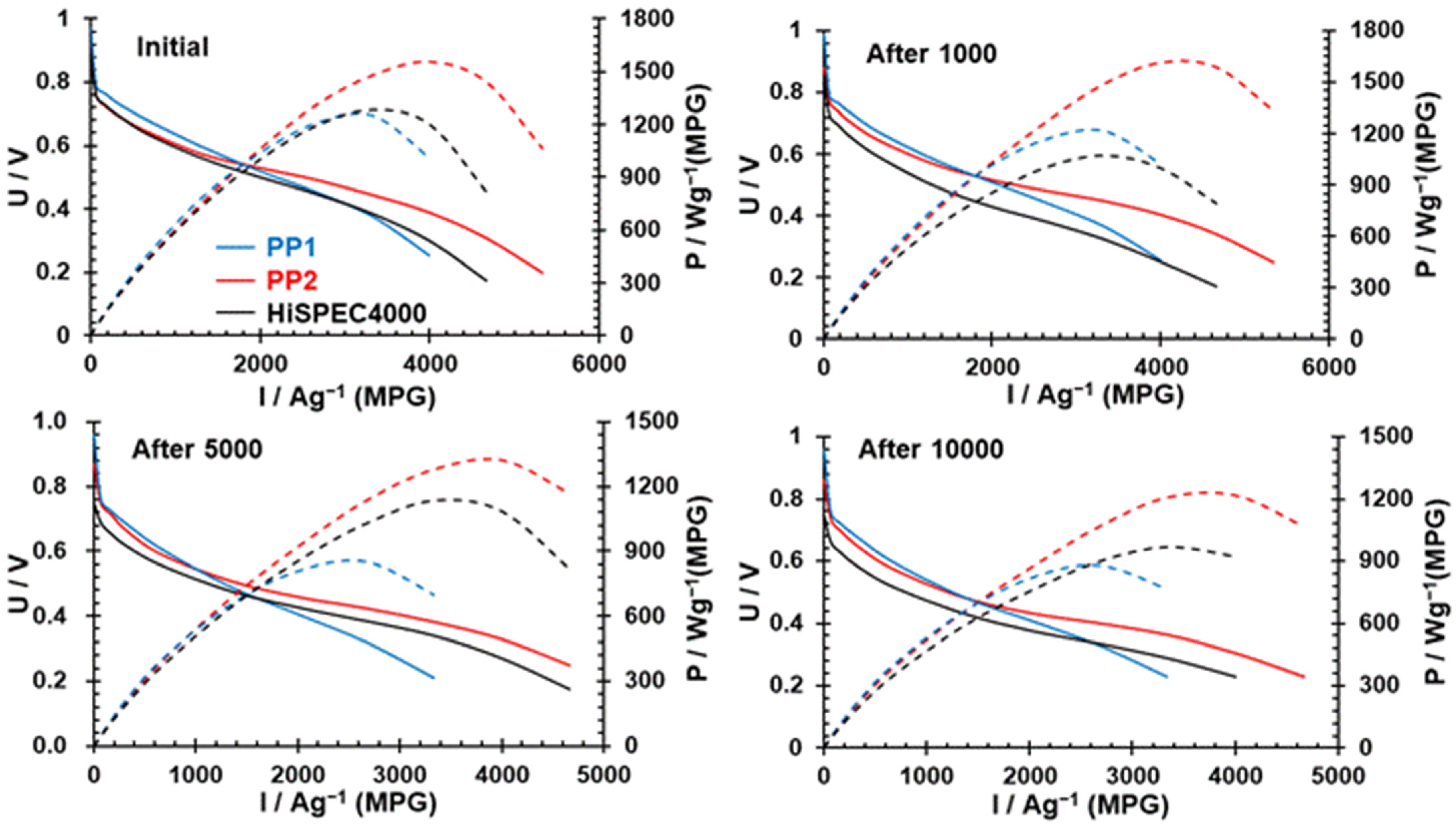
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoogers, G. (Ed.) Fuel Cell Technology Handbook; CRC Press LLC: Boca Raton, FL, USA, 2003; pp. 1–286. [Google Scholar]
- Staffell, I.; Scamman, D.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shahd, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Lipman, T.E.; Weber, A.Z. Fuel Cells and Hydrogen Production, a Volume in the Encyclopedia of Sustainability Science and Technology; Springer: New York, NY, USA, 2019. [Google Scholar] [CrossRef]
- Gröger, O.; Gasteiger, H.A.; Suchsland, J.P. Review—Electromobility: Batteries or Fuel Cells? J. Electrochem. Soc. 2015, 162, A2605–A2622. [Google Scholar] [CrossRef]
- Weitering, H. Aviation International News. Available online: https://www.ainonline.com/aviation-news/futureflight/2024-07-22/zeroavias-hydrogen-powertrain-attracts-new-applications (accessed on 25 September 2024).
- Terlouw, T.; Bauer, C.; McKenna, R.; Mazzotti, M. Large-Scale Hydrogen Production via Water Electrolysis: A Techno-Economic and Environmental Assessment. Energy Environ. Sci. 2022, 15, 3583–3602. [Google Scholar] [CrossRef]
- Ahmed, K.W.; Jang, M.J.; Park, M.G.; Chen, Z.; Fowler, M. Effect of Components and Operating Conditions on the Performance of PEM Electrolyzers: A Review. Electrochem 2022, 3, 581–612. [Google Scholar] [CrossRef]
- Khan, M.A.; Zhao, H.; Zou, W.; Chen, Z.; Cao, W.; Fang, J.; Xu, J.; Zhang, L.; Zhang, J. Recent Progresses in Electrocatalysts for Water Electrolysis. Electrochem. Energy R 2018, 1, 483–530. [Google Scholar] [CrossRef]
- Zhang, K.; Liang, X.; Wang, L.; Sun, K.; Wang, Y.; Xie, Z.; Wu, Q.; Bai, X.; Hamdy, M.S.; Chen, H.; et al. Status and Perspectives of Key Materials for PEM Electrolyzer. Nano Res. Energy 2022, 1, e9120032. [Google Scholar] [CrossRef]
- Böhm, H.; Goers, S.; Zauner, A. Estimating Future Costs of Power-to-Gas—A Component-Based Approach for Technological Learning. Int. J. Hydrog. Energy 2019, 44, 30789–30805. [Google Scholar] [CrossRef]
- Holdcroft, S. Fuel Cell Catalyst Layers: A Polymer Science Perspective. Chem. Mater. 2013, 26, 381–393. [Google Scholar] [CrossRef]
- Thompson, S.T.; Papageorgopoulos, D. Platinum group metal-free catalysts boost cost competitiveness of fuel cell vehicles. Nat. Catalysis 2019, 2, 558–561. [Google Scholar] [CrossRef]
- Adria, W.; Gregory, K.; Dimitrios, P. Department of Energy (DOE) Hydrogen and Fuel Cell Program Record 17007. 2017. Available online: https://www.hydrogen.energy.gov/pdfs/17007_fuel_cell_system_cost_2017.pdf (accessed on 25 September 2024).
- Borup, R.L.; Kusoglu, A.; Neyerlin, K.C.; Mukundan, R.; Ahluwalia, R.K.; Cullen, D.A.; More, K.L.; Weber, A.Z.; Myers, D.J. Recent developments in catalyst-related PEM fuel cell durability. Curr. Opin. Electrochem. 2020, 21, 192–200. [Google Scholar] [CrossRef]
- Sui, S.; Wang, X.; Zhou, X.; Su, Y.; Riffat, S.; Liu, C. A comprehensive review of Pt electrocatalysts for the oxygen reduction reaction: Nanostructure, activity, mechanism and carbon support in PEM fuel cells. J. Mater. Chem. A. 2017, 5, 1808–1825. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, X.; Pan, Y.; Peng, Z. A review of Pt-based electrocatalysts for oxygen reduction reaction. Front. Energy 2017, 11, 268–285. [Google Scholar] [CrossRef]
- Asanova, T.; Asanov, I.; Tur, V.; Gerasimov, E.; Brzhezinskaya, M. PtPd-nanoparticles supported by new carbon materials. J. Struct. Chem. 2016, 57, 1398–1406. [Google Scholar] [CrossRef]
- Appleby, A.J.; Dudley, R.; O’Grady, W.; Srinivasan, S. The Electrocatalysis of Fuel Cell Reactions. In The Electrochemical Society Softbound Proceedings Series; PV 79-2; The Electrochemical Society: Princeton, NJ, USA, 1979; p. 23. [Google Scholar]
- Nesselberger, M.; Ashton, S.; Meier, J.C.; Katsounaros, I.; Mayrhofer, K.J.J.; Arenz, M. The Particle Size Effect on the Oxygen Reduction Reaction Activity of Pt Catalysts: Influence of Electrolyte and Relation to Single Crystal Models. J. Am. Chem. Soc. 2011, 133, 17428–17433. [Google Scholar] [CrossRef]
- Tritsaris, G.A.; Greeley, J.; Rossmeisl, J.; Nørskov, J.K. Atomic-Scale Modeling of Particle Size Effects for the Oxygen Reduction Reaction on Pt. Catal. Letters 2011, 141, 909–913. [Google Scholar] [CrossRef]
- Suo, N.; Cao, L.; Qin, X.; Shao, Z. Research Progress of Pt and Pt-Based Cathode Electrocatalysts for Proton-Exchange Membrane Fuel Cells. Chin. Phys. B 2022, 31, 128108. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef]
- Mo, S.; Du, L.; Huang, Z.; Chen, J.; Zhou, Y.; Wu, P.; Meng, L.; Wang, N.; Xing, L.; Zhao, M.; et al. Recent Advances on PEM Fuel Cells: From Key Materials to Membrane Electrode Assembly. Electrochem. Energy Rev. 2023, 6. [Google Scholar] [CrossRef]
- Sasaki, K.; Kuttiyiel, K.A.; Adzic, R.R. Designing high performance Pt monolayer core-shell electrocatalysts for fuel cells. Curr. Opin. Electrochem. 2020, 22, 9–16. [Google Scholar] [CrossRef]
- Suter, T.A.M.; Smith, K.; Hack, J.; Rasha, L.; Rana, Z.; Angel, G.M.A.; Shearing, R.; Miller, T.S.; Brett, D.J.L. Engineering Catalyst Layers for Next-Generation Polymer Electrolyte Fuel Cells: A Review of Design. Adv. Energy Mat. 2021, 11, 2101025. [Google Scholar] [CrossRef]
- Mukerjee, S. Particle size and structural effects in platinum electroCatalysis. J. Appl. Electrochem. 1990, 20, 537–548. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B 2005, 56, 9–35. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, H.; Zhong, H.; Lu, Q.; Wang, Y.; Su, D. Effect of particle size on the activity and durability of the Pt/C electrocatalyst for proton exchange membrane fuel cells. Appl. Catal. B Environ. 2012, 111–112, 264–270. [Google Scholar] [CrossRef]
- Rossi, K.; Asara, G.G.; Baletto, F. Structural Screening and Design of Platinum Nanosamples for Oxygen Reduction. ACS Catal. 2020, 10, 3911–3920. [Google Scholar] [CrossRef]
- Guterman, V.E.; Belenov, S.V.; Alekseenko, A.A.; Lin, R.; Tabachkova, N.Y.; Safronenko, O.I. Activity and Stability of Pt/C and Pt-Cu/C Electrocatalysts. Electrocatalysis 2018, 9, 550–562. [Google Scholar] [CrossRef]
- Li, H.; Zhao, H.; Tao, B.; Xu, G.; Gu, S.; Wang, G.; Chang, H. Pt-Based Oxygen Reduction Reaction Catalysts in Proton Exchange Membrane Fuel Cells: Controllable Preparation and Structural Design of Catalytic Layer. Nanomaterials 2022, 12, 4173. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Yang, J.; Lei, Y.; Wang, C.; Wang, J.; Tanga, Y.; Mao, Z. Recent advances in Pt-based electrocatalysts for PEMFCs. RSC Adv. 2021, 11, 13316–13328. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Qu, Y.; Yuan, T.; Wang, W.; Wu, Y.; Li, Y. Review of Metal Catalysts for Oxygen Reduction Reaction: From Nanoscale Engineering to Atomic Design. Chem 2019, 5, 1486–1511. [Google Scholar] [CrossRef]
- Moriau, L.J.; Hrnjić, A.; Pavlišič, A.; Kamšek, A.R.; Petek, U.; Ruiz-Zepeda, F.; Šala, M.; Pavko, L.; Šelih, V.S.; Bele, M.; et al. Resolving the nanoparticles’ structure-property relationships at the atomic level: A study of Pt-based electrocatalysts. Iscience 2021, 24, 102102. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, P.; Markovic, N.M.; Stamenkovic, V.R. Shaping electrocatalysis through tailored nanomaterials. Nano Today 2016, 11, 587–600. [Google Scholar] [CrossRef]
- Antolini, E. Formation of carbon-supported PtM alloys for low temperature fuel cells: A Review. Mater. Chem. Phys. 2003, 78, 563–573. [Google Scholar] [CrossRef]
- Cho, K.Y.; Yeom, Y.S.; Seo, H.Y.; Lee, A.S.; Do, X.H.; Hong, J.P.; Jeong, H.K.; Baek, K.Y.; Yoon, H.G. Fine-sized Pt nanoparticles dispersed on PdPt bimetallic nanocrystals with non-covalently functionalized graphene toward synergistic effects on the oxygen reduction reaction. Electrochim. Acta 2017, 257, 412–422. [Google Scholar] [CrossRef]
- Kim, C.; Dionigi, F.; Beermann, V.; Wang, X.; Möller, T.; Strasser, P. Alloy Nanocatalysts for the Electrochemical Oxygen Reduction (ORR) and the Direct Electrochemical Carbon Dioxide Reduction Reaction (CO2RR). Adv. Mater. 2019, 31, 1805617–1805636. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, Y.; Wajrak, M.; Bhatelia, T.; Jiang, S.P.; Shao, Z. Grain Boundary Engineering: An Emerging Pathway toward Efficient ElectroCatalysis. InfoMat 2024, 6, e12608. [Google Scholar] [CrossRef]
- Oezaslan, M.; Heggen, M.; Strasser, P. Size-Dependent Morphology of Dealloyed Bimetallic Catalysts: Linking the Nano to the Macro Scale. J. Am. Chem. Soc. 2011, 134, 514–524. [Google Scholar] [CrossRef]
- Alekseenko, A.A.; Pavlets, A.S.; Belenov, S.V.; Pankov, I.V.; Guterman, V.E. The electrochemical activation mode as a way to exceptional ORR performance of nanostructured PtCu/C materials. Appl. Surf. Sci. 2022, 595, 153533–153543. [Google Scholar] [CrossRef]
- Pavlets, A.S.; Alekseenko, A.A.; Nikolskiy, A.V.; Kozakov, A.T.; Safronenko, O.I.; Pankov, I.V.; Guterman, V.E. Effect of the PtCu/C electrocatalysts initial composition on their activity in the de-alloyed state in the oxygen reduction reaction. Int. J. Hydrog. Energy 2022, 47, 30460–30471. [Google Scholar] [CrossRef]
- Ahn, C.Y.; Park, J.E.; Kim, S.; Kim, O.H.; Hwang, W.; Her, M.; Kang, S.Y.; Park, S.B.; Kwon, O.J.; Park, H.S.; et al. Differences in the Electrochemical Performance of Pt-Based Catalysts Used for Polymer Electrolyte Membrane Fuel Cells in Liquid Half- and Full-Cells, Review. Chem. Rev. 2021, 121, 15075–15140. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Pizzutilo, E.; Geiger, S.; Freakley, S.J.; Mingers, A.; Cherevko, S.; Hutchings, G.J.; Mayrhofer, K.J.J. Palladium electrodissolution from model surfaces and nanoparticles. Electrochim. Acta 2017, 229, 467–477. [Google Scholar] [CrossRef]
- Smiljanić, M.; Bele, M.; Moriau, L.; Ruiz-Zepeda, F.; Šala, M.; Hodnik, N. Electrochemical Stability and Degradation of Commercial Pd/C Catalyst in Acidic Media. J. Phys. Chem. C 2021, 125, 27534–27542. [Google Scholar] [CrossRef]
- Xiao, W.; Cordeiro, M.A.L.; Gong, M.; Han, L.; Wang, J.; Bian, C.; Zhu, J.; Xin, H.L.; Wang, D. Optimizing the ORR activity of Pd based nanocatalysts by tuning their strain and particle size. J. Mater. Chem. A 2017, 5, 9867–9872. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data; CRC Press: New York, NY, USA, 2014–2015; pp. 1–2666. [Google Scholar]
- Duan, H.; Xu, C. Nanoporous PtPd Alloy Electrocatalysts with High Activity and Stability toward Oxygen Reduction Reaction. Electrochim. Acta 2015, 152, 417–424. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Eid, K.; Alshehri, S.M.; Malgras, V.; Yamauchi, Y.; Wang, L. One-Step Synthesis of Dendritic Bimetallic PtPd Nanoparticles on Reduced Graphene Oxide and Its Electrocatalytic Properties. Electrochim. Acta 2016, 188, 845–851. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, Y.; Liu, C.; Xu, G.; Chen, Y.; Tang, Y.; Lu, T. Pd@Pt core–shell tetrapods as highly active and stable electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 20855–20860. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Lin, X.X.; Zhang, X.F.; Wang, A.J.; Zhu, X.Y.; Feng, J.J. Alloys and Compounds Bimetallic PtPd alloyed core-shell nanodendrites supported on reduced graphene oxide: One-pot green synthesis and efficient electrocatalytic performances for glycerol oxidation and hydrogen evolution. J. Alloys Compd. 2018, 735, 2123–2132. [Google Scholar] [CrossRef]
- Liu, J.; Yin, J.; Feng, B.; Li, F.; Wang, F. One-pot synthesis of unprotected PtPd nanoclusters with enhanced catalytic activity, durability, and methanol-tolerance for oxygen reduction reaction. Appl. Surf. Sci. 2019, 473, 318–325. [Google Scholar] [CrossRef]
- Xiao, Y.Y.; Ying, J.; Tian, G.; Tao, Y.; Wei, H.; Fan, S.Y.; Sun, Z.H.; Zou, W.J.; Hu, J.; Chang, G.G.; et al. Highly dispersed PtPd on graphitic nanofibers and its heavy d-π effect. Appl. Catal. B Environ. 2019, 259, 118080. [Google Scholar] [CrossRef]
- Wu, Z.P.; Caracciolo, D.T.; Maswadeh, Y.; Wen, J.; Kong, Z.; Shan, S.; Vargas, J.A.; Yan, S.; Hopkins, E.; Park, K.; et al. Alloying–realloying enabled high durability for Pt–Pd-3d-transition metal nanoparticle fuel cell catalysts. Nat. Commun. 2021, 12, 859–873. [Google Scholar] [CrossRef]
- Brouzgou, A.; Seretis, A.; Song, S.; Shen, P.K.; Tsiakaras, P. CO tolerance and durability study of PtMe(Me = Ir or Pd) electrocatalysts for H2-PEMFC application. Int. J. Hydrog. Energy 2021, 46, 13865–13877. [Google Scholar] [CrossRef]
- Available online: http://premetek.com/product-category/electrocatalysts-for-fuel-cells-sensors-electrolyzers-etc/pd-alloys-with-ni-ir-rh-au-and-pt/ (accessed on 25 September 2024).
- Available online: https://www.thermofisher.com/order/catalog/product/422980010?SID=srch-srp-422980010 (accessed on 25 September 2024).
- Available online: https://www.prometheusrd.com/?lang=en (accessed on 25 September 2024).
- Nagai, T.; Jahn, C.; Jia, H. Improved Accelerated Stress Tests for ORR Catalysts Using a Rotating Disk Electrode. J. Electrochem. Soc. 2019, 166, F3111–F3115. [Google Scholar] [CrossRef]
- Xu, R.; Kang, L.; Papanikolaou, K.G.; Wang, B.; Marlow, S.; He, Q.; Zhang, P.; Wang, J.; Brett, D.J.L.; Stamatakis, M.; et al. Improving the ORR performance by enhancing the Pt oxidation resistance. J. Catal. 2022, 416, 311–321. [Google Scholar] [CrossRef]
- Wang, R.; Wang, H.; Luo, F.; Liao, S. Core–Shell-Structured Low-Platinum Electrocatalysts for Fuel Cell Applications. Electrochem. Energ. Rev. 2018, 1, 324–387. [Google Scholar] [CrossRef]
- Luo, M.; Wei, L.; Wang, F.; Han, K.; Zhu, H. Gram-level synthesis of core–shell structured catalysts for the oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 2014, 270, 34–41. [Google Scholar] [CrossRef]
- Pryadchenko, V.V.; Belenov, S.V.; Shemet, D.B.; Srabionyan, V.V.; Avakyan, L.A.; Volochaev, V.A.; Mikheykin, A.S.; Bdoyan, K.E.; Zizak, I.; Guterman, V.E.; et al. Effect of Thermal Treatment on the Atomic Structure and Electrochemical Characteristics of Bimetallic PtCu Core–Shell Nanoparticles in PtCu/C Electrocatalysts. J. Phys. Chem. C 2018, 122, 17199–17210. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, X.Z.; Martin, J.J.; Wang, H.; Zhang, J.; Shen, J.; Wu, S.; Merida, W. A review of PEM fuel cell durability: Degradation mechanisms and mitigation strategies. J. Power Sources 2008, 184, 104–119. [Google Scholar] [CrossRef]
- Wallnöfer-Ogris, E.; Poimer, F.; Köll, R.; Macherhammer, M.G.; Trattner, A. Main degradation mechanisms of polymer electrolyte membrane fuel cell stacks – Mechanisms, influencing factors, consequences, and mitigation strategies. Int. J. Hydrog. Energy 2024, 50, 1159–1182. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X. A review of polymer electrolyte membrane fuel cell durability for vehicular applications: Degradation modes and experimental techniques. Energy Convers. Manag. 2019, 199, 112022–112044. [Google Scholar] [CrossRef]
- Paperzh, K.O.; Alekseenko, A.A.; Volochaev, V.A.; Pankov, I.V.; Safronenko, O.A.; Guterman, V.E. Stability and activity of platinum nanoparticles in the oxygen electroreduction reaction: Is size or uniformity of primary importance? Beilstein J. Nanotechnol. 2021, 12, 593–606. [Google Scholar] [CrossRef]
- Leontyev, I.N.; Leontyeva, D.V.; Kuriganova, A.B.; Popov, Y.V.; Maslova, O.A.; Glebova, N.V.; Nechitailov, A.A.; Zelenina, N.K.; Tomasov, A.A.; Hennet, L.; et al. Characterization of the electrocatalytic activity of carbon-supported platinum-based catalysts by thermal gravimetric analysis. Mendeleev Commun. 2015, 25, 468–469. [Google Scholar] [CrossRef]
- Belenov, S.; Pavlets, A.; Paperzh, K.; Mauer, D.; Menshikov, V.; Alekseenko, A.; Pankov, I.; Tolstunov, M.; Guterman, V. The PtM/C (M = Co, Ni, Cu, Ru) Electrocatalysts: Their Synthesis, Structure, Activity in the Oxygen Reduction and Methanol Oxidation Reactions, and Durability. Catalysts 2023, 13, 243. [Google Scholar] [CrossRef]
- He, W.; Liu, J.; Qiao, Y.; Zou, Z.; Zhang, X.; Akins, D.L.; Yang, H. Simple preparation of Pd–Pt nanoalloy catalysts for methanol-tolerant oxygen reduction. J. Power Sources 2010, 195, 1046–1050. [Google Scholar] [CrossRef]
- Zheng, J.N.; He, L.L.; Chen, F.Y.; Wang, A.J.; Xue, M.W.; Feng, J.J. Simple one-pot synthesis of platinum-palladium nanoflowers with enhanced catalytic activity and methanol-tolerance for oxygen reduction in acid media. Electrochim. Acta 2014, 137, 431–438. [Google Scholar] [CrossRef]
- He, W.; Chen, M.; Zou, Z.; Li, Z.; Zhang, X.; Jin, S.A.; You, D.J.; Pak, C.; Yang, H. Oxygen reduction on Pd3Pt1 bimetallic nanoparticles highly loaded on different carbon supports. Appl. Catal. B Environ. 2010, 97, 347–353. [Google Scholar] [CrossRef]
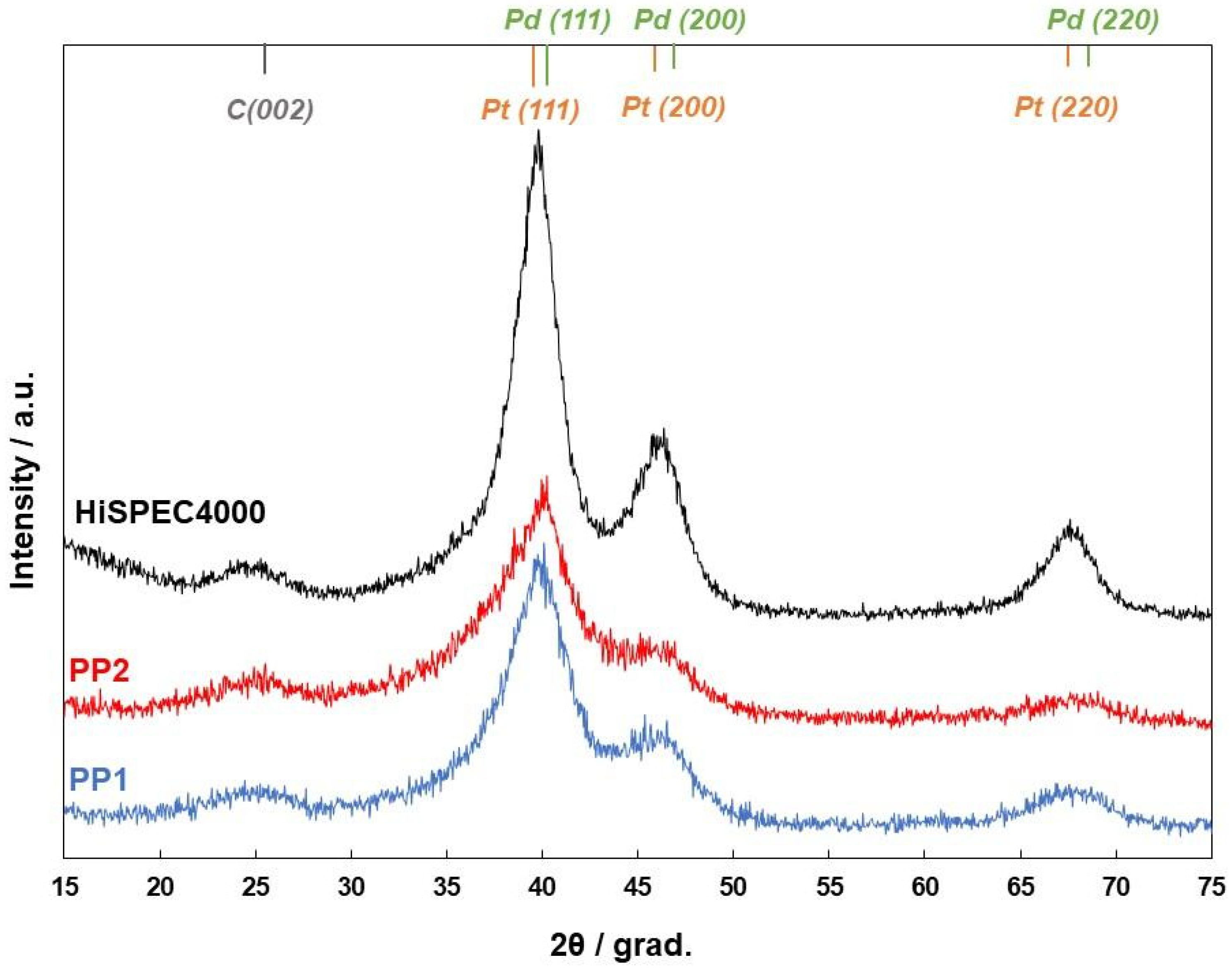
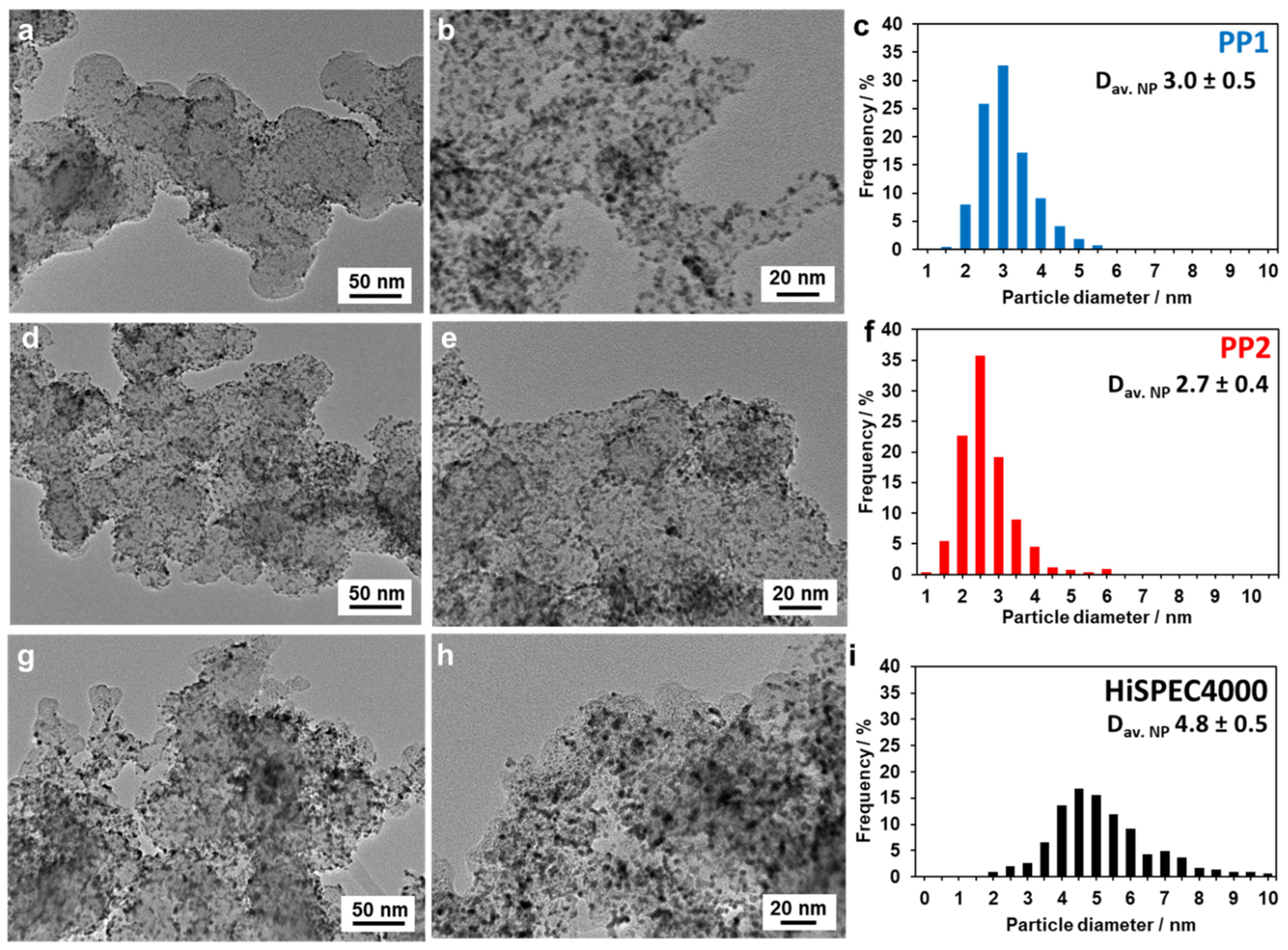

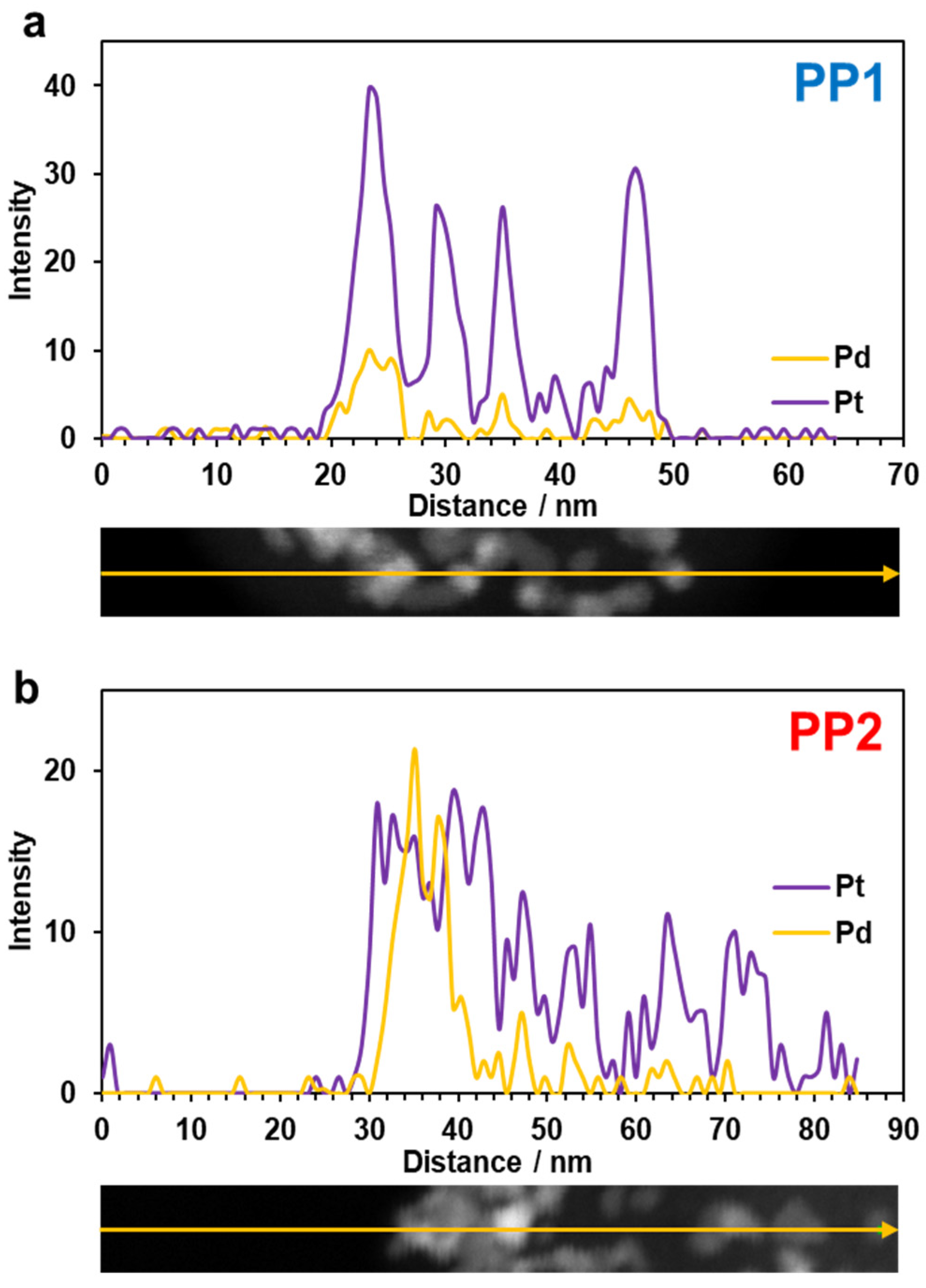



| Sample | Metals Loading, % wt. | Composition | DAv (cryst.), nm | DAv (NPs), nm | ECSA, m2/g (PGM) | MA, A/g (Pt) | SA, A/m2 | E1/2 at 1600 rpm, V | |
|---|---|---|---|---|---|---|---|---|---|
| TXRF | ICP AS | ||||||||
| PP1 | 37.5 | Pt78Pd22 | Pt73Pd27 | 2.3 | 3.1 | 85 | 322 | 3.8 | 0.90 |
| PP2 | 38.2 | Pt75Pd25 | Pt74Pd26 | 2.4 | 2.6 | 90 | 407 | 5.0 | 0.91 |
| HiSpec4000 | 40.0 | Pt | Pt | 3.8 | 4.8 | 60 | 243 | 4.0 | 0.90 |
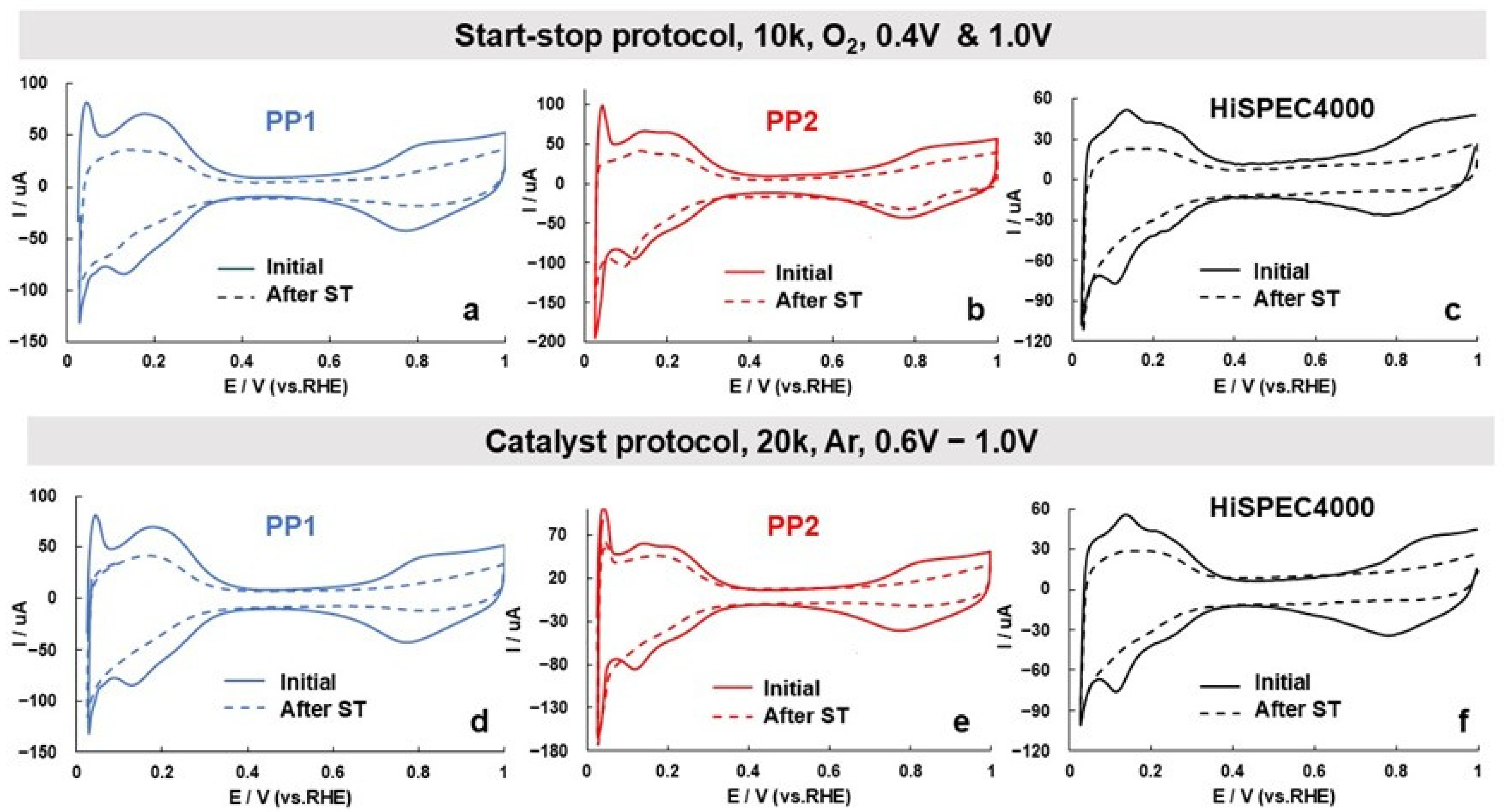
| Sample | Before ST | Arter ST | Degree of Degradation (ECSA), % | Degree of Degradation (MA), % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ECSA, m2/g (PGM) | Surface Activity, A/m2 | Mass Activity, A/g (PGM) | E1/2, V | ECSA, m2/g (PGM) | Surface Activity, A/m2 | Mass Activity, A/g (PGM) | E1/2, V | |||
| Start–stop protocol (O2, 10 k cycles, 0.4 & 1.0 V) | ||||||||||
| PP1 | 87 | 3.5 | 304 | 0.90 | 50 | 4.2 | 212 | 0.90 | 43 | 30 |
| PP2 | 91 | 4.4 | 403 | 0.91 | 59 | 5.0 | 295 | 0.90 | 35 | 27 |
| HiSPEC | 66 | 3.4 | 226 | 0.90 | 34 | 4.0 | 135 | 0.89 | 47 | 43 |
| Catalyst protocol (Ar, 20 k cycles, 0.6–1.0 V) | ||||||||||
| PP1 | 85 | 3.6 | 304 | 0.90 | 52 | 4.4 | 228 | 0.90 | 39 | 25 |
| PP2 | 85 | 4.9 | 420 | 0.90 | 57 | 5.4 | 307 | 0.90 | 33 | 27 |
| HiSPEC | 66 | 3.2 | 212 | 0.90 | 44 | 3.0 | 133 | 0.88 | 33 | 37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guterman, V.; Alekseenko, A.; Belenov, S.; Menshikov, V.; Moguchikh, E.; Novomlinskaya, I.; Paperzh, K.; Pankov, I. Exploring the Potential of Bimetallic PtPd/C Cathode Catalysts to Enhance the Performance of PEM Fuel Cells. Nanomaterials 2024, 14, 1672. https://doi.org/10.3390/nano14201672
Guterman V, Alekseenko A, Belenov S, Menshikov V, Moguchikh E, Novomlinskaya I, Paperzh K, Pankov I. Exploring the Potential of Bimetallic PtPd/C Cathode Catalysts to Enhance the Performance of PEM Fuel Cells. Nanomaterials. 2024; 14(20):1672. https://doi.org/10.3390/nano14201672
Chicago/Turabian StyleGuterman, Vladimir, Anastasia Alekseenko, Sergey Belenov, Vladislav Menshikov, Elizaveta Moguchikh, Irina Novomlinskaya, Kirill Paperzh, and Ilya Pankov. 2024. "Exploring the Potential of Bimetallic PtPd/C Cathode Catalysts to Enhance the Performance of PEM Fuel Cells" Nanomaterials 14, no. 20: 1672. https://doi.org/10.3390/nano14201672
APA StyleGuterman, V., Alekseenko, A., Belenov, S., Menshikov, V., Moguchikh, E., Novomlinskaya, I., Paperzh, K., & Pankov, I. (2024). Exploring the Potential of Bimetallic PtPd/C Cathode Catalysts to Enhance the Performance of PEM Fuel Cells. Nanomaterials, 14(20), 1672. https://doi.org/10.3390/nano14201672









